Abstract
We sought to investigate the relationship between newly identified genetic variants and vitamin D levels and fracture risk in healthy African American (Black) children. This case-control study included children of both sexes, ages 5 to 9 years, with and without forearm fractures. Serum 25-hydroxy vitamin D levels, bone mineral density, body mass index and calcium/vitamin D intake were measured in 130 individuals (n = 60 cases and n = 70 controls). The five variants tested were located in the GC gene (rs2282679), in the NADSYN1 gene (rs12785878 and rs3829251), and in the promoter region of the CYP2R1 gene (rs2060793 and rs104741657). Associations between single nucleotide polymorphisms (SNPs) and vitamin D levels were tested using an ANCOVA. Associations between SNPs and fracture status were tested using logistic regression. The GC gene variant was associated with vitamin D levels (p = 0.038). None of the SNPs were associated with fracture status in young Blacks. These results suggest that the variants tested, which are associated with circulating vitamin D levels in Whites, are not associated with fracture status in healthy Black children. Additional research is required to discover the genetics of fracture risk in Blacks.
Keywords: fracture risk, single nucleotide polymorphism, vitamin D levels, body mass index, bone mineral density
Introduction
Recent genome-wide association studies (GWAS) have been utilized to identify new genetic variants of vitamin D status using a hypothesis-free method.1-4 Specifically, variants in the Group-specific component (vitamin D binding protein) (GC) gene (rs2282679), in the Nicotinamide adenine dinucleotide synthetase 1 (NADSYN1) gene (rs12785878 and rs3829251), and in the promoter region of the Cytochrome P450, family 2, subfamily R, polypeptide 1 (CYP2R1) gene (rs2060793 and rs104741657) have been associated with serum 25-hydroxy vitamin D [25(OH)D] levels.2,4
These studies have been limited to adult White populations of European descent. 2,4 However, in comparison, African American (Black) populations may be more likely to have abnormal vitamin D levels as darker skin pigmentation is a risk factor for vitamin D deficiency.5 Similarly, recent studies document a high prevalence of vitamin D insufficiency in U.S. children and, in particular, Black children.6,7 Vitamin D insufficiency is associated with a multitude of clinical consequences, including bone fracture 8,9 and diminished bone mineral density.10
To date, no studies have evaluated the role of genetics in vitamin D status in a Black population. Likewise, few studies have evaluated the role of such variants in the context of fracture risk in this same population. In this paper, we hypothesized that variants identified via GWAS for circulating 25(OH)D levels in Whites would be associated with vitamin D levels and risk of forearm fracture (the most common long-bone pediatric fracture) 11-13 in a young Black cohort.
Materials and Methods
Study Participants
This prospective study included Black children, ages 5 to 9 years, with a parent or guardian fluent in English. Case patients had an isolated and radiographically demonstrated forearm fracture (radius, ulna or both bones) and control patients had no self-reported history of a prior bone fracture. Exclusion criteria for both groups included a current underlying bone mineralization disorder (osteomalacia, osteogenesis imperfecta), current or prior use of anti-epileptic medication, current or prior daily use of oral steroids for ≥ 1 month and the presence of a chronic illness affecting bone (sickle cell disease, cancer, kidney disease, GI malabsorption disease, cerebral palsy). A convenience sample of patients was enrolled between December 2005 and July 2010. The study was conducted in Washington, DC, at Children's National Medical Center (CNMC), a large, urban pediatric hospital.
Case patients were recruited through the outpatient Orthopaedic Clinic and the Emergency Department. Control patients were recruited through the Emergency Department, outpatient clinics, and using hospital-based advertisements.
Patients were studied in the CNMC General Clinical Research Center. The CNMC Institutional Review Board approved this study (#3682). All guardians provided informed consent and children between the ages of 7 and 9 provided assent.
Demographic data were recorded for all participants. Bone health evaluation included measurement of 25(OH)D level and BMD by dual energy x-ray absorptiometry scan. Body mass index (BMI) measurements were obtained using standardized procedures and percentiles were determined using Centers for Disease Control Body Mass Index Percentile Calculator.14 Study participants also completed a BLOCK Kids 8–17 Food Frequency Questionnaire (NutritionQuest, Berkeley, CA, USA), which calculated a daily dietary calcium nutrient density (mg calcium/total kcal intake) and dietary vitamin D intake.
SNP Selection
The five variants tested were located in the GC gene (rs2282679), in the NADSYN1 gene (rs12785878 and rs3829251), and in the promoter region of the CYP2R1 gene (rs2060793 and rs104741657). These genes were selected for analysis as two recent GWAS studies identified these variants in European populations as associated with circulating 25(OH)D.2,4 The GC gene encodes vitamin D binding protein, a serum glycoprotein in the albumin family, which binds to 25(OH) D and its metabolites and transports them in circulation to target organs. The NADSYN1 gene encodes NAD synthetase, an enzyme that functions as a catalyst for the final step of NAD synthesis and involves a coenzyme, 7-dehydrocholesterol reductase (DHCR7) which is vital in the synthetic pathway of vitamin D3, a precursor to 25(OH)D. The CYP2R1 gene encodes vitamin D 25-hydroxylase, a microsomal hepatic enzyme that catalyzes C-25 hydroxylation of vitamin D3 to an active vitamin D receptor ligand. A point mutation in this gene has been related to rickets.15
Genotyping
Variant genotypes were determined with assay-by-design Taqman genotyping assays (Applied Biosystems, Foster City, CA, USA) using allele discrimination assays that employ the 5′ nuclease activity of Taq polymerase to detect a fluorescent reporter signal (Supplemental Table 1A). Both alleles are detected simultaneously using SNP-specific oligonucleotides labeled with different fluorophores, and genotypes automatically determined by the ratio of the two fluorophores. The polymerase chain reaction (PCR) for the each genetic variant contained a minimum of 10 ng of DNA, 900 nM primers, 200 nM probes, and TaqMan Genotyping Universal PCR Master Mix (Applied Biosystems) in a final volume of 10 μL. PCR was performed on ABI thermocyclers (9700 or 2720) using the following PCR profile: 10 minutes at 95 °C (denaturation), 44 cycles of 15 seconds at 92 °C, and 1 minute at an annealing temperature of 60 °C. The post-PCR allele calling was completed using an ABI 7900HT (genotyping software SDS ver. 2.3) and checked manually.
Statistical Analysis
The observed frequency of each genotype was compared with the expected frequency of the population in the Hardy–Weinberg equilibrium using a chi square test with one degree of freedom. The normality of each quantitative phenotype was confirmed using the Shapiro-Wilk normality test.
Associations between SNPs and 25(OH)D levels were tested using ANCOVA models, with BMI and fracture status as covariates. BMI was included because an association between overweight status and vitamin D deficiency has been reported in children.16 These associations were tested in the overall cohort (cases and controls). Post-hoc pair-wise comparisons were performed for those ANCOVAs showing a significant genotype effect F-test, and the resulting p-values were adjusted for multiple comparisons using the Sidak method.
Associations between SNPs and fracture status were tested using logistic regression adjusted for quantitative BMI and 25(OH)D values as covariates. BMI was included because an association between overweight status and forearm fracture risk has been reported in children.17-19 All regression models used a full genetic model comparing homozygous common allele individuals to heterozygotes to homozygous rare allele individuals.
The nominal p-value for significance was 0.05 and all statistical tests were two-sided. All statistical analyses were performed using Stata V11 (College Station, TX).
Results
This analysis included 60 cases and 70 controls. Demographic and clinical data for participants are summarized in Table 1. Mean age and proportion of participants who were male did not differ between cases and controls. There was likewise no significant difference between cases and controls for mean 25(OH)D level, total BMD, weekly dietary calcium nutrient density, weekly dietary vitamin D intake, or proportion of patients enrolled during the winter/spring seasons. Cases had a significantly higher mean BMI percentile than controls (72.3 vs. 59.7, respectively, p=0.02).
Table 1. Demographics and clinical characteristics of study participants.
| Variable | Cases | Controls | Significance of comparison between cases and controls |
|---|---|---|---|
| N | 60 | 70 | |
| Sex: Proportion male | 35/60, 58.3% | 37/70, 52.8% | p=0.50 |
| Mean age in years ± SD | 7.0 ± 1.5 | 6.9 ± 1.5 | p=0.90 |
| Mean body mass index percentile ± SD | 72.3 ± 25.5 (n=53) | 59.7 ± 29.9 (n=69) | p=0.02 |
| Mean total body bone mineral density z score ± SD | 0.63 ± 1.0 (n=49) | 1.01 ± 1.1 (n=62) | p=0.06 |
| Mean 25-hydroxy vitamin D level (ng/ml) ± SD | 21.7 ± 7.0 (n=58) | 22.6 ± 7.3 (n=69) | p=0.50 |
| Dietary calcium nutrient density (mg Ca+ intake/ total kcal) ± SD | 0.40 ± 0.12 (n=48) | 0.39 ± 0.16 (n=61) | p=0.90 |
| Dietary vitamin D intake (IU) ± SD | 185.7 ± 104.0 (n=48) | 159.9 ± 105.0 (n=61) | p=0.20 |
| Season of enrollment: proportion winter/spring | 31/70 (44.3%) | 27/60 (45.0%) | p=0.90 |
All of the SNPs genotyped (Table 2) were in Hardy-Weinberg equilibrium (Supplemental Table 1B). We compared allele frequencies between the five SNPs genotyped in our Black cohort with the HapMap ASW (African ancestry in the southwest USA) and HapMap CEU (Utah residents with northern and western European ancestry from the CEPH collection) populations (Supplementary Table 1C). The most common allele was the same in all three populations except for rs12785878. The HapMap CEU cohort showed the common allele (T), and our cohort and the HapMap ASW showed the other allele as common (G).
Table 2. SNPs examined for association with vitamin D levels, fracture risk, BMI, and DXA Z-measurements of whole body, lumbar spine, and left hip.
| SNP | Gene (nearest Gene) | Location | Genomic Position | Risk Allele# | Ancestral Allele |
|---|---|---|---|---|---|
| rs2282679 | GC | 4q13.3 | 72608383 | C | A |
| rs12785878 | NADSYN1 | 11q13.4 | 71167449 | G | T |
| rs3829251 | NADSYN1 | 11q13.4 | 71194559 | A | G |
| rs2060793 | (CYP2R1) | 11p15.2 | 14915310 | A | G |
| rs10741657 | (CYP2R1) | 11p15.2 | 14914878 | G | G |
Risk allele has been previously associated with lower levels of vitamin D.
Circulating vitamin D levels in the entire cohort (cases and controls) were analyzed for association with five SNPs (Tables 3A and 3B). The SNP located in an intron of the GC gene (rs2282679) was associated with vitamin D levels (Figure 1). None of the other SNPs were associated with vitamin D levels in this cohort.
Table 3.
| Table 3A: Analysis of vitamin D levels and SNPs adjusted for BMI and fracture status | |||
|---|---|---|---|
| SNP | p-value for genotype effect | p-value for BMI effect | p-value for fracture status effect |
| rs2282679 | 0.0379 | 0.3398 | 0.3422 |
| rs12785878 | 0.2512 | 0.4750 | 0.5509 |
| rs3829251 | 0.5349 | 0.7791 | 0.3853 |
| rs2060793 | 0.9680 | 0.7811 | 0.5892 |
| rs10741657 | 0.6100 | 0.9270 | 0.7871 |
| Table 3B: Significant Association for rs2282679 with vitamin D levels | |||
|---|---|---|---|
| SNP | Genotype effect p-value | N; Adjusted mean ± SEM | p-value for significantly different means |
| rs2282679 | 0.0379 | AA (N=82; 23 ± 1)* AC (N=27; 19 ± 1)* |
*p=0.0379 |
Figure 1. A variant in the GC gene (rs2282679) is associated with vitamin D levels in cases and controls.
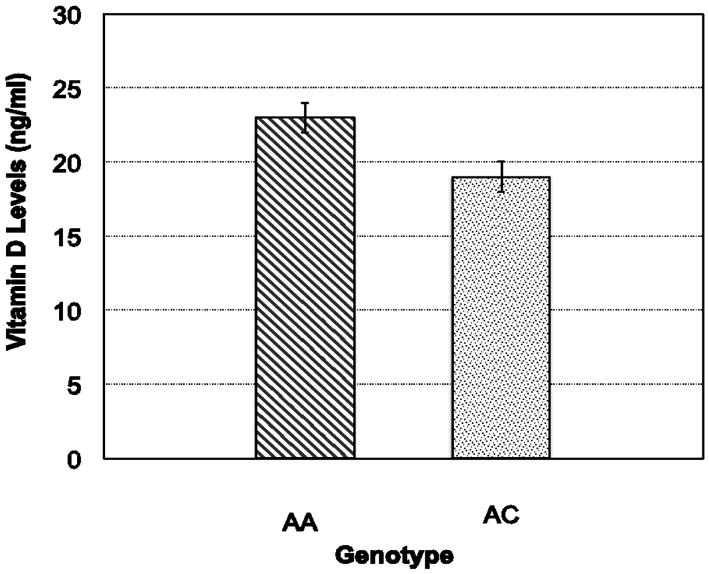
Individuals with the AA genotype (n=82: 23 ± 1 ng/ml) had significantly higher values of vitamin D than carriers of the C allele (n=27: 19 ± 1 ng/ml) (p=0.38). The ANCOVA was adjusted for BMI and fracture status.
None of the five SNPs was associated with fracture risk (Supplemental Table 2A). In addition, none of the five SNPs was associated with fracture risk while controlling for BMI between cases and controls (Supplemental Table 2B) or for vitamin D status between cases and controls (Supplemental Table 2C).
Discussion
In this prospective case-control study of Black children, we explored genetic associations between five genetic variants previously associated with vitamin D status in Caucasians2,4 and the vitamin D status of the study participants. Our study population represents a group at high risk for vitamin D deficiency5-7 that has not been included in prior studies. In addition, we examined these same variants for an association with forearm fracture status in our study population. Forearm fractures are the most common long-bone pediatric fracture in children.11-13 Little is known about the relationship between childhood forearm fracture risk and genetics because few clinical studies of pediatric fractures have included direct measures of genes. To our knowledge, this is the first study to evaluate the role of genetic factors on forearm fracture risk in children.
The five variants tested were located in the GC gene (rs2282679), in the NADSYN1 gene (rs12785878 and rs3829251), and in the promoter region of the CYP2R1 gene (rs2060793 and rs104741657). One of the variants was associated with vitamin D levels in a combined analysis of cases and controls (Figure 1). The SNP (rs2282679) is located in the 12th intron of the group-specific component gene (GC) that encodes a vitamin D-binding protein (DBP). The GC protein is a serum glycoprotein that belongs to the albumin family and binds to 25(OH)D. In addition, this protein binds other blood vitamin D sterol metabolites (including 25(OH)D and 1,25-dihydroxyvitamin D concentrations) and transports them to target organs.
This particular variant was found in both GWAS studies to have the strongest association 2,4 and was replicated in both GWAS studies with additional populations. We observed the same direction of association in our cohort: individuals with two copies of the common allele (A allele) had higher levels of vitamin D than did individuals with a copy of the rare allele (C allele). This is the first study to show an association between genetic variants of rs2282679 and circulating levels of vitamin D in a Black population.
Other studies have shown associations with non-synonymous SNPs in the GC gene and vitamin D levels 21-23, but the rs2282679 is an intronic SNP with an unknown function. It has been postulated this SNP might affect the GC binding of 25(OH)D,4 but this needs to be explored experimentally, which is beyond the scope of this paper. In addition, another potential mechanism by which rs2282679 may relate to circulating 25(OH)D levels is through its linkage disequilibrium with the non-synonymous SNP, rs7041.2,4 In this manner, rs2282679 may not have any biological activity but may be a marker for rs7041.
None of the GWAS variants associated with vitamin D levels were related to fracture status in our cohort. This was surprising to us, particularly since inadequate vitamin D levels have been associated with fractures, osteomalacia, and childhood rickets.20
Our case population had a significantly higher BMI and a trend toward a lower BMD than our control population. This is consistent with evidence from multiple clinical studies that supports an association between increased forearm fracture risk in children and obesity19,24 and lower BMD.18,24,25 Although these studies have also focused on Caucasian populations, there are many reasons why Black children may be a higher-risk population. In the United States, African American children have significantly higher rates of obesity than non-Hispanic White children.26,27 Additionally, a high prevalence of vitamin D insufficiency 6,7 and dietary calcium and vitamin D deficiencies 28 are reported in Black children, and these factors are known to negatively impact BMD.19,29
Therefore, we examined associations with SNPs from GWAS studies for circulating vitamin D and BMI (Table 3A and 3B).As noted, BMI was the only clinical characteristic that was significantly different between the cases and controls (Table 2), but our cohort was not powered for BMI. For this reason, we did not analyze the cases and controls separately for BMI or vitamin D levels.
This study has limitations. The size of the study sample is small compared to that of GWAS studies (>3,000 individuals). Similarly, because of power limitations we could not address potential gene-gene and gene-environment interactions. Despite being underpowered to assess some of the phenotypes, the genetic association identified by our study is sufficiently supported by the literature to suggest a plausible biological effect. We are now investigating the potential molecular impact of the associated variants and are collecting data on a second cohort in order to attempt to replicate our results. Similarly, given that rs2282679 is in linkage disequilibrium with rs7041, our future studies will also examine rs7041.
In conclusion, our study did not find genetic association between fracture risk and genetic variants that have previously been associated with vitamin D levels. However we did find that a SNP (rs2282679) associated with circulating levels of vitamin D in Caucasians was replicated in our young, Black cohort. Further study is needed to better understand if and how genetic factors contribute to forearm fracture risk in children and how this risk may vary among racial/ethnic groups.
Acknowledgments
Funding: This study is funded in part by the National Institutes of Health National Center for Research Resources (1K23 RR024467-01).
Footnotes
The authors have no conflicts of interest related to this article to disclose.
References
- 1.Styrkarsdottir U, Halldorsson BV, Gretarsdottir S, et al. New sequence variants associated with bone mineral density. Nat Genet. 2009;41:15–17. doi: 10.1038/ng.284. [DOI] [PubMed] [Google Scholar]
- 2.Wang TJ, Zhang F, Richards JB, et al. Common genetic determinants of vitamin D insufficiency: a genome-wide association study. Lancet. 2010;376:180–188. doi: 10.1016/S0140-6736(10)60588-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rivadeneira F, Styrkarsdottir U, Estrada K, et al. Twenty bone-mineral-density loci identified by large-scale meta-analysis of genome-wide association studies. Nat Genet. 2009;41:1199–1206. doi: 10.1038/ng.446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ahn J, Yu K, Stolzenberg-Solomon R, et al. Genome-wide association study of circulating vitamin D levels. Hum Mol Genet. 2010;19:2739–2745. doi: 10.1093/hmg/ddq155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Thomas MK, Demay MB. Vitamin D deficiency and disorders of vitamin D metabolism. Endocrinol Metab Clin. 2000;29:611–627. doi: 10.1016/s0889-8529(05)70153-5. [DOI] [PubMed] [Google Scholar]
- 6.Stein EM, Laing EM, Hall DB, et al. Serum 25-hydroxyvitamin D concentrations in girls aged 4-8 y living in the southeastern United States. Am J Clin Nutr. 2006;83:75–81. doi: 10.1093/ajcn/83.1.75. [DOI] [PubMed] [Google Scholar]
- 7.Cole CR, Grant FK, Tangpricha V, et al. 25-hydroxyvitamin D status of healthy, low-income, minority children in Atlanta, Georgia. Pediatrics. 2010;125:633–639. doi: 10.1542/peds.2009-1928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chapuy MC, Arlot ME, Duboeuf F, et al. Vitamin D3 and calcium to prevent hip fractures in elderly women. N Engl J Med. 1992;327:1637–1642. doi: 10.1056/NEJM199212033272305. [DOI] [PubMed] [Google Scholar]
- 9.LeBoff MS, Kohlmeier L, Hurwitz S, et al. Occult vitamin D deficiency in postmenopausal US women with acute hip fracture. JAMA. 1999;281:1505–1511. doi: 10.1001/jama.281.16.1505. [DOI] [PubMed] [Google Scholar]
- 10.Lehtonen-Veromaa MK, Mottonen TT, Nuotio IO, et al. Vitamin D and attainment of peak bone mass among peripubertal Finnish girls: a 3-y prospective study. Am J Clin Nutr. 2002;76:1446–1453. doi: 10.1093/ajcn/76.6.1446. [DOI] [PubMed] [Google Scholar]
- 11.Khosla S, Melton LJ, 3rd, Dekutoski MB, et al. Incidence of childhood distal forearm fractures over 30 years: a population-based study. JAMA. 2003;290:1479–1485. doi: 10.1001/jama.290.11.1479. [DOI] [PubMed] [Google Scholar]
- 12.Jones IE, Cannan R, Goulding A. Distal forearm fractures in New Zealand children: annual rates in a geographically defined area. N Z Med J. 2000;113:443–445. [PubMed] [Google Scholar]
- 13.Landin LA. Fracture patterns in children. Analysis of 8,682 fractures with special reference to incidence, etiology and secular changes in a Swedish urban population 1950-1979. Acta Orthop Scand Suppl. 1983;202:1–109. [PubMed] [Google Scholar]
- 14.Centers for Disease Control and Prevention. (2011) Division of Nutrition, Physical Activity and Obesity. National Center for Chronic Disease Prevention and Health Promotion, Centers for Disease Control and Prevention; [Accessed: July 30, 2011]. Basics about childhood obesity [Online] Available from : http://www.cdc.gov/obesity/childhood/basics.html. [Google Scholar]
- 15.Cheng JB, Levine MA, Bell NH, Mangelsdorf DJ, Russel DW. Genetic evidence that the human CYPR1 is a likely key vitamin D 25-D hydroxylase. Proc Natl Acad Sci USA. 2004;101:7711–7715. doi: 10.1073/pnas.0402490101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Smotkin-Tangorra M, Purushothaman R, Gupta A, et al. Prevalence of vitamin D insufficiency in obese children and adolescents. J Pediatr Endocrinol Metab. 2007;20:817–23. doi: 10.1515/jpem.2007.20.7.817. [DOI] [PubMed] [Google Scholar]
- 17.Goulding A, Cannan R, Williams SM, et al. Bone mineral density in girls with forearm fractures. J Bone Miner Res. 1998;13:143–148. doi: 10.1359/jbmr.1998.13.1.143. [DOI] [PubMed] [Google Scholar]
- 18.Goulding A, Jones IE, Taylor RW, et al. Bone mineral density and body composition in boys with distal forearm fractures: a dual-energy x-ray absorptiometry study. J Pediatr. 2001;139:509–515. doi: 10.1067/mpd.2001.116297. [DOI] [PubMed] [Google Scholar]
- 19.Goulding A, Rockell JE, Black RE, et al. Children who avoid drinking cow's milk are at increased risk for prepubertal bone fractures. J Am Diet Assoc. 2004;104:250–253. doi: 10.1016/j.jada.2003.11.008. [DOI] [PubMed] [Google Scholar]
- 20.Bouillon R, Carmeliet G, Verlinden L, et al. Vitamin D and human health: lessons from vitamin D receptor null mice. Endocr Rev. 2008;29:726–776. doi: 10.1210/er.2008-0004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lauridsen AL, Vestergaard P, Hermann AP, et al. Plasma concentrations of 25-hydroxy-vitamin D and 1,25-dihydroxy-vitamin D are related to the phenotype of Gc (vitamin D-binding protein): a cross-sectional study on 595 early postmenopausal women. Calcif Tissue Int. 2005;77:15–22. doi: 10.1007/s00223-004-0227-5. [DOI] [PubMed] [Google Scholar]
- 22.Sinotte M, Diorio C, Berube S, et al. Genetic polymorphisms of the vitamin D binding protein and plasma concentrations of 25-hydroxyvitamin D in premenopausal women. Am J Clin Nutr. 2009;89:634–640. doi: 10.3945/ajcn.2008.26445. [DOI] [PubMed] [Google Scholar]
- 23.Engelman CD, Fingerlin TE, Langefeld CD, et al. Genetic and environmental determinants of 25-hydroxyvitamin D and 1,25-dihydroxyvitamin D levels in Hispanic and African Americans. J Clin Endocrinol Metab. 2008;93:3381–3388. doi: 10.1210/jc.2007-2702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Goulding A, Taylor RW. Plasma leptin values in relation to bone mass and density and to dynamic biochemical markers of bone resorption and formation in postmenopausal women. Calcif Tissue Int. 1998;63:456–458. doi: 10.1007/s002239900557. [DOI] [PubMed] [Google Scholar]
- 25.Ma D, Morley R, Jones G. Risk-taking, coordination and upper limb fractures in children: a population based case-control study. Osteoporos Int. 2004;15:633–638. doi: 10.1007/s00198-003-1579-9. [DOI] [PubMed] [Google Scholar]
- 26.Ogden CL, Flegal KM, Carroll MD, Johnson CL. Prevalence and trends in overweight among US children and adolescents, 1999-2000. JAMA. 2002;288:1728–1732. doi: 10.1001/jama.288.14.1728. [DOI] [PubMed] [Google Scholar]
- 27.Ogden CL, Lamb MM, Carroll MD, Flegal KM. Obesity and socioeconomic status in children and adolescents: United States, 2005-2008. NCHS Data Brief. 2010;51:1–8. [PubMed] [Google Scholar]
- 28.Fulgoni V, 3rd, Nicholls J, Reed A, et al. Dairy consumption and related nutrient intake in African-American adults and children in the United States: continuing survey of food intakes by individuals 1994-1996, 1998, and the National Health And Nutrition Examination Survey 1999-2000. J Am Diet Assoc. 2007;107:256–264. doi: 10.1016/j.jada.2006.11.007. [DOI] [PubMed] [Google Scholar]
- 29.Black RE, Williams SM, Jones IE, Goulding A. Children who avoid drinking cow milk have low dietary calcium intakes and poor bone health. Am J Clin Nutr. 2002;76:675–680. doi: 10.1093/ajcn/76.3.675. [DOI] [PubMed] [Google Scholar]


