Abstract
Nanoparticle-based therapies are currently being explored for both the imaging and treatment of primary and metastatic cancers. Effective nanoparticle cancer therapy requires significant accumulations of nanoparticles within the tumor environment. Various techniques have been used to improve tumor nanoparticle uptake and biodistribution. Most notable of these techniques are the use of tumor-specific-peptide-conjugated nanoparticles and chemical modification of the nanoparticles with immune-evading polymers. Another strategy for improving the tumor uptake of the nanoparticles is modification of the tumor microenvironment with a goal of enhancing the enhanced permeability and retention effect inherent to solid tumors. We demonstrate a two-fold increase in the tumor accumulation of systemically delivered iron oxide nanoparticles following a single, 15 Gy radiation dose in a syngeneic mouse breast tumor model. This increase in nanoparticle tumor accumulation correlates with a radiation-induced decrease in tumor interstitial pressure and a subsequent increase in vascular permeability.
Keywords: ionizing radiation, nanoparticle, tumor, biodistribution, interstitial pressure
1. Background
A variety of hyperthermia-based techniques[1], including intratumoral magnetic nanoparticle (mNP) hyperthermia[2], have been used to treat tumors. The difference in mNP hyperthermia, as compared to conventional hyperthermia, is the ability tofocally heat according to mNP uptake and biodistribution in both primary and metastatic tumors. One of the most significant challenges is delivering an effective concentration ofnanoparticles to tumor cells. Investigators have relied on increasing nanoparticle circulation time through evasion of the reticuloendothelial system (RES)[2] by modifying nanoparticle surface coating[3], the enhanced permeability and retention (EPR) effect[4] and tumor-specific peptide conjugates to mNP[5] to increase nanoparticle accumulation in tumors.
High interstitial tumor pressure (ITP) has been shown to hinder diffusion of macromolecules into tumors[6]. Ionizing radiation has been shown lower ITP at doses above 10 GyI[7].
2. Materials Methods
2.1. Cell Culture
MTG-B mouse mammary adenocarcinoma cells were cultured in 150 cm2 cell culture flasks (Corning Inc., Lowell, MA) in Alpha MEM medium (10% fetal bovine serum, 1% penicillin-streptomycin, 1% L-glutamine; all from Thermo Fisher Scientific Inc., Waltham, MA., USA). Cells were then trypsinized (0.25% trypsin in EDTA, Mediatech, Inc., Manassas, Va) and resuspended in serum-free Alpha MEM at 107 cells/ml.
2.2. Murine tumor model
One hundred μL (106 cells) was implanted bilaterally in the flanks 6-8 week old female C3H mice (Charles River Laboratories, Wilmington, MA, USA). Mice were treated once tumors reached 150±40 mm3. All animal experimentation was approved by the Dartmouth Institutional Animal Care and Use Committee, in accordance with all federal, institutional and AAALAC guidelines.
2.3. Interstitial pressure measurements
Interstitial tumor pressure measurements in ten tumors were accomplished by placing a fiber optic pressure sensor (0.5 mm diameter FPI-HR, FISO Technologies Inc., Quebec, Canada) in the centers of thetumors, using a techniquesimilar to others[8].
2.4. Ionizing radiation
Within one hour of establishing a baseline ITP, half of the tumors received a single 15 Gy, 6 MeV electron radiationdose (surfaced based homogeneous dose,100 cm source-skin distance (SSD), 1.7 cm diameter circular lead cut-out, average tumor depth 0.5 cm and width 0.7 cm) using a Varian Clinac 2100C linear accelerator (Varian Medical Systems, Inc., Palo Alto, CA, USA). ITP measurements were repeated daily, for five days.
2.5. Tumor vascular permeability assessment
Three days following irradiation, a vascular permeability assay[9] was performed using Evans blue (Sigma Aldrich, St. Louis, MO, USA) suspended in phosphate buffered saline (PBS, Mediatech) at 1.25 mg/ml. The solution was injected into the left jugular vein to 10 mg/kg mouse. Two hours later the mouse was perfused with 20 ml PBS and the tumors removed, weighed and digested in 10 ml formamide (Sigma Aldrich) per gram of tissue. Three days following tumor removal, tumor dye concentration was determined using a Perkin Elmer MBA 2000 spectrophotometer.
2.6. Nanoparticle composition and quantification
Iron oxide nanoparticles (70 and 120 nm diameter, Micromod Partikeltechnologie GmbH, Rostock, Germany) with various coatings (Table 1) were purchasedsuspended in deionized water. NaCl (0.9%) was added to ensure an appropriate isotonic balance.
Table 1. Nanoparticle Characteristics.
Iron oxide nanoparticles and zeta potential measurements acquired from Micromod. Hydrodynamicdiameters (core plus coating)were acquired using a Malvern Instruments Ltd. (Westborough, MA) SPIO = superparamagnetic iron oxide; BNF = bionized nanoferrite ferromagnetic nanoparticles; PEG 200 = polyethylene glycol polymer(200 Da molecular weight); PEG 6000 = polyethylene glycol polymer (6000 Da); HES = hydroxyethyl starch.
| Nanoparticle Designation |
Average Particle Hydrodynamic Diameter (nm) |
Coating | Zeta Potential (mV) at pH 7 |
|---|---|---|---|
| SPIO | 74 | HES | −2.6 |
| SPIO PEG 200 | 72 | HES + PEG 200 | −7.9 |
| BNF | 116 | HES | −18 |
| BNF PEG 200 | 125 | HES + PEG 200 | −6.5 |
| BNF PEG 6000 | 132 | HES + PEG 6000 | −3.5 |
When an individual tumor reached treatment size, it was irradiated with the contralateral tumor serving as a non-irradiated control. MNPs or PBS (control) were injected into the left jugular vein at a concentration of 0.2 mg Fe/g mouse three days following irradiation. Six days following irradiation, the mice were perfused with 20 ml PBS and tumors removed. Half of each tumor was fixed in 10% buffered formalin and processed for microscopic assessment of iron (Prussian blue/non-heme iron) anda subset of these for vascular endothelium (anti-CD-31). The other half of each tumor was weighed, digested in 3:1 trace element grade Nitric Acid:HCl (Thermo Fisher Scientific) and analyzed for iron content via ICP-MS (Agilent 7500cx inductively coupled plasma mass spectrometer) .
2.7. Statistical analysis
Statistical significance was assessed with a two-tailed, two-sample t-test with Matlab software (The MathWorks, Inc. Natick, MA, USA).
3. Results
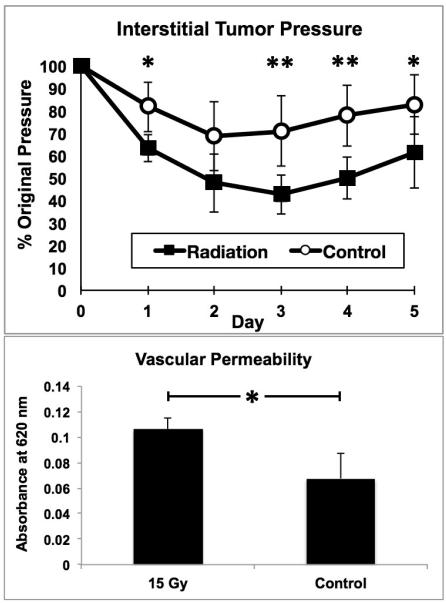
Interstitial tumor pressure (ITP) was decreased 3, 4 and 5 days post-15 Gy irradiation (Figure 1) by up to 40% as compared with controls. Three days post tumor irradiation, vascular permeability, as assessed by higher Evans blue absorbance, was increased by approximately 60% (Figure 1).
Figure 1.
Top:A single 15 Gy fraction of 6 MeV electron radiation significantly decreases interstitial tumor pressure as compared to non-irradiated controls. Bottom: Three days following irradiation vascular permeability is correspondingly increased (Evans blue spectrophotometry assessment). * = p < 0.05 and ** = p < 0.01. Error bars show standard deviations.
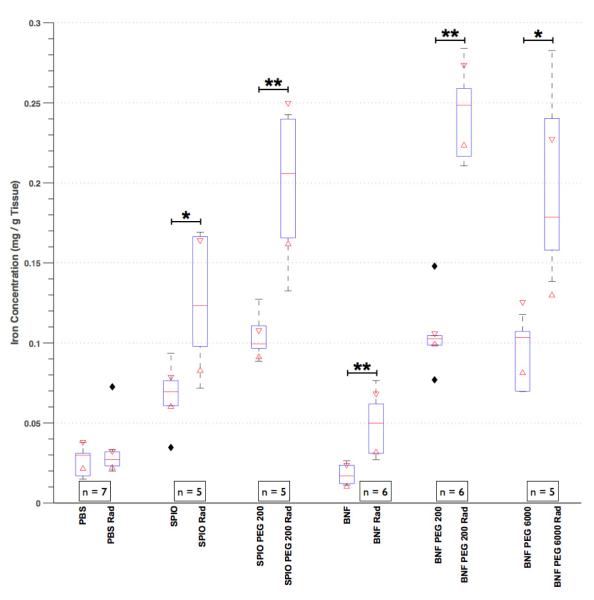
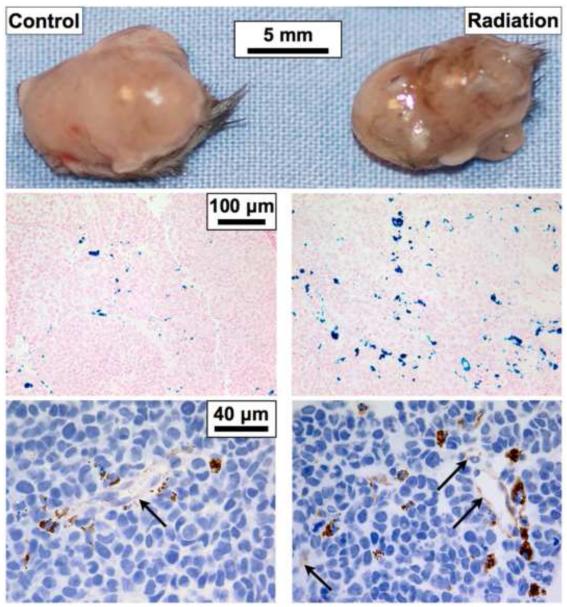
Three days following mNP administration the concentration of both 70 and 120 nm NPs was doubled in the irradiated tumors, as compared to controls (Figure 2). The median concentrations of systemically delivered PEG 200 coated BNF mNPs increased 2.5 fold in mouse flank tumors, as compared to non-coated mNPs (0.1 mg Fe/g vs 0.25 mg Fe/g). Prussian blue histology and iron quantification results were confirmed with ICP-MS (Figure 3). Mice receiving PBS instead of mNPs had ICP-MS iron levels less than 5% of the mNP treatment mice and no histologic iron staining.
Figure 2.
This boxplot of ICP-MS-based tumor iron quantification demonstrates that PEG coating increases nanoparticle accumulation in irradiated tumors. Nanoparticle size was irrelevant with respect to tumor accumulation when PEG-coated. When not PEG coated, smaller nanoparticles (SPIO) accumulated in tumors more than larger nanoparticles (BNF). The numbers in the figure legends (i.e. n=7) refers to number of animals in each group; each animal had anirradiated (Rad) and non-treated (control) tumor.The top of each box is the 75th percentile, the middle line the median and the bottom of the box the 25th percentile for each group. The errors bars show the range of data in each group and the filled diamonds show outliers. The 95% confidence interval for each group is the range between the two triangles overlaying each box. * = p < 0.05 and ** = p < 0.01.
Figure 3.
Bilateral flank tumors were excised from the same mouse three days after systemic administration of BNF PEG200 nanoparticles. Gross and histological analyses demonstrate an increased level of mNPs (brown in topand bottom, blue in middle) in irradiated tumors. IHC-stained endothelial cells (anti-CD31) demonstrate the close spatial relationship of tumor vessels (light brown, arrows) and nanoparticles (dark brown).
4. Discussion
Our results demonstrate tumor irradiation, PEG coating and small size (70 vs. 120 nm) increase mNP accumulation in a murine flank breast tumor model.This increased deposition of mNP within tumors due to addition of PEG to the surface is likely due to the increased circulation time and evasion of the RES which PEG confers to the mNP[10].
These results demonstrate that it is possible to increase accumulation of nanoparticles within tumors by modification of the tumor microenvironment with clinically relevant ionizing radiation. While modification of nanoparticles with targeting agents and RES-evading polymers also assists nanoparticle accumulation in tumors, these effects can be enhanced through modification of interstitial tumor pressure and tumor vascular permeability with the use of ionizing radiation.
Acknowledgments
This work was supported by the Dartmouth Center of Cancer Nanotechnology Excellence (NIH NCI grant 1U54CA151662-01). A.J. Giustini and A.A. Petryk gratefully acknowledge support from the Thayer School of Engineering Innovation Fellowship. The P. J. Hoopes laboratory shared a DOD grant (Award Number TSI-4029-08-78777) with Triton BioSystems (Now Aspen MediSys, LLC). The Hoopes laboratory has an ongoing collaboration with Aspen MediSys to use iron oxide nanoparticles for cancer therapy. Aspen MediSys did not, in any way, participate in the studies described in this manuscript or in the preparation of this manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial and competing interests disclosure: The authors report no conflicts of interest.
6. References
- 1.Dewhirst MW, Viglianti BL, Lora-Michiels M, Hanson M, Hoopes PJ. Basic principles of thermal dosimetry and thermal thresholds for tissue damage from hyperthermia. Int. J. of Hyperthermia. 2003 May;19(3):267–294. doi: 10.1080/0265673031000119006. [DOI] [PubMed] [Google Scholar]
- 2.Giustini AJ, Petryk AA, Cassim SM, Tate JA, Baker I, Hoopes PJ. Magnetic nanoparticle hyperthermia in cancer treatment. Nano LIFE. 2010;1(1 & 2):17–32. doi: 10.1142/S1793984410000067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Moghimi S, Hunter A, Murray J. Long-circulating and target-specific nanoparticles: theory to practice. Pharmacological reviews. 2001;53(2):283–318. [PubMed] [Google Scholar]
- 4.Greish K. Enhanced permeability and retention of macromolecular drugs in solid tumors: a royal gate for targeted anticancer nanomedicines. Journal of Drug Targeting. 2007;15(7-8):457–464. doi: 10.1080/10611860701539584. [DOI] [PubMed] [Google Scholar]
- 5.DeNardo SJ, DeNardo GL, Natarajan A, Miers LA, Foreman AR, Gruettner C, Adamson GN, Ivkov R. Thermal dosimetry predictive of efficacy of 111In-ChL6 nanoparticle AMF--induced thermoablative therapy for human breast cancer in mice. J Nucl Med. 2007 Mar.48(3):437–444. [PubMed] [Google Scholar]
- 6.Jain RK, Stylianopoulos T. Delivering nanomedicine to solid tumors. Nature reviews clinical oncology. 2010;7(11):653–664. doi: 10.1038/nrclinonc.2010.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Znati CA, Rosenstein M, Boucher Y, Epperly MW, Bloomer WD, Jain RK. Effect of radiation on interstitial fluid pressure and oxygenation in a human tumor xenograft. Cancer Res. 1996 Mar.56(5):964–968. [PubMed] [Google Scholar]
- 8.Ozerdem U, Hargens AR. A simple method for measuring interstitial fluid pressure in cancer tissues. Microvasc Res. 2005 Jul.70(1-2):116–120. doi: 10.1016/j.mvr.2005.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen B, Pogue BW, Luna JM, Hardman RL, Hoopes PJ, Hasan T. Tumor vascular permeabilization by vascular-targeting photosensitization: effects, mechanism, and therapeutic implications. Clin Cancer Res. 2006 Feb.12(3 Pt 1):917–923. doi: 10.1158/1078-0432.CCR-05-1673. [DOI] [PubMed] [Google Scholar]
- 10.Almeida JPM, Chen AL, Foster A, Drezek R. In vivo biodistribution of nanoparticles. Nanomedicine (Lond) 2011 Jul.6(5):815–835. doi: 10.2217/nnm.11.79. [DOI] [PubMed] [Google Scholar]