Summary
Background
Many people with epilepsy in low-income countries do not receive appropriate biomedical treatment. This epilepsy treatment gap might be caused by patients not seeking biomedical treatment or not adhering to prescribed antiepileptic drugs (AEDs). We measured the prevalence of and investigated risk factors for the epilepsy treatment gap in rural Kenya.
Methods
All people with active convulsive epilepsy identified during a cross-sectional survey of 232 176 people in Kilifi were approached. The epilepsy treatment gap was defined as the percentage of people with active epilepsy who had not accessed biomedical services or who were not on treatment or were on inadequate treatment. Information about risk factors was obtained through a questionnaire-based interview of sociodemographic characteristics, socioeconomic status, access to health facilities, seizures, stigma, and beliefs and attitudes about epilepsy. The factors associated with people not seeking biomedical treatment and not adhering to AEDs were investigated separately, adjusted for age.
Findings
673 people with epilepsy were interviewed, of whom 499 (74%) reported seeking treatment from a health facility. Blood samples were taken from 502 (75%) people, of whom 132 (26%) reported taking AEDs, but 189 (38%) had AEDs detectable in the blood. The sensitivity and specificity of self-reported adherence compared with AEDs detected in blood were 38·1% (95% CI 31·1–45·4) and 80·8% (76·0–85·0). The epilepsy treatment gap was 62·4% (58·1–66·6). In multivariable analysis, failure to seek biomedical treatment was associated with a patient holding traditional animistic religious beliefs (adjusted odds ratio 1·85, 95% CI 1·11–2·71), reporting negative attitudes about biomedical treatment (0·86, 0·78–0·95), living more than 30 km from health facilities (3·89, 1·77–8·51), paying for AEDs (2·99, 1·82–4·92), having learning difficulties (2·30, 1·29–4·11), having had epilepsy for longer than 10 years (4·60, 2·07–10·23), and having focal seizures (2·28, 1·50–3·47). Reduced adherence was associated with negative attitudes about epilepsy (1·10, 1·03–1·18) and taking of AEDs for longer than 5 years (3·78, 1·79–7·98).
Interpretation
The sensitivity and specificity of self-reported adherence is poor, but on the basis of AED detection in blood almost two-thirds of patients with epilepsy were not on treatment. Education about epilepsy and making AEDs freely available in health facilities near people with epilepsy should be investigated as potential ways to reduce the epilepsy treatment gap.
Funding
Wellcome Trust.
Introduction
More than 62 million people with epilepsy live in countries with low and middle incomes1 and most of them do not receive appropriate treatment, an issue known as the epilepsy treatment gap.2 The epilepsy treatment gap is defined as the number of people with active epilepsy who have not accessed biomedical services (ie, epilepsy not diagnosed) or who are not on treatment or are on inadequate treatment, expressed as a percentage of the total number with active epilepsy.2 In a systematic review of studies done in countries with low and middle incomes, the overall estimate of the epilepsy treatment gap based on self-reporting was 56%, but the 95% CI was wide, ranging from 31% to 100%, with a higher proportion in rural areas than in urban areas.3 A more recent review reported an epilepsy treatment gap of more than 75% in low-income countries compared with less than 10% in high-income countries.4
The epilepsy treatment gap could be caused by poor access to diagnosis and treatment of epilepsy or non-adherence to antiepileptic drugs (AEDs). Adherence to a drug regimen is defined as the extent to which patients take drugs as prescribed by their health-care providers.5 Unintentional non-adherence might be due to forgetfulness or inability to follow treatment instructions because of poor understanding or impairment (eg, poor eyesight), whereas intentional non-adherence arises when the patient rejects either the doctor's diagnosis or recommended treatment. Between 25% and 75% of people with epilepsy do not follow prescribed drug regimens, leading to uncontrolled seizures and reduced quality of life.6
Health-care seeking behaviour and adherence are complex issues affected by many factors. They can be explored with Andersen's behavioural model,7 which considers that health service use and adherence are a function of four categories: health-care system factors (eg, distance to health facilities); predisposing factors (eg, health beliefs); enabling factors (eg, transportation); and need, which refers to severity of illness and whether people judge their illness to be of sufficient magnitude to consult health services.
We determined the treatment gap, investigated the reliability of self-reporting of adherence, and examined factors associated with failure to seek biomedical treatment and non-adherence in people with epilepsy in a rural area of Kenya.
Methods
Participants
We undertook a cross-sectional survey and risk-factor analysis of the epilepsy treatment gap. The study was done in the Kilifi health and demographic surveillance system (KHDSS) with 233 881 residents in 2008.8 The residents are mainly Mijikenda, a Bantu group of nine tribes with Giriama (45%) dominating. The average per head income is about 700 Kenyan Shillings (KES; US$8) per month and about 55% of the population is regarded as poor. Most people (80%) depend on subsistence farming. Literacy is low (45%).9 KHDSS is served by one district hospital (Kilifi District Hospital, KDH), one health centre, and 12 dispensaries. KDH serves as a primary care centre and first-level referral facility for the district and stocks four AEDs: phenobarbital, phenytoin, carbamazepine, and sodium valproate. The health centres and dispensaries stock only phenobarbital.
People with epilepsy were identified in a three-stage cross-sectional survey that was done to establish the prevalence of active convulsive epilepsy as previously described.10 In the first stage, household heads were asked if anybody in the household had convulsions. People who were reported to have seizures were interviewed with an epilepsy-specific questionnaire11 and those who were classed as positive were invited to be assessed by a clinician. Active convulsive epilepsy was defined as two or more unprovoked tonic or clonic seizures in a lifetime, of which at least one seizure was within the 12 months preceding the survey, since this criterion is used for prescription of AEDs in Kenya,12 is used in other parts of Africa,13, 14 and is recommended by the International League Against Epilepsy for calculation of the treatment gap,2, 15 and there is poor documentation of previous seizures and recall beyond a year in this region. The study was reviewed and approved by the Kenya Medical Research Institute and national scientific and ethical review committees and the Swiss Tropical and Public Health Research Committee. All participants or their carers provided written informed consent.
Procedures
Questionnaires based on those used in previous studies of epilepsy in this area10, 16, 17, 18, 19 and elsewhere20, 21 were developed in English and translated into local languages. In addition to the outcomes of not seeking treatment and non-adherence to AEDs, we obtained data for several other items: demographic details of the person with epilepsy (age and sex), social characteristics (religion,22 education, occupation, and marital status) of either the person with epilepsy or their carer if the carer made decisions about seeking treatment and taking AEDs if prescribed; socioeconomic data for the households based on assets;23 accessibility factors (distance from home to health facility, and payment for AEDs); severity of epilepsy (duration of epilepsy, seizure frequency, type of seizures, and injury during a seizure); stigma; and beliefs and attitudes about epilepsy. People with learning difficulties were defined as those who were not orientated for person, place, or the period of day (morning or afternoon) and who had difficulty following instructions—eg, finger nose test—without a neurological deficit as assessed by the clinician. For analysis of non-adherence, details of prescribed regimen were obtained (number of prescribed AEDs, frequency of taking AEDs per day, side-effects of AEDs, and number of years a person with epilepsy has taken AEDs). The field staff administered questionnaires to all people with a confirmed diagnosis of active convulsive epilepsy after written informed consent. If the person with epilepsy was a child or cognitively impaired adult, a caregiver was interviewed. For the risk factor analysis, people who sought biomedical treatment were compared with those who had not sought treatment, and those who adhered (assessed by presence of AEDs in blood) were compared with those who did not have AEDs detected in their blood.
Data were obtained from households for assets and indicators of household characteristics such as livestock and source of energy for cooking and lighting. Principal component analysis was done to construct a homestead wealth assets index from the range of assets and household characteristics.24 Households were classified into socioeconomic status quintiles on the basis of this index.23
Perceived stigma and epilepsy beliefs and attitudes were measured with Likert scales. The Kilifi stigma scale for epilepsy had 15 questions and measured only perceived stigma.25 The Kilifi epilepsy beliefs and attitude scale had 34 questions, divided into five subscales: causes of epilepsy, biomedical treatment of epilepsy, cultural treatment of epilepsy, risk and safety concerns, and negative stereotypes about epilepsy.26
To calculate distance to health facilities, homesteads and health facilities were mapped with a global positioning system and the Euclidean distance was calculated with the coordinates as a reflection of the time taken to travel the distance. The health facility used was defined as the one where people with epilepsy reported seeking treatment most frequently. Treatment-seeking behaviour was established by asking whether the person had ever sought treatment for epilepsy from a health facility before the 2008 epidemiological survey. People answering “yes” were asked to identify the health facility from which they sought treatment.
Questions were asked about the AEDs that patients were currently taking (actual tablets were shown on a board to aid recognition). Reported adherence was assessed with the Morisky medication adherence scale, a four-item scale with high reliability and validity27 that has been used to measure adherence in epilepsy.28, 29
To measure adherence, blood samples (2 mL) were collected. Phenobarbital was measured in all samples, with phenytoin and carbamazepine measured in those from participants taking these AEDs, with a TDx FLx analyser (Abbott Laboratories, Abbott Park, IL, USA). An individual was defined as adherent if AEDs were detected in their blood, with detectable limits of 1·1 μg/mL for phenobarbital, 1·0 μg/mL for phenytoin, and 0·5 μg/mL for carbamazepine. Since the half-lives of phenytoin and carbamazepine (the AEDs with the shortest elimination half-lives used in this area) are greater than 5 h,30 and it takes at least five half-lives to clear the drug after one dose, the absence of AED in the blood suggests that the drug has not been taken within at least the 24 h before the blood sample was taken. The optimum therapeutic ranges were defined as 10–40 μg/mL for phenobarbital, 10–20 μg/mL for phenytoin, and 4–12 μg/mL for carbamazepine.31
Statistical analysis
Data were double-entered and verified with a MySQL V5 database. Statistical analyses were done with Stata versions 11 and 12. We investigated treatment seeking for all study participants interviewed, whereas non-adherence was investigated only in those who reported taking AEDs and gave a blood sample. The epilepsy treatment gap was defined as the number of people with active epilepsy who had not accessed biomedical services or who were not on treatment or were on inadequate treatment, expressed as a percentage of the total number with active epilepsy.2 The sensitivity, specificity, and confidence intervals were calculated in Stata. Concordance was assessed between self-reported adherence and AED detection in blood with the κ statistic. In exploratory analyses, we examined the distributions of the dependent variables before selecting the appropriate test statistic. We used Pearsons' χ2 test to measure associations between categorical sociodemographic characteristics and failure to seek treatment or non-adherence.
Univariate associations for two outcomes (failure to seek treatment or non-adherence to AED) were investigated with logistic regression, adjusted for age. We included variables with p≤0·20 in a multivariable model using a backward elimination process. Variables with p≤0·05 in multivariable analysis were retained in the final model. Attributable fractions were calculated with the final multivariable logistic regression model by a user-written command “punaf” in STATA version 12.32, 33
Role of the funding source
The sponsor of the study had no role in the study design, data collection, data analysis, data interpretation, or writing of the report. The corresponding author had full access to all the data in this study and had final responsibility for the decision to submit for publication.
Results
The epidemiological survey included 232 176 (99%) of 233 881 people living in the KHDSS region during 2008. 699 people with epilepsy were identified, of whom 673 (96%) were interviewed (figure). 392 (58%) participants were younger than 18 years. Many adults (133 [47%]) had no formal education.
Figure.
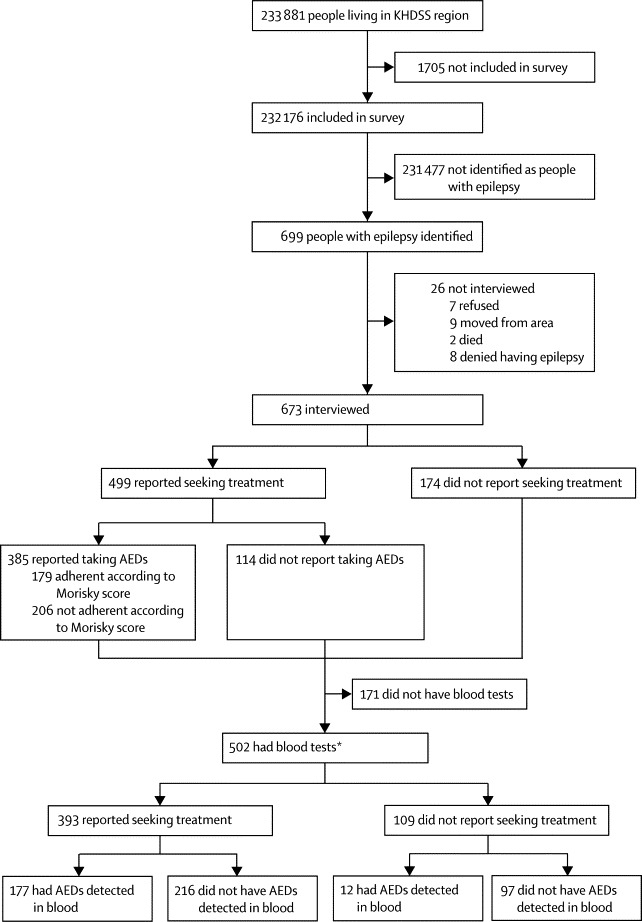
Study profile
KHDSS=Kilifi health and demographic surveillance system. AED=antiepileptic drug. *Complete data available for 341 participants: 172 judged to be adherent on the basis of AED detection in blood, 169 did not have AED detected in blood.
Of the 673 people with epilepsy who were interviewed, 499 (74%) reported seeking treatment for epilepsy from a health facility, of whom 395 (79%) used KDH as their primary source of AEDs (appendix p 1). 502 (75%) gave blood samples. People who did not give blood samples were significantly younger, were more likely to have traditional religious (animist) beliefs, were more likely to believe that epilepsy responds to biomedical treatment, were more likely to be married (if adults), less likely to pay for AEDs, had epilepsy for a shorter period, had less frequent seizures, and were less likely to injure themselves during the seizures compared with people who provided samples (appendix pp 2–3). Of the 502 people in whom blood concentrations of AEDs were measured, 393 reported seeking treatment, of whom 117 (40%) were on polytherapy. Phenobarbital was detected in the blood of 151 people with epilepsy, phenytoin in 50, and carbamazepine in 48. Four patients reported taking sodium valproate (which was not measured in blood), but two of these patients were taking one of the other AEDs, and the other two had not collected their drug from KDH (the only source of sodium valproate) within the past 3 months and were classified as non-adherent. The epilepsy treatment gap based on AEDs detected in blood samples was 62·4% (95% CI 58·1–66·6).
We studied 19 variables as potential predictors of people not seeking biomedical treatment (table 1). Of these, 16 had univariate p values of 0·20 or less and were included in the multivariable logistic regression model. From the multivariable analysis, people with epilepsy were less likely to seek treatment if they had traditional animistic religious beliefs, had negative attitudes about biomedical treatment, lived 20 or more km from where they sought health care, reported paying for AEDs, had epilepsy for longer than 10 years, had learning difficulties, and had focal seizures (table 2). Attributable fraction analysis indicated that treatment seeking could improve by 11% (95% CI 1–21) if people with epilepsy thought that biomedical treatment would help, 40% (21–54) if AEDs were free, and 12% (3–21) if the health facilities where people obtained AEDs were less than 20 km from their homes.32, 33
Table 1.
Univariate analysis of factors associated with failure to seek biomedical treatment
| Sought biomedical treatment (n=499) | Never sought biomedical treatment (n=174) | Odds ratio*(95% CI) | p value | |
|---|---|---|---|---|
| Sex | ||||
| Female | 239 (48%) | 93 (53%) | 1·0 | .. |
| Male | 260 (52%) | 81 (47%) | 0·81 (0·57–1·14) | 0·227 |
| Religion | ||||
| Christianity | 237 (47%) | 58 (33%) | 1·0 | .. |
| Islam | 58 (12%) | 23 (13%) | 1·65 (0·94–2·90) | 0·081 |
| Traditional | 204 (41%) | 93 (53%) | 1·83 (1·26–2·68) | 0·002 |
| Education | ||||
| None | 231 (46%) | 75 (43%) | 1·0 | .. |
| Primary | 227 (45%) | 89 (51%) | 1·28 (0·89–1·84) | 0·178 |
| Secondary | 31 (6%) | 9 (5%) | 0·96 (0·44–2·13) | 0·927 |
| Tertiary | 10 (2%) | 1 (1%) | 0·26 (0·03–2·09) | 0·207 |
| Marital status | ||||
| Single | 97 (19%) | 20 (11%) | 1·0 | .. |
| Married | 313 (63%) | 121 (70%) | 1·97 (1·16–3·34) | 0·012 |
| Separated | 9 (2%) | 2 (1%) | 0·94 (0·18–4·75) | 0·940 |
| Divorced | 27 (5%) | 12 (7%) | 2·18 (0·94–5·02) | 0·068 |
| Widowed | 53 (11%) | 19 (11%) | 1·65 (0·81–3·39) | 0·168 |
| Occupation | ||||
| Subsistence farmer | 271 (54%) | 102 (59%) | 1·0 | .. |
| Trader | 111 (22%) | 34 (20%) | 0·85 (0·54–1·33) | 0·473 |
| Casual | 41 (8%) | 20 (11%) | 1·30 (0·73–2·33) | 0·372 |
| Other | 76 (15%) | 18 (10%) | 0·60 (0·34–1·06) | 0·079 |
| Socioeconomic status | ||||
| Least poor | 110 (22%) | 24 (14%) | 1·0 | .. |
| Less poor | 95 (19%) | 39 (22%) | 0·99 (0·58–1·71) | 0·981 |
| Poor | 94 (19%) | 40 (23%) | 1·21 (0·71–2·01) | 0·489 |
| Very poor | 97 (19%) | 35 (20%) | 1·15 (0·68–1·97) | 0·599 |
| Most poor | 103 (21%) | 36 (21%) | 0·62 (0·35–1·11) | 0·108 |
| Beliefs about causes of epilepsy† | ||||
| Median | 8 (5–10) | 8 (4–10) | 0·90 (0·85–0·95) | 0·0002 |
| Beliefs about biomedical treatment† | ||||
| Median | 16 (15–16) | 16 (14–16) | 0·84 (0·78–0·91) | <0·0001 |
| Beliefs about cultural treatment† | ||||
| Median | 12 (8–15) | 12 (7–15) | 0·98 (0·94–1·01) | 0·243 |
| Risk and safety concerns about epilepsy† | ||||
| Median | 8 (8–8) | 8 (7–8) | 0·90 (0·79–1·02) | 0·109 |
| Stereotype about epilepsy† | ||||
| Median | 8 (6–12) | 8 (5–12) | 1·00 (0·96–1·04) | 0·915 |
| Stigma scores‡ | ||||
| Median | 7 (2–13) | 6 (2–12) | 0·98 (0·96–1·01) | 0·131 |
| Distance to health facility | ||||
| <10 km | 177 (35%) | 36 (21%) | 1·0 | .. |
| 10–19 km | 169 (34%) | 65 (37%) | 1·82 (1·15–2·89) | 0·011 |
| 20–30 km | 127 (25%) | 51 (29%) | 1·91 (1·18–3·11) | 0·009 |
| >30 km | 26 (5%) | 22 (13%) | 4·02 (2·04–7·89) | 0·021 |
| Payment for AEDs | ||||
| No | 195 (39%) | 32 (18%) | 1·0 | .. |
| Yes | 304 (61%) | 142 (82%) | 2·92 (1·91–4·48) | <0·0001 |
| Learning difficulties | ||||
| No | 346 (69%) | 146 (84%) | 1·0 | .. |
| Yes | 153 (31%) | 28 (16%) | 2·23 (1·42–3·50) | 0·0005 |
| Duration of epilepsy | ||||
| <1 year | 18 (4%) | 26 (15%) | 1·0 | .. |
| 1–5 years | 167 (33%) | 70 (40%) | 1·05 (0·62–1·78) | 0·086 |
| 6–10 years | 121 (24%) | 28 (16%) | 2·03 (1·32–3·19) | 0·001 |
| >10 years | 193 (39%) | 50 (29%) | 7·20 (3·57–14·5) | <0·0001 |
| Number of seizures in past 3 months | ||||
| None | 146 (29%) | 63 (36%) | 1·0 | .. |
| 1–3 | 166 (33%) | 54 (31%) | 0·73 (0·47–1·12) | 0·144 |
| 4–6 | 72 (14%) | 22 (13%) | 0·72 (0·41–1·23) | 0·246 |
| >6 | 115 (23%) | 35 (20%) | 0·71 (0·44–1·16) | 0·170 |
| Focal seizures | ||||
| No | 157 (31%) | 88 (51%) | 1·0 | .. |
| Yes | 342 (69%) | 86 (49%) | 2·15 (1·51–3·07) | <0·0001 |
| Injury during seizure | ||||
| No | 230 (46%) | 102 (59%) | 1·0 | .. |
| Yes | 269 (54%) | 72 (41%) | 0·50 (0·35–0·72) | 0·0002 |
Data are number of patients (%), median (IQR), odds ratio (95% CI), or p value. AED=antiepileptic drug. For the variables of religion, education, marital status, occupation, socioeconomic status, beliefs and attitudes, and stigma scores, the information pertains to either the person with epilepsy, or if the person with epilepsy was a child or cognitively impaired, to the carer, since the carer made the decision about treatment seeking and giving the AED.
Adjusted for age.
Scores of the Kilifi belief and attitude scale were based on a Likert scale (0=not at all, 1=believe a little, 2=totally believe), which were summated for the subscale score; higher scores represent more positive beliefs about biomedical treatment.26
Scores of the Kilifi stigma scale for epilepsy were based on a Likert scale (0=not at all, 1=sometimes, 2=always), which were summated; higher scores represent more stigmatisation.25
Table 2.
Multivariable analysis of factors associated with failure to seek biomedical treatment
| Odds ratio*(95% CI) | p value | ||
|---|---|---|---|
| Predisposing factors | |||
| Religion | |||
| Christian | 1·0 | .. | |
| Islam | 1·52 (0·79–2·91) | 0·211 | |
| Traditional | 1·85 (1·11–2·71) | 0·015 | |
| Negative beliefs about biomedical treatment | 0·86 (0·78–0·95) | 0·003 | |
| Health-care system factors | |||
| Distance to health facility | |||
| <10 km | 1·0 | .. | |
| 10–19 km | 1·56 (0·92–2·62) | 0·097 | |
| 20–30 km | 1·93 (1·11–3·35) | 0·019 | |
| >30 km | 3·89 (1·77–8·51) | 0·0007 | |
| Enabling factors | |||
| Payment for AEDs | |||
| No | 1·0 | .. | |
| Yes | 2·99 (1·82–4·92) | <0·0001 | |
| Need-specific and disease-specific factors | |||
| Learning difficulties | |||
| No | 1·0 | .. | |
| Yes | 2·30 (1·29–4·11) | 0·005 | |
| Duration of epilepsy | |||
| <1 year | 1·0 | .. | |
| 1–5 years | 0·72 (0·40–1·30) | 0·280 | |
| 6–10 years | 1·08 (0·63–1·87) | 0·764 | |
| >10 years | 4·60 (2·07–10·23) | <0·0001 | |
| Focal seizures | |||
| No | 1·0 | .. | |
| Yes | 2·28 (1·50–3·47) | 0·0001 | |
AED=antiepileptic drug. For the variables of religion, beliefs, and attitudes about epilepsy, the information pertains to either the person with epilepsy, or if the person with epilepsy was a child or cognitively impaired, to the carer, since the carer made the decision about treatment seeking and giving the AED.
Adjusted for age.
Of the 499 people who reported seeking biomedical treatment, only 385 (77%) were prescribed AEDs and the person with epilepsy or their carer were able to answer the questions on the Morisky scale. On the basis of Morisky scores, 179 (46%) people with epilepsy were adherent (score of 0) and 206 (54%) were non-adherent (score >0; table 3). Of the 502 who had AEDs measured in blood, 189 (38%) had AEDs detected of whom 72 reported taking AEDs. In the 313 who did not have any AED detected, 60 (19%) reported taking AEDs. 48 (25%) of the 189 people with AEDs detected in their blood had concentrations in the detectable but not optimum range and 141 (75 %) were in the optimum range. Overall, the sensitivity and specificity of reported adherence compared with AEDs detected in the blood were 38·1% (95% CI 31·1–45·4) and 80·8% (76·0–85·0), respectively. The concordance between self-reported adherence and detection in blood for the three prescribed AEDs occurred in 72 (55%) of 132 people with epilepsy. There was little agreement between adherence as measured by blood samples and the Morisky scores.
Table 3.
Adherence as assessed with the self-reported Morisky scale compared with that as assessed by detection of AEDs in blood samples
| Patients who answered “No”* | Patients with AED detected in blood who answered “No”† | κ‡ | |
|---|---|---|---|
| Do you ever forget to take your medication? | 112 (29%) | 41 (37%) | −0·048 |
| Are you careless at times about taking your medication? | 114 (30%) | 37 (32%) | 0·099 |
| When you feel better, do you sometimes stop taking your medication? | 44 (11%) | 19 (43%) | −0·004 |
| Sometimes, if you feel worse when you take your medication, do you stop taking it? | 29 (8%) | 13 (45%) | −0·022 |
Data are n (%), n, %, or κ. AED=antiepileptic drug.
Percentages of the 385 people with epilepsy who were prescribed AEDs and they or their carer was able to answer the questions on the Morisky scale.
Percentages of the participants who had answered “No” to the relevant Morisky question.
κ score of “No” on Morisky scale versus detectable blood concentrations of AEDs.
We studied 25 variables as potential predictors of non-adherence (table 4). 15 were retained in the multivariable logistic regression model. People with epilepsy were less likely to adhere if they had negative attitudes such as feeling that they were rejected or had taken AEDs for more than 5 years (table 5). Attributable fraction analysis indicated that adherence could be improved by 20% (95% CI 7–30) if attitudes could be modified.
Table 4.
Univariate analysis of factors associated with non-adherence to AEDs
| Non-adherent people (n=172) | Adherent people (n=169) | Odds ratio*(95% CI) | p value | |
|---|---|---|---|---|
| Sex | ||||
| Female | 74 (43%) | 82 (49%) | 1·0 | .. |
| Male | 98 (57%) | 87 (51%) | 1·25 (0·81–1·92) | 0·299 |
| Religion | ||||
| Christianity | 85 (49%) | 91 (54%) | 1·0 | |
| Islam | 23 (13%) | 18 (11%) | 1·38(0·70–2·75) | 0·352 |
| Traditional | 60 (36%) | 64 (37%) | 1·13 (0·71–1·79) | 0·613 |
| Education | ||||
| None | 70 (41%) | 74 (44%) | 1·0 | .. |
| Primary | 85 (49%) | 76 (45%) | 1·20 (0·76–1·88) | 0·433 |
| Secondary | 11 (6%) | 16 (9%) | 0·75 (0·32–1·74) | 0·502 |
| Tertiary | 6 (3%) | 3 (2%) | 2·03 (0·48–8·46) | 0·336 |
| Marital status | ||||
| Single | 34 (20%) | 52 (31%) | 1·0 | .. |
| Married | 108 (63%) | 82 (49%) | 2·02 (1·20–3·39) | 0·008 |
| Separated | 1 (1%) | 5 (3%) | 0·29 (0·03–2·60) | 0·267 |
| Divorced | 9 (5%) | 10 (6%) | 1·40 (0·52–3·81) | 0·508 |
| Widowed | 20 (12%) | 20 (12%) | 1·53 (0·72–3·26) | 0·270 |
| Occupation of household head | ||||
| Farmer | 90 (52%) | 87 (51%) | 1·0 | .. |
| Trader | 41 (24%) | 36 (21%) | 1·13 (0·66–1·93) | 0·665 |
| Casual | 18 (11%) | 17 (10%) | 1·03 (0·50–2·13) | 0·934 |
| Other | 23 (13%) | 29 (17%) | 0·77 (0·42–1·45) | 0·428 |
| Social economic status | ||||
| Least poor | 38 (22%) | 50 (30%) | 1·0 | .. |
| Less poor | 36 (21%) | 35 (21%) | 1·64 (0·77–3·48) | 0·196 |
| Poor | 36 (21%) | 34 (20%) | 1·11 (0·55–2·25) | 0·774 |
| Very poor | 35 (20%) | 22 (13%) | 1·08 (0·53–2·19) | 0·831 |
| Most poor | 27 (16%) | 28 (17%) | 0·79 (0·40–1·55) | 0·489 |
| Availability of family support | ||||
| No | 19 (11%) | 40 (24%) | 1·0 | .. |
| Yes | 143 (83%) | 129 (76%) | 1·58 (0·92–2·71) | 0·097 |
| Unknown | 10 (6%) | 0 | .. | .. |
| Beliefs about causes of epilepsy† | ||||
| Median | 8 (5–10) | 8 (6–10) | 0·94 (0·87–1·01) | 0·113 |
| Beliefs about biomedical treatment† | ||||
| Median | 16 (15–16) | 16 (15–16) | 0·92 (0·80–1·04) | 0·178 |
| Beliefs about cultural treatment† | ||||
| Median | 12 (8–16) | 13 (9–16) | 0·99 (0·95–1·04) | 0·716 |
| Risk and safety concerns about epilepsy† | ||||
| Median | 8 (7–8) | 8 (8–8) | 0·82 (0·68–0·98) | 0·034 |
| Beliefs about epilepsy† | ||||
| Median | 8 (4–11) | 10 (6–13) | 1·08 (1·03–1·15) | 0·002 |
| Stigma scores‡ | ||||
| Median | 7 (2–13) | 9 (4–15) | 0·97 (0·94–1·00) | 0·088 |
| Learning difficulties | ||||
| No | 127 (74%) | 107 (63%) | 1·0 | .. |
| Yes | 45 (26%) | 62 (37%) | 0·62 (0·39–0·99) | 0·047 |
| Distance to health facility | ||||
| <10 km | 44 (26%) | 63 (37%) | 1·0 | .. |
| 10–19 km | 61 (35%) | 45 (27%) | 1·92 (1·11–3·31) | 0·019 |
| 20–30 km | 58 (34%) | 49 (29%) | 1·70 (0·99–2·94) | 0·053 |
| >30 km | 9 (5%) | 12 (7%) | 1·07 (0·41–2·75) | 0·893 |
| Good relation with health-care provider | ||||
| No | 19 (11%) | 24 (14%) | 1·0 | .. |
| Yes | 153 (89%) | 145 (86%) | 1·34 (0·70–2·55) | 0·374 |
| Payment for AEDs | ||||
| No | 116 (67%) | 88 (52%) | 1 | .. |
| Yes | 56 (33%) | 81 (48%) | 1·97(1·26–3·06) | 0·003 |
| AEDs stored out of sight | ||||
| No | 127 (74%) | 107 (63%) | 1·0 | .. |
| Yes | 45 (26%) | 62 (37%) | 1·62 (1·02–2·57) | 0·043 |
| Duration of epilepsy | ||||
| <1 years | 4 (2%) | 1 (1%) | 1·0 | .. |
| 1–9 years | 91 (53%) | 87 (51%) | 0·26 (0·03–2·41) | 0·238 |
| 10–20 years | 51 (30%) | 60 (36%) | 0·21 (0·02–1·97) | 0·173 |
| >20 years | 26 (15%) | 21 (12%) | 0·30 (0·03–2·88) | 0·294 |
| Focal seizures | ||||
| No | 54 (31%) | 50 (30%) | 1·0 | .. |
| Yes | 118 (69%) | 119 (70%) | 0·91 (0·57–1·45) | 0·683 |
| Number of seizures in past 3 months | ||||
| None | 46 (27%) | 42 (25%) | 1·0 | .. |
| 1–3 | 68 (40%) | 44 (26%) | 1·41 (0·80–2·49) | 0·230 |
| 4–6 | 28 (16%) | 28 (17%) | 0·92 (0·47–1·81) | 0·811 |
| >6 | 30 (17%) | 55 (33%) | 0·50 (0·27–0·93) | 0·029 |
| Duration of AED treatment | ||||
| <1 year | 49 (28%) | 17 (10%) | 1·0 | .. |
| 1–3 years | 52 (30%) | 48 (28%) | 1·68 (0·83–3·39) | 0·147 |
| 4–5 years | 21 (12%) | 21 (12%) | 1·80 (1·06–3·04) | 0·029 |
| >5 years | 50 (29%) | 83 (49%) | 4·76 (2·47–9·15) | <0·0001 |
| Number of AEDs | ||||
| Monotherapy | 115 (67%) | 87 (51%) | 1·0 | |
| Polytherapy | 57 (33%) | 82 (49%) | 0·52 (0·34–0·81) | 0·004 |
| Reported side-effects of AEDs | ||||
| No | 156 (91%) | 157 (93%) | 1·0 | |
| Yes | 16 (9%) | 12 (7%) | 1·36 (0·62–2·96) | 0·460 |
| Injury during seizure | ||||
| No | 69 (40%) | 54 (32%) | 1·0 | |
| Yes | 103 (60%) | 115 (68%) | 0·65 (0·41–1·04) | 0·071 |
Data are number of patients (%), median (IQR), odds ratio (95% CI), or p value. The 341 patients included in this table are drawn from the 393 who reported seeking treatment, and had more than 90% data available for the variables examined. For the variables of religion, education, marital status, occupation, socioeconomic status, beliefs and attitudes, and stigma scores, the information pertains to either the person with epilepsy, or if the person with epilepsy was a child or cognitively impaired, to the carer, since the carer made the decision about treatment seeking and giving the AED. AED=antiepileptic drug.
Adjusted for age.
Scores of the Kilifi belief and attitude scale were based on a Likert scale (0=not at all, 1=believe a little, 2=totally believe), which were summated for the subscale score; higher scores represent more positive beliefs about biomedical treatment.26
Scores of the Kilifi stigma scale for epilepsy were based on a Likert scale (0=not at all, 1=sometimes, 2=always), which were summated; higher scores represent more stigmatisation.25
Table 5.
Multivariable analysis of factors associated with non-adherence to AEDs
| Odds ratio*(95% CI) | p value | |
|---|---|---|
| Attitudes | ||
| Negative attitudes about epilepsy | 1·10 (1·03–1·18) | 0·004 |
| Duration of AED treatment | ||
| <1 year | 1·0 | |
| 1–3 years | 1·75 (0·79–3·87) | 0·165 |
| 4–5 years | 1·79 (0·98–3·29) | 0·059 |
| >5 years | 3·78 (1·79–7·98) | 0·0005 |
AED=antiepileptic drug. For attitudes about epilepsy, the information pertains to either the person with epilepsy, or if the person with epilepsy was a child or cognitively impaired, to the carer, since the carer made the decision about treatment seeking and giving the AED.
Adjusted for age.
Discussion
In Kenyans with active convulsive epilepsy who had similar characteristics to people with epilepsy in other parts of Africa,3, 34 we found that the epilepsy treatment gap was 62% as measured by detection of AEDs in blood samples. This finding represents a reduction from 74% measured during a survey in 200310 and could be attributed to setting up an epilepsy clinic with a continuous supply of AEDs and sensitisation of the community. However, the sensitivity and specificity of self-reporting remained poor. The factors associated with reduced treatment seeking are different to those associated with reduced adherence to AEDs. Modifiable risk factors for failure to seek treatment are negative attitudes about biomedical treatment, payment for AEDs, and distance from health facilities providing AEDs. Adherence to AEDs could be improved by reduction of negative attitudes to epilepsy (panel).
Panel. Research in context.
Systematic review
We searched Medline (1966, to May, 2012), ISI Web of Science (1966, to May, 2012), and Cochrane reviews with the search term “epilepsy treatment gap”. Searches were restricted to human studies. We found 281 references, most of which we reviewed in a previous paper3 and a recent review.4 We reviewed the outstanding 31 references. We found that the epilepsy treatment gap was attributed to cultural beliefs, inadequate skilled manpower, cost of treatment, and unavailability of antiepileptic drugs.
Interpretation
This study showed that self-reporting of adherence to antiepileptic drugs (AEDs) might not be reliable in rural Africa, and that risk factors for the failure to seek biomedical treatment for epilepsy and the poor adherence to prescribed treatment are different. Treatment seeking could be improved by modification of attitudes to epilepsy and provision of free AEDs in health facilities near to the residence of the person with epilepsy. Adherence could be improved by changes in attitudes towards epilepsy.
People with epilepsy who held traditional animistic religious beliefs were less likely to seek biomedical treatment than were Christians and Muslims, possibly because of misconceptions and superstitions associated with epilepsy in Kilifi.35 People with epilepsy with negative beliefs and attitudes about biomedical treatment of epilepsy were less likely to seek treatment from health facilities, suggesting that modification of patients' beliefs and attitudes is an important step in improvement of treatment seeking.36, 37
Other factors associated with failure to seek treatment were distance to health facilities and payment for AEDs. The geographical proximity of health services to people's homes is an important factor that affects use of health services, particularly in resource-poor settings where density of biomedical health facilities is often low. The 1997 Kenyan health policy strategic framework states that all households should have access to health services within a 5 km range.38 In KHDSS, there is a high density of biomedical health facilities within reach by people with epilepsy, but only KDH has an epilepsy clinic with a continuous supply of AEDs and AEDs other than phenobarbital. The erratic supplies of AEDs in the peripheral clinics (JAC, unpublished) might have reduced the use of these peripheral facilities. People with epilepsy who reported paying for AEDs were less likely to seek treatment than were people who did not pay. The cost of AEDs in peripheral facilities was 10 KES (US$0·13) for a 1-month supply of phenobarbital in 2008, whereas at KDH it was 40 KES ($0·51). This cost of AEDs, although small, is significant when combined with indirect costs of seeking care—eg, transport costs.
Of the epilepsy-specific factors, people with epilepsy who had seizures for more than 10 years might be less likely to seek treatment compared with people with disease of shorter duration because they had learnt to cope with their disorder. People with focal seizures were less likely to seek treatment than were those with generalised seizures, although focal seizures are associated with symptomatic causes and are more likely to be associated with behavioural problems.39 Most of the seizures were focal becoming generalised, and being aware at the onset of seizure might have made people less inclined to seek treatment because of the stigma. People with epilepsy and learning difficulties were less likely to seek treatment, perhaps because they were not brought to biomedical facilities by their families or because of their difficulties in understanding the importance of treatment.
Patient self-reporting using reliable and valid questionnaires is thought to be an efficient and cost-effective method to assess adherence, although some patients might underestimate or overestimate their adherence.40 We found that the sensitivity and specificity of self-reporting compared with detection of AEDs in blood samples was low, suggesting that self-reporting is not a reliable measure of adherence in people with epilepsy in this community and probably in many countries with low and middle incomes. This finding questions the reliability of data in systematic reviews of the epilepsy treatment gap.3, 4 Furthermore, we did not find that the Morisky scale was useful for measuring adherence in this population. Although the scale has been used in other studies of epilepsy,28, 29 it has not been compared with AED detection in blood. There was no significant difference in adherence based on blood samples among the different AEDs (data not shown), despite the different half-lives (phenobarbital, 48–168 h; phenytoin, 7–42 h; carbamazepine, 5–26 h).30
Non-adherence was associated with established epilepsy, which is in accord with previous reports that people with epilepsy who had taken AEDs for a longer duration were less likely to adhere.41 Non-adherence was also associated with negative beliefs and attitudes about epilepsy as reported previously.6
Our study had several limitations. Non-adherence was reported at only one timepoint as opposed to monitoring over time, which might capture variations such as differences in timing of administration, missing doses of AEDs, or taking AEDs just before clinical assessment.42 We did not study people with non-convulsive epilepsy since the survey was designed to capture only those with active convulsive epilepsy. Recall and reporting bias of self-reported answers might have occurred, and there were significant differences between people who gave blood samples for measurement of adherence and those who did not. This study was done in an area where we have been studying epilepsy since 2003, although the treatment gap is still 62%. We did not control for quality of care, which might play a part in determining health service use.
In this rural area of Kenya, almost two-thirds of people with epilepsy were not adequately treated and the sensitivity and specificity of self-reported adherence were poor. Modification of beliefs and attitudes and provision of a free and constant supply of AEDs to nearby health facilities could substantially reduce the treatment gap.
Acknowledgments
Acknowledgments
The Wellcome Trust supported CKM under a training grant (No 08538) and funded this research through a Wellcome Trust Senior Fellowship in Clinical Tropical Medicine (No 083744) awarded to CRN. We thank Racheal Mapenzi, Joseph Muya, Isaac Fondo, Eddison Charo, and Eddie Chengo for data collection, Alex Maina for assisting in obtaining references, and the people with epilepsy and their caregivers for participating in this study.
Contributors
CKM and CRN conceptualised the study. CKM, PO, JAC, and CRN participated in proposal development. CKM implemented and managed the study. CKM, AKN, GF, TE, PO, JAC, and CRN provided input to the analytical plan. SNM did the assays of AEDs. CN drew the maps and RO managed the data. CKM drafted the report. AKN, GF, TE, SNM, PO, JAC, and CRN reviewed and edited the report. FI was involved in statistical analysis and editing of the report. All authors read and approved the final version of the report.
Conflicts of interest
We declare that we have no conflicts of interest.
Supplementary Material
References
- 1.Ngugi AK, Bottomley C, Kleinschmidt I, Sander JW, Newton CR. Estimation of the burden of active and life-time epilepsy: a meta-analytic approach. Epilepsia. 2010;51:883–890. doi: 10.1111/j.1528-1167.2009.02481.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Meinardi H, Scott RA, Reis R, Sander JW. The treatment gap in epilepsy: the current situation and ways forward. Epilepsia. 2001;42:136–149. doi: 10.1046/j.1528-1157.2001.32800.x. [DOI] [PubMed] [Google Scholar]
- 3.Mbuba CK, Ngugi AK, Newton CR, Carter JA. The epilepsy treatment gap in developing countries: a systematic review of the magnitude, causes, and intervention strategies. Epilepsia. 2008;49:1491–1503. doi: 10.1111/j.1528-1167.2008.01693.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Meyer AC, Dua T, Ma J, Saxena S, Birbeck G. Global disparities in the epilepsy treatment gap: a systematic review. Bull World Health Organ. 2010;88:260–266. doi: 10.2471/BLT.09.064147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Osterberg L, Blaschke T. Adherence to medication. N Engl J Med. 2005;353:487–497. doi: 10.1056/NEJMra050100. [DOI] [PubMed] [Google Scholar]
- 6.Buck D, Jacoby A, Baker GA, Chadwick DW. Factors influencing compliance with anti-epileptic drug regimens. Seizure. 1997;6:87–93. doi: 10.1016/s1059-1311(97)80060-x. [DOI] [PubMed] [Google Scholar]
- 7.Andersen R. Revisiting the behavioual model and access to medical care: does it matter? J Health Soc Behav. 1995;36:1–10. [PubMed] [Google Scholar]
- 8.Scott JA, Bauni E, Moisi JC. Profile: the Kilifi health and demographic surveillance system (KHDSS) Int J Epidemiol. 2012 doi: 10.1093/ije/dys062. published online April 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Government of Kenya. Kilifi District Development Plan 2000. 2000.
- 10.Edwards T, Scott AG, Munyoki G. Active convulsive epilepsy in a rural district of Kenya: a study of prevalence and possible risk factors. Lancet Neurol. 2008;7:50–56. doi: 10.1016/S1474-4422(07)70292-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Placencia M, Sander JW, Shorvon SD, Ellison RH, Cascante SM. Validation of a screening questionnaire for the detection of epileptic seizures in epidemiological studies. Brain. 1992;115:783–794. doi: 10.1093/brain/115.3.783. [DOI] [PubMed] [Google Scholar]
- 12.Kenyan Ministry of Health . In: Clinical guidelines. 2nd edn. Kimathi NA, Macheni JN, Muriithi A, editors. Ministry of Health, Government of Kenya; Nairobi: 2002. Central nervous system; pp. 55–60. [Google Scholar]
- 13.Birbeck GL, Kalichi EM. Epilepsy prevalence in rural Zambia: a door-to-door survey. Trop Med Int Health. 2004;9:92–95. doi: 10.1046/j.1365-3156.2003.01149.x. [DOI] [PubMed] [Google Scholar]
- 14.Longe AC, Osuntokun BO. Prevalence of neurological disorders in Udo, a rural community in southern Nigeria. Trop Geogr Med. 1989;41:36–40. [PubMed] [Google Scholar]
- 15.Thurman DJ, Beghi E, Begley CE. Standards for epidemiologic studies and surveillance of epilepsy. Epilepsia. 2011;52(suppl 7):2–26. doi: 10.1111/j.1528-1167.2011.03121.x. [DOI] [PubMed] [Google Scholar]
- 16.Mung'Ala-Odera V, White S, Meehan R. Prevalence, incidence and risk factors of epilepsy in older children in rural Kenya. Seizure. 2008;17:396–404. doi: 10.1016/j.seizure.2007.11.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Munyoki G, Edwards T, White S. Clinical and neurophysiologic features of active convulsive epilepsy in rural Kenya: a population-based study. Epilepsia. 2010;51:2370–2376. doi: 10.1111/j.1528-1167.2010.02653.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.El Sharkawy G, Newton C, Hartley S. Attitudes and practices of families and health care personnel toward children with epilepsy in Kilifi, Kenya. Epilepsy Behav. 2006;8:201–212. doi: 10.1016/j.yebeh.2005.09.011. [DOI] [PubMed] [Google Scholar]
- 19.Kendall-Taylor N, Kathomi C, Rimba K, Newton CR. Traditional healers and epilepsy treatment on the Kenyan coast. Epilepsia. 2008;49:1638–1639. doi: 10.1111/j.1528-1167.2008.01580_1.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Radhakrishnan K, Pandian JD, Santhoshkumar T. Prevalence, knowledge, attitude, and practice of epilepsy in Kerala, South India. Epilepsia. 2000;41:1027–1035. doi: 10.1111/j.1528-1157.2000.tb00289.x. [DOI] [PubMed] [Google Scholar]
- 21.Rwiza HT, Matuja WB, Kilonzo GP. Knowledge, attitude, and practice toward epilepsy among rural Tanzanian residents. Epilepsia. 1993;34:1017–1023. doi: 10.1111/j.1528-1157.1993.tb02127.x. [DOI] [PubMed] [Google Scholar]
- 22.Pelto PJ, Pelto GH. Studying knowledge, culture and behavior in applied medical anthropology. Med Anthropol Q. 1997;11:147–163. doi: 10.1525/maq.1997.11.2.147. [DOI] [PubMed] [Google Scholar]
- 23.Chuma J, Molyneux C. Estimating inequalities in ownership of insecticide treated nets: does the choice of socio-economic status measure matter? Health Policy Plan. 2009;24:83–93. doi: 10.1093/heapol/czn050. [DOI] [PubMed] [Google Scholar]
- 24.Vyas S, Kumaranayake L. Constructing socio-economic status indices: how to use principal components analysis. Health Policy Plan. 2006;21:459–468. doi: 10.1093/heapol/czl029. [DOI] [PubMed] [Google Scholar]
- 25.Mbuba CK, Abubakar A, Odermatt P, Newton CR, Carter JA. Development and validation of the Kilifi stigma scale for epilepsy in Kenya. Epilepsy Behav. 2012 doi: 10.1016/j.yebeh.2012.02.019. published online April 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mbuba CK, Abubakar A, Hartley S, Odermatt P, Newton CR, Carter JA. Development and validation of the Kilifi epilepsy beliefs and attitude scale. Epilepsy Behav (in press). [DOI] [PMC free article] [PubMed]
- 27.Morisky DE, Green LW, Levine DM. Concurrent and predictive validity of a self-reported measure of medication adherence. Med Care. 1986;24:67–74. doi: 10.1097/00005650-198601000-00007. [DOI] [PubMed] [Google Scholar]
- 28.McAuley JW, McFadden LS, Elliott JO, Shneker BF. An evaluation of self-management behaviors and medication adherence in patients with epilepsy. Epilepsy Behav. 2008;13:637–641. doi: 10.1016/j.yebeh.2008.07.005. [DOI] [PubMed] [Google Scholar]
- 29.Sweileh WM, Ihbesheh MS, Jarar IS. Self-reported medication adherence and treatment satisfaction in patients with epilepsy. Epilepsy Behav. 2011;21:301–305. doi: 10.1016/j.yebeh.2011.04.011. [DOI] [PubMed] [Google Scholar]
- 30.Faught E. Pharmacokinetic considerations in prescribing antiepileptic drugs. Epilepsia. 2001;42(suppl 4):19–23. [PubMed] [Google Scholar]
- 31.Gomes MM, Maia Filho HS, Noe RA. Anti-epileptic drug intake adherence. The value of the blood drug level measurement and the clinical approach. Arq Neuropsiquiatr. 1998;56:708–713. doi: 10.1590/s0004-282x1998000500002. [DOI] [PubMed] [Google Scholar]
- 32.Greenland S, Drescher K. Maximum likelihood estimation of the attributable fraction from logistic models. Biometrics. 1993;49:865–872. [PubMed] [Google Scholar]
- 33.Newson R. PUNAF: Stata module to compute population attributable fractions for cohort studies. 24-12-2010. Boston College Department of Economics. Statistical Software Components S457193.
- 34.Preux PM, Druet-Cabanac M. Epidemiology and aetiology of epilepsy in sub-Saharan Africa. Lancet Neurol. 2005;4:21–31. doi: 10.1016/S1474-4422(04)00963-9. [DOI] [PubMed] [Google Scholar]
- 35.Kendall-Taylor NH, Kathomi C, Rimba K, Newton CR. Comparing characteristics of epilepsy treatment providers on the Kenyan coast: implications for treatment-seeking and intervention. Rural Remote Health. 2009;9:1253. [PubMed] [Google Scholar]
- 36.Albert R. Exploring patient beliefs. Arch Intern Med. 1983;143:1773–1775. doi: 10.1001/archinte.143.9.1773. [DOI] [PubMed] [Google Scholar]
- 37.Ngubane H. Aspects of clinical practice and traditional organization of indigenous healers in South Africa. Soc Sci Med B. 1981;15:361–365. doi: 10.1016/0160-7987(81)90061-2. [DOI] [PubMed] [Google Scholar]
- 38.Ministry of Health . Health Policy Framework. Government of Kenya; Nairobi: 1997. [Google Scholar]
- 39.Kariuki SM, Abubakar A, Holding PA. Behavioral problems in children with epilepsy in rural Kenya. Epilepsy Behav. 2012;23:41–46. doi: 10.1016/j.yebeh.2011.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Thorbecke R. Measurement of compliance through patient interviews. Epilepsy Res Suppl. 1988;1:79–83. [PubMed] [Google Scholar]
- 41.French J. The long-term therapeutic management of epilepsy. Ann Intern Med. 1994;120:411–422. doi: 10.7326/0003-4819-120-5-199403010-00010. [DOI] [PubMed] [Google Scholar]
- 42.Farmer KC. Methods for measuring and monitoring medication regimen adherence in clinical trials and clinical practice. Clin Ther. 1999;21:1074–1090. doi: 10.1016/S0149-2918(99)80026-5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


