Abstract
Background/Aims
High-fat diets contribute to pancreatic fibrogenesis, but the pathogenesis remains unclear. This study investigated the role of nuclear factor kappa B (NF-κB) in high-fat diet-induced pancreatic fibrosis in rats.
Methods
Male Wistar rats were fed a high-fat diet or standard normal chow for 20 weeks. Pancreatic fibrosis was determined by Sirius red staining. Immunohistochemical staining, reverse transcription-polymerase chain reaction and Western blotting were used to identify NF-κB-associated genes or protein expressions.
Results
Inflammation, fat deposition, pancreatic stellate cell activation and fibrosis were observed in the pancreases of the high-fat diet group. NF-κB subunit p65 (NF-κB/p65) expression was localized to the nucleus, and intercellular adhesion molecule 1 (ICAM-1) was over-expressed. Pancreatic gene expression levels of NF-κB/p65, ICAM-1 and tumor necrosis factor α were all elevated significantly in rats fed a high-fat diet compared with control rats. Western blotting also revealed significantly increased levels of ICAM-1 and nuclear NF-κB/p65 in rats fed high-fat diets comparison with control rats.
Conclusions
NF-κB is involved in high-fat diet-related pancreatic fibrosis.
Keywords: High-fat diet, Pancreatic fibrosis, NF-kappa B, Intercellular adhesion molecule 1, Tumor necrosis factor-alpha
INTRODUCTION
Prolonged high-fat diets intake is found harmful to pancreas. According to previous studies, high-fat diets can induce pancreatic endocrine and exocrine abnormalities,1-3 increased inflammatory cytokines in pancreatic tissues,4,5 and pancreatic stellate cell (PSC) activation and fibrogenesis.6,7
High-fat diets incite oxidative stress in pancreas,6,7 which has been shown to be involved in PSCs activation and pancreatic fibrosis.8,9 Although elevated levels of platelet-derived growth factor type beta and transforming growth factor beta 1 (TGF-β1) have been found in the pancreas in an animal model after high fat-diet feeding,7 however, the regulatory mechanisms and signaling pathways involved in this oxide damage process have not been elucidated and our knowledge remains limited.
Nuclear factor kappa B (NF-κB) is an oxidative stress-sensitive transcription factor which modulates a wide variety of genes, including pro-inflammatory cytokines and adhesion molecules such as tumor necrosis factor α (TNF-α) and intercellular adhesion molecule 1 (ICAM-1).10-12 Quiescent PSCs can be stimulated by cytokines, growth factors and reactive oxygen species (ROS)9,13 to subsequently synthesize and secrete increased amounts of extracellular matrix. Activated PSCs promote autocrine factors including ICAM-1, TNF-α, and TGF-β in turn.9,14,15
In view of the above considerations, we hypothesize that NF-κB might be involved in the deleterious effects on the pancreas that are due to chronic high-fat diets. In this present study, we fed rats a high-fat diet singly for 20 weeks, observed histological alterations, investigated some molecules expression that related to the NF-κB signaling pathways in the pancreas, and discuss the underlying implications of our results.
MATERIALS AND METHODS
1. Animal models
This study had the approval of the Ethics Committee of Shandong University. Twenty-four male Wistar rats (weighing 167 to 188 g, obtained from Shandong University Laboratory Animal Center) were used in the experiment. They were maintained in accordance with the Laboratory Animal Care and Use Regulations of Shandong University. The rats received a regular rat chow for 1 week to acclimatize to their new environment, and were then divided into two dietary groups based comparable body weight. Rats in the control group (n=10) received a regular chow; rats in the treatment group (n=12) were fed a high-fat diet (2% cholesterol, 10% lard, and 88% regular chow as for the control group). All rats were fed for 20 weeks from the beginning of the experiment. Animals were sacrificed after fasting overnight and anesthetized by intra-peritoneal injection with pentobarbital sodium (50 mg per kg body weight), at which time pancreas tissues were obtained.
2. Hematoxylin and eosin (H&E) and Sirius red staining
Samples of pancreas were formalin-fixed, paraffin-embedded, and cut into 5 µm thick sections and stained with H&E for histological observations. Inflammation score and fat deposition was evaluated as follows: 0, 0%; 1, 0% to 25%; 2, 25% to 50%; 3, >50%.
For collagen detection, sections were deparaffined and immersed for 25 minutes in saturated aqueous picric acid containing 0.5% Sirius red to stain collagen fibers, and exposed to Harris hematoxylin for 3 minutes to stain nuclei. Under these conditions, the collagen fibrils appear red and the non-fibrotic areas appear blue. The fibrotic area was measured by ImageJ analysis software version 1.39n (National Institutes of Health, Bethesda, MD, USA) (http://rsb.info.nih.gov/ij/), and was expressed as fibrotic index (fibrotic index=area of pancreatic fibrosis/total area of specimen×100%).
To evaluate histological changes, three pancreas sections were randomly selected from each rat, and five non-overlapping fields were captured in every section for observation.
3. Immunohistochemical staining
Sections of pancreas were incubated with primary mouse anti-rat IgG antibodies (Santa Cruz Biotechnology, Santa Cruz, CA, USA) at 4℃ overnight, and then incubated with biotinylated goat anti-mouse secondary antibodies and HRP-conjugated streptavidin (Santa Cruz Biotechnology) at room temperature for 15 minutes. Distilled water with 0.4% tween 20 phosphate buffered saline (PBS) (v/v) was used as a rinsing solution. Negative controls were done in the absence of primary antibodies. When stained for NF-κB/p65, the primary antibody was diluted with 0.3% triton in PBS (v/v), and both cytoplasm- and nucleus-stained cells were considered positive. For observation, three pancreas sections were randomly selected from each rat and five non-overlapping fields were captured in every section for further analyze. The average number of NF-κB/p65 or ICAM-1 positive stained cells (brown) per high power field was reported. Areas staining positive for α smooth muscle actin (α-SMA) were measured by ImageJ software, and expressed as percentage of total area.
4. Gene expression
Gene expression was determined by reverse transcription-polymerase chain reaction (RT-PCR). PCR was performed with reaction mixtures containing dNTP, sense and antisense primers and TaqDNA polymerase (Takara, Shiga, Japan). β-actin was used as an internal standard control. PCR primers and conditions are as follows: NF-κB/p65: sense primer: 5'-ATGGACGATCTGTTTCCC-3', antisense primer: 5'-GTCTTAGTGGTATCTGTGCT-3', fragment size: 170 bp, PCR condition: 94℃, 45"; 60℃, 45"; 72℃, 45", 35 cycles; ICAM-1: sense primer: 5'-AGCCTCAGGCCTAAGAGGAC-3', antisense primer: 5'-AGGGGTCCCAGAGAGGTCTA-3', fragment size: 496, PCR condition: 94℃, 45"; 58℃, 45"; 72℃, 45", 35 cycles; TNF-α: sense primer: 5'-TCGTAGCAAACCACCAAG-3', antisense primer: 5'-CTGACGGTGTGGGTGA-3', fragment size: 193 bp, PCR condition: 94℃, 45"; 50℃, 45"; 72℃, 45", 35 cycles; β-actin: sense primer: 5'-AAGATCCTGACCGAGCGTGG-3', antisense primer: 5'-CAGCACTGTGTTGGCATAGAGG-3' fragment size: 327 bp, PCR condition: 94℃, 45"; 58℃, 45"; 72℃, 45", 35 cycles.
PCR products were separated by gel electrophoresis (1.5% agarose stained with ethidium bromide). Specific bands were visualized with an image system (FluorChem 9900; Alpha Innotech, San Leandro, CA, USA). The intensity of the bands was analyzed using ImageJ software and standardized to the β-actin signal.
5. Western blotting
Pancreatic tissues were cut into small pieces, washed with PBS (pH7.4), and then homogenized in ice-cold lysis buffer (10 mM Hepes, 10 mM KCl, 1.5 mM MgCl2, 1 mM dithiothreitol (DTT), 1.5 mM phenylmethylsulfonyl fluoride (PMSF), 20 mM NaF, 200 µM Na3VO4, and protease inhibitor cocktail). After keeping the sample on ice for 20 minutes, NP-40 was added to a final concentration of 0.5%, and then samples were incubated on ice for another 20 minutes and centrifuged at 10,000×g for 2 minutes at 4℃. The supernatants were used as cytosolic extracts for measurements of ICAM-1. The pellets were washed with PBS and re-suspended in nuclear extraction buffer (20 mM Hepes, 420 mM NaCl, 1.5 mM MgCl2, 0.2 mM EDTA, 1 mM DTT, 1.5 mM PMSF, 20 mM NaF, 200 µM Na3VO4, and protease inhibitor cocktail) for 20 minutes on ice and centrifuged at 15,000×g for 15 minutes at 4℃. The supernatants containing nuclear protein were collected for NF-κB/p65 detection.
An equal amount of protein (20 µg) was loaded into different lanes and was separated by 12% sodium dodecyl sulfate-polyacrylamide gel electrophoresis. The primary antibodies (Santa Cruz Biotechnology) were NF-κB/p65 (1:150) and ICAM-1 (1:200). A peroxidase-conjugated secondary antibody (1:1,250; Santa Cruz Biotechnology) was used, and the membrane was visualized by enhanced chemiluminescence. The intensity of the bands was quantified using ImageJ software and standardized to the signal of the control.
6. Statistical analysis
All values are presented as means±SD. The significance of differences between the two experimental groups was analyzed with the independent samples t-test using SPSS software version 15.0 (SPSS Inc., Chicago, IL, USA). Probability values less than 0.05 were considered significant.
RESULTS
1. Body weight and pancreatic oxidative stress
All animals gained body weight during the experimental period. But the body weight of rats fed high-fat diets was 122.1% of that of control rats at the end of experiment. The level of malondialdehyde increased whereas the activity of superoxide dismutase decreased significantly in pancreas of rats fed high-fat diet compared with those of control rats (data were not shown) as we reported in our previous study.6
2. H&E and Sirius red staining
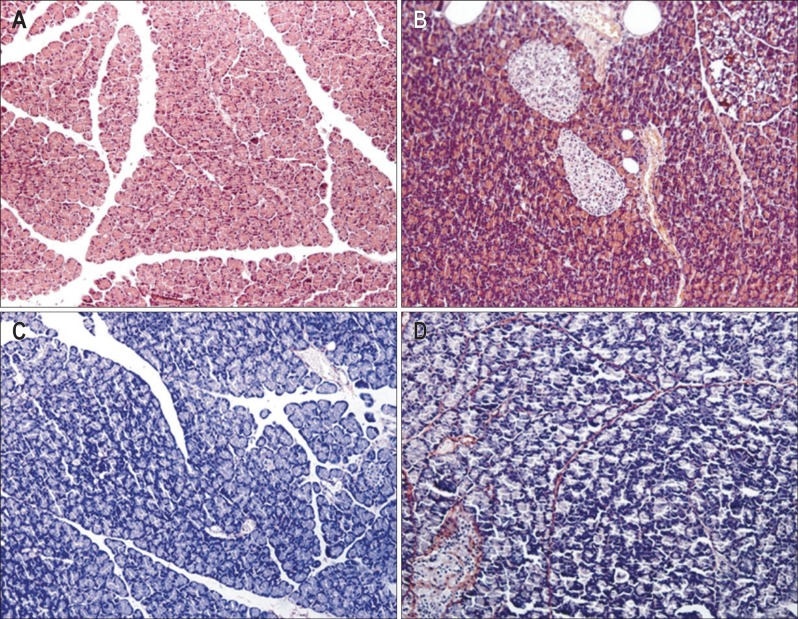
H&E stained sections from rats fed high-fats diet revealed fat deposition in partial acinar and islet cells as well as cell atrophy. Lymphocytes infiltration was also observed in these samples. In addition, the pancreatic samples of rats fed high-fat diets exhibited obvious Sirius red-stained collagen deposition surrounding the lobules and in periacinar areas of the pancreatic parenchyma. In contrast, for samples taken from the control animals no or only slight collagen deposition was presented (Table 1 and Fig. 1).
Table 1.
Histological Changes in the Pancreatic Tissue in Rats at Week 20
Data are presented as mean±SD. Histological changes were evaluated as described in Materials and Methods.
*p<0.001 vs control group.
Fig. 1.
(A, B) Hematoxylin-eosin (H&E stain, ×200) and (C, D) Sirius red staining (×200) in pancreatic samples. The collagen fibrils appear red, and the non-fibrotic areas appear blue after Sirius red staining. The stained sections display none of the obvious histopathological changes observed in the control group (A, C), but fat deposition in acinar cells (cytoplasmic vacuolization), lymphocyte infiltration and collagen deposition were observed in the high-fat diet group (B, D), indicating the presence of fibrogenesis in the pancreas after prolonged ingestion of a high-fat diet. Control, n=10; High-fat, n=12.
3. Immunohistochemical staining
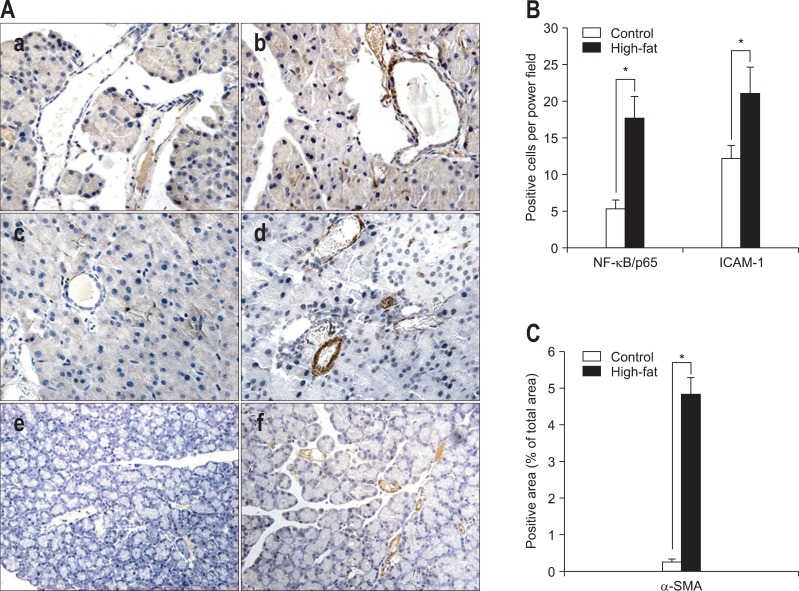
Immunohistochemical staining was used to observe the location and intensity of expressions of NF-κB/p65, ICAM-1 and α-SMA. As a result, NF-κB/p65 was found obviously expressed in both cytoplasm and the nucleus of acinar, vascular endothelial and ductal epithelial cells in samples obtained from rats fed high-fat diets (5.40±1.18 vs 17.80±2.83, p<0.001; control group vs high-fat group) (Fig. 2Aa, b, and B). ICAM-1 was expressed mainly in vascular endothelial cells and some acinar cells, and its expression was stronger in rats fed high-fat diets than in control rats (12.27±1.75 vs 21.07±3.65, p<0.001; control group vs high-fat group) (Fig. 2Ac, d, and B). In high-fat diet rat pancreas samples, α-SMA-positive staining areas were observed, which mainly presented in periacinar space (0.26±0.07 vs 4.85±0.45, p<0.001; control group vs high-fat group) (Fig. 2Ae, f, and C).
Fig. 2.
Immunohistochemical staining for nuclear factor kappa B p65 (NF-κB/p65), intercellular adhesion molecule 1 (ICAM-1), and α smooth muscle actin (α-SMA) (×400) and the corresponding statistical analysis. (A) Representative immunohistochemistry for NF-κB/p65 (a, b), ICAM-1 (c, d) and α-SMA (e, f) (control, a, c, e; high-fat, b, d, f). For NF-κB/p65 staining, cells with stained cytoplasm and a stained nucleus (brown) were considered positive. (B, C) The corresponding statistical analysis. The average number of NF-κB/p65- or ICAM-1-positive stained (brown) cells per high power field is reported. Areas staining positive for α-SMA are expressed as percentages of the total area. Data are presented as means±SD. Control, n=10; High-fat, n=12.
*p<0.001, control versus high-fat diet group.
4. Gene expression by RT-PCR
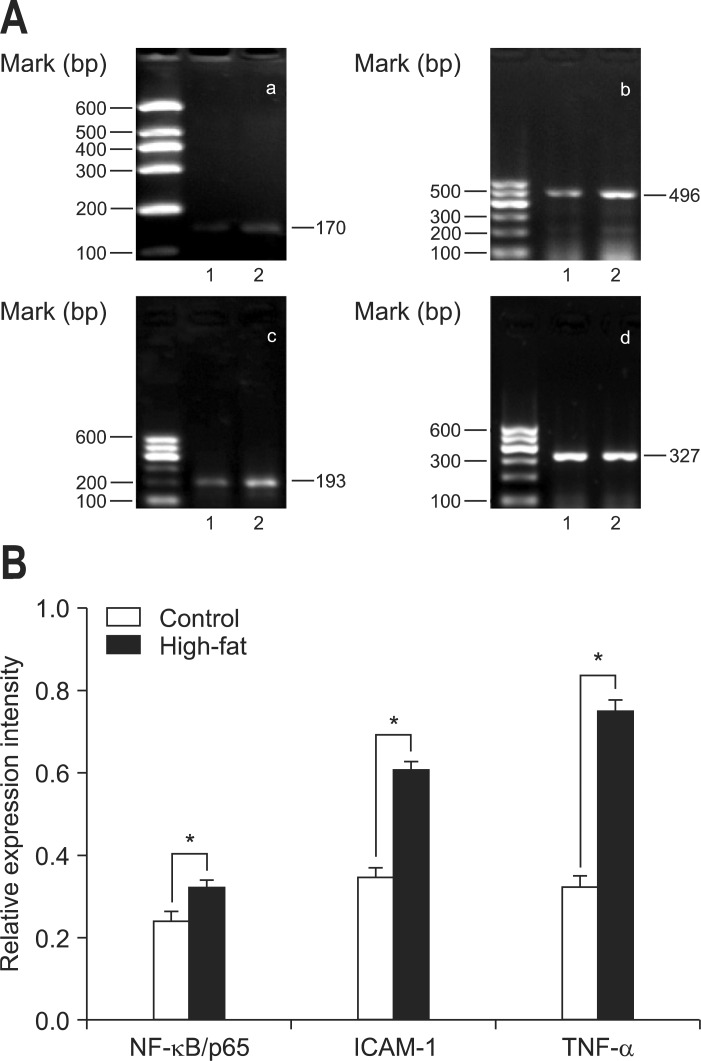
In order to observe the molecular events proceeding from a chronic high-fat diet, some suspected genes were evaluated by RT-PCR. The results demonstrated that expression of NF-κB/p65 mRNA was increased significantly in the samples taken from rats on the high-fat diet compared to those of the control rats (0.24±0.02 vs 0.32±0.02, p<0.001; control group vs high-fat group). Accordingly, both ICAM-1 and TNF-α mRNA, genes which are modulated directly by NF-κB, were also upregulated (0.35±0.02 vs 0.61±0.02, p<0.001; 0.32±0.02 vs 0.75±0.03, p<0.001; control group vs high-fat group) (Fig. 3).
Fig. 3.
Gene expression in pancreatic samples. (A) Reverse transcription-polymerase chain reaction (RT-PCR) results for nuclear factor kappa B p65 (NF-κB/p65) (a), intercellular adhesion molecule 1 (ICAM-1) (b), tumor necrosis factor α (TNF-α) (c), and β-actin (d). Representative RT-PCR results are displayed. (B) The relative intensity of PCR production bands was analyzed using ImageJ software (National Institutes of Health). Data are expressed as the ratio of each mRNA to the corresponding β-actin mRNA. Data are presented as means±SD. Control, n=10; High-fat, n=12.
*p<0.001, control (1) versus the high-fat (2) diet group.
5. Protein expression by Western blot
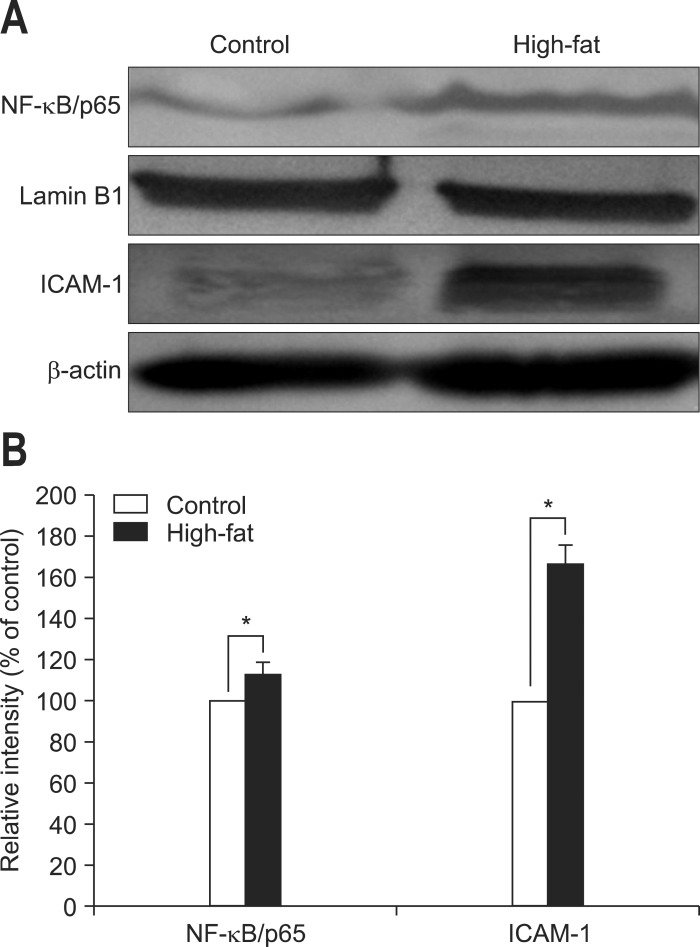
Western blotting was used to evaluate the level of NF-κB/p65 in nuclear proteins and the levels of ICAM-1 in cytosolic proteins. As a result, the amount of intra-nuclear NF-κB/p65 in samples of rats fed the high-fat diet was significantly higher than that of rats fed the normal diet (1.00±0.00 vs 1.13±0.17, p<0.001; control group versus high-fat group). In addition, the total amounts of ICAM-1was also elevated significantly in rats fed the high-fat diet in comparison to those in rats fed the normal diet (1.00±0.00 vs 1.67±0.09, p<0.001; control group vs high-fat group) (Fig. 4).
Fig. 4.
Protein expression in pancreatic samples. (A) Western blots displaying the expression of intra-nuclear protein nuclear factor kappa B (NF-κB)/p65 and cytosolic protein intercellular adhesion molecule 1 (ICAM-1). A representative image from three experiments is displayed. (B) The relative intensity of the Western blot bands was analyzed using ImageJ software. The data are expressed as percentages of the control values. Data are presented as means±SD. Control, n=10; High-fat, n=12.
*p<0.001, control versus high-fat diet.
DISCUSSION
Many researchers have found PSCs activation and TGF-β1 upregulation as well as pancreatic fibrosis in the pancreas after feeding animals high-fat diets, however, the exact mechanisms of high-fat diet-related pancreatic fibrogenesis is not well defined. Previous studies demonstrated increased oxidative stress in rat pancreases after feeding high-fat diets,2,6,16 and the pancreatic fibrosis was found to be ameliorated by inhibiting oxidative stress.17,18 These findings suggest a potent role for oxidative stress in high-fat diet-related pancreatic fibrogenesis.
Thus, NF-κB, a ROS-sensitive transcription factor, might be involved in the underlying molecular pathogenesis of this pancreatic abnormality. Activated NF-κB regulates the expression of many molecules, including cytokines and adhesion molecules. TNF-α, a pro-inflammatory cytokine, can effect the NF-κB pathway and promote its activation.19 In our present study, we found PSC activation and fibrogenesis in pancreatic tissues of rats fed the high-fat diet, and we also found elevated levels of NF-κB/p65 mRNA and higher intra-nuclear p65 protein expression in these samples according to RT-PCR, immunohistochemistry and Western blot. There is abundant evidence that oxidative stress-related NF-κB activation is associated with inflammation and fibrosis,20,21 and the blockade of NF-κB activity could prevent the progression of chronic pancreatitis through inhibition of ECM synthesis and pro-inflammatory cytokine production.22 Therefore, we suppose that NF-κB might be involved in not only pancreatic inflammation but also fibrosis in the rats fed high-fat diets.
On the other hand, we also found that both ICAM-1 and TNF-α, the downstream molecules of NF-κB, were upregulated. These findings suggest that a long-term high-fat diet results in an activated NF-κB signaling pathway in the pancreas. Elevated TNF-α in the pancreas may act as a positive feedback for reactivation of NF-κB in turn, and promote further development of this disease.
As we know, TGF-β is a pro-fibrogenetic factor and a major mediator of pancreatic fibrosis which can promote PSC activation and ECM secretion.9 Recent studies have demonstrated a crosstalk between NF-κB and TGF-β signaling pathways: 1) NF-κB can elicit TGF-β gene transcription;23 2) the suppression of NF-κB decreases the autocrine of PSCs by TGF-β1;24 3) over-expressed Smad7 blocks NF-κB activation and inflammatory cytokine and ICAM-1 expression, and inhibits fibrosis.25,26 These results suggest a close relationship between NF-κB activation and TGF-β expression. Accordingly in our study, we have found over-expressed NF-κB/p65 in pancreatic tissues taken from the rats fed high-fat diets. This upregulation of NF-κB might contribute to TGF-β1 expression.
Therefore, based on the results of our study, we suppose that the mechanism of high-fat diet-associated pancreatic fibrosis might be partly due to NF-κB activation following over-expression of pro-inflammatory cytokines, which can incite interactions of different molecules, and initiate the pancreatic fibrogenesis.
We acknowledge that there are limitations in our study. It is unclear whether the pancreatic histological changes would be ameliorated automatically if the high-fat diet were stopped. Moreover, there are other molecules that are involved in NF-κB activation, such as I-κB kinase and other proteins. Other signalling pathways which are associated with PSCs activation, such as MAPKs and JAK/STAT pathways were not investigated in our study. But our study may have significant clinical implications. We are facing a global epidemic of obesity and there is already widespread appreciation for the links between obesity and metabolic disorders. Future clinical and experimental studies to investigate the profound underlying relationships between a high-fat diet, obesity and pancreatic damage should be encouraged.
ACKNOWLEDGEMENTS
The authors would like to thank Dr. William Tam at Royal Adelaide Hospital, Australia for critical reading of the manuscript. This work was supported by grants from the Department of Science & Technology and the Department of Public Health of Shandong Province, China (No. 2009HZ069).
Footnotes
No potential conflict of interest relevant to this article was reported.
References
- 1.Popov D, Simionescu M, Shepherd PR. Saturated-fat diet induces moderate diabetes and severe glomerulosclerosis in hamsters. Diabetologia. 2003;46:1408–1418. doi: 10.1007/s00125-003-1185-6. [DOI] [PubMed] [Google Scholar]
- 2.Qiu L, List EO, Kopchick JJ. Differentially expressed proteins in the pancreas of diet-induced diabetic mice. Mol Cell Proteomics. 2005;4:1311–1318. doi: 10.1074/mcp.M500016-MCP200. [DOI] [PubMed] [Google Scholar]
- 3.Fraulob JC, Ogg-Diamantino R, Fernandes-Santos C, Aguila MB, Mandarim-de-Lacerda CA. A mouse model of metabolic syndrome: insulin resistance, fatty liver and non-alcoholic fatty pancreas disease (NAFPD) in C57BL/6 mice fed a high fat diet. J Clin Biochem Nutr. 2010;46:212–223. doi: 10.3164/jcbn.09-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Borst SE, Conover CF. High-fat diet induces increased tissue expression of TNF-alpha. Life Sci. 2005;77:2156–2165. doi: 10.1016/j.lfs.2005.03.021. [DOI] [PubMed] [Google Scholar]
- 5.Mathur A, Marine M, Lu D, et al. Nonalcoholic fatty pancreas disease. HPB (Oxford) 2007;9:312–318. doi: 10.1080/13651820701504157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yan MX, Li YQ, Meng M, Ren HB, Kou Y. Long-term high-fat diet induces pancreatic injuries via pancreatic microcirculatory disturbances and oxidative stress in rats with hyperlipidemia. Biochem Biophys Res Commun. 2006;347:192–199. doi: 10.1016/j.bbrc.2006.06.063. [DOI] [PubMed] [Google Scholar]
- 7.Zhang X, Cui Y, Fang L, Li F. Chronic high-fat diets induce oxide injuries and fibrogenesis of pancreatic cells in rats. Pancreas. 2008;37:e31–e38. doi: 10.1097/MPA.0b013e3181744b50. [DOI] [PubMed] [Google Scholar]
- 8.Asaumi H, Watanabe S, Taguchi M, Tashiro M, Otsuki M. Externally applied pressure activates pancreatic stellate cells through the generation of intracellular reactive oxygen species. Am J Physiol Gastrointest Liver Physiol. 2007;293:G972–G978. doi: 10.1152/ajpgi.00018.2007. [DOI] [PubMed] [Google Scholar]
- 9.Omary MB, Lugea A, Lowe AW, Pandol SJ. The pancreatic stellate cell: a star on the rise in pancreatic diseases. J Clin Invest. 2007;117:50–59. doi: 10.1172/JCI30082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Leung PS, Chan YC. Role of oxidative stress in pancreatic inflammation. Antioxid Redox Signal. 2009;11:135–165. doi: 10.1089/ars.2008.2109. [DOI] [PubMed] [Google Scholar]
- 11.Shi H, Kokoeva MV, Inouye K, Tzameli I, Yin H, Flier JS. TLR4 links innate immunity and fatty acid-induced insulin resistance. J Clin Invest. 2006;116:3015–3025. doi: 10.1172/JCI28898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim F, Pham M, Luttrell I, et al. Toll-like receptor-4 mediates vascular inflammation and insulin resistance in diet-induced obesity. Circ Res. 2007;100:1589–1596. doi: 10.1161/CIRCRESAHA.106.142851. [DOI] [PubMed] [Google Scholar]
- 13.Mews P, Phillips P, Fahmy R, et al. Pancreatic stellate cells respond to inflammatory cytokines: potential role in chronic pancreatitis. Gut. 2002;50:535–541. doi: 10.1136/gut.50.4.535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shek FW, Benyon RC, Walker FM, et al. Expression of transforming growth factor-beta 1 by pancreatic stellate cells and its implications for matrix secretion and turnover in chronic pancreatitis. Am J Pathol. 2002;160:1787–1798. doi: 10.1016/s0002-9440(10)61125-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Masamune A, Sakai Y, Kikuta K, Satoh M, Satoh A, Shimosegawa T. Activated rat pancreatic stellate cells express intercellular adhesion molecule-1 (ICAM-1) in vitro. Pancreas. 2002;25:78–85. doi: 10.1097/00006676-200207000-00018. [DOI] [PubMed] [Google Scholar]
- 16.Czakó L, Szabolcs A, Vajda A, et al. Hyperlipidemia induced by a cholesterol-rich diet aggravates necrotizing pancreatitis in rats. Eur J Pharmacol. 2007;572:74–81. doi: 10.1016/j.ejphar.2007.05.064. [DOI] [PubMed] [Google Scholar]
- 17.Gómez JA, Molero X, Vaquero E, Alonso A, Salas A, Malagelada JR. Vitamin E attenuates biochemical and morphological features associated with development of chronic pancreatitis. Am J Physiol Gastrointest Liver Physiol. 2004;287:G162–G169. doi: 10.1152/ajpgi.00333.2003. [DOI] [PubMed] [Google Scholar]
- 18.Tasci I, Deveci S, Isik AT, et al. Allopurinol in rat chronic pancreatitis: effects on pancreatic stellate cell activation. Pancreas. 2007;35:366–371. doi: 10.1097/mpa.0b013e31806dbaaa. [DOI] [PubMed] [Google Scholar]
- 19.Chen F, Castranova V, Shi X, Demers LM. New insights into the role of nuclear factor-kappaB, a ubiquitous transcription factor in the initiation of diseases. Clin Chem. 1999;45:7–17. [PubMed] [Google Scholar]
- 20.Jokelainen K, Reinke LA, Nanji AA. Nf-kappab activation is associated with free radical generation and endotoxemia and precedes pathological liver injury in experimental alcoholic liver disease. Cytokine. 2001;16:36–39. doi: 10.1006/cyto.2001.0930. [DOI] [PubMed] [Google Scholar]
- 21.Parola M, Robino G. Oxidative stress-related molecules and liver fibrosis. J Hepatol. 2001;35:297–306. doi: 10.1016/s0168-8278(01)00142-8. [DOI] [PubMed] [Google Scholar]
- 22.Hisada S, Shimizu K, Shiratori K, Kobayashi M. Peroxisome proliferator-activated receptor gamma ligand prevents the development of chronic pancreatitis through modulating NF-kappaB-dependent proinflammatory cytokine production and pancreatic stellate cell activation. Rocz Akad Med Bialymst. 2005;50:142–147. [PubMed] [Google Scholar]
- 23.Strauch ED, Yamaguchi J, Bass BL, Wang JY. Bile salts regulate intestinal epithelial cell migration by nuclear factor-kappa B-induced expression of transforming growth factor-beta. J Am Coll Surg. 2003;197:974–984. doi: 10.1016/S1072-7515(03)00720-8. [DOI] [PubMed] [Google Scholar]
- 24.Shinozaki S, Mashima H, Ohnishi H, Sugano K. IL-13 promotes the proliferation of rat pancreatic stellate cells through the suppression of NF-kappaB/TGF-beta1 pathway. Biochem Biophys Res Commun. 2010;393:61–65. doi: 10.1016/j.bbrc.2010.01.078. [DOI] [PubMed] [Google Scholar]
- 25.Ka SM, Huang XR, Lan HY, et al. Smad7 gene therapy ameliorates an autoimmune crescentic glomerulonephritis in mice. J Am Soc Nephrol. 2007;18:1777–1788. doi: 10.1681/ASN.2006080901. [DOI] [PubMed] [Google Scholar]
- 26.He J, Sun X, Qian KQ, Liu X, Wang Z, Chen Y. Protection of cerulein-induced pancreatic fibrosis by pancreas-specific expression of Smad7. Biochim Biophys Acta. 2009;1792:56–60. doi: 10.1016/j.bbadis.2008.10.010. [DOI] [PubMed] [Google Scholar]