Abstract
Objective
For patients with rheumatoid arthritis (RA) and comorbid cardiovascular disease (CVD), diabetes, or hyperlipidemia, annual lipid testing is recommended to reduce morbidity and mortality from comorbidities. Given trends encouraging complex patients to receive care in “medical homes,” we examined associations between regularly seeing a primary care provider (PCP) and lipid testing in RA patients with cardiovascular-related comorbidities.
Methods
We performed a retrospective cohort study examining a 5% random US Medicare sample (2004–2006) of beneficiaries over 65 years old with RA and concomitant CVD, diabetes, or hyperlipidemia (N=16,893). We examined the relationship between receiving lipid testing in 2006, and having at least one PCP visit per year in 2004, 2005, and 2006 using multivariate regression.
Results
90% of patients had prevalent CVD, 46% had diabetes, and 64% had hyperlipidemia; however, annual lipid testing was only performed in 63% of these RA patients. Thirty percent of patients saw a PCP less than once per year, despite frequent visits (mean >9) with other providers. Patients without at least one annual PCP visit were 16% less likely to have lipid testing. Increased age, complexity scores, hospitalization, and large town residence predicted decreased lipid testing.
Conclusions
Despite comorbid CVD, diabetes, or hyperlipidemia, 30% of Medicare RA patients saw a PCP less than once per year, and one in three lacked annual lipid testing. Findings support advocating primary care visits at least once per year. Remaining gaps in lipid testing suggest the need for additional strategies to improve lipid testing in at-risk RA patients.
INTRODUCTION
Older adults with rheumatoid (RA) frequently have comorbid cardiovascular disease (CVD), diabetes mellitus (hereafter diabetes), and/or hyperlipidemia as indications for annual lipid testing. For these comorbid conditions, the importance of lipid management for reducing morbidity and mortality is well-recognized. Furthermore, RA itself compounds CVD risk, making aggressive management of traditional CVD risk factors vital for this population (1). Results from previous studies examining the influence of comorbidity would predict poor performance on such routine testing tasks for patients with RA (2). Little is known regarding the optimal roles of primary and specialty care and the actual performance for monitoring lipids among patients with both RA and recognized CVD, diabetes, or hyperlipidemia.
Quality care and health maintenance for RA patients generally requires input from both rheumatologists and primary care providers (3–5), leading to challenges in care coordination and determining scope of practice. In many modern health care systems, patients with complex comorbidities are increasingly encouraged to receive care in “patient centered primary care medical homes” (6). Not all RA patients see a primary care provider (PCP), but primary care is highly recommended (4), particularly for those with comorbid CVD, diabetes, or hyperlipidemia. Traditionally, prevention has been considered a primary care role and therefore, within the “scope” of primary care practice (7). In the case of lipid testing, the amplification of CVD risk by RA may raise concern among the rheumatologists who frequently encounter these patients. The European League Against Rheumatism (EULAR) has even gone so far as to advocate annual CVD-risk assessment for all RA patients (8). However, prevention of CVD, including lipid testing, has not traditionally been assumed to fall within the rheumatologist’s scope of practice (9). In contrast, national US quality of care measures for PCPs routinely report annual low density lipoprotein (LDL) testing and lipid performance for patients with CVD or diabetes, relying on the assumption that regular primary care visits are occurring for such care (10, 11).
Given the likely importance of primary care for managing patients with RA and CVD risk, we sought to examine the influence of primary care visits on the occurrence of annual lipid testing. We specifically examined associations between seeing a PCP at least once each year in 2004, 2005, and 2006 as a predictor for receipt of lipid testing in 2006 among RA patients with diabetes, hyperlipidemia, or prevalent CVD.
METHODS
Setting and Participants
In this retrospective cohort study, beneficiaries age 65 and older continuously enrolled and alive in 2004–2006 were identified from a 5% random US Medicare sample obtained from the Medicare Chronic Condition Warehouse dataset (12). Patients were determined to have RA if they had two or more International Classification of Diseases, Ninth Edition (ICD-9) codes for RA (714.0–714.33) on inpatient or outpatient claims at least two months apart during a 24-month period (2004–2005) based upon a previously validated algorithm (4, 13). Enrollment and claims data (2004–2006) were extracted for patients meeting the RA definition. The Medicare denominator file was used to exclude beneficiaries without continuous Medicare Part A or B coverage, or with supplemental health maintenance organization (HMO) or railroad benefits. We also excluded patients without any outpatient encounters 2004–2006 or who died prior to 12/31/06. The Institutional Review Board at the University of Wisconsin approved this study with a waiver of consent.
Patients were included if they were eligible for annual lipid testing due to pre-existing CVD or diabetes as indicated by a flag for those conditions before 1/1/2004 in the Medicare Chronic Condition Warehouse dataset, or if they had baseline hyperlipidemia. The Chronic Condition Warehouse contains flags created using validated algorithms applied bi-annually since 1999 to define 21 chronic diseases (12, 14–18). Flags from the Chronic Condition Warehouse dataset denoted pre-existing CVD (myocardial infarction, stroke, heart failure, or ischemic heart disease) (14–17) or diabetes, a CVD risk equivalent (12, 18). Baseline hyperlipidemia was identified by presence of more than one ICD-9 code (272.0–272.4) in 24 months 2004–2005 (16, 19). Among more than 25,000 potentially eligible RA patients, 67% met inclusion for baseline CVD, diabetes, or hyperlipidemia. Ultimately, this group of 16,893 RA patients from this data source represented a random 5% sample of over 339,000 Medicare RA patients nationwide.(Figure 1)
Figure 1.
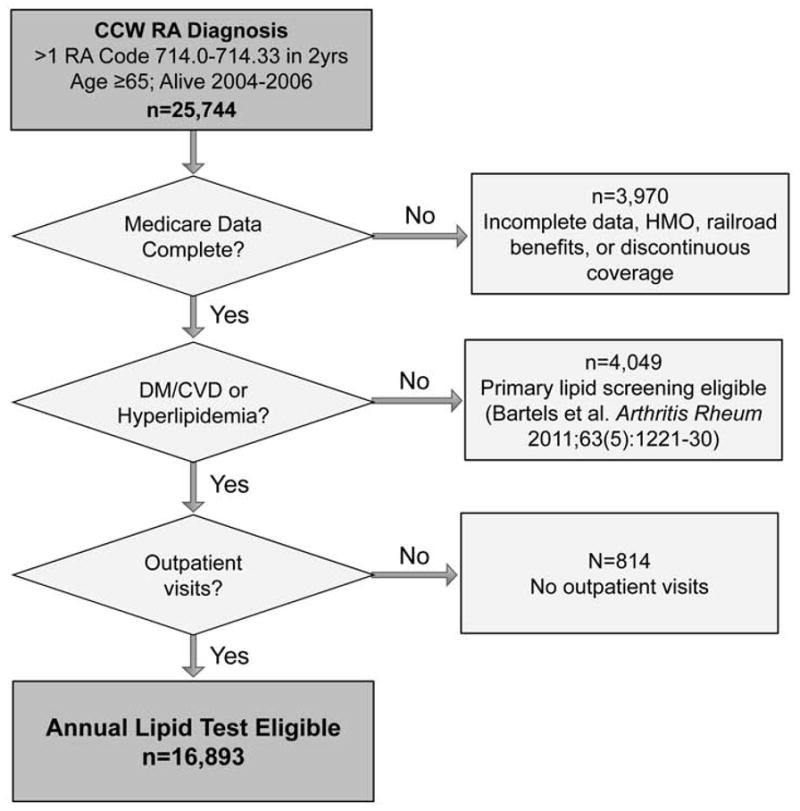
Consort diagram outlining cohort eligibility beginning with n=25, 744 patients meeting criteria for RA in 5% national Medicare sample, and n=16,893 eligible for annual lipid testing due to comorbid cardiovascular disease, diabetes, or hyperlipidemia.
Variables
All variables were obtained from Medicare data. The main dependent variable was receiving lipid testing (i.e., LDL cholesterol testing) during 2006. Lipid testing was identified by current procedural terminology (CPT) codes indicating lipid panel testing (80061), LDL cholesterol (83721), electrophoretic lipoprotein (83715), high resolution lipoprotein (e.g., NMR) (83716), electrophoretic or high resolution lipoproteins (83700, 83701, or 83704), or calculated LDL components (82465, 83718, and 84478) (20). The patient was considered to have been lipid tested if any of these CPT codes was present at least once in 2006.
The main explanatory variable was meeting a minimum number of primary care visits. Visits were identified through carrier claims including encounter dates and provider specialty codes. Primary care providers were defined as family medicine or internal medicine physicians, nurse practitioners, or physician assistants (21, 22). We created two variables to test the impact of minimum primary care visits. The first required at least one PCP visit in each calendar year 2004–2006. The second limit that we tested required at least two visits per year. Our conclusions did not differ using these two thresholds; only the results distinguishing those with and without at least one annual PCP visit are presented.
Individual sociodemographic and clinical characteristics were included as other potential explanatory variables. These included baseline (2004) age, sex, race, designation of ever receiving Medicaid, and zip code residence grouping using US Department of Agriculture census-based Rural Urban Commuting Area (RUCA) codes (urban, suburban, large town, or small town based upon population census and commuting flows) (23). Acknowledging the limits of administrative data or comorbidity measures to capture RA disease severity or physical limitations, we have used history of an orthopedic surgery or gait device claims as respective surrogates (24, 25) (code lists available upon request). Additionally, patient risk/complexity and risk adjustment was addressed using the Centers for Medicare and Medicaid Services - Hierarchical Condition Categories (CMS-HCC) community risk score (26). The CMS-HCC score is the risk adjustment system used by CMS that samples inpatient and outpatient condition codes and demographics over the prior year (2004 here) to predict cost (average score=1.0, with higher scores indicating greater cost risk/complexity) (26). It has recently demonstrated superior prediction of mortality over the Charlson (27, 28) or Elixhauser (29) co-morbidity measures (30). CMS-HCC’s inclusion of a full spectrum of diagnoses, inclusion of inpatient and outpatient factors, and evidence of strong ability to predict mortality led to our selection of this method to control for patient complexity. It has performed well in many older adult disease populations, although it has not been extensively studied in RA. Measures of utilization included mean annual number of outpatient visits and total number of unique providers 2004–2006, as well as ever being hospitalized in 2004–2006. We used billing dates within the carrier file to determine mean number of visits. Provider specialty codes were used to count unique provider totals and distinguish primary care, rheumatology, and non-rheumatology specialist visits.
Statistical Analysis
Logistic regression with robust estimates of the variance was used to analyze the relationship between explanatory variables and receiving at least one annual lipid test. Adjusted and unadjusted probabilities of screening were estimated by whether patients met minimum primary care visit thresholds. Age; gender; race; Medicaid status; prior hospitalization status; prior orthopedic surgery; prior gait-assistance device; baseline presence of CVD, diabetes, and hyperlipidemia; CMS-HCC score; rural/urban residence; total number of unique providers; and average annual visits were initially included within logistic models based upon theoretical importance.
Analyses were conducted using SAS version 9.1.3 (SAS Institute, Inc., Cary, NC) and Stata version 10.0 (StataCorp, College Station, TX). Results of logistic regression were reported as adjusted predicted probabilities, adjusted risk ratios (ARR), and 95% confidence intervals (95% CI) (31, 32). Adjusted predicted probabilities were estimated based on the recycled predictions approach using the Stata margins command. This approach predicts the outcome (lipid testing) assuming that everyone in the dataset was treated as if they met a certain visit threshold profile. Confidence intervals were calculated using the delta method and allowed correlation among observations (analogous to the robust option) to estimate the logistic regression (32).
RESULTS
Descriptive Characteristics
Among over 25,000 Medicare RA patients, 16,893 (67%) had CVD, diabetes, or hyperlipidemia, and thus were included in our sample of those eligible for annual lipid testing. Seventy-five percent of the sample was female, 84% were white/Caucasian, and the mean age was 74.6 years (Table 1). Of those with RA and at least one qualifying co-morbid condition, 90% had baseline CVD, 46% had diabetes, and 64% had hyperlipidemia. During the observed period, 64% were hospitalized at least once. The 2004 baseline CMS-HCC risk/complexity score was 1.24, suggesting that these annual lipid testing eligible RA patients were predicted to have higher than average Medicare expenditures.
Table 1.
Characteristics of RA annual lipid test-eligible by PCP visit threshold (N=16,893)
| Characteristic | All Patients (n=16,893) | <1 PCP Visit/yr (n=5,098) | ≥1 PCP Visit/yr (n=11,795) |
|---|---|---|---|
| Age, % (n) | |||
| 65–74 years | 48.4 (8,184) | 43.4 (2,212) | 50.6 (5,972) |
| 75–84 years | 42.0 (7,104) | 43.9 (2,237) | 41.26 (4,867) |
| 85+ years | 9.5 (1,605) | 12.73 (649) | 8.11 (956) |
| Female, % (n) | 75.29 (12,719) | 78.56 (4,005) | 73.88 (8,714) |
| Race/ethnicity, % (n) | |||
| White | 83.54 (14,113) | 83.93 (4,279) | 83.37 (9,834) |
| Black | 9.32 (1,574) | 10.34 (527) | 8.88 (1,047) |
| Other | 7.14 (1,206) | 5.73 (292) | 7.75 (914) |
| Medicaid, % ever (n) | 20.27 (3,425) | 22.30 (1,137) | 19.40 (2,288) |
| Baseline CVD, % (n) | 89.75 (15,161) | 89.29 (4,552) | 89.94 (10,609) |
| Baseline diabetes, % (n) | 45.80 (7,737) | 42.88 (2,186) | 47.06 (5,551) |
| Baseline Hyperlipidemia, % (n) | 64.42 (10,883) | 53.90 (2,748) | 68.97 (8,135) |
| HCC score, mean (SD) | 1.24 (0.83) | 1.16 (.91) | 1.28 (.80) |
| Hospitalization, % ever (n) | 63.68 (10,757) | 65.42 (3,335) | 62.92 (7,422) |
| Orthopedic surgery, % (n) | 26.08 (4,406) | 21.93 (1,118) | 27.88 (3,288) |
| Gait device, % ever (n) | 31.99 (5,404) | 32.33 (1,648) | 31.84 (3,756) |
RA=Rheumatoid arthritis; PCP=Primary care provider; CVD = Cardiovascular disease; HCC=Hierarchical Condition Categories scale; SD=Standard deviation
Thirty percent of sample patients did not meet the minimum visit threshold of at least one PCP visit per year in years 2004, 2005, and 2006 (N=5,098). Patients who did not meet the single annual PCP minimum visit threshold were older, female, had fewer comorbidities, lower CMS-HCC scores, were more likely to use a gait-assistance device, and were more often from small towns (Table 2).
Table 2.
Urbanization/utilization of RA annual lipid test-eligible by PCP visit threshold (N=16,893)
| Characteristic | All Patients (n=16,893) Mean (SD) |
<1 PCP Visit/yr (n=5,098) Mean (SD) |
≥1 PCP Visit/yr (n=11,795) Mean (SD) |
|---|---|---|---|
| RUCA category % (n) | |||
| Urban | 68.09 (11,296) | 65.96 (3,292) | 69.01 (8,004) |
| Suburban | 8.43 (1,399) | 7.43 (371) | 8.86 (1,028) |
| Large town | 11.67 (1,936) | 11.58 (578) | 11.71 (1,358) |
| Small town | 11.81 (1,959) | 15.03 (750) | 10.42 (1,209) |
| Total unique providers | 8.4 (5.2) | 6.8 (4.1) | 9.1 (5.5) |
| Unique PCPs | 2.8 (2.9) | 1.8 (1.7) | 3.2 (3.2) |
| Unique rheumatologists | 0.7 (0.7) | 0.8 (0.7) | 0.7 (0.8) |
| Unique other specialty | 4.9 (3.6) | 4.3 (3.3) | 5.2 (3.7) |
| Total outpatient visits | 13.5 (8.6) | 9.7 (7.2) | 15.1 (8.7) |
| PCP visits | 6.1 (5.3) | 2.6 (2.9) | 7.6 (5.4) |
| Rheumatology visits | 2.1 (3.1) | 2.2 (3.2) | 2.1 (3.0) |
| Other specialty visits | 5.2 (5.3) | 4.9 (5.4) | 5.4 (5.2) |
RA=Rheumatoid arthritis; PCP=Primary care provider; SD=Standard deviation; RUCA=Rural Urban Commuting Area
Visit and Provider Patterns
Overall, patients saw an average of eight unique providers over three years in an average of 14 annual visits, including a mean of six PCP visits annually (Table 2). Even those without one primary care visit per year averaged more than nine annual visits with seven unique providers. Their average number of primary care visits per year in 2004–6 was just under three (2.6) despite not meeting a minimum of at least one visit in each calendar year. This potentially reflects clustered episodes of care rather than consistent annual visits. Total visits to “other non-rheumatology specialists” outnumbered rheumatology visits, at 5±5 SD versus 2±3 SD overall. Other specialty visits outnumbered rheumatology visits for both those with and without at least one annual PCP visit.
Lipid Testing Performance
Lipid testing was performed in 63% of sample patients with RA and comorbid CVD, diabetes, or hyperlipidemia in whom annual lipid testing was indicated (Table 3). After adjusting for age, complexity/risk score, sociodemographics, and RA severity, the predicted probability of lipid testing for those with at least one PCP visit per year was 65% compared to 57% for those without at least one PCP visit per year (ARR=1.16, 95% CI=[1.12, 1.19]). Tested patients were more likely to have comorbid diabetes or hyperlipidemia. Coding for hyperlipidemia in particular predicted much higher lipid testing (ARR=1.73, 95% CI=[1.67–1.78]) as expected. Patients were less likely to be tested if they were older, ever hospitalized, had higher CMS-HCC risk/complexity scores, or lived in a large town (Tables 3 & 4).
Table 3.
Multivariate adjusted probability predicting lipid testing by PCP visit threshold (n=16,893)
| Characteristic | Raw Probability (%) | Adjusted* Predicted Probability (%) | 95% CI | Adjusted Risk Ratio | 95% CI |
|---|---|---|---|---|---|
| All Patients | 62.6 | 62.7 | |||
| <1 PCP visit each year | 50.2 | 56.6 | (55.2, 58.0) | 1.00 | (Reference) |
| ≥1 PCP visit each year | 68.02 | 65.4 | (64.5, 66.3) | 1.16 | (1.12, 1.19) |
| Age: 65–74 years | 69.1 | 65.5 | (64.5, 66.6) | 1.00 | (Reference) |
| 75–84 years | 59.8 | 61.5 | (60.4, 62.5) | 0.94 | (.92, .96) |
| 85+ years | 42.2 | 54.3 | (52.0, 56.6) | 0.83 | (.79, .87) |
| Female | 61.7 | 62.5 | (61.7, 63.4) | 0.99 | (.97, 1.01) |
| Race/ethnicity: White | 62.1 | 62.7 | (61.9, 63.5) | 1.00 | (Reference) |
| Black | 61.2 | 61.4 | (59.1, 63.8) | 0.98 | (.94, 1.02) |
| Other | 70.3 | 64.3 | (61.5, 67.2) | 1.03 | (.98, 1.07) |
| Medicaid (ever) | 61.5 | 62.5 | (60.8, 64.1) | 1.00 | (.97, 1.03) |
| Hospitalization (ever) | 58.1 | 59.5 | (58.6, 60.4) | 0.87 | (.85, .89) |
| Orthopedic surgery (ever) | 64.7 | 63.8 | (62.4, 65.2) | 1.02 | (1.00, 1.05) |
| Gait-assistance device (ever) | 57.3 | 60.4 | (59.1, 61.6) | 0.95 | (.92, .97) |
| Baseline CVD | 62 | 62.7 | (61.9, 63.5) | 1.01 | (.96, 1.05) |
| Baseline diabetes | 68.9 | 66.6 | (65.5, 67.7) | 1.12 | (1.09, 1.14) |
| Baseline Hyperlipidemia | 76.1 | 74.1 | (73.3, 75.0) | 1.73 | (1.67, 1.78) |
| HCC: Lowest quartile | 67.3 | 67.9 | (66.5, 69.3) | 1.00 | (Reference) |
| Second quartile | 63.5 | 62.3 | (61.0, 63.7) | 0.92 | (.89, .95) |
| Third quartile | 63.5 | 62.5 | (61.1, 63.9) | 0.92 | (.89, .95) |
| Highest quartile | 56.2 | 58.2 | (56.7, 59.6) | 0.86 | (.83, .89) |
PCP=Primary Care Provider; CVD=Cardiovascular Disease; HCC=Hierarchical Condition Categories scale
Logistic model included: Rural/urban residence, and average numbers of providers and annual visits, in addition to all items listed above.
Table 4.
Urbanization/utilization covariates for predicting lipid testing by PCP visit threshold (n=16,893)
| Characteristic | Raw Probability (%) | Adjusted* Predicted Probability (%) | 95% CI | Adjusted Risk Ratio | 95% CI |
|---|---|---|---|---|---|
| RUCA category | |||||
| Urban | 65.2 | 63.6 | (62.7, 64.5) | 1.00 | (Reference) |
| Suburban | 61.8 | 62.2 | (59.9, 64.5) | 0.98 | (.94, 1.02) |
| Large town | 55.9 | 59.3 | (57.3, 61.3) | 0.93 | (.90, .97) |
| Small town | 55.6 | 61.3 | (59.3, 63.2) | 0.96 | (.93, 1.00) |
| Annual total visit quartiles | |||||
| Lowest quartile | 48 | 55.7 | (54.0, 57.4) | 0.83 | (.79, .86) |
| Second quartile | 63.5 | 62.6 | (61.2, 64.0) | 0.93 | (.90, .96) |
| Third quartile | 68.2 | 65.9 | (64.4, 67.4) | 0.98 | (.95, 1.01) |
| Highest quartile | 71.9 | 67.4 | (65.8, 69.0) | 1.00 | (Reference) |
| Total provider quartiles | |||||
| Lowest quartile | 52.5 | 62.0 | (60.3, 63.7) | 1.00 | (Reference) |
| Second quartile | 60.7 | 62.8 | (61.5, 64.1) | 1.01 | (.98, 1.04) |
| Third quartile | 65.5 | 62.1 | (60.6, 63.4) | 1.00 | (.96, 1.04) |
| Highest quartile | 70.6 | 63.7 | (62.1, 65.3) | 1.03 | (.99, 1.07) |
RUCA=Rural Urban Commuting Area
Logistic model included: Age, gender, race, Medicaid status, prior hospitalization status prior orthopedic surgery, prior gait-assistance device, HCC score, baseline presence diabetes, hyperlipidemia, CVD rural/urban residence, and average numbers of providers andannual visits.
DISCUSSION
In our study, two-thirds of Medicare RA patients had indications for annual lipid testing including 90% with prevalent CVD, 46% with diabetes, and 64% with hyperlipidemia. More than one-third of eligible patients lacked appropriate lipid testing despite the presence of both traditional CVD risk factors and RA compounding CVD risk. Moreover, all of these patients should be seen in primary care at least once per year to assess comorbidities, yet 30% were not. Patients with RA who did see their PCP at least once per year were 16% more likely to receive lipid testing than those without regular PCP contact. Still poor overall annual lipid screening (63%) was observed, rising only to 65% among those with regular primary care.
In our study, observed lipid testing rates were low compared to previously studied HMO populations or Medicare patients with diabetes or CVD, without RA. Publically reported quality of care measures, including the Healthcare Effectiveness Data and Information Set (HEDIS) and Physician Quality Reporting Initiative (PQRI), report annual testing rates, a process measure, and lipid management performance, outcomes measures, as a standard of care for patients with diabetes and coronary artery disease (10, 11). Though HEDIS measures generally apply to younger HMO/insurance populations, annual lipid testing rates reach 85% in coronary artery disease and 77% for diabetes (33), clearly exceeding our observed rates of 63%. Reported lipid testing rates in the Medicare population in 2003 were 68–78% for coronary artery disease and diabetes, and even among “general” Medicare patients’ lipid screening approached 55% each year (34–36). Our observed rate only marginally exceeds general testing rates despite the high-risk profile of our sample with RA plus CVD, diabetes, and/or hyperlipidemia. While we have compared our observed lipid testing rates to published reports, contemporaneous comparison populations will be examined in future work. We also acknowledge that actual lipid treatment rates and the impact of treatments on prospective CVD events would offer more direct outcome measures of care quality, and these should be examined in the future.
The idea of RA itself as an indication for lipid testing is gathering momentum. Beyond EULAR (8), both the American Heart Association’s Guidelines for Prevention of CVD in Women (37) and the Canadian Cardiovascular Society’s Guidelines for the Diagnosis and Treatment of Dyslipidemia (38) list RA as a major risk factor indicating lipid testing. The optimal intervals for such testing, therapeutic goals, and optimal provider roles for ordering or managing lipid results for RA patients remain unanswered.
The finding that 30% of these high-risk RA patients did not see a PCP at least annually, despite an average of nine outpatient encounters each year, suggests that advocating primary care visits may be a simple intervention to improve lipid testing. Among those meeting the threshold of at least one annual PCP visit, lipid testing performance improved by 16%. This allows us to conclude that if rheumatologists were to prescriptively advise patients with RA to see their PCP at least once per year, LDL testing rates might increase. Still, one in three RA patients lacked testing even when regularly seeing their primary care provider, suggesting that advocating yearly primary care visits alone may be inadequate.
The remaining gap in lipid testing rates calls attention to the need to improve delivery of CVD preventive care for patients with RA. In this sample with amplified risk from RA and traditional risk factors or prevalent CVD, care fell significantly short in nearly 40% of patients. Factors involved in this lack of appropriate testing may include issues related to patient preferences, provider factors, communication, and health systems. Many advocate for improved partnerships between rheumatologists and PCPs to deliver appropriate CVD risk management (9). In new partnerships, roles might shift to include rheumatologists annually assessing CVD risk factors per EULAR recommendations, rheumatologists actively co-managing CVD risks including lipid ordering, or use of novel non-physician systems for CVD preventive care that could be activated by either a rheumatologist or primary care provider. Future research and quality improvement efforts should address these issues and options.
Strengths of this study include a large, nationally representative sample of Medicare patients with RA, and extensive demographic, comorbidity, and utilization data; however, a few limitations should be noted when interpreting results. First, there is the potential for misclassification of RA and other diagnoses using administrative data. To address this concern, previously validated algorithms of RA and key conditions were used (4, 13). Though the strictest validation study used rheumatologist-reported RA coding demonstrating high correlation with audited American College of Rheumatology criteria, we adopted the convention of subsequent authors citing more than one RA code in 24 months (3–5) to ensure inclusion of RA patients exclusively receiving primary care. Similarly, hyperlipidemia was defined based upon presence of more than one ICD-9 code (19). Sample definitions might have been improved with inclusion of pharmacy information, but that was not available. Moreover, results in patients on Medicare with RA may not be generalizable to non-Medicare populations. Performance since 2006 may also have temporally improved with increasing awareness of RA as a CVD risk factor. Nevertheless, in this large representative sample, we see that among RA Medicare patients with key cardiovascular-related comorbidities, annual lipid testing was suboptimal.
CONCLUSION
Despite high rates of prevalent CVD, diabetes, and/or hyperlipidemia as indications for annual lipid testing in Medicare RA patients, we found that one in three lacked testing, and more than one in four did not see a primary care provider at least once per year. This suggests that efforts to enhance delivery of primary care services to patients with RA and established CVD and/or hyperlipidemia are needed. We hypothesized that meeting a minimum primary care threshold would improve lipid testing, and some found evidence to support advocating at least one annual PCP visit. Nevertheless, the impact of having at least one PCP visit each year is likely insufficient to achieve a substantial increase in overall lipid testing performance. Future work should investigate strategies to improve delivery of appropriate lipid testing and other CVD preventive care for patients with RA, including optimal use of lipid therapies.
Acknowledgments
ROLE OF THE FUNDING SOURCE: This project was supported by a training grant and partnership with the Health Innovation Program and the Community-Academic Partnerships core of the University of Wisconsin Institute for Clinical and Translational Research (UW-ICTR), grant 1UL1RR025011 from the Clinical and Translational Science Award (CTSA) program of the National Center for Research Resources, National Institutes of Health. Additional support was provided by the UW School of Medicine and Public Health from the Wisconsin Partnership Program. The Health Innovation Program assisted with data management and manuscript preparation. No other funding source had a role in the study design; collection, analysis, and interpretation of the data; writing of the manuscript; or the decision to submit the manuscript for publication.
The authors would like to thank Robert Purvis for meticulous data preparation, and Lauren Fahey and Colleen Brown for manuscript preparation.
ABBREVIATIONS USED
- ARR
Adjusted Risk Ratio
- CMS-HCC
Centers for Medicare and Medicaid Services - Hierarchical Condition Categories
- CPT
Current Procedural Terminology
- CVD
Cardiovascular Disease
- EULAR
European League Against Rheumatism
- HEDIS
Healthcare Effectiveness Data and Information Set
- HMO
Health Maintenance Organization
- ICD-9
International Classification of Diseases, 9th Edition
- LDL
Low Density Lipoprotein
- PCP
Primary Care Provider
- RA
Rheumatoid Arthritis
- RUCA
Rural Urban Commuting Area
- SD
Standard Deviation
Footnotes
CONFLICTS OF INTEREST/DISCLOSURES: None.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Gabriel SE. Cardiovascular morbidity and mortality in rheumatoid arthritis. Am J Med. 2008;121(10 Suppl 1):S9–14. doi: 10.1016/j.amjmed.2008.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Turner BJ, Hollenbeak CS, Weiner M, Ten Have T, Tang SS. Effect of unrelated comorbid conditions on hypertension management. Ann Intern Med. 2008;148(8):578–586. doi: 10.7326/0003-4819-148-8-200804150-00002. [DOI] [PubMed] [Google Scholar]
- 3.Schmajuk G, Schneeweiss S, Katz JN, Weinblatt ME, Setoguchi S, Avorn J, et al. Treatment of older adult patients diagnosed with rheumatoid arthritis: improved but not optimal. Arthritis Rheum. 2007;57(6):928–934. doi: 10.1002/art.22890. [DOI] [PubMed] [Google Scholar]
- 4.MacLean CH, Louie R, Leake B, McCaffrey DF, Paulus HE, Brook RH, et al. Quality of care for patients with rheumatoid arthritis. JAMA. 2000;284(8):984–992. doi: 10.1001/jama.284.8.984. [DOI] [PubMed] [Google Scholar]
- 5.Lacaille D, Anis AH, Guh DP, Esdaile JM. Gaps in care for rheumatoid arthritis: a population study. Arthritis Rheum. 2005;53(2):241–248. doi: 10.1002/art.21077. [DOI] [PubMed] [Google Scholar]
- 6.Rosenthal TC. The medical home: growing evidence to support a new approach to primary care. J Am Board Fam Med. 2008;21(5):427–440. doi: 10.3122/jabfm.2008.05.070287. [DOI] [PubMed] [Google Scholar]
- 7.Rosenblatt RA, Hart LG, Baldwin LM, Chan L, Schneeweiss R. The generalist role of specialty physicians: is there a hidden system of primary care? JAMA. 1998;279(17):1364–1370. doi: 10.1001/jama.279.17.1364. [DOI] [PubMed] [Google Scholar]
- 8.Peters MJ, Symmons DP, McCarey D, Dijkmans BA, Nicola P, Kvien TK, et al. EULAR evidence-based recommendations for cardiovascular risk management in patients with rheumatoid arthritis and other forms of inflammatory arthritis. Ann Rheum Dis. 2010;69(2):325–331. doi: 10.1136/ard.2009.113696. [DOI] [PubMed] [Google Scholar]
- 9.Burgos PI, Alarcon GS. Preventive health services for systemic lupus erythematosus patients: whose job is it? Arthritis Res Ther. 2010;12(3):124. doi: 10.1186/ar3040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.National Committee for Quality Assurance. HEDIS® 2006 Summary Table of Measures and Product Lines. Washington, DC: National Committee for Quality Assurance; 2006. [updated 2006 cited 2011 10 February]; Available from: http://www.ncqa.org/Portals/0/HEDISQM/Archives/2006/MeasuresList.pdf. [Google Scholar]
- 11.Centers for Medicare & Medicaid Services. Physician Quality Reporting Initiative: 2007 PQRI Quality Measures. Baltimore, MD: Centers for Medicare & Medicaid Services; 2007. [updated 2007; cited 2011 10 February]; Available from: https://www.cms.gov/PQRI/Downloads/2007PQRIQualityMeasures.zip. [Google Scholar]
- 12.Buccaneer Computer Systems & Service, Inc. Chronic Condition Data Warehouse User Manual. Warrenton, VA: Buccaneer Computer Systems & Service, Inc; 2009. [updated 2009; cited 2010 13 April]; Available from: http://ccwdata.org/downloads/CCW%20User%20Manual.pdf. [Google Scholar]
- 13.Katz JN, Barrett J, Liang MH, Bacon AM, Kaplan H, Kieval RI, et al. Sensitivity and positive predictive value of Medicare Part B physician claims for rheumatologic diagnoses and procedures. Arthritis Rheum. 1997;40(9):1594–1600. doi: 10.1002/art.1780400908. [DOI] [PubMed] [Google Scholar]
- 14.Goldstein LB. Accuracy of ICD-9-CM coding for the identification of patients with acute ischemic stroke: effect of modifier codes. Stroke. 1998;29(8):1602–1604. doi: 10.1161/01.str.29.8.1602. [DOI] [PubMed] [Google Scholar]
- 15.Kiyota Y, Schneeweiss S, Glynn RJ, Cannuscio CC, Avorn J, Solomon DH. Accuracy of Medicare claims-based diagnosis of acute myocardial infarction: estimating positive predictive value on the basis of review of hospital records. Am Heart J. 2004;148(1):99–104. doi: 10.1016/j.ahj.2004.02.013. [DOI] [PubMed] [Google Scholar]
- 16.Rector TS, Wickstrom SL, Shah M, Thomas Greeenlee N, Rheault P, Rogowski J, et al. Specificity and sensitivity of claims-based algorithms for identifying members of Medicare+Choice health plans that have chronic medical conditions. Health Serv Res. 2004;39(6 Pt 1):1839–1857. doi: 10.1111/j.1475-6773.2004.00321.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tirschwell DL, Longstreth WT., Jr Validating administrative data in stroke research. Stroke. 2002;33(10):2465–2470. doi: 10.1161/01.str.0000032240.28636.bd. [DOI] [PubMed] [Google Scholar]
- 18.Hebert PL, Geiss LS, Tierney EF, Engelgau MM, Yawn BP, McBean AM. Identifying persons with diabetes using Medicare claims data. Am J Med Qual. 1999;14(6):270–277. doi: 10.1177/106286069901400607. [DOI] [PubMed] [Google Scholar]
- 19.Borzecki AM, Wong AT, Hickey EC, Ash AS, Berlowitz DR. Identifying hypertension-related comorbidities from administrative data: what’s the optimal approach? Am J Med Qual. 2004;19(5):201–206. doi: 10.1177/106286060401900504. [DOI] [PubMed] [Google Scholar]
- 20.Halanych JH, Wang F, Miller DR, Pogach LM, Lin H, Berlowitz DR, et al. Racial/ethnic differences in diabetes care for older veterans: accounting for dual health system use changes conclusions. Med Care. 2006;44(5):439–445. doi: 10.1097/01.mlr.0000207433.70159.23. [DOI] [PubMed] [Google Scholar]
- 21.Druss BG, Marcus SC, Olfson M, Tanielian T, Pincus HA. Trends in care by nonphysician clinicians in the United States. N Engl J Med. 2003;348(2):130–137. doi: 10.1056/NEJMsa020993. [DOI] [PubMed] [Google Scholar]
- 22.Pham HH, Schrag D, Hargraves JL, Bach PB. Delivery of preventive services to older adults by primary care physicians. JAMA. 2005;294(4):473–481. doi: 10.1001/jama.294.4.473. [DOI] [PubMed] [Google Scholar]
- 23.Washington State Department of Health. Guidelines for using rural-urban classification systems for public health assessment: A four-tier consolidation of the RUCA system at the sub-county level. Olympia, WA: Washington State Department of Health; 2009. [updated 2009; cited April 13, 2010]; Available from: http://www.doh.wa.gov/data/Guidelines/RuralUrban2.htm#fourtier. [Google Scholar]
- 24.Weiss RJ, Ehlin A, Montgomery SM, Wick MC, Stark A, Wretenberg P. Decrease of RA-related orthopaedic surgery of the upper limbs between 1998 and 2004: data from 54,579 Swedish RA inpatients. Rheumatology (Oxford) 2008;47(4):491–494. doi: 10.1093/rheumatology/ken009. [DOI] [PubMed] [Google Scholar]
- 25.Weiss RJ, Stark A, Wick MC, Ehlin A, Palmblad K, Wretenberg P. Orthopaedic surgery of the lower limbs in 49,802 rheumatoid arthritis patients: results from the Swedish National Inpatient Registry during 1987 to 2001. Ann Rheum Dis. 2006;65(3):335–341. doi: 10.1136/ard.2005.039420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pope GC, Kautter J, Ellis RP, Ash AS, Ayanian JZ, Lezzoni LI, et al. Risk adjustment of Medicare capitation payments using the CMS-HCC model. Health Care Financ Rev. 2004;25(4):119–141. [PMC free article] [PubMed] [Google Scholar]
- 27.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 28.Deyo RA, Taylor VM, Diehr P, Conrad D, Cherkin DC, Ciol M, et al. Analysis of automated administrative and survey databases to study patterns and outcomes of care. Spine (Phila Pa 1976) 1994;19(18 Suppl):2083S–2091S. doi: 10.1097/00007632-199409151-00011. [DOI] [PubMed] [Google Scholar]
- 29.Elixhauser A, Steiner C, Harris DR, Coffey RM. Comorbidity measures for use with administrative data. Med Care. 1998;36(1):8–27. doi: 10.1097/00005650-199801000-00004. [DOI] [PubMed] [Google Scholar]
- 30.Li P, Kim MM, Doshi JA. Comparison of the performance of the CMS Hierarchical Condition Category (CMS-HCC) risk adjuster with the Charlson and Elixhauser comorbidity measures in predicting mortality. BMC Health Serv Res. 2010;10:245. doi: 10.1186/1472-6963-10-245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kleinman LC, Norton EC. What’s the Risk? A simple approach for estimating adjusted risk measures from nonlinear models including logistic regression. Health Serv Res. 2009;44(1):288–302. doi: 10.1111/j.1475-6773.2008.00900.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.StataCorp. Stata Statistical Software: Release 10. College Station, TX: StataCorp; 2007. [Google Scholar]
- 33.Wilson AR, Rodin H, Garrett NA, Bargman EP, Harris LA, Pederson MK, et al. Comparing quality of care between a consumer-directed health plan and a traditional plan: an analysis of HEDIS measures related to management of chronic diseases. Popul Health Manag. 2009;12(2):61–67. doi: 10.1089/pop.2008.0018. [DOI] [PubMed] [Google Scholar]
- 34.Chang VW, Asch DA, Werner RM. Quality of care among obese patients. JAMA. 2010;303(13):1274–1281. doi: 10.1001/jama.2010.339. [DOI] [PubMed] [Google Scholar]
- 35.Fiscella K, Holt K, Meldrum S, Franks P. Disparities in preventive procedures: comparisons of self-report and Medicare claims data. BMC Health Serv Res. 2006;6:122. doi: 10.1186/1472-6963-6-122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Holmboe ES, Wang Y, Meehan TP, Tate JP, Ho SY, Starkey KS, et al. Association between maintenance of certification examination scores and quality of care for Medicare beneficiaries. Arch Intern Med. 2008;168(13):1396–1403. doi: 10.1001/archinte.168.13.1396. [DOI] [PubMed] [Google Scholar]
- 37.Mosca L, Benjamin EJ, Berra K, Bezanson JL, Dolor RJ, Lloyd-Jones DM, et al. Effectiveness-based guidelines for the prevention of cardiovascular disease in women--2011 update: a guideline from the American Heart Association. J Am Coll Cardiol. 2011;57(12):1404–1423. doi: 10.1016/j.jacc.2011.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Genest J, McPherson R, Frohlich J, Anderson T, Campbell N, Carpentier A, et al. 2009 Canadian Cardiovascular Society/Canadian guidelines for the diagnosis and treatment of dyslipidemia and prevention of cardiovascular disease in the adult - 2009 recommendations. Can J Cardiol. 2009;25(10):567–579. doi: 10.1016/s0828-282x(09)70715-9. [DOI] [PMC free article] [PubMed] [Google Scholar]


