Abstract
Background/Aims
We have a limited understanding of the effect of nonalcoholic fatty liver disease (NAFLD) on the development of type 2 diabetes.
Methods
The study subjects included male who had received biennial medical check-ups between 2005 and 2009 and who had been diagnosed with fatty liver disease. The subjects with sustained NAFLD (FL, n=107) and sustained non-NAFLD (NFL, n=1,054) were followed to determine the development of type 2 diabetes.
Results
In the FL group, there were more subjects with impaired fasting glucose (IFG), type 2 diabetes and high HOMA-IR than there were in the NFL group during the 5-year follow-up period (32.7 vs. 17.6%, 1.9 vs. 0.3%, 17.9 vs. 5.2% respectively, p<0.05). The FL group showed a higher risk than NFL group for abnormal glucose metabolism as determined using IFG (odds ratio [OR], 2.13; confidence interval [CI], 1.36 to 3.35), type 2 diabetes (OR, 7.63; 95% CI, 1.03 to 56.79) and high HOMA-IR (OR, 3.25; 95% CI, 1.79 to 5.91) and metabolic parameters such as body mass index (OR, 3.35; 95% CI, 1.87 to 6.02), triglyceride (OR, 3.05; 95% CI, 1.92 to 4.86) and fasting blood sugar (OR, 2.18; 95% CI, 1.39 to 3.41).
Conclusions
Sustained NAFLD appears to be associated with an increased risk for the development of type 2 diabetes and deterioration of metabolic parameters in non-obese, non-diabetic Korean men.
Keywords: Nonalcoholic fatty liver disease, Type 2 diabetes mellitus, Metabolic syndrome
INTRODUCTION
Nonalcoholic fatty liver disease (NAFLD) includes a wide spectrum of liver damage ranging from simple steatosis to nonalcoholic steatohepatitis (NASH), advanced fibrosis and hepatic cancer and is the most commonly encountered chronic liver disease.1 One study reported the prevalence of NAFLD in Korea as 18% among adults,2 and the prevalence is increasing due to high fat and high calorie diets, ageing of the population, lack of physical exercise, change in life style, and/or increased obesity.3 Recently, it was reported that quite a number of people who received health screening tests were found to have NAFLD.
NAFLD is considered to be a hepatic component of metabolic syndrome (MetS).4,5 It is associated with obesity, dyslipidemia, and type 2 diabetes,6-8 and increased level of serum fatty acid, and it also predicts the clustering of risk factors for cardiovascular disease.9-11
More and more studies have reported that the presence of NAFLD plays a role as an independent risk factor for other systemic metabolic diseases. Also, another study has suggested that NAFLD is associated with insulin resistance, independent of obesity.12 Numerous studies have reported that the presence of NAFLD independently exacerbates associated systemic metabolic disease.
We retrospectively reviewed cases diagnosed with NAFLD over a period of 5 years to determine the effects of NAFLD on the development of type 2 diabetes and metabolic parameters.
MATERIALS AND METHODS
1. Subjects
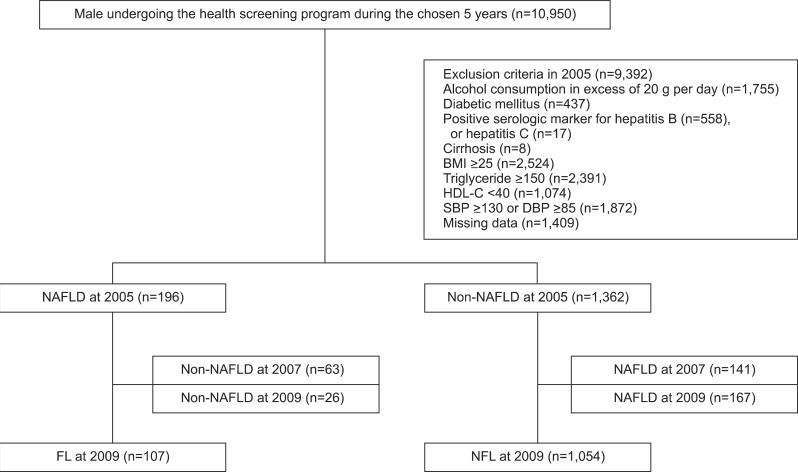
Among the 10,950 male subjects who participated in the health screening program at the Health Promotion Center in Kangbuk Samsung Hospital in 2005, we excluded subjects with a history of drinking 20 g or more alcohol per day (n=1,755), diabetes (n=437), chronic liver diseases such as viral hepatitis B (based on serology test or history) (n=558), viral hepatitis C (n=17) or liver cirrhosis (n=8), those with missing data (n=1,409) and those who had any one of the components of MetS according to the ATP III criteria13 (n=7,861). As a consequence, the initial cohort comprised 1,558 participants who were followed-up every 2 years until 2009.
The ultrasonography performed in 2005 identified 196 subjects with NAFLD and 1,362 subjects with non-NAFLD. Among these subjects, 107 showed sustained NAFLD both in 2007 and 2009 based on ultrasonography findings (sustained NAFLD, FL), and 1,054 subjects were found to be non-NAFLD on the two consecutive biennial ultrasounds (sustained non-NAFLD, NFL) (Fig. 1).
Fig. 1.
Flow of subjects through the study.
BMI, body mass index; HDL-C, high-density lipoprotein cholesterol; SBP, systolic blood pressure; DBP, diastolic blood pressure; NAFLD, nonalcoholic fatty liver disease; FL, sustained NAFLD; NFL, sustained non-NAFLD.
This study was approved by the Institutional Review Board at Kangbuk Samsung Hospital. The informed consent requirement for this study was exempted by the Institutional Review Board because researchers only accessed the database for analysis purposes, not to obtain personal information.
2. Medical evaluation
All subjects underwent a questionnaire survey, interview, history taking, blood sampling and ultrasonography a total of three times biennially from 2005 to 2009 at the Health Promotion Center in Kangbuk Samsung Hospital.
3. Interview
A questionnaire survey and interview were completed for all subjects to determine the current history of diabetes or hypertension, past medical history, alcohol intake, smoking status, and family history.
Height and body weight were measured (FA-94H; Fanics, Seoul, Korea) with the patient in a light gown with feet bare. The measured height and weight were recorded to the nearest 0.1 cm and 0.1 kg, respectively. The body mass index (BMI) index was calculated as body weight divided by squared height in meters (kg/m2).
4. Laboratory assessments
A blood sample was taken from the antecubital vein after a minimum of 12 hours fasting, and serum glucose, uric acid, lipid profile (total cholesterol, triglyceride [TG], high-density lipoprotein cholesterol [HDL-C], low-density lipoprotein cholesterol [LDL-C]), HbA1c, and insulin levels were measured.
The fasting glucose level was measured using the hexokinase method, and the fasting serum insulin level was measured with an immune-radiometric assay (BioSource, Nivelles, Belgium). The intra- and inter-assay coefficients of variation were 2.1% to 4.5% and 4.7% to 12.2%, respectively.
Serum total cholesterol and TG were measured with an enzymatic calorimetric test. HDL-C was measured with a selective inhibin test, and LDL-C was measured with a homogenous enzymatic calorimetric test (Advia 1650 Autoanalyzer; Byer Diagnostics, Leverkusen, Germany).
Homeostatic model assessment insulin resistance (HOMA-IR) was used as the index of insulin resistance based on the following formula:
HOMA-IR=(fasting serum insulin [µIU/mL]×fasting serum glucose [mmol/L]/22.5).
5. Definitions
The metabolic components used to define MetS include abdominal obesity (>102 cm), hypertension (blood pressure ≥130/85 mm Hg), high TG (TG ≥150 mg/dL), glycemia (fasting blood sugar [FBS] ≥100 mg/dL), low HDL (HDL-C <40 mg/dL) and presence of any of at least three components. However, in our study, we replaced abdominal obesity with BMI (BMI ≥25 kg/m2) because abdominal circumference was not measured.
Type 2 diabetes was defined by the presence of one of two criteria: HbA1c ≥6.5% or FBS level 126 mg/dL, following the American Diabetes Association guideline 2010,14 in which the level of HbA1c was newly added as a criterion for type 2 diabetes.
Abdominal ultrasonography (ASPEN; Acuson, Malvern, PA, USA) was performed with a 3.5 MHz probe by one of three radiologists to evaluate the presence of hepatic steatosis in all subjects. The diagnosis of fatty liver was made based on the following criteria: a diffuse hyper-echoic echotexture, hepatorenal echo contrast in reference to the cortex of the right kidney, vascular blurring and deep-echo attenuation. When making the diagnosis of NAFLD, the result of the liver function test was not taken into consideration.15
6. Statistical analyses
We used IBM SPSS version 19.0 statistics package (IBM, New York, NY, USA) to analyze the data. The Student's t-test was used for comparison. Odds ratios (ORs) and 95% confidence interval (CI) were calculated using univariable logistic regression, multivariable logistic regression, and multinomial logistic regression. A p-value less than 0.05 was considered statistically significant.
RESULTS
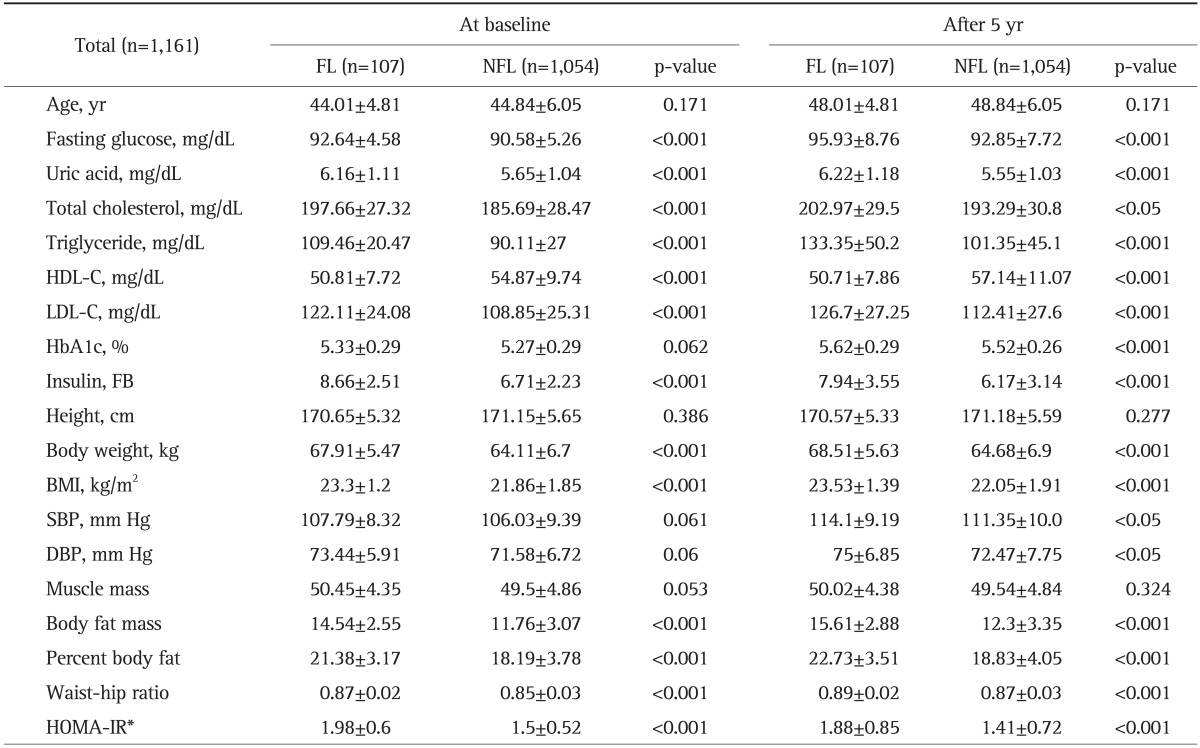
Among those who showed consistent results of either FL or NFL on three consecutive ultrasounds in 2005, 2007, and 2009, we compared the demographic and biochemical parameters at baseline in 2005 and after 5 years in 2009 between the FL (n=107) and NFL (n=1,054) groups. At baseline, the FL group showed a higher average BMI, higher levels of fasting glucose, insulin and uric acid, and a worse lipid profile (higher levels of total cholesterol, TG and LDL-C but lower HDL-C) than did the NFL group at baseline. These tendencies still remained after 5 years when the same parameters were compared between those two groups. In addition to these findings, the FL group at year 5 showed a higher HbA1c level, systolic blood pressure, and diastolic blood pressure (Table 1).
Table 1.
Clinical, Laboratory, and Metabolic Data for Sustained NAFLD (FL) and Sustained Non-NAFLD (NFL) Patients at Baseline and 5 Years
Data are presented as mean±SD or medians (interquartile ranges) for skewed variables and the number of patients and prevalence for categorical variables. The differences were assessed using the t-test for continuous variables.
HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; FB, fasting blood; BMI, body mass index; SBP, systolic blood pressure; DBP, diastolic blood pressure; HOMA-IR, homeostasis model assessment insulin resistance.
*HOMA-IR ≥2.6.
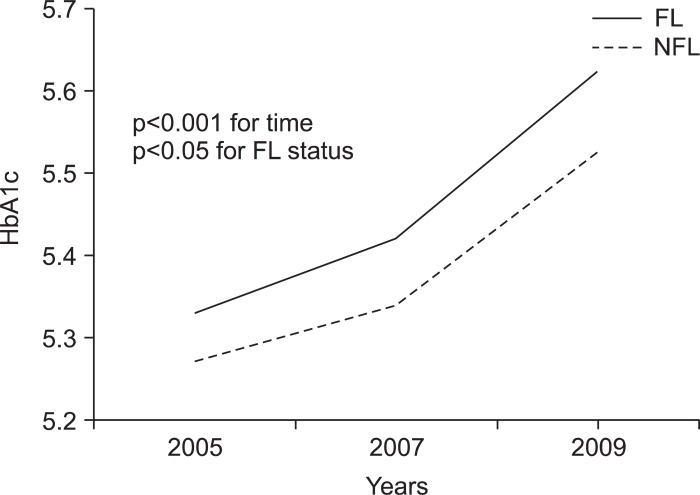
We examined the impacts of FL and NFL on HbA1c level. Fig. 2 shows the comparisons of HbA1c level between the FL and NFL groups in years 2005, 2007, and 2009 after adjusting for age and BMI. The HbA1c level was significantly different depending on the NAFLD status at each time point (p<0.001 for time, p<0.05 for FL status). The HbA1c level increased in the FL group with time.
Fig. 2.
Correlation between HbA1c level and time in the sustained nonalcoholic fatty liver disease (NAFLD) (FL) and sustained non-NAFLD (NFL) groups after adjustments for age and body mass index during the 5-year follow-up period. The HbA1c and FL statuses were evaluated using one-way repeated measures ANCOVA. The p-values are for the post-hoc analysis of variance comparisons.
To assess the impact of NAFLD on the development of type 2 diabetes and MetS, we compared HOMA-IR indices and the incidences (adjusted for age and BMI) of impaired fasting glucose (IFG) and type 2 diabetes during 2005 and 2009. The FL group was large in number of cases with high HOMA-IR (≥2.6) and a higher number of new cases of IFG and type 2 diabetes during the 5-year follow-up than did the NFL group (32.7% vs 17.6%, 1.9% vs 0.3%, and 17.9% vs 5.2%, respectively; p<0.05).
In addition, the FL group, when compared with the NFL group, showed significantly higher ORs for parameters related to abnormal glucose metabolism; IFG 2.13 (95% CI, 1.36 to 3.35), type 2 diabetes 7.63 (95% CI, 1.03 to 56.79), and HOMA-IR 3.25 (95% CI, 1.79 to 5.91) (Table 2).
Table 2.
Multivariate Relationship between Glucose Metabolism and Nonalcoholic Fatty Liver Disease (NAFLD) during the 5-Year Follow-Up
Chi-square test or Fisher's exact test. Univariate logistic regression, multivariate logistic regression, multinomial logistic regression analysis. Adjusted for body mass index and age.
OR, odds ratio; CI, confidence interval; IFG, impaired fasting glucose; HOMA-IR, homeostasis model assessment insulin resistance.
*IFG, impaired fasting glucose (100-126 mg/dL); †HOMA-IR ≥2.6.
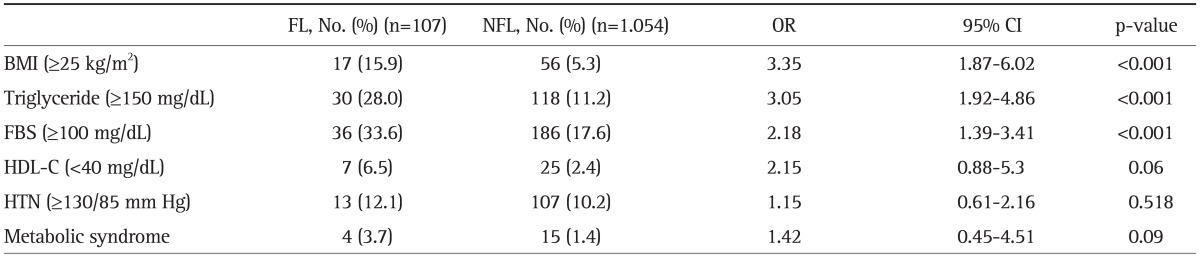
Other metabolic parameters that also showed significantly higher ORs included BMI (3.35; 95% CI, 1.87 to 6.02), TG level (3.05; 95% CI, 1.92 to 4.86) and FBS level (2.18; 95% CI, 1.39 to 3.41), whereas the ORs for HDL-C level (2.15; 95% CI, 0.88 to 5.3), incidence of hypertension (1.15; 95% CI, 0.61 to 2.16) and incidence of MetS (1.42; 95% CI, 0.45 to 4.51) were not statistically significant (Table 3).
Table 3.
Multivariate Relationships between the Metabolic Parameters and Nonalcoholic Fatty Liver Disease (NAFLD) during the 5-Year Follow-Up
Chi-square test or Fisher's exact test. Univariate logistic regression, multivariate logistic regression. Adjusted for age.
OR, odds ratio; CI, confidence interval; BMI, body mass index; FBS, fasting blood sugar; HDL-C, high-density lipoprotein cholesterol; HTN, hypertension.
DISCUSSION
In this study, we observed that the subjects with FL for at least 5 years had a higher risk of developing type 2 diabetes and worsened metabolic parameters compared to those of subjects with NFL. Although both groups showed changes in metabolic parameters with time, the differences were more apparent in the FL group. At baseline, no male subjects met the criteria for MetS in either group; however, after 5 years of follow-up, there were more subjects with obesity and dyslipidemia in the FL group than there were in the NFL group. Also, the FL group showed a higher HOMA-IR, HbA1c level and higher risk for developing type 2 diabetes. These findings imply that the sustained presence of NAFLD has an impact on the development of insulin resistance and diabetes. Therefore, NAFLD might not merely be the hepatic manifestation of MetS, but may also directly promote the occurrence and development of metabolic problems.16-18 NAFLD is an insulin-resistant state, and subjects with NAFLD should be screened regularly for metabolic disorders.17,19,20
Around 90% of NAFLD patients had at least one component of MetS, and around 33% of patients met the criteria for MetS.7 In addition, MetS increased the risk of development of NAFLD.21
Insulin resistance and hyperinsulinemia are the two of major players in the pathogenesis of NAFLD.8 The degree of insulin resistance is positively associated with the progression of intrahepatic fibrosis, whereas a decrease in insulin resistance with weight control, exercise or medication produced improvements in NAFLD.22-24 Several studies performed in animals and humans showed that insulin resistance has an important role in the development of NAFLD.3,25,26
The incidence of MetS was higher in the group with NAFLD compared to that in the group without NAFLD, which implies that the presence of NAFLD contributes to the development of systemic insulin resistance or might be an early manifestation of systemic insulin resistance. This implication is in line with suggestions from several previous clinical and experimental studies.16,17,27,28
Type 2 diabetes is closely related to both the development and progression of NAFLD. In addition to type 2 diabetes, pre-diabetic conditions (such as impaired glucose tolerance, or IFG) were also associated with NAFLD, and a family history of type 2 diabetes increased the risk of developing NAFLD.8
Cohort studies that followed patients with NAFLD for 5 to 8 years found that the risks for type 2 diabetes, pre-diabetes, hyperlipidemia, and hypertension increased, independent of obesity or factors that can affect metabolic disease.17,29,30 The present study found similar results except for the association between hypertension and MetS. This is probably because the 5-year follow-up period was not sufficient to observe the significant difference in the incidences of hypertension and MetS.
These findings suggest that we need to consider NAFLD as a potential early predictor of MetS.31 Follow-up studies are needed to better understand the clinical significance of our findings.
In this study, the diagnosis of NAFLD was made using ultrasonography rather than invasive liver biopsy. This is one of the limitations of this study in that ultrasonography is less sensitive to detecting a fatty liver with low fat accumulation and can also suffer from inter-observer variation.15 In addition, an ultrasound cannot assess the degrees of hepatic inflammation or fibrosis, so we could not differentiate NASH. This study only followed male subjects for 5 years, so the findings of this study cannot be applied to women.
Despite these limitations, we found that NAFLD is clearly associated with the development of type 2 diabetes and metabolic disorders. In particular, the findings of this study suggest that the presence of NAFLD may be a strong predictor for the development of type 2 diabetes.
The fact that NAFLD is closely related to obesity, dyslipidemia, insulin resistance, type 2 diabetes, and glucose metabolism calls for the recognition of NAFLD as a systemic metabolic disease rather that just as a hepatic manifestation of metabolic disease.
To better understand the pathogenesis of NAFLD and its causal relationship with insulin resistance, type 2 diabetes and metabolic disease, prospective and long-term follow-up studies are required.
Footnotes
No potential conflict of interest relevant to this article was reported.
References
- 1.Brunt EM. Nonalcoholic steatohepatitis: definition and pathology. Semin Liver Dis. 2001;21:3–16. doi: 10.1055/s-2001-12925. [DOI] [PubMed] [Google Scholar]
- 2.Park SH, Jeon WK, Kim SH, et al. Prevalence and risk factors of non-alcoholic fatty liver disease among Korean adults. J Gastroenterol Hepatol. 2006;21(1 Pt 1):138–143. doi: 10.1111/j.1440-1746.2005.04086.x. [DOI] [PubMed] [Google Scholar]
- 3.Farrell GC. Non-alcoholic steatohepatitis: what is it, and why is it important in the Asia-Pacific region? J Gastroenterol Hepatol. 2003;18:124–138. doi: 10.1046/j.1440-1746.2003.02989.x. [DOI] [PubMed] [Google Scholar]
- 4.Choudhury J, Sanyal AJ. Clinical aspects of fatty liver disease. Semin Liver Dis. 2004;24:349–362. doi: 10.1055/s-2004-860864. [DOI] [PubMed] [Google Scholar]
- 5.Eckel RH, Grundy SM, Zimmet PZ. The metabolic syndrome. Lancet. 2005;365:1415–1428. doi: 10.1016/S0140-6736(05)66378-7. [DOI] [PubMed] [Google Scholar]
- 6.Fan JG, Zhu J, Li XJ, et al. Prevalence of and risk factors for fatty liver in a general population of Shanghai, China. J Hepatol. 2005;43:508–514. doi: 10.1016/j.jhep.2005.02.042. [DOI] [PubMed] [Google Scholar]
- 7.Marchesini G, Bugianesi E, Forlani G, et al. Nonalcoholic fatty liver, steatohepatitis, and the metabolic syndrome. Hepatology. 2003;37:917–923. doi: 10.1053/jhep.2003.50161. [DOI] [PubMed] [Google Scholar]
- 8.Marchesini G, Brizi M, Bianchi G, et al. Nonalcoholic fatty liver disease: a feature of the metabolic syndrome. Diabetes. 2001;50:1844–1850. doi: 10.2337/diabetes.50.8.1844. [DOI] [PubMed] [Google Scholar]
- 9.Bedogni G, Miglioli L, Masutti F, Tiribelli C, Marchesini G, Bellentani S. Prevalence of and risk factors for nonalcoholic fatty liver disease: the Dionysos nutrition and liver study. Hepatology. 2005;42:44–52. doi: 10.1002/hep.20734. [DOI] [PubMed] [Google Scholar]
- 10.Knobler H, Schattner A, Zhornicki T, et al. Fatty liver: an additional and treatable feature of the insulin resistance syndrome. QJM. 1999;92:73–79. doi: 10.1093/qjmed/92.2.73. [DOI] [PubMed] [Google Scholar]
- 11.Seppälä-Lindroos A, Vehkavaara S, Häkkinen AM, et al. Fat accumulation in the liver is associated with defects in insulin suppression of glucose production and serum free fatty acids independent of obesity in normal men. J Clin Endocrinol Metab. 2002;87:3023–3028. doi: 10.1210/jcem.87.7.8638. [DOI] [PubMed] [Google Scholar]
- 12.Day CP, Saksena S. Non-alcoholic steatohepatitis: definitions and pathogenesis. J Gastroenterol Hepatol. 2002;17(Suppl 3):S377–S384. doi: 10.1046/j.1440-1746.17.s3.31.x. [DOI] [PubMed] [Google Scholar]
- 13.Exert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. Executive summary of the third report of the National Cholesterol Education Program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (Adult Treatment Panel III) JAMA. 2001;285:2486–2497. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 14.American Diabetes Association. Standards of medical care in diabetes: 2010. Diabetes Care. 2010;33(Suppl 1):S11–S61. doi: 10.2337/dc10-S011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Saadeh S, Younossi ZM, Remer EM, et al. The utility of radiological imaging in nonalcoholic fatty liver disease. Gastroenterology. 2002;123:745–750. doi: 10.1053/gast.2002.35354. [DOI] [PubMed] [Google Scholar]
- 16.Fan JG, Zhu J, Li XJ, et al. Fatty liver and the metabolic syndrome among Shanghai adults. J Gastroenterol Hepatol. 2005;20:1825–1832. doi: 10.1111/j.1440-1746.2005.04058.x. [DOI] [PubMed] [Google Scholar]
- 17.Friis-Liby I, Aldenborg F, Jerlstad P, Rundström K, Björnsson E. High prevalence of metabolic complications in patients with non-alcoholic fatty liver disease. Scand J Gastroenterol. 2004;39:864–869. doi: 10.1080/00365520410006431. [DOI] [PubMed] [Google Scholar]
- 18.Fan JG, Fang JW, Lu YS, Qian Y, Cai XB. A study of the changes of hepatic gene expression in the process of nonalcoholic fatty liver disease development using U230A oligonucleotide microarray. Zhonghua Gan Zang Bing Za Zhi. 2005;13:597–601. [PubMed] [Google Scholar]
- 19.Ekstedt M, Franzén LE, Mathiesen UL, Holmqvist M, Bodemar G, Kechagias S. Survival and causes of death in patients with elevated liver enzymes associated with non-alcoholic fatty liver disease (NAFLD) J Hepatol. 2006;44(Suppl 2):S40–S41. [Google Scholar]
- 20.Singh SP. Non-alcoholic fatty liver disease: the unfolding monster? J Gastroenterol Hepatol. 2006;21(1 Pt 2):199–201. doi: 10.1111/j.1440-1746.2006.04206.x. [DOI] [PubMed] [Google Scholar]
- 21.Hamaguchi M, Kojima T, Takeda N, et al. The metabolic syndrome as a predictor of nonalcoholic fatty liver disease. Ann Intern Med. 2005;143:722–728. doi: 10.7326/0003-4819-143-10-200511150-00009. [DOI] [PubMed] [Google Scholar]
- 22.Paradis V, Perlemuter G, Bonvoust F, et al. High glucose and hyperinsulinemia stimulate connective tissue growth factor expression: a potential mechanism involved in progression to fibrosis in nonalcoholic steatohepatitis. Hepatology. 2001;34:738–744. doi: 10.1053/jhep.2001.28055. [DOI] [PubMed] [Google Scholar]
- 23.Ozcan U, Cao Q, Yilmaz E, et al. Endoplasmic reticulum stress links obesity, insulin action, and type 2 diabetes. Science. 2004;306:457–461. doi: 10.1126/science.1103160. [DOI] [PubMed] [Google Scholar]
- 24.Svegliati-Baroni G, Ridolfi F, Di Sario A, et al. Insulin and insulin-like growth factor-1 stimulate proliferation and type I collagen accumulation by human hepatic stellate cells: differential effects on signal transduction pathways. Hepatology. 1999;29:1743–1751. doi: 10.1002/hep.510290632. [DOI] [PubMed] [Google Scholar]
- 25.Sanyal AJ, Campbell-Sargent C, Mirshahi F, et al. Nonalcoholic steatohepatitis: association of insulin resistance and mitochondrial abnormalities. Gastroenterology. 2001;120:1183–1192. doi: 10.1053/gast.2001.23256. [DOI] [PubMed] [Google Scholar]
- 26.Marchesini G, Brizi M, Morselli-Labate AM, et al. Association of nonalcoholic fatty liver disease with insulin resistance. Am J Med. 1999;107:450–455. doi: 10.1016/s0002-9343(99)00271-5. [DOI] [PubMed] [Google Scholar]
- 27.Bae JC, Cho YK, Lee WY, et al. Impact of nonalcoholic fatty liver disease on insulin resistance in relation to HbA1c levels in nondiabetic subjects. Am J Gastroenterol. 2010;105:2389–2395. doi: 10.1038/ajg.2010.275. [DOI] [PubMed] [Google Scholar]
- 28.Stefan N, Kantartzis K, Häring HU. Causes and metabolic consequences of fatty liver. Endocr Rev. 2008;29:939–960. doi: 10.1210/er.2008-0009. [DOI] [PubMed] [Google Scholar]
- 29.Fan JG, Li F, Cai XB, Peng YD, Ao QH, Gao Y. Effects of nonalcoholic fatty liver disease on the development of metabolic disorders. J Gastroenterol Hepatol. 2007;22:1086–1091. doi: 10.1111/j.1440-1746.2006.04781.x. [DOI] [PubMed] [Google Scholar]
- 30.Shibata M, Kihara Y, Taguchi M, Tashiro M, Otsuki M. Nonalcoholic fatty liver disease is a risk factor for type 2 diabetes in middle-aged Japanese men. Diabetes Care. 2007;30:2940–2944. doi: 10.2337/dc07-0792. [DOI] [PubMed] [Google Scholar]
- 31.Kim HJ, Lee KE, Kim DJ, et al. Metabolic significance of nonalcoholic fatty liver disease in nonobese, nondiabetic adults. Arch Intern Med. 2004;164:2169–2175. doi: 10.1001/archinte.164.19.2169. [DOI] [PubMed] [Google Scholar]