Abstract
It is widely accepted that aerobic exercise enhances hippocampal plasticity. Often, this plasticity co-occurs with gains in hippocampal-dependent memory. Cross-sectional work investigating this relationship in preadolescent children has found behavioral differences in higher versus lower aerobically fit participants for tasks measuring relational memory, which is known to be critically tied to hippocampal structure and function. The present study tested whether similar differences would arise in a clinical intervention setting where a group of preadolescent children were randomly assigned to a nine-month after school aerobic exercise intervention versus a wait-list control group. Performance measures included eye-movements as a measure of memory, based on recent work linking eye-movement indices of relational memory to the hippocampus. Results indicated that only children in the intervention increased their aerobic fitness. Compared to the control group, those who entered the aerobic exercise program displayed eye-movement patterns indicative of superior memory for face-scene relations, with no differences observed in memory for individual faces. The results of this intervention study provide clear support for the proposed linkage among the hippocampus, relational memory, and aerobic fitness, as well as illustrating the sensitivity of eye-movement measures as a means of assessing memory.
Keywords: hippocampus, aerobic fitness, eye-tracking, item memory, development
Introduction
Converging research has established that the hippocampus is vital for relational memory, which refers to the ability to form and use memory representations among the constituent elements of an event or scene (Cohen and Eichenbaum, 1993; Davachi, 2006). To accomplish its role in memory, the hippocampus demonstrates remarkable plasticity; it displays a disproportionate capacity for synaptic modulation, and is one of two known brain regions to undergo adult neurogenesis (Kaplan and Hinds, 1977; Neve et al., 1988). Multiple lifestyle factors amplify the rate and magnitude of plasticity in the hippocampus, including living in an enriched environment and partaking in aerobic exercise (Kempermann et al., 1997; van Praag et al., 1999b). This latter finding has been demonstrated multiple times across various species. For instance, rodents that run on a running wheel display greater numbers of newly born neurons in the dentate gyrus of the hippocampus, and these neurons are integrated into the functional circuitry of the hippocampus (van Praag et al., 1999a; van Praag et al., 2002). In human adults, aerobic exercise interventions have resulted in increased cerebral blood volume in the hippocampus, which is an in vivo marker of neurogenesis (Pereira et al., 2007). Moreover, a recent report suggests that exercise may attenuate the hippocampal volume loss associated with healthy aging (Erickson et al., 2011). Further, this plasticity occurring from aerobic exercise often accompanies improvements in memory (van Praag et al., 1999a; Pereira et al., 2007; Erickson et al., 2011).
This line of inquiry has recently been extended to preadolescent children in an effort to understand how aerobic exercise can modify the hippocampus and confer mnemonic benefits upon this special population. In a recent neuroimaging study, Chaddock et al. (2010) employed a cross-sectional design to compare a group of nine- and-ten-year-old children that were high in aerobic fitness to a group of their peers measuring low in aerobic fitness. The results indicated that children in the high-fit group had larger hippocampal volumes. Furthermore, group accuracy differences were observed on a relational memory task, with no differences on an item memory task, and performance on the relational memory task was selectively correlated with hippocampal volume. This latter result is in line with the view that the hippocampus is particularly suited for relational memory, while memory for individual items is more dependent on other brain regions, likely the medial temporal lobe cortex (Eichenbaum and Cohen, 2001). Critically, these findings identify a relationship between aerobic exercise, the hippocampus, and relational memory in preadolescent children. However, what is unknown is whether similar results would occur in children due to increased physical activity in a randomized intervention setting, or if they only are found when two disparate groups far apart on the fitness spectrum are compared.
Additionally, the question of aerobic exercise and fitness effects on eye-movement measures of memory remains entirely uninvestigated. In the past decade, the method of using eye-movements has proven to be an illuminating cognitive neuroscience technique in the study of memory (see Hannula et al., 2010 for review). This is true in part due to the nature of the method, which allows for a more objective and quantitative analysis of memory that is not solely limited to subjective report. Furthermore, in relational memory paradigms, eye-movement measures such as the proportion of viewing time allocated to a specific region of interest are linked to hippocampal structure and function (Ryan et al., 2000; Hannula et al., 2007; Hannula and Ranganath, 2009). Thus, eye-movements provide a sensitive measure for investigating hippocampal function and integrity, particularly in populations where overt behavioral responses are either not possible or are less reliable.
In this study we tested whether a group of preadolescent children randomly assigned to an aerobic exercise program would exhibit differences in behavioral and eye-movement measures of memory compared to a group of children who remained sedentary. We hypothesized that group differences would be selective to relational memory, consistent with our previous work in children and the abundant literature on both hippocampal functioning and aerobic exercise-induced plasticity in the hippocampus.
Methods
Participants
Fifty-one preadolescent children were randomly assigned to a nine-month aerobic exercise intervention group (AE) or a wait-list control group (C). The results reported here contain data from 44 children, as data from seven participants were discarded due to failure to adhere to task instructions (n = 5), an inability to obtain eye-movement data because of eyeglasses (n = 1), or the use of psychotropic medication (n = 1). The AE group’s final sample included 25 participants, while the C group contained 19 children. This study was approved by the University of Illinois at Urbana Champaign Institutional Review Board, and all children signed an assent form while legal guardians signed an informed consent document.
Factors affecting cognition such as age and IQ were taken into account during the randomization process to ensure the two groups were equal on these measures. Participants completed the Kaufman Brief Intelligence Test (K-BIT; Kaufman & Kaufman, 1990) to obtain an IQ measure that includes both crystallized and fluid measures of intelligence. In addition to this, data on the participant’s socioeconomic status (SES) was obtained by having the legal guardian complete an index measuring SES across three factors: 1) participation in a free or reduced-price meal program at school, 2) the highest level of education attained by the child’s mother and father, and 3) the number of parents who work full time (Birnbaum et al., 2002). Finally, an index of pubertal timing was collected for 42 of the participants by having children and guardians collaboratively complete a modified version of the Tanner Staging System, which assesses pubertal timing on a five-point scale, with scores of two or below corresponding to prepubescence (Taylor et al., 2001). Table 1 displays the mean scores for the two groups on these variables, and confirms the groups did not differ on these demographic factors.
Table 1.
Participant Demographics and Fitness Data: Mean (SD)
| Measured Variable | Aerobic Exercise | Control |
|---|---|---|
| n | 25 (9 female) | 19 (10 female) |
| Age | 9.4 (.63) | 9.5 (.44) |
| KBIT | 115.2 (12.3) | 122.2 (17) |
| SES | 1.9 (.86) | 2.1 (.74) |
| Pubertal timing | 1.4 (.41) | 1.6 (.67) |
| Pre-VO2max percentile | 20.8 (22) | 34.6 (24.8) |
| Post- VO2max percentile | 29 (28.3) | 28.3 (23.1) |
| Δ VO2max percentile* | 8.2 (21) | -6.3 (19.7) |
p < .05
Note: KBIT, Kaufman Brief Intelligence Exam; SES, socioeconomic status; VO2max, maximum oxygen uptake measured in mL/kg/min and converted to normative percentiles for age and gender taken before and after intervention
Aerobic exercise intervention and fitness testing
The AE group was enrolled in the Fitness Improves Thinking (FITKids) after-school intervention program (Castelli et al., 2011; Kamijo et al., 2011), while the C group was placed on a wait-list and continued their normal after-school activities. The FITKids intervention ran for nine months, corresponding to the academic year; sessions were held after every school day and lasted two hours. The overarching goal of the program was to improve the aerobic fitness of the participants. This was achieved by having individuals perform at least 70 minutes of moderate to vigorous physical activity per session, recorded by heart rate monitors worn by each participant (E600 Polar heart rate monitor; Polar Electro, Finland). A typical FITKids session consisted of an instant activity, followed by a healthy snack and an educational component, and then 40–60 minutes of physical activity at various stations. For the remainder of the time, participants took part in low organizational games that were aerobically demanding, but centered on developing a specific skill theme (i.e. dribbling); this provided an opportunity for motor skill refinement in addition to increasing aerobic capacity.
Prior to the intervention period and directly after, both groups had aerobic fitness levels calculated via a graded exercise test during which each participant’s maximal oxygen consumption (VO2max) level was obtained. VO2 max values correspond to one’s maximum ability to consume and utilize oxygen during physical exertion, with higher values indicating greater aerobic fitness. VO2 max was assessed via a computerized indirect calorimetry system (ParvoMedics True Max 2400) during a modified Balke treadmill test (American College of Sports Medicine, 2006). The heart rate of each participant was monitored constantly using a Polar heart rate monitor (Polar WearLink® +31, Polar Electro, Finland) and a measure of perceived exertion was attained every two minutes using the children’s OMNI scale of perceived exertion (Utter et al., 2002).
During the maximal exercise test, participants initially walked on a motor-driven treadmill for a warm-up period of two minutes, during which time the speed gradually increased. After this warm-up period the test began. Participants ran on the treadmill at a constant speed and the grade increased 2.5% every two minutes. Averages for oxygen uptake (VO2) were assessed every 30 seconds. Maximum oxygen consumption (VO2 max) was measured in milliliters per kilogram per minute (mL/kg/min), and based on a maximal effort, which was defined by accomplishing two of the following four criteria: 1) a heart rate within ten beats/min of the age predicted maximum, 2) a respiratory exchange ratio (the ratio between carbon dioxide and oxygen percentage) greater than 1.0 (Bar-Or, 1983), 3) a rating greater than eight on the children’s OMNI scale of perceived exertion, and/or 4) a plateau in VO2 despite an increase in workload. Values for VO2 max reported below are percentile rankings calculated from each participants VO2 max value originally measured in mL/kg/min. These percentile rankings are based on normative data (Shvartz and Reibold, 1990), which consider age and gender.
Eye-tracking Apparatus
Eye-movements were recorded with an Eyelink 1000 eye-tracking system (SR Research, Ontario, Canada). The eye-tracking system was calibrated on every block in between the study and test phases of the memory paradigm. To reduce head movement, participants placed their head in a desk mounted chin rest while performing the test phases of the paradigm. Stimulus presentation and behavioral data collection were accomplished through Presentation software (Neurobehavioral Systems, http://nbs.neuro-bs.com), and all stimuli were displayed on a 21″ full color monitor.
Memory paradigm
On a separate day from fitness testing, participants completed a memory task inspired by Hannula et al. (2007), which contained a relational memory condition (RM) and an item memory condition (IM) divided into eight study-test blocks (RM = 6, IM = 2). Stimuli were comprised of 108 male and 108 female color faces selected from a face database used in a previous study (Althoff and Cohen, 1999). Face images measured 480 × 480 pixels and were displayed on a black background. 110 color images of real world scenes taken from Brand X photography were also used; scenes measured 1280 × 1024 pixels. Figure 1 illustrates the design and timing of the task.
Figure 1.
The RM condition consisted of the participant encoding and subsequently recognizing face-scene pairs; there were 18 unique face-scene pairs in the study phase of an RM block. To encourage the encoding of the face and scene as a pair, participants were instructed to make a judgment via a button press as to whether the person depicted would be expected to be found in that scene. Following the study phase of the RM block, the test phase occurred and contained six trials. In the test phase a previously shown scene was paired with three faces from the study phase, one of which had been displayed with that scene during study, and participants were instructed to find the face originally paired with that scene. Thus, all faces and scenes were equally familiar, emphasizing the need for relational memory to successfully complete this condition.
In the study phase of an IM block, participants saw the same scene with a different face on each trial, and decided via a button press if the person in the scene “looked younger than 25 years of age.” Similar to an RM block, the study phase of an IM block contained 18 trials. In the test phase of an IM block, participants saw three faces, one face from the study phase and two novel faces; the instructions were to find the face shown during the study phase, hence this condition could be solved solely on the basis of familiarity. Since every face could be used as a target in the test phase of the IM condition, the test phases were comprised of 18 trials.
In both the study and test phases of each condition, a trial began with a fixation cross, followed by the scene alone, and then finally a one or three face display superimposed on the scene. In the test phase of both conditions, participants were instructed to press a button during the viewing period when they knew which of the three faces was correct. Following the viewing period, an answer screen appeared with numbers corresponding to the locations of the faces, and participants verbalized which face they thought was correct and also made a confidence judgment on this answer on a 1–3 scale. The values of the confidence scale translated into: 1 = “Not Sure”; 2 = “Kind of Sure”; 3 = “Very Sure.”
The inclusion of six RM blocks and two IM blocks yielded a total of 36 test trials in each condition. Extensive counterbalancing took place to minimize the effect of extraneous variables on performance. First, the pool of 216 faces used in the experiment was separated into two equal lists in a quasi-random fashion such that within each list there were an equal number of male and female faces, as well as faces bearing a smiling versus a neutral expression. For approximately half of the participants within a group, one list was used in the IM condition while faces from the other list appeared in the RM condition; this was reversed for the other participants, thus ensuring each face had an equal probability of appearing in either condition. Also, to counterbalance for order of conditions, the experiment utilized two orders, which were equally dispersed across the groups. The two orders of the eight study-test blocks were as follows: “RM, RM, RM, IM, RM, RM, RM, IM” or “IM, RM, RM, RM, IM, RM, RM, RM.” Also, the location of the target was equally likely in any of the three positions of the test display, and also had an identical probability of containing a neutral or smiling expression. Finally, within each block, participants only saw faces from one gender.
Data Analyses
The behavioral outcome measures were accuracy, as well as reaction time (RT) and confidence ratings to correct trials, while the main outcome measure from the eye-movement data was the proportion of viewing time allocated to a correctly identified face during the three-face viewing period. Proportion of viewing time was defined as the amount of time spent viewing the correctly chosen face divided by the amount of time spent looking at all faces on a trial. There were no significant differences on any of the reported measures in the Results section for any combination of order and list, and thus all analyses were collapsed across list and order. In an effort to remove either fixations or trials that were outliers and could adversely affect the data, fixations that were less than 83 ms were discarded, as were trials in which participants did not spend at least 33% of possible viewing time (2000 ms) to the test display (Warren et al., 2010). On average fewer than one trial per condition had to be discarded for each participant in the eye-tracking analysis due to imposing the 33% viewing time minimum (RM condition mean = 0.55, SD = 1; IM condition mean = 0.82, SD = 1.9). Finally, out of 352 blocks of eye-movement data collected in this sample of 44 participants, only six blocks were discarded (less than 2%) due to improper calibration of the eye-tracker resulting in inaccurate data.
Results
Aerobic fitness data
To establish the efficacy of the aerobic exercise intervention, we subjected the VO2max data to a two-way mixed measures ANOVA with group as a between-subjects factor and time (before and after intervention period) as a within-subjects factor. The results indicated a significant group × time interaction, F (1,42) = 5.47, P = .024, reflecting the AE group’s increased VO2max percentile rankings and the C group’s slightly decreased values. The pre and post VO2max percentiles as well as the difference score are listed in Table 1.
Behavioral data
The accuracy data were analyzed in a two-way mixed measures ANOVA with group as a between-subjects factor and memory condition (relational vs. item) as a within-subjects factor. The accuracy data contained a main effect of memory condition, with accuracy scores higher in the item condition than the relational condition F (1,42) = 22.16, P < .001. However, there was no group effect for accuracy (P = .203), nor was there a group × condition interaction (P = .613), as both groups performed with similar accuracy in the conditions (RM accuracy: AE group mean = 66.28%, SD = 13.1; C group mean = 69.29%, SD = 12.9; IM accuracy: AE group mean = 75%, SD = 11.6; C group mean = 80.12%, SD = 11.6). RT and confidence measures were also subjected to similar memory condition × group ANOVAs. No group differences emerged for RT, though there was a significant main effect of memory type such that both groups were faster in the RM condition, F(1,42) = 8.7, P = .005; all other P values > .4 (RM RT: AE group mean = 2691 ms, SD = 734 ms; C group mean = 2550 ms, SD = 633 ms; IM RT: AE group mean = 2909 ms, SD = 625 ms; C group mean = 2742 ms, SD = 606 ms). The ANOVA examining the confidence data revealed no significant effects, as all P values were > .2 (RM confidence ratings: AE group mean = 2.58, SD = .28; C group mean = 2.57, SD = .36; IM confidence ratings: AE group mean = 2.51, SD = .34; C group mean = 2.56, SD = .34).
Eye-movement data
Prior to analyzing the main variable of interest, proportion of viewing time allocated to the correctly identified face, we analyzed total duration of viewing to the test display to ensure the groups did not differ on this basic measure. Total duration of viewing was analyzed in a group × memory condition mixed measures ANOVA. This analysis revealed no significant effects, as all P values were > .25 (RM total viewing duration: AE group mean = 4715 ms, SD = 471; C group mean = 4630 ms, SD = 512 ms; IM total viewing duration: AE group mean = 4694 ms, SD = 435 ms; C group mean = 4713 ms, SD = 462 ms).
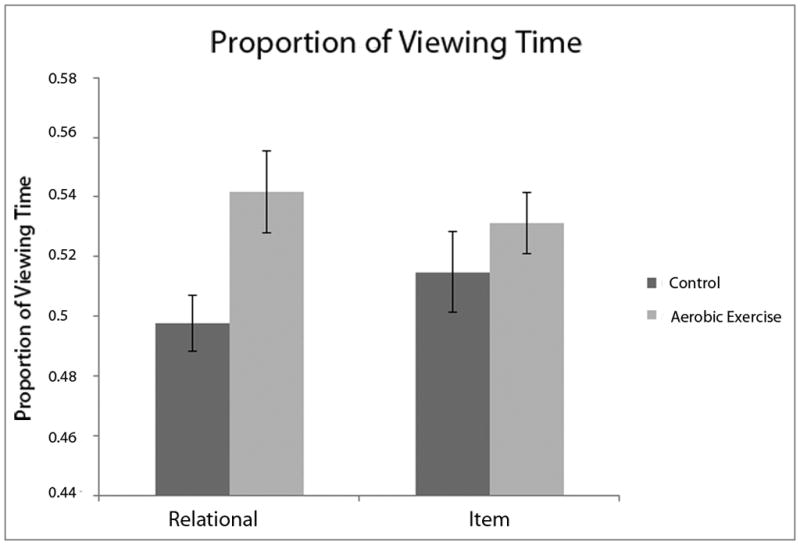
The data for the proportion of viewing time allocated to a correctly identified face were analyzed in a group × memory condition mixed measures ANOVA, revealing a near-significant effect of group F (1,42) = 3.97, P = .053, and a marginally significant group × memory condition interaction F (1,42) = 2.84, P = .099. To further understand the nature of these effects, particularly given the a priori hypothesis of group differences selective for the relational memory condition, group contrasts were performed on the proportion of viewing time variable for the relational and item conditions. These follow-up tests revealed that the AE group differed significantly in proportion of viewing time selectively for the RM condition (RM condition: t (42) = 2.47, P = .017; IM condition: t (42) = .982, P = .331) (see Figure 2).
Figure 2.
Discussion
To summarize, compared to children in the wait-list control group, children randomly assigned to an aerobic exercise intervention displayed significant differences on an eye-movement measure of memory selectively for the RM condition. The greater amount of viewing displayed by the AE group to the faces that had previously been paired with the test scenes suggests that these individuals had a stronger representation of relational memory for studied face-scene pairs. Previous work has shown that these particular eye-movement measures of relational memory are critically linked to hippocampal structure and function (Hannula et al., 2007; Hannula and Ranganath, 2009). Accordingly, the group differences observed here may index beneficial changes in hippocampal functioning in the AE group resulting from increased aerobic fitness, consistent with a wealth of animal and human literature on the relationship among hippocampus, physical activity or fitness, and relational memory. Importantly, these results are congruent with our previous work documenting differences between aerobically low-versus high-fit children on relational, but not item memory performance, correlated with differences in hippocampal volume (Chaddock et al., 2010; Chaddock et al., 2011).
The current report strengthens the causal link between aerobic exercise and hippocampal-dependent memory in preadolescent children. The previous reports on this topic studied children who were pre-experimentally different on measures of aerobic fitness, leaving open the possibility that there were other correlated factors influencing performance that could account for the observed memory effects. Here, children were randomly assigned to the AE or C group, greatly reducing the possibility that there were extraneous, uncontrolled variables contaminating the results. Thus, random assignment to an exercise intervention induced increased aerobic fitness in the AE group versus the C group, and in turn elicited an advantage for the AE group selectively for relational memory, but not item memory, permitting us to conclude that increases in aerobic fitness elicit benefits to hippocampal-dependent relational memory.
The absence of group differences on overt accuracy despite group differences on the eye-movement measure of memory is intriguing. Recent work by Hannula and Ranganath (2009) found that while hippocampal activity predicted proportion of viewing time to a correct face, it was not enough to predict if a participant would choose the correct face with an overt response. The authors found that correct relational memory decisions were correlated with increased lateral left prefrontal cortex (PFC) activity, and increased hippocampal-PFC functional connectivity. These results indicate that while proportion of viewing time seems to be linked to the hippocampus, correct memory decisions are dependent on a more distributed network, including the hippocampus and PFC.
Our previous work documenting effects of aerobic fitness on relational memory was based on comparing aerobically low- and-high-fit children, two groups on opposite ends of the aerobic fitness spectrum. In the current sample, despite significant effects of the physical activity intervention on measurements of aerobic fitness, the mean normative percentile ranking for VO2max scores for both groups fell within the “aerobically low-fit” classification and only seven participants in the total sample were categorized as “aerobically high-fit.” Thus, it is not unexpected that the two groups would perform similarly on the accuracy measure given their overall low levels of aerobic fitness.
Nonetheless, significant group differences were seen on the eye-movement measure, suggesting that the hippocampus, given its disproportionate capacity for plasticity, responds in a malleable fashion to any amount of exercise, and this can be detected when sufficiently sensitive outcome measures are employed. But, more sustained exercise leading to high aerobic fitness levels may be needed to modify the PFC and hippocampal-PFC functional connectivity, and thereby drive exercise-related modulation of behavioral manifestations of relational memory. Indeed, other recent work from our group suggests aerobic fitness is a source of variance in PFC-MTL functional connectivity, and higher-fit children perform better and have more efficient neuroelectric indices of attention on tasks putatively reliant on the PFC (Voss et al., 2010; Pontifex et al., 2011). Future studies containing a sample of children with a wider range of baseline VO2max scores will be able to further illuminate the contributions of aerobic exercise to memory function.
One limitation of the current work is the lack of pre-test data on the memory task. It is conceivable that the two groups’ eye-movement patterns may have been different prior to the intervention, and that the findings here cannot be attributed to the aerobic exercise intervention; however, we believe this is unlikely. Children were randomly assigned to the two groups and were matched on variables that affect cognition. Additionally, it seems more likely that if the groups did differ prior to the intervention, this would be apparent in both memory conditions due to a more general propensity for making longer fixation durations to a correct face, rather than a pre-existing group divergence in the specific and predicted way that is reported here. Nevertheless, it would be beneficial if future work included memory data collected prior to the intervention to allow for a true pre and post comparison.
One finding may warrant additional explanation. Given the significant result of higher overt accuracy in the IM condition, one might have expected significantly faster RTs in this condition as well; however, the opposite effect was found. Rather than reflecting speed-accuracy tradeoffs in the data, it is likely that faster RTs to correct trials in the RM condition occurred because the scene preview that appeared just prior to the three-face test display conveyed information that aided in completing the task, whereas by design, the scene preview in the IM condition afforded no information to aid participants in their response.
The aerobic exercise intervention resulted in differences on eye-movement measures of memory that were selective to hippocampal-dependent relational memory. This finding is consistent with the wealth of animal and human literature describing an up-regulation of hippocampal plasticity and enhanced memory resultant from exercise, and helps set the stage for future research directed at understanding how exercise modifies the various components of hippocampal-based memory.
Acknowledgments
Grant Sponsor: NIH; Grant Number: HD055352
We would like to thank Joel Voss and Patrick Watson for technical and data analysis assistance, Bonnie Hemrick for her help in participant recruitment, and Sebastian Wraight for his contributions to data collection. This work was supported by NIH grant HD055352 awarded to Charles H. Hillman.
References
- Althoff RR, Cohen NJ. Eye-movement-based memory effect: A reprocessing effect in face perception. JEP: LMC. 1999;25:997–1010. doi: 10.1037//0278-7393.25.4.997. [DOI] [PubMed] [Google Scholar]
- American College of Sports Medicine. ACSM’s guidelines for exercise testing and prescription. 7. New York: Lippincott, Williams, and Watkins; 2006. [Google Scholar]
- Bar-Or O. Pediatric sports medicine for the practitioner: From physiologic principles to clinical application. New York: Springer-Verlag; 1983. [Google Scholar]
- Birnbaum AS, Lytle LA, Murray DM, Story M, Perry CL, Boutelle KN. Survey development for assessing correlates of young adolescents’ eating. Am J Health Behav. 2002;26:284–295. doi: 10.5993/ajhb.26.4.5. [DOI] [PubMed] [Google Scholar]
- Castelli DM, Hillman CH, Hirsch J, Hirsch A, Drollette E. FIT Kids: Time in target heart zone and cognitive performance. Prev Med. 2011;52(Suppl 1):S55–9. doi: 10.1016/j.ypmed.2011.01.019. [DOI] [PubMed] [Google Scholar]
- Chaddock L, Erickson KI, Prakash RS, Kim JS, Voss MW, Vanpatter M, Pontifex MB, Raine LB, Konkel A, Hillman CH, Cohen NJ, Kramer AF. A neuroimaging investigation of the association between aerobic fitness, hippocampal volume, and memory performance in preadolescent children. Brain Res. 2010;1358:172–183. doi: 10.1016/j.brainres.2010.08.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaddock L, Hillman CH, Buck SM, Cohen NJ. Aerobic fitness and executive control of relational memory in preadolescent children. Med Sci Sports Exerc. 2011;43:344–349. doi: 10.1249/MSS.0b013e3181e9af48. [DOI] [PubMed] [Google Scholar]
- Cohen NJ, Eichenbaum H. Memory, Amnesia and the Hippocampal System. Cambridge: MIT Press; 1993. [Google Scholar]
- Davachi L. Item, context and relational episodic encoding in humans. Curr Opin Neurobiol. 2006;16:693–700. doi: 10.1016/j.conb.2006.10.012. [DOI] [PubMed] [Google Scholar]
- Eichenbaum H, Cohen NJ. From Conditioning to Conscious Recollection: Multiple Memory Systems in the Brain. New York: Oxford University Press; 2001. [Google Scholar]
- Erickson KI, Voss MW, Prakash RS, Basak C, Szabo A, Chaddock L, Kim JS, Heo S, Alves H, White SM, Wojcicki TR, Mailey E, Vieira VJ, Martin SA, Pence BD, Woods JA, McAuley E, Kramer AF. Exercise training increases size of hippocampus and improves memory. Proc Natl Acad Sci U S A. 2011;108:3017–3022. doi: 10.1073/pnas.1015950108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannula DE, Ryan JD, Tranel D, Cohen NJ. Rapid onset relational memory effects are evident in eye movement behavior, but not in hippocampal amnesia. J Cogn Neurosci. 2007;19:1690–1705. doi: 10.1162/jocn.2007.19.10.1690. [DOI] [PubMed] [Google Scholar]
- Hannula DE, Ranganath C. The eyes have it: hippocampal activity predicts expression of memory in eye movements. Neuron. 2009;63:592–599. doi: 10.1016/j.neuron.2009.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannula DE, Althoff RR, Warren DE, Riggs L, Cohen NJ, Ryan JD. Worth a glance: using eye movements to investigate the cognitive neuroscience of memory. Front Hum Neurosci. 2010;4:166. doi: 10.3389/fnhum.2010.00166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamijo K, Pontifex MB, O’Leary KC, Scudder MR, Wu C, Castelli DM, Hillman CH. The effects of an afterschool physical activity intervention on working memory in preadolescent children. Dev Sci. 2011;14:1046–1058. doi: 10.1111/j.1467-7687.2011.01054.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan MS, Hinds JW. Neurogenesis in the adult rat: electron microscopic analysis of light radioautographs. Science. 1977;197:1092–1094. doi: 10.1126/science.887941. [DOI] [PubMed] [Google Scholar]
- Kempermann G, Kuhn HG, Gage FH. More hippocampal neurons in adult mice living in an enriched environment. Nature. 1997;386:493–495. doi: 10.1038/386493a0. [DOI] [PubMed] [Google Scholar]
- Neve RL, Finch EA, Bird ED, Benowitz LI. Growth-associated protein GAP-43 is expressed selectively in associative regions of the adult human brain. Proc Natl Acad Sci U S A. 1988;85:3638–3642. doi: 10.1073/pnas.85.10.3638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira AC, Huddleston DE, Brickman AM, Sosunov AA, Hen R, McKhann GM, Sloan R, Gage FH, Brown TR, Small SA. An in vivo correlate of exercise-induced neurogenesis in the adult dentate gyrus. Proc Natl Acad Sci U S A. 2007;104:5638–5643. doi: 10.1073/pnas.0611721104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pontifex MB, Raine LB, Johnson CR, Chaddock L, Voss MW, Cohen NJ, Kramer AF, Hillman CH. Cardiorespiratory fitness and the flexible modulation of cognitive control in preadolescent children. J Cogn Neurosci. 2011;23:1332–1345. doi: 10.1162/jocn.2010.21528. [DOI] [PubMed] [Google Scholar]
- Ryan JD, Althoff RR, Whitlow S, Cohen NJ. Amnesia is a deficit in relational memory. Psychol Sci. 2000;11:454–461. doi: 10.1111/1467-9280.00288. [DOI] [PubMed] [Google Scholar]
- Shvartz E, Reibold RC. Aerobic fitness norms for males and females aged 6 to 75 years: a review. Aviat Space Environ Med. 1990;61:3–11. [PubMed] [Google Scholar]
- Taylor SJ, Whincup PH, Hindmarsh PC, Lampe F, Odoki K, Cook DG. Performance of a new pubertal self-assessment questionnaire: A preliminary study. Pediatric and Perinatal Epidem. 2001;15:88–94. doi: 10.1046/j.1365-3016.2001.00317.x. [DOI] [PubMed] [Google Scholar]
- van Praag H, Christie BR, Sejnowski TJ, Gage FH. Running enhances neurogenesis, learning, and long-term potentiation in mice. Proc Natl Acad Sci U S A. 1999a;96:13427–13431. doi: 10.1073/pnas.96.23.13427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Praag H, Kempermann G, Gage FH. Running increases cell proliferation and neurogenesis in the adult mouse dentate gyrus. Nat Neurosci. 1999b;2:266–270. doi: 10.1038/6368. [DOI] [PubMed] [Google Scholar]
- van Praag H, Schinder AF, Christie BR, Toni N, Palmer TD, Gage FH. Functional neurogenesis in the adult hippocampus. Nature. 2002;415:1030–1034. doi: 10.1038/4151030a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voss MW, Erickson KI, Prakash RS, Chaddock L, Malkowski E, Alves H, Kim JS, Morris KS, White SM, Wojcicki TR, Hu L, Szabo A, Klamm E, McAuley E, Kramer AF. Functional connectivity: a source of variance in the association between cardiorespiratory fitness and cognition? Neuropsychologia. 2010;48:1394–1406. doi: 10.1016/j.neuropsychologia.2010.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Utter AC, Robertson RJ, Nieman DC, Kang J. Children’s OMNI scale of perceived exertion: Walking/running evaluation. Med Sci Sports Exerc. 2002;34:139–144. doi: 10.1097/00005768-200201000-00021. [DOI] [PubMed] [Google Scholar]
- Warren DE, Duff MC, Tranel D, Cohen NJ. Medial temporal lobe damage impairs representation of simple stimuli. Front Hum Neurosci. 2010;4:35. doi: 10.3389/fnhum.2010.00035. [DOI] [PMC free article] [PubMed] [Google Scholar]



