Abstract
Fish oil, enriched in bioactive n-3 polyunsaturated fatty acids (PUFA), has been shown to play a role in prevention of colon cancer. The effects of n-3 PUFA are pleiotropic and multifaceted, resulting in an incomplete understanding of their molecular mechanisms of action. Here, we focus on a highly conserved mechanism of n-3 PUFA, which is the alteration of the organization of the plasma membrane. We highlight recent work demonstrating that enrichment of n-3 PUFA in the plasma membrane alters the lateral organization of membrane signaling assemblies (i.e. lipid rafts). This mechanism is central for n-3 PUFA regulation of downstream signaling, T-cell activation, transcriptional activation, and cytokine secretion. We conclude that these studies provide strong evidence for a predominant mechanism by which n-3 PUFA function in colon cancer prevention.
Keywords: n-3 polyunsaturated fatty acids, DHA, EPA, lipid rafts, colon cancer, chemoprevention, T-lymphocytes
1. Introduction
Colon cancer is a prolific disease, with an estimated 140,000 new cases occurring in the past year [1]. Therefore, developing strategies for prevention is a foremost public health concern. Many modifiable factors have been associated with risk of colon cancer, including diet [2]. One major dietary component that has been associated with prevention of colon cancer is fish oil. Experimental, clinical, and epidemiological studies have strongly indicated a role for fish oil in colon cancer prevention [3,4,5,6,7,8,9,10,11,12]. However, the exact mechanisms of action of fish oil have not yet been fully elucidated. Two of the most abundant bioactive lipids enriched in fish oil, eicosapentaenoic acid (EPA, 20:5 n-3) and docosahexaenoic acid (DHA, 22:6 n-3), have been shown to have pleiotropic effects. One central molecular target involves alterations to the plasma membrane. DHA has also been shown to have significant effects on plasma membrane properties, including altering membrane fluidity, phase behavior, permeability, fusion, flip-flop, and resident protein activity [13,14]. Recent evidence suggests that DHA can perturb specialized regions of the plasma membrane known as lipid rafts [15,16,17]. Lipid rafts are small (10–200 nm), heterogeneous microdomains that are enriched in cholesterol, sphingolipids, and saturated acyl chains [18]. DHA, due to its high degree of unsaturation, is sterically incompatible with cholesterol [14], which could be the cause of the disruption in lipid rafts caused by this fatty acid. Lipid rafts serve as signaling platforms by compartmentalizing plasma membrane proteins and lipids. In response to stimuli, nanometer-scale domains can coalesce and display high molecular order [19]. Several important processes involve lipid rafts, including T-cell activation, signal transduction, and protein and lipid trafficking [20]. Many of these lipid raft mediated processes play an integral role in the process of colon tumorigenesis. Signaling pathways emanating from lipid rafts mediate a variety of mitogenic, metastatic, and other tumor-promoting cellular activities, and these pathways are often hyperactivated in cancer [21]. Additionally, chronic inflammation, central to the process of tumorigenesis [22], involves excessive T-cell activation, which is regulated by lipid rafts. Our lab has made numerous recent discoveries highlighting the role of n-3 polyunsaturated fatty acids (PUFA) in the regulation of lipid rafts and lipid raft-mediated signaling. Our central hypothesis is that n-3 PUFA manifest chemoprotective properties by inducing changes in membrane composition resulting in altered cell function.
2. n-3 PUFA alter lipid raft size
Lipid raft size is integral for dynamic lateral segregation of signaling proteins into microdomains. Partitioning of proteins into rafts can increase specific protein-protein collision rates to facilitate efficient signaling. However, to maximize this essential, biologically relevant function, rafts must be mobile and small, with a diameter up to 14 nm [23]. Our lab assessed the effect of n-3 PUFA on lipid raft size in T-cells. We compared the size of lipid rafts in splenic T-cells from wild type and fat-1 transgenic mice, which express n-3 fatty acid desaturase cloned from C. elegans [24]. This enzyme can catalyze the production of endogenous n-3 PUFA by introducing a double bond into fatty acyl chains. The fat-1 mice allow us to assess the biological properties of n-3 PUFA without having to incorporate these fatty acids into the diet. In combination with the utilization of Laurdan, a dye that is sensitive to the ordering properties of the membrane, it is possible to visualize rafts within the T-cell plasma membrane. Using this approach, we found that n-3 PUFA actually enhanced the clustering of lipid rafts to form large raft domains [16]. This is likely caused by a lipid-driven mechanism for lateral phase separation of cholesterol- or sphingolipid-rich lipid microdomains from n-3 PUFA due to very poor affinity. By increasing the size of lipid rafts, n-3 PUFA impairs efficient functioning of lipid rafts in T-cells. This in turn suppresses T-cell activation [16], which is a potential mechanism by which n-3 PUFA function as anti-inflammatory agents. We observed similar results in HeLa cells, a human cancer cell line. HeLa cells treated with DHA exhibited enhanced clustering of lipid raft domains compared to untreated cells [25]. Importantly, increasing lipid raft size has implications for a role of n-3 PUFA in regulation of multiple signaling events which emanate from lipid rafts.
3. n-3 PUFA alter lipid raft composition
The composition of lipid rafts is important for lipid raft function. Lipid rafts provide a more ordered lipid environment than the bulk membrane due to interactions between cholesterol, sphingolipids, and phospholipids containing saturated fatty acyl chains [26], and extraction of certain lipids from lipid rafts, including cholesterol, has been shown to perturb lipid raft function. Therefore, we have assessed the effect of n-3 PUFA on lipid composition of rafts in multiple cell types. Upon feeding mice a diet enriched in n-3 PUFA, the cholesterol content of lipid rafts in colonocytes was reduced by 46% compared to mice fed a diet enriched in n-6 PUFA [27]. In mouse splenic T-cells, we found that dietary n-3 PUFA reduced lipid raft sphingolipid content by ~45% [28]. Specifically, we demonstrated that raft sphingomyelin content was decreased by 30% in T-cells from mice fed n-3 PUFA [29]. These n-3 PUFA induced modifications of lipid raft composition are significant because cholesterol and sphingomyelin are major building blocks of lipid rafts that promote the formation of hydrophobic liquid-ordered molecular packing.
In addition to lipid components, certain proteins are known to be enriched in lipid rafts. Many proteins involved in cell signaling, including receptors and G proteins, localize to lipid rafts [30,31]. Several of these lipid raft localized proteins require raft localization for proper functioning. One such lipid raft sequestered protein is Ras. Ras is an important signaling mediator, and one of its major isoforms, H-Ras, is well known to localize to lipid raft domains [32]. Activation of H-Ras and downstream signal transduction has been shown to be dependent on lipid raft localization [33,34,35]. We assessed the localization of Ras both in vivo and in vitro to determine the effect of n-3 PUFA on lipid raft localization of H-Ras [27]. Feeding mice a diet enriched in n-3 PUFA and treating immortalized young adult mouse colonocytes (YAMC) with DHA decreased the localization of H-Ras to lipid raft domains [27]. Upon further examination of the effect of n-3 PUFA on localization of lipid raft proteins in colonocytes, our lab found that DHA inhibited the plasma membrane targeting of lipidated proteins, including H-Ras [36]. We also assessed the effect of n-3 PUFA on the lipid raft localization of the epidermal growth factor receptor (EGFR). EGFR is a master signal involved in colonic transformation, and lipid rafts are required for efficient EGFR signaling [20,37,38]. We observed in YAMC cells that DHA reduced EGFR lipid raft localization [39]. We also determined the effect of n-3 PUFA on lipid raft partitioning of key proteins involved in T-cell activation, including PKCθ, PLCγ-1, and F-actin. We found that dietary fish oil significantly suppressed the recruitment of PKCθ to lipid rafts following stimulation [29]. In a complementary study, we noted that recruitment of PKCθ, PLCγ-1, and F-actin to lipid raft domains is suppressed in T-cells isolated from fat-1 transgenic mice compared to wild-type mice [16]. Overall, these data clearly demonstrate that n-3 PUFA modify the lipid raft microenvironment.
4. n-3 PUFA perturb lipid raft regulated signaling
Our initial observations on the effect of n-3 PUFA on lipid raft size and composition led us to hypothesize that n-3 PUFA would suppress lipid raft mediated cell signaling. We therefore assessed the effect of n-3 PUFA on activation of Ras in the mouse colon. We found that n-3 PUFA feeding suppressed EGF-induced activation of H-Ras, but not K-Ras [27]. Interestingly, H-Ras signaling has been shown to be sensitive to lipid raft perturbations, whereas K-Ras signaling is largely insensitive [40]. Additionally, we have observed that DHA suppresses EGF-induced activation of several downstream pathways, including ERK1/2, STAT3, and mTOR, in mouse colonocytes both in vivo and in vitro [39]. These data strongly suggest that n-3 PUFA suppress lipid raft mediated signaling in colonocytes. We also determined the role of n-3 PUFA in mediating lipid raft regulated signaling in T-cells because upon activation, lipid rafts compartmentalize signal-transducing molecules to provide an environment conducive to signal transduction [41]. Phosphorylation of PLCγ-1 is a lipid raft dependent process that occurs in the very early stages of T-cell activation [42]. We found that stimulation-induced PLCγ-1 phosphorylation is inhibited in cells from fat-1 transgenic mice compared to wild-type mice [16]. Additionally, lipid rafts have been shown to both integrate and amplify signaling processes that lead to activation of transcription factors, including AP-1 and NF-κB, in T-cells. Binding sites for these transcription factors have been found in the promoters of many genes encoding cytokines and chemokines, and activation of NF-κB and AP-1 has been shown to be essential for induction of a response to immune and inflammatory challenges [43,44]. Therefore, we assessed the effect of n-3 PUFA on receptor-induced activation of these important pathway modifiers. We found that both fish oil and purified DHA suppressed the binding activity of both of AP-1 and NF-κB [29]. Together, these findings are consistent with our central hypothesis that changes in membrane composition induced by n-3 PUFA have functional consequences with regard to cell signaling.
5. n-3 PUFA suppress lipid raft mediated cell function
Lipid rafts play a role in mediating numerous cell functions. T-cell activation requires lipid raft coalescence and translocation of key proteins, ultimately leading to the production of cytokines and cell cycle progression. We assessed the effect of n-3 PUFA on production of IL-2, which functions in both a paracrine and autocrine manner to induce T-cell proliferation. We observed that n-3 PUFA inhibited activation-induced production of IL-2 and ultimately suppressed T-cell proliferation [29]. In a separate experiment, we assessed the proliferation of T-cells from fat-1 mice in response to multiple stimuli. We found that T-cell proliferation was suppressed in cells isolated from fat-1 mice compared to wild-type mice [16]. Furthermore, many lipid raft mediated processes, including EGFR signaling, mediate cell proliferation in colonocytes. We have observed that DHA suppresses cell proliferation in YAMC cells [39]. These data signify the implicit role of n-3 PUFA in regulation of lipid raft mediated cell processes.
6. Conclusions
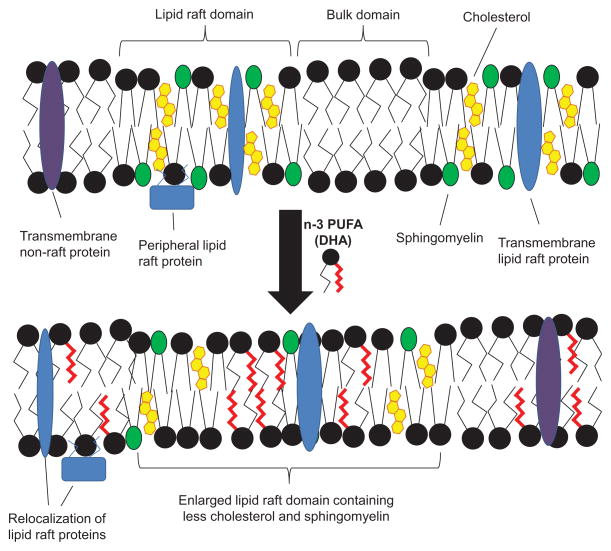
Because of the broad-acting effects of n-3 PUFA on mammalian physiology, it has been postulated that these dietary fatty acids act at a fundamental level common to all cells, i.e., by altering the physical properties of biological membranes [14,15,17,45]. A wealth of published literature supports the hypothesis that n-3 PUFA play an important role in mediating lipid raft composition. Our lab has clearly demonstrated that n-3 PUFA modify lipid raft organization, leading to altered cell signaling and function (Fig. 1).
Fig. 1. Putative model for the effect of n-3 PUFA on lipid rafts.
Lipid rafts are nanoscale regions of the plasma membrane, enriched in cholesterol, sphingomyelin, and phospholipids containing saturated acyl chains. Both transmembrane and peripheral membrane proteins can be localized to lipid rafts. Upon treatment with a combination of n-3 PUFA or DHA alone, these PUFA are incorporated into phospholipids which are inserted into both raft and non-raft regions of the plasma membrane. This results in enhanced clustering of lipid raft regions, which are depleted of cholesterol and sphingomyelin. Additionally, many lipid raft associated proteins “mislocalize” to the bulk membrane domain. This results in a suppression of lipid raft mediated processes, including T-cell activation and downstream signal transduction.
One should always be conscious of the physiological relevance of the dose of n-3 PUFA utilized in studies. Typically, our fish oil-enriched diets contain 1.4% energy as DHA, and diets using purified DHA contain 2.2% energy as DHA. In contrast, corn oil control diets contain only trace amounts of EPA and DHA. The amount of these lipids consumed by human populations varies greatly. The Japanese typically consume 1–2% of energy in the diet from DHA [46], whereas those in most European countries and the United States consume 0.1–0.2% of energy as n-3 PUFA [47]. Therefore, our experimental diets are within the range that can be consumed in the human diet. In cell culture experiments, we typically utilize low, physiologically relevant doses of DHA (up to 50 μM), which replicates our in vivo results without inducing apoptosis [48]. Duration of treatment is another important issue. Typically, we treat cell cultures with n-3 PUFA for at least 3 days, and feed animals diets enriched in n-3 PUFA for a minimum of two weeks. Since upon supplementation, DHA is very rapidly incorporated into the phospholipids of the plasma membrane of many tissues [49], relatively little time is required in order to observe an effect of n-3 PUFA exposure. Additionally, removal of n-3 PUFA supplementation has been shown to result in a rapid release of n-3 PUFA from the plasma membrane and reversal of induced effects [36]. This demonstrates the need for constant consumption of n-3 PUFA in order to maintain an effect on lipid rafts.
A significant proportion of the literature describing the effects of n-3 PUFA on lipid rafts utilizes either fish oil, purified DHA, or a combination of EPA and DHA [25,29,50]. Fish oils from different sources contain variable mixtures of EPA and DHA, with most commercially available fish oils containing a 2:1 ratio of EPA to DHA. However, whether the effects of n-3 PUFA supplementation on lipid raft perturbation are due to EPA, DHA, or both remain to be determined. EPA is both shorter and less unsaturated than DHA, suggesting that it may not be as efficient as DHA at perturbing lipid rafts. However, it remains to be fully determined whether the structural differences between these two fatty acids are enough to result in functional differences in regard to lipid raft perturbation. Based on the current literature, it is clearly evident that DHA has a significant effect on lipid raft organization [17,45]. The effect of EPA on lipid rafts is less well documented. Some previous work has indicated a differential effect of EPA and DHA on lipid rafts [17], with DHA, but not EPA, modifying the size and distribution of lipid rafts. Additionally, our lab has generated data indicating that DHA uniquely modifies the lateral organization of lipid raft localized proteins [39]. These studies suggest a specificity of DHA in disruption of lipid rafts. However, further work is needed in order to clarify the unique lipid raft perturbing effects of EPA and DHA. From a nutritional perspective, based on the differential effects of EPA and DHA in regulating multiple aspects of health, it is likely best to consume a combination of these n-3 PUFA.
Lipid rafts are involved in multiple, sundry cellular processes, which require diversity in the composition of membrane domains [51]. It is evident that cell membranes incorporate a plethora of structure–function relationships to generate a lateral diversity [52]. Both lipid-protein and lipid-lipid associations have the potential to organize features of the membrane, resulting in a heterogeneous population of lipid rafts. This heterogeneity is likely to result in differential effects of n-3 PUFA on lipid rafts. Therefore, the role of n-3 PUFA in the regulation of processes emanating from different types of lipid rafts is an ambiguous area that requires further investigation. Functional raft-based membrane heterogeneity is dependent upon both lipid and protein physical parameters [52], and we have observed that n-3 PUFA can alter lipid raft composition of both lipids and proteins. Based on the interactions within certain raft domains, n-3 PUFA could be expected to perturb the function of some lipid raft domains while leaving others unaffected. Future research should be directed toward discerning the effects of n-3 PUFA on different types of lipid rafts.
The role for n-3 PUFA in prevention and treatment of colon cancer is well documented, but the efficacy of n-3 PUFA is still a source of contention. Many current prevention and treatment options are aimed at suppression of chronic inflammation and cell proliferation. We have demonstrated a role for innocuous dietary fatty acids in the regulation of these processes by modifying the organization of lipid rafts. Lipid rafts play a central role in multiple cellular processes involved in colonic tumorigenesis. A number of studies have indicated that cholesterol accumulates in various tumors, and it has been proposed that progressive increases in membrane cholesterol contributes to the expansion of lipid rafts to potentiate oncogenic cell signaling pathways [53,54]. Additionally, some cancer cell lines have been shown to contain elevated levels of lipid rafts, resulting in increased sensitivity to cell death induced by lipid raft disruption relative to their normal counterparts [54]. These studies suggest that lipid rafts play a functional role during tumorigenesis of multiple types of cancer, indicating a therapeutic role for n-3 PUFA since these fatty acids modulate lipid raft structure/function. This mechanism of action could potentially impact complex disease states that rely on lipid raft-mediated processes for potentiation of the pathophysiology, including Alzheimer’s disease, Parkinson’s disease, cardiovascular diseases, HIV, and many others [55]. Overall, the basic knowledge obtained from our studies provides a solid mechanistic underpinning for the role of n-3 PUFA in the resolution of chronic inflammation and colon cancer prevention.
Acknowledgments
Sources of support: This work was supported by National Institute of Health grants CA59034, CA129444, by USDA 2010-34402-20875 (Chapkin RS).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 2.Martínez ME, Jacobs ET. Diet and Environment, Role in Colon Cancer. In: Leonard J, editor. Encyclopedia of Gastroenterology. Elsevier; New York: 2004. pp. 593–597. [Google Scholar]
- 3.Anti M, Armelao F, Marra G, et al. Effects of different doses of fish oil on rectal cell proliferation in patients with sporadic colonic adenomas. Gastroenterology. 1994;107:1709–1718. doi: 10.1016/0016-5085(94)90811-7. [DOI] [PubMed] [Google Scholar]
- 4.Anti M, Marra G, Armelao F, Bartoli GM, et al. Effect of omega-3 fatty acids on rectal mucosal cell proliferation in subjects at risk for colon cancer. Gastroenterology. 1992;103:883–891. doi: 10.1016/0016-5085(92)90021-p. [DOI] [PubMed] [Google Scholar]
- 5.Bartram HP, Gostner A, Scheppach W, et al. Effects of fish oil on rectal cell proliferation, mucosal fatty acids, and prostaglandin E2 release in healthy subjects. Gastroenterology. 1993;105:1317–1322. doi: 10.1016/0016-5085(93)90135-y. [DOI] [PubMed] [Google Scholar]
- 6.Caygill CP, Charlett A, Hill MJ. Fat, fish, fish oil and cancer. Br J Cancer. 1996;74:159–164. doi: 10.1038/bjc.1996.332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chang WL, Chapkin RS, Lupton JR. Fish oil blocks azoxymethane-induced rat colon tumorigenesis by increasing cell differentiation and apoptosis rather than decreasing cell proliferation. J Nutr. 1998;128:491–497. doi: 10.1093/jn/128.3.491. [DOI] [PubMed] [Google Scholar]
- 8.Courtney ED, Matthews S, Finlayson C, et al. Eicosapentaenoic acid (EPA) reduces crypt cell proliferation and increases apoptosis in normal colonic mucosa in subjects with a history of colorectal adenomas. Int J Colorectal Dis. 2007;22:765–776. doi: 10.1007/s00384-006-0240-4. [DOI] [PubMed] [Google Scholar]
- 9.Pot GK, Geelen A, van Heijningen EM, Siezen CL, van Kranen HJ, Kampman E. Opposing associations of serum n-3 and n-6 polyunsaturated fatty acids with colorectal adenoma risk: an endoscopy-based case-control study. Int J Cancer. 2008;123:1974–1977. doi: 10.1002/ijc.23729. [DOI] [PubMed] [Google Scholar]
- 10.Geelen A, Schouten JM, Kamphuis C, et al. Fish consumption, n-3 fatty acids, and colorectal cancer: a meta-analysis of prospective cohort studies. Am J Epidemiol. 2007;166:1116–1125. doi: 10.1093/aje/kwm197. [DOI] [PubMed] [Google Scholar]
- 11.Hall MN, Chavarro JE, Lee IM, Willett WC, Ma J. A 22-year prospective study of fish, n-3 fatty acid intake, and colorectal cancer risk in men. Cancer Epidemiol Biomarkers Prev. 2008;17:1136–1143. doi: 10.1158/1055-9965.EPI-07-2803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim S, Sandler DP, Galanko J, Martin C, Sandler RS. Intake of polyunsaturated fatty acids and distal large bowel cancer risk in whites and African Americans. Am J Epidemiol. 2010;171:969–979. doi: 10.1093/aje/kwq032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stillwell W, Wassall SR. Docosahexaenoic acid: membrane properties of a unique fatty acid. Chem Phys Lipids. 2003;126:1–27. doi: 10.1016/s0009-3084(03)00101-4. [DOI] [PubMed] [Google Scholar]
- 14.Wassall SR, Brzustowicz MR, Shaikh SR, Cherezov V, Caffrey M, Stillwell W. Order from disorder, corralling cholesterol with chaotic lipids. The role of polyunsaturated lipids in membrane raft formation. Chem Phys Lipids. 2004;132:79–88. doi: 10.1016/j.chemphyslip.2004.09.007. [DOI] [PubMed] [Google Scholar]
- 15.Chapkin RS, McMurray DN, Davidson LA, Patil BS, Fan YY, Lupton JR. Bioactive dietary long-chain fatty acids: emerging mechanisms of action. Br J Nutr. 2008;100:1152–1157. doi: 10.1017/S0007114508992576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim W, Fan YY, Barhoumi R, Smith R, McMurray DN, Chapkin RS. n-3 polyunsaturated fatty acids suppress the localization and activation of signaling proteins at the immunological synapse in murine CD4+ T cells by affecting lipid raft formation. J Immunol. 2008;181:6236–6243. doi: 10.4049/jimmunol.181.9.6236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shaikh SR, Rockett BD, Salameh M, Carraway K. Docosahexaenoic acid modifies the clustering and size of lipid rafts and the lateral organization and surface expression of MHC class I of EL4 cells. J Nutr. 2009;139:1632–1639. doi: 10.3945/jn.109.108720. [DOI] [PubMed] [Google Scholar]
- 18.Pike LJ. Rafts defined: a report on the Keystone Symposium on Lipid Rafts and Cell Function. J Lipid Res. 2006;47:1597–1598. doi: 10.1194/jlr.E600002-JLR200. [DOI] [PubMed] [Google Scholar]
- 19.Lingwood D, Simons K. Lipid rafts as a membrane-organizing principle. Science. 2010;327:46–50. doi: 10.1126/science.1174621. [DOI] [PubMed] [Google Scholar]
- 20.Pike LJ. Growth factor receptors, lipid rafts and caveolae: an evolving story. Biochim Biophys Acta. 2005;1746:260–273. doi: 10.1016/j.bbamcr.2005.05.005. [DOI] [PubMed] [Google Scholar]
- 21.Patra SK. Dissecting lipid raft facilitated cell signaling pathways in cancer. Biochim Biophys Acta. 2008;1785:182–206. doi: 10.1016/j.bbcan.2007.11.002. [DOI] [PubMed] [Google Scholar]
- 22.Ullman TA, Itzkowitz SH. Intestinal inflammation and cancer. Gastroenterology. 2011;140:1807–1816. doi: 10.1053/j.gastro.2011.01.057. [DOI] [PubMed] [Google Scholar]
- 23.Nicolau DV, Jr, Burrage K, Parton RG, Hancock JF. Identifying optimal lipid raft characteristics required to promote nanoscale protein-protein interactions on the plasma membrane. Mol Cell Biol. 2006;26:313–323. doi: 10.1128/MCB.26.1.313-323.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kang JX, Wang J, Wu L, Kang ZB. Transgenic mice: fat-1 mice convert n-6 to n-3 fatty acids. Nature. 2004;427:504. doi: 10.1038/427504a. [DOI] [PubMed] [Google Scholar]
- 25.Chapkin RS, Wang N, Fan YY, Lupton JR, Prior IA. Docosahexaenoic acid alters the size and distribution of cell surface microdomains. Biochim Biophys Acta. 2008;1778:466–471. doi: 10.1016/j.bbamem.2007.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brown DA, London E. Structure and function of sphingolipid- and cholesterol-rich membrane rafts. J Biol Chem. 2000;275:17221–17224. doi: 10.1074/jbc.R000005200. [DOI] [PubMed] [Google Scholar]
- 27.Ma DW, Seo J, Davidson LA, et al. n-3 PUFA alter caveolae lipid composition and resident protein localization in mouse colon. FASEB J. 2004;18:1040–1042. doi: 10.1096/fj.03-1430fje. [DOI] [PubMed] [Google Scholar]
- 28.Fan YY, McMurray DN, Ly LH, Chapkin RS. Dietary (n-3) polyunsaturated fatty acids remodel mouse T-cell lipid rafts. J Nutr. 2003;133:1913–1920. doi: 10.1093/jn/133.6.1913. [DOI] [PubMed] [Google Scholar]
- 29.Fan YY, Ly LH, Barhoumi R, McMurray DN, Chapkin RS. Dietary docosahexaenoic acid suppresses T cell protein kinase C theta lipid raft recruitment and IL-2 production. J Immunol. 2004;173:6151–6160. doi: 10.4049/jimmunol.173.10.6151. [DOI] [PubMed] [Google Scholar]
- 30.Pike LJ. Lipid rafts: bringing order to chaos. J Lipid Res. 2003;44:655–667. doi: 10.1194/jlr.R200021-JLR200. [DOI] [PubMed] [Google Scholar]
- 31.Smart EJ, Graf GA, McNiven MA, et al. Caveolins, liquid-ordered domains, and signal transduction. Mol Cell Biol. 1999;19:7289–7304. doi: 10.1128/mcb.19.11.7289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Prior IA, Muncke C, Parton RG, Hancock JF. Direct visualization of Ras proteins in spatially distinct cell surface microdomains. J Cell Biol. 2003;160:165–170. doi: 10.1083/jcb.200209091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Prior IA, Harding A, Yan J, Sluimer J, Parton RG, Hancock JF. GTP-dependent segregation of H-ras from lipid rafts is required for biological activity. Nat Cell Biol. 2001;3:368–375. doi: 10.1038/35070050. [DOI] [PubMed] [Google Scholar]
- 34.Mineo C, James GL, Smart EJ, Anderson RG. Localization of epidermal growth factor-stimulated Ras/Raf-1 interaction to caveolae membrane. J Biol Chem. 1996;271:11930–11935. doi: 10.1074/jbc.271.20.11930. [DOI] [PubMed] [Google Scholar]
- 35.Eisenberg S, Shvartsman DE, Ehrlich M, Henis YI. Clustering of raft-associated proteins in the external membrane leaflet modulates internal leaflet H-ras diffusion and signaling. Mol Cell Biol. 2006;26:7190–7200. doi: 10.1128/MCB.01059-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Seo J, Barhoumi R, Johnson AE, Lupton JR, Chapkin RS. Docosahexaenoic acid selectively inhibits plasma membrane targeting of lipidated proteins. FASEB J. 2006;20:770–772. doi: 10.1096/fj.05-4683fje. [DOI] [PubMed] [Google Scholar]
- 37.Matveev SV, Smart EJ. Heterologous desensitization of EGF receptors and PDGF receptors by sequestration in caveolae. Am J Physiol Cell Physiol. 2002;282:C935–946. doi: 10.1152/ajpcell.00349.2001. [DOI] [PubMed] [Google Scholar]
- 38.Ringerike T, Blystad FD, Levy FO, Madshus IH, Stang E. Cholesterol is important in control of EGF receptor kinase activity but EGF receptors are not concentrated in caveolae. J Cell Sci. 2002;115:1331–1340. doi: 10.1242/jcs.115.6.1331. [DOI] [PubMed] [Google Scholar]
- 39.Turk HF, Barhoumi R, Chapkin RS. Alteration of EGFR spatiotemporal dynamics suppresses signal transduction. doi: 10.1371/journal.pone.0039682. Submitted for publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Roy S, Luetterforst R, Harding A, et al. Dominant-negative caveolin inhibits H-Ras function by disrupting cholesterol-rich plasma membrane domains. Nat Cell Biol. 1999;1:98–105. doi: 10.1038/10067. [DOI] [PubMed] [Google Scholar]
- 41.Leitenberg D, Balamuth F, Bottomly K. Changes in the T cell receptor macromolecular signaling complex and membrane microdomains during T cell development and activation. Semin Immunol. 2001;13:129–138. doi: 10.1006/smim.2000.0304. [DOI] [PubMed] [Google Scholar]
- 42.Zhang W, Samelson LE. The role of membrane-associated adaptors in T cell receptor signalling. Semin Immunol. 2000;12:35–41. doi: 10.1006/smim.2000.0205. [DOI] [PubMed] [Google Scholar]
- 43.Ben-Neriah Y, Karin M. Inflammation meets cancer, with NF-kappaB as the matchmaker. Nat Immunol. 2011;12:715–723. doi: 10.1038/ni.2060. [DOI] [PubMed] [Google Scholar]
- 44.Hess J, Angel P, Schorpp-Kistner M. AP-1 subunits: quarrel and harmony among siblings. J Cell Sci. 2004;117:5965–5973. doi: 10.1242/jcs.01589. [DOI] [PubMed] [Google Scholar]
- 45.Wassall SR, Stillwell W. Docosahexaenoic acid domains: the ultimate non-raft membrane domain. Chem Phys Lipids. 2008;153:57–63. doi: 10.1016/j.chemphyslip.2008.02.010. [DOI] [PubMed] [Google Scholar]
- 46.Nagata C, Takatsuka N, Shimizu H. Soy and fish oil intake and mortality in a Japanese community. Am J Epidemiol. 2002;156:824–831. doi: 10.1093/aje/kwf118. [DOI] [PubMed] [Google Scholar]
- 47.Gibney MJ. Incorporation of n-3 polyunsaturated fatty acids into processed foods. Br J Nutr. 1997;78:193–195. doi: 10.1079/bjn19970138. [DOI] [PubMed] [Google Scholar]
- 48.Turk HF, Kolar SS, Fan YY, Cozby CA, Lupton JR, Chapkin RS. Linoleic acid and butyrate synergize to increase Bcl-2 levels in colonocytes. Int J Cancer. 2011;128:63–71. doi: 10.1002/ijc.25323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Arterburn LM, Hall EB, Oken H. Distribution, interconversion, and dose response of n-3 fatty acids in humans. Am J Clin Nutr. 2006;83:1467S–1476S. doi: 10.1093/ajcn/83.6.1467S. [DOI] [PubMed] [Google Scholar]
- 50.Schley PD, Brindley DN, Field CJ. (n-3) PUFA alter raft lipid composition and decrease epidermal growth factor receptor levels in lipid rafts of human breast cancer cells. J Nutr. 2007;137:548–553. doi: 10.1093/jn/137.3.548. [DOI] [PubMed] [Google Scholar]
- 51.Pike LJ. Lipid rafts: heterogeneity on the high seas. Biochem J. 2004;378:281–292. doi: 10.1042/BJ20031672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lingwood D, Kaiser HJ, Levental I, Simons K. Lipid rafts as functional heterogeneity in cell membranes. Biochem Soc Trans. 2009;37:955–960. doi: 10.1042/BST0370955. [DOI] [PubMed] [Google Scholar]
- 53.Freeman MR, Solomon KR. Cholesterol and prostate cancer. J Cell Biochem. 2004;91:54–69. doi: 10.1002/jcb.10724. [DOI] [PubMed] [Google Scholar]
- 54.Li YC, Park MJ, Ye SK, Kim CW, Kim YN. Elevated levels of cholesterol-rich lipid rafts in cancer cells are correlated with apoptosis sensitivity induced by cholesterol-depleting agents. Am J Pathol. 2006;168:1107–1118. doi: 10.2353/ajpath.2006.050959. quiz 1404–1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Michel V, Bakovic M. Lipid rafts in health and disease. Biol Cell. 2007;99:129–140. doi: 10.1042/BC20060051. [DOI] [PubMed] [Google Scholar]