Abstract
Intracranial meningiomas are often indolent tumors which typically grow over years to decades. Nonetheless, meningiomas that progress after maximum safe resection and radiation therapy pose a significant therapeutic challenge and effective therapies have yet to be identified. Preclinical studies implicate angiogenesis in the pathophysiology of more aggressive meningiomas, suggesting that anti-angiogenic therapies may be of utility in this setting. We performed a retrospective review of fourteen patients with recurrent meningioma treated at Duke University Medical Center with bevacizumab, a humanized monoclonal antibody against vascular endothelial growth factor, administered either alone or in combination with chemotherapy. Most patients were heavily pre-treated. Progression-free survival at 6 months was 86 % and was comparable regardless of meningioma grade and whether bevacizumab was administered as monotherapy or in combination with chemotherapy. Most toxicities were mild however single patients developed CNS hemorrhage (grade 1) and intestinal perforation (grade 4), respectively. Bevacizumab can be administered safely to patients with meningioma and appears to be associated with encouraging anti-tumor effect when administered as either a single agent or in combination with chemotherapy. Phase II trials investigating bevacizumab in patients with progressive/recurrent meningioma are warranted.
Keywords: Meningioma, Angiogenesis, Vascular endothelial growth factor, Bevacizumab
Introduction
Meningiomas account for 33.8 % of all primary brain tumors, and therefore are the most common primary tumor of the central nervous system. According to the Central Brain Tumor Registry of the United States (CBTRUS), 53,455 patients developed meningiomas between 2004 and 2006 and the annual incidence is estimated at 6.29 cases per 100,000 person-years [1]. Eighty percent of meningiomas are benign (World Health Organization [WHO] grade I), while nearly 20 % are atypical (WHO grade II) and 1–2 % are anaplastic (WHO grade III) [2]. Initial therapy for symptomatic or growing benign meningiomas is maximum safe resection, while radiation therapy is usually added for atypical and anaplastic lesions or for inoperable, progressive grade I lesions [3, 4]. Nonetheless, effective therapy for meningiomas that recur following radiation therapy has not been identified. In particular, overall outcome for patients with progressive grade II and III meningiomas remains poor, with most series reporting 5-year survival rates of 28–61 % [5]. Results with several chemotherapy agents have been disappointing, although hydroxyurea has demonstrated modest anti-tumor activity in some series [6–11]. Targeted therapies that inhibit specific activators of growth factor signaling pathways, such as the epidermal growth factor receptor (EGFR) and platelet-derived growth factor receptor (PDGFR), have proven ineffective in clinical trials to date among non-enriched progressive meningioma patients [12, 13]. In summary, effective therapy for patients with meningiomas that recur or progress following resection and radiotherapy remains a major challenge and a current unmet need in neuro-oncology.
Angiogenesis, or the process of new blood vessel formation, is a critical adaptation of aggressive cancers [14] and is predominantly mediated by vascular endothelial growth factor (VEGF) [15]. Growing evidence also supports the role of VEGF-mediated angiogenesis among some meningiomas, particularly higher grade subtypes [16, 17]. The rationale for targeting angiogenesis therapeutically for recurrent/progressive meningioma patients is based on several factors. First, as described above, effective therapies for such patients are currently lacking. Second, bevacizumab is effective for most patients with recurrent glioblastoma, another highly aggressive primary CNS tumor [18]. Third, levels of VEGF, VEGF-R, and microvessel density increase with meningioma grade and may provide prognostic significance [16, 19, 20]. Pistolesi et al. [16] performed reverse transcriptase polymerase chain reaction (RT-PCR) and immunohistochemical staining (IHC) on 40 samples of intracranial meningioma, and determined that microvessel density and VEGF expression were significantly associated with grade II and III meningiomas. Interestingly, Maiuri et al. [21] demonstrated a lack of correlation of VEGF expression with recurrence among patients with grade I meningiomas following complete resection. This study however, excluded patients with grade II or grade III meningiomas, as well as patients whose resection was subtotal.
Levels of VEGF and VEGF-R have also been positively correlated with extent of peritumoral brain edema in meningioma patients [20, 22–26]. Ding et al. evaluated biopsy material from areas of associated peritumoral edema adjacent to intracranial meningiomas obtained from 37 patients. The biopsy material was tested for VEGF mRNA and protein. Of note, VEGF mRNA was essentially absent, but the protein was present, suggesting that VEGF produced and secreted by the tumor may extend outward into the associated tumor microenvironment [27]. We performed a retrospective review of all patients with meningioma who received bevacizumab therapy at our institution in order to assess whether formal evaluation of bevacizumab-based therapy for recurrent/progressive meningioma patients is warranted.
Materials and methods
We retrospectively reviewed records of all patients with histopathologically confirmed meningioma treated at Duke University Medical Center between December 2008 and January 2011. During this time period, fifteen patients with recurrent/progressive meningioma were prescribed bevacizumab. One of these patients was lost to follow-up after the initial recommendation of bevacizumab therapy. Administration of bevacizumab could not be confirmed and hence this patient is not included in the current analysis. The remaining fourteen patients were confirmed to have received bevacizumab therapy and their medical records were reviewed for demographic and prior treatment characteristics, adverse events and outcome.
All patients were over 18 years of age and had radiologic evidence of either progressive or recurrent tumor after prior therapy. All patients submitted archival tumor material for histopathologic confirmation of tumor and grade assessment (R. E. M., neuropathologist). There was no limit on the number or type of prior treatment. Patients who received prior radiation therapy including radiosurgery were not required to have histologic confirmation of tumor prior to initiating bevacizumab salvage therapy. Patients received bevacizumab according to published dosing guidelines, with or without chemotherapy [28, 29]. Patients were evaluated by physical examination and MRI scans every 8 weeks. Assessment of response was based on Radiologic Assessment in Neuro-Oncology (RANO) criteria for malignant gliomas that included evaluation of both enhancing and non-enhancing imaging findings as well as clinical changes [30]. Routine laboratory studies were assessed each month or sooner if medically indicated. Toxicities were graded according to the National Cancer Institute’s Common Terminology Criteria for Adverse Events, version 4.0 (evs.nci.nih.gov/ftp1/CTCAE/About.html).
Statistical methods
The primary objective was to estimate 6-month progression-free survival (PFS-6) among adults with progressive/recurrent meningioma treated with bevacizumab. Secondary objectives included evaluation of the safety and tolerability of this regimen and of radiographic response, PFS and overall survival (OS) in this patient population.
Kaplan–Meier curves were generated to describe PFS and OS, with each measured from the date bevacizumab therapy began. Progression-free survival was defined as the time until death, initial disease progression, or last follow-up, assuming the patient remained alive without disease progression. Overall survival was defined as the time until death.
Results
Fourteen patients (8 female; 6 male) with recurrent/progressive meningioma were initiated on bevacizumab treatment (Table 1). The median age at bevacizumab initiation was 53.5 years (range, 20–70 years). Nearly 80 % of patients had a Karnofsky performance status (KPS) ≥80 as well as 1–2 meningioma tumors. Twenty percent of patients had 3 or more meningiomas. Five patients (36 %) had grade I meningioma, five (36 %) had grade II (atypical) meningioma, and three patients (21 %) had grade III (anaplastic) meningioma. One patient had a confirmed histologic diagnosis of meningioma, but grade was not possible to specify.
Table 1.
Patient characteristics and treatment outcome
| Patient | Age at diagnosis (years) | Grade | Number lesions | Age BV treatment (years) | KPS | Prior therapies | BV partner | Months of BV treatment | Reason BV discontinued | Time to progression (months) |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 30 | I | 2 | 45 | 80 | Resection × 3, tamoxifen/RT, imatinib/hydroxyurea, SRS, octreotide/celebrex | Etoposide | 29.5 | PD | 29.5 |
| 2 | 44 | III | 1 | 53 | 90 | Resection × 3, temozolomide/RT, octreotide, imatinib/hydroxyurea, 5-day temozolomide | Etoposide, sirolimus | 5.9 | PD | 5.9 |
| 3 | 51 | II | 1 | 57 | 80 | Resection × 3, hydroxyurea, SRS, imatinib, pasireotide | Etoposide | 7.0 | Ongoing | None |
| 4 | 28 | III | 1 | 43 | 90 | Resection × 2, RT, SRS, 5-day temozolomide | Etoposide | 19.4 | PD | 19.4 |
| 5 | 52 | II | 1 | 54 | 80 | Resection × 2, temozolomide/RT, SRS | Etoposide | 2.5 | Compliance | None |
| 6 | 46 | II | 1 | 54 | 80 | Resection × 1, RT, SRS | Temozolomide (daily) | 10.0 | Ongoing | None |
| 7 | 53 | I | 1 | 57 | 90 | Resection × 2, imatinib/hydroxyurea, daily temozolomide | None | 13.3 | PD | 13.3 |
| 8 | 47 | III | 3 | 52 | 80 | Resection × 2, temozolomide/RT, etoposide/5-day temozolomide, imatinib/hydroxyurea | None | 17.1 | PD | 17.1 |
| 9 | 30 | I | 4 | 50 | 70 | Resection × 2, RT, octreotide | Temozolomide (5-day) | 9.2 | PD | 9.2 |
| 10 | 65 | II | 1 | 66 | 70 | Resection × 2, RT/temozolomide | None | 7.0 | Ongoing | None |
| 11 | 42 | Unknown | 1 | 59 | 80 | Resection × 2; RT; SRS | None | 7.0 | Toxicity | None |
| 12 | 19 | I | 1 | 20 | 90 | Resection; SRS; RT | Temozolomide (5-day) | 8.0 | Ongoing | None |
| 13 | 37 | I | 3 | 51 | 60 | Resection; imatinib/hydroxyurea; temozolomide | Etoposide | 0.5 | Toxicity | None |
| 14 | 77 | II | 1 | 79 | 90 | Resection | Temozolomide (5-day) | 0.5 | Toxicity | None |
BV bevacizumab, KPS Karnofsky performance status, NA not applicable, PD progressive disease, RT radiation therapy, SRS stereotactic radiosurgery
Most patients were heavily pretreated. All patients had undergone prior surgical resection including four patients (29 %) with one prior resection, seven patients (50 %) with two prior resections and three patients (21 %) with three prior resections. Ten patients (77 %) had received prior fractionated radiotherapy and seven patients (50 %) had also undergone stereotactic radiosurgery. Nine patients (64 %) had received prior chemotherapy including five patients treated with one prior chemotherapeutic, 3 patients who received two prior chemotherapies and one patient who was treated with three prior chemotherapies. Seven patients (50 %) received prior biologic targeted therapy including octreotide (n = 3) or pasireotide (SOM230), a multi-ligand somatostatin receptor analogue (n = 1), imatinib mesylate (n = 6), tamoxifen (n = 1) and cele-coxib (n = 1). Only one patient received bevacizumab therapy without prior radiation, chemotherapy or biologic therapy.
Bevacizumab was administered as single agent in four patients (29 %), while ten patients (71 %) received bevacizumab with chemotherapy. Chemotherapeutic agents combined with bevacizumab included daily etoposide (n = 5), daily temozolomide plus sirolimus (n = 1), and temozolomide (5-day schedule, n = 3; daily schedule, n = 1).
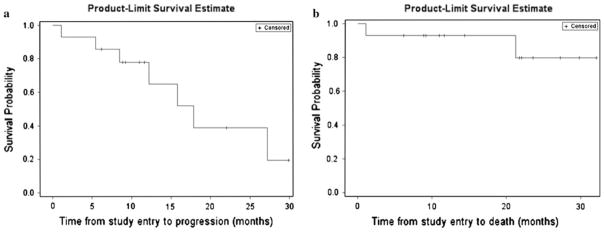
The median follow-up for all patients was 21.8 months (95 % CI, 9.2, 27.3). At the time this manuscript was prepared, four patients (29 %) were continuing on bevacizumab therapy while ten patients (71 %) had discontinued bevacizumab. The reason for bevacizumab discontinuation included progressive disease (n = 6), toxicity (n = 3) and lack of compliance (n = 1). Although no patients achieved a complete response (CR), one patient with multifocal disease achieved a partial response (PR; Fig. 1), 11 patients achieved stable disease (SD) and 2 patients had progressive disease (PD) as their best response. Clinically, neurologic function and KPS were preserved in general among patients who achieved stable disease or radiographic response. The median PFS and PFS-6 were 17.9 months (95 % CI: 8.5, ∞) and 85.7 % (95 % CI: 53.9, 96.2), respectively. Median PFS and PFS-6 were 12.2 months (95 % CI: 1.1, 27.2) and 80 % (95 % CI: 20.4, 96.9), respectively, for patients with grade I meningiomas, and 15.8 months (95 % CI: 5.5, 17.9) and 87.5 % (38.7, 98.1) for patients with grade II/III meningiomas, respectively. The median PFS and PFS-6 were 15.8 months (95 % CI: 12.2, ∞) and 100 %, respectively for patients treated with single-agent bevacizumab (n = 4), and 17.9 (95 % CI: 1.1, 27.2) and 80 % (95 % CI: 40.9, 94.6), respectively for patients treated with bevacizumab plus chemotherapy (n = 10). Median OS for all patients has not been reached (Fig. 2). Due to the heterogeneity of the patient population we examined, it is difficult to speculate how and if specific prior treatments and varying histologic grades impacted response to treatment with bevacizumab. There is a general trend toward increased PFS in patients who had received stereotactic radiotherapy as part of their prior treatment regimen; in general these patients (7 total) seemed to do well on bevacizumab. As nearly half of the patients have not yet demonstrated disease progression as of this writing, the long-term implications and associations are yet to be determined.
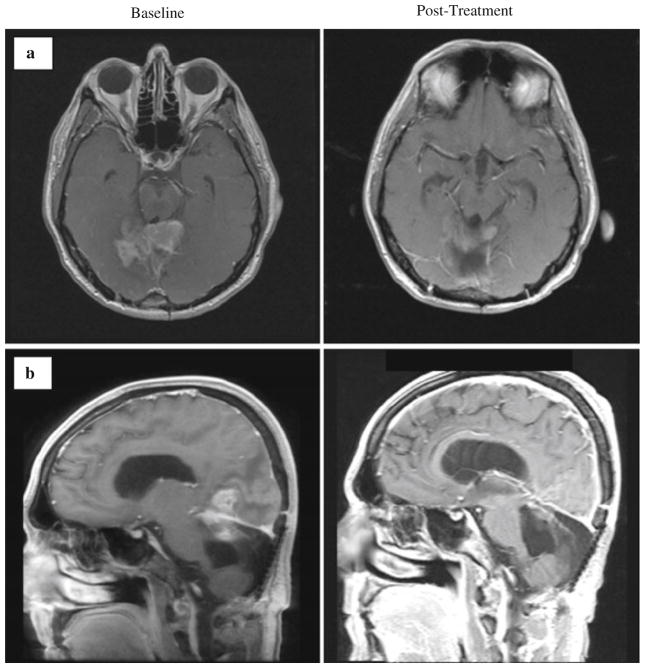
Fig. 1.
Magnetic resonance imaging demonstrating a partial response of a patient with recurrent atypical (grade II) meningioma following four cycles of bevacizumab with daily oral etoposide. The patient (Patient 13, Table 1) initially presented with grade I fibroblastic meningioma treated with resection, adjuvant imatinib/hydroxyurea, and then temozolomide following first progression and did not receive prior radiotherapy. a Post-contrast axial T1-weighted images. b Post-contrast sagittal T1-weighted images
Fig. 2.
Kaplan–Meier plots of progression-free survival (a) and overall survival (b) for all patients
Hematologic toxicity was limited to grade 1 thrombocytopenia (n = 1) attributed most likely to prior extensive chemotherapy treatment and grade 3 thrombocytopenia (n = 1) which was attributed to concurrent temozolomide therapy. Non-hematologic toxicity included proteinuria (grade 1, n = 3; grade 2, n = 3), hypertension (grade 1, n = 4; grade 2, n = 1) and craniotomy site cellulitis (grade 2, n = 1) which responded to oral antibiotics. In addition, five patients experienced hemorrhage while receiving bevacizumab. All of these events were grade 1, and included hematochezia (n = 1), microscopic hematuria (n = 3) and bleeding into a meningioma along the vermis (grade 1; n = 1). Bevacizumab was discontinued in three patients (21 %) due to toxicity including the previously described patient with hemorrhage into a vermis meningioma, a patient with grade 4 intestinal perforations and one patient with grade 5 pneumonia/sepsis.
Discussion
Meningiomas are the most common primary CNS tumor [1]. Most meningiomas are grade I and respond durably to surgical resection [4]. Atypical and anaplastic meningiomas account for 20–30 % of these tumors and usually recur following surgery and radiation therapy [2]. Currently there are no effective therapies for meningiomas that recur after surgery and radiation therapy, thus such patients represent an unmet need in oncology at present. In the current report, we describe the outcome of fourteen recurrent meningioma patients treated with bevacizumab.
Limited data have been reported to date describing the anti-tumor activity of anti-angiogenic agents among patients with recurrent/progressive meningioma. Puchner et al. reported significant regression of an anaplastic meningioma that recurred following prior surgery and radiation therapy that was durable and ongoing after 6 months of bevacizumab therapy [31]. DeBoer recently reported preliminary results of a phase II trial incorporating PTK787, an oral tyrosine kinase inhibitor against VEGF-R2 and PDGFR, among twelve patients with recurrent grade II/III meningioma. One patient (8 %) achieved a radiographic response and nine patients (69 %) achieved stable disease with a median time-to-progression of 15.7 weeks, and a median PFS-6 of 46 % [32].
Our retrospective review of fourteen recurrent meningioma patients suggests that bevacizumab may have activity in this indication. Most patients in our series were heavily pretreated, having undergone multiple prior surgical resections as well as treatment with conventional external beam radiotherapy and chemotherapy. In addition, half of the patients received prior stereotactic radiosurgery and half also were previously treated with biological targeted therapies. The median PFS and PFS-6 compare favorably to outcome achieved using salvage chemotherapy [33–37], targeted therapeutics against PDGFR [12] and EGFR [13], interferon-α [38], somatostatin inhibitors [39, 40] and hormonal agents (Table 2) [41, 42]. Nonetheless, conclusions from our series are limited by its small size and retrospective nature. We also elected to utilize RANO criteria to assess response. This choice poses further potential limitations of our findings given that the RANO criteria were specifically drafted to assess response among malignant glioma patients and not meningioma patients. Nonetheless, given the complexity of response assessment observed among malignant glioma patients treated with bevacizumab, we felt that our assessment should include both the enhancing as well as non-enhancing radiographic components of the tumors, as is specified in the RANO criteria. An additional limitation of our findings is that patients, who received prior radiotherapy, including radiosurgery, may have had radionecrosis rather than true tumor progression at the time bevacizumab therapy was initiated.
Table 2.
Outcome of systemic therapies from selected published recurrent meningioma series
| Treatment | Study type | Number patients | Median PFS (months) | PFS-6 (%) | Citation |
|---|---|---|---|---|---|
| Bevacizumab | Retrospective | 14 | 18 | 86 | Current series |
| Hydroxyurea | Phase II | 12 (grade 1, n = 12) | 13 | NR | Loven et al. [10] |
| Hydroxyurea | Retrospective | 60 (grade I) | 4 | 10 | Chamberlain and Johnston [34] |
| Hydroxyurea + RT | Phase II | 21 (grade I, n = 13; grade II/III, n = 4; unknown grade, n = 4) | 15 | NR | Hahn et al. [36] |
| Temozolomide | Phase II | 16 (grade I, n = 16) | 5 | 0 | Chamberlain et al. [37] |
| Irinotecan | Phase II | 16 (grade I, n = 16) | 5 | 6 | Chamberlain et al. [35] |
| Imatinib | Phase II | 23 (grade I, n = 13; grade II, n = 5; grade III, n = 5) | 2 | 29 | Wen et al. [12] |
| Imatinib + Hydroxyurea | Phase II | 21 (grade 1, n = 8; grade II, n = 9; grade III, n = 4) | 7 | 62 | Reardon et al. [33] |
| Erlotinib/Gefitinib | Phase II | 25 (grade I, n = 8; grade II, n = 9; grade III, n = 8) | 2.5 | 28 | Norden et al. [13] |
| Interferon-α | Phase II | 35 (grade I, n = 35) | 7 | 54 | Chamberlain and Glantz[38] |
| Tamoxifen | Phase II | 19 (grade not reported) | 15 | NR | Goodwin et al. [41] |
| Mifepristone | Phase II | 90 (grade unknown) | 10 | NR | Grunberg et al. [42] |
| Octreotide | Phase II | 11 (grade I, n = 3; grade II, n = 3; grade III, n = 5) | 4 | NR | Johnson et al. [39] |
| Sandostatin LAR | Phase II | 16 (grade I, n = 8; grade II, n = 3; grade III, n = 5) | 5 | 44 | Chamberlain et al. [40] |
n Number, NR not reported, PFS progression-free survival, PFS-6 progression-free survival at 6 months, RT radiation therapy
Advanced-grade meningiomas express higher levels of VEGF and exhibit increases in microvessel density [16, 19, 20], thus potentially making them more susceptible to VEGF inhibition with bevacizumab. This correlation has been demonstrated in preclinical studies to be most prominent in grades II and III meningiomas [16]. In addition, alleviation of peritumoral brain edema, which may propagate VEGF distribution in vivo [27], may also be alleviated by bevacizumab. We hypothesize the patients with higher grade meningioma tumors respond most effectively to bevacizumab due to higher levels of VEGF expression and subsequent response to inhibition. Further examination of this issue is warranted and may provide utility in prognostication and determination of which patients will respond most effectively.
Most patients in our series tolerated bevacizumab well and observed toxicities were similar in type, severity and frequency to those reported among GBM patients treated with bevacizumab [28, 29]. Serious toxicities leading to bevacizumab discontinuation in our study occurred in 3 patients (21 %) including one intracranial hemorrhage (grade 1), one episode of intestinal perforation (grade 4) and one episode of pneumonia/sepsis (grade 5).
There are currently several ongoing clinical trials incorporating VEGF/VEGFR-directed therapy for patients with recurrent, progressive meningioma, including a multicenter phase II trial combining bevacizumab with the mTOR inhibitor everolimus (Clinicaltrial.gov identifier: NCT00972335), and separate phase II study evaluating single-agent bevacizumab (Clinicaltrials.gov identifier: NCT01125046. Additional clinical trials evaluating multikinase inhibitors targeting VEGFR and PDGFR, including sunitinib and vatalanib, are also underway for recurrent/progressive meningioma patients.
In summary, effective therapy for patients with recurrent/progressive meningioma after surgery and radiation therapy represents an unmet need in neuro-oncology. Pre-clinical studies have suggested that microvessel density and VEGF expression appear to increase with increasing meningioma grade [16, 21], suggesting that anti-VEGF therapies may be active in this setting. Our retrospective series of recurrent/progressive meningioma patients suggests that bevacizumab, administered as single-agent or in combination with chemotherapy, has activity for these patients and can be safely administered. However, our findings are limited by the overall small number of patients evaluated and the retrospective nature of our analysis. Prospective studies of anti-VEGF/VEGFR therapeutics are warranted for recurrent/progressive meningioma patients.
Acknowledgments
This work was supported by NIH Grants 5P50-NS-20023 and 5 R37 CA11898; NIH Grant MO1 RR 30, GCRC Program, NCRR; and NCI SPORE 1 P20 CA096890; and a grant from Genentech Pharmaceuticals.
The authors thank Wendy Gentry for her excellent secretarial support in the development of this manuscript.
Contributor Information
Emil Lou, Department of Surgery, The Preston Robert Tisch Brain Tumor Center at Duke, Duke University Medical Center, Durham, NC 27710, USA.
Ashley L. Sumrall, Department of Surgery, The Preston Robert Tisch Brain Tumor Center at Duke, Duke University Medical Center, Durham, NC 27710, USA
Scott Turner, Department of Surgery, The Preston Robert Tisch Brain Tumor Center at Duke, Duke University Medical Center, Durham, NC 27710, USA.
Katherine B. Peters, Departments of Medicine, The Preston Robert Tisch Brain Tumor Center at Duke, Duke University Medical Center, Durham, NC 27710, USA
Annick Desjardins, Departments of Medicine, The Preston Robert Tisch Brain Tumor Center at Duke, Duke University Medical Center, Durham, NC 27710, USA.
James J. Vredenburgh, Departments of Medicine, The Preston Robert Tisch Brain Tumor Center at Duke, Duke University Medical Center, Durham, NC 27710, USA
Roger E. McLendon, Department of Pathology, The Preston Robert Tisch Brain Tumor Center at Duke, Duke University Medical Center, Durham, NC 27710, USA
James E. Herndon, II, Cancer Center Biostatistics, The Preston Robert Tisch Brain Tumor Center, Duke University Medical Center, Durham, NC 27710, USA.
Frances McSherry, Cancer Center Biostatistics, The Preston Robert Tisch Brain Tumor Center, Duke University Medical Center, Durham, NC 27710, USA.
Julie Norfleet, Department of Surgery, The Preston Robert Tisch Brain Tumor Center at Duke, Duke University Medical Center, Durham, NC 27710, USA.
Henry S. Friedman, Department of Surgery, The Preston Robert Tisch Brain Tumor Center at Duke, Duke University Medical Center, Durham, NC 27710, USA. Department of Pediatrics, The Preston Robert Tisch Brain Tumor Center at Duke, Duke University Medical Center, Durham, NC 27710, USA
David A. Reardon, Email: david_reardon@dfci.harvard.edu, Department of Surgery, The Preston Robert Tisch Brain Tumor Center at Duke, Duke University Medical Center, Durham, NC 27710, USA. Department of Pediatrics, The Preston Robert Tisch Brain Tumor Center at Duke, Duke University Medical Center, Durham, NC 27710, USA. Center for Neuro-Oncology, Dana-Farber Cancer Institute, 450 Brookline Avenue, SW-460G, Boston, MA 02215, USA
References
- 1.CBTRUS. CBTRUS: 2010 statistical report: primary brain and central nervous system tumors diagnosed in the United States in 2004–2006. 2010. [Google Scholar]
- 2.Mawrin C, Perry A. Pathological classification and molecular genetics of meningiomas. J Neurooncol. 2010;99:379–391. doi: 10.1007/s11060-010-0342-2. [DOI] [PubMed] [Google Scholar]
- 3.Alexiou GA, Gogou P, Markoula S, Kyritsis AP. Management of meningiomas. Clin Neurol Neurosurg. 2010;112:177–182. doi: 10.1016/j.clineuro.2009.12.011. [DOI] [PubMed] [Google Scholar]
- 4.Wen PY, Quant E, Drappatz J, Beroukhim R, Norden AD. Medical therapies for meningiomas. J Neurooncol. 2010;99:365–378. doi: 10.1007/s11060-010-0349-8. [DOI] [PubMed] [Google Scholar]
- 5.Hanft S, Canoll P, Bruce JN. A review of malignant meningiomas: diagnosis, characteristics, and treatment. J Neurooncol. 2010;99:433–443. doi: 10.1007/s11060-010-0348-9. [DOI] [PubMed] [Google Scholar]
- 6.Mason WP, Gentili F, Macdonald DR, Hariharan S, Cruz CR, Abrey LE. Stabilization of disease progression by hydroxyurea in patients with recurrent or unresectable meningioma. J Neurosurg. 2002;97:341–346. doi: 10.3171/jns.2002.97.2.0341. [DOI] [PubMed] [Google Scholar]
- 7.Newton HB. Hydroxyurea chemotherapy in the treatment of meningiomas. Neurosurg Focus. 2007;23:E11. doi: 10.3171/FOC-07/10/E11. [DOI] [PubMed] [Google Scholar]
- 8.Newton HB, Scott SR, Volpi C. Hydroxyurea chemotherapy for meningiomas: enlarged cohort with extended follow-up. Br J Neurosurg. 2004;18:495–499. doi: 10.1080/02688690400012392. [DOI] [PubMed] [Google Scholar]
- 9.Rosenthal MA, Ashley DL, Cher L. Treatment of high risk or recurrent meningiomas with hydroxyurea. J Clin Neurosci. 2002;9:156–158. doi: 10.1054/jocn.2001.1019S0967586801910197. [DOI] [PubMed] [Google Scholar]
- 10.Loven D, Hardoff R, Sever ZB, Steinmetz AP, Gornish M, Rappaport ZH, Fenig E, Ram Z, Sulkes A. Non-resectable slow-growing meningiomas treated by hydroxyurea. J Neurooncol. 2004;67:221–226. doi: 10.1023/b:neon.0000021827.85754.8e. [DOI] [PubMed] [Google Scholar]
- 11.Schrell UM, Rittig MG, Anders M, Koch UH, Marschalek R, Kiesewetter F, Fahlbusch R. Hydroxyurea for treatment of unresectable and recurrent meningiomas. II. Decrease in the size of meningiomas in patients treated with hydroxyurea. J Neurosurg. 1997;86:840–844. doi: 10.3171/jns.1997.86.5.0840. [DOI] [PubMed] [Google Scholar]
- 12.Wen PY, Yung WK, Lamborn KR, Norden AD, Cloughesy TF, Abrey LE, Fine HA, Chang SM, Robins HI, Fink K, Deangelis LM, Mehta M, Di Tomaso E, Drappatz J, Kesari S, Ligon KL, Aldape K, Jain RK, Stiles CD, Egorin MJ, Prados MD. Phase II study of imatinib mesylate for recurrent meningiomas (North American Brain Tumor Consortium study 01-08) Neuro-oncology. 2009;11:853–860. doi: 10.1215/15228517-2009-010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Norden AD, Raizer JJ, Abrey LE, Lamborn KR, Lassman AB, Chang SM, Yung WK, Gilbert MR, Fine HA, Mehta M, Deangelis LM, Cloughesy TF, Robins HI, Aldape K, Dancey J, Prados MD, Lieberman F, Wen PY. Phase II trials of erlotinib or gefitinib in patients with recurrent meningioma. J Neurooncol. 2010;96:211–217. doi: 10.1007/s11060-009-9948-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Folkman J. Tumor angiogenesis: therapeutic implications. N Engl J Med. 1971;285:1182–1186. doi: 10.1056/NEJM197111182852108. [DOI] [PubMed] [Google Scholar]
- 15.Hicklin DJ, Ellis LM. Role of the vascular endothelial growth factor pathway in tumor growth and angiogenesis. J Clin Oncol. 2005;23:1011–1027. doi: 10.1200/JCO.2005.06.081. [DOI] [PubMed] [Google Scholar]
- 16.Pistolesi S, Boldrini L, Gisfredi S, De Ieso K, Camacci T, Caniglia M, Lupi G, Leocata P, Basolo F, Pingitore R, Parenti G, Fontanini G. Angiogenesis in intracranial meningiomas: immunohistochemical and molecular study. Neuropathol Appl Neurobiol. 2004;30:118–125. doi: 10.1046/j.0305-1846.2003.00516.x. [DOI] [PubMed] [Google Scholar]
- 17.Rogers L, Gilbert M, Vogelbaum MA. Intracranial meningiomas of atypical (WHO grade II) histology. J Neurooncol. 2010;99:393–405. doi: 10.1007/s11060-010-0343-1. [DOI] [PubMed] [Google Scholar]
- 18.Cohen MH, Shen YL, Keegan P, Pazdur R. FDA drug approval summary: bevacizumab (Avastin) as treatment of recurrent glioblastoma multiforme. Oncologist. 2009;14:1131–1138. doi: 10.1634/theoncologist.2009-0121. [DOI] [PubMed] [Google Scholar]
- 19.Barresi V, Tuccari G. Evaluation of neo-angiogenesis in a case of chordoid meningioma. J Neurooncol. 2009;95:445–447. doi: 10.1007/s11060-009-9942-0. [DOI] [PubMed] [Google Scholar]
- 20.Provias J, Claffey K, delAguila L, Lau N, Feldkamp M, Guha A. Meningiomas: role of vascular endothelial growth factor/vascular permeability factor in angiogenesis and peritumoral edema. Neurosurgery. 1997;40:1016–1026. doi: 10.1097/00006123-199705000-00027. [DOI] [PubMed] [Google Scholar]
- 21.Maiuri F, De Caro MB, Esposito F, Cappabianca P, Strazzullo V, Pettinato G, de Divitiis E. Recurrences of meningiomas: predictive value of pathological features and hormonal and growth factors. J Neurooncol. 2007;82:63–68. doi: 10.1007/s11060-005-9078-9. [DOI] [PubMed] [Google Scholar]
- 22.Otsuka S, Tamiya T, Ono Y, Michiue H, Kurozumi K, Daido S, Kambara H, Date I, Ohmoto T. The relationship between peritumoral brain edema and the expression of vascular endothelial growth factor and its receptors in intracranial meningiomas. J Neurooncol. 2004;70:349–357. doi: 10.1007/s11060-004-9164-4. [DOI] [PubMed] [Google Scholar]
- 23.Yoshioka H, Hama S, Taniguchi E, Sugiyama K, Arita K, Kurisu K. Peritumoral brain edema associated with meningioma: influence of vascular endothelial growth factor expression and vascular blood supply. Cancer. 1999;85:936–944. doi: 10.1002/(sici)1097-0142(19990215)85:4<936::aid-cncr23>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 24.Ragel BT, Jensen RL. Aberrant signaling pathways in meningiomas. J Neurooncol. 2010;99:315–324. doi: 10.1007/s11060-010-0381-8. [DOI] [PubMed] [Google Scholar]
- 25.Goldman CK, Bharara S, Palmer CA, Vitek J, Tsai JC, Weiss HL, Gillespie GY. Brain edema in meningiomas is associated with increased vascular endothelial growth factor expression. Neurosurgery. 1997;40:1269–1277. doi: 10.1097/00006123-199706000-00029. [DOI] [PubMed] [Google Scholar]
- 26.Kalkanis SN, Carroll RS, Zhang J, Zamani AA, Black PM. Correlation of vascular endothelial growth factor messenger RNA expression with peritumoral vasogenic cerebral edema in meningiomas. J Neurosurg. 1996;85:1095–1101. doi: 10.3171/jns.1996.85.6.1095. [DOI] [PubMed] [Google Scholar]
- 27.Ding YS, Wang HD, Tang K, Hu ZG, Jin W, Yan W. Expression of vascular endothelial growth factor in human meningiomas and peritumoral brain areas. Ann Clin Lab Sci. 2008;38:344–351. [PubMed] [Google Scholar]
- 28.Friedman HS, Prados MD, Wen PY, Mikkelsen T, Schiff D, Abrey LE, Yung WK, Paleologos N, Nicholas MK, Jensen R, Vredenburgh J, Huang J, Zheng M, Cloughesy T. Bevacizumab alone and in combination with irinotecan in recurrent glioblastoma. J Clin Oncol. 2009;27:4733–4740. doi: 10.1200/JCO.2008.19.8721. [DOI] [PubMed] [Google Scholar]
- 29.Kreisl TN, Kim L, Moore K, Duic P, Royce C, Stroud I, Garren N, Mackey M, Butman JA, Camphausen K, Park J, Albert PS, Fine HA. Phase II trial of single-agent bevacizumab followed by bevacizumab plus irinotecan at tumor progression in recurrent glioblastoma. J Clin Oncol. 2009;27:740–745. doi: 10.1200/JCO.2008.16.3055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wen PY, Macdonald DR, Reardon DA, Cloughesy TF, Sorensen AG, Galanis E, Degroot J, Wick W, Gilbert MR, Lassman AB, Tsien C, Mikkelsen T, Wong ET, Chamberlain MC, Stupp R, Lamborn KR, Vogelbaum MA, van den Bent MJ, Chang SM. Updated response assessment criteria for high-grade gliomas: response assessment in neuro-oncology working group. J Clin Oncol. 2010;28:1963–1972. doi: 10.1200/JCO.2009.26.3541. [DOI] [PubMed] [Google Scholar]
- 31.Puchner MJ, Hans VH, Harati A, Lohmann F, Glas M, Herrlinger U. Bevacizumab-induced regression of anaplastic meningioma. Ann Oncol. 2010;21:2445–2446. doi: 10.1093/annonc/mdq634. [DOI] [PubMed] [Google Scholar]
- 32.DeBoer R, Grimm SA, Chandler J, Gallot L, Kennedy S, Burns K, Rice L, Cabreza C, Muro K, Raizer JJ. A phase II trial of PTK-787 (PTK/ZK) in recurrent or progressive meningiomas. J Clin Oncol; 2008; ASCO annual meeting proceedings (post-meeting edition); 2008. p. 2060. [Google Scholar]
- 33.Reardon DA, Norden AD, Desjardins A, Vredenburgh JJ, Herndon JE, II, Coan A, Sampson JH, Gururangan S, Peters KB, McLendon RE, Norfleet JA, Lipp ES, Drappatz J, Wen PY, Friedman HS. Phase II study of Gleevec((R)) plus hydroxyurea (HU) in adults with progressive or recurrent meningioma. J J Neurooncol. 2011 doi: 10.1007/s11060-011-0687-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chamberlain MC, Johnston SK. Hydroxyurea for recurrent surgery and radiation refractory meningioma: a retrospective case series. J Neurooncol. 2011 doi: 10.1007/s11060-011-0541-5. [DOI] [PubMed] [Google Scholar]
- 35.Chamberlain MC, Tsao-Wei DD, Groshen S. Salvage chemotherapy with CPT-11 for recurrent meningioma. J Neurooncol. 2006;78:271–276. doi: 10.1007/s11060-005-9093-x. [DOI] [PubMed] [Google Scholar]
- 36.Hahn BM, Schrell UM, Sauer R, Fahlbusch R, Ganslandt O, Grabenbauer GG. Prolonged oral hydroxyurea and concurrent 3d-conformal radiation in patients with progressive or recurrent meningioma: results of a pilot study. J Neurooncol. 2005;74:157–165. doi: 10.1007/s11060-004-2337-3. [DOI] [PubMed] [Google Scholar]
- 37.Chamberlain MC, Tsao-Wei DD, Groshen S. Temozolomide for treatment-resistant recurrent meningioma. Neurology. 2004;62:1210–1212. doi: 10.1212/01.wnl.0000118300.82017.f4. [DOI] [PubMed] [Google Scholar]
- 38.Chamberlain MC, Glantz MJ. Interferon-alpha for recurrent World Health Organization grade 1 intracranial meningiomas. Cancer. 2008;113:2146–2151. doi: 10.1002/cncr.23803. [DOI] [PubMed] [Google Scholar]
- 39.Johnson DR, Kimmel DW, Burch PA, Cascino TL, Giannini C, Wu W, Buckner JC. Phase II study of subcutaneous octreotide in adults with recurrent or progressive meningioma and meningeal hemangiopericytoma. Neuro Oncol. 2011;13:530–535. doi: 10.1093/neuonc/nor044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chamberlain MC, Glantz MJ, Fadul CE. Recurrent meningioma: salvage therapy with long-acting somatostatin analogue. Neurology. 2007;69:969–973. doi: 10.1212/01.wnl.0000271382.62776.b7. [DOI] [PubMed] [Google Scholar]
- 41.Goodwin JW, Crowley J, Eyre HJ, Stafford B, Jaeckle KA, Townsend JJ. A phase II evaluation of tamoxifen in un-resectable or refractory meningiomas: a Southwest Oncology Group study. J Neurooncol. 1993;15:75–77. doi: 10.1007/BF01050266. [DOI] [PubMed] [Google Scholar]
- 42.Grunberg SM, Rankin C, Townsend J, et al. Phase III double-blind randomized placebo-controlled study of mifepristone (RU) for the treatment of unreasectable meningioma. Proc Am Soc Clin Oncol. 2001;20:222. [Google Scholar]