Abstract
Social challenges during the perinatal period influence the mother-infant relationship in nonhuman primates and may affect the offspring’s response to later social challenge(s). Relocation of a breeding colony of monkeys (Macaca radiata) created two groups of infants: one group experienced social group relocation to a new housing facility during the perinatal period (ATYPICAL) and the second group developed within a constant environment (TYPICAL). At a mean age of 25 mo., all animals were removed from their natal group and placed in same sex adolescent social groups. Behavioral observations were collected after group formation or introduction to a new group. ATYPICAL subjects showed increased aggression and reduced affiliation compared to TYPICAL subjects. Hair cortisol in male subjects collected 6 months after introduction was elevated in the ATYPICAL subjects compared to TYPICAL subjects. These findings demonstrate that early life challenges affect behavior as well as stress hormone responses to social challenge in adolescence.
Keywords: hair cortisol, development, social challenge, Macaca radiata, aggression, affiliation, risk, early environment, early adversity, maternal relationship, perinatal stress
INTRODUCTION
Early stressful events experienced by a mother during her pregnancy, or within the immediate postnatal period following birth, create changes in the regulation of multiple systems of her offspring (Cameron, Shahrokh, Del Coro, Dhir, Szyf, Champagne, & Meany, 2008; Davis, Glynn, Waffman, & Sandman, 2011; Glover, 2011). These effects may be evidenced at different times throughout the life span (Lupien, McEwen, Gunnar, & Heim, 2009; Veenema & Veenema, 2009; Yehuda, & Bierer, 2008). The factors and experiences that contribute to individual differences represent a central theme in developmental science (Rutter, 1997). Early trauma and adverse experiences may increase an individual’s stress reactivity, alter coping and behavioral strategies in adolescence, and continue into adulthood with potentially profound adverse consequences.
The epigenetic impacts of maternal style on stress reactivity have been demonstrated through elegant work in rodents (Caldji, Diorio, & Meaney, 2000). In nonhuman primates, early challenges to the mother-infant dyad affecting the maternal relationship with her offspring can significantly affect later stress reactivity (Rosenblum & Andrews, 1994). In both human and nonhuman primates, early social challenges and experiences affect immune, neuroendocrine, and behavioral regulation in adolescence and adulthood (for reviews see Bennett & Pierre, 2010 or Coe & Laudenslager, 2007). Early challenges in children contribute to the etiology of posttraumatic stress disorder with significant consequences for adult behavioral and physiological regulation (Breslau, Davis, & Andreski, 1995; Hoge, Austin, & Pollack, 2007; Laudenslager. Assal, Adler, Berger, Montgomery, Sandberg, Wahlberg, Wilkins, Zweig, & Reite, 1998; Laudenslager, Berger, Boccia, & Reite, 1996).
The longitudinal study of early social challenges is complex in humans for many reasons, including a multifaceted set of potential contributing factors (including SES, culture, parental absence, peer groups, etc.) which has led to significant policy statements regarding early development and toxic stress (Garner et al., 2012; Shonkoff, 2010). Animal studies provide opportunities for experimental approaches aimed at better understanding of the long-term consequences of early experience. In addition to their close genetic, physiological, social and developmental similarity to humans, nonhuman primate maturation parallels humans but proceeds at a more rapid pace (Bennett & Pierre, 2010; Machado & Bachevalier, 2003; Nelson & Barlow, 2009). In this brief report, we describe the impact of two administratively directed colony relocations that occurred during the perinatal period of bonnet macaque infants on later adolescent behavior and neuroendocrine responses. The present subjects were described previously (Laudenslager, Natvig, Mikulich-Gilbertson, Blevins, Corcoran, Pierre, & Bennett, 2010) in which we noted that social group relocations (ATYPICAL early experience) during the perinatal period were significantly associated with less time in contact of the infant with its mother and more time interacting with social group members between 2–4 months of age compared to offspring experiencing no relocations (TYPICAL early experience).
Early experiences such as the introduction of a new male into a social group can affect existing mother-offspring relationships among vervets (Fairbanks & McGuire, 1993; Fairbanks & McGuire, 1988). Rhesus macaque mothers normally detach themselves from their offspring during mating season leading to distress in the offspring, the magnitude of which is negatively related to later immune challenge (Laudenslager, Rasmussen, Berman, Suomi, & Berger, 1993). Bonnet and pigtail macaque offspring experiencing greater agonistic interactions with their mother before 6 months of age show increased rates of aggression during social group introduction or formation as adolescents (Weaver, Richardson, Worlein, De Waal, & Laudenslager, 2004). These observations led us to suspect that relocations associated with altered mother-infant relationship might also affect later responses to challenge. However not all experiences, such as brief maternal absence(s), are associated with adverse outcomes on biobehavioral development. For example, when they occur frequently under controlled laboratory settings, early brief maternal separation events in squirrel monkeys result in increased resilience in the offspring to later challenge, as well as different patterns of brain development and cognitive abilities (Lyons & Macri, 2011; Lyons, Parker, & Schatzberg, 2010).
The present study investigated the impact of perinatal relocation on the later response of TYPICAL and ATYPICAL subject’s behavioral and endocrine response to introduction or formation of a new peer group. We hypothesized that ATYPICAL offspring would be more aggressive and less affiliative during social group formation (a new social group created de novo) or introduction (addition of subjects to an established social group) at two years of age. Hair cortisol serves as a proxy marker of prior cumulative HPA activity (Davenport, Tiefenbacher, Novak, & Meyer, 2006). The recent development of a reliable protocol for measuring cortisol in hair by our group (D’Anna-Hernandez, Ross, Natvig, & Laudenslager, 2011; Fairbanks, Jorgensen, Bailey, Breidenthal, Grzywa, Laudenslager, 2011; Laudenslager, Jorgensen, Grzywa, & Fairbanks, 2011) permitted inclusion of hair cortisol levels from a subpopulation of males who experienced adolescent group introduction. We predicted that subjects which were more reactive in the new group would show higher hair cortisol following introduction.
METHODS
Subjects
A total of 22 bonnet macaque (Macaca radiata) monkey offspring (13 males and 9 females) were observed during the process of group formation or introduction. Subjects were born in social groups comprised of a single adult male, 3–5 adult females and their offspring. All animals were reared by their mothers until they were moved into same sex social groups (mean age = 25.0 ± 5.7 mo). Housing consisted of interconnecting quadrant style pens with two indoor and two outdoor sections (2.4 M × 2.4 M × 2.7M/quadrant or a total of 23 M2 floor space) for each social group as described previously (Laudenslager et al, 2010). Outdoor quadrants were restricted when temperature fell below 5°C or during pen cleaning. Indoor pens were heated during the winter months and maintained between 16 and 24°C. Pens were exposed to natural lighting from the outside and inside pens were maintained on a 12:12 light cycle. Feeding (Purina Monkey Biscuits #5038) took place approximately 2–3 hrs. prior to morning (AM) observations and 30 min prior to afternoon (PM) observations. Foraging materials were distributed in the afternoon following observations as environmental enrichment.
For the present analysis, subjects were defined as ATYPICAL (n=14) based on the experience of perinatal social group relocation with respect to estimated conception and early postnatal housing. Subjects were included as TYPICAL (n=8) only if conception occurred more than four months following colony relocation (Laudenslager, et al., 2010). ATYPICAL subjects’ mothers experienced relocation to a new facility either during pregnancy or within a month of estimated conception date after relocation (Laudenslager, et al., 2010). This study was approved by the Wake Forest University School of Medicine Institutional Animal Care and Use Committee and all experimental procedures were conducted in accordance with the National Institute of Health Guide for the Care and Use of Animal Subjects.
Social Challenge
Subjects were entered into the study during different birth years, matured in different calendar years, and either formed a new group or were introduced into an existing group. As an opportunistic study, random assignment to group was not possible as there was no control over the administrative relocations or birth cohort timing. ATYPICAL subjects experienced either Introduction or Formation. TYPICAL subjects were introduced to existing groups because they entered the cohort when the colony location was stable. The social challenge included either placing 4–6 monkeys together simultaneously into a same sex housing pen (Formation) or introducing 2 additional monkeys to a previously established group (Introduction) which had been stable for over one year. Group Formation or Introduction occurred at 1000 hr. and behavioral observations began immediately after the monkeys were transferred. All TYPICAL subjects (n = 8) experienced Group Introduction whereas seven ATYPICAL animals experienced a Group Introduction and seven ATYPICAL animals experienced Group Formation, thus creating three groups for analysis.
Behavioral observations
Five minute focal animal observations were collected using commercially-available software (The Observer 5.0, Noldus, Inc.) to record the behaviors of each subject in the new group (Formation) or only subjects introduced to the existing group (Introduction) using a previously described ethogram (Laudenslager et al, 2010). Observations of all focal animals were coded in real-time by one of two reliable observers (Cohen’s kappa= 0.82) sitting in full view of the subjects. On the first day of new group Formation or social group Introduction (always on Monday) observations of each focal animal were collected twice in the morning and twice in the afternoon to fully characterize the day of transition. On Tuesday through Friday after the day of Introduction or Formation, two 5 min. observations were collected, one in the morning and one in the afternoon, each day. During the second week, two observations each day were collected on Monday, Wednesday and Friday distributed between morning and afternoon epochs. Total duration of observation was either 90 or 160 min over the two weeks following introductions. The initial seven animals were observed more frequently (160 min) but the remaining 15 were observed 90 min. We compared mean scores for the shorter (90 min) and longer (160 min) observation periods and found no differences in mean behavioral measures based on length of observation period. The ethogram included behaviors recorded as events to reflect social interactions between the focal subject and other members of the social group (Laudenslager et al, 2010). The behaviors of play, groom, affiliation, aggression and submit included modifiers that indicated initiate (I), receive (R), or unknown (U) with respect to dyadic interactions.
Hair cortisol
The hair cortisol assay was developed by our group later in the study and included for a subset of study subjects. A 5 cm2 patch of hair was collected 5.7 ± 1.4 months after introduction to an existing social group in 5 ATYPICAL and 5 TYPICAL males. Because of the lack of equivalently timed samples females are not included. Hair was pulverized in a ball mill, extracted in HPLC grade methanol, reconstituted in assay buffer and processed for cortisol content in duplicate as previously described (Davenport, et al,, 2006; Fairbanks et al, 2011). Any duplicates exceeding a CV of 10% were rerun in triplicate and the triplicate median reported. Intra-assay coefficient of variation (CV) was less than 9% and inter assay CV was less than 5%. The low end detection limit for hair cortisol was 0.4 pg/mg.
Statistical Analyses
All analyses were performed using SPSS (IBM Inc, Version 19.0.0.1). Behaviors were first tested for differences between morning and afternoon observations by dependent t-tests. Observations collected in morning and afternoon did not differ and were collapsed into daily observations. Because many behaviors occurred infrequently, they were summed across all observation periods for the two weeks after group Formation or Introduction as mean frequency/min for aggression, affiliation, groom, submit, and play for each subject. As these means were not normally distributed, square root transformation normalized distributions of behaviors observed following social group challenge. Play was not observed in females and was dropped from further analysis.
Three groups were created based on ATYPICAL Formation, ATYPICAL Introduction, and TYPICAL Introduction. They were evaluated by multivariate ANOVA (MANOVA applying Pillai’s Trace) followed by post hoc Bonferroni comparisons of each group. Sex was unequally distributed across these groups and treated as a covariate due to well-known effects of sex on nonhuman primate behavior (Bernstein, Judge, & Ruehlmann, 1993). Covariance matrices for dependent variables were equivalent by Box’s M (sig = .191).
RESULTS
After controlling for the significant effects of sex [F(4,15) = 3.992, p=0.021], a multivariate test for effect of the three groups on the dependent variables of Aggression, Submit, Affiliation, and Groom was significant [F(8,32) = 5.476, p <.001]. Univariate ANOVAs showed that three dependent variables differed significantly among groups: Aggression [F(2,18) = 23.193, p <.001], Submit [F(2,18) = 13.673, p <.001], and Affiliation [F(2,18) = 5.647, p = .012]; Groom was non-significant [F(2,18) = 2.626, p = .226] but removing it from the model did not improve the multivariate test result [F(6,34) = 5.610, P <.001].
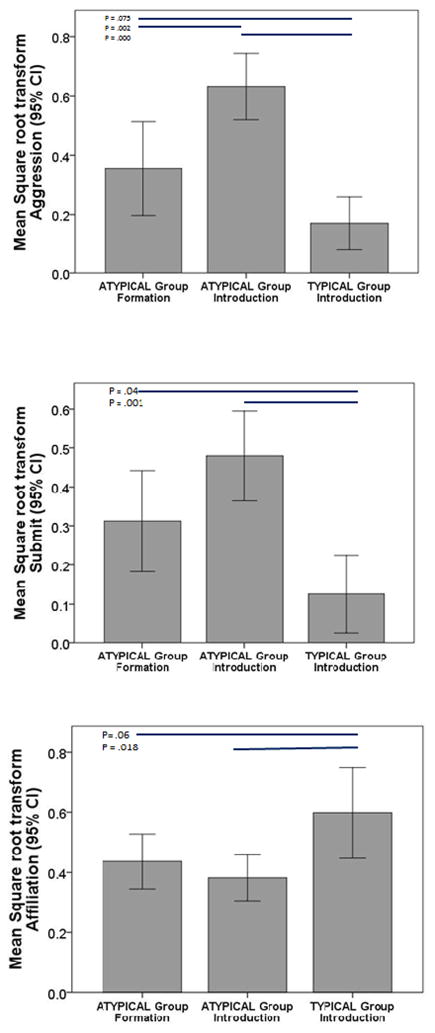
Post hoc tests (Bonferroni) evaluating differences between groups (Figure 1) indicated that the TYPICAL group showed the lowest rate of aggression and submission compared to ATYPICAL subjects. By contrast, the TYPICAL subjects showed a higher rate of affiliation during social group introduction compared to the ATYPICAL subjects. This generally held for either Introduction or Formation challenges experienced by the ATYPICAL subjects as indicated in Figure 1.
Figure 1.
Mean (95% CI) square root transformed summary scores observed in young bonnet macaques summed for observations occurring during two weeks following group formation or introduction from TYPICAL and ATYPICAL subjects. Differences between groups based on Bonferroni post hoc comparisons are shown as straight lines in the top part of each panel alongside corresponding p levels.
We have noted a sex dependent difference in developmental patterns of hair cortisol such that with earlier maturing females, hair cortisol levels decline developmentally before male vervets (Laudenslager et al, in review). We focused on males to avoid developmental differences in this age range. Hair cortisol was limited to males for which samples were collected 5.7 ± 1.4 months following group introduction. The experience has been noted to be associated with significant increases in hair cortisol associated with a prior social challenge (Davenport et al, 2006; Fairbanks et al, 2011). Hair cortisol was significantly higher in the ATYPICAL males compared to the TYPICAL males after entering time since group introduction as a covariate [F(1,7) = 8.350, p = .023] (Table 1). Although it did not reach statistical significance, hair cortisol was positively correlated with aggression (r = .49) and submissions (r = .64) and negatively correlated with grooming (r=−.46) during the two weeks after transition to new groups in directions expected by greater HPA activation associated with these behaviors (Laudenslager & Kennedy, 2007), that is, higher cortisol was associated with agonistic behaviors and lower cortisol with grooming.
Table 1.
Hair cortisol (pg/mg)
| Group | Mean | Std. Error | 95% Confidence Interval
|
|
|---|---|---|---|---|
| Lower Bound | Upper Bound | |||
|
| ||||
| ATYPICAL Group Introduction | 137.840 | 12.340 | 109.384 | 166.295 |
| TYPICAL Group Introduction | 89.733 | 12.340 | 61.278 | 118.189 |
DISCUSSION
The influences of the early developmental milieu of nonhuman primates has a significant influence on the nature of later responses to environmental challenge and it contributes significantly to individual differences in responses to those challenges (Lyons & Macri 2011; Rosenblum & Andrews, 1994). The impact later in development may be either debilitating or associated with resilience. This opportunistic data set provides further support that early experience affects later response to challenge(s). Groups were not randomly assigned but differences emerged in adolescence that were associated with uncontrolled events experienced by the ATYPICAL groups during the perinatal period but not by the TYPICAL group that was conceived and reared in a stable environment.
The present data suggest that perinatal events experienced by the mother and her offspring may impact behavioral responses to social challenge in adolescence. In the present observations, an ATYPICAL early experience was associated with both initiating and receiving more aggression and submission, as well as less affiliation, during the two week period following peer group introduction or formation. The small sample size in the present cohort does not permit a meditational analysis of the role of the mother-infant relationship in infancy. However these results may parallel previous observations in bonnet and pigtail macaques, wherein adolescents displayed greater levels aggression during group formation and introduction when they received relatively greater agonistic and fewer affiliative interactions from their mother at 6 mo. (Weaver et al, 2004). We cannot point to the exact etiology of greater aggression and reduced affiliation in the ATYPICAL group. It does alert us to look more closely at early mother-infant relationships as possible mediators or moderators of adolescent behavior patterns in future studies.
Hair cortisol levels in the ATYPICAL males were higher following group introduction. Data was available for these subjects approximately 6 mo. after group introduction because the collection of hair samples began as an added value protocol for the present longitudinal project. Hair cortisol is a retrospective proxy marker of hypothalamic pituitary adrenal activity (Davenport, Tiefenbacher, Lutz, Novak, & Meyer, 2006; D’Anna-Hernandez, Ross, Natvig, & Laudenslager, 2011). The present data suggests that ATYPICAL offspring had greater activation of the HPA axis in conjunction with the social challenge of peer group introduction. In vervets and rhesus monkeys, relocation is associated with a significant increase in hair cortisol after 6 mo in the new situation, (Davenport, Lutz, Tiefenbacher, Novak, & Meyer, 2008; Fairbanks et al., 2011). The present results provide preliminary evidence that higher hair cortisol may have been associated with greater aggressive interactions in the social group and lower hair cortisol was associated with greater grooming activity. These pilot observations suggest important behavioral characteristics to follow in developing foundations of individual differences in hair cortisol.
A number of studies have shown converging evidence for ties between observed behavior and underlying physiological measures of arousal and other homeostatic systems. For example lower heart rates are associated with grooming in socially-housed macaques following agonistic interactions (Boccia, Reite, & Laudenslager, 1989). Grooming serves as an important buffer during social group introduction as well as other shorter term distresses in macaques (Aureli & Yates, 2010; Boccia, et al., 1989). Additionally, behavioral responses in macaques during group disruptions are related to immune response changes (Kaplan, Heise, Manuck, Shively, Cohen, Rabin, & Kasprowicz, 1991) as well as viral resistance (Capitanio & Lerche, 1998). In rodents, social defeat behaviors as opposed to reciprocal aggression during territorial intrusion are associated with lower antibody responses to antigenic challenge (Fleshner, Laudenslager, Simons, & Maier, 1989). Furthermore, novelty seeking behaviors are related to hair cortisol levels such that lower hair cortisol was associated with greater novelty seeking in vervets (Laudenslager, et al., 2011). However the present relationships of behavior to hair cortisol must be viewed with caution as the sample size in this brief report was limited. The full meaning of hair cortisol remains an important area in HPA investigations (Russell, Koren, Rieder, & Van Uum, in press).
The present results provide support for the role of early experience on subsequent responses to social challenges and regulation of neuroendocrine and other systems (Lupien et al., 2009). In the present study we provide evidence that social group relocation during the perinatal period not only affects early mother-infant interactions (Laudenslager et al., 2010) but these offspring are more likely to participate in aggressive interactions as adolescents when challenged by introduction to a new same sex peer group. How the mother-infant relationship in the present situation is related to the behavior of the adolescent remains unclear. Previous work in rhesus monkeys has demonstrated that early rearing conditions influence social and other behaviors later in life (Fahlke, Lorenz, Long, Champoux, Soumi, & Higley, 2000; Lupien, et al, 2009; Rosenblum & Andrews, 1994). Rearing conditions affect relative social dominance rank in the juvenile and peri-adolescent period (Bastian, et al., 2003). Unfortunately, we do not have data to inform final dominance status of the TYPICAL and ATYPICAL subjects in their adolescent peer groups.
Adolescent monkeys experiencing natal social group relocation during the perinatal period showed increased activation of the HPA axis as reflected in hair cortisol 6 months after this transition to the new group. The role of maternal stress hormones in this relationship has been repeatedly suggested as a critical factor in establishing regulatory patterns of offspring (Davis, Waffarn, & Sandman, 2011) suggesting that maternal hair cortisol durng colony relocation would have been useful information for the present study. In summary, early perinatal experience of bonnet macaque mothers are associated with behavioral differences toward her offspring in infancy as well as in adolescence in her absence. The findings underscore the need for longitudinal developmental designs beginning prenatally and continuing through adulthood in order to improve our understanding of individual differences in stress reactivity and to identify core factors that contribute to resilience and vulnerability following early life challenge.
Acknowledgments
This project and its preparation were supported in part by NIH research grants R01AA013973 (MLL). R01AA013995 (AJB), P20AA11997 (AJB), P01AA0170456-016688 (AJB, PJP), and Administration for Children and Families (ACF) grant YR0058. The authors would also like to thank Tara Chavanne for her assistance in live observation of the animals included in this study and Rachel Cobb for processing the hair cortisol samples. We are also grateful to the animal care technicians and veterinary staff at Wake Forest University Health Sciences and the University of Colorado Anschutz Medical Campus for care of the animals.
References
- Aureli F, Yates K. Distress prevention by grooming others in crested black macaques. Biology Letters. 2010;6(1):27–29. doi: 10.1098/rsbl.2009.0513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barr CS, Newman TK, Lindell S, Becker ML, Shannon C, Champoux M, Higley JD. Early experience and sex interact to influence limbic-hypothalamic-pituitary-adrenal-axis function after acute alcohol administration in rhesus macaques (Macaca mulatta) Alcoholism: Clinical & Experimental Research. 2004;28(7):1114–1119. doi: 10.1097/01.alc.0000130973.94350.8c. [DOI] [PubMed] [Google Scholar]
- Bastian ML, Sponberg AC, Suomi SJ, Higley JD. Long-term effects of infant rearing condition on the acquisition of dominance rank in juvenile and adult rhesus macaques (Macaca mulatta) Developmental Psychobiology. 2003;42(1):44–51. doi: 10.1002/dev.10091. [DOI] [PubMed] [Google Scholar]
- Bennett AJ, Pierre PJ. Nonhuman Primate Research Contributions to Understanding Genetic and Environmental Influences on Phenotypic Outcomes Across Development. In: Hood Kathryn, Halpern Carolyn Tucker, Greenberg Gary, Lerner Richard., editors. The Handbook of Developmental Science, Behavior, and Genetics. John Wiley & Sons, Inc; 2010. pp. 353–399. [Google Scholar]
- Bernstein IS, Judge PG, Ruehlmann TE. Sex Differences in Adolescent Rhesus Monkey (Macaca Mulatta) Behavior. American Journal of Primatology. 1993;31:197–210. doi: 10.1002/ajp.1350310305. [DOI] [PubMed] [Google Scholar]
- Boccia ML, Reite M, Laudenslager M. On the physiology of grooming in a pigtail macaque. Physiol Behav. 1989;45:667–670. doi: 10.1016/0031-9384(89)90089-9. [DOI] [PubMed] [Google Scholar]
- Breslau N, Davis GC, Andreski P. Risk factors for PTSD-related traumatic events: A prospective analysis. American Jounral of Psychiatry. 1995;152:529–535. doi: 10.1176/ajp.152.4.529. [DOI] [PubMed] [Google Scholar]
- Caldji C, Diorio J, Meaney MJ. Variations in maternal care in infancy regulate the development of stress reactivity. Biological Psychiatry. 2000;48(12):1164–1174. doi: 10.1016/s0006-3223(00)01084-2. [DOI] [PubMed] [Google Scholar]
- Cameron NM, Shahrokh D, Del Corpo A, Dhir SK, Szyf M, Champagne FA, Meaney MJ. Epigenetic programming of phenotypic variations in reproductive strategies in the rat through maternal care. Journal of Neuroendocrinology. 2008;20(6):795–801. doi: 10.1111/j.1365-2826.2008.01725.x. [DOI] [PubMed] [Google Scholar]
- Capitanio JP, Lerche NW. Social separation, housing relocation, and survival in simian AIDS: a retrospective analysis. Psychosomatic Medicine. 1998;60(3):235–244. doi: 10.1097/00006842-199805000-00001. [DOI] [PubMed] [Google Scholar]
- Coe CL, Laudenslager ML. Psychosocial influences on immunity, including effects on immune maturation and senescence. Brain Behavior Immunity. 2007;21(8):1000–1008. doi: 10.1016/j.bbi.2007.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Anna-Hernandez KL, Ross RG, Natvig CL, Laudenslager ML. Hair cortisol levels as a retrospective marker of hypothalamic-pituitary axis activity throughout pregnancy: comparison to salivary cortisol. Physiology & Behavior. 2011;104(2):348–353. doi: 10.1016/j.physbeh.2011.02.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davenport MD, Lutz CK, Tiefenbacher S, Novak MA, Meyer JS. A rhesus monkey model of self-injury: effects of relocation stress on behavior and neuroendocrine function. Biological Psychiatry. 2008;63(10):990–996. doi: 10.1016/j.biopsych.2007.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davenport MD, Tiefenbacher S, Lutz CK, Novak MA, Meyer JS. Analysis of endogenous cortisol concentrations in the hair of rhesus macaques. General & Comparative Endocrinology. 2006;147(3):255–261. doi: 10.1016/j.ygcen.2006.01.005. [DOI] [PubMed] [Google Scholar]
- Davis EP, Waffarn F, Sandman CA. Prenatal treatment with glucocorticoids sensitizes the hpa axis response to stress among full-term infants. Developmental Psychobiology. 2011;53(2):175–183. doi: 10.1002/dev.20510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fahlke C, Lorenz JG, Long J, Champoux M, Suomi SJ, Higley JD. Rearing experiences and stress-induced plasma cortisol as early risk factors for excessive alcohol consumption in nonhuman primates. Alcoholism: Clinical & Experimental Research. 2000;24(5):644–650. [PubMed] [Google Scholar]
- Fairbanks LA, McGuire ML. Maternal protectiveness and response to the unfamilar in vervet monkeys. American Journal of Primatology. 1993;30:119–129. doi: 10.1002/ajp.1350300204. [DOI] [PubMed] [Google Scholar]
- Fairbanks LA, McGuire MT. Long-term effects of early mothering behavior on responsiveness to the environment in vervet monkeys. Developmental Psychobiology. 1988;21(7):711–724. doi: 10.1002/dev.420210708. [DOI] [PubMed] [Google Scholar]
- Fairbanks LA, Jorgensen MJ, Bailey JN, Breidenthal SE, Grzywa R, Laudenslager ML. Heritability and genetic correlation of hair cortisol in vervet monkeys in low and higher stress environments. Psychoneuroendocrinology. 2011;36(8):1201–1208. doi: 10.1016/j.psyneuen.2011.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleshner M, Laudenslager ML, Simons L, Maier SF. Reduced serum antibodies associated with social defeat in rats. Physiology & Behavior. 1989;45(6):1183–1187. doi: 10.1016/0031-9384(89)90107-8. [DOI] [PubMed] [Google Scholar]
- Garner AS, Shonkoff JP, Siegel BS, Dobbins MI, Earls MF, McGuinn L, et al. Early childhood adversity, toxic stress, and the role of the pediatrician: translating developmental science into lifelong health. Pediatrics. 2012;129(1):e224–231. doi: 10.1542/peds.2011-2662. [DOI] [PubMed] [Google Scholar]
- Glover V. Annual Research Review: Prenatal stress and the origins of psychopathology: an evolutionary perspective. Journal of Child Psychology & Psychiatry & Allied Disciplines. 2011;52(4):356–367. doi: 10.1111/j.1469-7610.2011.02371.x. [DOI] [PubMed] [Google Scholar]
- Grant KA, Bennett AJ. Advances in nonhuman primate alcohol abuse and alcoholism research. Pharmacology & Therapeutics. 2003;100(3):235–55. doi: 10.1016/j.pharmthera.2003.08.004. [DOI] [PubMed] [Google Scholar]
- Hoge EA, Austin ED, Pollack MH, Hoge EA, Austin ED, Pollack MH. Resilience: research evidence and conceptual considerations for posttraumatic stress disorder. Depression & Anxiety. 2007;24(2):139–152. doi: 10.1002/da.20175. [DOI] [PubMed] [Google Scholar]
- Kaplan JR, Heise ER, Manuck SB, Shively CA, Cohen S, Rabin BS, Kasprowicz AL. The Relationship of Agonistic and Affiliative Behavior Patterns to Cellular Immune Function Among Cynomolgus Monkeys (Macaca fascicularis) Living in Unstable Social Groups. American Jounral of Primatology. 1991;25:157–173. doi: 10.1002/ajp.1350250303. [DOI] [PubMed] [Google Scholar]
- Laudenslager ML, Kennedy S. Social dominance and immunity in animals. In: Ader R, editor. Psychoneuroimmunology. 4. Vol. 1. San Diego: Elsevier; 2007. pp. 475–496. [Google Scholar]
- Laudenslager ML, Aasal R, Adler L, Berger CL, Montgomery PT, Sandberg E, Wahlberg L, Wilkins R, Zweig l, Reite ML. Elevated cytotoxicity in combat veterans with long-term post-traumatic stress disorder: preliminary observations. Brain Behavior Immunity. 1998;12(1):74–79. doi: 10.1006/brbi.1997.0513. [DOI] [PubMed] [Google Scholar]
- Laudenslager ML, Berger CL, Boccia ML, Reite ML. Natural cytotoxicity toward K562 cells by macaque lymphocytes from infancy through puberty: effects of early social challenge. Brain, Behavior, and Immunity. 1996;10(3):275–287. doi: 10.1006/brbi.1996.0024. [DOI] [PubMed] [Google Scholar]
- Laudenslager ML, Jorgensen MJ, Grzywa R, Fairbanks LA. A novelty seeking phenotype is related to chronic hypothalamic-pituitary-adrenal activity reflected by hair cortisol. Physiology & Behavior. 2011;104(2):291–295. doi: 10.1016/j.physbeh.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laudenslager ML, Natvig C, Mikulich-Gilbertson SM, Blevins M, Corcoran C, Pierre PJ, Bennett AJ. Challenges to bonnet monkey (Macaca radiata) social groups: Mother-infant dyad and infant social interactions. Developmental Psychobiology. 2010;52(5):465–474. doi: 10.1002/dev.20449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laudenslager ML, Rasmussen KL, Berman CM, Suomi SJ, Berger CB. Specific antibody levels in free-ranging rhesus monkeys: relationships to plasma hormones, cardiac parameters, and early behavior. Developmental Psychobiology. 1993;26(7):407–420. doi: 10.1002/dev.420260704. [DOI] [PubMed] [Google Scholar]
- Laudenslager M, Capitanio JP, Reite M. Possible effects of early separation experiences on subsequent immune function in adult macaque monkeys. American Journal of Psychiatry. 1985;142:862–864. doi: 10.1176/ajp.142.7.862. [DOI] [PubMed] [Google Scholar]
- Lupien SJ, McEwen BS, Gunnar MR, Heim C. Effects of stress throughout the lifespan on the brain, behaviour and cognition. Nature Reviews Neuroscience. 2009;10(6):434–445. doi: 10.1038/nrn2639. [DOI] [PubMed] [Google Scholar]
- Lyons DM, Macri S. Resilience and adaptive aspects of stress in neurobehavioral development. Neuroscience and Biobehavioral Reviews. 2011;35(7):1451. doi: 10.1016/j.neubiorev.2010.09.004. [DOI] [PubMed] [Google Scholar]
- Lyons DM, Parker KJ, Schatzberg AF. Animal models of early life stress: Implications for understanding resilience. Developmental Psychobiology. 2010;52(5):402–410. doi: 10.1002/dev.20429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machado CJ, Bachevalier J. Non-human primate models of childhood psychopathology: The promise and the limitations. Journal of Child Psychology and Psychiatry. 2003;44:64–87. doi: 10.1111/1469-7610.00103. [DOI] [PubMed] [Google Scholar]
- Nelson EE, Winslow JT. Non-human primates: Model animals for developmental psychopathology. Neuropsychopharmacology. 2009;34:90–105. doi: 10.1038/npp.2008.150. [DOI] [PubMed] [Google Scholar]
- Rosenblum LA, Andrews MW. Influences of environmental demand on maternal behavior and infant development. Acta Paediatrica, Supplement. 1994;397:57–63. doi: 10.1111/j.1651-2227.1994.tb13266.x. [DOI] [PubMed] [Google Scholar]
- Russell E, Koren G, Rieder M, Van Uum S. Hair cortisol as a biological marker of chronic stress: Current status, future directions and unanswered questions. Psychoneuroendocrinology. doi: 10.1016/j.psyneuen.2011.09.009. (in press) [DOI] [PubMed] [Google Scholar]
- Rutter M. Child psychiatric disorder - Measures, causal mechanisms, and interventions. Arch Gen Psychiatry. 1997;54(9):785–789. doi: 10.1001/archpsyc.1997.01830210021002. [DOI] [PubMed] [Google Scholar]
- Shonkoff JP. Building a new biodevelopmental framework to guide the future of early childhood policy. Child Development. 2010;81(1):357–367. doi: 10.1111/j.1467-8624.2009.01399.x. [DOI] [PubMed] [Google Scholar]
- Veenema AH, Veenema AH. Early life stress, the development of aggression and neuroendocrine and neurobiological correlates: what can we learn from animal models? Frontiers in Neuroendocrinology. 2009;30(4):497–518. doi: 10.1016/j.yfrne.2009.03.003. [DOI] [PubMed] [Google Scholar]
- Weaver A, Richardson R, Worlein J, De Waal F, Laudenslager M. Response to social challenge in young bonnet (Macaca radiata) and pigtail (Macaca nemestrina) macaques is related to early maternal experiences. American Journal of Primatology. 2004;62(4):243–259. doi: 10.1002/ajp.20019. [DOI] [PubMed] [Google Scholar]
- Yehuda R, Bierer LM, Yehuda R, Bierer LM. Transgenerational transmission of cortisol and PTSD risk. Progress in Brain Research. 2008;167:121–135. doi: 10.1016/S0079-6123(07)67009-5. [DOI] [PubMed] [Google Scholar]