Abstract
Objective
Pregnancy is a common indication for initiation of highly active antiretroviral therapy (HAART) in sub-Saharan Africa. Our objective was to evaluate how pregnancy at treatment initiation predicts virologic response to HAART.
Methods
We evaluated an open cohort of 9,173 patients who initiated HAART between April 2004 and September 2009 in the Themba Lethu Clinic in Johannesburg, South Africa. Risk ratios were estimated using log-binomial regression; hazard ratios were estimated using Cox proportional hazards models; time ratios were estimated using accelerated failure time models. We controlled for calendar date, age, ethnicity, employment status, history of smoking, tuberculosis, WHO stage, weight, body mass index, hemoglobin, CD4 count and CD4 percent, and whether clinical care was free. Extensive sensitivity and secondary analyses were performed.
Results
During follow-up, 822 non-pregnant women and 70 pregnant women experienced virologic failure. In adjusted analyses, pregnancy at baseline was associated with reduced risk of virologic failure by six months (risk ratio 0.66, 95% confidence limits [CL] 0.35, 1.22) and with reduced hazard of virologic failure over follow-up (hazard ratio 0.69, 95% CL 0.50, 0.95). The adjusted time ratio for failure was 1.44 (95% CL 1.13, 1.84), indicating 44% longer time to event among women pregnant at baseline. Sensitivity analyses generally confirmed main findings.
Conclusion
Pregnancy at HAART initiation is not associated with increased risk of virologic failure at six months or during longer follow-up.
Keywords: Pregnancy, HIV, highly active antiretroviral therapy (HAART), South Africa
INTRODUCTION
Women of child-bearing age constitute the single largest group of people living with HIV in sub-Saharan Africa.1 In South Africa and elsewhere, pregnancy is a common indication for the initiation of highly active antiretroviral therapy (HAART), for the prevention of mother to child transmission;2, 3 among pregnant women, HIV prevalence has been estimated as high as 40% in some settings.4, 5
There are many reasons to hypothesize that response to HAART may be affected by pregnancy. Changes in body mass and blood volume may lead to drug underdosing. Pregnancy may lead to changes in metabolism of nevirapine and lopinavir.6-9 Rising levels of beta-estradiol during pregnancy may attenuate the effect of stavudine.10, 11 Finally, social and personal issues including stigma and intimate partner violence (negative) and desire to protect an unborn child (positive) may affect adherence.12
While a number of studies have examined prevention of mother to child transmission of HIV and subsequent response to HAART,13, 14 issues of fertility during HAART,15 and more generally the effects of pregnancy on HIV disease progression in the pre-HAART era,16 there are few published reports examining the impact of pregnancy on maternal response to HAART in sub-Saharan Africa.12, 17, 18 Here, we examine pregnancy at time of HAART initiation as well as biological sex as predictors of virologic outcomes of HAART.
METHODS
Study population and design
We performed an observational cohort study in the database of the Themba Lethu Clinic3. The Themba Lethu Clinic (TLC) Cohort comprises adults initiating HAART in Johannesburg, South Africa. TLC is located within Helen Joseph Hospital in urban Johannesburg, and is the largest single clinic providing HAART in South Africa.
Patients starting HAART between 1 April 2004 and 30 September 2009 were selected for study inclusion; end of follow-up was set on 31 March 2010, due to last visit, transfer of care, administrative reasons, drop-out or death. Only antiretroviral therapy-naïve patients were selected. 19 Subjects were also limited to ages 18-45, because the database included only a single woman over age 45 who initiated HAART while pregnant.18
Typical first-line HAART in South Africa included stavudine, lamivudine, and efavirenz; due to concerns about teratogenicity, women pregnant at baseline are typically placed on the boosted protease inhibitor Kaletra (lopinavir and ritonavir) rather than efavirenz. Non-pregnant women with declared pregnancy intention are typically placed on nevirapine rather than efavirenz due to concerns about teratogenicity of efavirenz.20 Other aspects of the TLC clinical database have been described previously.3, 18, 21
Definitions and data
The main exposure in this study was first biological sex (male or female), and then among females, prevalent pregnancy: that is, pregnancy present at baseline, the time of HAART initiation. This is distinguished from incident pregnancy which occurs subsequent to HAART initiation for a woman's health;15, 18 prevalent pregnancy is in general the cause for a recognition of an HIV infection requiring treatment, especially for initiation of HAART. Incident pregnancies were ignored in this analysis, but have been addressed elsewhere.18 For this analysis, we conducted additional review of the records of women who were on Kaletra at baseline but were not marked as pregnant in the database.
The main outcome in this study was time to virologic failure defined as either a failure to achieve virologic suppression of plasma HIV-1 RNA to less than 400 copies/ml within six months of HAART initiation, or a viral rebound to above 400 copies at any time after initial suppression.18, 22 A second outcome was virologic failure by six months (a dichotomous outcome). When possible, confirmation of outcome by a second viral load test within 30 days was obtained, but we included failures from patients missing a confirmatory sample.
Statistical analysis
We used simple descriptive statistics for baseline characteristics of individuals. We examined time to virologic failure using Kaplan-Meier-type cumulative incidence curves. We estimated relative risk of failure to suppress by six months using log-binomial (risk) regression (and logistic regression where noted). Last, we used accelerated failure time models to estimate relative time to virologic failure.
In multivariable analyses, we considered the following confounders based on previous literature and plausible biological mechanism: calendar date of HAART initiation, age, ethnicity, employment status, history of smoking, tuberculosis, WHO stage, weight, body mass index, hemoglobin, CD4 count and CD4 percent, and whether there was any charge for being seen in clinic (discontinued as of October 2006). We used restricted cubic splines to flexibly control for age, body mass index, weight, CD4 count, and time-on-study.
We did not control for baseline viral load because it is collected in less than 25% of participants; instead, we performed a sensitivity analysis restricted to women who had suppressed virus by six months of follow-up to eliminate impacts of baseline viral load. We likewise did not control for baseline or time-updated drug regimen in main analysis (but did so in sensitivity analysis), because drug regimen is determined chiefly by pregnancy status (as well as pregnancy intentions), and is thus may be part of an effect of pregnancy on the outcome.
RESULTS
The study population comprised 9,173 men and women at time of HAART initiation, of which approximately two-thirds (n=5,997) were women. Of the 5,997 women, 587 were pregnant at baseline. These 9,173 individuals were followed up for a median of 18 (interquartile range [IQR] 8, 37) months until virologic failure, death, loss-to-follow-up, transfer, or administrative end of follow-up; maximum follow-up time was 72 months. At baseline HAART initiation, about 14% of pregnant women were in their first trimester, 43% were in their second trimester, and 43% were in their last trimester.
Baseline characteristics of all subjects are described in Table 1. In general, men and non-pregnant women were similar at baseline, although men generally had indicators of more advanced disease status. For example, comparing men to non-pregnant women, men had lower median body mass index (BMI; 20.1 vs. 22.2 kg/m2), more active tuberculosis (22.1% vs. 17.7%), and were more likely to have CD4 count ≤ 50 cells/mm3 (40.5% vs. 32.9%). However, as expected, pregnant women were substantially healthier than either men or non-pregnant women. For example, median CD4 cell count at baseline was 74 cells/mm3 among men, 92 cells/mm3 among non-pregnant women, and 156 cells/mm3 among pregnant women. And likewise as expected, only 1.6% of pregnant women had a BMI < 18.5 kg/m2, compared to 27.1% and 17.9% of men and non-pregnant women, respectively.
Table 1.
Characteristics of men and non-pregnant and pregnant women at time of HAART initiation.
| Demographics | Male (n=3,176) | Female, non-pregnant (n = 5,410) | Female, pregnant (n = 587) |
|---|---|---|---|
| Follow-up time months | 16 (7, 36) | 19 (9, 37) | 22 (8, 48) |
| Age years | 36 (32, 40) | 34 (29, 38) | 30 (26, 33) |
| Employed | 1642 (51.7) | 2194 (40.6) | 218 (37.1) |
| History of smoking | 593 (18.7) | 264 (4.9) | 26 (4.4) |
| Clinical | |||
| HAART regimen | |||
| d4t-3TC-EFV | 2761 (86.9) | 4580 (84.7) | 38 (6.5) |
| d4t-3TC-NVP | 177 (5.6) | 473 (8.7) | 58 (9.9) |
| d4t-3TC-LPVr | 48 (1.5) | 80 (1.5) | 479 (81.6) |
| Weight kg | 59 (53, 66) | 57 (49, 65) | 68 (60, 77) |
| Body mass index kg/m2 | 20.1 (18.3, 22.3) | 22.2 (19.4, 25.5) | 26.5 (23.3, 29.7) |
| Body mass index category | |||
| < 18.5 | 818 (27.1) | 927 (17.9) | 9 (1.6) |
| 18.5-24.9 | 1901 (63.0) | 2813 (54.3) | 198 (35.9) |
| 25.0-29.9 | 252 (8.4) | 961 (18.6) | 221 (40.1) |
| ≥ 30 | 46 (1.5) | 478 (9.2) | 123 (22.3) |
| WHO stage III or IV | 1521 (47.9) | 2355 (43.5) | 105 (17.9) |
| Current tuberculosis | 701 (22.1) | 957 (17.7) | 22 (3.8) |
| Laboratory | |||
| Hemoglobin, low‡ | 1581 (51.1) | 2884 (54.8) | 131 (31.0) |
| CD4 count cells/mm3 | 74 (22, 149) | 92 (35, 162) | 156 (103, 200) |
| CD4 count category | |||
| ≤ 50 | 1245 (40.5) | 1727 (32.9) | 48 (8.8) |
| 51-100 | 593 (19.3) | 1062 (20.2) | 85 (15.6) |
| 101-200 | 937 (30.5) | 1843 (35.1) | 280 (51.3) |
| 201-350 | 261 (8.5) | 508 (9.7) | 124 (22.7) |
| > 350 | 40 (1.3) | 115 (2.2) | 9 (1.7) |
| CD4 % | 6.3 (2.8, 10.5) | 7.2 (3.7, 11.5) | 11.6 (7.8, 15.5) |
| Viral load†log10 copies/ml | 4.3 (3.6, 4.6) | 4.2 (3.5, 4.6) | 3.8 (1.7, 4.1) |
| Viral load category | |||
| ≤ 400 | 163 (17.9) | 289 (19.1) | 31 (41.9) |
| 401-10,000 | 131 (14.4) | 244 (16.1) | 20 (27.0) |
| > 10,000 | 615 (67.7) | 979 (64.8) | 23 (31.1) |
Categorical variables expressed as number (% total); continuous variables as median (interquartile range).
After adjustment for altitude, lower limit of normal hemoglobin is 12.35 g/dl for men and 11.35 (10.35) g/dl for non-pregnant (pregnant) women.
Viral load was missing in 71% of men, 72% of non-pregnant women, and 88% of pregnant women.
During all of follow-up, a total of 472 men (14.9%), 822 (15.2%) non-pregnant women, and 70 (11.9%) prevalent pregnant women experienced the outcome of failure to suppress or subsequent virologic failure. The clear majority of these failures (65%) were unconfirmed within 30 days. Over follow-up, 408 (7.5%) non-pregnant women, 297 (9.3%) men, and 17 (2.9%) pregnant women died; while 1298 (24.0%) non-pregnant women, 919 (28.9%) men, and 234 (39.9%) pregnant women become lost to follow-up.
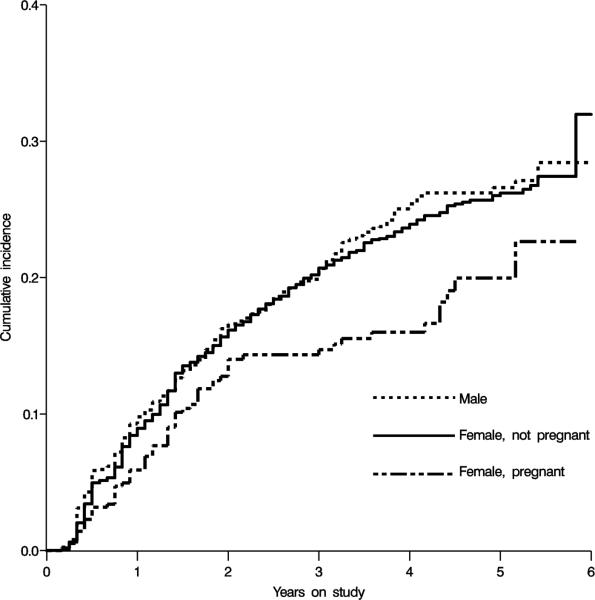
The cumulative incidence of virologic failures in non-pregnant women, pregnant women, and men are shown in Figure 1. There was no difference in hazard of failure comparing non-pregnant women to men (crude hazard ratio [HR]=0.95, 95% confidence limit [CL] 0.85, 1.07; adjusted HR=0.98, 95% CL 0.84, 1.14); accordingly, men were excluded from further analysis.
Figure 1.
Crude cumulative incidence curves for the effect of pregnancy on time to virologic failure among men, non-pregnant women, and pregnant women initiating HAART in Johannesburg, South Africa.
Results comparing pregnant women to non-pregnant women are reported in Table 2. The crude HR for the outcome of virologic failure comparing only pregnant women to non-pregnant women over all follow-up was 0.73 (95% CL 0.57, 0.93), and the adjusted was similar at 0.69 (95% CL 0.50, 0.95). The time ratios comparing relative time to virologic failure among pregnant and non-pregnant women were calculated in crude accelerated failure time models under gamma, Weibull, and exponential distributions. All distributions yielded similar inferences; the crude time ratio under an exponential distribution was 1.44 (95% CL 1.13, 1.84), and an adjusted time ratio was similar but less precise.
Table 2.
Association of prevalent pregnancy with virologic failure from main and sensitivity analyses. All models compare prevalent pregnant women with non-pregnant women.
| Model | Effect | Estimate | 95% CL |
|---|---|---|---|
| All of follow-up | |||
| Crude | Hazard ratio | 0.73 | 0.57, 0.93 |
| Adjusted† | Hazard ratio | 0.69 | 0.50, 0.95 |
| Adjusted† | Time ratio | 1.44 | 1.13, 1.84 |
| Six-month | |||
| Crude | Risk ratio | 0.66 | 0.41, 1.06 |
| Adjusted† | Risk ratio | 0.66 | 0.35, 1.22 |
| Sensitivity analysis† | |||
| No failure by six months | Hazard ratio | 0.69 | 0.47, 1.02 |
| Confirmed failures only | Hazard ratio | 0.54 | 0.30, 0.97 |
| By trimester | |||
| First trimester | Hazard ratio | 0.72 | 0.42, 1.22 |
| Second trimester | Hazard ratio | 0.60 | 0.34, 1.05 |
| Third trimester | Hazard ratio | 0.86 | 0.43, 1.75 |
| Adjusting for drug regimen | |||
| Six-month | Risk ratio | 0.41 | 0.16, 1.00 |
| All of follow-up | Hazard ratio | 0.50 | 0.30, 0.85 |
| Adjusting for adherence | Hazard ratio | 0.70 | 0.50, 0.97 |
| By baseline CD4 count | |||
| CD4 > 200 cells/mm3 | Hazard ratio | 0.30 | 0.10, 0.87 |
| CD4 ≤ 200 cells/mm3 | Hazard ratio | 0.76 | 0.54, 1.07 |
| Started HAART for health | Hazard ratio | 0.73 | 0.51, 1.03 |
CL, confidence limits.
All adjusted models (including all sensitivity analyses) control for calendar date of HAART initiation, age, ethnicity, employment status, history of smoking, tuberculosis, WHO stage, weight, body mass index, hemoglobin, CD4 count and CD4 percent, and whether there was any charge for being seen in clinic (discontinued as of October 2006).
Considering only the first six months, 251 (4.6%) of non-pregnant women and only 18 (3.1%) of pregnant women had experienced virologic failure. Again among women only, the crude risk ratio (RR) for the effect of prevalent pregnancy on risk of failure to suppress virus by six months was 0.66 (95% CL 0.41, 1.06); the adjusted RR was 0.66 (95% CL 0.35, 1.22); the adjusted HR was similar.
Sensitivity analyses
We performed several sensitivity analyses, all adjusted for confounding and summarized in Table 2. First, we limited analyses to women who had not failed HAART by six months of follow-up, and examined time to virologic failure from six months onward as in the main analysis. We found results very similar to the main “all follow-up” analysis (adjusted HR=0.69, 95% CL 0.47, 1.02). Second, we analyzed only confirmed virologic failures (295/822 in non-pregnant women; 19/70 in pregnant women) and found a stronger though less precise effect (HR=0.54, 95% CL 0.30, 0.97) than in main analysis. Third, we analyzed by trimester of pregnancy at HAART initiation; this analysis yielded similar though less precise results overall, with no clear temporal trends. Fourth and fifth, we controlled for whether initial drug regimen contained nevirapine or Kaletra versus efavirenz. In the six-month analysis (analysis 4), we found RR=0.41 (95% CL 0.16, 1.00) (estimated using logistic regression); in the all-follow-up analysis (analysis 5) we found HR=0.50 (95% CL 0.30, 0.85). Sixth, we controlled for time-updated adherence, to see if this might be a pathway by which baseline pregnancy affected virologic failure; results suggested that it was not (HR=0.70, 95% CL 0.50, 0.97), although residual confounding of the mediator-outcome relationship may be present.23 Seventh, we examined time to virologic failure by baseline CD4 count. In women with baseline CD4 > 200 cells/mm3, the association of pregnancy with virologic failure was stronger (HR=0.30, 95% CL 0.10, 0.87); in women with baseline CD4 ≤ 200 cells/mm3, the effect was slightly weaker (HR=0.76, 95% CL 0.54, 1.07), consistent with the overall findings. Last, we analyzed only among women who were indicated to start HAART for their own health: those with CD4<200 cells/mm3, or in WHO stage IV, finding HR=0.73 (95% CL 0.51, 1.03) in this group.
DISCUSSION
The WHO has stated that women's health should be “the overarching priority in decisions about ARV treatment during pregnancy.”24 But despite this, relatively little remains known about how or whether pregnancy predicts response to HAART. Here we found that, among women who remain alive and in care in our cohort, pregnancy is associated with lower risks and hazards of virologic failure over follow-up. Notably, time to virologic failure was 44% longer among (prevalent) pregnant women than among non-pregnant women. These results were generally confirmed in sensitivity analyses addressing likely alternative scenarios (Table 2).
One possible reason for this observed effect might be dynamic observation, or diagnostic, bias. In particular, pregnant women might have fewer viral loads, or have more time between viral loads, than non-pregnant women; for example, if antenatal care visits during pregnancy caused visits to our clinic to be scheduled further apart. If this were the case, then the observed longer time to failure might be the result of less detection of failure (rather than less failure, per se). In the first six months (the period in which these women were pregnant), the HR comparing timing of new viral loads between pregnant and non-pregnant women was 0.84 (95% CL 0.75, 0.95); and after the first six months, the HR was essentially null at 0.97 (95% CL 0.91, 1.04). Since the association of pregnancy on virologic failure after six months (first sensitivity analysis) was identical to the main analysis, diagnostic bias seems unlikely to explain away these findings.
ART Guidelines in South Africa during the study period20 stated that pregnant women should initiate HAART at a CD4 count <200 cells/mm3 or a WHO stage IV. More recent guidelines25 recommend starting pregnant women at CD4 ≤ 350 cells/mm3. However, in this study 24% of pregnant women who initiated HAART had both CD4 > 200 cells/mm3 and WHO stage of III or lower, suggesting that clinician preferences played some role in determining HAART initiation. Sensitivity analysis which found somewhat different associations of pregnancy with time to virologic failure by baseline CD4 cell count may speak to such clinician preferences; but in neither baseline CD4 cell count stratum did the point estimate indicate an increased risk of virologic failure among pregnant women.
Rates of lost to follow-up were high among these subjects, and indeed were substantially higher among pregnant than non-pregnant women, similar to what was seen in a previous study from South Africa.17 Among non-pregnant women and men many of these losses are likely to be deaths,19 but among pregnant women “lost to follow-up” often does not mean “lost from care” (such as from death, or leading rapidly to death as antiretroviral therapy is abandoned), but instead transfer to perinatal care centers that were not tracked. The observed relation of trimester of pregnancy to hazard of lost to follow-up is consistent with this, where the earlier in pregnancy women enter care, the more likely they are to be lost to follow-up. Compared to non-pregnant women, the HR for becoming lost is 2.3 (95% CL 1.7, 3.1) among women who initiate HAART in their first trimester, 1.9 (95% CL 1.4, 2.6) among women who initiate in their second trimester, and 1.2 (95% CL 0.6, 2.1) among women who initiate in their third trimester. Thus, comparing rates of loss to follow-up between these two populations may be misleading.
While associated with lower risks of virologic failure, it is likely not the case that pregnancy (in this case, prevalent pregnancy) causes lower rates of failure. Indeed, previous work in this cohort suggests that incident pregnancy increases the risk of virologic failure.18 In this study, many women who initiate HAART while pregnant are initiating because of pregnancy, while those who initiate while non-pregnant are, universally, initiating because they are sick. As importantly, pregnant women with low CD4 counts or who are in WHO stage IV were nonetheless healthy enough to become pregnant in the first place. Thus, even exposure groups comparable in measured health status may remain fundamentally non-comparable due to indication, arguing against a causal interpretation of pregnancy in this report. Instead, we argue that current standard of care for women initiating HAART at baseline appears to be adequate in this setting, not associated with increased risks of virologic failure.
Notably, there was little confounding apparent in this analysis: crude and adjusted models were similar, despite control for an extensive list of confounders. This may reflect that there are few factors which affect risk of virologic failure beyond adherence to drugs and drug regimen itself, and that neither factor could be a confounder of the prevalent pregnancy-virologic response relationship because both occur after the exposure begins (in this analysis, women were pregnant before initiating a particular HAART regimen, and before their adherence can be measured). Nonetheless, we explored whether the effect of pregnancy on virologic response changed while controlling for these post-pregnancy factors as a kind of mediation analysis, albeit one whose causal interpretability may be limited (as discussed above). Controlling for adherence did not alter the main effect estimates; the model controlling for drug regimen suggested that increased use of Kaletra or nevirapine during pregnancy (rather than efavirenz) may lead to relatively increased risks of virologic failure among pregnant women. Thus, both models failed to explain the overall reduced risks observed in main analysis; determining why prevalent pregnancy is associated with lower rates of virologic failure should be a priority in future investigations.
In conclusion, pregnancy at time of HAART initiation was not associated with increased hazards or risks of virologic failure over follow-up, leading us to conclude that current clinical management of women who initiate HAART during pregnancy is likely to be adequate. However, there is preliminary evidence that choice of drug regimen associated with pregnancy may be leading to somewhat elevated risks of virologic failure.
ACKNOWLEDGEMENTS
We thanks the patients of the Themba Lethu Clinic.
FUNDING: The clinical activities of the Helen Joseph Hospital are supported by the National and Gauteng Department of Health and the United States President's Emergency Plan for AIDS Relief (PEPFAR) in a grant by USAID to Right to Care and the Institution (674-A-00-08-00007-00). The research activities of this publication are supported by the National Institutes of Health in a grant from the Eunice Kennedy Shriver NICHD 4R00-HD-06-3961-03 and NIAID 2P30-AI064518-06 Duke Center for AIDS Research. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Footnotes
HUMAN SUBJECTS APPROVAL
This research was declared exempt from review by both the University of the Witwatersrand and Duke University.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.UNAIDS [6 July 2010];AIDS Epidemic Update. 2009 http://wwwunaidsorg/en/dataanalysis/epidemiology/2009aidsepidemicupdate/ 2009; Available from.
- 2.Black V, Hoffman RM, Sugar CA, Menon P, Venter F, Currier JS, et al. Safety and efficacy of initiating highly active antiretroviral therapy in an integrated antenatal and HIV clinic in Johannesburg, South Africa. J Acquir Immune Defic Syndr. 2008;49(3):276–81. doi: 10.1097/QAI.0b013e318189a769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sanne IM, Westreich D, Macphail AP, Rubel D, Majuba P, Van Rie A. Long term outcomes of antiretroviral therapy in a large HIV/AIDS care clinic in urban South Africa: a prospective cohort study. J Int AIDS Soc. 2009;12:38. doi: 10.1186/1758-2652-12-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pettifor AE, Rees HV, Kleinschmidt I, Steffenson AE, MacPhail C, Hlongwa-Madikizela L, et al. Young people's sexual health in South Africa: HIV prevalence and sexual behaviors from a nationally representative household survey. Aids. 2005;19(14):1525–34. doi: 10.1097/01.aids.0000183129.16830.06. [DOI] [PubMed] [Google Scholar]
- 5.UNAIDS/WHO . AIDS Epidemic Update 2007. Geneva: 2007. [Google Scholar]
- 6.Mirochnick M, Capparelli E. Pharmacokinetics of antiretrovirals in pregnant women. Clin Pharmacokinet. 2004;43(15):1071–87. doi: 10.2165/00003088-200443150-00002. [DOI] [PubMed] [Google Scholar]
- 7.Aweeka F, Tierney C, Stek A, Sun X, Cohn S, Coombs R, et al. ACTG 5153s: Pharmacokinetic Exposure and Virological Response in HIV-1-infected Pregnant Women Treated with PI.. 14th Conference on Retroviruses and Opportunistic Infections; Los Angeles, CA. 2007; Abstract 739. [Google Scholar]
- 8.Floridia M, Giuliano M, Palmisano L, Vella S. Gender differences in the treatment of HIV infection. Pharmacol Res. 2008;58(3-4):173–82. doi: 10.1016/j.phrs.2008.07.007. [DOI] [PubMed] [Google Scholar]
- 9.Roustit M, Jlaiel M, Leclercq P, Stanke-Labesque F. Pharmacokinetics and therapeutic drug monitoring of antiretrovirals in pregnant women. Br J Clin Pharmacol. 2008;66(2):179–95. doi: 10.1111/j.1365-2125.2008.03220.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Prins M, Meyer L, Hessol NA. Sex and the course of HIV infection in the pre- and highly active antiretroviral therapy eras. Aids. 2005;19(4):357–70. doi: 10.1097/01.aids.0000161765.75663.27. [DOI] [PubMed] [Google Scholar]
- 11.Zhang M, Huang Q, Huang Y, Wood O, Yuan W, Chancey C, et al. beta-Estradiol attenuates the anti-HIV-1 efficacy of Stavudine (D4T) in primary PBL. Retrovirology. 2008;5:82. doi: 10.1186/1742-4690-5-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.MacCarthy S, Laher F, Nduna M, Farlane L. Responding to Her Question: A Review of the Influence of Pregnancy on HIV Disease Progression in the Context of Expanded Access to HAART in Sub-Saharan Africa. AIDS Behavior. 2009 doi: 10.1007/s10461-009-9541-2. (ePub ahead of print 2009) [DOI] [PubMed] [Google Scholar]
- 13.Guay LA, Musoke P, Fleming T, Bagenda D, Allen M, Nakabiito C, et al. Intrapartum and neonatal single-dose nevirapine compared with zidovudine for prevention of mother-to-child transmission of HIV-1 in Kampala, Uganda: HIVNET 012 randomised trial. Lancet. 1999;354(9181):795–802. doi: 10.1016/S0140-6736(99)80008-7. [DOI] [PubMed] [Google Scholar]
- 14.Chi BH, Sinkala M, Stringer EM, Cantrell RA, Mtonga V, Bulterys M, et al. Early clinical and immune response to NNRTI-based antiretroviral therapy among women with prior exposure to single-dose nevirapine. Aids. 2007;21(8):957–64. doi: 10.1097/QAD.0b013e32810996b2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Myer L, Carter RJ, Katyal M, Toro P, El-Sadr WM, Abrams EJ. Impact of antiretroviral therapy on incidence of pregnancy among HIV-infected women in Sub-Saharan Africa: a cohort study. PLoS Med. 2010;7(2):e1000229. doi: 10.1371/journal.pmed.1000229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.French R, Brocklehurst P. The effect of pregnancy on survival in women infected with HIV: a systematic review of the literature and meta-analysis. Br J Obstet Gynaecol. 1998;105(8):827–35. doi: 10.1111/j.1471-0528.1998.tb10226.x. [DOI] [PubMed] [Google Scholar]
- 17.Kaplan R, Orrell C, Zwane E, Bekker LG, Wood R. Loss to follow-up and mortality among pregnant women referred to a community clinic for antiretroviral treatment. Aids. 2008;22(13):1679–81. doi: 10.1097/QAD.0b013e32830ebcee. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Westreich D, Cole S, Nagar S, Maskew M, van der Horst C, Sanne I. Pregnancy and Virologic Response to Antiretroviral Therapy in South Africa. PLOS ONE. 2011;6(8):e22778. doi: 10.1371/journal.pone.0022778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fox MP, Brennan A, Maskew M, MacPhail P, Sanne I. Using vital registration data to update mortality among patients lost to follow-up from ART programmes: evidence from the Themba Lethu Clinic, South Africa. Trop Med Int Health. 2010;15(4):405–13. doi: 10.1111/j.1365-3156.2010.02473.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.South African National Department of Health National Antiretroviral Treatment Guidelines. 2004 http://www.doh.gov.za/docs/factsheets/guidelines/artguidelines04/
- 21.Westreich D, MacPhail P, Van Rie A, Malope-Kgokong B, Ive P, Rubel D, et al. Effect of pulmonary tuberculosis on mortality in patients receiving HAART. AIDS. 2009;23(6):707–15. doi: 10.1097/QAD.0b013e328325d115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Riddler SA, Haubrich R, DiRienzo AG, Peeples L, Powderly WG, Klingman KL, et al. Class-sparing regimens for initial treatment of HIV-1 infection. N Engl J Med. 2008;358(20):2095–106. doi: 10.1056/NEJMoa074609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cole SR, Hernan MA. Fallibility in estimating direct effects. Int J Epidemiol. 2002;31(1):163–5. doi: 10.1093/ije/31.1.163. [DOI] [PubMed] [Google Scholar]
- 24.WHO . Recommendations for a public health approach. Geneva: 2010. Antiretroviral drugs for treating pregnant women and preventing HIV infection in infants. [PubMed] [Google Scholar]
- 25.South African National Department of Health The South African Antiretroviral Treatment Guidelines. 2010 http://www.doh.gov.za/docs/factsheets/guidelines/art.pdf.