Abstract
Glucocorticoid (GC) hormones are used in the treatment of hematopoietic malignancies. When the GC binds to the glucocorticoid receptor (GR) protein, c-Myb and GR are recruited at the Glucocorticoid Response Unit in the DNA. Here we demonstrate that c-Myb interacts with the GR and that decreasing c-Myb amounts reduces the levels of GR transcripts and protein in 697 pre-B-acute lymphoblastic leukemia (ALL) cells. Furthermore, the auto-upregulation of GR promoter 1C and promoter 1D is blunted at reduced c-Myb levels. Taken together, these data show that c-Myb is a direct, key regulator of the GR. Unexpectedly, the reduction in c-Myb levels increased the sensitivity of the cells to steroid-mediated apoptosis. This was because the reduction in c-Myb itself decreases cell viability, and the residual GR remained above the threshold needed to trigger apoptosis. These studies show the mutual importance of c-Myb and the GR in controlling survival of pre-B ALL cells.
Keywords: c-Myb, glucocorticoid receptor, acute lymphoblastic leukemia, auto-upregulation
1. INTRODUCTION
Glucocorticoids are steroid hormones synthesized in the adrenal cortex that affect many different functions in the body such as development, differentiation, metabolism and the immune response (Bamberger et al., 1996). Glucocorticoids diffuse through the cell membrane and bind to their intracellular binding protein, the glucocorticoid receptor (GR), which belongs to the nuclear steroid receptor family of transcription factors. Upon GC binding, the GR undergoes a conformational change and subsequently translocates to the nucleus and functions as a transcription factor. The GC-GR complex then binds to specific DNA sequences [GC response element (unit), GRE or GRU] in target gene promoters and alters target gene expression (Adcock, 2000, Webster and Cidlowski, 1999). GCs broadly influence the activities and functions of most cells and tissues and may elicit opposite responses in different cell types (Gross and Cidlowski, 2008, Keith, 2008).
Glucocorticoid (GC) steroid hormones are used to treat hematopoietic malignancies, especially acute lymphoblastic leukemia (ALL), because of their pro-apoptotic effects in malignant cells (Nanni et al., 1982, Pui et al., 2008). Interestingly, GC sensitivity is a major prognostic factor in childhood ALL, as in vitro resistance to GCs at initial diagnosis is correlated to an unfavorable event free survival (Hongo et al., 1997, Kaspers et al., 1998, Kaspers et al., 1997, Pieters et al., 1991). In vitro studies demonstrate that leukemic blasts in patients with relapsed ALL are 300 times more resistant to GCs than blasts taken at initial diagnosis (Klumper et al., 1995), and glucocorticoid resistance is present in almost all cases of ALL relapse (Kaspers et al., 1998, Kofler et al., 2003). However, it is difficult to understand the mechanism of relapse after hormone treatment in these patients, because the precise mechanistic link from GCs to apoptosis remains unclear. Furthermore, many studies show that the auto-upregulation of the GR is required for GC sensitivity (Ashraf et al., 1991, Miller et al., 2007, Pedersen and Vedeckis, 2003). We have recently shown that there is a threshold level of GR transcripts/protein that has to be present in a cell for it to respond to GC therapy (Schwartz et al., 2010). Thus, the amount of GR in the cells is a critical factor in determining the GC sensitivity of the cells.
We have discovered a novel glucocorticoid response unit (GRU), which contains a GR/c-Myb cassette (Geng et al., 2008, Geng and Vedeckis, 2005) that is present in at least three promoters for the GR gene. Recently, we have shown that c-Myb and GR are recruited to GR promoters 1C and 1D in the 697 pre-B-ALL cell line (Geng and Vedeckis, 2011). c-Myb is the cellular progenitor of the v-Myb oncogene carried by the avian myeloblastosis virus (AMV) and E26 retroviruses (Klempnauer et al., 1982, Leprince et al., 1983). The c-Myb transcription factor is highly expressed in immature hematopoietic cells and down-regulated during differentiation. c-Myb plays a direct role in lineage fate selection, cell cycle progression, and differentiation of myeloid and B- and T-lymphoid progenitor cells (Anfossi et al., 1989, Caracciolo et al., 1990, Emambokus et al., 2003, Gewirtz et al., 1989, Gewirtz and Calabretta, 1988, Lieu et al., 2004, Nakata et al., 2007, Oh and Reddy, 1999, Thomas et al., 2005, Vegiopoulos et al., 2006, Weston, 1998, Xiao et al., 2007). The involvement of c-Myb in the auto-upregulation of GR promoters may be significant, because c-Myb is present at a high level in many ALL cells that auto-upregulate the GR, while c-Myb is absent in more mature lymphoblastic lineages (e.g., IM-9 B-lymphoblastoid cells) that usually down-regulate GR expression. However, the precise role of c-Myb in the regulation of the GR protein remains unknown and needs to be elucidated. Also, it is not known if there is a physical interaction between the two transcription factors, GR and c-Myb. The recruitment of GR and c-Myb to GR promoter 1C and 1D led us to hypothesize that the c-Myb transcription factor regulates the level of GR transcripts and protein in the cells, and thus c-Myb affects the auto-upregulation of GR in pre-B-ALL cells. In the present study, we wished to determine if c-Myb binds to the GR and if c-Myb affects the level of GR and the sensitivity of the cells to GC therapy. We used GST fusion protein pulldown and co-immunoprecipitation assays to determine if there is an interaction between c-Myb and GR. Further, we used a controllable lentiviral based shRNA, specific for c-Myb, as a tool to manipulate c-Myb levels and test if c-Myb regulates the level of GR transcripts and protein in the 697 pre-B-ALL cell line. Our results indicate that the decreases in c-Myb reduced the levels of GR transcripts and protein. Taken together, our data suggests a fundamental importance of c-Myb in the regulation of GR levels in these cells.
2. MATERIALS AND METHODS
2.1 Cell lines and cell culture
The human T-ALL cell line, CEM-C7, was a gift from Dr. E. Brad Thompson, University of Texas Medical Branch, Galveston, TX. The human IM-9 B-lymphoblastoid cell line was obtained from the American Type Culture Collection (ATCC, CCL-159, Manassas, VA). The human pre-B-ALL cell line, 697, was obtained from Dr. Noreen M. Robertson, Drexel University School of Medicine, Philadelphia, PA. These cell lines and their transduced derivatives (see below) were grown in RPMI 1640 culture medium supplemented with 10% fetal bovine serum and 2 mM L-glutamine, and they were incubated at 37 °C and 5% CO2. The 697-sh-GR2 cell line has been described previously (Schwartz et al., 2010). The E8.2 cell line was obtained from Dr. Paul R. Housley, University of South Carolina School of Medicine. E8.2 is a glucocorticoid receptor deficient mouse fibroblast cell-line that was derived from mouse L929 fibroblasts (Housley and Forsthoefel, 1989). COS-7 cells are a kind gift of Dr. Wanguo Liu (LSU Health Sciences Center, New Orleans). E8.2 cells, COS-7 cells, and HEK-293T cells (ATCC, CRL-11268, Manassas, VA) were grown in DMEM supplemented with 10% fetal bovine serum (Invitrogen, Carlsbad, CA) and 4 mM L-glutamine, and they were incubated at 37 °C and 5% CO2.
2.2 Plasmids
The human c-Myb expression construct, pcDNA3-c-Myb-HA was provided by Dr. Giuseppe Raschella (Ente Nuove Tecnologie Energia Ambiente, Rome, Italy). The human GR expression construct, pcDNA3-GR-Flag was synthesized by inserting GR cDNA at the HindIII and BamHI sites, and a synthetic Flag sequence at the NotI and XhoI sites, of the pcDNA3 vector. The hGRα protein expression vector, pCYGR was a kind gift from Dr. John Cidlowski (National Institute of Envirnonmental Health Sciences, Research Triangle Park, NC). The constructs pXP-1C and pXP-1D have been described previously (Geng and Vedeckis, 2011).
2.3 Protein Expression and GST pulldown assays
Full length GST-c-Myb protein (Catalog No. H00004602-P01) was obtained from Novus Biologicals (Littleton, CO). GST fusion proteins prebound to glutathione sepharose beads (GE Healthcare, Wauwatosa, Wisconsin) were incubated for 1 h with recombinant GR protein (Protein-one, Rockville, MD, USA) and 1 μM dexamethasone in 1x binding buffer (20 mM Tris pH 7.4, 50 mM NaCl, 2% NP-40). Beads were washed four times in 1 ml of binding buffer and proteins eluted in 25 μl of 2x SDS loading buffer. Proteins were separated by SDS-PAGE and detected by immunoblotting using an anti-GR antibody (sc-8992) from Santa Cruz Biotechnologies, Santa Cruz, CA.
2.4 Transient Transfection and Immunoprecipitation
697 pre-B-ALL cells and CEM-C7 cells were treated with Dex for 18 h prior to lysis. HEK-293T cells, E8.2 cells, and COS-7 cells cultured on 100 mm dishes were transfected with 4.5 μg of HA-tagged c-Myb expression vector and 4.5 μg of Flag tagged GR expression vector for 48 h. Lipofectamine (Invitrogen, Carlsbad, CA) was used to transfect the cells according to the manufacturer’s instructions. These cells were treated with Dex 24 h after transfection and were collected after an additional 24 h of incubation.
The cells were washed twice with PBS, harvested and lysed with 500 μl lysis buffer containing 50 mM Tris-Hcl pH 7.5, 150 mM NaCl, 0.1% Triton X-100, 15 mM EDTA and a complete protease inhibitor mixture. Samples were centrifuged for 15 min at 14000 × g and the supernatant was collected. The protein concentration was measured using a DC Protein Assay (Bio-Rad, Hercules, CA). Protein G Sepharose beads (GE Healthcare Wauwatosa, Wisconsin) were incubated with 2.5 μg of anti-GR antibody (in case of 697 cells and CEM-C7 cells) or anti-Flag antibody (in case of E8.2, COS-7 and HEK-293T cells) and control IgG antibody overnight at 4 °C. The beads were washed three times with lysis buffer. 1 μM Dex was added to the cell lysates and they were incubated with the beads at 4°C with gentle rotation for 3 h. The resin was collected by centrifugation and washed three times each with 500 μl of lysis buffer, and the proteins were then eluted in 40 μl 2x SDS loading buffer. Proteins were separated by SDS-PAGE and probed using anti-GR and anti-c-Myb antibodies. The anti-Flag antibody (F3165) was obtained from Sigma (St. Louis, MO). The anti-HA antibody (AB9119) was obtained from Abcam (Cambridge, MA). Anti-c-Myb (sc-7874) and anti-hGR (sc-8992) were from Santa Cruz Biotechnologies (Santa Cruz, CA). A mouse monoclonal anti-c-Myb antibody (clone 1-1 catalog no. 05-175) was obtained from Millipore (Billerica, MA) and was used for detecting c-Myb in 697 and CEM-C7 cells because we used an anti-GR (Rabbit) antibody to pull down endogenous GR protein in these cells.
2.5 Lentivirus production, titering, and transduction of target cell lines
Five constitutive c-Myb shRNAmir lentiviral constructs (oligo ID numbers, V2LHS_36795, V2LHS_36796, V2LHS_36797, V2LHS_36798, V2LHS_36799; Open Biosystems, Huntsville, AL) were tested for the ability to knock down c-Myb levels. While all worked initially (indicating target specificity), stable knockdown after cell culture passage was not retained. Therefore, we transferred two of these shRNA mirs (oligo ID numbers, V2LHS_36796 and V2LHS_36798) into the Expression Arrest™ TRIPZ lentiviral shRNAmir system (Open Biosystems, Huntsville, AL) to produce lentiviral particles containing Doxy-controllable c-Myb-specific shRNA constructs, according to the manufacturer’s protocol, via HEK-293T transfection. We also obtained another c-Myb shRNAmir inducible construct (oligo ID number, V2LHS_36794) from the same company. While all three of these exhibited the controllable reduction of c-Myb levels (data not shown), the V2LHS_36794-containing construct had the best knockdown, and so it was chosen for the remainder of the experiments. After transfection, HEK-293T cells were plated in a 100 mm culture dish at a density of 5.5 × 106 cells/plate. Twenty-four hours following initial plating, serum-containing medium was aspirated and replaced with serum-free media containing the transfection mixture. The transfection mixture was composed of the transfer plasmid that contained the specific shRNAmir sequence, a mixture of packaging plasmids (supplied by manufacturer), and the Arrest-In™ transfection reagent in a 1:5 DNA:Arrest-In™ ratio. The transfection duration in serum-free media was 6 h. Following transfection, the serum-free medium with the transfection reagents was aspirated and replaced with media containing serum. Lentiviral particles were collected at two time intervals (48 and 72 h) following the addition of fresh serum-containing media. Media containing the lentiviral particles were combined, centrifuged, filtered, and concentrated via ultracentrifugation (70,000 × g for 1.5 h at 4 °C). The parental HEK-293T cells were used to calculate a functional titer of the viral stocks using the red fluorescent protein (RFP) within the transfer construct as a marker for microscopic visualization. After serially diluting an aliquot of concentrated virus, HEK-293T cells were transduced with each dilution of virus. Forty-eight hours following transduction, the number of RFP-positive colonies per well were counted via fluorescence microscopy. Transducing units per milliliter (TU/ml) were calculated by the following equation: #RFP+ colonies × dilution factor × 40; multiplying by a factor of 40 converts microliters of virus used in the transduction to milliliters for TU/ml. 697 parental cell lines were transduced with a multiplicity of infection (MOI) of 0.3, and they were subsequently selected via puromycin treatment (shRNA-containing provirus contains a puromycin resistance gene) to produce 2 new cell lines, 697 sh-c-Myb and 697 sh-non-silencing control (697 sh-NSC). A puromycin killing curve was completed on the parental cell line 697 cells to determine the correct puromycin selection dosage, which was 1.0 μg/ml. Clonal populations of each new cell line were obtained by flow cytometric cell sorting using the BD FACSAria instrument (BD Biosciences, San Jose, CA).
2.6 Western blotting
Cells were collected, washed with PBS, and lysed with 1× Laemmli sample buffer containing 1/100 volume of protease inhibitor cocktail (Sigma, St. Louis, MO) and 1 mM PMSF. Proteins were resolved by SDS-PAGE using 10% separating and 4% stacking gels and transferred to 0.45 μm nitrocellulose membranes. The membranes were blocked with 5% non-fat milk and blotted with protein-specific antibodies. The antibodies, anti-c-Myb (sc-7874), anti-GAPDH (sc-25778) and anti-hGR (sc-8992) were from Santa Cruz Biotechnologies (Santa Cruz, CA). The anti-TurboRFP (AB321) antibody was from Evrogen (Moscow, Russia).
2.7 Real time quantitative reverse transcription polymerase chain reaction (QRT-PCR)
The PCR DNA primer and Taqman® probe sequences specific for GR transcripts containing the exon 5/6 junction, GR promoter 1C, GR promoter 1D, and c-Myb were previously described (Geng and Vedeckis, 2011, Pedersen and Vedeckis, 2003). QRT-PCR reactions were normalized to the concentration of their internal 18S rRNA concentration. The relative amplification units are relative to the 18S rRNA concentration. Real time analysis was completed with the iScript™ One-Step RT-PCR Kit for Probes on the Bio-Rad (Hercules, CA) CFX96 system following the manufacturer’s instructions. Total RNA used in QRT-PCR reactions was isolated from cell samples using the Qiagen RNeasy Mini RNA isolation kit. All RNA samples were treated with DNase I according to the manufacturer’s (Qiagen, Valencia, CA) on-column digestion instructions.
2.8 Transient Transfection and Luciferase Reporter Assay
Electroporation was used to transfect 697 sh-c-Myb cells. Cells grown in log phase were collected by centrifugation and washed. The cells were resuspended at 1 × 107 cells/ml in RPMI 1640 medium containing 20% FBS. 400 μl aliquots(4 × 106 cells) were mixed with 20 μg of promoter vector plasmid plus 20 μg of β-galactosidase expression vector (to normalize transfection efficiency) and transferred into 0.4 cm gap electroporation cuvettes (Bio-Rad, Hercules, CA) on ice. The cuvettes were electroporated using 500 μF and 0.3 kV with a Gene Pulser II (Bio-Rad, Hercules, CA) eletroporator and then cooled on ice for 5 minutes. The cells were resuspended in 3 ml (per cuvette) of RPMI-1640 medium supplemented with 10% FBS and cultured overnight. The cells were then treated with 1 μg/ml of Doxy for 6 h to induce c-Myb shRNA followed by treatment with 50 nM Dex or EtOH for 18 h. The total time of Doxy treatment was 24 h. Cell lysates were collected, lysed and assayed for luciferase and β-galactosidase activity on an Ascent luminometer (Lab System, Franklin, MA) as described previously (Geng et al., 2008, Geng and Vedeckis, 2005, 2011).
2.9 Cell viability assays
The Vybrant® Apoptosis Assay Kit #4 (Invitrogen, Carlsbad, CA) was used to determine cell viability. The cells were collected by centrifugation and washed two times with PBS. After washing, the cells were resuspended in 1 ml PBS, and the reagents were added and incubated according to manufacturer’s instructions. Cell viability was measured by flow cytometry (FACSCalibur, BD Biosciences, Franklin Lakes, NJ).
3. RESULTS
3.1 c-Myb interacts with GR in vitro and in vivo
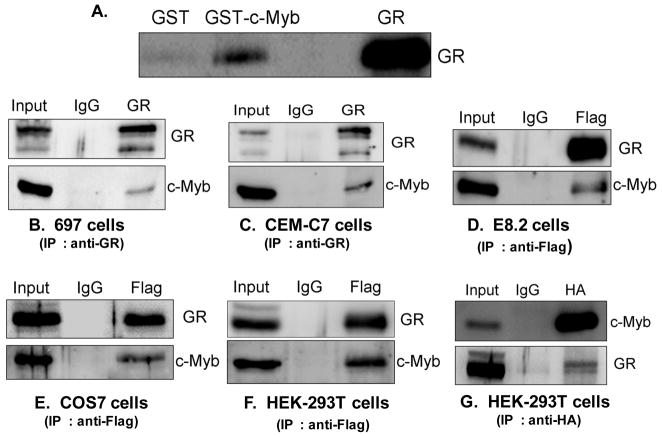
When the GC binds to the GR, the GR interacts at the GRU in concert with the c-Myb transcription factor to auto-upregulate the GR mRNA and protein levels in the cell. Recently, we showed that c-Myb and GR are recruited to GR promoter 1C and 1D in the 697 pre-B-ALL cell line (Geng and Vedeckis, 2011). However, it is not known if there is a direct interaction between these two transcription factors. To test if c-Myb and GR interact directly, GST pulldown experiments were performed with recombinantly expressed GR protein in the presence of 1 μ Dex. Full length GR was analyzed for binding to GST-c-Myb and to GST alone as a control. Figure 1A shows the immunoblotting for GR using an anti-GR antibody. The fourth lane is the input used in the pulldown assay. An interaction between GR and GST-c-Myb was observed (Fig. 1A), confirming that the two proteins have affinity for each other and interact in vitro.
Figure 1. c-Myb interacts with the GR in vitro and in vivo.
A.) Full length GST-c-Myb and GST alone (control) were incubated with recombinant GR protein and Dex. GR retained on glutathione–sepharose beads was identified by SDS-page and immunoblotting with an anti-GR antibody. The fourth lane shows the input used in the pulldown assay B, C.) Lysates from 697 cells and CEM-C7 cells were immunoprecipitated with an anti-GR antibody. D, E, F.) Lysates from E8.2 cells (D) and COS-7 cells (E) and HEK-293T cells (F) transfected with plasmids encoding HA-tagged c-Myb and FLAG-tagged GR were immunoprecipitated with an anti-FLAG antibody. G.) Lysates from HEK-293T cells transfected with plasmids encoding HA-tagged c-Myb and FLAG-tagged GR were immunoprecipitated with an anti-HA antibody. After SDS/PAGE of the immunoprecipitates of all these cell lines, immunoblot analysis was performed using anti-GR and anti-c-Myb antibodies. 5% of the input (total cell extract) used for immunoprecipitation was loaded as a reference. Mouse IgG antibody was used as negative control of immunoprecipitation.
To test if c-Myb and GR were associated under physiological conditions in vivo, we performed co-immunoprecipitation experiments in lysates from 697 pre-B-ALL (Fig. 1B) and CEM-C7 T-ALL (Fig. 1C) cell lines that express both GR and c-Myb. A band of 75 kDa, corresponding to c-Myb, was detected after immunoprecipitation with the anti-GR antibody (Fig. 1B, 1C). This demonstrates that c-Myb interacts with GR under physiological conditions in these ALL cell lines. Furthermore, we also tested if c-Myb and GR can interact in cell lines that don’t express GR or express very low levels of GR. Thus, we performed co-immunoprecipitation experiments in lysates from E8.2 cells, COS-7 cells and HEK-293T cells cotransfected with plasmids encoding HA-tagged-c-Myb and Flag-tagged GR. HEK-293T cells and COS-7 cells express very low levels of GR whereas the E8.2 cells do not express any GR. A band of 75 kDa, corresponding to c-Myb, was detected after immunoprecipitation with the anti-Flag antibody in E8.2 cells, COS-7 cells and HEK-293T cells (Fig. 1D, 1E, and 1F), while it was not detected when the cells were only transfected with HA-tagged-c-Myb (data not shown). This shows that the Flag antibody was not non-specifically immunoprecipitating c-Myb, and that the c-Myb/GR interaction is specific. Mouse IgG antibody was used as negative control of immunoprecipitation in these experiments. Also, we performed an opposite pulldown, where we used an HA tagged antibody to pull down c-Myb from lysates of HEK-293T cells co-transfected with plasmids encoding HA-tagged c-Myb and Flag-tagged-GR. A band of 95 kDa, corresponding to hGR, was detected after immunoprecipitation with the anti-HA antibody in HEK-293T cells. These experiments, in five different cell lines, demonstrate that c-Myb can interact with GR in vivo.
3.2 Doxycycline controllable reduction of c-Myb in 697 pre-B-ALL cells
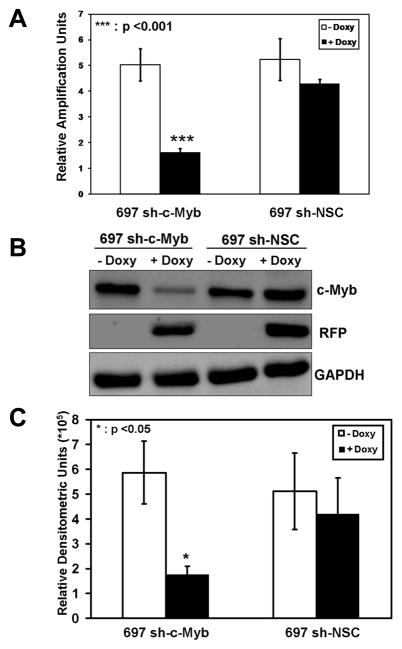
While our efforts to constitutively reduce the expression of c-Myb in 697 pre-B-ALL cells gave positive results for 5 different c-Myb shRNAmir constructs at short times after transduction, this was lost with extended times of cell culture (data not shown). Therefore, we decided to use a doxycycline (Doxy) controllable system to lower the expression of c-Myb. Transduction of parental 697 cells with lentiviral particles containing a c-Myb specific, Doxy controllable shRNAmir sequence resulted in the stable cell line, 697 sh-c-Myb. In addition, another cell line was produced that contained an integrated provirus with a controllable shRNAmir sequence that was not specific for any human gene - the non-silencing control (NSC) cell line, 697 sh-NSC. When 697 sh-c-Myb cells were pre-treated with Doxy for 48 h, c-Myb RNA transcript levels were lowered, whereas no significant decrease occurred in the 697 sh-NSC cell line (Fig. 2A). In addition, c-Myb protein levels (Fig. 2B, 2C) were reduced in the 697 sh-c-Myb cell line, whereas Doxy did not change the c-Myb expression levels in the 697 sh-NSC cell line. (The expression of TurboRFP is a marker for Doxy induction and was used to validate that the Doxy inducible system was functioning correctly in these cells.) These data demonstrate that the reduction of c-Myb is controllable via Doxy treatment in the 697 sh-c-Myb cell line.
Figure 2. Controllable reduction of c-Myb in 697 pre-B-ALL cells.
A.) c-Myb mRNA transcript measurements were performed via QRT-PCR with a probe and primer set specific for c-Myb transcripts on 697 cells stably transduced with a c-Myb specific shRNAmir lentivirus (697 sh-c-Myb) or with a non-silencing control vector (697 sh-NSC). The data are normalized to endogenous 18S rRNA levels. The relative amplification units are relative to 18S RNA levels. Total RNA was isolated following 48 h of Doxy treatment. B.) 697 cells stably transduced with a c-Myb specific shRNAmir lentivirus and with non-silencing control vector were treated with 1 μg/ml of Doxy for 48 h. Expression levels of c-Myb, TurboRFP and GAPDH proteins were analyzed by immunoblotting. A representative blot is shown. C.) Quantitative western blotting of the c-Myb protein bands. For A and C, three separate experiments were performed and the values are the average ± SEM. *, p < 0.05 and ***, p <0.001 for Doxy treated samples vs. untreated samples.
3.3 Controllable lowering of c-Myb in 697 pre-B-ALL cells reduces the basal and Dex induced levels of GR transcripts and protein
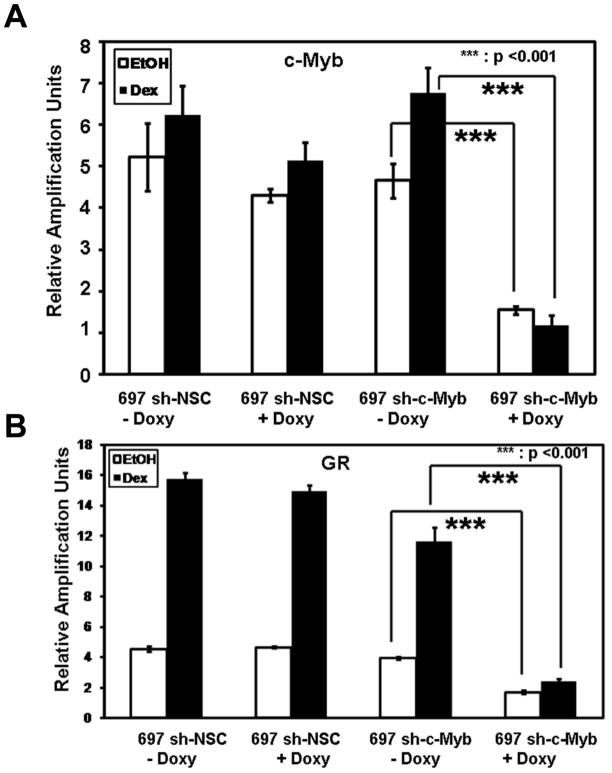
When the GC binds to the GR protein, the GR interacts at the GRU in concert with the c-Myb transcription factor to auto-upregulate the GR mRNA and protein levels in the cell. We wanted to test if c-Myb regulates the level of GR and affects the auto-upregulation of GR after Dex treatment in these newly-derived cell lines. When 697 sh-c-Myb cells were pre-treated for 48 h with Doxy and 18 h with EtOH or Dex, c-Myb RNA transcript levels were reduced (Fig. 3A). c-Myb knockdown also resulted in a significant decrease in both the basal and Dex induced total GR mRNA levels, compared to the control 697 sh-NSC cell line (Fig. 3B), in which a robust Dex-mediated upregulation of GR transcripts still occurred. These data show that the loss of intracellular c-Myb leads to a reduction in the basal and Dex induced levels of total GR transcripts.
Figure 3. Controllable decreases of c-Myb levels in 697 pre-B-ALL cells reduces the basal and Dex induced levels of GR transcripts.
A.) c-Myb mRNA transcript measurements were performed via QRT-PCR with a probe and primer set specific for c-Myb transcripts. The data are normalized to endogenous 18S RNA levels. The relative amplification units are relative to 18S rRNA levels. Total RNA was isolated following 48 h of Doxy treatment and 18 h of EtOH or Dex treatment. B.) GR mRNA transcript measurements were performed via QRT-PCR with a probe and primer set specific for GR RNA transcripts (containing the exon 5/6 junction). The data are normalized to endogenous 18S RNA levels. The relative amplification units are relative to 18S RNA levels. Three separate experiments were performed and the values are the average ± SEM. ***, p <0.001 for Doxy treated samples vs. untreated samples.
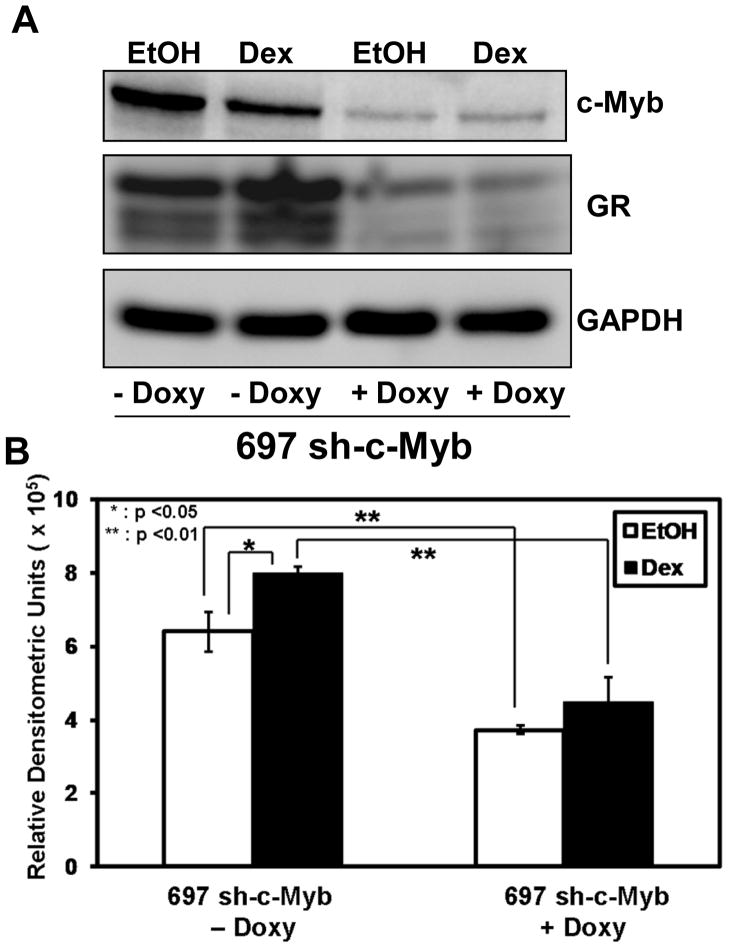
To test if the reduction of c-Myb decreased the level of GR protein in 697 cells, a Doxy titration experiment was performed. In general, there was a decrease in both c-Myb and GR protein levels with increasing Doxy amounts (data not shown). To more closely analyze the decrease in c-Myb and GR protein levels in 697 sh-c-Myb cells the highest concentration of Doxy (1 μg/ml) was used. The cells were treated with or without Doxy for 48 h followed by treatment with Dex or EtOH for 18 h. Samples were subjected to western blotting and densitometry was performed for the GR blots. A significant decrease in the amount of basal GR protein, as well as GR protein levels after Dex treatment, is seen after the reduction of c-Myb (Fig 4A, B), suggesting an important role of c-Myb in the regulation of GR in these cells.
Figure 4. Controllable lowering of c-Myb in 697-pre-B-ALL cells reduces the basal and Dex induced levels of GR protein.
A.) 697 sh-c-Myb cells were treated with or without 1 μg/ml Doxy followed by treatment with 1 μM Dex or EtOH for 18 hr. Expression levels of c-Myb, GR, and GAPDH proteins were analyzed by immunoblotting. B.) Quantitative western blotting of the GR protein bands. Three separate experiments were performed and the values are the average ± SEM. *, p < 0.05 and **, p <0.01.
3.4 c-Myb is critical to the regulation of promoter 1C and 1D in 697 cells
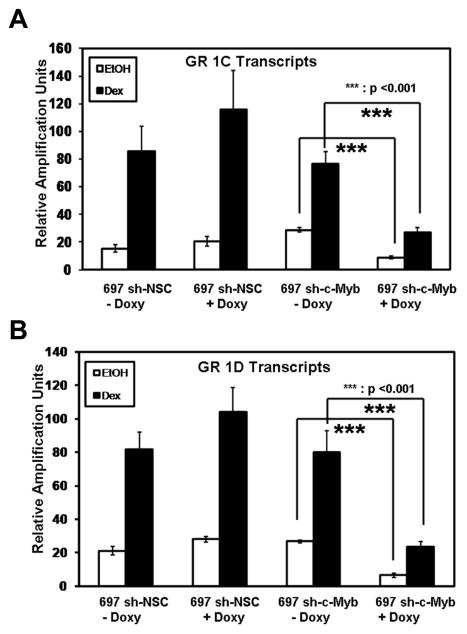
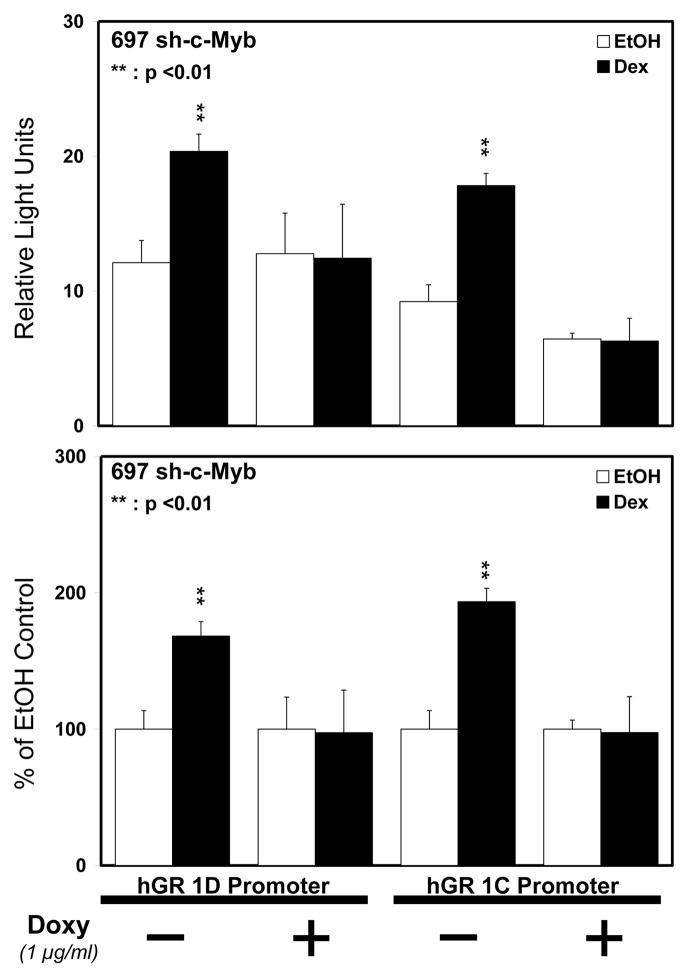
As c-Myb and GR are recruited to GR promoters 1C and 1D in 697 pre-B-ALL cells (Geng and Vedeckis, 2011), we wanted to test if c-Myb regulates the level of GR promoter 1C- and 1D-derived transcripts in these cells. 697 sh-c-Myb cells and 697 sh-NSC cells were pre-treated for 48 h with Doxy and 18 h with EtOH or Dex and mRNA was isolated from the cells. QRT-PCR was then performed using primers specific for GR promoter 1C and 1D transcripts. The knockdown of c-Myb resulted in a significant decrease in both the basal and Dex induced transcript levels of GR promoters 1C as well as 1D compared to the control 697 sh-NSC cell line (Fig. 5A and 5B), in which there were no changes in the transcript levels even after the addition of Doxy. These data show that the loss of intracellular c-Myb leads to a reduction in the basal and Dex induced levels of endogenous GR promoter 1C- and 1D-derived transcripts. To directly test if the downregulation of c-Myb affects the promoter activity of GR promoters 1C and 1D, we performed luciferase reporter gene analysis. pXP-1 luciferase reporters containing promoter sequences upstream of exons 1C (−2525/−2986) and 1D (−4525/−4898), as described previously (Geng and Vedeckis, 2011), were transfected into 697 sh-c-Myb cells. The cells were then pre-treated with or without Doxy for 6 h and followed by treatment with EtOH vehicle or 50 nM Dex for 18 h. The loss of c-Myb blunted the auto-upregulation of GR promoter 1C and 1D (Fig. 6A and 6B). These data further suggest the importance of c-Myb in the regulation of GR promoters 1C and 1D.
Figure 5. Controllable decreases of c-Myb in 697 pre-B-ALL cells reduces the basal and Dex induced levels of GR 1C and 1D transcripts.
GR 1C (Fig 5A) and GR 1D (Fig 5B) mRNA transcript measurements were performed via QRT-PCR with a probe and primer set specific for GR 1C and 1D exon-containing transcripts. The data are normalized to endogenous 18S RNA levels. The relative amplification units are relative to 18S RNA levels. Three separate experiments were performed and the values are the average ± SEM. ***, p <0.001 for Doxy treated samples vs. untreated samples.
Figure 6. Controllable reduction of c-Myb blunts the auto-upregulation of GR promoter 1C and 1D in 697 pre-B-ALL.
697 sh-c-Myb cells were transfected with GR 1C and GR 1D luciferase reporter genes and promoter activities were measured via luciferase reporter analysis. The cells were pretreated with Doxy for 24 h and 50 nM Dex or EtOH for 18 h. Three separate experiments were performed and the values are the average ± SEM. **, p <0.01 for Dex treated samples vs. EtOH treated samples.
3.5 The knockdown of c-Myb increases the sensitivity of pre-B-ALL 697 cells to steroid hormone
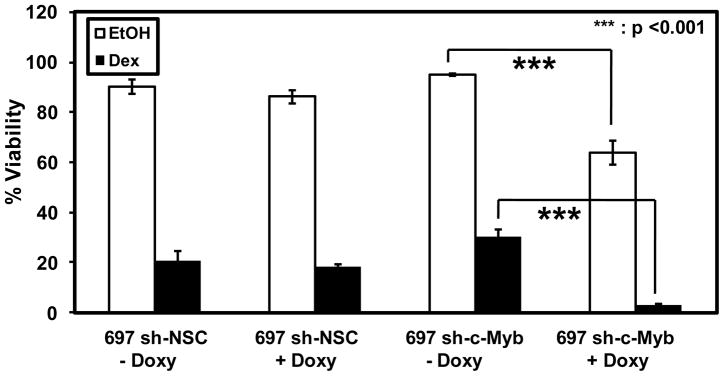
The primary aim of this study was to test the role of c-Myb in GC mediated apoptosis. Our model indicates that GR and c-Myb cooperate in the auto-upregulation of GR gene transcription (Geng et al., 2008, Geng and Vedeckis, 2011). The c-Myb knockdown experiments shown above (Figs. 3–6) supported this contention. Thus, we expected that c-Myb and GR knockdown would render the 697 sh-c-Myb cells resistant to Dex-mediated apoptosis. The cell-lines 697 sh-NSC and 697 sh-c-Myb were pre-treated with Doxy for 48 h and EtOH or Dex for 36 h, and cell viability was then assessed by flow cytometry using the Vybrant® Apoptosis Assay Kit #4. Contrary to our expectations, there was a significant decrease in viability in 697 sh-c-Myb cells after Dex treatment when c-Myb was knocked down, while this was not seen in the control 697 sh-NSC cell line (Fig. 7, filled bars). This suggested that the knockdown of c-Myb actually increased the sensitivity of pre-B-ALL 697 cells to steroid hormone-mediated apoptosis. Indeed, the knockdown of c-Myb alone, without Dex treatment, reduced the viability of the 697 sh-c-Myb cells, suggesting that c-Myb is a cell viability factor in this cell line (Fig. 7, open bars).
Figure 7. The knockdown of c-Myb increases the sensitivity of pre-B-ALL 697 cells to steroid hormone treatment.
697 sh-c-Myb cells and 697 sh-NSC cells were treated with 1 μg/ml Doxy for 48 h and 1 μM Dex or EtOH for 36 hr. Cell viability was then measured by flow cytometry. Three separate experiments were performed and the error bars represent the standard error of the mean. ***, p <0.001 for Doxy treated samples vs. untreated samples.
3.6 The residual GR after knockdown of c-Myb is above the threshold level of GR required for GC mediated apoptosis
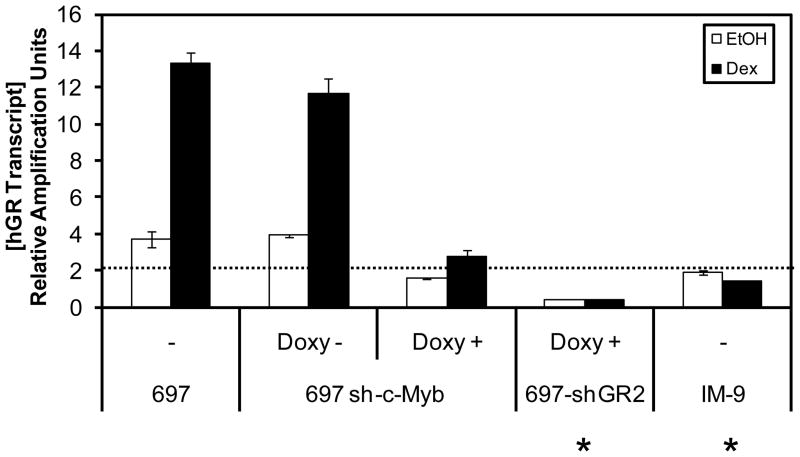
One remaining question was why the 697-sh-c-Myb cells were still sensitive to steroid-mediated apoptosis, even though the levels of the GR transcripts and protein were lowered. We have demonstrated the importance of the amount of GR required for GC mediated apoptosis by suggesting that there is a threshold level of GR that is required for the cells to respond to steroid therapy (Schwartz et al., 2010). Therefore, the possibility existed that the residual GR present after c-Myb knockdown still exceeded the minimal threshold amount needed for steroid sensitivity. To answer this, we compared the amount of GR transcripts after the knockdown of c-Myb to that of the 697-shGR2 knockdown cell line, as well as to IM-9 B-lymphoblastoid cells that are resistant to GC therapy. At a [Doxy] of 250 ng/ml, the 697-shGR2 cells are resistant to GC treatment. The parental 697 cells are sensitive to GC treatment, while IM-9 B-lymphoblastoid cells are resistant to GC treatment. Figure 8 shows the results of QRT-PCR assays for the GR transcripts in these cell lines. The dotted line marks the proposed threshold, i.e., the minimal level of GR mRNA that appears to be necessary, either basally or after GR auto-upregulation, to trigger apoptosis. We observed that the amount of GR transcript after c-Myb knockdown and GC-mediated auto-upregulation in the 697 sh-c-Myb cell line is still above the threshold level that is required for GC mediated apoptosis. Therefore, because the GR transcripts were not suppressed below the threshold level, the 697 sh-c-Myb cells were still sensitive to steroid therapy.
Figure 8. The residual GR mRNA after knockdown of c-Myb is above the postulated threshold level required for GC mediated apoptosis.
697 sh-c-Myb cells were treated with 1 μg/ml Doxy for 48 h and 1 μM Dex/EtOH for 18 hr. 697 sh-GR cells were treated with 250 ng/ml Doxy for 48 h followed by 1 μM Dex/EtOH treatment for 18 hr. 697 cells and IM-9 cells were treated with 1 μM Dex/EtOH treatment for 18 hr. GR mRNA transcript measurements were performed via QRT-PCR with a probe and primer set specific for GR mRNA transcripts (containing the exon 5/6 junction). The data are normalized to endogenous 18S RNA levels. Error bars represent the standard error of the mean of three separate experiments. (*) denotes the cells that are resistant to GC-mediated apoptosis. The dotted line marks the apparent threshold level of GR transcripts that are required to trigger GC mediated apoptosis.
4. DISCUSSION
Glucocorticoids are effective agents in the treatment of both acute and chronic lymphoblastic leukemia and a variety of different lymphomas, as they trigger apoptosis in the sensitive cells. Although patients with lymphoid malignancies are treated successfully with current protocols, some do not respond well to therapy initially and many do not respond well to therapy at relapse. Importantly, in vitro and in vivo GC sensitivity are major prognostic factors in childhood ALL, and patients who respond well to GC monotherapy have good outcomes. While a recent study implicates the GSK3 protein in connecting upstream GR signals with the downstream proapoptotic effector, Bim (Spokoini et al., 2010), the precise mechanism of GC mediated apoptosis is not completely understood. One factor that may be important in steroid-mediated apoptosis is the auto-upregulation of GR promoters (Geng et al., 2008, Geng and Vedeckis, 2005). Many studies have shown that the auto-upregulation of the GR is required for GC-sensitivity (Ashraf et al., 1991, Miller et al., 2007, Pedersen and Vedeckis, 2003, Ramdas et al., 1999, Riml et al., 2004). We have recently shown that there is a threshold level of GR that has to be present in the cell for it to respond to GC therapy (Schwartz et al., 2010). Thus, the level of GR in the cells plays a critical role in determining the GC sensitivity of the cells and hence it is essential to identify the mechanisms of GR regulation.
We previously reported that c-Myb and the GR were recruited to GR promoter 1C and 1D, which exhibit steroid-mediated auto-upregulation in the 697 pre-B-ALL cell line (Geng and Vedeckis, 2011). Using this 697 pre-B-ALL cell line with the conditional knockdown of c-Myb protein, we further determined the essential roles of c-Myb in hormone responsive upregulation of GR in these cells, by identifying the functional direct interaction of the transcription factors GR and c-Myb in the present study. This is the first study to demonstrate that c-Myb interacts directly with the GR, suggesting that this interaction may be important for GR gene promoter auto-activation, perhaps by stabilizing a GR/c-Myb/GRU ternary complex. The experiments performed after the knockdown of c-Myb by the addition of Doxy clearly showed that c-Myb regulates the basal and Dex induced levels of total GR transcripts and protein in pre-B-ALL cells. Furthermore, the transcript levels as well as the activity of GR promoters 1C and 1D, which are important for GR auto-upregulation in 697 pre-B-ALL cells, were reduced after decreasing intracellular c-Myb amounts, suggesting an important role for c-Myb in the process of GC mediated apoptosis. This led to the prediction that the knockdown of c-Myb in these pre-B-ALL cells would render the cells steroid resistant, because the GR would no longer be able to trigger steroid-mediated apoptosis. Surprisingly, we observed two unexpected results: the cells were still sensitive to the steroid, and, even more striking, we found that the knockdown of c-Myb actually augments the steroid sensitivity of 697 pre-B-ALL cells. The first quandary was resolved when we measured the amount of remaining GR transcript present in the 697 sh-c-Myb cells after c-Myb knockdown and found that it was still above the threshold level of GR required for GC mediated apoptosis (after induction) that we had postulated previously. The reason that c-Myb knockdown actually increases steroid sensitivity was resolved by studies on the effect of c-Myb itself on the 697 pre-B-ALL cell line, which suggest that c-Myb is essential for the viability of pre-B-ALL cells (Sarvaiya et al, submitted).
In summary, these findings suggest that c-Myb plays a critical role in regulating GR levels, which are important in determining the steroid sensitivity of lymphoid cells. However, the role of c-Myb is more complex, as the knockdown of c-Myb can augment the steroid sensitivity of 697 pre-B-ALL cells. In the present case, it appears that c-Myb knockdown reduces, in a steroid hormone-independent manner, cell viability, while allowing the cells to remain steroid sensitive, because of the residual level of GR which is above the therapeutic threshold level. Getting a detailed understanding of the components that regulate the GR will pave the way to a better understanding of the mechanism of GC mediated apoptosis and eventually improve the response of steroid resistant ALL patients to hormone therapy. Finally, c-Myb may be another molecule that can be targeted in the future to improve therapy of acute lymphoblastic leukemia, although the expression of other proapoptotic proteins, such as the GR, and the interplay and crosstalk between these molecules, must be carefully considered in any clinical strategies.
RESEARCH HIGHLIGHTS.
The transcription factor c-Myb interacts with the glucocorticoid receptor (GR)
The knockdown of c-Myb reduces the levels of GR transcripts and protein
The knockdown of c-Myb lowers the levels of GR promoter 1C and 1D transcripts
The knockdown of c-Myb blunts the autoupregulation of GR promoters 1C and 1D
Reduced c-Myb levels, by itself, decreases cell viability
Acknowledgments
We thank Dr. Beatriz F. Finkel-Jimenez for flow cytometry assistance. We thank Dr. E. Brad Thompson for providing CEM-C7 cells, Dr. Paul R. Housley, University of South Carolina School of Medicine for providing E8.2 cells, Dr. Wanguo Liu (LSUHSC, New Orleans) for the kind gift of COS-7 cells, Dr. Giuseppe Raschella (Ente Nuove Tecnologie Energia Ambiente, Rome, Italy) for providing the human c-Myb expression construct pcDNA3-c-Myb-HA and Dr. John Cidlowski (National Institute of Envirnonmental Health Sciences, Research Triangle Park, NC) for providing the hGRα protein expression vector, pCYGR. This work was supported by NCI grant CA116042 (to W.V.V).
Abbreviations
- ALL
acute lymphoblastic leukemia
- Dex
dexamethasone
- Doxy
doxycycline
- GC
glucocorticoid
- GR
glucocorticoid receptor
- GRE
Glucocorticoid Response Element
- GRU
Glucocorticoid Response Unit
- NSC
Non-Silencing Control
Footnotes
This work was supported by NCI grant CA116042 to W.V.V
DISCLOSURE
The authors have nothing to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adcock IM. Molecular mechanisms of glucocorticosteroid actions. Pulm Pharmacol Ther. 2000;13:115–26. doi: 10.1006/pupt.2000.0243. [DOI] [PubMed] [Google Scholar]
- Anfossi G, Gewirtz AM, Calabretta B. An oligomer complementary to c-myb-encoded mRNA inhibits proliferation of human myeloid leukemia cell lines. Proc Natl Acad Sci U S A. 1989;86:3379–83. doi: 10.1073/pnas.86.9.3379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashraf J, Kunapuli S, Chilton D, Thompson EB. Cortivazol mediated induction of glucocorticoid receptor messenger ribonucleic acid in wild-type and dexamethasone-resistant human leukemic (CEM) cells. J Steroid Biochem Mol Biol. 1991;38:561–8. doi: 10.1016/0960-0760(91)90313-t. [DOI] [PubMed] [Google Scholar]
- Bamberger CM, Schulte HM, Chrousos GP. Molecular determinants of glucocorticoid receptor function and tissue sensitivity to glucocorticoids. Endocr Rev. 1996;17:245–61. doi: 10.1210/edrv-17-3-245. [DOI] [PubMed] [Google Scholar]
- Caracciolo D, Venturelli D, Valtieri M, Peschle C, Gewirtz AM, Calabretta B. Stage-related proliferative activity determines c-myb functional requirements during normal human hematopoiesis. J Clin Invest. 1990;85:55–61. doi: 10.1172/JCI114433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emambokus N, Vegiopoulos A, Harman B, Jenkinson E, Anderson G, Frampton J. Progression through key stages of haemopoiesis is dependent on distinct threshold levels of c-Myb. Embo J. 2003;22:4478–88. doi: 10.1093/emboj/cdg434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geng CD, Schwartz JR, Vedeckis WV. A conserved molecular mechanism is responsible for the auto-up-regulation of glucocorticoid receptor gene promoters. Mol Endocrinol. 2008;22:2624–42. doi: 10.1210/me.2008-0157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geng CD, Vedeckis WV. A new, lineage specific, autoup-regulation mechanism for human glucocorticoid receptor gene expression in 697 pre-B-acute lymphoblastic leukemia cells. Mol Endocrinol. 2011;25:44–57. doi: 10.1210/me.2010-0249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geng CD, Vedeckis WV. c-Myb and members of the c-Ets family of transcription factors act as molecular switches to mediate opposite steroid regulation of the human glucocorticoid receptor 1A promoter. J Biol Chem. 2005;280:43264–71. doi: 10.1074/jbc.M508245200. [DOI] [PubMed] [Google Scholar]
- Gewirtz AM, Anfossi G, Venturelli D, Valpreda S, Sims R, Calabretta B. G1/S transition in normal human T-lymphocytes requires the nuclear protein encoded by c-myb. Science. 1989;245:180–3. doi: 10.1126/science.2665077. [DOI] [PubMed] [Google Scholar]
- Gewirtz AM, Calabretta B. A c-myb antisense oligodeoxynucleotide inhibits normal human hematopoiesis in vitro. Science. 1988;242:1303–6. doi: 10.1126/science.2461588. [DOI] [PubMed] [Google Scholar]
- Gross KL, Cidlowski JA. Tissue-specific glucocorticoid action: a family affair. Trends Endocrinol Metab. 2008;19:331–9. doi: 10.1016/j.tem.2008.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hongo T, Yajima S, Sakurai M, Horikoshi Y, Hanada R. In vitro drug sensitivity testing can predict induction failure and early relapse of childhood acute lymphoblastic leukemia. Blood. 1997;89:2959–65. [PubMed] [Google Scholar]
- Housley PR, Forsthoefel AM. Isolation and characterization of a mouse L cell variant deficient in glucocorticoid receptors. Biochem Biophys Res Commun. 1989;164:480–7. doi: 10.1016/0006-291x(89)91745-2. [DOI] [PubMed] [Google Scholar]
- Kaspers GJ, Pieters R, Van Zantwijk CH, Van Wering ER, Van Der Does-Van Den Berg A, Veerman AJ. Prednisolone resistance in childhood acute lymphoblastic leukemia: vitro-vivo correlations and cross-resistance to other drugs. Blood. 1998;92:259–66. [PubMed] [Google Scholar]
- Kaspers GJ, Veerman AJ, Pieters R, Van Zantwijk CH, Smets LA, Van Wering ER, Van Der Does-Van Den Berg A. In vitro cellular drug resistance and prognosis in newly diagnosed childhood acute lymphoblastic leukemia. Blood. 1997;90:2723–9. [PubMed] [Google Scholar]
- Keith BD. Systematic review of the clinical effect of glucocorticoids on nonhematologic malignancy. BMC Cancer. 2008;8:84. doi: 10.1186/1471-2407-8-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klempnauer KH, Gonda TJ, Bishop JM. Nucleotide sequence of the retroviral leukemia gene v-myb and its cellular progenitor c-myb: the architecture of a transduced oncogene. Cell. 1982;31:453–63. doi: 10.1016/0092-8674(82)90138-6. [DOI] [PubMed] [Google Scholar]
- Klumper E, Pieters R, Veerman AJ, Huismans DR, Loonen AH, Hahlen K, Kaspers GJ, van Wering ER, Hartmann R, Henze G. In vitro cellular drug resistance in children with relapsed/refractory acute lymphoblastic leukemia. Blood. 1995;86:3861–8. [PubMed] [Google Scholar]
- Kofler R, Schmidt S, Kofler A, Ausserlechner MJ. Resistance to glucocorticoid-induced apoptosis in lymphoblastic leukemia. J Endocrinol. 2003;178:19–27. doi: 10.1677/joe.0.1780019. [DOI] [PubMed] [Google Scholar]
- Leprince D, Gegonne A, Coll J, de Taisne C, Schneeberger A, Lagrou C, Stehelin D. A putative second cell-derived oncogene of the avian leukaemia retrovirus E26. Nature. 1983;306:395–7. doi: 10.1038/306395a0. [DOI] [PubMed] [Google Scholar]
- Lieu YK, Kumar A, Pajerowski AG, Rogers TJ, Reddy EP. Requirement of c-myb in T cell development and in mature T cell function. Proc Natl Acad Sci U S A. 2004;101:14853–8. doi: 10.1073/pnas.0405338101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller AL, Garza AS, Johnson BH, Thompson EB. Pathway interactions between MAPKs, mTOR, PKA, and the glucocorticoid receptor in lymphoid cells. Cancer Cell Int. 2007;7:3. doi: 10.1186/1475-2867-7-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakata Y, Shetzline S, Sakashita C, Kalota A, Rallapalli R, Rudnick SI, Zhang Y, Emerson SG, Gewirtz AM. c-Myb contributes to G2/M cell cycle transition in human hematopoietic cells by direct regulation of cyclin B1 expression. Mol Cell Biol. 2007;27:2048–58. doi: 10.1128/MCB.01100-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nanni P, Nicoletti G, Prodi G, Galli MC, De Giovanni C, Grilli S, Lollini PL, Gobbi M, Cavo M, Tura S. Glucocorticoid receptor and in vitro sensitivity to steroid hormones in human lymphoproliferative diseases and myeloid leukemia. Cancer. 1982;49:623–32. doi: 10.1002/1097-0142(19820215)49:4<623::aid-cncr2820490403>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- Oh IH, Reddy EP. The myb gene family in cell growth, differentiation and apoptosis. Oncogene. 1999;18:3017–33. doi: 10.1038/sj.onc.1202839. [DOI] [PubMed] [Google Scholar]
- Pedersen KB, Vedeckis WV. Quantification and glucocorticoid regulation of glucocorticoid receptor transcripts in two human leukemic cell lines. Biochemistry. 2003;42:10978–90. doi: 10.1021/bi034651u. [DOI] [PubMed] [Google Scholar]
- Pieters R, Huismans DR, Loonen AH, Hahlen K, van der Does-van den Berg A, van Wering ER, Veerman AJ. Relation of cellular drug resistance to long-term clinical outcome in childhood acute lymphoblastic leukaemia. Lancet. 1991;338:399–403. doi: 10.1016/0140-6736(91)91029-t. [DOI] [PubMed] [Google Scholar]
- Pui CH, Robison LL, Look AT. Acute lymphoblastic leukaemia. Lancet. 2008;371:1030–1043. doi: 10.1016/S0140-6736(08)60457-2. [DOI] [PubMed] [Google Scholar]
- Ramdas J, Liu W, Harmon JM. Glucocorticoid-induced cell death requires autoinduction of glucocorticoid receptor expression in human leukemic T cells. Cancer Res. 1999;59:1378–85. [PubMed] [Google Scholar]
- Riml S, Schmidt S, Ausserlechner MJ, Geley S, Kofler R. Glucocorticoid receptor heterozygosity combined with lack of receptor auto-induction causes glucocorticoid resistance in Jurkat acute lymphoblastic leukemia cells. Cell Death Differ. 2004;11(Suppl 1):S65–72. doi: 10.1038/sj.cdd.4401413. [DOI] [PubMed] [Google Scholar]
- Schwartz JR, Sarvaiya PJ, Vedeckis WV. Glucocorticoid receptor knock down reveals a similar apoptotic threshold but differing gene regulation patterns in T-cell and pre-B-cell acute lymphoblastic leukemia. Mol Cell Endocrinol. 2010;320:76–86. doi: 10.1016/j.mce.2010.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spokoini R, Kfir-Erenfeld S, Yefenof E, Sionov RV. Glycogen synthase kinase-3 plays a central role in mediating glucocorticoid-induced apoptosis. Mol Endocrinol. 2010;24:1136–50. doi: 10.1210/me.2009-0466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas MD, Kremer CS, Ravichandran KS, Rajewsky K, Bender TP. c-Myb is critical for B cell development and maintenance of follicular B cells. Immunity. 2005;23:275–86. doi: 10.1016/j.immuni.2005.08.005. [DOI] [PubMed] [Google Scholar]
- Vegiopoulos A, Garcia P, Emambokus N, Frampton J. Coordination of erythropoiesis by the transcription factor c-Myb. Blood. 2006;107:4703–10. doi: 10.1182/blood-2005-07-2968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webster JC, Cidlowski JA. Mechanisms of Glucocorticoid-receptor-mediated Repression of Gene Expression. Trends Endocrinol Metab. 1999;10:396–402. doi: 10.1016/s1043-2760(99)00186-1. [DOI] [PubMed] [Google Scholar]
- Weston K. Myb proteins in life, death and differentiation. Curr Opin Genet Dev. 1998;k8:76–81. doi: 10.1016/s0959-437x(98)80065-8. [DOI] [PubMed] [Google Scholar]
- Xiao C, Calado DP, Galler G, Thai TH, Patterson HC, Wang J, Rajewsky N, Bender TP, Rajewsky K. MiR-150 controls B cell differentiation by targeting the transcription factor c-Myb. Cell. 2007;131:146–59. doi: 10.1016/j.cell.2007.07.021. [DOI] [PubMed] [Google Scholar]