Abstract
Sleep is maximal during early postnatal life when rapid and extensive synapse remodeling occurs. It remains unknown whether and how sleep affects synapse development and plasticity. Using transcranial two-photon microscopy, we examined the formation and elimination of fluorescently-labeled dendritic spines and filopodia of layer 5 pyramidal neurons in the barrel cortex of 3-week old mice during wakefulness and sleep. We observed high turnover of dendritic protrusions over 2 hours in both wake and sleep states. The formation rate of dendritic spines or filopodia over 2 hours was comparable between the two states. The elimination rate of dendritic spines or filopodia was lower during 2-hour wakefulness than during 2-hour sleep. Similar results were observed on dendritic protrusion dynamics over 12-hour light/dark cycle when mice spent more time asleep or awake. The substantial remodeling of dendritic protrusions during the sleep state supports the notion that sleep plays an important role in the development and plasticity of synaptic connections in the mouse cortex.
Keywords: Sleep, two-photon imaging, dendritic spine, filopodia
INTRODUCTION
Infants spend more than half of their time asleep in the first few postnatal months when there is an explosion of synapse formation in the brain (Hobson, 2009). The fact that extensive synaptogenesis occurs during early development when sleep amounts are the greatest, suggests that sleep may be crucial for synapse development and plasticity. It has been shown that sleep deprivation reduces ocular dominance plasticity during a critical period of the visual cortex development in kittens (Frank et al., 2001; Jha et al., 2005). Sleep is also important for the process of imprinting in domestic chick (Jackson et al., 2008) and song development in juvenile zebra finches (Shank and Margoliash, 2009). These lines of evidence suggest a view that sleep may play an important role in promoting or enhancing synaptic plasticity. On the other hand, sleep may be involved in synaptic downscaling that may be important for brain function during subsequent wakefulness (Gilestro et al., 2009; Liu et al., 2010; Vyazovskiy et al., 2008). Long periods (6 hour but not 3 hour) of sleep deprivation modestly increase the synapse density of hypocretin neuron in zebrafish larvae (Appelbaum et al., 2010). In Drosophila, it has been reported that sleep phase is associated with loss of terminals at neuromuscular synapses (Donlea et al., 2009). Thus, sleep may also play an important role in downscaling the strength and number of synaptic connections in the brain.
To better understand the role of sleep in synapse development and plasticity, we examined the formation and elimination of dendritic protrusions (spines and filopodia) in the primary somatosensory cortex during sleep and wake states in 3-week-old mice. Using transcranial two-photon microscopy and transgenic mice overexpressing yellow fluorescent protein (YFP) in layer 5 pyramidal neurons (Grutzendler et al., 2002; Yang et al., 2010), we tracked dendritic protrusion dynamics over a period of 2–4 hours in sleeping and awake mice. We found that the formation rate of dendritic protrusions over hours is comparable between sleep and wake states. The elimination of dendritic spines or filopodia in the sleep state is higher than that during wakefulness. These findings support a view that sleep is actively involved in the formation and maintenance of synaptic connections in the developing brain.
METHODS
Experimental animals
Transgenic mice overexpressing YFP in layer 5 pyramidal neurons (H line) were purchased from the Jackson Laboratory (Bar Harbor, Maine) and housed in Skirball animal facility in New York University Medical Center. All experiments were done in accordance with the institutional guidelines. 3-week-old animals were used for all the experiments.
Surgery
Surgical preparation for EEG/EMG recording and transcranial two-photon imaging was performed in the following three steps: electrode implantation, attachment of a head holder, and creation of a thinned-skull window.
Electrode implantation
Two electrodes were used for recording epidural EEG and two for recording EMG. Each electrode was made by soldering one end of an Epoxy coated silver wire (0.005 inch in diameter) to a connector pin. To implant the electrodes, mice were deeply anesthetized with an intraperitoneal injection of Ketamine (85 μg/g) and Xylazine (8.5 μg/g). The mouse head was shaved and the skull surface was exposed with a midline scalp incision. The periosteum tissue over the skull surface was removed without damaging the temporal and occipital muscles. One EEG electrode was placed over the left frontal cortex (2 mm lateral to midline, 2 mm anterior to bregma) and another over the cerebellum (at midline, 1 mm posterior of lambdoid suture). Before electrode implantation, a small area of skull (each ~0.2 mm in diameter) was thinned with a high speed drill and carefully removed with forceps. The electrodes were bent at 1 mm from the tip of the silver wire and carefully inserted under the skull above the dural matter. The electrodes were fixed by cyanoacrylate-based glue and further stabilized by dental cement. Two electrodes for EMG recording were placed on the nuchal muscle.
Attachment of a head holder
A head holder composed of two parallel micro-metal bars was attached to the animal’s skull to help restrain the animal’s head and reduce motion-induced artifact during imaging. A small skull region (~0.2 mm in diameter) was located over the right primary somatosensory cortex based on stereotaxic coordinates (1.1 mm posterior from bregma and 3.4 mm lateral from the midline) and marked with a pencil. A thin layer of cyanoacrylate-based glue was first applied to the top of entire skull surface, and the head holder was then mounted on top of the skull with dental acrylic cement (Lang Dental Manufacturing Co., IL, USA) such that the marked skull region was exposed between the two bars. Precaution was taken not to cover the marked region with dental acrylic cement.
Creation of a thinned-skull window
After the dental cement was completely dry, the head holder was screwed to two metal cubes that were attached to a solid metal base, and a cranial window was created over the previously marked region. The procedures for preparing a thinned skull cranial window for two-photon imaging has been described in detail in previous publications (Grutzendler et al., 2002; Yang et al., 2010). Briefly, a high-speed drill was used to carefully reduce the skull thickness by approximately 50% under a dissecting microscope. The skull was immersed in artificial cerebrospinal fluid during drilling. Skull thinning was completed by carefully scraping the cranial surface with a microsurgical blade to ~20 μm in thickness. A high quality picture of the brain vasculature was taken with a CCD camera attached to a stereo dissecting microscope. Completed cranial window was covered with silicon elastomer (World Precision Instruments, Sarasota, FL) and the animals were returned to their own cages to recover.
Two-photon imaging of dendritic spines and filopodia in awake head-restrained mice
Mice were given at least four hours to recover after the surgery, and then habituated for 2–3 times (10 minutes each time) in the imaging apparatus to minimize potential stress effects of head restraining and imaging. After surgery and habituation, dendritic protrusions were imaged repeatedly over a period of 2–4 hours in the next morning (typically starting at ~8am). To image un-anesthetized mice, the head holder was screwed to two metal cubes attached to a solid metal base. The silicon elastomer covering the thinned skull window was removed and the skull was immersed in artificial cerebrospinal fluid. The head-restrained animal was then placed on the stage of a two-photon microscope. The area of interest was selected and marked on the CCD vasculature map taken previously. After tuning the two-photon laser to the wavelength of ~920 nm, images were acquired using 1.1 NA 60X water-immersion objectives. A low magnification stack (200 μm × 200 μm; 512 × 512 pixel) of fluorescently labeled neural processes was taken and used as a map for relocation of the same area at later time points, in addition to the marked brain vasculature map. Two to three stacks of image planes (66.7 μm × 66.7 μm; 512 pixel × 512 pixel) within a depth of 70 μm from the pial surface were collected at each time point, yielding a full three-dimensional data set of dendrites in the area of interest. Based on our visual inspection, the animal appeared awake during the period of image acquisition, which lasts ~10 minutes. After imaging, the animals were immediately released to its original cage and stayed there until the next imaging sessions.
Imaging data analysis
Data analysis was performed with NIH ImageJ software as described previously (Grutzendler et al., 2002; Xu et al., 2007; Zuo et al., 2005). To determine changes of dendritic protrusions over time, the same dendritic segments were identified from three-dimensional image stacks with high image quality (ratio of signal to background noise > 4) taken from each time points. Dendritic protrusions were identified in each view when their length was more than one-third of dendritic shaft diameter. Three-dimensional stacks were used to ensure that tissue movements and rotation between imaging intervals do not influence the analysis. Filopodia were identified as long, thin structures without enlarged heads (generally twice longer than the average spine length; head diameter: neck diameter < 1.2; length: neck diameter > 3). The rest of the protrusions were classified as spines. Spines or filopodia were considered the same (“stable”) between two views based on their spatial relationship to adjacent landmarks and their relative position to immediately adjacent spines. Spines or filopodia in the second view were considered different if they were more than 0.7 μm away from their expected positions based on the first view. The degree of dendritic plasticity was calculated as the number of protrusions (spines or filopodia) added, eliminated or stable divided by the number of pre-existing spines/filopodia/protrusions. The total number of protrusions was pooled from dendritic segments from different animals.
EEG/EMG recording and analysis
Between imaging sessions, EEG/EMG was recorded using A-M systems™ (Sequim, WA) with band pass setting of 0.1–100 Hz and digitized at 10 KHz through PolyVIEW™ data acquisition & analysis system (Grass Technologies, West Warwick, RI). EEG/EMG data were visually scored for the states of wake and sleep with the Grass Rodent Sleep Stager program (Grass Technologies, West Warwick, RI). Wake state was identified by lower amplitude and higher frequency (> 10 Hz) of EEG activity, and medium to high muscle activity. The rest of the states were identified as the sleep state. No different stages of sleep were analyzed.
Statistics
All imaging data were presented as mean ± S.D. Tests for differences between groups were performed using one-way ANOVA and student’s t-tests. Significant levels were set at P ≤ 0.05. All statistical analyses were performed using the SigmaPlot software (Systat Software Inc, Chicago, USA).
RESULTS
The formation rate of dendritic spines or filopodia over 2 hours is comparable between wake and sleep states
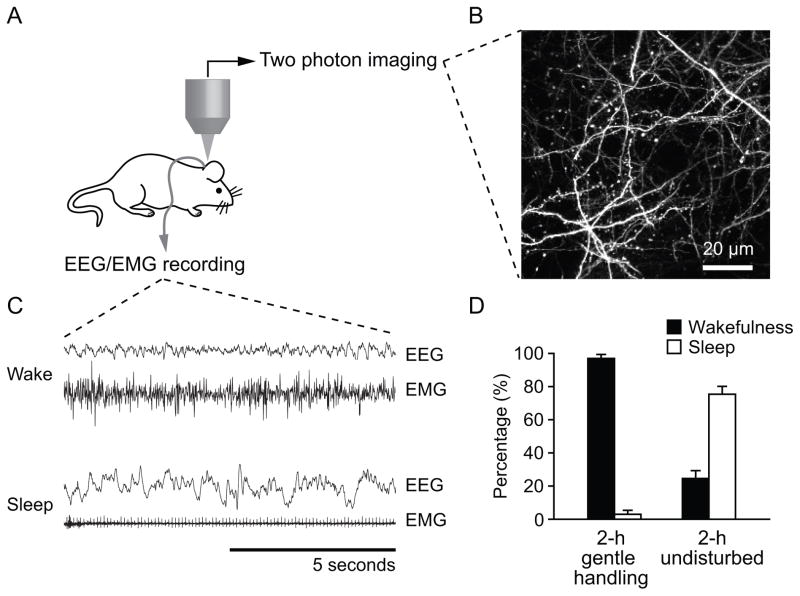
To investigate the effect of sleep on the development and plasticity of neuronal connections, we repeatedly imaged dendritic protrusions over a period of 2–4 hours in unanesthetized/awake, behaving mice at 3 weeks of age, and recorded EEG and EMG activity from the same animals [Fig. 1(A–C)]. The experiments were carried in the morning when mice typically spent most of their time sleeping. EEG and EMG recording showed that mice spent ~97% time awake over 2 hours when they were exposed to novel objects and/or subjected to gentle handing. Gentle handling was only applied when exposure to novel objects was not sufficient to keep the mice awake. In contrast, mice spent ~75% of time asleep in the next 2 hours when they were left undisturbed [Fig. 1(C–D)]. For the sake of simplicity, we designated this undisturbed state as the sleep state in the remaining part of the paper.
Figure 1.
(A) Diagram of transcranial two-photon imaging and EEG/EMG recording in the same animal without anesthesia. (B) Two dimensional projections of a three dimensional stack of dendritic branches and axons in the somatosensory cortex of an awake mouse. Scale bar, 20 μm. (C) Examples of EEG and EMG signals from 10 s epoch of wake and sleep. (D) Percentage of time that animals spent on wakefulness and sleep when they were subject to gentle handling or left undisturbed. EEG recording showed that in the morning animals were mostly awake during 2-h gentle handling, and mostly asleep when left undisturbed after previous gentle handling. Data are presented as mean ± S.E.
Two groups of mice were first kept awake for 2 hours. Following the first 2 hours of wakefulness, one group of mice (WS group) was left undisturbed while another group (WW group) continued to be kept awake for an additional 2 hours [Fig. 2(A)]. When the formation rate of dendritic protrusions was measured over 2-h wakefulness or sleep in the same animal (WS group), we found that the formation rate of dendritic protrusions during 2-h wakefulness (W: 0 h to 2 h) was slightly but not significantly higher than that during 2-h sleep (S: 2 h to 4 h) (15.0 ± 2.6% versus 13.5 ± 3.6%, 6 animals; P > 0.4) [Fig. 2(B–C)]. Furthermore, mice that stayed awake for a total of 4 h (WW group) had a formation rate of dendritic protrusions that was comparable between the first and second 2-h of wakefulness (P > 0.9) [Fig. 2(C)].
Figure 2.
Dendritic protrusions were formed at a similar rate during both wakefulness and sleep. (A) Experimental protocol: Two groups of mice were imaged at 0, 2 and 4 h, with the first imaging session typically starting early in the morning. Both groups were kept awake from 0 h to 2 h. From 2 h to 4 h, WS group was allowed to sleep and WW group was kept awake. (B) In vivo time-lapse imaging of the same dendritic segment every 2 h in the primary somatosensory cortex of P21 mouse that was awake from 0 h to 2 h and sleep from 2 h to 4 h. Filled arrow heads indicate newly-formed dendritic protrusions in the past 2 h and open arrow heads indicate dendritic protrusions that were eliminated in the next 2 h. Scale bar, 2 μm. (C) Percentages of dendritic protrusions that were formed over 2 h. The formation rate of dendritic protrusions was comparable between 2-h sleep and 2-h wakefulness. Percentages were calculated as the number of protrusions formed divided by the number of pre-existing protrusions. (D) Percentages of spines and filopodia relative to the total dendritic protrusions at P21. (E, F) Percentages of spines/filopodia formed over 2 h. There is no significant difference in spine and filopodia formation between 2-h sleep and wakefulness. Percentages were calculated as the number of spines/filopodia formed divided by the number of pre-existing spines/filopodia. Data are presented as mean ± S.D.
Dendritic branches are covered by two types of protrusions, dendritic spines and filopodia. Unlike spines, dendritic filopodia are long, thin protrusions without bulbous heads. They are highly abundant during development and serve as the precursors of dendritic spines (Bhatt et al., 2009; Dailey and Smith, 1996; Ziv and Smith, 1996). In the barrel cortex of 3-week-old mice, we found that 71.4 ± 6.7% of total dendritic protrusions (3294 protrusions, 15 animals) were spines and 28.6 ± 6.7% were filopodia [Fig. 2(D)]. To further investigate the effect of sleep, we measured the formation rates of dendritic spines and filopodia during sleep and wake states respectively. Similar to the total protrusions, the formation rates of spines are comparable between sleep and wake states (P > 0.2) [Fig. 2(E)]. Furthermore, there is no significant difference in the formation of dendritic filopodia between the two states (P > 0.7) [Fig. 2(F)].
We also examined the survival of dendritic protrusions that were formed during 2-h sleep and wake states. We found that 44.6 ± 12.7% of new dendritic protrusions formed in 2-h sleep state persist in the next 2 h of wakefulness. This survival rate is not significantly different from that of new protrusions formed in 2-h wake state (52.6 ± 9.8%, P > 0.4). Taken together, these results indicate that the formation and persistence of new dendritic spines and filopodia over a period of 2 hours are similar between the sleep and wake states.
The elimination rate of dendritic spines or filopodia is lower during wakefulness than during sleep
To further understand the impact of sleep on dendritic spine/filopodium development, we measured the elimination rate of dendritic protrusions over 2 h of sleep or wake states in the same animal (WS group), we found that 10.7 ± 3.6% of existing dendritic protrusions were eliminated during 2-h wakefulness, which was significantly lower than the elimination rate of protrusions during 2-h sleep (18.2 ± 2.3%; P < 0.01) [Fig. 3(A, B)]. In addition, the elimination rate of dendritic protrusions during the second 2-h period (2 h to 4 h) in the WW group was significantly lower than that in the WS group (12.2 ± 2.4% versus 18.2 ± 2.3%; P < 0.001). Because the formation rates of dendritic protrusions are comparable between two states, the net number of total protrusions increased over 2-h wake (104.3 ± 2.1%) and decreased over 2-h sleep (95.3 ± 2.7%)[Fig. 3(C)].
Figure 3.
The elimination of dendritic spines and filopodia over 2 h is higher during sleep. (A) Experimental protocol: Mice were imaged at 0, 2 and 4 h. Mice in WS group were awake from 0 h to 2 h and asleep from 2 h to 4 h; mice in WW group were kept awake from 0 h to 4 h. (B) Percentages of eliminated dendritic protrusions over 2 h. The elimination rate of dendritic protrusions was significantly higher during 2-h sleep than wakefulness. (C) The net number of total protrusions increased after 2-h wakefulness and decreased after 2-h sleep. (D, E) Percentages of spines/filopodia eliminated over 2 h. There is a significant increase in spine and filopodia elimination during 2-h sleep. Data are presented as mean ± S.D. *** P < 0.001, ** P < 0.01, * P < 0.05.
We also analyzed the elimination of dendritic spines and filopodia separately during the 2-h period of sleep or wakefulness. While most of dendritic spines persisted over 2 h, there was a slight but significant increase of spine elimination during sleep (S: 5.1 ± 1.6%; 1st W: 1.6 ± 0.6%, P < 0.01; 2nd W: 2.6 ± 0.7%, P < 0.05) [Fig. 3(D)]. Furthermore, the elimination rate of dendritic filopodia was significantly higher over 2-h sleep (54.1 ± 10.4%, 6 animals) as compared with either the first or second 2-h wakefulness (1st W: 34.8 ± 12.5%, P < 0.05; 2nd W: 40.4 ± 10.8%, P < 0.05) [Fig. 3(E)]. Together, these results indicate that the elimination rate of dendritic spines or filopodia during 2-h sleep is higher than that during 2-h wakefulness.
Dendritic protrusion dynamics and density differ between 12-hour dark and light cycle
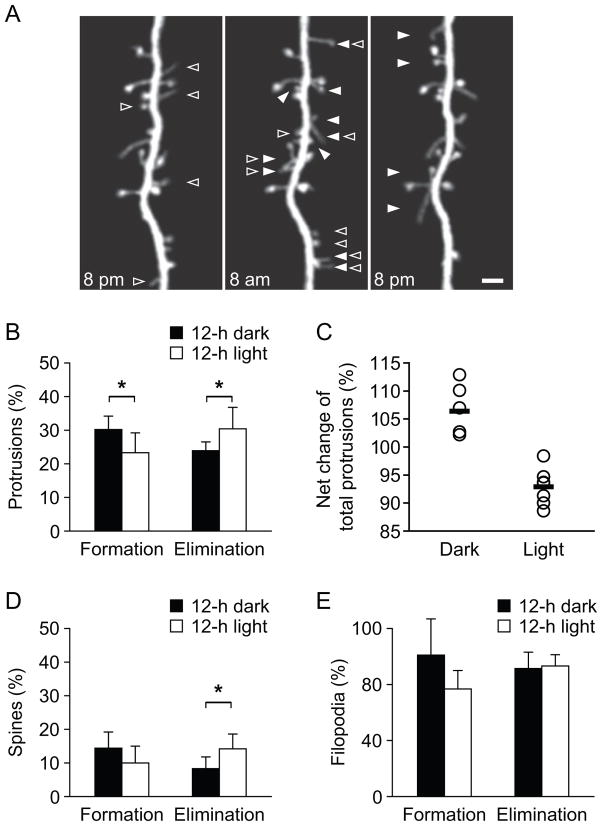
Mice sleep in both 12-hour light/dark cycles but spend more time sleeping in the light cycle (~65%) than in the dark cycle (~35%) (Huber et al., 2000). To further investigate the effect of sleep, we imaged the formation and elimination of dendritic protrusions over the 12-h dark and 12-h light cycles [Fig. 4]. We found that the elimination rate of dendritic protrusions during the 12-h dark cycle was significantly lower than that during the 12-h light cycle (23.9 ± 2.6% versus 30.4 ± 6.4%; P < 0.05) [Fig. 4(A–B)]. The formation rate of dendritic protrusions was significantly higher during the 12-h dark cycle than that during the 12-h light cycle (30.2 ± 4.0% versus 23.3 ± 5.9%; P < 0.05) [Fig. 4(A–B)]. As a result, there was a significant increase in the total number of dendritic protrusions during the dark cycle and a decrease during the light cycle (P < 0.05) [Fig. 4(C)].
Figure 4.
Dynamics and density of dendritic protrusions differ during the 12-hour light and dark cycle. (A) In vivo time-lapse imaging of the same dendritic branch at 8 pm, 8 am and 8 pm in the mouse primary somatosensory cortex at 3 weeks of age. The dendritic protrusions including spines and filopodia underwent rapid turnover during 12-hour light/dark cycle. Filled arrow heads indicate newly formed dendritic protrusions in past 12 h, and open arrow heads indicate eliminated dendritic protrusions in next 12 h. Scale bar, 2 μm. (B) Percentage of formed and eliminated dendritic protrusions over 12-h night and 12-h day respectively. There are more new dendritic protrusions accumulated during the 12 hour dark cycle, and more existing protrusions eliminated during the 12 hour light cycle. Percentages were calculated as the number of protrusions formed or eliminated divided by the number of pre-existing protrusions. (C) The net number of dendritic protrusions increased after 12-h dark cycle and decreased after 12-h light cycle. Each circle represented one animal and the thick line represented the average. (D–E) Percentages of formed and eliminated dendritic spines (D) and filopodia (E) over 12-h night and 12-h day. Data are presented as mean ± S.D. * P < 0.05.
We further measured the formation and elimination rates of dendritic spines and filopodia during the light and dark cycles separately. Similar to the total protrusions, dendritic spines were eliminated at a significantly higher rate during the 12-h light cycle than during the 12-h dark cycle (14.2 ± 4.4% versus 8.3 ± 3.5%, P < 0.05) [Fig. 4(D)]. The formation rate of spines during the light cycle was lower than that during the dark cycle, although the difference was not statistically significant (10.0 ± 5.0% versus 14.4 ± 4.8%, P = 0.1) [Fig. 4(D)]. Consistent with the short average lifetime of filopodia, we found the majority of dendritic filopodia were eliminated over a period of 12 hours. There is no significant difference in the elimination of dendritic filopodia between the 12-h light and 12-h dark cycles (77.7 ± 6.7% versus 76.2 ± 9.7%, P > 0.7) [Fig. 4(E)]. The formation rate of filopodia during the light cycle tended to be lower than during the dark cycle, although the difference did not reach statistical significance (formation: 64.0 ± 11.0% versus 84.2 ± 21.5%, P = 0.07) [Fig. 4(E)]. Taken together, these results indicate that the dynamics of the total dendritic protrusions and dendritic spines differ between the 12-h dark and 12-h light cycles. The elimination of protrusions/spines tends to be higher while their formation tends to be lower in the 12-h light cycle when animals spend more time in sleep.
DISCUSSION
In this study, we examined dynamics of dendritic protrusions in the developing somatosensory cortex of awake and sleeping mice using transcranial two-photon microscopy. Combined with EEG/EMG recording of sleep-wake patterns, we were able to, for the first time, examine structural changes of dendritic spines and filopodia in sleep and wake states without complication from anesthesia. Our results indicate that (1) the formation rate of dendritic protrusions (dendritic spines and filopodia) was comparable between 2-h sleep and 2-h wakefulness; (2) the elimination rate of dendritic spines or filopodia was significantly lower during 2-h wakefulness than during 2-h sleep. (3) In agreement with the findings made over 2 hours, the formation and elimination of dendritic protrusions differ over 12-hour light/dark cycle. These findings suggest that the sleep state contributes to both formation and elimination of synaptic connections during cortical development.
In the mouse somatosensory cortex, extensive synaptogenesis in the first 3 weeks of postnatal life is followed by a progressive decline in the densities of spines and filopodia between 3–8 weeks of age (Zuo et al., 2005; Yang et al., 2009). Our results show that the degree of new dendritic spines or filopodia formed during 2-h sleep is similar to that during 2-h wakefulness in 3-week-old mice. Furthermore, the persistence of these new protrusions formed during the two states is comparable in the subsequent 2 hours. Thus, during the developmental stage (3 weeks of age) when the total number of cortical connections are reducing (Zuo et al., 2005; Yang et al., 2009), sleep contributes to the generation of new persistent spines as effectively as wakefulness. This finding suggests that sleep may play an important and active role in establishing new synaptic connections and supports the memory consolidation theory that sleep promotes neural plasticity (Jackson et al., 2008).
Our study shows that the elimination rate of both dendritic spines and filopodia during 2-h sleep is higher as compared to that during 2-h wakefulness. Because a substantial loss of spines and filopodia occurs in the primary somatosensory cortex around 3 weeks of age (Zuo et al., 2005; Yang et al., 2009), wakefulness may enhance the survival of dendritic protrusions while sleep may not. Such a scenario would suggest a permissive role of sleep in spine elimination. Alternatively, sleep may play an active role in the elimination of dendritic protrusions. A higher elimination rate of dendritic protrusions during sleep may reflect the downscaling of synaptic strengths and the reduction of synapse number that are increased during the previous wake state (Gilestro et al., 2009; Liu et al., 2010; Vyazovskiy et al., 2008). Many lines of evidence have indicated that sensory and learning experiences promote the elimination of dendritic spines in the developing mouse cortex (Bhatt et al., 2009; Yang et al., 2009). A higher elimination rate of dendritic protrusions may also indicate that sleep is actively involved in experience-dependent modification of synaptic circuits via elimination of existing dendritic protrusions. A better understanding of mechanisms underlying spine elimination associated with sleep and wakefulness would help to determine the function of sleep in synapse development.
Sleep is not a homogeneous state and contains rapid eye movement (REM) sleep and non-REM (NREM) sleep (Aserinsky and Kleitman, 1953). REM sleep is maximal in the fetus and the neonate and has been proposed to function as an inducer of the central nervous system development (Roffwarg et al., 1966). It would be interesting to investigate whether REM and NREM sleep may have different effects on the formation and elimination of dendritic protrusions at different stages of development. Recent studies in zebrafish larvae have suggested the importance of the circadian clock in regulating the number of presynaptic terminals of hypocretin/orexin (HCRT) neurons (Appelbaum et al., 2010). Although our experimental design (WS and WW, imaging spine dynamics in the morning) suggests that our findings are not due to pure circadian effects, it would be interesting to examine how circadian rhythms, rather than sleep per se, impact on synaptic plasticity in the mammalian cortex. Lastly, it is important to point out that children with neurodevelopmental disabilities such as autism and attention deficit hyperactivity disorder often have sleep disorders (Dorris et al., 2008; Halbower et al., 2006; O’Brien and Gozal, 2004). Sleep disruption during development has also been connected to neuropsychiatric illness (Wulff et al., 2010). As changes of synaptic connections are important for the development and function of nervous system, it is of great interest to investigate how sleep disorder may affect synapse development and function in neuropsychiatric diseases.
Acknowledgments
This work was supported by grants from AFAR and the Alzheimer’s Association (NIRG-11-205362) to GY; NIH (R01 NS047325) to WBG. We thank members of the Gan laboratory for their critical comments.
Footnotes
Contributions
G.Y. and W.B.G. designed the experiments. G.Y. carried out the experiments and analyzed the data. G.Y. and W.B.G. wrote the paper.
References
- Appelbaum L, Wang G, Yokogawa T, Skariah GM, Smith SJ, Mourrain P, Mignot E. Circadian and homeostatic regulation of structural synaptic plasticity in hypocretin neurons. Neuron. 2010;68:87–98. doi: 10.1016/j.neuron.2010.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aserinsky E, Kleitman N. Regularly occurring periods of eye motility, and concomitant phenomena, during sleep. Science. 1953;118:273–4. doi: 10.1126/science.118.3062.273. [DOI] [PubMed] [Google Scholar]
- Bhatt DH, Zhang S, Gan WB. Dendritic spine dynamics. Annu Rev Physiol. 2009;71:261–82. doi: 10.1146/annurev.physiol.010908.163140. [DOI] [PubMed] [Google Scholar]
- Dailey ME, Smith SJ. The dynamics of dendritic structure in developing hippocampal slices. J Neurosci. 1996;16:2983–94. doi: 10.1523/JNEUROSCI.16-09-02983.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donlea JM, Ramanan N, Shaw PJ. Use-dependent plasticity in clock neurons regulates sleep need in Drosophila. Science. 2009;324:105–8. doi: 10.1126/science.1166657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorris L, Scott N, Zuberi S, Gibson N, Espie C. Sleep problems in children with neurological disorders. Dev Neurorehabil. 2008;11:95–114. doi: 10.1080/17518420701860149. [DOI] [PubMed] [Google Scholar]
- Frank MG, Issa NP, Stryker MP. Sleep enhances plasticity in the developing visual cortex. Neuron. 2001;30:275–87. doi: 10.1016/s0896-6273(01)00279-3. [DOI] [PubMed] [Google Scholar]
- Gilestro GF, Tononi G, Cirelli C. Widespread changes in synaptic markers as a function of sleep and wakefulness in Drosophila. Science. 2009;324:109–12. doi: 10.1126/science.1166673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grutzendler J, Kasthuri N, Gan WB. Long-term dendritic spine stability in the adult cortex. Nature. 2002;420:812–6. doi: 10.1038/nature01276. [DOI] [PubMed] [Google Scholar]
- Halbower AC, Degaonkar M, Barker PB, Earley CJ, Marcus CL, Smith PL, Prahme MC, Mahone EM. Childhood obstructive sleep apnea associates with neuropsychological deficits and neuronal brain injury. PLoS Med. 2006;3:e301. doi: 10.1371/journal.pmed.0030301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hobson JA. REM sleep and dreaming: towards a theory of protoconsciousness. Nat Rev Neurosci. 2009;10:803–13. doi: 10.1038/nrn2716. [DOI] [PubMed] [Google Scholar]
- Jackson C, McCabe BJ, Nicol AU, Grout AS, Brown MW, Horn G. Dynamics of a memory trace: effects of sleep on consolidation. Curr Biol. 2008;18:393–400. doi: 10.1016/j.cub.2008.01.062. [DOI] [PubMed] [Google Scholar]
- Jha SK, Jones BE, Coleman T, Steinmetz N, Law CT, Griffin G, Hawk J, Dabbish N, Kalatsky VA, Frank MG. Sleep-dependent plasticity requires cortical activity. J Neurosci. 2005;25:9266–74. doi: 10.1523/JNEUROSCI.2722-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu ZW, Faraguna U, Cirelli C, Tononi G, Gao XB. Direct evidence for wake-related increases and sleep-related decreases in synaptic strength in rodent cortex. J Neurosci. 2010;30:8671–5. doi: 10.1523/JNEUROSCI.1409-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Brien LM, Gozal D. Sleep in children with attention deficit/hyperactivity disorder. Minerva Pediatr. 2004;56:585–601. [PubMed] [Google Scholar]
- Roffwarg HP, Muzio JN, Dement WC. Ontogenetic development of the human sleep-dream cycle. Science. 1966;152:604–19. doi: 10.1126/science.152.3722.604. [DOI] [PubMed] [Google Scholar]
- Shank SS, Margoliash D. Sleep and sensorimotor integration during early vocal learning in a songbird. Nature. 2009;458:73–7. doi: 10.1038/nature07615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vyazovskiy VV, Cirelli C, Pfister-Genskow M, Faraguna U, Tononi G. Molecular and electrophysiological evidence for net synaptic potentiation in wake and depression in sleep. Nat Neurosci. 2008;11:200–8. doi: 10.1038/nn2035. [DOI] [PubMed] [Google Scholar]
- Wulff K, Gatti S, Wettstein JG, Foster RG. Sleep and circadian rhythm disruption in psychiatric and neurodegenerative disease. Nat Rev Neurosci. 2010;11:589–99. doi: 10.1038/nrn2868. [DOI] [PubMed] [Google Scholar]
- Xu HT, Pan F, Yang G, Gan WB. Choice of cranial window type for in vivo imaging affects dendritic spine turnover in the cortex. Nat Neurosci. 2007;10:549–51. doi: 10.1038/nn1883. [DOI] [PubMed] [Google Scholar]
- Yang G, Pan F, Parkhurst CN, Grutzendler J, Gan WB. Thinned-skull cranial window technique for long-term imaging of the cortex in live mice. Nat Protoc. 2010;5:201–8. doi: 10.1038/nprot.2009.222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziv NE, Smith SJ. Evidence for a role of dendritic filopodia in synaptogenesis and spine formation. Neuron. 1996;17:91–102. doi: 10.1016/s0896-6273(00)80283-4. [DOI] [PubMed] [Google Scholar]
- Zuo Y, Lin A, Chang P, Gan WB. Development of long-term dendritic spine stability in diverse regions of cerebral cortex. Neuron. 2005;46:181–9. doi: 10.1016/j.neuron.2005.04.001. [DOI] [PubMed] [Google Scholar]