Abstract
Estrogen receptor-beta (ERβ) has been suggested to exert anti-inflammatory and anti-tumorigenic effects in the colon, providing a translational potential to prevent and/or treat inflammatory bowel disease (IBD) and its progression to colitis-associated colorectal cancer (CAC). However, the specific direct role of ERβ in CAC has not yet been tested. We assessed the effects of ERβ deficiency in the azoxymethane (AOM)/dextran sodium sulfate(DSS)-induced CAC model using ERβ knockout (βERKO) mice and wild-type (WT) littermates. These mice were injected with AOM followed by one week of DSS treatment, and sacrificed on weeks 9 or 16.βERKO mice developed more severe clinical colitis compared to WT mice, as evidenced by significantly higher disease activity index after DSS treatment, weight to length ratio of the colons, inflammation score, and grade of dysplasia. ERβ-deficient colons presented greater number and size of polyps at weeks 9 and 16, respectively, and were characterized by a significant increase in interleukin (IL)-6, IL-17, tumor necrosis factor alpha, and interferon-gamma mRNA levels. Furthermore, higher protein expression levels of nuclear factor-kappa B, inducible nitric oxide synthase, β-catenin, proliferating cell nuclear antigen, mucin-1, and significantly lower caveolin-1 and mucin-2 protein levels were shown in βERKO mice compared to WT mice. These data suggested a possible anti-inflammatory and anti-neoplastic mechanism of action of ERβ in CAC. These results demonstrate for the first time that ERβ provides protection in the AOM/DSS-induced CAC model in mice, suggesting a preventive and/or therapeutic potential for the use of ERβ-selective agonists in IBD.
Keywords: Estrogen receptor-beta, azoxymethane, dextran sodium sulfate, inflammatory bowel disease, colon cancer
Introduction
Inflammatory bowel disease (IBD) comprises the two main chronic inflammatory disorders of the intestine, ulcerative colitis (UC) and Crohn's disease (CD), which often lead to colitis-associated colorectal cancer (CAC).1 CAC accounts for approximately 15% of IBD-related deaths2 and it affects individuals at a younger age than does sporadic colorectal cancer in the general population.3 Overall, the risk of developing CAC in UC patients was estimated to be 2%, 8%, and 18% after 10, 20, and 30 years of disease onset, respectively4, and 2.9%, 5.6%, and 8.3% for the same time intervals in CD patients.5 These estimates emphasize development of effective therapies for IBD as an important public health priority and justify a need to find means to prevent or treat this disease and inhibit its progression to CAC.
IBD-induced colon cancers do not have a well-defined genetic etiology; their genesis appears to be a result of chronic inflammation.6 Supportive evidence showed that the risk of developing colon tumors increases with longer duration of colitis, as well as with the extent and/or severity of inflammation.7 In addition, production of pro-inflammatory cytokines (e.g., interleukin (IL)-6, IL-17, and tumor necrosis factor alpha [TNFα]) and activation of inflammation-related pathways (e.g., nuclear factor-kappa B [NFκB]), as a result of chronic inflammation, have been shown to promote proliferative and anti-apoptotic processes that are believed to contribute to CAC.8 Therefore, identifying molecular targets that control inflammation and carcinogenesis may provide a therapeutic strategy to control symptoms of IBD and its progression to colon cancer.
Multiple lines of evidence have been presented in the literature ascribing anti-inflammatory and anti-tumorigenic effects of estrogens in the colon.9,10 Furthermore, hormone replacement therapy was shown to reduce the risk of developing colon cancer in postmenopausal women11 and to reduce disease activity in postmenopausal women with IBD.12 Cellular signaling of estrogens is mediated by its association with either estrogen receptor-alpha (ERα) or ER-beta (ERβ). In human colonic epithelium, ERβ is the predominant functional ER subtype expressed13 and its levels are reduced in colorectal tumors compared to normal colonic tissues14, which suggest that estrogens’ protective role in the colon may be exerted via ERβ signaling. Supporting these observations, we demonstrated that ERβ deficiency increased the incidence of pre-cancerous lesions in the colon15. Similarly, the use of an ERβ-selective agonist was shown to prevent intestinal tumorigenesis in ApcMin/+ mice16 and to exert beneficial effects in the HLA-B27 transgenic rat model of IBD.17 More recently, ERβ overexpression in colon cancer cell lines was shown to downregulate the expression of pro-inflammatory molecules.18 These data form the basis for the translational significance of using ERβ-selective agonists to prevent the development of CAC in IBD patients. However, the specific direct role of ERβ in CAC has not yet been evaluated and its mechanism of action remains to be explored. In order to address these questions we evaluated the effects of azoxymethane (AOM)/dextran sodium sulfate(DSS)-induced CAC in ERβ knockout (βERKO) and wild-type (WT) mice. The AOM/DSS protocol is a well established in vivo model used to study the pathogenesis and chemoprevention of CAC.19,20 The use of βERKO mice allowed us to assess the direct effects of ERβ deficiency on the development of IBD-related phenotype, the progression of IBD to colon cancer, and the expression of inflammation-related molecules. The studies presented in this report elucidate the significance of ERβ signaling pathway in IBD-induced colon cancer.
Materials and Methods
Animals
TheβERKO (B6.129P2-Esr2tm1Unc/J) and the background-WT (C57BL/6J) female mice were purchased from The Jackson Laboratory (Bar Harbor, ME). The genotype of these mice was confirmed by polymerase chain reaction (PCR). Animals were quarantined for 7 days and housed under conventional conditions with a 12-hour light-dark cycle, at a temperature range of 20–26°C, and a relative humidity of 50% (± 20%). A red mouse igloo (Bio-Serv, Frenchtown, NJ) was placed in each cage as an environmental enrichment. Drinking water and diet were supplied ad libitum. Animals were fed the LabDietR 5001 (PMIR Nutrition International, St. Louis, MO) throughout the study. This study was approved by the IITRI Institutional Animal Care and Use Committee.
Induction of CAC by AOM/DSS treatment
Eight to ten-week-old female βERKO (18–23 g) and WT littermates (16–21 g) were allocated to different treatment groups by randomized block design according to age and weight. For the AOM/DSS treatment group, mice were injected once subcutaneously with AOM at 10mg/kg body weight (BW) (NCI Chemical Carcinogen Reference Standards Repository, Kansas City, MO) on day 1 of the first week (wk). One wk later, these mice were exposed orally to 2.5% DSS (MP Biomedicals, LLC, Solon, OH) in their drinking water ad libitum for 7 days, after which the water was changed back to regular drinking water. A negative control group was used for each genotype in order to assess potential differences in clinical observations and estradiol production between AOM/DSS-treated and vehicle-treated mice. For this, the chemical treatment was substituted by treatment with the vehicle only, saline (0.90% NaCl) or regular drinking water for AOM and DSS, respectively. Mice were sacrificed by CO2 asphyxiation on wk 9 and 16, or when the mice developed anal prolapse and/or lost ≥ 20% BW.
Evaluation of Clinical Colitis
Clinical severity of colitis was evaluated by monitoring BW, stool consistency, rectal bleeding, and anal prolapse throughout the study. Disease activity index (DAI) was determined by combining the scores of weight loss, stool consistency, and bleeding divided by three as described by Cooper et al.21
Tissue Collection
Colons were harvested, washed, opened along the long axis, the length measured, and the weight determined. After macroscopic assessment of the number and size in diameter of polyps, a small section (approximately 0.5 cm) of the distal part of each colon was cut and flash frozen in liquid nitrogen for quantitative reverse transcription-PCR (qRT-PCR) analyses. The rest of the colon was fixed flat in 10% buffered formalin (Fisher Scientific, Fair Lawn, NJ) overnight and tissue sections containing the polyps were then embedded in paraffin. These colonic sections were stained with hematoxylin and eosin (H&E) and adjacent sections were used for immunohistochemistry analyses.
Histological Evaluation of Colitis
Colonic H&E sections were examined and scored for inflammation, dysplasia, and neoplasia by a gastrointestinal pathologist (J.H.) blinded to mouse genotype, following established criteria.21,22,23 Briefly, inflammation was scored from 0 to 4, with normal colonic mucosa = 0, shortening and loss of the basal one-third of the crypts with mild inflammation in the mucosa = 1, loss of the basal two-thirds of the crypts with moderate inflammation in the mucosa = 2, loss of the entire crypts, but not the surface epithelium with severe inflammation in the mucosa = 3, and loss of both entire crypts and surface epithelium with severe inflammation in the mucosa = 4. Grade of dysplasia was scored from 0 to 3, with normal colonic epithelium = 0, adenoma of low grade dysplasia = 1, adenoma of mild grade dysplasia = 2, and adenoma or carcinoma in situ of high-grade dysplasia = 3. Adenomas were identified as neoplastic lesions presenting aberrant glandular architecture with branching or elongated crypts. Cytologically, these lesions showed hyperchromatic, elongated and pseudo stratified nuclei with slightly increased nuclear to cytoplasmic ratios. Adenomas with carcinoma in situ presented marked glandular complexity, as well as areas with a cribriform configuration and cytological atypia characterized by large irregular nuclei and loss of nuclear polarity.
qRT-PCR Analyses
Flash-frozen distal colon sections were homogenized using the BBX24B Bullet Blender (Next Advance Inc., Averill Park, NY). Total RNA was isolated from the homogenized tissue by using Trizol reagent and following the manufacturer’s instructions (Invitrogen, Carlsbad, CA). Quantification of RNA concentration was performed using the Spectronic BioMate 5 UV-Visible Spectrophotometer (Thermo Electron Corporation, Madison, WI) and 2 μg of total RNA was reversed transcribed using M-MLV reverse transcriptase (Invitrogen). qRT-PCR was performed on cDNA product in a MyiQ Single Color Real-time PCR Detection System (Bio-Rad, Hercules, CA) by using iQ SYBR Green PCR Supermix (Bio-Rad) as instructed by the supplier. For cDNA quantification of inflammation markers the following mouse gene-specific primer pairs were used: 18S ribosomal RNA (18S) forward 5’-CATGGCCGTTCTTAGTTGGT-3’ and reverse 5’-GAACGCCACTTGTCCCTCTA-3’, IL-6 forward 5’-CCGGAGAGGAGACTTCACAG-3’ and reverse 5’-CAGAATTGCCATTGCACAAC-3’; IL-17 forward 5’-TCCAGAAGGCCCTCAGACTA-3’ and reverse 5’-TGAGCTTCCCAGATCACAGA-3’, TNFα forward 5’-CCCCAAAGGGATGAGAAGTT-3’ and reverse 5’-CACTTGGTGGTTTGCTACGA-3’; and interferon-gamma (IFNγ) forward 5’-GCGTCATTGAATCACACCTG-3’ and reverse 5’-TGAGCTCATTGAATGCTTGG-3’. The expression levels of each gene were normalized to that of 18S in all respective samples. Samples were run in duplicate and the expression of each inflammation marker gene was calculated as the mean per group. Each reaction was repeated at least three times.
Quantitative Immunohistochemistry analyses
Tissue sections (4 μm thick) were processed for immunohistochemistry as previously described.24 Briefly, slides were deparaffinized in xylene (Fisher Scientific, Fair Lawn, NJ) and rehydrated in decreasing concentrations of ethanol. Antigen retrievals for the following proteins (antibodies): inducible nitric oxide synthase (iNOS), mucin-1 (MUC1), mucin-2 (MUC2) (Abcam Inc., Cambridge, MA), NFκB/p65 (Rel A) (NFκB) (Thermo Fisher Scientific, Fremont, CA), β-catenin, proliferating cell nuclear antigen (PCNA) (Santa Cruz Biotechnology, Inc., Santa Cruz, CA), and caveolin-1 (Cav-1) (Cell Signaling Technology, Inc., Danvers, MA) were performed by boiling slides for 15 minutes in antigen unmasking citrate-based solution (Vector Laboratories, Inc., Burlingame, CA). Endogenous peroxidase activity was quenched by incubating slides for 5 minutes in 3% hydrogen peroxide solution (Fisher Scientific, Fair Lawn, NJ). Slides were blocked for unspecific binding with Ultra V Block (Thermo Fisher Scientific, Fremont, CA) and then incubated with primary antibodies either for one hour at room temperature in a humidified chamber for iNOS (1:250) and NFκB (1:100), or overnight at 4°C temperature in a humidified chamber forβ-catenin (1:100), PCNA (1:100), MUC2 (1:50), MUC1 (1:50), and Cav-1 (1:200). Antibody detection was performed using the Ultravision Mouse Tissue Detection Kit (Lab Vision Corporation, Fremont, CA) per manufacturer’s instructions. For all the sections, negative controls were obtained following the same procedure, but omitting the primary antibody.
Expression levels of each protein were quantified by calculating the immunostaining total score, which is equal to the sum of the proportion score and the intensity score (range 0, 2–8).25 The proportion score was determined by the percentage of positive staining cells per section: 0% = 0, 1% = 1, 2–10% = 2, 11–33% = 3, 34–66% = 4, > 67% = 5. The intensity score was determined by the average staining intensity: negative = 0, weak (staining seen at high power) = 1, intermediate (distinct color seen at low power) = 2, strong (dense, dark color seen at low power) = 3. The scores were determined by an observer blinded to mice genotype from microscope images captured with an Olympus DP70 camera. Scores represent the mean immunostaining of colonic sections from three randomly chosen mice per group.
Enzyme-Linked Immunosorbent Assay (ELISA) of Serum Estradiol
Blood was collected from each animal by cardiac puncture and allowed to clot for a minimum of 30 minutes in tubes with serum separator (BD Microtainer, Franklin Lakes, NJ). The blood was then centrifuged for 3 minutes at 5100 rpm, and the serum was collected and stored at −80°C. To assess mouse serum estradiol levels we used the Mouse/Rat Estradiol ELISA kit (Calbiotech Inc., Spring Valley, CA) and followed the manufacturer’s instructions. The absorbance was read at 450 nm with the ELx800 absorbance microplate reader (BioTek Instruments, Inc., Winooski, VT). To calculate the concentration of estradiol per group, a standard curve was constructed by plotting the mean absorbance obtained for each reference standard against its concentration. Interpolation of absorbance values of each sample with the standard curve allowed the determination of the corresponding concentration of estradiol in pg/mL. The results are displayed as the mean values of the samples for each group.
Statistical Analysis
All statistical analyses were performed using the GraphPad InStat 3 program (GraphPad Software Inc, La Jolla, CA). Student t-test was used for comparison between only two groups. One-way analysis of variance (ANOVA) was used to compare more than two groups followed by Bonferroni Multiple Comparison correction. Differences were considered statistically significant when p-values were less than 0.05.
Results
Colitis clinical severity was augmented in βERKO mice
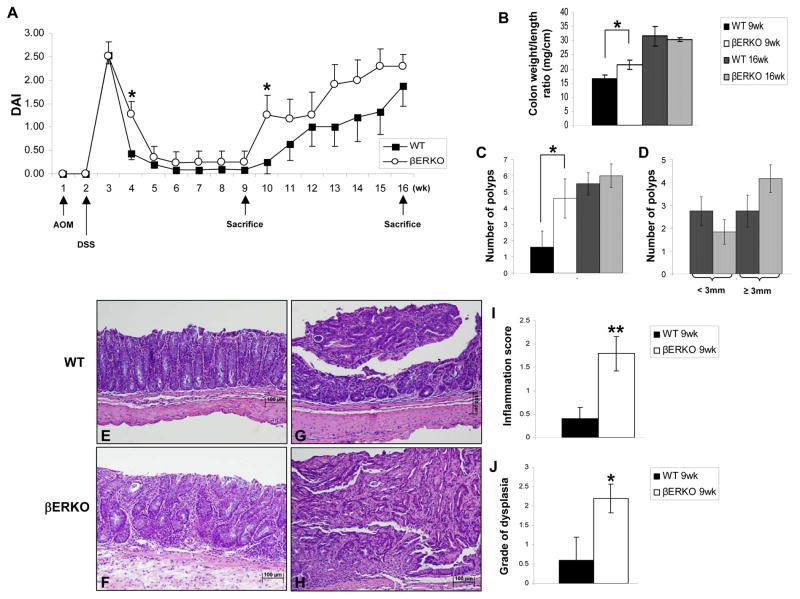
The clinical severity of colitis was determined by assessing changes in BW, differences in colon weight, length, weight to length ratio, the occurrence of diarrhea, and rectal bleeding throughout the study. DSS treatment, after one AOM injection, induced colitis characterized by weight loss, diarrhea and bleeding, which was not observed in vehicle-treated mice. We observed occurrence of diarrhea and bleeding in all the mice and a significant decrease in BW for both WT and βERKO groups one wk after DSS treatment characterized by an increase in DAI (wk 3, Fig. 1A). On wk 4, DAI was significantly higher in βERKO mice (Fig. 1A) that had not yet recovered the loss of BW (p < .05) and were still suffering from diarrhea or passing loose stools, compared to the WT mice that had recovered from the weight loss (p > .05) and were passing formed stools. DAI was also significantly increased in βERKO mice at wk 10, which started losing weight, presented loose stools, and rectal bleeding earlier than WT mice. At wk 16, the BW of βERKO mice (21.5 ± 1.1 g) was significantly decreased compared to its respective control group (25.20 ± 0.71 g; p = .0154). For the WT mice, no difference in final BW was observed between AOM/DSS treated (23.2 ± 0.7g) and control group (23.17 ± 0.43 g; p = .4199). Macroscopic analyses of the colons revealed that ERβ deficient colons presented a greater weight to length ratio than WT colons (Fig. 1B) at the 9 wk time point. This might be explained by the development of an inflamed mucosa and/or greater tumor burden. Furthermore, βERKO mice presented significantly higher multiplicity of polyps at wk 9 than WT mice (Fig. 1C, p < .05) and at wk 16 the number of polyps with a diameter equal or greater than 3mm was increased in ERβ deficient colons compared to WT colons (Fig. 1D). These data demonstrated that the severity of AOM/DSS induced-CAC was augmented in βERKO compared to WT mice.
Figure 1.
ERβ deficiency is associated with increased severity of clinical colitis.
(A) Disease Activity Index (DAI) was calculated on a weekly basis for WT and βERKO mice treated with AOM and DSS. Black arrows indicate the start of treatments and when animals were sacrificed as described in the Material and Methods. The values are means ± SE. *p < 0.05 (t-test) [n=13/group from wk 1 to 9, n=8/group from wk 10 to 16]. (B) Colon weight to length ratio assessed at wk 9 and 16 after AOM/DSS treatment. (C) Number of polyps per colon assessed at wk 9 and 16 after AOM/DSS treatment. (D) Differences in diameter of the polyps of βERKO and WT mice at wk 16. (B-D) Bars are means ± SE. *p < 0.05 (t-test between groups for the same time point) [n=5/group and n=8/group for the 9wk and 16wk time period, respectively]. (E-H) Representative H&E-stained colonic sections are presented. (E) WT normal colonic mucosa (inflammation score 0); (F) Loss of the basal two-thirds of the crypts with moderate inflammation in the mucosa of βERKO mice (inflammation score 2); (G) WT adenoma of low-grade dysplasia; (H) ERβ-deficient carcinoma in situ with high-grade dysplasia. Original magnifications = x10. (I) Inflammation score assessed at the 9wk time point. (J) Grade of dysplasia determined at the 9wk time point. Bars are means ± SE. *p < .05; **p < .01 (t-test) [n=5/group].
Colitis-associated histological features were manifested earlier in the absence of ERβ
Colitis-associated histological features were examined on H&E stained colonic sections (Fig. 1C–F). ERβ-deficient mice demonstrated higher inflammation scores (Fig. 1G, p < .01) and grade of dysplasia (Fig. 1H, p < .05) at the 9 wk time point. At wk 16, these colitis-associated morphologic features were identical between WT and βERKO mice, which suggested that the expression of ERβ in the colon might delay inflammation and neoplastic transformation.
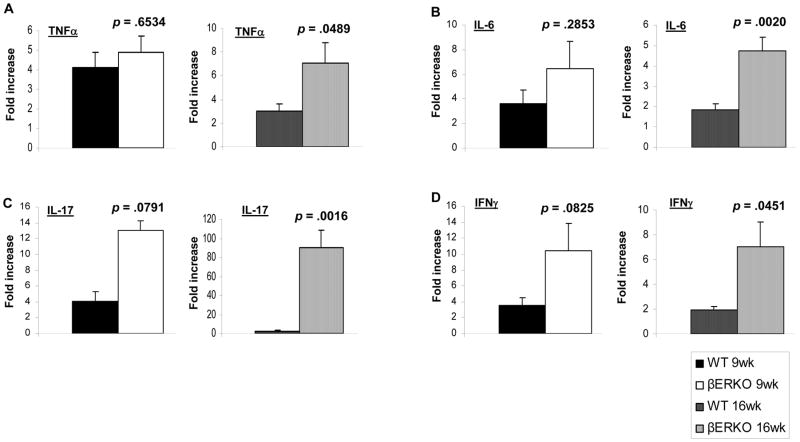
βERKO mice showed increased expression of inflammation-related molecules
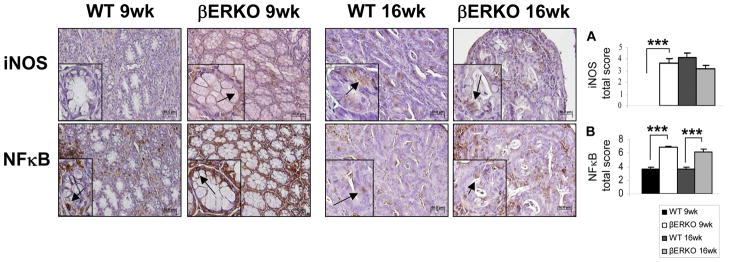
To support this potential preventive effect of ERβ against IBD-associated colon cancer and investigate its possible mechanism of action, we examined the expression of several inflammation markers in colonic tissues from βERKO and WT mice. We assessed the expression patterns of the pro-inflammatory molecules TNFα, IL-6, IL-17, and IFNγ, which have been highly correlated with IBD.8 The mRNA levels of these molecules were elevated but not significantly higher in βERKO compared to WT colons at wk 9 (Fig. 2A–D, left panel). At 16 wk, these inflammatory markers were significantly increased in ERβ-deficient colons (Fig. 2A–D, right panel), suggesting a potential role for ERβ signaling in the regulation of these inflammation-related molecules in the colon. Moreover, iNOS, a pro-inflammatory mediator in IBD involved in inflammation and dysplasia7, was identified in colons of ERβ-deficient but not WT mice at the 9 wk time point (p < .001) (Fig. 3A). At the 16 wk time point, both genotypes showed identical levels of iNOS expression (Fig. 3A). Additionally, Greten et al.26 demonstrated that the activation of NFκB signaling plays a key role in the initiation of CAC in the AOM/DSS model. In our study, we observed a significant increase in nuclear staining of NFκB in βERKO colonic epithelium compared to the WT mice at both time points (p < .001) (Fig. 3B). In summary, with respect to iNOS and NFκB, ERβ deficiency seems to be correlated with increased activation of inflammation-related pathways in the colon following AOM/DSS treatment.
Figure 2.
Effects of AOM/DSS treatment on transcript expression of inflammation markers in colons of WT and βERKO mice. The mRNA levels of (A) TNFα, (B) IL-6, (C) IL-17, and (D) IFNγ expressed in colonic tissues isolated from WT andβERKO mice at wk 9 (left panel) and 16 (right panel) were determined by qRT- PCR and normalized to 18S mRNA. Results are represented as means + SE of three independent experiments performed in duplicate for each inflammation marker expressed in colonic tissues. p-values (t-test) represent statistical differences between WT and βERKO groups [n=4/group and n=6/group for the 9 and16 wk time point, respectively].
Figure 3.
AOM/DSS treatment induces earlier iNOS expression and increased NFκB activation in ERβ-deficient colons. The expression of (A) iNOS and (B) NFκB proteins was assessed by immunohistochemical staining on colonic sections of WT and βERKO mice. Shown are representative stained colonic sections from each group. Original magnification = x20. The inset within each image represents a higher magnification of a stained area of interest indicated by the black arrows. Expression levels of each protein per group were quantified by calculating the immunostaining total score per stained sections (range 0, 2-8) represented in the graphs as means + SE. ***p < .001 (ANOVA followed by Bonferroni Multiple Comparison correction) [n=3/group].
βERKO mice showed increased β-catenin, and decreased caveolin-1 levels
Up-regulation of nuclear β-catenin, which drives increased proliferation, has been correlated with the genesis of sporadic, as well as colitis-associated colon tumors.8 On the other hand, caveolin-1, previously identified as an ERβ-target gene in colon cancer cell lines, exerts anti-tumorigenic, pro-apoptotic, and anti-inflammatory effects.18 The results obtained showed a significant increase in levels of β-catenin (p < .01 for 9 wk and p < .001 for 16 wk) (Fig. 4A) and a significant decrease in levels of caveolin-1 (p < .05 for 9 wk and p < .01 for 16 wk) (Fig. 4B) in colons of βERKO compared to the WT mice. β-catenin was visualized mainly in the membrane by 9 wk and had translocated to the nucleus by 16 wk (Fig. 4), indicating an activated state for this transcription factor.8 To confirm the potential increase in cellular proliferation due to β-catenin activation, we performed PCNA immunostaining. Figure 4C shows that PCNA expression was significantly higher at wk 16 in the absence of ERβ (p < .05) compared to WT colons. Based on differences in colonicβ-catenin, PCNA, and caveolin-1 expression in ERβ and WT mice, we suggest that the absence of ERβ may lead to increased cellular proliferation, decreased cellular apoptosis, and activation of inflammation-related pathways.
Figure 4.
ERβ-deficient colons expressed higher β-catenin and lower Cav-1 levels after AOM/DSS treatment. The expression of (A) β-catenin, (B) Cav-1, and (C) PCNA proteins was assessed by immunohistochemical staining on colonic sections of WT and βERKO mice. Shown are representative stained colonic sections from each group. Original magnification = x20. The inset within each image represents a higher magnification of a stained area of interest indicated by the black arrows. Expression levels of each protein per group were quantified by calculating the immunostaining total score per stained sections (range 0, 2-8) represented in the graphs as means + SE. *p < .05; **p < .01; ***p < .001 (ANOVA followed by Bonferroni Multiple Comparison correction) [n=3/group].
AOM/DSS treatment induced greater loss of cellular differentiation in ERβ-deficient colons
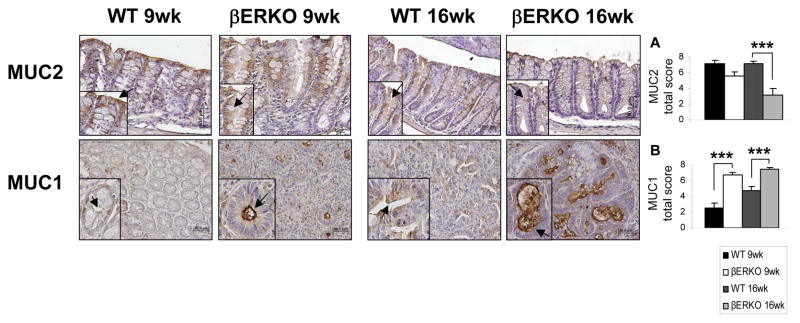
MUC2 is secreted by differentiated goblet cells, which contribute to the lining of the colonic crypts epithelium, forming a protective mucus layer in the colon. Alterations in MUC2 secretion are associated with colonic carcinogenesis and IBD.27,28 Furthermore, MUC2 KO mice are more susceptible to colonic tumorigenesis.29 Significant decreases in MUC2 levels were identified in ERβ-deficient colons at the 16 wk time point (p < .001) (Fig. 5A). In addition, we evaluated the expression of MUC1, a transmembrane mucin, which is correlated with poor colon cancer differentiation, tumor progression, and worse survival.30 TheβERKO mice expressed significantly higher colonic levels of MUC1 at both end-time points of our study (p < .001 for 9 and 16 wk) (Fig. 5B). Decreases in MUC2 and increases in MUC1 in ERβ-deficient mice indicate loss of cellular differentiation and in human disease would portend a worse prognosis.
Figure 5.
AOM/DSS-induced CAC in βERKO mice is characterized by a decrease in MUC2 and an increase in MUC1 levels. The expression of (A) MUC2 and (B) MUC1 proteins was assessed by immunohistochemical staining on colonic sections of WT and βERKO mice. Shown are representative stained colonic sections from each group. Original magnification = x20. The inset within each image represents a higher magnification of a stained area of interest indicated by the black arrows. Expression levels of each protein per group were quantified by calculating the immunostaining total score per stained sections (range 0, 2-8) represented in the graphs as means + SE. ***p < .001 (ANOVA followed by Bonferroni Multiple Comparison correction) [n=3/group].
Serum estradiol levels are similar between WT and βERKO mice
To determine whether ERβ related differences in inflammation and dysplasia observed in this study might be influenced by changes in estradiol, we measured serum estradiol levels. The mean values of the groups ranged from 7.20 (± 1.22) pg/mL to 9.53 (± 1.51) pg/mL for the 9 wk time point, and from 9.00 (± 1.68) pg/mL to 12.91 (± 1.81) pg/mL for the 16 wk time point. There were no statistical differences observed between the AOM/DSS and non-treated groups. These results confirm previously published findings that ovaries fromβERKO mice produce normal serum levels of estradiol31 and also shows that the AOM and DSS treatments do not affect estradiol production.
Discussion
In this study, we demonstrated that the AOM/DSS treatment increased the severity of colitis and accelerated progression to CAC in βERKO mice. First, ERβ-deficiency was associated with a significant increase in colitis disease activity after DSS treatment and greater number and size of colonic polyps. Second, it reduced the latency period between the disease onset and the manifestation of inflammation-associated features and molecules, evidenced by the significantly increased colon weight to length ratio, inflammation score, grade of dysplasia, iNOS, and NFκB expression at the earliest time point of this study. Lastly, the absence of ERβ was associated with greater proliferation, reduced protective mucus-layer, and less differentiated colonic tissue as demonstrated by increases in PCNA, nuclearβ-catenin and up-regulated MUC1, along with reduced caveolin-1 and MUC2 expressions. These observations indicate thatβERKO mice are more susceptible to AOM/DSS treatment, and that the molecular changes observed in these mice more closely resemble the molecular changes occurring as a result of chronic intestinal inflammation in humans.8 Taken together, these findings validate the use of the βERKO mouse model as a powerful genetic tool to investigate the role of ERβ in IBD-associated colon cancer.
Chronic inflammation has been strongly associated with colon carcinogenesis in IBD patients.32 For example, IFNγ and IL-17 are well known pro-inflammatory cytokines that drive intestinal inflammation in IBD.8,33 Moreover, patients with active UC, dysplasia, and cancer had significantly higher IL-6 levels in the colonic epithelium compared to normal individuals or those with quiescent UC.34 TNFα was shown to induce IL-6 secretion and to regulate inflammation-related pathways in CAC.35 NFκB signaling was demonstrated to be activated in inflamed mucosa of IBD patients. This master regulator of inflammatory cytokines stimulates iNOS, other pro-inflammatory, and pro-survival genes, providing a link between inflammation and carcinogenesis.35 Furthermore, it is believed that inflammation promotes multiple hallmarks of cancer, such as sustained proliferative signaling, resistance to cell death, and induction of angiogenesis.36 Therefore, finding new molecules that can target inflammatory pathways, usually activated in CAC, has an enormous translational potential in prevention and therapy of this type of cancer. Previously, ERβ re-expression in SW480 colon cancer cells was shown to down-regulate IL-6 and downstream networks, suggesting a possible anti-inflammatory role of ERβ in chronic intestinal inflammation.18 Our results demonstrated the up-regulation of IL-6 expression in the absence of ERβ signaling in vivo, as well as enhanced expression of TNFα, IL-17, and IFNγ, and offered mechanistic insights into the protective role of ERβ against the development of CAC (Fig. 6). The cytokine profiles became more significantly divergent at 16 wk when the AOM/DSS-induced inflammation was healing compared to the 9 wk time point (Fig. 2), suggesting that these cytokines may also be important for driving neoplastic transformation. Further supporting a protective effect of ERβ in IBD, Looijer-van Langen et al.37 demonstrated that ERβ mRNA levels were reduced in animal models of colitis and in colonic biopsies from IBD patients. Furthermore, these authors suggested that the regulation of colonic epithelial permeability might in fact be mediated by ERβ signaling. Moreover, ERB-041, a selective ERβ agonist, was shown to suppress NFκB signaling and inhibit iNOS production in LPS-activated peritoneal macrophages of endometriosis.38 Our studies also suggest increased activation of NFκB and iNOS production in the absence of ERβ signaling in the colon. These findings highlight the need to further explore the potential use of ERβ agonists or inducers as suppressers of NFκB and other inflammation-related pathways that are activated in CAC and in other types of inflammatory diseases.
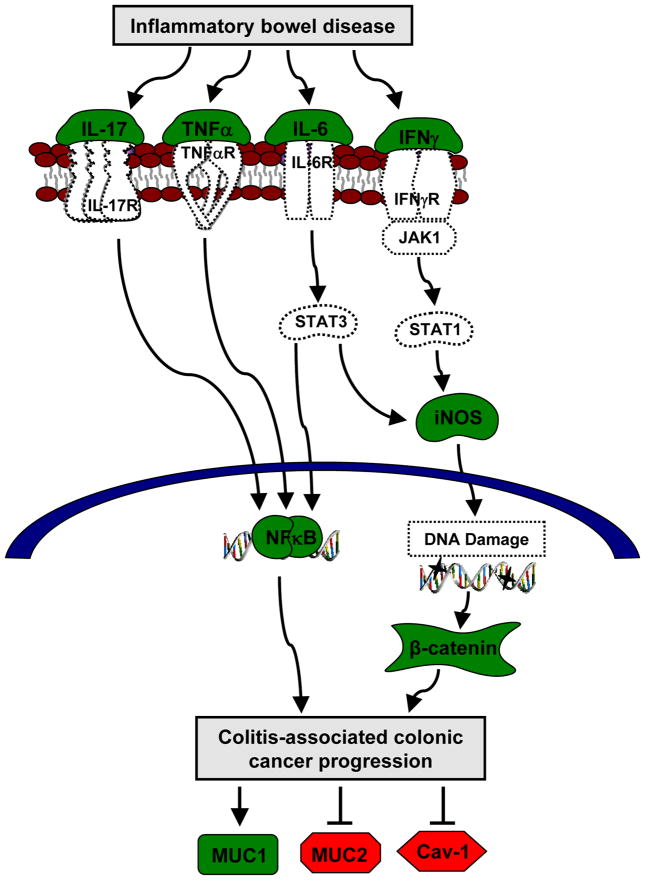
Figure 6.
Proposed regulatory mechanism of ERβ in colitis-associated colon cancer. In inflammatory bowel disease, pro-inflammatory cytokines, such as IL-17, TNFα, IL-6, and IFNγ, activate the NFκB signaling pathway and increase iNOS levels. In turn, iNOS is involved in initiating inflammation and inducing DNA damage that activate β-catenin. Both NFκB and β-catenin signaling pathways enhance the progression of colitis-associated colonic tumorigenesis. Poor tumor prognosis is characterized by an increase in MUC1, and a decrease in MUC2 and Cav-1. Our results suggest a potential role for ERβ in the regulation of several molecules highlighted in this model. ERβ signals appear to suppress AOM/DSS-induced IL-17, TNFα, IL-6, IFNγ, iNOS, NFκB, and MUC1 represented in green, and to preserve MUC2 and Cav-1 expressions represented in red. Molecules represented in white were implicated in the literature in IBD-induced colon cancer development.
In conclusion, these results revealed a possible anti-inflammatory and anti-neoplastic mechanism of action of ERβ in CAC and provided support for the potential use of ERβ inducers, given that some inducers (e.g., ERB-041) are already undergoing Phase I trials39, to prevent or treat IBD.
Brief statement on novelty and impact.
Our studies represent the first direct evidence of estrogen receptor-beta (ERβ) protective role in colitis-associated colon cancer model, and provide rationale for the potential use of ERβ inducers to prevent and/or treat IBD and its progression to colon cancer.
Acknowledgments
This work was supported by Fulbright Portugal Commission and Fundação para a Ciência e Tecnologia SFRH/BD/33544/2008 to D.S.; and National Institutes of Health (K01 CA103861 to G.M., R01 CA121157 to R.G.M., and R01 CA122299-01 RVB). This project was performed, in part, using the compound AOM provided by the National Cancer Institute’s Chemical Carcinogen Reference Standards Repository operated under contract by Midwest Research Institute, NO. N02-CB-66600. Part of this work has been previously reported at the 2011 American Association for Cancer Research 102nd Annual Meeting in Orlando, and published in the 2011 Proceedings of the American Association for Cancer Research. There are no conflicts of interest to be disclosed.
Abbreviations
- AOM
Azoxymethane
- BW
body weight
- CAC
colitis-associated colorectal cancer
- Cav-1
caveolin-1
- CRC
colorectal cancer
- CD
Crohn’s disease
- DAI
disease activity index
- DSS
dextran sodium sulfate
- 18S
18S ribosomal RNA
- ER
estrogen receptor
- H&E
hematoxylin and eosin
- iNOS
inducible nitric oxide synthase
- IBD
inflammatory bowel disease
- IFN
interferon
- IL
interleukin
- KO
knockout
- mRNA
messenger RNA
- MUC
mucin
- NFκB
nuclear factor-kappa B
- PCNA
proliferating cell nuclear antigen
- qRT-PCR
quantitative reverse transcription-polymerase chain reaction
- SE
standard error of the mean
- TNF
tumor necrosis factor
- UC
ulcerative colitis
- wk
week(s)
- WT
wild-type
References
- 1.Mattar MC, Lough D, Pishvaian MJ, Charabaty A. Current management of inflammatory bowel disease and colorectal cancer. Gastrointest Cancer Res. 2011;4:53–61. [PMC free article] [PubMed] [Google Scholar]
- 2.Munkholm P. Review article: the incidence and prevalence of colorectal cancer in inflammatory bowel disease. Aliment Pharmacol Ther. 2003;18:1–5. doi: 10.1046/j.1365-2036.18.s2.2.x. [DOI] [PubMed] [Google Scholar]
- 3.Lutgens MW, Vleggaar FP, Schipper ME, Stokkers PC, van der Woude CJ, Hommes DW, de Jong DJ, Dijkstra G, van Bodegraven AA, Oldenburg B, Samsom M. High frequency of early colorectal cancer in inflammatory bowel disease. Gut. 2008;57:1246–1251. doi: 10.1136/gut.2007.143453. [DOI] [PubMed] [Google Scholar]
- 4.Eaden JA, Abrams KR, Mayberry JF. The risk of colorectal cancer in ulcerative colitis: a meta-analysis. Gut. 2001;48:526–535. doi: 10.1136/gut.48.4.526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Canavan C, Abrams KR, Mayberry J. Meta-analysis: colorectal and small bowel cancer risk in patients with Crohn's disease. Aliment Pharmacol Ther. 2006;23:1097–1104. doi: 10.1111/j.1365-2036.2006.02854.x. [DOI] [PubMed] [Google Scholar]
- 6.Danese S, Mantovani A. Inflammatory bowel disease and intestinal cancer: a paradigm of the Yin-Yang interplay between inflammation and cancer. Oncogene. 2010;29:3313–3323. doi: 10.1038/onc.2010.109. [DOI] [PubMed] [Google Scholar]
- 7.Itzkowitz SH, Yio X. Inflammation and cancer IV. Colorectal cancer in inflammatory bowel disease: the role of inflammation. Am J Physiol Gastrointest Liver Physiol. 2004;287:G7–G17. doi: 10.1152/ajpgi.00079.2004. [DOI] [PubMed] [Google Scholar]
- 8.Terzić J, Grivennikov S, Karin E, Karin M. Inflammation and colon cancer. Gastroenterology. 2010;138:2101–2114. doi: 10.1053/j.gastro.2010.01.058. [DOI] [PubMed] [Google Scholar]
- 9.Harnish DC, Albert LM, Leathurby Y, Eckert AM, Ciarletta A, Kasaian M, Keith JC., Jr Beneficial effects of estrogen treatment in the HLA-B27 transgenic rat model of inflammatory bowel disease. Am J Physiol Gastrointest Liver Physiol. 2004;286:G118–25. doi: 10.1152/ajpgi.00024.2003. [DOI] [PubMed] [Google Scholar]
- 10.Kennelly R, Kavanagh DO, Hogan AM, Winter DC. Oestrogen and the colon: potential mechanisms for cancer prevention. Lancet Oncol. 2008;9:385–91. doi: 10.1016/S1470-2045(08)70100-1. [DOI] [PubMed] [Google Scholar]
- 11.Grodstein F, Newcomb PA, Stampfer MJ. Postmenopausal hormone therapy and the risk of colorectal cancer: a review and meta-analysis. AM J Med. 2009;106:574–582. doi: 10.1016/s0002-9343(99)00063-7. [DOI] [PubMed] [Google Scholar]
- 12.Kane SV, Reddy D. Hormonal replacement therapy after menopause is protective of disease activity in women with inflammatory bowel disease. Am J Gastroenterol. 2008;103:1193–1196. doi: 10.1111/j.1572-0241.2007.01700.x. [DOI] [PubMed] [Google Scholar]
- 13.Campbell-Thompson M, Lynch IJ, Bhardwaj B. Expression of estrogen receptor (ER) subtypes and ERbeta isoforms in colon cancer. Cancer Res. 2001;61:632–640. [PubMed] [Google Scholar]
- 14.Konstantinopoulos PA, Kominea A, Vandoros G, Sykiotis GP, Andricopoulos P, Varakis I, Sotiropoulou-Bonikou G, Papavassiliou AG. Oestrogen receptor beta (ERβ) is abundantly expressed in normal colonic mucosa, but declines in colon adenocarcinoma paralleling the tumour's dedifferentiation. Eur J Cancer. 2003;39:1251–1258. doi: 10.1016/s0959-8049(03)00239-9. [DOI] [PubMed] [Google Scholar]
- 15.Saleiro D, Murillo G, Lubahn DB, Kopelovich L, Korach KS, Mehta RG. Enhanced induction of mucin-depleted foci in estrogen receptor {beta} knockout mice. Cancer Prev Res (Phila) 2010;3:1198–1204. doi: 10.1158/1940-6207.CAPR-10-0044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Giroux V, Bernatchez G, Carrier JC. Chemopreventive effect of ERβ-Selective agonist on intestinal tumorigenesis in Apc(Min/+) mice. Mol Carcinog. 2011;50:359–369. doi: 10.1002/mc.20719. [DOI] [PubMed] [Google Scholar]
- 17.Harris HA, Albert LM, Leathurby Y, Malamas MS, Mewshaw RE, Miller CP, Kharode YP, Marzolf J, Komm BS, Winneker RC, Frail DE, Henderson RA, Zhu Y, et al. Evaluation of an estrogen receptor-beta agonist in animal models of human disease. Endocrinology. 2003;144:4241–4249. doi: 10.1210/en.2003-0550. [DOI] [PubMed] [Google Scholar]
- 18.Edvardsson K, Ström A, Jonsson P, Gustafsson JÅ, Williams C. Estrogen receptorβ induces antiinflammatory and antitumorigenic networks in colon cancer cells. Mol Endocrinol. 2011;25:969–979. doi: 10.1210/me.2010-0452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tanaka T, Kohno H, Suzuki R, Yamada Y, Sugie S, Mori H. A novel inflammation-related mouse colon carcinogenesis model induced by azoxymethane and dextran sodium sulfate. Cancer Sci. 2003;94:965–973. doi: 10.1111/j.1349-7006.2003.tb01386.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.De Robertis M, Massi E, Poeta ML, Carotti S, Morini S, Cecchetelli L, Signori E, Fazio VM. The AOM/DSS murine model for the study of colon carcinogenesis: From pathways to diagnosis and therapy studies. J Carcinog. 2011;10:9. doi: 10.4103/1477-3163.78279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cooper HS, Murthy SN, Shah RS, Sedergran DJ. Clinicopathologic study of dextran sulfate sodium experimental murine colitis. Lab Invest. 1993;69:238–249. [PubMed] [Google Scholar]
- 22.Riddell RH, Goldman H, Ransohoff DF, Appelman HD, Fenoglio CM, Haggitt RC, Ahren C, Correa P, Hamilton SR, Morson BC, Sommers SD, Yardley JH. Dysplasia in inflammatory bowel disease: standardized classification with provisional clinical applications. Hum Pathol. 1983;14:931–968. doi: 10.1016/s0046-8177(83)80175-0. [DOI] [PubMed] [Google Scholar]
- 23.Suzuki R, Kohno H, Sugie S, Tanaka T. Sequential observations on the occurrence of preneoplastic and neoplastic lesions in mouse colon treated with azoxymethane and dextran sodium sulfate. Cancer Sci. 2004;95:721–727. doi: 10.1111/j.1349-7006.2004.tb03252.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Matusiak D, Murillo G, Carrol RE, Mehta RG, Benya RV. Expression of vitamin D receptor and 25-hydroxyvitamin D3-1α-hydroxylase in normal and malignant human colon. Cancer Epidemiol Biomarkers Prev. 2005;14:2370–2376. doi: 10.1158/1055-9965.EPI-05-0257. [DOI] [PubMed] [Google Scholar]
- 25.Allred DC, Harvey JM, Berardo M, Clark GM. Prognostic and predictive factors in breast cancer by immunohistochemical analysis. Mod Pathol. 1998;11:155–168. [PubMed] [Google Scholar]
- 26.Greten FR, Eckmann L, Greten TF, Park JM, Li ZW, Egan LJ, Kagnoff MF, Karin M. IKKbeta links inflammation and tumorigenesis in a mouse model of colitis-associated cancer. Cell. 2004;118:285–296. doi: 10.1016/j.cell.2004.07.013. [DOI] [PubMed] [Google Scholar]
- 27.Hollingsworth MA, Swanson BJ. Mucins in cancer: protection and control of the cell surface. Nat Rev Cancer. 2004;4:45–60. doi: 10.1038/nrc1251. [DOI] [PubMed] [Google Scholar]
- 28.Andrianifahanana M, Moniaux N, Batra SK. Regulation of mucin expression: mechanistic aspects and implications for cancer and inflammatory diseases. Bioch Bioph Acta. 2006;1765:189–222. doi: 10.1016/j.bbcan.2006.01.002. [DOI] [PubMed] [Google Scholar]
- 29.Velcich A, Yang W, Heyer J, Fragale A, Nicholas C, Viani S, Kucherlapati R, Lipkin M, Yang K, Augenlicht L. Colorectal cancer in mice genetically deficient in the mucin Muc2. Science. 2002;295:1726–1729. doi: 10.1126/science.1069094. [DOI] [PubMed] [Google Scholar]
- 30.Byrd JC, Bresalier RS. Mucins and mucin binding proteins in colorectal cancer. Cancer Metastasis Rev. 2004;23:77–99. doi: 10.1023/a:1025815113599. [DOI] [PubMed] [Google Scholar]
- 31.Curtis Hewitt S, Couse JF, Korach KS. Estrogen receptor transcription and transactivation: Estrogen receptor knockout mice: what their phenotypes reveal about mechanisms of estrogen action. Breast Cancer Res. 2000;2:345–352. doi: 10.1186/bcr79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420:860–867. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Szkaradkiewicz A, Marciniak R, Chudzicka-Strugała I, Wasilewska A, Drews M, Majewski P, Karpiński T, ZwoŸdziak B. Proinflammatory cytokines and IL-10 in inflammatory bowel disease and colorectal cancer patients. Arch Immunol Ther Exp (Warsz) 2009;57:291–294. doi: 10.1007/s00005-009-0031-z. [DOI] [PubMed] [Google Scholar]
- 34.Li Y, de Haar C, Chen M, Deuring J, Gerrits MM, Smits R, Xia B, Kuipers EJ, van der Woude CJ. Disease-related expression of the IL6/STAT3/SOCS3 signalling pathway in ulcerative colitis and ulcerative colitis-related carcinogenesis. Gut. 2010;59:227–235. doi: 10.1136/gut.2009.184176. [DOI] [PubMed] [Google Scholar]
- 35.Goel GA, Kandiel A, Achkar JP, Lashner B. Molecular pathways underlying IBD-associated colorectal neoplasia: therapeutic implications. Am J Gastroenterol. 2011;106:719–730. doi: 10.1038/ajg.2011.51. [DOI] [PubMed] [Google Scholar]
- 36.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 37.Looijer-van Langen M, Hotte N, Dieleman LA, Albert E, Mulder C, Madsen KL. Estrogen receptor-β signaling modulates epithelial barrier function. Am J Physiol Gastrointest Liver Physiol. 2011;300:G621– G626. doi: 10.1152/ajpgi.00274.2010. [DOI] [PubMed] [Google Scholar]
- 38.Xiu-li W, Wen-jun C, Hui-hua D, Su-ping H, Shi-long F. ERB-041, a selective ER beta agonist, inhibits iNOS production in LPS-activated peritoneal macrophages of endometriosis via suppression of NF-kappaB activation. Mol Immunol. 2009;46:2413–2418. doi: 10.1016/j.molimm.2009.04.014. [DOI] [PubMed] [Google Scholar]
- 39.Roman-Blas JA, Castañeda S, Cutolo M, Herrero-Beaumont G. Efficacy and safety of a selective estrogen receptor βagonist, ERB-041, in patients with rheumatoid arthritis: a 12-week, randomized, placebo-controlled, phase II study. Arthritis Care Res (Hoboken) 2010;62:1588–1593. doi: 10.1002/acr.20275. [DOI] [PubMed] [Google Scholar]