Abstract
Objective
Growing evidence indicates that non-clinical psychotic-like experiences occur in otherwise healthy individuals, suggesting that psychosis may occur on a continuum. However, little is know about how the diathesis for formal psychosis maps on to individuals at the non-clinical side of this continuum. Our current understanding of the pathophysiology of schizophrenia implicates certain key factors such as early developmental abnormalities and fronto-striatal dysfunction. To date, no studies have examined these core factors in the context of non-clinical psychosis.
Method
A total of 221 young adults were assessed for distressing attenuated positive symptoms (DAPS), dermatoglyphic asymmetries (a marker of early developmental insult), and procedural memory (a proxy for fronto-striatal function).
Results
Participants reporting DAPS (n=16; 7.2%) and no-DAPS (n=205; 92.7%) were split into two groups. The DAPS group showed significantly elevated depression, elevated dermatoglyphic asymmetries, and a pattern of procedural learning consistent with other studies with formally psychotic patients.
Conclusion
The results indicate that the non-clinical side of the psychosis continuum also shares key vulnerability factors implicated in schizophrenia, suggesting that both early developmental disruption and abnormalities in fronto-striatal function are core aspects underlying the disorder.
Keywords: dermatoglyphic asymmetries, prenatal, procedural learning, non-clinical psychosis, fronto-striatal dysfunction
INTRODUCTION
Accumulating evidence shows that symptoms of non-clinical psychosis (e.g., fleeting auditory hallucinations) are commonly experienced by otherwise healthy individuals (1, 2) and suggests that psychosis may occur as a continuous phenotype. However, to date, it remains unclear how the non-clinical side of the continuum is linked to formal psychosis, and if it reflects an underlying continuum of putative markers as well.
Two core factors widely implicated in the etiology of formal psychosis include an early developmental insult and abnormalities in fronto-striatal functioning. Although these signs of vulnerability are key factors in leading etiological models (3-5), to date we have little conception if and how they present on the non-clinical side of the psychosis continuum. Understanding which factors are unique and shared along the continuum may highlight variables crucial to the onset of psychosis. Further, this knowledge is integral to understanding the full continuum of vulnerability, particularly in a population without serious confounds such as years of illness or neurotoxicity, and may help to elucidate our conceptions of Axis-I psychotic disorders such as schizophrenia.
Signs of developmental dysfunction have been of interest to researchers since early conceptualizations of schizophrenia (6), and several aspects unique to these signs may shed light on a continuum of vulnerability among individuals reporting symptoms of non-clinical psychosis. Minor physical anomalies (MPAs), particularly in the face, hands and feet, appear to reflect disruptions in early fetal development (7). Within this context, dermatoglyphics (the epidermal ridge patterns that form on the fingerprints and palms during prenatal development) are of particular interest. Although dermatoglyphics are not usually included in the construct of MPAs (8), evidence suggests that they are in the same family, as both physical features and fingerprints develop out of the same fetal ectodermal tissue, and both MPAs and dermatoglyphics are enduring reflections of an early prenatal environment (8, 9). It has been reliably demonstrated that schizophrenia patients have more dermatoglyphic asymmetries than healthy controls (10). Dermatoglyphics are excellent candidates for investigating non-clinical psychosis, in contrast to other indices of developmental upset (e.g., dichotomous obstetric complication-type events), because the slight aberrations in the symmetry of fingerprints occurs on a continuum, and in a continuous fashion across the normal population (11). They are also not prone to a recall bias associated with self-reports of pregnancy complications. Further, the timing of epidermal ridge formation limits potential contributing factors to the early prenatal period, when the outer layer of the embryo (i.e., ectoderm) forms both the skin and the central nervous system (CNS) (8). Finally, dermatoglyphics are excellent for understanding early developmental disruption in non-clinical psychosis because similar markers have been observed in other populations at the lower end of the spectrum including schizotypal and prodromal samples (12, 13).
Cognitive deficits, occurring early in childhood among individuals who later develop psychosis as adults, are believed to be a core feature and reflective of brain pathology underlying psychosis (14). Consistent with what has been reliably observed in patients with schizophrenia, several studies have found that individuals reporting symptoms of non-clinical psychosis show lower IQ scores than controls (15-17). However, to date, there has been little research aimed at understanding specific cognitive processes in non-clinical psychosis and there have been no studies examining procedural learning. Procedural learning, in contrast to declarative (knowledge based) learning, is when the quality of speed of a task performance increases as a function of the amount of practice, while the subject is not required to consciously remember any facts (18). Several early studies have indicated that schizophrenia patients show some degree of impairment in procedural learning (19, 20), and this process is highly relevant to the study of psychosis, as it is believed to reflect fronto-striatal function (21, 22). Within the context of understanding vulnerability in individuals reporting non-clinical psychosis, fronto-striatal processing is highly relevant, as dysregulation is implicated in dopamine hypothesis of schizophrenia, and has been observed in a venue of lower spectrum populations including groups with schizotypal personality disorder (23), as well as the prodrome (24).
Aims of the study
In the present study, 221 non-clinical young adults were assessed for distressing attenuated positive symptoms, and administered clinical scales as well as dermatoglyphic and procedural learning assessments to test the hypothesis that when compared to a group showing low levels of non-clinical psychosis, those individuals showing high levels of non-clinical psychosis would also exhibit elevated symptoms and vulnerability factors key to etiological conceptions of formal psychosis.
MATERIAL AND METHODS
Participants
Participants were recruited at the University of Colorado Boulder’s Adolescent Development and Preventive Treatment (ADAPT) research program. All participants were young adults (aged 18 and older) in the University of Colorado Boulder’s human subject recruitment pool (consisting of students and community members from the general population), and there were no exclusion criteria. The protocol and informed consent procedures were approved by the University Institutional Review Board. See Table 1 for demographic characteristics of the sample.
Table 1.
Demographic and Clinical Characteristics for Participants
| No-DAPS | DAPS | Total Group | |
|---|---|---|---|
| Total | 205 | 16 | 221 |
| Males | 110(53.7%) | 12(75%) | 122 |
| Females | 95(46.3%) | 4 (25%) | 99 |
| Age (yrs.) | |||
| M (SD) | 19.97 (1.95) | 19.63 (1.62) | 19.94 1.93 |
| Parental Education (yrs.) | |||
| M (SD) | 15.56 (1.94) | 14.68 (2.20) | 15.50 (1.96) |
| Beck Depression Inventory | |||
| M (SD) | 7.64 (6.59) | 20.50 (11.79) | 8.57 (7.81) |
| Prodromal Questionnaire-Brief | |||
| Total Scalea | |||
| M (SD) | 4.01 (3.56) | 8.68 (3.84) | 4.35 (3.77) |
| Weighted Scaleb | |||
| M (SD) | 9.47 (10.07) | 31.75 (17.16) | 11.09 (12.15) |
Note: Distressing Attenuated Positive Symptoms (DAPS)
Total possible PQB scores range from 0-21
Total weighted score range from 0-105
Clinical Symptoms
Symptoms of non-clinical psychosis were assessed with the Prodromal Questionnaire-Brief (PQ-B) (25, 26), a 21-dichotomous item self-report questionnaire comprised of attenuated positive symptoms items, each with an accompanying weighted impact scale (“When this happens, I feel frightened, concerned, or it causes problems for me”) ranging from 1-5 (no-strongly agree). Using the most conservative approach, participants scoring a five on the distress scale (strongly agree), were considered as having experienced a definite experience. To accurately reflect this strategy, the term “distressing attenuated positive symptoms” (DAPS) is used to refer to symptoms of non-clinical psychosis endorsed in this sample. The total PQB score was calculated using the sum of the endorsed 21 items (range 0-21). Subjects were asked to indicate experiences in the past month, and asked to not include experiences that occurred only while under the influence of alcohol, drugs, or medications that were not prescribed. This instrument has been used in recent studies to examine attenuated positive symptoms in healthy populations (27, 28). The participants also completed the Beck Depression Inventory (BDI), a widely used and well-validated, measure of depression that contains 21 items (29).
Pursuit Rotor
We used a computerized version of the pursuit rotor task (Life Science Associates, New York, New York) that has been validated and used in other studies with schizophrenia samples as well as with college age populations (30, 31). Participants were instructed to follow a moving target around a rectangular track with a mouse held in their preferred hand. Each trial lasted 20 seconds with a five-second interval between trials. Subjects were given three blocks of three trials each, interspersed with 20 minutes rest periods after each block. Employing a widely used titration strategy (30, 32), the initial level of performance for each participant was equated during practice trials, and speed of the target stimulus was subsequently adjusted so that each subject reached a criterion of being able to maintain contact with the target 20%– 25% of the time.
Dermatoglyphics
Dermatoglyphic asymmetry was assessed for homologous fingers of the right and left hands of the participants. Fingerprints were obtained by utilizing digital scans of the actual hand from a high-definition photo scanner (Epson Perfection V500 Photo Scanner). Dermatoglyphics were rated by coders utilizing Adobe Photoshop (CS3), which allowed high-level zoom/enlargement and demarcation. Because of this technology, the prints were of very high quality, and consequently it was not necessary to code partial prints or use subjects with missing fingers due to poor print quality. Employing a widely-adopted procedure described by Holt (11), the number of dermal ridges crossing a line drawn between the center of the pattern and the triradius of each fingerprint was counted for all fingers on both hands. The number of ridges on each finger of the left hand was subtracted from the number of ridges on the homologous finger of the right. The total ridge count asymmetry score was calculated by summing the absolute values of the differences observed for each homologous finger pair, and taking the mean for each participant (i.e., the total discrepant ridge count divided by five). Five raters, blind to all clinical and procedural memory test information, coded the participant’s prints. Raters trained by practicing on sets of handprints for a one-month period, and continued until intra-class correlation coefficients (ICCs) > .80. Quality was periodically assessed by a gold standard rater (D.D.); a print from every 20 participants was coded again, and reliability for the rescored/original prints exceeded .90.
Data Analyses
Independent t-tests and chi-square tests were employed to examine differences in continuous and categorical demographic variables (respectively) between groups. Independent t-tests were also used to examine group differences in symptoms and dermatoglyphic asymmetries. If necessary, results were adjusted for inequalities of variance as tested by Levene’s test. To test for procedural learning, a series of 3 (trial blocks) × 2 (diagnostic group) mixed repeated measures ANOVA was conducted. Bivariate correlations were used to check for any potential associations between the vulnerability markers. One-tailed tests were employed for directional hypotheses (i.e., group differences in clinical symptoms, dermatoglyphic asymmetries, and procedural learning), and two-tailed tests were employed in all other cases (i.e., comparisons of demographic variables between groups, comparisons between groups on rotation speed for the pursuit rotor task, exploratory analyses examining correlations between vulnerability characteristics and between domains of symptoms for the entire sample).
RESULTS
Of the 221 participating young adults, a total of 16 (7.3%) reported at least one DAPS over the past one month (indexed by a score of five on the weighted scale) and 205 (92.7%) did not. Based on these results, the participants were split into DAPS and no-DAPS groups. There were no significant differences between the groups on demographic characteristics including gender, age, and parental education. Please see Table 1 for a demographic breakdown of the participants in the DAPS and no-DAPS group. Kolmogorov-Smirnov tests revealed that distributions of target dermatoglyphic and procedural learning variables were normal and as a result, analyses were conducted with parametric statistics. Consistent with other recent studies utilizing the PQ-B (26), the distribution of PQ-B total scale was non-normally distributed, with skewness of 1.05 (SE = .16) and kurtosis of .83 (SE = .32); this was also the case for the BDI total with skewness of 1.72 (SE = .16) and kurtosis of 4.05 (SE = .32).
Clinical Symptoms
An independent t-test indicated that the DAPS group (mean = 8.68, SD = 3.84) endorsed a significantly higher level of items on PQB total scale, t(219) = -5.02, p </= .01, when compared to the no-DAPS group (mean = 4.01, SD = 3.05). The most commonly endorsed items were: “Do you find yourself feeling mistrustful or suspicious of other people?”(2.7%), “Do people sometimes find it hard to understand what you are saying?” (1.4%), “Do you worry at times that something may be wrong with your mind?” (1.8%), “Have you had the sense that some person or force is around you, although you couldn’t see anyone?” (.9%), “Do you have difficulty getting your point across, because you ramble or go off the track a lot when you talk?” (.9%), “Have you ever felt that you don’t exist, the world does not exist, or that you are dead?” (.9%), and “Have you seen unusual things like flashes, flames, blinding light, or geometric figures?” (.9%).
Although both groups showed subclinical levels of depression, there were notable group differences on the Beck Depression scale. Specifically, an independent t-test indicated that the DAPS group (mean = 20.5, SD = 11.79) showed significant elevations on the BDI when compared to the no-DAPS group (mean = 7.64, SD = 6.59), t(219) = -4.30, p </= .01 (see Table 1 for group means and standard deviations). It should be noted that the results for group differences on PQB-Total and BDI scales remained the same when non-parametric tests were employed.
Prenatal Disruption: Dermatoglyphic Asymmetry
Discrepancy scores for each participant were calculated using the absolute value of the right finger ridge count minus the left finger ridge count for each participant’s five homologous pairs. The mean ridge count discrepancy (the average of each participant’s five discrepancy scores) was used as an index of overall asymmetry. An independent sample t-test showed significant group differences, t(219)=-2.01, p </= .05, where the no-DAPS group (mean = 3.15, SD = 1.30) reported significantly fewer asymmetries (ridge discrepancies) than the DAPS group (mean = 3.65, SD = .95). Figure 1 shows an example of a highly discrepant ridge count between a pair of corresponding fingerprints.
Figure 1. Asymmetrical Dermatoglyphic Patterns.
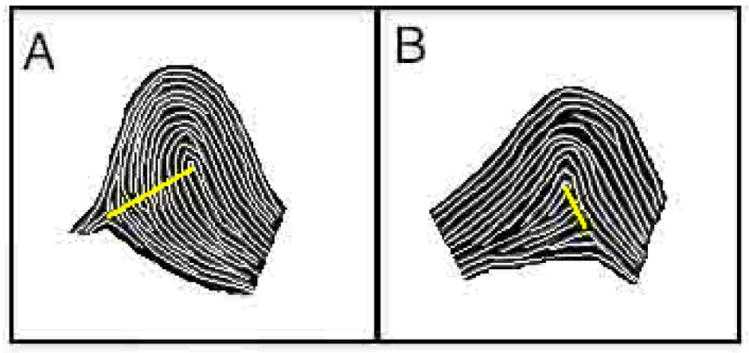
In this example of an asymmetrical print pair, taken from a non-participant model, the ridge count between the triradius (meeting of patterns on the periphery of the print) and core (center of print) for the left finger L3 (A) is markedly higher than that of the respective homologous right finger R3 (B).
Procedural Learning: Pursuit Rotor
As noted, the participants were titrated, so that each individual reached a criterion of being able to maintain contact with the target approximately 20%– 25% of the time. Each of the subjects was able to meet this criterion. Analyses of the subjects’ eventual target rotation speed revealed that the DAPS group started with a trend towards a lower rate of rotation than the no-DAPS group, t(219) = 1.68, p </= .10. Following the titration process, participants began three trial blocks at 0, 20, and 40 minutes, each consisting of three 20-second trials (separated by 5-second rest intervals). Procedural learning was computed by calculating the mean of the three trials during each block, to yield a single index of performance for each block.
A 2 (group) × 3 (mean score trial block) mixed repeated measures ANOVA with percent time on target as the dependent variable was conducted. Results revealed a significant main effect for group F(1,217)=4.62, p </= .05, indicating that the DAPS group performed significantly poorer than the no-DAPS group (despite the trend towards slower target speed). There was a significant effect for trial block F(2,217)=133.05, p </= .01, and post-hoc repeated measures t-tests conducted for both the separate groups indicated statistically significant changes between each trial block (p < .01), indicating that both groups improved on the task over time. The interaction effect did not approach significance indicating that while the DAPS group performed consistently more poorly, both groups showed evidence of procedural learning (Figure 2 illustrates performance by group, across the trial blocks).
Figure 2. Pursuit Rotor Task Performance for Groups of Participants Who Report and Who Do Not Report Distressing Attenuated Positive Symptoms (DAPS).
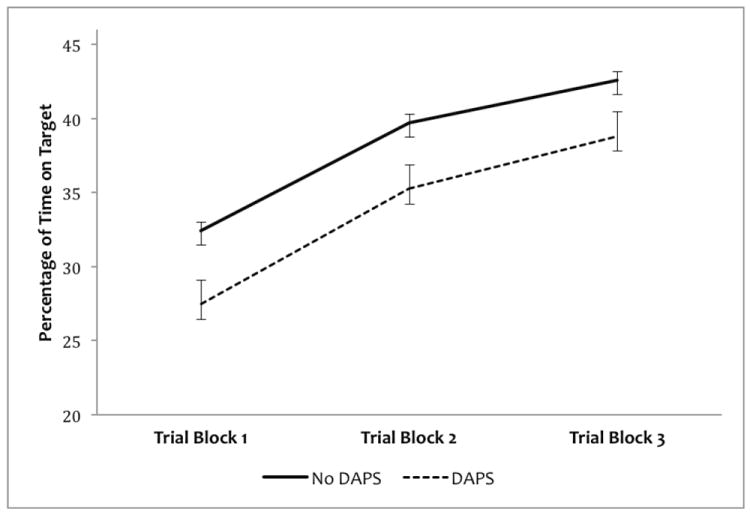
Although both groups showed indication of procedural learning over three time-spaced trials, the percentage time on target for participants experiencing a distressing attenuated positive symptom was significantly less than those participants without such an experience. Error Bars represent mean standard error.
Correlations Between Vulnerability Characteristics
Bivariate correlations were used to examine potential interrelationships between vulnerability and symptom measures among the entire. There was no significant relationship between the dermatoglyphic asymmetry score and performance on the procedural memory task for any trial block. BDI scores were not associated with dermatoglyphic or pursuit rotor variables. However, BDI was significantly associated with both total PQB score (r = .53, p < /= .01) and weighted impact total (r = .63, p </= .01).
DISCUSSION
Consistent with recent reports (33), the present findings suggest that symptoms of non-clinical psychosis are common in young adults. Taken together, the results of this study suggest that early developmental and core cognitive deficits characterizing key points in diathesis-stress conceptualizations of psychosis are also present on the non-clinical side of the psychosis spectrum. To our knowledge, the current study is the first to examine dermatoglyphics and procedural learning in a sample of participants reporting psychotic-like experiences. The findings show that among this large group of young adults, those reporting DAPS showed elevated dermatoglyphic asymmetries and a pattern of procedural learning uniquely distinct from controls, and consistent with studies of patients with formal psychosis (22, 30, 34).
Prevalence rates of DAPS in the current sample (7.3%) are highly consistent with a recent reports suggesting an overall rate of 5-8% for this phenomenon in the general population (35, 36). The mean BDI scores were significantly elevated in the DAPS group, which also appears consistent with other recent reports (37, 38). In addition, BDI scores were associated with elevated reports of DAPS among the entire sample. Taken together, the results are consistent with a growing recent body of literature that suggests that non-clinical symptoms of psychosis appear to be associated with other domains of clinical dysfunction (37-39). However, epidemiological or prospective longitudinal designs are necessary to determine any potential causal relationship.
Early developmental disruption, reflecting a complex relationship between genetic and environmental factors (5), has characterized risk across the psychosis spectrum ranging from adolescents with schizotypal personality disorder (40), young adults with a prodromal syndrome (41), and first-episode and chronic populations (42). The present results are consistent with other investigations finding that symptoms of non-clinical psychosis are associated with obstetric complications such as maternal infection, and maternal diabetes (43). However, it is also important to note that the study of early developmental insults is highly complex, and other studies examining non-clinical psychosis have found that factors such as paternal age, or preeclampsia or birth season (a proxy for prenatal exposure to viral infection) have not been found to be associated (43-45). The present findings may be helpful in narrowing the time window of developmental insult, and implicating brain structures also in ascendance during the formation of the skin. More specifically, abnormalities in fingerprints reflect disruption during gestational weeks 14-22 (46), and within this context, it is important to note that this narrow window represents the second trimester, a critical time for medial temporal brain structures highly implicated in psychosis (42).
To date, research on the cognitive functioning at the non-clinical end of the psychosis-spectrum has painted a complex figure. Researchers have observed specific deficits in receptive language (15), processing speed/executive functioning, motor (47), and verbal fluency (48). However, studies of expressive language (15), attention, memory, or abstract reasoning (47) have not found significant differences. It remains highly important to understand the neuropsychological functioning of this group, as cognitive dysfunction is considered a core feature of vulnerability, and therefore may be key to understanding the etiology of psychosis (14).
Procedural learning does not require conscious recollection; investigators have described this process as automatic, in that it can occur in the absence of directed attention (49). Early studies have implicated a procedural memory deficit in schizophrenia (19, 20), but recent studies have painted a more nuanced picture. Specifically, researchers have observed that while there are deficits in performance in each trial block (where patients with psychosis show poorer performance than controls, despite having the benefit of slower stimulus rotation), the overall slope of learning is consistent across clinical and control groups (22, 30, 34, 50). One interpretation is that while procedural memory is relatively intact (the patients show the ability to learn), motor deficits (also prevalent in schizophrenia and tied to fronto-striatal dysregulation) (3) result in an overall poorer task performance. In the present context, it is noteworthy that deficits on motor procedural learning tasks are linked with abnormalities in fronto-striatal functioning (19, 20). Taken together, the DAPS group’s performance in the present study was consistent with the noted larger body of literature, showing that fronto-striatal dysfunction also occurs at the non-clinical end of a psychosis continuum. These results are also consistent with an important study that reported structural and connective abnormalities in components of the basal ganglia circuits in adolescents who had reported significant psychotic-like experiences (51) and a recent study that observed dyskinesias in this group as well (28).
A neural developmental diathesis-stress model of schizophrenia suggests that an early constitutional vulnerability (reflecting genetic and environmental liability factors), later interacts with environmental triggers and neural development to result in a psychotic outcome (4). Dysmorphic features, found to be more prevalent in patients with schizophrenia (10), arise during the formation of the craniofacial region, limbs, and hands, which critically, co-occurs with the development of the CNS. As such, dermatoglyphic asymmetries are enduring representations, reflecting developmental disruption to neural structures widely implicated in psychosis (3). Procedural memory deficits, representing a fronto-striatal abnormality highly implicated in formal psychosis (3), reflect core pathology underlying the diathesis of psychosis. Findings that developmental and fronto-striatal abnormalities are present in a non-clinical sample reporting DAPS support the diathesis-stress model and elucidate our understanding of constitutional vulnerability. Perhaps, in the absence of a sufficient diathesis or stressor, the vulnerability in non-clinical populations contributes to these phenomena. However, it is also important to note that notion of vulnerability is complex, and involves numerous interrelated and distinct factors including environmental influences such as social disadvantage (52) and cannabis use (53); this complexity is evident in the current investigation, as dermatoglyphic and procedural memory variables were not found associated in this sample.
Overall, the present study adds to our understanding of liability by showing that early developmental disruption and fronto-striatal dysfunction are core elements of pathophysiology underlying psychosis, as these signs also characterize the non-clinical end of a continuum, which is not obfuscated by confounds that come along with studies in samples with formal psychosis. One significant limitation of the study involves single indices of developmental disruption and procedural learning. Future work utilizing non-motor based procedural learning tests can help to elucidate our understanding of both motor and pure procedural learning contributions to deficits seen in individuals reporting symptoms of non-clinical psychosis. In addition, future studies should be conducted to explore factors underlying psychosis risk in a continuous fashion. Further, because the present study is the first investigation in this area, has a relatively small DAPS group (limiting ability to detect subtle effects), and does not explicitly test for formal psychotic disorders, the present findings should be considered as preliminary until future studies control for these potential confounds and replicate the results in a larger and unselected general population sample. However, it is unlikely that a subgroup of formally psychotic individuals accounted for the observed effect, as no subjects scored in the upper 25% of the possible attenuated symptom weighted distress score range of 1-5. Finally, it should be noted that although a recent series of papers has utilized the PQ-B to assess non-clinical psychosis (27, 28), future studies are necessary to formally validate the measure in this context. As we continue to map out the psychosis continuum, future studies examining subtypes of non-clinical psychosis and a greater variety of designs (e.g., longitudinal) and developmental markers are warranted.
Significant Outcomes.
Elevated dermatoglyphic asymmetries, characteristic of individuals with formal psychosis, were observed in a non-clinical sample of individuals reporting attenuated positive symptoms.
Fronto-striatal dysfunction, observed as unique patterns of procedural learning, was found among non-clinical individuals reporting attenuated positive symptoms.
Vulnerability signs associated with schizophrenia may also occur along a continuum, as they are apparent in the non-clinical side of the psychosis-spectrum as well.
Limitations.
Lacking multiple indices of measurement for early developmental disruption.
Used a single instrument to test procedural learning.
The group reporting symptoms of non-clinical psychosis (n = 16) was relatively small.
Acknowledgments
This work was supported by National Institutes of Health Grants R01MH094650 and MH08725 to Dr. Mittal.
Footnotes
Declaration of Interest
None.
Contributor Information
Vijay A. Mittal, Department of Psychology and Neuroscience, Center for Neuroscience, University of Colorado at Boulder.
Derek J. Dean, Department of Psychology and Neuroscience, University of Colorado at Boulder
Andrea Pelletier, Department of Psychology and Neuroscience, University of Colorado at Boulder.
References
- 1.VAN OS J, HANSSEN M, BIJL RV, VOLLEBERGH W. Prevalence of psychotic disorder and community level of psychotic symptoms: an urban-rural comparison. Arch Gen Psychiatry. 2001;58:663–8. doi: 10.1001/archpsyc.58.7.663. [DOI] [PubMed] [Google Scholar]
- 2.AHMED AO, BUCKLEY PF, MABE PA. Latent structure of psychotic experiences in the general population. Acta Psychiatrica Scandinavica. 2012;125:54–65. doi: 10.1111/j.1600-0447.2011.01800.x. [DOI] [PubMed] [Google Scholar]
- 3.ROBBINS TW. The case of frontostriatal dysfunction in schizophrenia. Schizophr Bull. 1990;16:391–402. doi: 10.1093/schbul/16.3.391. [DOI] [PubMed] [Google Scholar]
- 4.WALKER E, MITTAL VA, TESSNER K. Stress and the hypothalamic pituitary adrenal axis in the developmental course of schizophrenia. Annu Rev Clin Psychol. 2008;4:189–216. doi: 10.1146/annurev.clinpsy.4.022007.141248. [DOI] [PubMed] [Google Scholar]
- 5.MITTAL VA, ELLMAN LM, CANNON TD. Gene-environment interaction and covariation in schizophrenia: the role of obstetric complications. Schizophr Bull. 2008;34:1083–94. doi: 10.1093/schbul/sbn080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.KRAEPELIN E. Dementia Praecox and Paraphrenia. Livingstone; Edinburgh: 1896. [Google Scholar]
- 7.ISMAIL B, CANTOR-GRAAE E, MCNEIL TF. Minor physical anomalies in schizophrenia: cognitive, neurological and other clinical correlates. J Psychiatr Res. 2000;34:45–56. doi: 10.1016/s0022-3956(99)00034-5. [DOI] [PubMed] [Google Scholar]
- 8.COMPTON MT, WALKER EF. Physical manifestations of neurodevelopmental disruption: are minor physical anomalies part of the syndrome of schizophrenia? Schizophr Bull. 2009;35:425–36. doi: 10.1093/schbul/sbn151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.KING S, MANCINI-MARIE A, BRUNET A, WALKER E, MEANEY MJ, LAPLANTE DP. Prenatal maternal stress from a natural disaster predicts dermatoglyphic asymmetry in humans. Dev Psychopathol. 2009;21:343–53. doi: 10.1017/S0954579409000364. [DOI] [PubMed] [Google Scholar]
- 10.REILLY JL, MURPHY PT, BYRNE M, et al. Dermatoglyphic fluctuating asymmetry and atypical handedness in schizophrenia. Schizophr Res. 2001;50:159–68. doi: 10.1016/s0920-9964(00)00044-x. [DOI] [PubMed] [Google Scholar]
- 11.HOLT SB. Genetics of dermal ridges; the relation between total ridge-count and the variability of counts from finger to finger. Ann Hum Genet. 1958;22:323–39. doi: 10.1111/j.1469-1809.1958.tb01426.x. [DOI] [PubMed] [Google Scholar]
- 12.WEINSTEIN DD, DIFORIO D, SCHIFFMAN J, WALKER E, BONSALL R. Minor physical anomalies, dermatoglyphic asymmetries, and cortisol levels in adolescents with schizotypal personality disorder. Am J Psychiatry. 1999;156:617–23. doi: 10.1176/ajp.156.4.617. [DOI] [PubMed] [Google Scholar]
- 13.MITTAL VA, DHRUV S, TESSNER KD, WALDER DJ, WALKER EF. The relations among putative biorisk markers in schizotypal adolescents: minor physical anomalies, movement abnormalities, and salivary cortisol. Biol Psychiatry. 2007;61:1179–86. doi: 10.1016/j.biopsych.2006.08.043. [DOI] [PubMed] [Google Scholar]
- 14.CORNBLATT BA, LENCZ T, SMITH CW, CORRELL CU, AUTHER AM, NAKAYAMA E. The schizophrenia prodrome revisited: a neurodevelopmental perspective. Schizophr Bull. 2003;29:633–51. doi: 10.1093/oxfordjournals.schbul.a007036. [DOI] [PubMed] [Google Scholar]
- 15.CANNON M, CASPI A, MOFFITT TE, et al. Evidence for early-childhood, pan-developmental impairment specific to schizophreniform disorder: results from a longitudinal birth cohort. Arch Gen Psychiatry. 2002;59:449–56. doi: 10.1001/archpsyc.59.5.449. [DOI] [PubMed] [Google Scholar]
- 16.JOHNS LC, CANNON M, SINGLETON N, et al. Prevalence and correlates of self-reported psychotic symptoms in the British population. Br J Psychiatry. 2004;185:298–305. doi: 10.1192/bjp.185.4.298. [DOI] [PubMed] [Google Scholar]
- 17.HORWOOD J, SALVI G, THOMAS K, et al. IQ and non-clinical psychotic symptoms in 12-year-olds: results from the ALSPAC birth cohort. Br J Psychiatry. 2008;193:185–91. doi: 10.1192/bjp.bp.108.051904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.COHEN NJ, SQUIRE LR. Preserved learning and retention of pattern-analyzing skill in amnesia: dissociation of knowing how and knowing that. Science. 1980;210:207–10. doi: 10.1126/science.7414331. [DOI] [PubMed] [Google Scholar]
- 19.HUSTON PE, SHAKOW D. Learning capacity in schizophrenia; with special reference to the concept of deterioration. Am J Psychiatry. 1949;105:881–8. doi: 10.1176/ajp.105.12.881. [DOI] [PubMed] [Google Scholar]
- 20.EYSENCK HJ, FRITH CD. Reminiscence, motivation, and personality. Plenum; New York: 1977. [Google Scholar]
- 21.SARAZIN M, DEWEER B, MERKL A, VON POSER N, PILLON B, DUBOIS B. Procedural learning and striatofrontal dysfunction in Parkinson’s disease. Mov Disord. 2002;17:265–73. doi: 10.1002/mds.10018. [DOI] [PubMed] [Google Scholar]
- 22.GRANHOLM E, BARTZOKIS G, ASARNOW RF, MARDER SR. Preliminary associations between motor procedural learning, basal ganglia T2 relaxation times, and tardive dyskinesia in schizophrenia. Psychiatry Res. 1993;50:33–44. doi: 10.1016/0925-4927(93)90022-a. [DOI] [PubMed] [Google Scholar]
- 23.MITTAL VA, NEUMANN C, SACZAWA M, WALKER EF. Longitudinal progression of movement abnormalities in relation to psychotic symptoms in adolescents at high risk of schizophrenia. Arch Gen Psychiatry. 2008;65:165–71. doi: 10.1001/archgenpsychiatry.2007.23. [DOI] [PubMed] [Google Scholar]
- 24.HOWES OD, MONTGOMERY AJ, ASSELIN MC, et al. Elevated striatal dopamine function linked to prodromal signs of schizophrenia. Arch Gen Psychiatry. 2009;66:13–20. doi: 10.1001/archgenpsychiatry.2008.514. [DOI] [PubMed] [Google Scholar]
- 25.LOEWY R, CANNON T. The Prodromal Questionnaire, Brief Version (PQ-B) University of California; 2010. [Google Scholar]
- 26.LOEWY RL, PEARSON R, VINOGRADOV S, BEARDEN CE, CANNON TD. Psychosis risk screening with the Prodromal Questionnaire - Brief Version (PQ-B) Schizophr Res. 2011;129:42–6. doi: 10.1016/j.schres.2011.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.LOEWY RL, JOHNSON JK, CANNON TD. Self-report of attenuated psychotic experiences in a college population. Schizophr Res. 2007;93:144–51. doi: 10.1016/j.schres.2007.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.MITTAL VA, DEAN DJ, PELLETIER A, CALIGIURI M. Associations between spontaneous movement abnormalities and psychotic-like experiences in the general population. Schizophr Res. 2011;132:194–6. doi: 10.1016/j.schres.2011.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.BECK AT, STEER RA. Internal consistencies of the original and revised Beck Depression Inventory. J Clin Psychol. 1984;40:1365–7. doi: 10.1002/1097-4679(198411)40:6<1365::aid-jclp2270400615>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 30.GOMAR JJ, POMAROL-CLOTET E, SARRO S, SALVADOR R, MYERS CE, MCKENNA PJ. Procedural learning in schizophrenia: reconciling the discrepant findings. Biol Psychiatry. 2011;69:49–54. doi: 10.1016/j.biopsych.2010.07.013. [DOI] [PubMed] [Google Scholar]
- 31.HARRISON EL, FILLMORE MT. Social drinkers underestimate the additive impairing effects of alcohol and visual degradation on behavioral functioning. Psychopharmacology. 2005;177:459–64. doi: 10.1007/s00213-004-1964-x. [DOI] [PubMed] [Google Scholar]
- 32.KERN RS, GREEN MF, WALLACE CJ. Declarative and procedural learning in Schizophrenia: A test of the integrity of divergent memory systems. Cogn Neuropsychiatry. 1997;2:39–50. doi: 10.1080/135468097396405. [DOI] [PubMed] [Google Scholar]
- 33.SCOTT J, WELHAM J, MARTIN G, et al. Demographic correlates of psychotic-like experiences in young Australian adults. Acta psychiatrica Scandinavica. 2008;118:230–7. doi: 10.1111/j.1600-0447.2008.01214.x. [DOI] [PubMed] [Google Scholar]
- 34.CLARE L, MCKENNA PJ, MORTIMER AM, BADDELEY AD. Memory in schizophrenia: what is impaired and what is preserved? Neuropsychologia. 1993;31:1225–41. doi: 10.1016/0028-3932(93)90070-g. [DOI] [PubMed] [Google Scholar]
- 35.VAN OS J, LINSCOTT RJ, MYIN-GERMEYS I, DELESPAUL P, KRABBENDAM L. A systematic review and meta-analysis of the psychosis continuum: evidence for a psychosis proneness-persistence-impairment model of psychotic disorder. Psychol Med. 2009;39:179–95. doi: 10.1017/S0033291708003814. [DOI] [PubMed] [Google Scholar]
- 36.GALE CK, WELLS JE, MCGEE MA, BROWNE MA. A latent class analysis of psychosis-like experiences in the New Zealand Mental Health Survey. Acta psychiatrica Scandinavica. 2011;124:205–13. doi: 10.1111/j.1600-0447.2011.01707.x. [DOI] [PubMed] [Google Scholar]
- 37.ARMANDO M, NELSON B, YUNG AR, et al. Psychotic-like experiences and correlation with distress and depressive symptoms in a community sample of adolescents and young adults. Schizophr Res. 2010;119:258–65. doi: 10.1016/j.schres.2010.03.001. [DOI] [PubMed] [Google Scholar]
- 38.VARGHESE D, SCOTT J, WELHAM J, et al. Psychotic-like experiences in major depression and anxiety disorders: a population-based survey in young adults. Schizophr Bull. 2011;37:389–93. doi: 10.1093/schbul/sbp083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.NISHIDA A, SASAKI T, NISHIMURA Y, et al. Psychotic-like experiences are associated with suicidal feelings and deliberate self-harm behaviors in adolescents aged 12-15 years. Acta Psychiatrica Scandinavica. 2010;121:301–7. doi: 10.1111/j.1600-0447.2009.01439.x. [DOI] [PubMed] [Google Scholar]
- 40.MITTAL VA, SACZAWA ME, WALKER E, WILLHITE R, WALDER D. Prenatal exposure to viral infection and conversion among adolescents at high-risk for psychotic disorders. Schizophr Res. 2008;99:375–6. doi: 10.1016/j.schres.2007.11.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.MITTAL VA, WILLHITE R, NIENDAM T, et al. Obstetric complications and risk for conversion to psychosis among Individuals at high clinical risk. Early Interv Psychiatry. 2009;3:226–30. doi: 10.1111/j.1751-7893.2009.00137.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.O’CALLAGHAN E, BUCKLEY P, MADIGAN C, et al. The relationship of minor physical anomalies and other putative indices of developmental disturbance in schizophrenia to abnormalities of cerebral structure on magnetic resonance imaging. Biol Psychiatry. 1995;38:516–24. doi: 10.1016/0006-3223(94)00381-C. [DOI] [PubMed] [Google Scholar]
- 43.ZAMMIT S, ODD D, HORWOOD J, et al. Investigating whether adverse prenatal and perinatal events are associated with non-clinical psychotic symptoms at age 12 years in the ALSPAC birth cohort. Psychol Med. 2009;39:1457–67. doi: 10.1017/S0033291708005126. [DOI] [PubMed] [Google Scholar]
- 44.ZAMMIT S, HORWOOD J, THOMPSON A, et al. Investigating if psychosis-like symptoms (PLIKS) are associated with family history of schizophrenia or paternal age in the ALSPAC birth cohort. Schizophr Res. 2008;104:279–86. doi: 10.1016/j.schres.2008.04.036. [DOI] [PubMed] [Google Scholar]
- 45.POLANCZYK G, MOFFITT TE, ARSENEAULT L, et al. Etiological and clinical features of childhood psychotic symptoms: results from a birth cohort. Arch Gen Psychiatry. 2010;67:328–38. doi: 10.1001/archgenpsychiatry.2010.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.VAN VALEN L. A study of fluctuating asymmetry. Evolution. 1962;16:125–42. [Google Scholar]
- 47.BLANCHARD MM, JACOBSON S, CLARKE MC, et al. Language, motor and speed of processing deficits in adolescents with subclinical psychotic symptoms. Schizophr Res. 2010;123:71–6. doi: 10.1016/j.schres.2010.05.028. [DOI] [PubMed] [Google Scholar]
- 48.KRABBENDAM L, MYIN-GERMEYS I, HANSSEN M, VAN OS J. Familial covariation of the subclinical psychosis phenotype and verbal fluency in the general population. Schizophr Res. 2005;74:37–41. doi: 10.1016/j.schres.2004.08.009. [DOI] [PubMed] [Google Scholar]
- 49.TULVING E. Multiple memory systems and consciousness. Hum Neurobiol. 1987;6:67–80. [PubMed] [Google Scholar]
- 50.SCHERER H, STIP E, PAQUET F, BEDARD MA. Mild procedural learning disturbances in neuroleptic-naive patients with schizophrenia. J Neuropsychiatry Clin Neurosci. 2003;15:58–63. doi: 10.1176/jnp.15.1.58. [DOI] [PubMed] [Google Scholar]
- 51.JACOBSON S, KELLEHER I, HARLEY M, et al. Structural and functional brain correlates of subclinical psychotic symptoms in 11-13 year old schoolchildren. Neuroimage. 2010;49:1875–85. doi: 10.1016/j.neuroimage.2009.09.015. [DOI] [PubMed] [Google Scholar]
- 52.MORGAN C, FISHER H, HUTCHINSON G, et al. Ethnicity, social disadvantage and psychotic-like experiences in a healthy population based sample. Acta Psychiatr Scand. 2009;119:226–35. doi: 10.1111/j.1600-0447.2008.01301.x. [DOI] [PubMed] [Google Scholar]
- 53.SKINNER R, CONLON L, GIBBONS D, MCDONALD C. Cannabis use and non-clinical dimensions of psychosis in university students presenting to primary care. Acta Psychiatr Scand. 2011;123:21–7. doi: 10.1111/j.1600-0447.2010.01546.x. [DOI] [PubMed] [Google Scholar]


