Abstract
Background/Aims
There has been debate on whether a sodium-restricted diet (SRD) should be used in cirrhotic patients with ascites in China in recent years. The purpose of this study was to compare the effect of sodium-restricted and unrestricted diets on plasma renin activity (PRA), renal blood flow (RBF) and ascites in patients with liver cirrhosis.
Methods
Two hundred cirrhotic patients with ascites were randomly divided into two groups (98 cases in the sodium-unrestricted diet [SUD] group and 102 cases in the SRD group); 95 patients (96.94%) in the SUD group and 97 patients (95.1%) in the SRD group had post-hepatitis B cirrhosis.
Results
Blood sodium and RBF were higher in SUD group than in SRD group (p<0.001), while PRA were significantly lower in SUD group than the SRD group 10 days after treatment (p<0.001). Renal impairment caused by low blood sodium was higher in SRD group than in SUD group (p<0.01). Ascites disappeared in higher proportion of patients in SUD group than in SRD group (p<0.001).
Conclusions
SUD can increase the level of blood sodium and RBF, and be beneficial to diuresis and ascite reduction and disappearance.
Keywords: Liver cirrhosis, Ascites, Sodium-unrestricted diet, Albumin, Renal circulation
INTRODUCTION
Diagnostic guide1 for cirrhotic ascites made by the International Ascites Association and treatment guide2 made by American Association for the Study of Liver Diseases have described that sodium-restricted diet (SRD) should be used in the treatment of cirrhotic ascites in adults. However, there has been debate on whether SRD should be used in cirrhotic patients with ascites in China in recent years. Those who are in favor of sodium restriction hold that retention of sodium and water is an important link and one of the causes of ascites formation of cirrhotic patients. Increase of 1 g sodium intake would at least increase 200 mL water retention, resulting in generation or increase of ascites.3 On the contrary, studies found that patients with cirrhotic ascites had lower blood sodium level, while using diuretics, sodium restriction would result in further decrease of blood sodium volume. Which make diuretic effect weak, it is not beneficial to ascites disappearance. In addition, low blood sodium may induce renal impairment, making ascites subsiding even more difficult. So while diuretics are used to treat cirrhotic patients with ascites, sodium should not be restricted.4-7 In this study, 200 cirrhotic patients with ascites were randomly divided into two groups, the group of sodium-unrestricted diet (SUD) group and the group of SRD. Blood and urine sodium, plasma renin activity (PRA), angiotensin II (A II), aldosterone (ALD), renal blood flow (RBF), renal impairment, diuretic effect, serum albumin (ALB) and the volume of ascites were compared between SUD group and SRD group to explore whether sodium should be restricted in cirrhotic patients with ascites.
MATERIALS AND METHODS
1. Patients
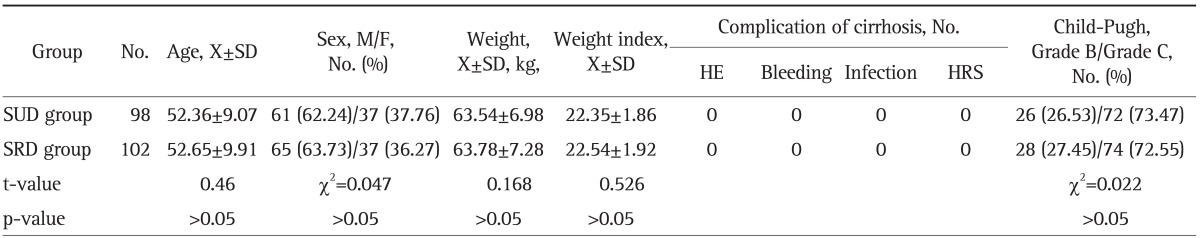
A total of 271 cirrhotic patients with ascites diagnosed based on clinical manifestation, laboratory examination and B-type ultrasound were inpatients of our hospital admitted between January 2007 and May 2010. All patients were from Wuxi, Jiangsu Province, China, Inclusion criteria consisted of cirrhotic patients with ascites caused by various causes, increased or normal alanine transaminase and total bilirubin. Exclusion criteria included hepatic failure, combination with hepatic encephalopathy, upper digestive tract bleeding, spontaneous bacterial peritonitis, hepatorenal syndrome, renal disease, hepatic cancer, shock, and heart and lung insufficiency. Two hundred and 10 cases conform to the inclusive standard, of which 10 cases refused to take part in the trial and 200 cases took part in the trial. Each patient signed informed consent to participate in the trial. The doctors in charge of this trial were familiar with the principles of randomized controlled trial. Random numeral table was used to assign patients randomly. When cirrhotic patients with ascites conform to the inclusion criteria (determined by the investigators), the investigators informed the statistician who controls the random numeral table, the statistician would assign the patients according to the table. Two hundred patients were randomly assigned to a group consumed SUD (SUD group, n=98) and a group where only SRD (SRD group, n=102) was allowed. Because salt content in diets of the two groups was different, the patients could taste whether it was salty, so blinding method could not be used. In SUD group, 61 patients were men and 37 women (mean age, 52.36±9.07 years); 95 patients (96.94%) had post hepatitis B cirrhosis and the causes of cirrhosis were unclear in 3 patients (3.06%). In SRD group, 65 patients were men and 37 women (mean age, 52.65±9.91 years); 97 patients (95.1%) had post hepatitis B cirrhosis and cause of disease was unclear in 5 patients (4.90%, p>0.05). Normal control (NC) group consisted of 30 healthy blood donors of whom 19 were men and 11 women with a mean age of 51.98±8.53 years. Comparison of SUD and SRD groups showed that there were no significant differences in sex and age (p>0.05). For comparison of demographic and clinical baseline features of the two treatment groups (Table 1). Reporting of the study conforms to CONSORT statement.
Table 1.
Comparison of Demographic and Clinical Baseline Characteristics between Treatment Groups
HE, hepatic encephalopathy; HRS, hepatorenal syndrome; SUD, sodium-unrestricted diet; SRD, sodium-restricted diet.
In the SUD and SRD groups, the calories-intake was 1,043.15±225.03 kcal/day versus 1,044.25±213.12 kcal/day, ascites 2.59±1.13 cm (in supine position by ultrasonic examination, no echo area in anterior liver 2.59±1.13 cm) versus 2.48±1.12 cm, 20 cases (20.41%) of SUD group and 21 cases (20.59%) of SRD group had light edema of lower limbs (χ2=0.001, p>0.05). Urine volume 1,243.75±201.12 mL/day versus 1,248.12±382.11 mL/day, Child-Pugh classification class B was found in 26 cases (26.53%) versus 28 cases (27.45%) and Child-Pugh classification class C in 72 cases (73.47%) versus 74 cases (72.55%) in SUD group and SRD group, respectively. Abnormal liver function was present in both groups before treatment, but p>0.05 for all these comparisons. There were no significant differences in blood sodium and chloride, urine sodium and chloride, PRA, A II, ALD, and RBF between the two groups of patients (Tables 2 and 3).
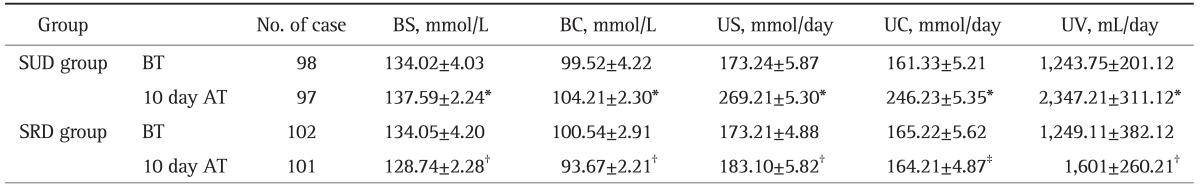
Table 2.
Blood Sodium and Chloride, Urine Sodium and Chloride, and Urine Volume in the Two Groups before and after Treatment (X±SD)
BS, normal value, 135-155 mmol/L; BC, normal value, 98-108 mmol/L; US, normal value, 27-387 mmol/day; UC, normal value, 170-255 mmol/day.
BS, blood sodium; BC, blood chloride; US, urine sodium; UC, urine chloride; UV, urine volume; BT, before treatment; AT, after treatment; SUD, sodium-unrestricted diet group; SRD, sodium-restricted diet.
*Compared with that before treatment and of the SRD group 10 days after treatment, t=4.09-50.21, p<0.001; †Compared with that of the SRD group before treatment, t=3.89-10.85, p<0.001; ‡Compared with that of the SRD group before treatment, t=0.98, p>0.05.
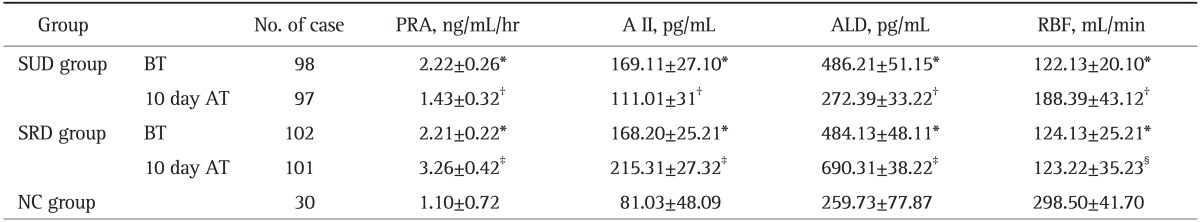
Table 3.
PRA, AII, ALD, and RBF in the Two Groups before and after Treatment (X±SD)
PRA, plasma renin activity; A II, angiotensin II; ALD, aldosterone; RBF, renal blood flow; SUD, sodium-unrestricted diet; SRD, sodium-restricted diet; NC, normal control; BT, before treatment; AT, after treatment.
*Compared with that of NC group, t=7.26-19.61, p<0.001; †Compared with that before treatment and that of SRD group 10 days after treatment, t=4.99-40.31, p<0.001; ‡Compared with those before treatment t=5.87-20.03, p<0.001; §Compared with that before treatment, t=0.99, p>0.05.
2. Therapeutic methods
Both groups were treated with the same preparations of silymarin (Zhong Xing Pharmaceutical Co., Ltd., Jiangsu, China) to protect the liver function, ALB for intravenous infusion 5 to 10 g, 3 times a week (25 g in a week), furosemide (20 mg, twice daily) and spironolactone (40 mg twice daily) were given orally and their dosages were adjusted depending on urine and ascites volume. In both groups water-intake was limited properly and daily weight loss was controlled under 0.5 kg. One case in SUD group had 3,600 mL/day urine, body weight decreased by 0.7 kg/day, 1 case in SRD group had 3,500 mL/day urine, body weight decreased by 0.6 kg/day, furosemide and spironolactone were used in half dosage for the two cases. In 14 cases of SRD group, when low blood sodium resulted in renal damage, furosemide, and spironolactone were used in half dosage. For other patients, dosage of diuretics was not changed. Total amount of furosemide was 176.3 g in SUD group, 238.34 g in SRD group, and total amount of spironolactone was 352.57 g in SUD group, 461.92 g in SRD group. Mean amount of furosemide was 1.82±0.24 g in SUD group, which was less than that in SRD group (2.36±0.44 g) (t=10.8, p<0.001). Mean amount of spironolactone was 3.63±0.47 g in SUD group, less than that in SRD group (4.57±1.25 g) (t=6.96, p<0.001). Mean amount of furosemide and spironolactone in SRD group was more than that in SUD group, because the length of hospital stay of SRD group was longer than that in SUD group. In SUD group (not limited), the amount of salt in diet was allowed to be the same as that of daily life (sodium chloride intake 5,000 to 6,500 mg/day). If urine volume increased or the level of blood sodium decreased, the amount of salt in diet was increased appropriately (1 case had 2,800 mL/day urine volume, blood sodium level decreased from 134 to 130 mmol/L, sodium chloride intake increased from 5,000 to 7,000 mg/day). In SRD group, only low salt diet (sodium chloride <5,000 mg/day) was allowed. Intravenous sodium was not used in any group. Professional dieticians were responsible for the patients' diet and doctors and nurses supervised to insure the patients had the correct diets. Total amount of NaCl in the diet was 25,531.1 g in SUD group and 12,656.2 g in SRD group. Mean amount of NaCl in the diet was 263.21±41.06 g in SUD group, more than that in SRD group (125.31±29.94 g) (t=27.08, p<0.001). Spontaneous bacterial peritonitis occurred in 1 case of SUD group on the fifth day after treatment. Upper digestive tract bleeding occurred in 1 case of SRD group on the third day after treatment and the two cases withdrew from the trial. The rest continued the trial until discharge from the hospital or death.
3. Laboratory examination
Blood sodium and chloride, and 24-hour urine sodium and chloride were determined by ion selective electrode method using dielectric analyzer (Medica Co., Bedford, MA, USA) and the reagent kit was purchased from Shanghai Fusheng Analytical Instrument Factory (Shanghai, China). Blood was taken from the ulnar vein at 7:00 AM and placed into pre-cooled test tube with anticoagulant agent and enzyme inhibitor containing 50 µL of 0.3 mol/L EDTA, 50 µL of 0.34 mol/L 8-quinolinol and 25 µL of 0.32 mol/L british anti-lewisite, and then plasma was separated using a centrifuge and stored at -20℃ for testing PRA and A II by radioimmunoassay competitive inhibition method using GC-911 type gamma-counter according to the manufacturers' instructions. All reagents were purchased from Beifang Biotechnology Institute (Beijing, China). Blood was taken in the same way as that for PRA and heparin was applied as anticoagulant, and then plasma was separated and stored at -20℃ for testing ALD according to instructions from the manufacturer. The used instruments and the source of reagents were the same as PRA. PRA, A II, and ALD were determined in NCs (healthy blood donors). All the tests were performed before treatment and on the 10th day after treatment. Blood sodium and chloride were determined once every 2 to 7 days, 10 days after treatment, renal function (urea nitrogen and creatinine) were determined once every 2 to 7 days, and liver function was determined once every 5 to 7 days using Japan OLYMPUS AU600 automatic biochemical analyzer (Olympus Optical Co., Tokyo, Japan). All the above-mentioned laboratory tests were performed by special laboratory technicians.
4. RBF
The patients were assigned in supine and lateral decubitus position. US CELOGIO-7 color Doppler ultrasound machine was used to detect renal coronary section or cross section blood flow, the renal hilum was thoroughly displayed, the renal artery was chosen and its internal diameter was measured at 1 cm over the renal hilum, and then the pulse Doppler was put in the lumen of the renal artery with sampling angle <60°, drawing frequency spectrum enveloping line along frequency spectrum edge using a vernier and average blood velocity and flow volume were automatically calculated using a computer. Blood flow volume was calculated with the following formula: (V)=π×(internal diameter of artery/2)2×average blood flow velocity×heart rate.8,9 RBF tests were performed by special ultrasonic technicians.
5. Determination of urine and ascites volume
Twenty-four hour-urine volume was recorded every day, ascites was measured twice a week using B-type ultrasound, and then once every 7 to 10 days after ascites disappearance. Ascites volume was measured by special ultrasonic technicians. Ascites volume was determined by cm of no echo area in anterior liver while patients were in supine lying position.
6. Statistical analysis
Statistical analysis was performed using SPSS software version 12.0 (SPSS Inc., Chicago, IL, USA). Chi-square test was used for comparison of enumeration data and t-test for measurement data. Statistical significance was set at p<0.05.
RESULTS
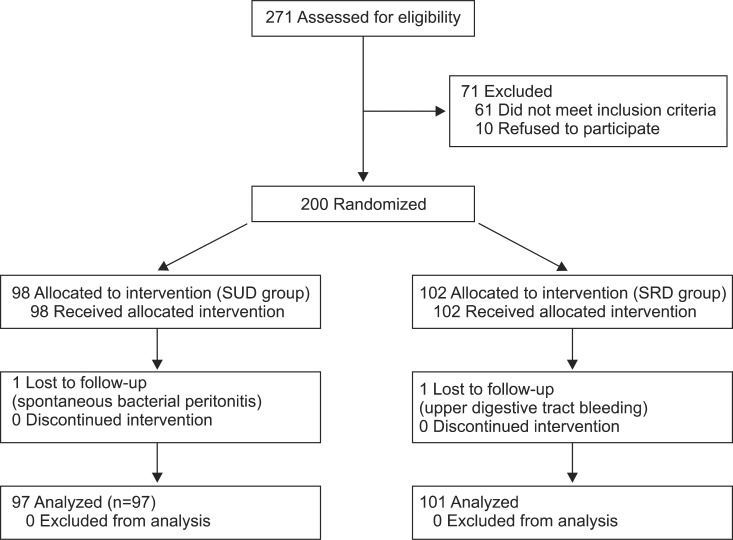
In the course of trial, spontaneous bacterial peritonitis occurred in 1 case of SUD group on the fifth day after treatment. Upper digestive tract bleeding occurred in 1 case of SRD group on the third day after treatment and the two cases withdrew from the trial. Ninety-seven cases in SUD group and 101 cases in SRD group accomplished the trial until discharge or death (Fig. 1).
Fig. 1.
Randomized controlled trial of effect of sodium-unrestricted diet (SUD) on ascites in patients with hepatic cirrhosis. Progressive flow at different stages. Our hospital have received 271 cases of cirrhosis ascites assessed for the eligibility from January 2007 to May 2010. Of all the cases, 71 patients are excluded, 61 cases not meeting the inclusion criteria and 10 cases refusing to participate. The left 200 patients were randomly divided into two groups, 98 cases of SUD and 102 cases of sodium-restricted diet (SRD). The patients in both SUD and SRD groups receive intervention. Spontaneous bacterial peritonitis occurred in 1 case of SUD group on the fifth day after treatment. Upper digestive tract bleeding occurred in 1 case of SRD group on the third day after treatment and the two cases withdrew from the trial. Ninety-seven cases in SUD group and 101 cases in SRD group accomplished the trial until discharge or death.
1. Calories-intake
Calories-intake in SUD group was 1,043.15±225.03/day before treatment and 2,081.15±121.19/day 30 days after treatment, while in SRD group 1,044.25±213.12/day and 1,529.45±113.96/day. Calories-intake in SUD group 30 days after treatment was significantly more than that in SUD group before treatment (t=28.941, p<0.001) and than that in SRD group 30 days after treatment (t=23.69, p<0.001). Calories-intake in SRD group after treatment was more than that in SRD group before treatment (t=14.741, p<0.001).
2. Blood sodium and chloride, urine sodium and chloride, and urine volume
In SUD group blood sodium before treatment was 134.02±4.03 mmol/L and 49 patients (50%) had low blood sodium, while in SRD group 134.05±4.20 mmol/L and 50 patients (49.02%, p>0.05). Table 2 shows that blood sodium and chloride in SUD group 10 days after treatment were increased compared with those before diuretics treatment and those in SRD group 10 days after treatment (t=4.09, 50.21, p<0.001). Blood sodium and chloride in SRD group 10 days after treatment were significantly lower than that before treatment (t=3.89, 10.85, p<0.001).
3. ALB, PRA, A II, and ALD
ALB (33.54±1.86 g/L) in SUD group 30 days after treatment was higher than that (30.97±4.42 g/L) before treatment and that (31.22±3.31 g/L) in SRD group 30 days after treatment (t=3.88, 3.99, p<0.001). The change in ALB was not significant in SRD group 30 days after treatment compared with that (30.59±2.84 g/L) before treatment (t=0.97, p>0.05). Table 3 shows that PRA, A II, and ALD in both groups before treatment were higher than those in NC group (t=7.26, 13.78, p<0.001); PRA, A II, and ALD in SUD group 10 days after treatment were significantly decreased compared with those before treatment and those in SRD group 10 days after treatment (t=6.57 to 40.31, p<0.001); and PRA, A II, and ALD in SRD group 10 days after treatment were significantly higher than those before treatment (t=5.87 to 20.03, p<0.001).
4. RBF
RBF in both groups before treatment was lower than that of NC group (t=19.51, 19.61, p<0.001). RBF in SUD group 10 days after treatment was increased compared with that before treatment and that in SRD group 10 days after treatment (t=4.99, 6.87, p<0.001).
5. Renal impairment and ascites
Renal impairment caused by low blood sodium occurred in 14 patients (13.86%) of SRD group but in none of SUD group (χ2=14.47, p<0.01; blood sodium was 127.35±2.14 mmol/L, urea nitrogen 11.49±1.24 mmol/L [the normal value, 2.8 to 8.2 mmol/L], and creatinine 199.57±34.11 umol/L [the normal value, 44 to 133 µmol/L]) in the 14 patients. Of the 14 patients, 8 died of renal failure. Creatinine rose to 550±15.16 µmol/L. Hemodialysis and other medical therapies were used for the 8 cases, but they died from hepatorenal syndrome at last. Ascites disappeared in higher proportion of patients in SUD group (44 cases, 45.36%) than in SRD group (16 cases, 15.84%) (χ2=20.42, p<0.001). The time to disappearance of ascites was shorter in SUD group (30.24±3.12 days) than in SRD group (47.19±9.22 days) (t=8.96, p<0.001). The length of hospital stay (45.66±7.10 days) in SUD group was shorter than that (62.49±8.06 days) in SRD group (t=6.99, p<0.001).
Adverse events such as hypertension, heart failure and cerebral edema did not occur in any group.
DISCUSSION
The traditional view is that sodium should be strictly restricted to prevent retention of sodium and water in patients with cirrhosis with ascites even though when patients are under diuretic treatment. It has been found that sodium retention has already existed before appearance of cirrhotic ascites accompanied by increased total amount of sodium, at this time increased sodium-intake would lead to appearance of ascites or aggravate ascites.10,11 The total amount of sodium in the body did not decrease in many patients with hyponatremia because hyponatremia is mainly due to dilution of sodium caused by water retention.12,13 Sterns14 found that most of their 62 patients with chronic severe hyponatremia (blood sodium <110 mmol/L) could tolerate low sodium, and the short-term mortality was only 8% and the death was not caused by hyponatremia. If low sodium was corrected quickly, complications of nervous system such as demyelination would occur. Another study showed that compared with appropriate sodium restriction, although serum sodium was not decreased obviously when sodium was not restricted, ascites was refractory; while sodium restriction could significantly shorten the time to control ascites and improve survival rate of patients with ascites.15-17 The studies mentioned above indicate that sodium restriction should be applied in treatment of cirrhotic ascites. However, some studies have shown that hyponatremia is common in cirrhotic patients with ascites18 and too low a level of serum sodium may lead to nausea, vomiting, lethargy and lassitude, and severe sodium deficiency can cause acute hyponatremia syndrome,19 hyponatremic encephalopathy, renal impairment and hepatorenal syndrome,20 which may increase mortality of cirrhotic patients with ascites. The main cause of hyponatremia in cirrhotic patients with ascites is due to inappropriate restriction of sodium-intake and diuretic use. Therefore, sodium should not be restricted strictly in the treatment of cirrhotic ascites, particularly when the patients are treated with diuretics. In contrast, appropriate sodium-intake should be assured to prevent too low a blood sodium level to reduce mortality. In this study, we observed whether no sodium restriction can lead to water-sodium retention. In this study, level of blood sodium was low in both SUD and SRD groups before treatment and the incidences of hyponatremia were 50% and 49.02% in SUD and SRD groups, respectively, which are consistent with that reported by other researchers.18 In this study, amount of NaCl in diet was significantly higher in SUD group than that in SRD group. So, blood sodium and chloride in SUD group 10 days after treatment was increased compared with those before treatment and those in SRD group 10 days after treatment. Therefore, urine sodium and chloride, and urine volume in SUD group 10 days after treatment increased compared with those before treatment and those in SRD group 10 days after treatment. Blood sodium and chloride in SRD group 10 days after treatment were significantly lower than those seen before treatment.
Activation of renin-angiotensin-aldosterone system (RAAS) is not caused by sodium retention in cirrhotic patients with ascites, but is related to low plasma level of sodium. The lower the plasma sodium is, the higher the RAAS activity will be. With the correction of plasma low sodium, RAAS activity is inhibited.7 After treatment with sodium restriction and diuretics, plasma sodium and osmotic pressure decrease, extracellular water shifts into cells, effective blood circulation volume decreases, blood pressure may drop, low renal blood perfusion stimulates the pressure receptor of afferent glomerular arterioles, renin is secreted and RAAS is activated on this basis. In this study, blood sodium and RBF in SUD group 10 days after treatment were higher than those before treatment and those in SRD group 10 days after treatment; PRA, A II, and ALD in SUD group were lower than those before treatment; while blood sodium in SRD group 10 days after treatment was lower than that before treatment and that in SUD group 10 days after treatment; therefore PRA, A II, and ALD in SRD group 10 days after treatment were higher than those measured before treatment and those in SUD group measured 10 days after treatment.
RAAS activation, renal cortical vasoconstriction, decreased glomerular filtration rate, decreased urine volume and sodium, and increased blood urea nitrogen and creatinine can induce hepatorenal syndrome.21 Hyponatremia and high activity of plasma renin are independent predictive factors of type I hepatorenal syndrome in cirrhotic patients with ascites.20 In this study, renal impairment caused by low blood sodium was less severe in SUD group than in SRD group.
Most cirrhotic patients with ascites have anorexia, which may be worsened by low salt diet, and therefore calories intake can be further reduced. In this study, calories intake in SUD group after treatment was more than that before treatment and that in SRD group after treatment. The increased calories intake may be beneficial to elevation of ALB. In this study, after treatment, ALB in SUD group was higher than that in SRD group. Increased ALB is conducive to ascites disappearance. In this study, ascites disappeared in higher proportion of patients in SUD group than in SRD group; the time of ascites disappearance was shorter in SUD group than in SRD group; and hospital stay in SUD group was shorter than that in SRD group.
In summary, cirrhotic patients with ascites have lower blood sodium. Diuretic use and SRD will further decrease blood sodium, which make diuretic effect weak and ascites disappear slowly. Decreased blood sodium will result in reduction of RBF and increase in PRA, A II, and ALD, which can lead to renal impairment, reduction of urine volume and slow ascites disappearance. Moreover, renal impairment can be fatal in severe patients. On the contrary, appropriate sodium-intake will improve diuretic effect and prevent renal impairment caused by low blood sodium. SUD is beneficial to calories-intake, elevation of ALB, and ascites disappearance. However, confirmation of this conclusion needs further studies on larger sample size with long-term follow-up. Most of the patients in this study (>90%) were cases of post hepatitis B cirrhosis, the difference between our study and results of some other studies might result from different etiology of cirrhosis. Subjects of present study had no cirrhotic complications, so the results of this study are only applicable to cases of post hepatitis B cirrhosis without other complications. It is necessary to study post hepatitis B cirrhosis with complications and cirrhosis resulted from other causes.
Footnotes
No potential conflict of interest relevant to this article was reported.
References
- 1.Moore KP, Wong F, Gines P, et al. The management of ascites in cirrhosis: report on the consensus conference of the International Ascites Club. Hepatology. 2003;38:258–266. doi: 10.1053/jhep.2003.50315. [DOI] [PubMed] [Google Scholar]
- 2.Runyon BA Practice Guidelines Committee, American Association for the Study of Liver Diseases (AASLD) Management of adult patients with ascites due to cirrhosis. Hepatology. 2004;39:841–856. doi: 10.1002/hep.20066. [DOI] [PubMed] [Google Scholar]
- 3.Zeng X, Lin Y, Xie WF. My view of sodium restriction in the treatment of cirrhotic ascites. Chin J Dig. 2007;27:331–333. [Google Scholar]
- 4.Gu XB, Liu XY, Xu YQ, et al. Influence of diet with or without sodium restriction on renal flow and ascites subsidence of patients with cirrhosis. Chin J Dig. 2008;28:61–62. [Google Scholar]
- 5.Gu XB, Chen HK, Zhu YF, Pei H, Liu XY. Influence of Na supplement and limitation on blood PRA, AII, ALD and renal function in patients with liver cirrhosis. Zhonghua Gan Zang Bing Za Zhi. 2004;12:370. [PubMed] [Google Scholar]
- 6.Gu XB, Chen HK, Zhu YF, Pei H. Influence of sodium supplement and restriction on subsidence of cirrhotic ascites. Chin J Infect Dis. 2004;22:129–130. [Google Scholar]
- 7.Liu JJ, Wu XY, Zhi H, Li N. Sodium restriction to correct cirrhotic ascites and application of hypertonic NaCl in ascites patients. Clin Focus. 2000;15:1141–1143. [Google Scholar]
- 8.Wang XC, Sun Q, Yu GL, Gao PJ, Pu YF. Color Doppler study on hepatorenal syndrome and renal artery hemodynamics in non-compensation period of cirrhosis. J Clin Hepatobiliary Dis. 1996;12:212–213. [Google Scholar]
- 9.Liang CX, Yu LD, Zhang Q, Chen XH, Guan YF. Color Doppler study on renal artery hemodynamics of normal adults. J Ultrasonic Med. 1995;11:103–104. [Google Scholar]
- 10.Domenicali M, Caraceni P, Principe A, et al. A novel sodium overload test predicting ascites decompensation in rats with CCl4-induced cirrhosis. J Hepatol. 2005;43:92–97. doi: 10.1016/j.jhep.2005.01.034. [DOI] [PubMed] [Google Scholar]
- 11.Wong F, Liu P, Blendis L. The mechanism of improved sodium homeostasis of low-dose losartan in preascitic cirrhosis. Hepatology. 2002;35:1449–1458. doi: 10.1053/jhep.2002.33637. [DOI] [PubMed] [Google Scholar]
- 12.Arroyo V, Rodés J, Gutiérrez-Lizárraga MA, Revert L. Prognostic value of spontaneous hyponatremia in cirrhosis with ascites. Am J Dig Dis. 1976;21:249–256. doi: 10.1007/BF01095898. [DOI] [PubMed] [Google Scholar]
- 13.Ginès P, Jiménez W. Aquaretic agents: a new potential treatment of dilutional hyponatremia in cirrhosis. J Hepatol. 1996;24:506–512. doi: 10.1016/s0168-8278(96)80174-7. [DOI] [PubMed] [Google Scholar]
- 14.Sterns RH. Severe symptomatic hyponatremia: treatment and outcome. A study of 64 cases. Ann Intern Med. 1987;107:656–664. doi: 10.7326/0003-4819-107-5-656. [DOI] [PubMed] [Google Scholar]
- 15.Reynolds TB, Lieberman FL, Goodman AR. Advantages of treatment of ascites without sodium restriction and without complete removal of excess fluid. Gut. 1978;19:549–553. doi: 10.1136/gut.19.6.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gauthier A, Levy VG, Quinton A, et al. Salt or no salt in the treatment of cirrhotic ascites: a randomised study. Gut. 1986;27:705–709. doi: 10.1136/gut.27.6.705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Desai HG. Salt restriction in ascites with cirrhosis of liver: will enhanced salt restriction increase longevity? J Assoc Physicians India. 2006;54:504. [PubMed] [Google Scholar]
- 18.Qiu ZG. Hyponatremia of cirrhotic ascites. J Clin Hepatobiliary Dis. 1994;10:165–166. [Google Scholar]
- 19.Gu XB. 36 cases of acute low sodium syndrome complicated by cirrhotic ascites. J Sino-Jpn Friendsh Hosp. 2000;14:192. [Google Scholar]
- 20.Wong F, Blendis L. New challenge of hepatorenal syndrome: prevention and treatment. Hepatology. 2001;34:1242–1251. doi: 10.1053/jhep.2001.29200. [DOI] [PubMed] [Google Scholar]
- 21.Liu JJ, Zhi H. Disadvantage of sodium restriction in the treatment of cirrhotic ascites and measures. New Med. 2003;34:123. [Google Scholar]