Highlights
▸ Event related potential (ERP) and behavioral measures of emotional reactivity. ▸ Smaller ERP effects and no significant behavior effect for emotional faces. ▸ Increased ERPs and slower reaction times for emotional images. ▸ Enhanced ERP effect over occipital sites for younger children.
Keywords: Late positive potential, Event-related potentials, Emotion, Childhood, Adolescence
Abstract
The late positive potential (LPP) is an event-related potential (ERP) component that indexes sustained attention toward motivationally salient information. The LPP has been observed in children and adults, however little is known about its development from childhood into adolescence. In addition, whereas LPP studies examine responses to images from the International Affective Picture System (IAPS; Lang et al., 2008) or emotional faces, no previous studies have compared responses in youth across stimuli. To examine how emotion interacts with attention across development, the current study used an emotional-interrupt task to measure LPP and behavioral responses in 8- to 13-year-olds using unpleasant, pleasant, and neutral IAPS images, as well as sad, happy, and neutral faces. Compared to older youth, younger children exhibited enhanced LPPs over occipital sites. In addition, sad but not happy faces elicited a larger LPP than neutral faces; behavioral measures did not vary across facial expressions. Both unpleasant and pleasant IAPS images were associated with increased LPPs and behavioral interference compared to neutral images. Results suggest that there may be developmental differences in the scalp distribution of the LPP, and compared to faces, IAPS elicit more robust behavioral and electrocortical measures of attention to emotional stimuli.
1. Introduction
Because emotions are multi-faceted constructs, psychophysiological measures can be useful in highlighting processes that may not be observable through behavioral or subjective measures (Larsen et al., 2008, Santerre and Allen, 2007). In particular, event-related potentials (ERPs) are especially useful for studying early neural responses to emotional stimuli across development because they are non-invasive, temporally sensitive and can be reliably measured across childhood (Banaschewski and Brandeis, 2007, Nelson and McCleery, 2008). The late positive potential (LPP) is an ERP component that indexes sustained attention toward, and elaborative processing of, emotional stimuli (Cuthbert et al., 2000, Hajcak et al., 2011, Olofsson et al., 2008). The LPP may provide insight into the ways in which emotional processing interacts with attention across both typical and atypical development. However, in order to apply ERP methods to understanding these processes from a developmental psychopathology perspective, it is first necessary to identify factors that influence behavioral and LPP measures of emotional processing across typical development.
1.1. Late positive potential
Numerous studies have examined the LPP as a measure of emotional reactivity in adults. The LPP is a sustained ERP component that begins approximately 200 ms after stimulus onset and continues throughout stimulus duration and even beyond offset (Cuthbert et al., 2000, Hajcak and Olvet, 2008). The LPP is enhanced for emotional compared to neutral stimuli and appears to reflect selective and sustained attention toward emotional stimuli, and activation of motivational systems that respond to salient information (Cuthbert et al., 2000, Foti et al., 2009, Hajcak et al., 2011, Olofsson et al., 2008). It has been linked to activation in the extrastriate visual system, which is likely to form a feedback loop with the neural circuitry underlying motivational systems, including the amygdala (Lang and Bradley, 2010, Sabatinelli et al., 2007). In addition, recent work in adults suggests that emotional processing, as indexed by the LPP, is associated with stronger connectivity between occipito-parietal cortex and frontal networks relative to the processing of neutral information (Moratti et al., 2011).
In adults, many studies of the LPP have used images from the International Affective Picture System (IAPS; Lang et al., 2008; e.g., Cuthbert et al., 2000, Foti et al., 2009, Weinberg and Hajcak, 2010); however, the LPP has also been observed in response to emotional faces (e.g., Schupp et al., 2004), words (e.g., Naumann et al., 1992), and even gestures (Flaisch et al., 2011). Images from IAPS can be grouped into broad categories of unpleasant, pleasant and neutral. Though IAPS images differ in visual complexity, ranging from simple figure-ground images to complex scenes, there is evidence that the LPP is more sensitive to motivational salience than perceptual organization (Bradley et al., 2007).
Although many studies have used passive viewing paradigms to examine electrocortical reactivity to emotional images in children and adults (e.g., Foti et al., 2009, Hajcak and Dennis, 2009, Kujawa et al., 2012, Weinberg and Hajcak, 2010), paradigms that incorporate active responses allow for the simultaneous examination of electrocortical and behavioral measures. For example, the emotional interrupt paradigm involves the presentation of an emotional image both before and after a target that the participant must categorize (Mitchell et al., 2006). Though ERPs are typically only examined in response to the pre-target image, presenting the emotional image before and after the target temporally surrounds the target by an emotional distracter. Trials involving emotional images (both pleasant and unpleasant) have been associated with slower reaction times to the target. In addition, recent work links delayed response times in this paradigm with enhanced LPPs (Weinberg and Hajcak, 2011), suggesting that sustained attention to the emotional stimulus, as measured by the LPP, interferes with subsequent processing of the target.
1.2. Development of the LPP
The LPP has been identified in response to IAPS images in early to middle childhood (Hajcak and Dennis, 2009) and in response to emotional faces in a large sample of 6-year-old children (Kujawa et al., 2012). Though there is some evidence of developmental changes in the LPP, the nature and timing of the changes remain unclear. The LPP in adults is typically apparent over centro-parietal electrode sites, whereas early findings suggest that the LPP in early to middle childhood may be maximal over more occipital sites (Hajcak and Dennis, 2009, Kujawa et al., 2012). In addition, there is some evidence of a developmental shift in the scalp distribution of LPPs in response to IAPS images, with younger adolescents showing enhanced positivities at occipital sites compared to older adolescents and young adults (Gao et al., 2010). Evidence from functional magnetic resonance imaging (fMRI) studies suggests that the neural circuitry underlying emotional processing may change from childhood to adolescence, with a shift from enhanced processing in subcortical regions to greater involvement of the prefrontal cortex (Monk, 2008, Yurgelun-Todd and Killgore, 2006). Nonetheless, the ways in which developmental changes in cortical processing of emotional information translate to ERP measures remain unclear. Thus, the current study examined the LPP and behavioral measures in both middle childhood and adolescence to identify potential developmental changes. In addition, though both emotional faces and images from IAPS are commonly used to measure the LPP, the ways in which different types of emotional information influence attention in children and adolescents are not well understood. Among adults, there is evidence that IAPS images are more arousing than emotional faces (Britton et al., 2006), suggesting that IAPS images may also be associated with enhanced LPPs compared to emotional faces. However, previous studies have not directly compared electrocortical responses to, or behavioral interference from, different sets of emotional images.
1.3. Current study
In order to evaluate the ways in which emotional stimuli interfere with attention across development, the current study examined behavioral and electrocortical reactivity to emotional faces and IAPS images in middle childhood (ages 8–10) and late childhood/early adolescence (ages 11–13). The first goal was to examine developmental changes in the scalp distribution and magnitude of the LPP from childhood to early adolescence. Based on previous work, we hypothesized that younger children would show LPPs over more occipital sites compared to older children/adolescents. The second goal was to compare electrocortical reactivity and behavioral interference effects across stimuli to determine the extent to which emotional faces and IAPS images elicit LPPs and are associated with behavioral interference in children and adolescents. We hypothesized that emotional IAPS images would be associated with a larger LPP and greater behavioral interference than emotional faces.
2. Methods
2.1. Participants
Participants were recruited from Stony Brook and the surrounding community using a commercial mailing list. Sixty-seven youth between the ages of 8 and 13 participated in the study. For the faces version of the task, data were excluded for 3 participants due to poor EEG data quality, 5 participants due to poor behavioral performance (accuracy below 75%), and 1 participant due to a technical error. Therefore, data from 58 children were included in analyses of the faces version of the task. For the IAPS version, 9 parents refused participation due to the nature of the images. In addition, data were excluded for 1 participant due to poor EEG data quality, 3 participants due to low accuracy, and 1 participant due to a technical error. Thus, data for 53 children were included in analyses of the IAPS version. For analyses focusing on a single task, all children with useable data on that task were included. A total of 51 children had useable data for both tasks and were included in analyses across tasks. Mean age and racial/ethnic distribution for each set of analyses are presented in Table 1.
Table 1.
Demographics and behavioral results for children included in analyses of the faces and IAPS versions of the task.
| Faces version (N = 58) | IAPS version (N = 53) | |
|---|---|---|
| M (SD) | M (SD) | |
| Age | 10.81 (1.33) | 10.96 (1.53) |
| Faces version (N = 58) | IAPS version (N = 53) | |
|---|---|---|
| % | % | |
| Caucasian | 86.2 | 84.9 |
| African-American | 1.7 | 1.9 |
| Asian-American | 5.2 | 5.7 |
| Hispanic/Latino | 6.9 | 7.5 |
| Age 10 and under | 43.1 | 37.7 |
| Faces version (N = 58) | IAPS version (N = 53) | ||
|---|---|---|---|
| M (SD) | M (SD) | ||
| Neutral faces RT | 576.00 (205.84) | Neutral IAPS RT | 571.55 (187.48) |
| Sad faces RT | 572.78 (205.82) | Unpleasant IAPS RT | 587.03 (192.52) |
| Happy faces RT | 573.16 (202.04) | Pleasant IAPS RT | 583.73 (188.97) |
| Neutral faces accuracy | 95.35 (4.47) | Neutral IAPS accuracy | 95.19 (5.28) |
| Sad faces accuracy | 95.40 (4.58) | Unpleasant IAPS accuracy | 93.26 (5.41) |
| Happy faces accuracy | 96.10 (3.72) | Pleasant IAPS accuracy | 94.76 (4.34) |
2.2. Emotional-interrupt task
2.2.1. Faces version
The task was administered using Presentation software (Neurobehavioral Systems, Inc.).
A total of 60 emotional face images1 were selected from the Karolinska dataset (Lundqvist et al., 1998). Twenty actors (10 males, 10 females) were selected, each with an image of a happy expression, neutral expression, and sad expression. Each image was randomly presented once in each of two blocks for a total of 120 trials. Each trial began with an 800 ms fixation (+), then an image was presented for 1000 ms followed by a target (< or >) presented for 150 ms and the same picture presented for an additional 400 ms. The target was an arrow pointed to the left or to the right, and participants were required to press either the left or right button on a mouse to indicate the direction of the arrow. The intertrial interval varied randomly between 1500 and 2000 ms.
2.2.2. IAPS version
A total of 60 developmentally appropriate pictures were selected from the IAPS (Lang et al., 2008). Of these, 20 depicted pleasant scenes (e.g., children playing, cute animals, babies), 20 depicted neutral scenes (e.g., people in neutral situations, neutral outdoor scenes, household objects), and 20 depicted unpleasant scenes (e.g., sad or angry people, weapons, aggressive animals).2 Other than the stimuli used, the IAPS version of the task was identical to the faces version described above.
2.3. Procedure
After assent was obtained from child participants and written informed consent was obtained from parents, electroencephalograph (EEG) sensors were attached and participants completed a total of four tasks, followed by an additional paradigm measuring the startle reflex. The current study focuses only on data from the emotional-interrupt paradigm, and the results of other tasks administered during the same experimental session are presented elsewhere (Bress et al., 2012, Glenn et al., 2011, Meyer et al., 2012). All tasks were counterbalanced, such that the order of the tasks varied across participants, and all possible combinations of orders were presented; however, the two versions of the emotional-interrupt task always followed one another. At the start of each emotional-interrupt task, the participant was instructed to press the left or right button on a mouse to indicate the direction of the arrow on the screen. Prior to the start of each task, participants completed 10 practice trials.
2.4. Psychophysiological recording, data reduction and analysis
Throughout the task, the continuous EEG was recorded using a 34-channel Biosemi system based on the 10/20 system (32 channel cap with the addition of Iz and FCz). Two electrodes were placed on the left and right mastoids, and the electrooculogram (EOG) generated from eye blinks and movements was recorded from four facial electrodes: two approximately 1 cm above and below the participant's left eye, one approximately 1 cm to the left of the left eye and one approximately 1 cm to the right of the right eye. The ground electrode during acquisition was formed by the Common Mode Sense active electrode and the Driven Right Leg passive electrode. The data were digitized using ActiView software at 24-bit resolution with a LSB value of 31.25 nV and a sampling rate of 1024 Hz, using a low-pass fifth order sinc filter with a half-power cutoff of 204.8 Hz. Off-line analysis was performed using Brain Vision Analyzer software (Brain Products). All data were converted to a mastoid reference and band-pass filtered with cutoffs of 0.1 and 30 Hz. The EEG was segmented for each trial, beginning 200 ms before each picture onset and continuing for 1000 ms after onset. The EEG was corrected for eye blinks using the method developed by Gratton et al. (1983). Artifact rejection was completed using semi-automated procedures and the following criteria: a voltage step of more than 50 μV between sample points, a voltage difference of 300 μV within a trial, and a maximum voltage difference of less than 0.5 μV within 100 ms intervals. Visual inspection was then used to reject trials in which additional artifacts were observed.
ERPs were constructed by separately averaging the picture types (i.e., happy, neutral, and sad faces; pleasant, neutral, and unpleasant IAPS). Only correct trials with responses between 150 and 2100 ms after target onset were included in average (see average accuracy rates in Table 1). For each ERP average, the average activity in the 200 ms window prior to picture onset served as the baseline. The LPP was scored as the mean activity 400 and 1000 ms after the onset of the pre-target image averaged across midline parietal (P3, P4, and Pz) and occipital sites (O1, O2, and Oz). Behavioral analyses were calculated with each participant's mean reaction time (RT) for correct trials and overall accuracy for each emotion type. Responses with RTs quicker than 150 ms or slower than 2100 ms after target onset were counted as errors. For analyses comparing responses across tasks, data from 51 participants were available. To maximize power to detect effects, follow up analyses focusing on a single task included all available data for that task (i.e., 58 participants for faces and 53 participants for IAPS).
3. Results
3.1. Effects of task, valence, and age on the LPP
A 2(Task) × 3(Valence) × 2(Electrode pooling) × 2 (age 10 and under vs. over 10) mixed-design ANOVA was performed to examine the effects of task and stimulus valence at each set of parietal and occipital sites as a function of age. The main effects of task, F(1, 49) = 27.45, p < .001, ηp2 = .36, valence, F(1, 98) = 32.70, p < .001, ηp2 = .40, and electrode pooling, F(1, 49) = 355.53, p < .001, ηp2 = .88, were significant. In addition, the task × age, F(1, 49) = 10.42, p < .01, ηp2 = .18, electrode pooling × age, F(1, 49) = 14.00, p < .001, ηp2 = .22, task × valence, F(2, 98) = 13.32, p < .001, ηp2 = .21, task × electrode pooling, F(1, 49) = 68.48, p < .001, ηp2 = .58, task × electrode pooling × age, F(1, 49) = 25.50, p < .001, ηp2 = .34, valence × electrode pooling × age, F(2, 98) = 11.65, p < .001, ηp2 = .19, and task × valence × electrode pooling, F(1, 98) = 4.52, p < .05, ηp2 = .08, interactions were significant. These effects were qualified, however, by a significant four-way interaction, F(2, 98) = 5.42, p < .01, ηp2 = .10. The main effect of age, as well as the valence × age, task × valence × age, and valence × electrode pooling interactions were not significant.
To interpret the four-way interaction, 3(Valence) × 2(Electrode pooling) × 2(Age) ANOVAs were calculated for each task. For the faces version of the task, the main effect of face valence was significant, F(2, 112) = 3.95, p < .05, ηp2 = .07, as was the main effect of electrode pooling, F(1, 56) = 297.78, p < .001, ηp2 = .84, and the electrode pooling × age interaction, F(1, 56) = 9.13, p < .01, ηp2 = .14. The main effect of age and the face valence × age, face valence × electrode pooling, and face valence × electrode pooling × age interactions were not significant.
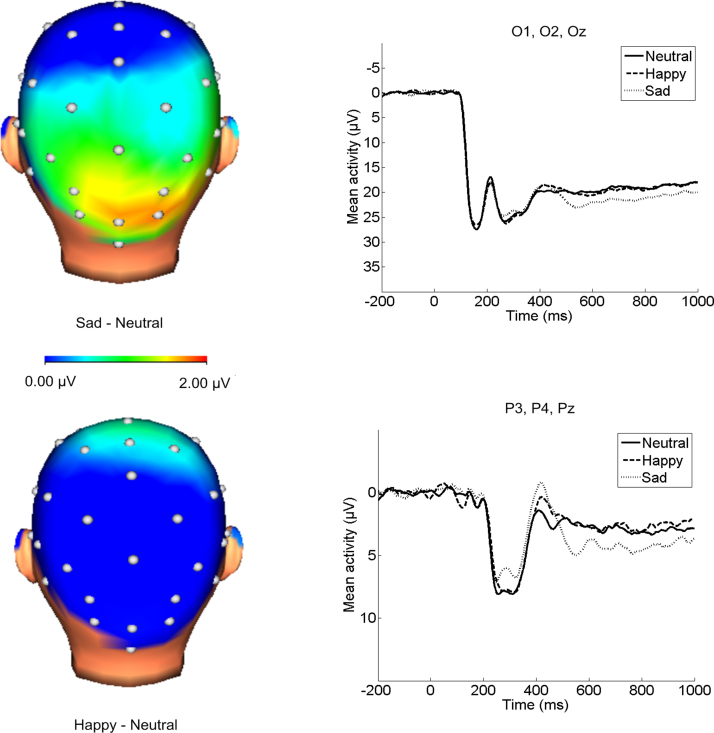
Paired samples t-tests comparing each emotional face type to neutral faces were calculated to interpret the main effect of face valence. Mean activity in response to sad faces was significantly more positive than neutral at occipital, t(57) = 2.74, p < .01, but not parietal sites, t(57) = 1.61, p = .11. No significant differences were found for happy compared to neutral faces at either occipital, t(57) = 0.11, p > .05, or parietal sites, t(57) = −0.54, p > .05 (Fig. 1).
Fig. 1.
Scalp distributions of the difference between emotional and neutral faces (400–1000 ms after stimulus onset), and ERPs (negative up) at occipital (top) and parietal (bottom) electrode sites. Sad faces significantly differed from neutral at occipital sites. No significant effects were found for happy faces. Note: ERPs at occipital sites are presented on a different scale than ERPs at parietal sites.
The electrode pooling × age interaction for the faces tasks indicated that younger children show increased reactivity (overall M = 21.39, SD = 10.87) compared to older children (overall M = 18.72, SD = 7.74) at occipital sites, while older children (overall M = 4.42, SD = 7.06) show greater activity at parietal sites compared to younger children (overall M = 1.01, SD = 5.16); however, the lack of a significant face valence × electrode pooling × age interaction indicates that this effect is not modulated by emotional valence. In addition, independent-samples t-tests indicated that the age groups significantly differed at parietal, t(56) = −2.03, p < .05, but not occipital sites, t(56) = 1.09, p > .05.
For the IAPS version of the task, the main effect of image valence was significant, F(2, 102) = 39.21, p < .001, ηp2 = .44, as was the main effect of electrode pooling, F(1, 51) = 400.22, p < .001, ηp2 = .89, the electrode pooling × age interaction, F(1, 51) = 23.85, p < .001, ηp2 = .32, and the valence × electrode pooling × age interaction, F(2, 102) = 13.68, p < .001, ηp2 = .21. The image valence × age, F(2, 102) = 2.70, p = .07, and image valence × electrode pooling interactions, F(2, 102) = 3.04, p = .052, did not reach significance. The main effect of age was not significant.
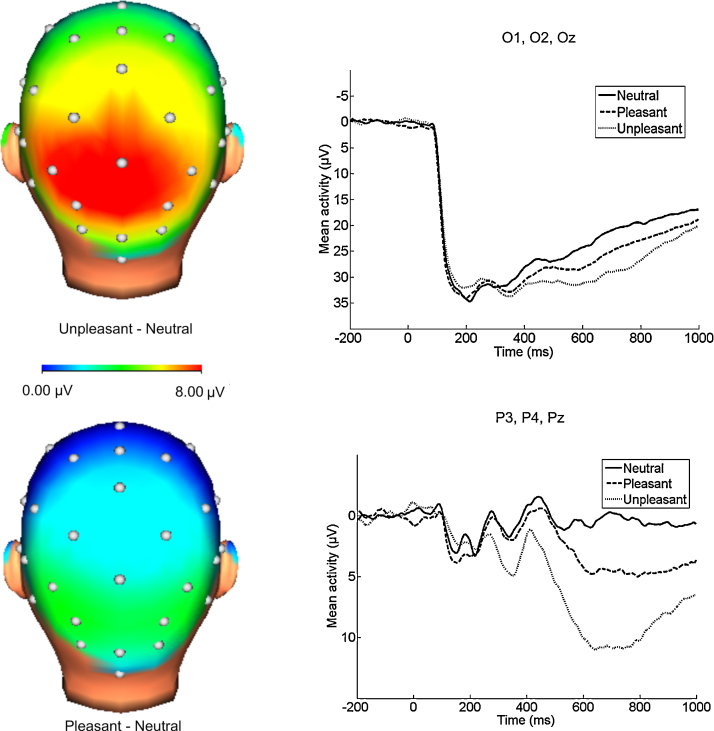
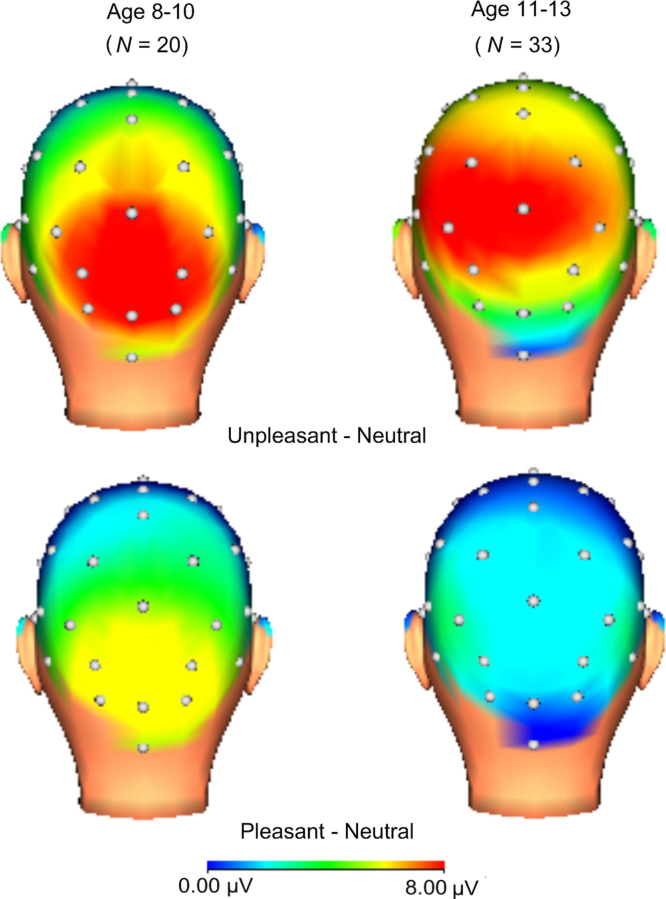
To interpret the three-way interaction for the IAPS version of the task, 3(Image Valence) × 2(Age) ANOVAs were performed at each set of electrode sites. At occipital sites, the main effects of image valence, F(2, 102) = 31.84, p < .001, ηp2 = .38, and age, F(1, 51) = 11.40, p < .01, ηp2 = .18, were significant, as was the image valence × age interaction, F(2, 102) = 7.12, p < .01, ηp2 = .12. The effect of image valence at occipital sites was then examined separately using one-way ANOVAs for each age group. For younger children, the main effect of image valence was significant, F(2, 38) = 16.34, p < .001, ηp2 = .46, and planned comparisons indicated that both unpleasant, F(1, 19) = 36.35, p < .001, ηp2 = .66, and pleasant images, F(1, 19) = 15.08, p < .01, ηp2 = .44, were associated with increased positivities compared to neutral at occipital sites. For older children, the main effect of image valence at occipital sites was significant but the effect size was smaller, F(2, 64) = 13.80, p < .001, ηp2 = .30, and only unpleasant images were associated with significant positivities compared to neutral, F(1, 32) = 25.94, p < .001, ηp2 = .45. No significant differences were found between pleasant and neutral images at occipital sites for older children, F(1, 32) = 1.13, p > .05.
At parietal sites, the main effect of image valence was significant, F(2, 102) = 36.94, p < .001, ηp2 = .42, and planned comparisons indicated that both unpleasant images, F(1, 51) = 74.93, p < .001, ηp2 = .59, and pleasant images, F(1, 51) = 12.67, p < .01, ηp2 = .20, were associated with significant positivities compared to neutral images. The image valence × age interaction, F(2, 102) = 1.52, p > .05, and main effect of age, F(1, 51) = 0.28, p > .05, were not significant (Fig. 2, Fig. 3).
Fig. 2.
Scalp distributions (400–1000 ms after stimulus onset) of the difference between emotional and neutral IAPS images, and ERPs (negative up) at occipital (top) and parietal (bottom) electrode sites. Significant effects of image valence were found for IAPS images at both parietal and occipital sites. Note: ERPs at occipital sites are presented on a different scale than ERPs at parietal sites.
Fig. 3.
Scalp distributions (400–1000 ms after stimulus onset) of the difference between emotional and neutral IAPS images for younger children (left) and older children/adolescents (right).
3.2. Effects of task, valence, and age on RT and accuracy
Average RTs and accuracy rates for each condition are presented in Table 1. A 2(Task) × 3(Valence) × 2(Age) ANOVA was performed to examine the effect of task, stimulus valence, and age on RT. Overall, RT was faster in the faces version of the task compared to the IAPS version, F(2, 98) = 7.60, p < .01, ηp2 = .13, which was qualified by a significant interaction between task and picture valence, F(2, 98) = 3.09, p=.05, ηp2 = .06. The main effect of age, F(1, 49) = 2.93, p = .09, did not reach significance. The main effect of valence, valence × age, task × age, and valence × task × age interactions were not significant.
To further examine the task × valence interaction, the role of picture valence was examined separately for each task. For the faces task, the main effect of valence was not significant, F(2, 114) = .25, p > .05, but for the IAPS version, the main effect of valence was significant, F(2, 104) = 4.20, p < .05, ηp2 = .08. Planned comparisons indicated that both unpleasant, F(1, 52) = 6.52, p < .05, ηp2 = .11, and pleasant images, F(1, 52) = 6.03, p < .05, ηp2 = .10, were associated with significantly slower RTs compared to neutral images.
A 2(Task) × 3(Valence) × 2(Age) ANOVA was performed to examine the effect of task, stimulus valence, and age on accuracy. The IAPS version of the task was associated with poorer accuracy overall compared to the faces version, F(1, 49) = 8.78, p < .01, ηp2 = .15. The main effects of valence and age and all interactions were not significant.
3.3. Correlations between ERP and behavioral measures within and between tasks
Table 2 presents correlations between ERP and behavioral measures within each valence on the faces and IAPS versions of the tasks. Occipital and parietal LPP measures were significantly correlated for each valence; however, few associations were found between ERP and behavioral measures, with the exception of a significant association between occipital LPP and accuracy for pleasant trials on the IAPS version, such that larger LPPs were associated with lower accuracy.
Table 2.
Correlations between LPP, reaction time, and accuracy within each valence for faces (top) and IAPS (bottom).
| Faces version | Sad LPP (parietal) | Sad RT | Sad accuracy |
|---|---|---|---|
| Sad LPP (occipital) | .54*** | −.01 | −.22 |
| Sad LPP (parietal) | – | −.05 | 0.09 |
| Sad RT | − | .16 |
| Happy LPP (parietal) | Happy RT | Happy accuracy | |
|---|---|---|---|
| Happy LPP (Occipital) | .56*** | −.08 | −.00 |
| Happy LPP (parietal) | – | −.16 | .06 |
| Happy RT | – | .26* |
| Neutral LPP (parietal) | Neutral RT | Neutral accuracy | |
|---|---|---|---|
| Neutral LPP (occipital) | .50*** | −.04 | −.03 |
| Neutral LPP (parietal) | – | −.10 | .03 |
| Neutral RT | – | .22 |
| IAPS version | Unpleasant LPP (parietal) | Unpleasant RT | Unpleasant accuracy |
|---|---|---|---|
| Unpleasant LPP (occipital) | .45*** | −.10 | −.04 |
| Unpleasant LPP (parietal) | – | −.11 | −.04 |
| Unpleasant RT | – | – | −.07 |
| Pleasant LPP (Parietal) | Pleasant RT | Pleasant accuracy | |
|---|---|---|---|
| Pleasant LPP (occipital) | .66*** | −.17 | −.33* |
| Pleasant LPP (parietal) | – | −.19 | −.08 |
| Pleasant RT | – | −.02 |
| Neutral LPP (parietal) | Neutral RT | Neutral accuracy | |
|---|---|---|---|
| Neutral LPP (occipital) | .60*** | −.12 | −.02 |
| Neutral LPP (parietal) | – | −.03 | .10 |
| Neutral RT | – | .22 |
p < .001.
p < .05.
Table 3 presents correlations between faces variables and IAPS variables matched on valence. Similar to the within task correlations, there were no significant associations across behavioral and ERP measures; however, all correlations between corresponding measures across tasks were significant, with the exception of the correlation between accuracy on neutral face trials and neutral IAPS trials. Across subjects, LPP amplitudes and behavioral measures correspond with the same measures in different tasks; however, LPP amplitudes are not consistently associated with behavioral measures in this sample.
Table 3.
Correlations between physiological/behavioral measures on the faces version and IAPS version of tasks.
| Unpleasant LPP (parietal) | Unpleasant LPP (occipital) | Unpleasant RT | Unpleasant accuracy | |
|---|---|---|---|---|
| Sad LPP (parietal) | .62*** | .27 | −.05 | −.07 |
| Sad LPP (occipital) | .33* | .68*** | −.16 | −.14 |
| Sad RT | −.12 | −.02 | .89*** | .09 |
| Sad accuracy | .04 | −.16 | .20 | .42** |
| Pleasant LPP (parietal) | Pleasant LPP (occipital) | Pleasant RT | Pleasant accuracy | |
|---|---|---|---|---|
| Happy LPP (parietal) | .66*** | .37** | −.12 | .04 |
| Happy LPP (occipital) | .43** | .70*** | −.21 | −.19 |
| Happy RT | −.15 | −.11 | .88*** | −.06 |
| Happy accuracy | .08 | −.19 | .17 | .42** |
| Neutral IAPS LPP (parietal) | Neutral IAPS LPP (occipital) | Neutral IAPS RT | Neutral IAPS accuracy | |
|---|---|---|---|---|
| Neutral face LPP (parietal) | .68*** | .36* | −.05 | .12 |
| Neutral face LPP (occipital) | .45** | .75*** | −.14 | .03 |
| Neutral face RT | −.09 | −.12 | .87*** | .16 |
| Neutral face accuracy | −.20 | −.18 | .16 | .20 |
p < .001.
p < .01.
p < .05.
4. Discussion
The objectives of the current study were to examine the ways in which emotion and attention interact across development by measuring behavioral and electrocortical responses to emotional faces and IAPS images in a modified emotional interrupt task. Our hypotheses were supported insofar as younger children showed enhanced positivities to IAPS images over occipital sites that were less observable among older children, and older children relative to younger children showed increased activation over parietal sites during the faces version of the task. In addition, LPP and behavioral measures were impacted by both pleasant and unpleasant IAPS images, whereas only a relatively small electrocortical effect was observed for sad emotional faces; emotional faces had no impact on behavioral measures.
4.1. Developmental changes in the LPP
The results of the current study suggest that although the LPP is apparent over occipital sites in middle childhood, occipital positivities are less observable in late childhood/early adolescence. This is consistent with previous findings that the LPP is more occipitally maximal in childhood than observed in adults (Hajcak and Dennis, 2009, Kujawa et al., 2012). Interestingly, the developmental shift in the LPP magnitude appears specific to IAPS images, with sad faces associated with significant LPPs only over occipital sites regardless of age. As the LPP in adults has been linked to connectivity between occipito-parietal cortex and frontal networks (Moratti et al., 2011), it may be that the LPP in middle childhood is marked by increased engagement of occipital regions, with a shift to a reliance on more fronto-parietal networks in adolescence. This possibility is supported by fMRI studies indicating that the development of emotional processing involves transitions from a reliance on subcortical regions to more involvement of the prefrontal cortex (Monk, 2008).
It is important to note that the LPP in the current study was not consistently associated with behavioral responses across children, although previous research in adults has linked enhanced LPPs to slower reaction times (Weinberg and Hajcak, 2011). Interestingly, the LPP in the adult study was derived using principal components analysis and peaked close to 1000 ms after stimulus onset. It is possible that the LPP isolated in the current study did not sufficiently capture the later, ongoing elaboration observed, and additional research using more advanced data analysis methods is needed to determine whether a similar component is observable among children.
There is some evidence that the LPP may be a useful measure for developmental psychopathology research. For example, reduced LPPs to emotional faces have been linked to risk for depressive disorders (Kujawa et al., 2012). In addition, the LPP may index sensitivity to rewards among children with attention-deficit hyperactivity disorder (van Meel et al., 2011) and reactivity to phobic stimuli among children with spider phobias (Leutgeb et al., 2010). When using LPP measures for individual differences and developmental psychopathology questions, it will be important to take into consideration normative developmental changes in the nature of the LPP, especially as a function of stimulus type employed.
4.2. Comparison of IAPS and faces
Among children and adolescents, IAPS images elicit electrocortical and behavioral effects that are not observed in response to emotional faces. Sad faces were associated with a small electrocortical positivity compared to neutral faces but only at occipital sites; no significant differences were found between neutral and happy faces. In addition, no behavioral effects were found for emotional faces. On the other hand, both unpleasant and pleasant IAPS images were associated with enhanced electrocortical positivities compared to neutral images, as well as significantly slower reaction times to targets that followed pictures. In addition, the IAPS version of the task was associated with poorer accuracy overall compared to the faces version, indicating that the presentation of the salient IAPS images has a particularly robust effect on behavioral performance. These results are consistent with work suggesting that IAPS images are rated as more arousing than emotional faces among adults (Britton et al., 2006), as well as a previous study of the LPP in young children that found a relatively small difference between emotional and neutral faces (Kujawa et al., 2012).
There are a number of processes that may contribute to the larger effects observed for IAPS images compared to emotional faces. As implied above, one possibility is that the content of IAPS images is more arousing and motivationally salient than emotional faces, which is consistent with subjective ratings in adults, as well as interpretations of the LPP as indexing salience (Britton et al., 2006, Weinberg and Hajcak, 2010). However, the content of emotional stimuli may have also played a role. While the negative emotional faces were all sad images, the stimuli selected for unpleasant IAPS images include some sad images (e.g., a young boy crying), along with a number of fear-related images (e.g., weapons and animals attacking). Thus, it is possible that the larger effects observed in response to IAPS relates to the inclusion of threatening stimuli. In our previous study examining the LPP in response to emotional faces, however, we found that sad, fearful, and angry faces elicited comparable LPPs in young children (Kujawa et al., 2012), suggesting that similar results may have been found even if threatening faces had been used in the current study. Lastly, it is possible that visual complexity of the images contributed to the results, as emotional faces may be processed more quickly than the complex IAPS scenes. Previous research in adults has examined the effect of complexity within the IAPS on the LPP and suggests that the LPP is primarily modulated by motivational salience (Bradley et al., 2007). While complexity was found to have some effects on the LPP in that study, the direction of the results indicated that relatively simple emotional images were associated with enhanced LPPs relative to more complex images (Bradley et al., 2007).
One advantage of the current study is the simultaneous assessment of behavioral and ERP measures that converge on the same point: IAPS images capture attention better than emotional faces. As the magnitude of the difference between emotional and neutral stimuli is substantially larger for IAPS images, these stimuli may be particularly useful for examining individual differences in the LPP. It is important to note, however, that approximately 13% of children in the current study did not participate in the IAPS task due to parental refusal. This raises the importance of balancing the selection of images that are arousing enough to measure emotional processing but also readily accepted by children and parents. Additional work is needed to establish emotional stimuli that are appropriate for use across development.
5. Conclusions
The LPP appears to be a useful measure of emotional processing in children than can be applied to developmental psychopathology research. The results of the current study suggest that there are developmental changes in the nature of the LPP, with younger children showing positivities over more occipital sites compared to older children/early adolescents.
In addition, IAPS images are more effective in eliciting emotion-related modulations of the LPP in children than emotional faces, and an active paradigm can offer a behavioral measure that complements the electrocortical measure.
Conflicts of Interest
The authors have no conflicts of interest to declare.
Acknowledgments
This work was supported by National Institute of Mental Health Grants RO1 MH069942 to Daniel N. Klein and F31 MH09530701 to Autumn Kujawa.
Footnotes
Faces used were: happy (AF01HAS, AF05HAS, AF07HAS, AF09HAS, AF13HAS, AF19HAS, AF22HAS, AF26HAS, AF31HAS, AF32HAS, AM08HAS, AM10HAS, AM11HAS, AM13HAS, AM14HAS, AM17HAS, AM25HSA, AM31HAS, BM05HAS, BM16HAS); neutral (AF01NES, AF05NES, AF09NES, AF13NES, AF19NES, AF22NES, AF26NES, AF31NES, AM08NES, AM10NES, AM11NES, AM13NES, AM14NES, AM17NES, AM25NES, AM31NES, BF07NES, BF32NES, BM05NES, BM16NES); sad (AF01SAS, AF05SAS, AF07SAS, AF09SAS, AF13SAS, AF19SAS, AF22SAS, AF26SAS, AF31SAS, AF32SAS, AM08SAS, AM11SAS, AM13SAS, AM14SAS, AM17SAS, AM25SAS, AM31SAS, BM05SAS, BM10SAS, BM16SAS).
IAPS pictures used were: pleasant (1463, 1710, 1750, 1811, 2070, 2091, 2092, 2224, 2340, 2345, 2347, 7325, 8031, 8200, 8461, 8496, 8497, 8370, 7400, 7330); neutral (5395, 7026, 7130, 7190, 7175, 2514, 7038, 2580, 5390, 7090, 5500, 5731, 5740, 7100, 5900, 7000, 7002, 7009, 7010, 7039); unpleasant (1050, 1052, 6571, 1205, 1200, 1300, 1304, 1930, 2458, 9600, 2691, 2703, 2800, 2811, 2900, 3022, 6190, 6213, 6231, 6510).
References
- Banaschewski T., Brandeis D. Annotation: what electrical brain activity tells us about brain function that other techniques cannot tell us – a child psychiatric perspective. Journal of Child Psychology and Psychiatry and Allied Disciplines. 2007;48(5):415–435. doi: 10.1111/j.1469-7610.2006.01681.x. [DOI] [PubMed] [Google Scholar]
- Bradley M.M., Hamby S., Löw A., Lang P.J. Brain potentials in perception: picture complexity and emotional arousal. Psychophysiology. 2007;44(3):364–373. doi: 10.1111/j.1469-8986.2007.00520.x. [DOI] [PubMed] [Google Scholar]
- Bress J.N., Smith E., Foti D., Klein D.N., Hajcak G. Neural response to reward and depressive symptoms in late childhood to early adolescence. Biological Psychology. 2012;89:156–162. doi: 10.1016/j.biopsycho.2011.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Britton J.C., Taylor S.F., Sudheimer K.D., Liberzon I. Facial expressions and complex IAPS pictures: common and differential networks. NeuroImage. 2006;31(2):906–919. doi: 10.1016/j.neuroimage.2005.12.050. [DOI] [PubMed] [Google Scholar]
- Cuthbert B.N., Schupp H.T., Bradley M.M., Birbaumer N., Lang P.J. Brain potentials in affective picture processing: covariation with autonomic arousal and affective report. Biological Psychology. 2000;52(2):95–111. doi: 10.1016/s0301-0511(99)00044-7. [DOI] [PubMed] [Google Scholar]
- Flaisch T., Häcker F., Renner B., Schupp H.T. Emotion and the processing of symbolic gestures: an event-related brain potential study. Social Cognitive and Affective Neuroscience. 2011;6(1):109–118. doi: 10.1093/scan/nsq022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foti D., Hajcak G., Dien J. Differentiating neural responses to emotional pictures: evidence from temporal–spatial PCA. Psychophysiology. 2009;46(3):521–530. doi: 10.1111/j.1469-8986.2009.00796.x. [DOI] [PubMed] [Google Scholar]
- Gao P.-X., Liu H.-J., Ding N., Guo D.-J. An event-related-potential study of emotional processing in adolescence. Acta Psychologica Sinica. 2010;42(3):342–351. [Google Scholar]
- Glenn C., Klein D.N., Lissek S., Britton J.C., Pine D.S., Hajcak G. The development of fear learning and generalization in 8- to 13-year-olds. Developmental Psychobiology. 2011 doi: 10.1002/dev.20616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gratton G., Coles M.G., Donchin E. A new method for off-line removal of ocular artifact. Electroencephalography and Clinical Neurophysiology. 1983;55(4):468–484. doi: 10.1016/0013-4694(83)90135-9. [DOI] [PubMed] [Google Scholar]
- Hajcak G., Dennis T.A. Brain potentials during affective picture processing in children. Biological Psychology. 2009;80(3):333–338. doi: 10.1016/j.biopsycho.2008.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajcak G., Olvet D.M. The persistence of attention to emotion: brain potentials during and after picture presentation. Emotion. 2008;8(2):250–255. doi: 10.1037/1528-3542.8.2.250. [DOI] [PubMed] [Google Scholar]
- Hajcak G., Weinberg A., MacNamara A., Foti D. ERPs and the study of emotion. In: Luck S.J., Kappenman E., editors. Handbook of Event-related Potential Components. Oxford University Press; New York: 2011. [Google Scholar]
- Kujawa A.J., Hajcak G., Torpey D., Kim J., Klein D.N. Electrocortical reactivity to emotional faces in young children and associations with maternal and paternal depression. Journal of Child Psychology and Psychiatry and Allied Disciplines. 2012;53(2):207–215. doi: 10.1111/j.1469-7610.2011.02461.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang P.J., Bradley M.M. Emotion and the motivational brain. Biological Psychology. 2010;84(3):437–450. doi: 10.1016/j.biopsycho.2009.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang, P.J., Bradley, M.M., Cuthbert, B.N., 2008. International Affective Picture System (IAPS): affective ratings of pictures and instructional manual. Technical Report A-8. University of Florida, Gainesville, FL.
- Larsen J.T., Berntson G.G., Poehlmann K.M., Ito T.A., Cacioppo J.T. The psychophysiology of emotion. In: Lewis M., Haviland-Jones J.M., Barrett L.F., editors. Handbook of Emotions. 3rd ed. Guilford Press; New York, NY, US: 2008. pp. 180–195. [Google Scholar]
- Leutgeb V., Schäfer A., Köchel A., Scharmüller W., Schienle A. Psychophysiology of spider phobia in 8- to 12-year-old girls. Biological Psychology. 2010;85(3):424–431. doi: 10.1016/j.biopsycho.2010.09.004. [DOI] [PubMed] [Google Scholar]
- Lundqvist, D., Flykt, A., Ohman, A., 1998. Karolinska Directed Emotional Faces [Database of Standardized Facial Images]. Psychology Section, Department of Clinical Neuroscience, Karolinska Hospital, S-171 76 Stockholm, Sweden.
- Meyer A., Weinberg A., Klein D.N., Hajcak G. The development of the error-related negativity and its relationship with anxiety: evidence from 8- to 13-year-olds. Developmental Cognitive Neuroscience. 2012;2:152–161. doi: 10.1016/j.dcn.2011.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell D.G.V., Richell R.A., Leonard A., Blair R.J.R. Emotion at the expense of cognition: psychopathic individuals outperform controls on an operant response task. Journal of Abnormal Psychology. 2006;115(3):559–566. doi: 10.1037/0021-843X.115.3.559. [DOI] [PubMed] [Google Scholar]
- Monk C.S. The development of emotion-related neural circuitry in health and psychopathology. Development and Psychopathology. 2008;20(4):1231–1250. doi: 10.1017/S095457940800059X. [DOI] [PubMed] [Google Scholar]
- Moratti S., Saugar C., Strange B.A. Prefrontal–occipitoparietal coupling underlies late latency human neuronal responses to emotion. Journal of Neuroscience. 2011;31(47):17278–17286. doi: 10.1523/JNEUROSCI.2917-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naumann E., Bartussek D., Diedrich O., Laufer M.E. Assessing cognitive and affective information processing functions of the brain by means of the late positive complex of the event-related potential. Journal of Psychophysiology. 1992;6(4):285–298. [Google Scholar]
- Nelson C.A., McCleery J.P. Use of event-related potentials in the study of typical and atypical development. Journal of the American Academy of Child and Adolescent Psychiatry. 2008;47(11):1252–1261. doi: 10.1097/CHI.0b013e318185a6d8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olofsson J.K., Nordin S., Sequeira H., Polich J. Affective picture processing: an integrative review of ERP findings. Biological Psychology. 2008;77(3):247–265. doi: 10.1016/j.biopsycho.2007.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabatinelli D., Lang P.J., Keil A., Bradley M.M. Emotional perception: correlation of functional MRI and event-related potentials. Cerebral Cortex. 2007;17(5):1085–1091. doi: 10.1093/cercor/bhl017. [DOI] [PubMed] [Google Scholar]
- Santerre C., Allen J.J.B. Methods for studying the psychophysiology of emotion. In: Rottenberg J., Johnson S.L., editors. Emotion and Psychopathology: Bridging Affective and Clinical Science. American Psychological Association; Washington, DC, US: 2007. pp. 53–79. [Google Scholar]
- Schupp H.T., Öhman A., Junghöfer M., Weike A.I., Stockburger J., Hamm A.O. The facilitated processing of threatening faces: an ERP analysis. Emotion. 2004;4(2):189–200. doi: 10.1037/1528-3542.4.2.189. [DOI] [PubMed] [Google Scholar]
- van Meel C.S., Heslenfeld D.J., Oosterlaan J., Luman M., Sergeant J.A. ERPs associated with monitoring and evaluation of monetary reward and punishment in children with ADHD. Journal of Child Psychology and Psychiatry. 2011;52(9):942–953. doi: 10.1111/j.1469-7610.2010.02352.x. [DOI] [PubMed] [Google Scholar]
- Weinberg A., Hajcak G. Beyond good and evil: the time-course of neural activity elicited by specific picture content. Emotion. 2010;10(6):767–782. doi: 10.1037/a0020242. [DOI] [PubMed] [Google Scholar]
- Weinberg A., Hajcak G. The late positive potential predicts subsequent interference with target processing. Journal of Cognitive Neuroscience. 2011;23(10):2994–3007. doi: 10.1162/jocn.2011.21630. [DOI] [PubMed] [Google Scholar]
- Yurgelun-Todd D.A., Killgore W.D.S. Fear-related activity in the prefrontal cortex increases with age during adolescence: a preliminary fMRI study. Neuroscience Letters. 2006;406(3):194–199. doi: 10.1016/j.neulet.2006.07.046. [DOI] [PubMed] [Google Scholar]