Abstract
Thimet oligopeptidase (TOP) and prolyl endopeptidase (PEP) are neuropeptidases involved in the hydrolysis of gonadotropin-releasing hormone, a key component of the hypothalamic-pituitary-gonadal axis. GnRH is regulated in part by feedback from steroid hormones such as estradiol. Previously, we demonstrated that TOP levels are down-regulated by estradiol in reproductively-relevant regions of the female rodent brain. The present study supports these findings by showing that TOP enzyme activity, as well as protein levels, in the ventromedial hypothalamic nucleus of female mice are controlled estradiol. We further demonstrate that PEP levels in this same brain region are down-regulated by estradiol in parallel with those of TOP. These findings provide evidence that these neuropeptidases are part of the fine control of hormone levels in the HPG axis.
Keywords: thimet oligopeptidase, estradiol, hypothalamus, GnRH, prolyl endopeptidase, ventromedial nucleus
1. Introduction
Thimet oligopeptidase (TOP; EC 3.4.24.15, or EP24.15) is a thiol-sensitive metallopeptidase, widely distributed throughout many organs but in highest concentration in brain and reproductive tissue (Pierotti et al., 1991; Shrimpton et al., 2002). TOP catalyzes the degradation of many peptides involved in important physiological processes (reviewed in Shrimpton et al., 2002). Prolyl endopeptidase (PEP), also known as prolyl oligopeptidase or POP (E.C. 3.4.21.26), is another endopeptidase present throughout the body, with high enzymatic activity in the brain (Brandt et al., 2007). Both neuropeptidases are involved in the degradation of gonadotropin-releasing hormone (GnRH) (Lew et al., 1997; Chu et al., 1985), a peptide that regulates the production and release of gonadal steroids. TOP catalyzes hydrolysis of the Try5-Gly6 bond, the rate-limiting step in GnRH degradation (Lew et al., 1997; Cleverly et al 2010)), while PEP facilitates TOP’s action by removal of the N-terminal glycinamide of GnRH (Lew et al., 1994).
TOP is found in brain areas associated with production and release of GnRH (Massarelli et al., 1999; Wu et al., 1997; Cyr et al., 2010). Furthermore, one of the products of TOP action on GnRH, GnRH1-5, is bioactive in promoting lordosis and in GnRH auto-regulation (reviewed in Roberts et al., 2007; Wu et al., 2009). The involvement of TOP and other neuropeptidases in breakdown of GnRH suggests that these enzymes may be another component of the hypothalamic-pituitary-gonadal HPG axis and therefore candidates for regulation by the end products, steroid hormones. We have previously demonstrated through immunohistochemistry of hypothalamic sections from ovariectomized female mice that TOP and estrogen receptor-α (ERα) are expressed in the same cells, providing a mechanism for estradiol action on enzyme levels. We found that levels of TOP are decreased in the ERα.rich caudal portion of the ventrolmedial nucleus of the hypothalamus (VMHvl) when animals are treated with estradiol (Cyr et al., 2010). The present study was designed to investigate if enzymatic activity, as well as protein levels of TOP, are regulated by estradiol, and to determine if control of the enzyme PEP, which is also involved in GnRH degradation, is coordinated with TOP regulation.
2. Materials and methods
2.1 Animal Care and Brain Tissue Collection
Female C57BL/6J mice were bred at Wellesley College, Wellesley, MA and housed together in groups in a controlled environment of 25 °C with a reverse light cycle (12L:12D). Food and water were provided ad libitum. All animal procedures were approved by the Wellesley College Institutional Animal Care and Use Committee (Wellesley, MA, USA).
As described in Cyr et al., (2010), mice were ovariectomized at approximately 7 weeks of age. One week after surgery, the mice were injected subcutaneously with estradiol benzoate (EB, 2 µg, in 0.1 ml sesame seed oil, n=15) or vehicle (sesame seed oil, n=15). Two days later, the mice were euthanized with CO2 gas. For those studies involving enzyme assays, each brain was collected, snap-frozen on dry ice, and stored at - 80 °C before obtaining micropunches of tissue from specific brain regions. Micropunches and subsequent tissue samples were stored in −80 °C. Tissue collection for immunohistochemistry is described below.
2.2 Tissue Preparation
Tissue micropunches (1 mm) were collected using a cryostat at −25 °C and sonicated in lysis buffer (25 mM Tris, 125 mM KCl, 1 µM ZnCl2, 10 mM NaF, and 1 mM PMSF added fresh, pH 7.8). The resulting sonicate was centrifuged at 16,000 × g for 30 min at 4 °C, and the supernatant obtained. Protein concentration was determined via micro Bradford assay using a Bio-Tek MicroQuant plate reader.
2.3 Quenched-Fluorescence Assay
TOP activity assays were conducted with a Cary Eclipse Spectrofluorimeter (Wolfson et al., 1996). Total volume for each sample was 2 mL in assay buffer (25 mM Tris, 10% glycerol, 125 mM KCl, 1 µM ZnCl2, 1 mM TCEP, pH 7.8) including 10 µL of enzyme or tissue homogenate and 10 µL of substrate. Enzyme and buffer were pre-incubated at room temperature before addition of substrate to initiate the reaction. Reaction was monitored for 90 sec at room temperature. All assay reactions were performed in triplicate or quadruplicate.
The substrate for TOP was 7-methoxycoumarin-4-acetyl-Pro-Leu-Gly-Pro-Lys-dinitrophenol (MCA), 2.8 mM stock solution. Settings for the TOP assay were: emission wavelength, 400 nm; emission slit width, 10 nm; excitation wavelength, 325 nm; and excitation slit width, 5 nm. Reaction was carried out in the presence or absence of a specific inhibitor of TOP, cFPAAF-pAB (N-[1-(R,S)-carboxy-3-phenylpropyl]-Ala-Ala-Phe-pAB (cFP), 16.5 µM. When inhibitor was added, the inhibitor was present for the pre-incubation step with enzyme.
2.4 Immunohistochemistry
For immunohistochemical analysis, mice were ovariectomized and treated with either EB (2µg, n=9) or vehicle (n=8). Forty-eight hours later the mice were injected i.p. with an overdose of sodium pentobarbital (0.1ml, 390 mg/ml) and perfused intracardially with saline (0.9%) for 1 minute followed by 4% paraformaldehyde in 0.1 M sodium phosphate buffer (pH = 7.2) for 8 min at a flow rate of 8 ml/min. Brains were immediately removed, blocked, and incubated in 0.1 M sodium phosphate buffer (pH = 7.2) containing 20% sucrose at 4 °C overnight. Using a freezing rotary microtome, 40 µm coronal sections were collected from the anterior commissure (Fig 30) through the periaqueductal gray (Fig 53), following the mouse brain atlas (Paxinos et al., 2004). Brain sections were stored in cryoprotectant at −20 °C until processing.
Immunofluorescence was used to detect cells expressing PEP and TOP, as well as ERα, to determine if TOP and/or PEP are expressed in estrogen-sensitive hypothalamic neurons. All sections were run through the immunofluorescence protocol simultaneously. Brain sections were incubated in 0.05% TBS buffer (0.05 M Tris-HCl, 0.15 M NaCl, pH 7.6) followed by a 90 min incubation in 0.05% TBS buffer containing donkey anti-mouse IgG antibody (0.02 µg/µl) to reduce non-specific background staining. The sections were incubated in 0.05% TBS containing 2% non-immune donkey serum and 1% w/v BSA for 20 min to reduce non-specific staining. Then, sections were incubated at 4 °C overnight in a cocktail containing antibodies goat anti-PEP (1:100, ab110857, Abcam), rabbit anti-TOP (1:5000, generous gift from Marc Glucksman), and rat-anti-ERα (1:100, H222, Santa Cruz,) in 0.05 M TBS/NaN3 buffer (0.02% Triton X-100, 0.02% NaN3, 0.1% gelatin, pH 7.6) with 1% non-immune donkey serum.
The following day, sections were rinsed in 0.05M TBS/NaN3 buffer, incubated for 90 min in 0.05M TBS/NaN3 buffer containing the secondary antibodies Alexa Flour 647 donkey anti-goat IgG (1:100, A-21447, Invitrogen) used to visualize PEP, donkey anti-rabbit IgG (1:100, A21207, Invitrogen) used to visualize TOP, and Alexa Flour 488 donkey anti-rat IgG (1:100, A-21208, Invitrogen) used to visualize ERα. Sections were rinsed in 0.05M TBS/NaN3 buffer, stained for 30 min with the nucleic acid stain, DAPI (1:100,000, D3571, Invitrogen), and then rinsed in 0.05 M TBS buffer. Sections were mounted on gelatin-coated slides, left to dry, cleared in distilled water and coverslipped with Gel/Mount mounting medium (Clear Mount, cat# 17985-12, Electron Microscopy Sciences, Hatfield, PA.).
Controls for immunohistochemistry included the omission of primary or secondary antibodies. Furthermore, the PEP antibody was preadsorbed with the respective immunogen (ab50437, Invitrogen). TOP and ERα antibody preadsorbtion controls were performed previously in our lab (Cyr et al., 2010).
2.5 Immunofluorescence imaging and analysis
Brain sections were matched to the ventromedial nucleus of the hypothalamus VMH (plate 46, following the mouse brain atlas; Paxinos et al., 2004) and one representative matched section per mouse was used. Immunofluorescent images of each matched brain region per mouse were captured at 200X magnification using a Leica TCS SP5 laser confocal microscope, equipped with HeNe 10mw/HeNe 2mw/Ar/UV 405 lasers that excite at 633 nm (PEP), 594 nm (TOP), 488 nm (ERα), and UV 405 (DAPI). Leica software was used to capture images and all images with scale bars were saved as TIFF files. A FieldMaster (Coherent) was used to monitor laser output in mV at time points throughout the experiment for standardization. For each brain region, sections from all animals were imaged on the same day using the same gain and offset settings for each laser with minimal laser power fluctuations.
Images were then analyzed using NIS-Elements AR 2.30 software by an investigator blind to treatment group. The scale generated from the confocal was used to convert pixels to microns in NIS element. A circular region of interest (ROI) was created for analysis of the VMH (29948.3 µm2) and cells identified within the ROI were restricted by circularity (0.2-1), area (> 35 µm) and intensity (>20 for TOP-immunoreactive cells, >30 for PEP-immunoreactive cell, and >1 for ERα-immunoreactive cells). For the intensity measure, an intensity value was assigned by the program for each pixel within a cell based on a 0-256 scale for each 8 bit TIFF file which provided an intensity value for each pixel in each cell. The sum of the intensity values for each cell in each region (optical integrated density) was then measured for each animal providing a measure of the optical integrated density of TOP, PEP, and ERα staining within the VMH. The intensity cut-off for each protein was determined as the minimum intensity above background staining. The number of cells immunoreactive for both ERα and TOP, as well as both ERα and PEP, in the VMH brain region were examined in control mice. To determine whether estradiol alters TOP and/or PEP expression, the number of immunoreactive cells, total area of immunoreactivity (total area of immunoreactive cells (µm2) within the ROI), and total intensity (total optical integrated density) for TOP/PEP within the ROI was measured in the brain area of interest for EB and control mice.
2.6 Statistical analyses
JMP 7.0 (SAS Institute Inc., Cary, NC) was used for all statistical analyses. A two-way t-test was used to analyze differences between groups for each experiment. A Shapiro-Wilk W goodness of fit test was used to test for normal distributions and Levene’s test was used to test for homogenous variances in all variables. For variables with non-normal distributions and unequal variances, a nonparametric Mann-Whitney U test was used. All means are presented ± their standard errors, and p < 0.05 was considered statistically significant.
3. Results and discussion
3.1 Effect of estradiol treatment on enzyme activity
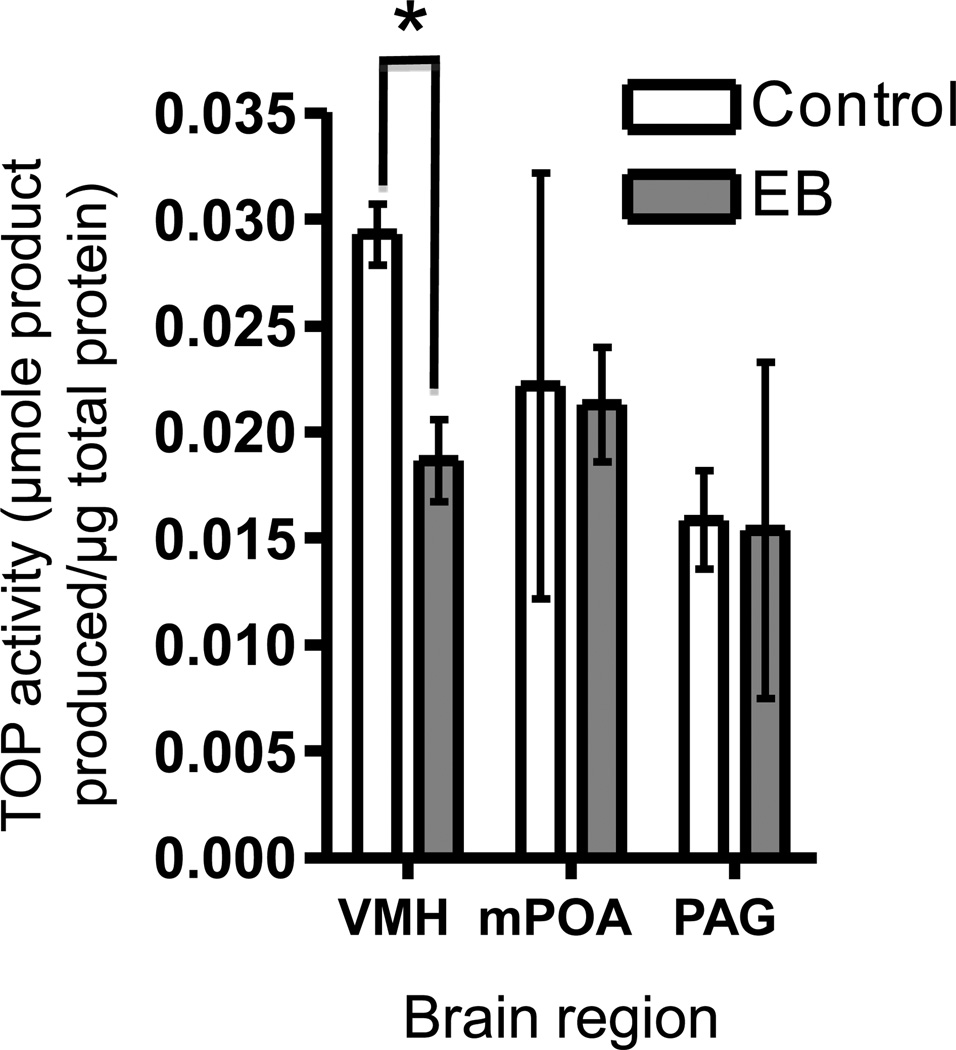
As seen in Figure 1, TOP enzyme activity was significantly decreased in the VMH of animals treated with estradiol benzoate but not changed in other brain regions by hormone treatment. These results are consistent with previously published immunoreactivity data and correspond to the decrease in TOP-immunoreactive cells in the VMH observed in our earlier studies (Cyr et al., 2010). Because the VMH is the site of the most significant change in both immunoreactivity and enzymatic activity, we focused on PEP regulation in this brain area.
Figure 1.
TOP enzyme activity in the VentroMedial Nucleus of the hypothalamus (VMH), Medial Preoptic Area (mPOA), and Periaqueductal Grey (PAG) from female mice treated with estradiol benzoate (EB) or oil control. Asterisk indicates significant difference (p<0.05). Data presented as the mean +/− SE.
3.2 Parallel regulation of TOP and PEP immunoreactivity in the VMH
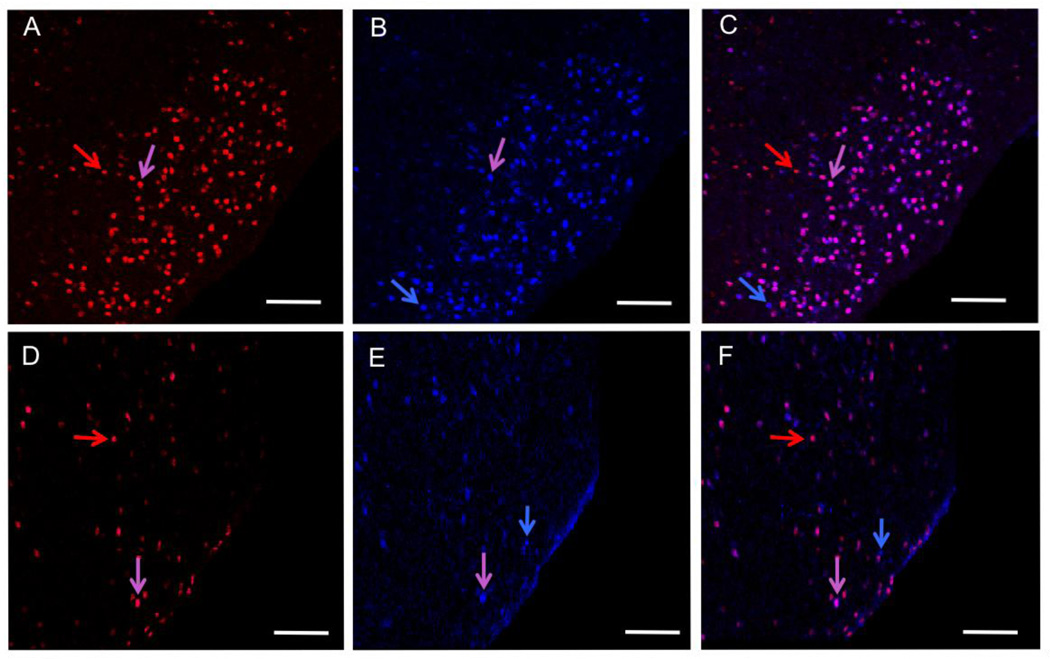
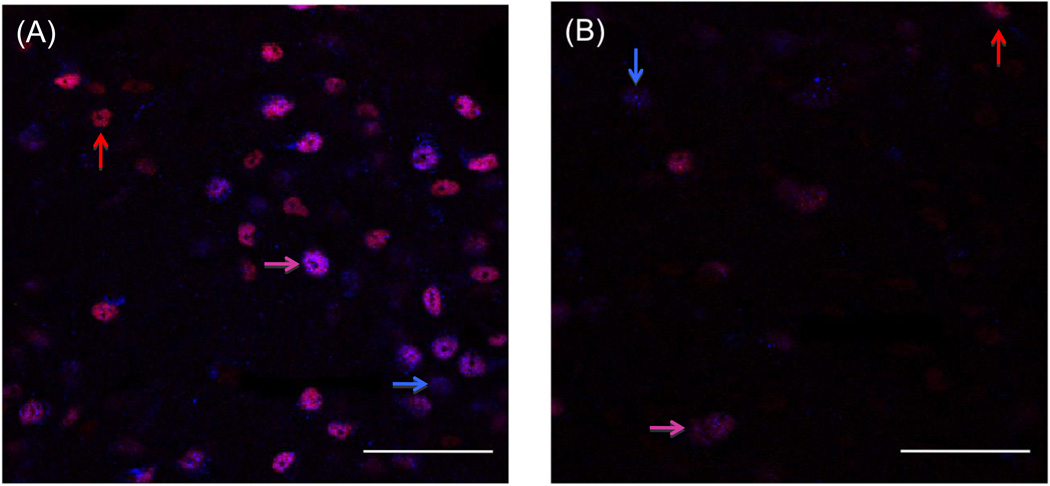
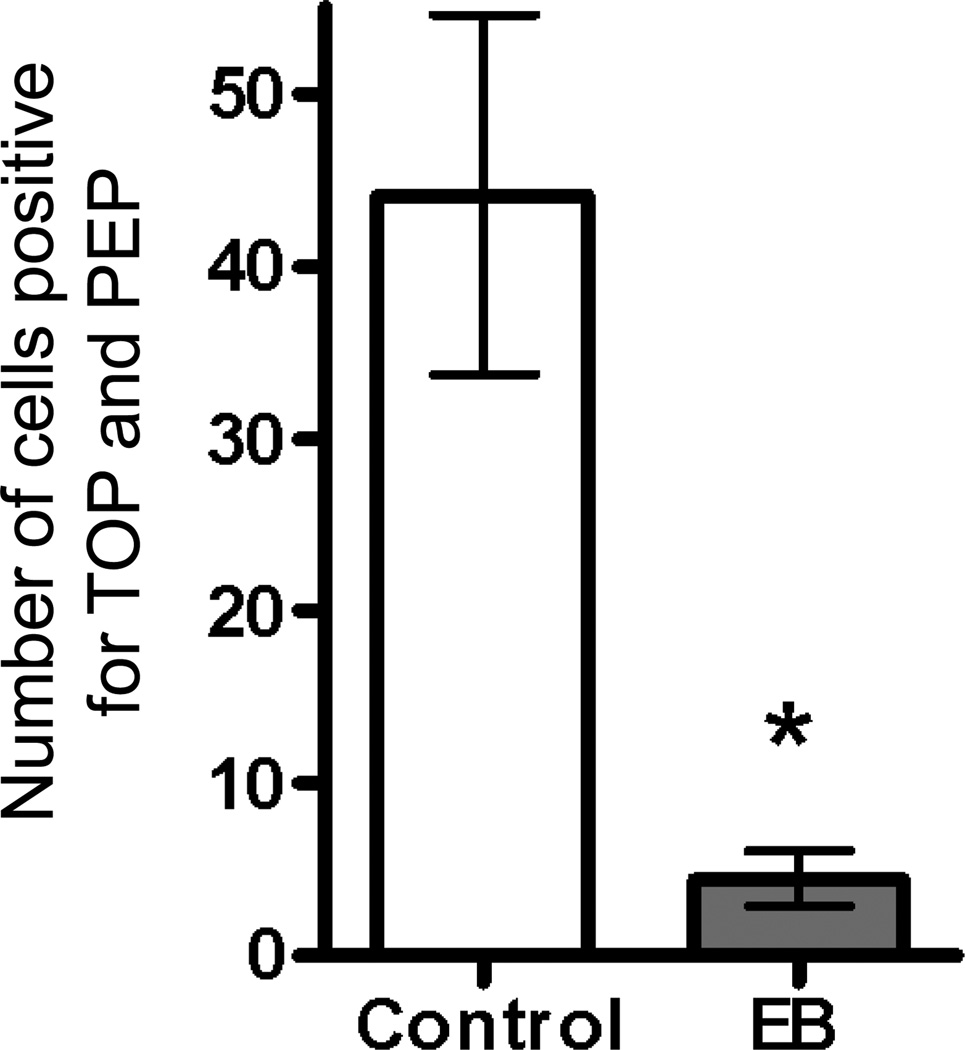
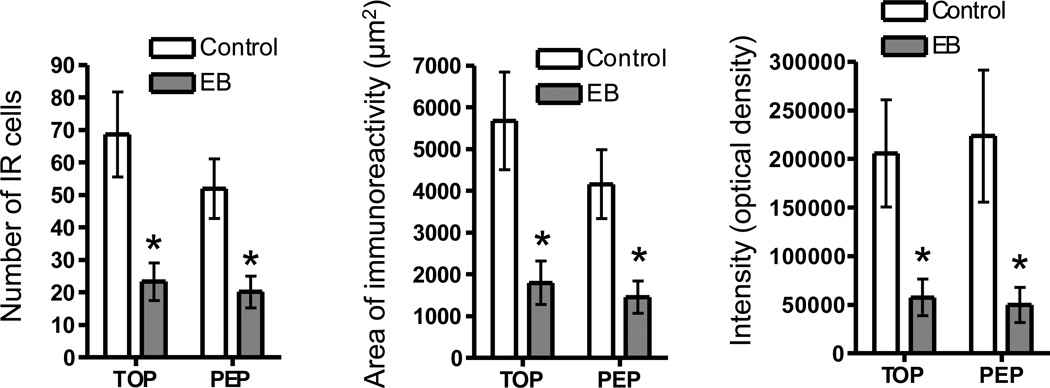
Cells in the ER-rich caudal region of the ventromedial hypothalamic nucleus (VMH) express both TOP and PEP, and these levels are decreased by estradiol treatment (Figures 2 and 3). For both TOP and PEP, estradiol treatment decreased the number of immunoreactive cells (a), the area of immunoreactivity (b), and total intensity (optical density) (c) in the VMH (Figure 2). Figure 5 demonstrates the large decrease in peptidase levels in the ventromedial hypothalamic nucleus after treatment with estradiol. The number of cells immunoreactive for TOP and PEP after estradiol treatment is approximately one-tenth the number for control cells.
Figure 2.
Cells in the ventromedial hypothalamic nucleus (VMH) coexpress TOP (red) and PEP (blue) from a control animal (A–C) and an estradiol-treated animal (D–F). TOP alone (A, D), PEP alone (B, E) and merged image of cells expressing both TOP and PEP (C, F). Cells expressing TOP only (red arrows), PEP only (blue arrows) and both TOP and PEP (pink arrows). Images were taken at 200X magnification. Scale bars = 100 µm.
Fig. 3.
Cells in the ventromedial hypothalamic nucleus (VMH) stained for TOP (red) and PEP (blue) from a control animal (A) and an estradiol- treated animal, (B). Co-expressed cells are pink for both treatment groups. Images were taken at 800x magnification, scale bars 50 µm.
Fig. 5.
Number of cells coexpressing thimet oligopeptidase (TOP) and prolyl endopeptidase (PEP) in VMH of female mouse brain treated with (shaded bar) and without (open bar) estradiol treatment. * indicates significance (p< 0.05)
Although intensity of confocal images may not provide an absolute quantity of a particular protein, it may be used for comparing relative amounts (Bogusz et al, 2008). The data clearly demonstrate the number of cells expressing each of the peptidases, as well as the number co-expressing TOP and PEP, is lower with estradiol treatment.
The present results are consistent with previous results for TOP (Cyr et al., 2010) and indicate that the two enzymes are present in the same cells, are subject to coordinated actions, and are affected by the presence of steroid hormones in specific brain regions. Our particular focus, the VMH, is of interest because this subregion contains ERα and controls female sexual behavior. The decreased levels of PEP and TOP when estradiol levels are high should result in elevated GnRH levels and decreased levels of the metabolite GnRH1-5. Our findings suggest that estradiol regulation of TOP and PEP contribute to the fine-tuning of GnRH levels and subsequent LH and FSH secretion following the estradiol surge.
Previous studies, using both enzymatic methods and immunohistochemistry, have established that both TOP and PEP are found in high concentrations in brain (Ferro et al., 2004; Myöhänen et al., 2008). The present study demonstrates that the two enzymes are co-expressed in the same hypothalamic cells. Lew et al. (2007) posited a two-step mechanism for breakdown of GnRH by PEP and TOP but did not establish the cellular location for the pathway. Earlier results, along with those from the present study indicate that 79.7%±5.88 of cells from untreated controls that were immunoreactive for ERα also expressed TOP and PEP (Cyr et al, 2010; Wolfson et al, in press). That is, ERα is found in the same cells as TOP and PEP, providing neuroanatomical evidence for a direct mechanism for estradiol regulation of enzyme levels and activity. Given that estradiol treatment is known to interfere with binding of the ERα antibody used in the present study (Blaustein et al., 1992), it is likely that the number of ERα containing cells identified in estradiol-treated animals here are an underestimate compared to that of the untreated controls.
Our findings do not address the question of how enzyme levels of TOP and PEP could affect GnRH levels in other areas of the hypothalamus. There are few GnRH neurons in the VMH, and we saw no change in peptidase levels with estradiol treatment in the MPOA, where most GnRH neurons are found. It is possible that the median eminence (ME) is a potential site for TOP and PEP to influence GnRH levels, which will be important to investigate in future studies. It has been reported in other species that direct projections from VMH neurons to GnRH neurons of the POA exist (Goubillon et l, 2002), but this is controversial (Pompolo et al, 2001). Additionally, there is some evidence that TOP is found in nerve terminals (Yamamoto et al, 2003), so that the peptidases themselves might be transported, rather than their substrates. Exogenous GnRH administered into the rodent VMH does affect lordosis (Dudley and Moss, 1988), so that peptidases in this brain region are relevant to female sexual behavior.
In this and previous studies (Cyr et al., 2010), we report that both TOP and PEP expression and TOP enzyme activity are decreased by estradiol administration in a brain-region specific manner. In future experiments it will be important to extend the findings with more quantitative measures of protein levels. Although PEP and TOP cooperate in hydrolysis of GnRH, and may function separately or in concert in protection from amyloid toxicity (Arif et al., 2009), each has distinct roles in other physiological processes in the brain as well as peripherally. TOP may act in brain to play a role in lordosis in female rodents (Wu et al., 2009), is implicated in neurotensin inactivation (Ferro et al., 2004), and its central role in bradykinin breakdown may extend to regulation of the cerebral microvasculature (Norman et al., 2001). PEP is thought to contribute to control of the cell cycle, learning, memory, mood, and several neurodegenerative diseases (Männisto et al., 2007; Rossner et al., 2005). As reviewed in Garcia-Horseman et al. 2007 (2007), the function and regulation of PEP depends on its location, and functions of PEP may not depend solely on its enzymatic hydrolytic activity (Myöhänen et al., 2008). Therefore, it is not surprising that we observe coordinate regulation of the two enzymes in a distinct subpopulation of hypothalamic cells. In fact, the target substrate may be a peptide other than GnRH, since both peptidases act on other, reproductively relevant, peptides such as neurotensin (Oliveria et al, 2001). The results presented in this study add neuroanatomical evidence to the growing case that peptidases, particularly TOP, may play a role in regulation of reproductive function, in both the central nervous system and periphery.
Figure 4.
TOP-IR and PEP-IR analysis of number of cells, area of immunoreactivity, and intensity of staining (optical density) for VMH brain region in female mice with either oil (control) or estradiol benzoate (EB). Asterisk indicates significant difference (p<.05). Data presented as the mean +/− SE.
Acknowledgments
We are grateful to Marc Glucksman (Rosalind Franklin School of Medicine) for the gifts of purified TOP and TOP antibody. Kelsey Diederich provided technical assistance, and Patricia Carey and Valerie LePage provided expert assistance with animal care and surgery. This work was supported by Wellesley College (Brachman Hoffman Fund) and by institutional grants from the Howard Hughes Medical Institute and NSF-REU to Wellesley College and the National Institute of Diabetes and Digestive and Kidney Diseases, R01DK061935 (MJT)
Abbreviations
- TOP
thimet oligopeptidase
- PEP
prolyl endopeptidase
- ERα
estrogen receptor alpha
- GnRH
Gonadotropin-Releasing Hormone
- VMH
ventromedial nucleus of the hypothalamus
- HPG
Hypothalamic-pituitary-gonadal
- EB
Estradiol benzoate
- IR
Immunoreactivity
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Arif M, Chikuma T, Ahmed MM, Nakazato M, Smith MA, Kato T. Effects of memantine on soluble Alphabeta(25–35)-induced changes in peptidergic and glial cells in Alzheimer's disease model rat brain regions. Neuroscience. 2009;164:1199–1209. doi: 10.1016/j.neuroscience.2009.08.063. [DOI] [PubMed] [Google Scholar]
- Blaustein JD. Cytoplasmic estrogen receptors in rat brain: immunocytochemical evidence using three antibodies with distinct epitopes. Endocrinology. 1992;131:1336–1342. doi: 10.1210/endo.131.3.1380440. [DOI] [PubMed] [Google Scholar]
- Bogusz AL, Hardy SL, Lehman MN, Connors JM, Hileman SM, Sliwowska JH, Billings HJ, McManus CJ, Valent M, Singh SR, Nestor CC, Coolen LM, Goodman RL. Evidence that gamma-aminobutyric acid is part of the neural circuit mediating estradiol negative feedback in anestrous ewes. Endocrinology. 2008;149:2762–2772. doi: 10.1210/en.2007-1362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandt I, Scharpé S, Lambeir AM. Suggested functions for prolyl oligopeptidase: a puzzling paradox. Clin Chim Acta. 2007;377:50–61. doi: 10.1016/j.cca.2006.09.001. [DOI] [PubMed] [Google Scholar]
- Chu TG, Orlowski M. Soluble metalloendopeptidase from rat brain: action on enkephalin-containing peptides and other bioactive peptides. Endocrinology. 1985;116:1418–1425. doi: 10.1210/endo-116-4-1418. [DOI] [PubMed] [Google Scholar]
- Cleverly K, Wu TJ. Is the metalloendopeptidase EC 3.4.24.15 (EP24.15), the enzyme that cleaves luteinizing hormone-releasing hormone (LHRH), an activating enzyme? Reproduction. 2010;139:319–330. doi: 10.1530/REP-09-0117. [DOI] [PubMed] [Google Scholar]
- Cyr NE, Kua LH, Bruce LA, Chadwick JG, Tetel MJ, Wolfson AJ. Nuclear Thimet oligopeptidase is coexpressed with oestrogen receptor alpha in hypothalamic cells and regulated by oestradiol in female mice. J Neuroendocrinol. 2010;22:936–943. doi: 10.1111/j.1365-2826.2010.02009.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudley CA, Moss RL. Facilitation of lordosis in female rats by CNS-site specific infusions of an LH-RH fragment, Ac-LH-RH-(5–10) Brain Res. 1988;441:161–167. doi: 10.1016/0006-8993(88)91394-7. [DOI] [PubMed] [Google Scholar]
- Ferro ES, Carreno FR, Goni C, Garrido PA, Guimaraes AO, Castro LM, Oliveira V, Araujo MC, Rioli V, Gomes MD, Fontenele-Neto JD, Hyslop S. The intracellular distribution and secretion of endopeptidases 24.15 (EC 3.4.24.15) and 24.16 (EC 3.4.24.16) Protein Pept Lett. 2004;11:415–421. doi: 10.2174/0929866043406706. [DOI] [PubMed] [Google Scholar]
- García-Horsman JA, Männistö PT, Venäläinen JI. On the role of prolyl oligopeptidase in health and disease. Neuropeptides. 2007;41:1–24. doi: 10.1016/j.npep.2006.10.004. [DOI] [PubMed] [Google Scholar]
- Goubillon ML, Caraty A, Herbison AE. Evidence in favour of a direct input from the ventromedial nucleus to gonadotropin-releasing hormone neurones in the ewe: an anterograde tracing study. J Neuroendocrinol. 2002;14:95–100. doi: 10.1046/j.0007-1331.2001.00739.x. [DOI] [PubMed] [Google Scholar]
- Lew RA, Cowley M, Clarke IJ, Smith AI. Peptidases that degrade gonadotropin-releasing hormone: influence on LH secretion in the ewe. J Neuroendocrinol. 1997;9:707–712. doi: 10.1046/j.1365-2826.1997.00628.x. [DOI] [PubMed] [Google Scholar]
- Lew RA, Tetaz TJ, Glucksman MJ, Roberts JL, Smith AI. Evidence for a two-step mechanism of gonadotropin-releasing hormone metabolism by prolyl endopeptidase and metalloendopeptidase EC 3.4.24.15 in ovine hypothalamic extracts. J Biol Chem. 1994;269:12626–12632. [PubMed] [Google Scholar]
- Massarelli EE, Casatti CA, Kato A, Camargo AC, Bauer JA, Glucksman MJ, Roberts JL, Hirose S, Ferro ES. Differential subcellular distribution of neurolysin (EC 3.4.24.16) and thimet oligopeptidase (EC 3.4.24.15) in the rat brain. Brain Res. 1999;851:261–265. doi: 10.1016/s0006-8993(99)02135-6. [DOI] [PubMed] [Google Scholar]
- Myöhänen TT, Venäläinen JI, Garcia-Horsman JA, Piltonen M, Männistö PT. Cellular and subcellular distribution of rat brain prolyl oligopeptidase and its association with specific neuronal neurotransmitters. J Comp Neurol. 2008;507:1694–1708. doi: 10.1002/cne.21642. [DOI] [PubMed] [Google Scholar]
- Myöhänen TT, Venäläinen JI, García-Horsman JA, Piltonen M, Männistö PT. Distribution of prolyl oligopeptidase in the mouse whole-body sections and peripheral tissues. Histochem Cell Biol. 2008;130:993–1003. doi: 10.1007/s00418-008-0468-x. [DOI] [PubMed] [Google Scholar]
- Männisto PT, Venäläinen J, Jalkanen A, García-Horsman JA. Prolyl oligopeptidase: a potential target for the treatment of cognitive disorders. Drug News Perspect. 2007;20:293–305. doi: 10.1358/dnp.2007.20.5.1120216. [DOI] [PubMed] [Google Scholar]
- Norman MU, Smith AI, Hickey MJ. Metalloendopeptidases EC 3.4.24.15 and EC 3.4.24.16: potential roles in vascular physiology. Letters in Peptide Science. 2001;8:195–199. [Google Scholar]
- Oliveira V, Campos M, Hemerly JP, Ferro ES, Camargo ACM, Juliano MA, Juliano L. Selective neurotensin-derived internally quenched fluorogenic substrates for neurolysin (EC 3.4.24.16): Comparison with thimet oligopeptidase (EC 3.4.24.15) and neprilysin (EC 3.4.24.11) % J Analytical Biochemistry. 2001;292:257–265. doi: 10.1006/abio.2001.5083. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Franklin KBJ. The Mouse Brain in Steriotaxic Coordinates (Second ed.) Boston: Academic Press; 2004. [Google Scholar]
- Pierotti AR, Lasdun A, Ayala JM, Roberts JL, Molineaux CJ. Endopeptidase-24.15 in rat hypothalamic/pituitary/gonadal axis. Mol Cell Endocrinol. 1991;76:95–103. doi: 10.1016/0303-7207(91)90264-s. [DOI] [PubMed] [Google Scholar]
- Pompolo S, Rawson JA, Clarke IJ. Projections from the arcuate/ventromedial region of the hypothalamus to the preoptic area and bed nucleus of stria terminalis in the brain of the ewe; lack of direct input to gonadotropin-releasing hormone neurons. Brain Res. 2001;904:1–12. doi: 10.1016/s0006-8993(01)02372-1. [DOI] [PubMed] [Google Scholar]
- Roberts JL, Mani SK, Woller MJ, Glucksman MJ, Wu TJ. LHRH-(1–5): a bioactive peptide regulating reproduction. Trends Endocrinol Metab. 2007;18:386–392. doi: 10.1016/j.tem.2007.09.005. [DOI] [PubMed] [Google Scholar]
- Rossner S, Schulz I, Zeitschel U, Schliebs R, Bigl V, Demuth HU. Brain prolyl endopeptidase expression in aging, APP transgenic mice and Alzheimer's disease. Neurochem Res. 2005;30:695–702. doi: 10.1007/s11064-005-6863-y. [DOI] [PubMed] [Google Scholar]
- Shrimpton CN, Smith AI, Lew RA. Soluble metalloendopeptidases and neuroendocrine signaling. Endocr Rev. 2002;23:647–664. doi: 10.1210/er.2001-0032. [DOI] [PubMed] [Google Scholar]
- Wolfson AJ, Cyr NE, Bruce LA, Qiao JW, DeFries CC, Tetel MJ. The significance of oestrogen receptor expression in breast and oesophageal cancers (GC Chen, ed) Hauppauge NY: Nova Science Publishers; Regulation of neuropeptidases involved in reproductive physiology by estradiol. (in press) [Google Scholar]
- Wolfson AJ, Shrimpton CN, Lew RA, Smith AI. Differential activation of endopeptidase EC 3.4.24.15 toward natural and synthetic substrates by metal ions. J Biochemical and Biophysical Research Communications. 1996;229:341–348. doi: 10.1006/bbrc.1996.1803. [DOI] [PubMed] [Google Scholar]
- Wu TJ, Pagano E, Mani SK. A biological role for the gonadotrophin-releasing hormone (GnRH) metabolite, GnRH-(1–5) J Neuroendocrinol. 2009;21:293–298. doi: 10.1111/j.1365-2826.2009.01854.x. [DOI] [PubMed] [Google Scholar]
- Wu TJ, Pierotti AR, Jakubowski M, Sheward WJ, Glucksman MJ, Smith AI, King JC, Fink G, Roberts JL. Endopeptidase EC 3.4.24.15 presence in the rat median eminence and hypophysial portal blood and its modulation of the luteinizing hormone surge. J Neuroendocrinol. 1997;9:813–822. doi: 10.1046/j.1365-2826.1997.00637.x. [DOI] [PubMed] [Google Scholar]
- Yamamoto M, Chikuma T, Yamashita A, Yamaguchi M, Hojo H, Ozeki Y, Ahmed M, Kato T. Anterograde axonal transport of endopeptidase 24.15 in rat sciatic nerves. Neurochem Int. 2003;42:231–237. doi: 10.1016/s0197-0186(02)00092-x. [DOI] [PubMed] [Google Scholar]