Abstract
Objectives
Resistant viruses may emerge in the female genital tract during antiretroviral therapy (ART). Our objective was to identify predictors of drug-resistant HIV-1 RNA in genital secretions after initiation of non-nucleoside reverse transcriptase inhibitor (NNRTI)-based therapy.
Design
We conducted a prospective cohort study with periodic evaluation of plasma and genital swab samples for HIV-1 RNA levels and antiretroviral resistance mutations.
Methods
First-line ART was initiated in 102 women. Plasma and genital HIV-1 RNA levels were measured at months 0, 3, 6, and 12. Genotypic resistance testing was performed for samples from all participants with RNA >1,000 copies/mL at month 6 or 12. Cox regression analysis was used to identify factors associated with incident genital tract resistance.
Results
Detectable genital tract resistance developed in 5 women, all with detectable plasma resistance (estimated incidence, 5.5/100 person-years of observation). Treatment interruption >48 hours, adherence by pill count, adherence by visual analog scale, and baseline plasma viral load were associated with incident genital tract resistance. In multivariate analysis, only treatment interruption was associated with risk of detectable genital tract resistance (adjusted hazard ratio 14.2, 95% CI 1.3–158.4).
Conclusions
Treatment interruption >48 hours during NNRTI-based therapy led to a significantly increased risk of detecting genotypically resistant HIV-1 RNA in female genital tract secretions. Patient- and program-level interventions to prevent treatment interruptions could reduce the risk of shedding resistant HIV-1 during ART.
Keywords: HIV drug resistance, virus shedding, female, antiretroviral therapy, adherence
Introduction
First-line regimens including two nucleoside reverse transcriptase inhibitors (NRTI) and one non-nucleoside reverse transcriptase inhibitor (NNRTI) are the mainstay of the global antiretroviral therapy (ART) roll-out [1]. Because treatment options are limited in most developing countries, it is critical that first-line regimens maintain their effectiveness. Relatively high levels of adherence (>80%) to NNRTI-containing regimens are required to achieve effective virologic suppression [2-5]. When viral replication is not suppressed, resistance mutations can develop in both plasma and genital tract secretions [6]. These drug-resistant variants may be sexually transmitted, compromising treatment efficacy in newly infected individuals and increasing drug resistance on a population level [6, 7].
While plasma drug resistance has been studied extensively, little is known about the emergence of resistant isolates in the female genital tract. In particular, it is unknown whether genital tract resistance is determined mainly by the emergence of resistance in plasma or whether adherence to therapy has an independent influence on the emergence of resistance in genital secretions. Our objective for the current study was to determine the incidence of detectable genotypic resistance to antiretrovirals in cervical and vaginal secretions during the first 12 months after initiation of a WHO-recommended NNRTI-containing ART regimen, and to explore associations between potential cofactors and the emergence of genital tract resistance.
Methods
Study population and procedures
The study followed non-pregnant, HIV-1-seropositive Kenyan women who initiated ART in a research cohort based at the Ganjoni Municipal Clinic in Mombasa between February 2005 and January 2008. Women enrolled if they were eligible for ART according to Kenyan National Guidelines during this period (CD4 cell count ≤200 cells/ml or AIDS-defining illness) and willing to undergo monthly follow-up per protocol. All participants gave written informed consent. Ethical review committees of the Kenya Medical Research Institute, University of Washington, and Fred Hutchinson Cancer Research Center approved the study.
The standard ART regimen was stavudine or zidovudine, lamivudine, and nevirapine, according to Kenyan National Guidelines at the time.[8] During the first month, directly administered therapy was used to observe one of the two daily ART doses on each weekday. Pill box organizers and a monthly support group were used to promote adherence, which was monitored at each visit by pill count and a validated visual analog scale (VAS).[9] Refill dates and the quantity of drug dispensed were also recorded. Women received transportation reimbursements for clinic visits.
Women were screened for genital infections prior to ART initiation. At treatment initiation and monthly thereafter, research staff interviewed participants using standardized questionnaires about recent sexual behavior, contraceptive practices, and genitourinary symptoms. The physical examination included a pelvic speculum examination, during which specimens were collected for viral quantitation and diagnosis of genital infections. If women were menstruating, the physical examination was rescheduled. Blood was collected for CD4 counts and HIV-1 quantitation at baseline and every 3 months thereafter.
Vaginal specimens for HIV-1 detection were collected first, by rolling a dacron swab three full turns against the lateral vaginal wall. Vaginal secretions were then sampled to screen for vaginal infections. The first cervical specimens collected were Dacron swabs for HIV-1 quantitation, obtained by inserting the swab gently into the os and rotating two full turns. Next, swabs were collected to screen for cervical infections. This standardized swab collection protocol has good reproducibility compared to other methods of female genital tract sampling.[10]
Laboratory methods
HIV-1 serostatus was evaluated using an ELISA (Detect HIV 1/2, BioChem Immunosystems). Positives were confirmed with a second ELISA (Recombigen, Cambridge Biotech or Vironostika HIV-1 Uni-Form II Ag/Ab, bioMérieux). CD4 cell counts were determined by FACS Count (Becton Dickinson). Pregnancy testing was performed using a rapid β–human chorionic gonadotropin test (Plasmatec Laboratory Products).
After Gram staining of cervical secretions, the number of polymorphonuclear leukocytes in three non-adjacent oil immersion fields on microscopy was quantified. Yeast and T. vaginalis were detected by light microscopy of vaginal saline wet preparations. Bacterial vaginosis (BV) was evaluated by Nugent scoring of vaginal Gram stains. The presence of sperm on cervical Gram stain or vaginal wet preparation was recorded. Culture for N. gonorrhoeae was performed on modified Thayer-Martin medium. A nucleic acid amplification assay (Aptima Combo 2, Gen-Probe) was used to detect N. gonorrhoeae or Chlamydia trachomatis.
Plasma and genital specimens were frozen at −70 °C until shipment to Seattle on dry ice or in liquid nitrogen for HIV-1 RNA quantitation using the Gen-Probe HIV-1 viral load assay.[11] HIV-1 RNA results were not available in real time at the study site. Specimens tested for this study included blood and Dacron swabs of cervical and vaginal secretions from the pre-treatment baseline visit and from 3, 6, and 12 months after ART initiation. The lower limit of quantitation was 100 copies/swab in genital secretions and 100 copies/mL in plasma.
Genotypic resistance was evaluated using population-based sequencing in all samples from month 6 or month 12 for which HIV-1 RNA levels were >1,000 copies/mL or >1,000 copies/swab. If resistance was detected at either of these time-points, we attempted to amplify all the participant’s samples (baseline through month 12) for which HIV-1 RNA levels were >300 copies/mL or >300 copies/swab. A nested real-time polymerase chain reaction (RT-PCR) method designed for HIV-1 subtypes common in Kenya was used to amplify an approximately 800-base-pair fragment of the reverse transcriptase gene.[12] The PCR product was gel-purified and the sequence of the products of two independent RT-PCRs were evaluated; additional RT-PCRs were performed as needed to verify mutations detected in only one of two initial PCRs. The Stanford University HIV Drug Resistance Database (http://hivdb.stanford.edu) was used to identify drug resistance mutations.
If viral cDNA could not be amplified or HIV-1 RNA levels were ≤1,000 copies/mL or copies/swab at month 6 and month 12, it was assumed for the purposes of analysis that no drug-resistant variants were present. Phylogenetic trees were constructed for all sequences from each woman who developed genital tract resistance after ART initiation. For this purpose, sequences were aligned using Clustal X (Conway Institute UCD Dublin), including reference sequences representing different HIV-1 subtypes. Evolutionary relationships were determined by a neighbor-joining method employing Phylogenetic Analysis Using Parsimony (PAUP, Sinauer Associates, Inc.).
Statistical analysis
The Wilcoxon signed ranks test was used to compare the number of resistance mutations detected in plasma versus the genital tract. Fisher’s exact test was used to estimate the association of detection of resistance mutations in plasma with detection in genital secretions. Cox proportional hazards regression was performed to identify predictors of time to first detection of genital tract resistance after ART initiation. Participants were censored at the first of: death, withdrawal, loss to follow-up, switch to second-line therapy, or the month 12 visit. Time-varying covariates for each time interval (0-3 months, 3-6 months, and 6-12 months) included detection of plasma resistance, mean adherence (by pill count or VAS), treatment interruption, hormonal contraception use, genital ulcer disease, and genital infections. Treatment interruption was defined as any ART discontinuation (by patient or provider) or a late refill (>48 hours without pills) during an interval. Time-dependent covariates were allowed to take on a different value during each time interval, which was represented by one observation (i.e., one line of data) identified by a subject number. Fixed covariates tested at baseline included CD4 count and log10-transformed plasma, cervical, and vaginal viral loads. Factors associated with genital tract resistance with p<0.10 on bivariate analysis were all included in a multivariate model to assess the independent effects of each of these factors. The proportional hazards assumption was tested and was not violated. Data were analyzed using Stata version 11.2 (StataCorp, College Station, Texas, USA).
Results
Baseline Characteristics
Between February 2005 and January 2008, 102 non-pregnant, HIV-1-seropositive women initiated ART. Their baseline characteristics are presented in Table 1. Two women had brief exposure to ART (1 day and 3 days) occurring 1 week and 4 months, respectively, prior to study entry.
Table 1.
Baseline characteristics of 102 women initiating ART
| Variable | Median (interquartile range) or number (%) |
|---|---|
| Age (years) | 36 (32-40) |
| Education (years) | 7 (6-9) |
| Widowed or divorced | 74 (72.5) |
| Works in a bar | 64 (62.7) |
| Contraception other than condoms | |
| Depot medroxyprogesterone acetate | 18 (17.6) |
| Tubal ligation or hysterectomy | 6 (5.9) |
| Oral contraceptive pills | 5 (4.9) |
| Norplant | 5 (4.9) |
| Sexual risk behavior in past week | |
| Number of partners | 0 (0-1) |
| Number of sex acts | 0 (0-1) |
| Sexually active | 32 (31.4) |
| 100% condom usea | 24 (75.0) |
| Genital infection | |
| Bacterial vaginosis | 71 (69.6) |
| Candidiasis | 43 (42.2) |
| Trichomoniasis | 12 (11.8) |
| Cervicitis | 9 (8.8) |
| CD4 cell count (cells/mm3) | 122 (78-164) |
| WHO clinical stage | |
| Stage I | 18 (17.6) |
| Stage II | 29 (28.4) |
| Stage III | 44 (43.1) |
| Stage IV | 11 (10.8) |
| Active tuberculosis | 19 (18.6) |
| Prior ART exposure for prevention of maternal-child transmission |
0 (0) |
| Prior ART exposure for treatmentb | 2 (2.0) |
| Plasma HIV-1 RNA (copies/mL) | 349,066 (149,106-919,173) |
| Cervical HIV-1 RNA (copies/swab) | 11,056 (2,858-45,159) |
| Vaginal HIV-1 RNA (copies/swab) | 9.403 (1,042-37,496) |
Among the 32 women who were sexually active.
Two women had brief exposure to ART (1 day and 3 days) occurring 1 week and 4 months, respectively, prior to entry into this study.
Follow-up, Adherence, and Clinical Outcomes
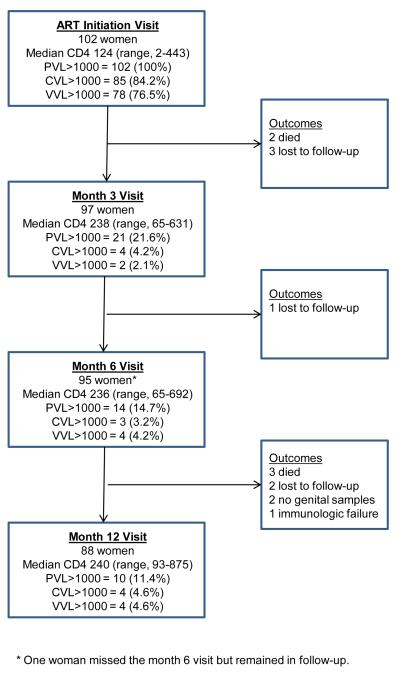
Of 102 women initiating ART, 97 (95.1%) remained in follow-up at month 3, 95 (93.1%) at month 6, and 90 (88.2%) at month 12. Two women had no genital samples available at month 12 and are excluded from analysis at this time-point. Figure 1 presents the flow of study participation and details on the response to therapy. No participant had isolated genital shedding >1,000 copies/swab without plasma viral load >1,000 copies/mL at 6 or 12 months.
Figure 1. Study profile, including treatment response.
CVL = cervical viral load, PVL = plasma viral load, VVL = vaginal viral load.
Median pill count adherence was 97.1% (interquartile range [IQR], 94.4% – 98.9%) and median VAS adherence was 99.8% (IQR, 99.1% – 100%) over the 12-month period. There were 40 treatment interruptions lasting from 3 to 142 days (median, 4 days; IQR, 3-18 days). Ten women discontinued ART at least once due to adverse drug events or intercurrent illness (one discontinued twice). Median pill count adherence at visits following treatment interruption was 83.1% (IQR, 66.7%-85.0%), compared to 98.3% (IQR, 95.2%-100%) at all other visits. One woman with adherence >95% throughout follow-up was diagnosed with immunologic treatment failure (CD4 count persistently below baseline) and started second-line therapy at 11 months. Her follow-up is, therefore, censored at month 6. Plasma and genital HIV-1 RNA levels were later found to be undetectable in stored samples from her month 6 visit and at the regimen switch date.
Antiretroviral Resistance Mutations
Antiretroviral resistance was examined in the 20 women with plasma HIV-1 RNA >1,000 copies/mL at either month 6 or month 12. Of the 86 samples for which resistance assays were attempted, 81 (94.2%) resulted in detectable amplification of the desired product: 45/49 for plasma samples, 19/20 for cervical secretion samples, and 17/17 for vaginal secretion samples. Of three samples with HIV-1 RNA between 300 and 1,000 copies/mL or copies/swab, two amplified (cervical load 758 copies/swab, plasma load 498 copies/mL) and one did not (cervical load 378 copies/swab).
Overall, nine women developed detectable genotypic resistance in plasma HIV-1 RNA (incidence 10.0/100 person-years of observation [pyo]), with mutations including M184V (7), K103N (6), G190A (3), Y181C (3), M184I (2), K101E (1), and V106A (1). Genotypic resistance in cervical HIV-1 RNA was detected in four women (4.4/100 pyo). Genotypic resistance in vaginal HIV-1 RNA was detected in five women (5.5/100 pyo), including all four with resistance in cervical secretions. Mutations identified in genital tract secretions included K103N (5), G190A (3), M184V (2), V106A (2), V108I (2), M184I (1), and Y181C (1). The number of resistance mutations detected in plasma versus genital tract secretions did not differ (p = 0.60).
HIV-1 RNA carrying the V108I mutation was detected in the cervical secretions of one woman at baseline. No other resistance mutations were detected in baseline samples. Four women who had resistant HIV-1 detected in plasma after ART initiation had cervical and vaginal HIV-1 RNA levels too low to amplify. Table 2 provides details of the time-points, viral loads, mutations detected at each site, and clinical history for each of the five participants in whom detectable antiretroviral resistance developed in genital tract secretions after ART initiation. Of note, none of these women had antiretroviral exposure prior to ART initiation. Over all visits, detection of resistance in genital secretions was associated with detection of resistance in plasma (p<0.001).
Table 2.
Characteristics of Individuals with Incident Genital Tract Resistance Detected after ART Initiation
| Study ID |
Time- point |
Subtype | CVL c/swab |
VVL c/swab |
PVL c/mL |
Cervical resistance |
Vaginal resistance |
Plasma resistance |
Comment |
|---|---|---|---|---|---|---|---|---|---|
| C648 | 0 | Rec | 29,021 | 48,606 | 572,601 | None | None | None | |
| 3 | <100 | <100 | 150 | Not tested | Not tested | Not tested | |||
| 6 | <100 | <100 | <100 | Not tested | Not tested | Not tested | |||
| 12 | Rec | 1,388 | 128 | 4,427 | K103N, M184V |
Not tested | K103N, M184V |
Adherence 90% months 11 and 12, late refill month 11 (3-day interruption) |
|
|
| |||||||||
| D717 | 0 | A | 139,395 | 23,857 | 4,662,773 | None | None | None | |
| 3 | A | 453,664 | 298,339 | 2,367,655 | None | None | None | TB diagnosed month 1, discontinued ART month 2 (unclear duration) |
|
| 6 | A | 73,568 | 26,306 | 235, 965 | K103N | K103N, G190A |
K103N | Regimen change month 3, discontinued ART month 4; died after month 6 visit |
|
|
| |||||||||
| F383 | 0 | A | 5,600 | 5,733 | 274,820 | None | None | None | |
| 3 | A | 35,452 | 141,027 | 264,191 | K103KN, V106AV, G190AG |
K103N | K103KN, V106AV |
Discontinued ART due to hepatitis month 2 |
|
| 6 | A | 11,818 | 31,785 | 511,554 | K103KN | None | K103KN | Remained in follow-up and off treatment | |
| 12 | A | 2,245 | 2,199 | 193,190 | K103N | K103N | K103N | Remained in follow-up and off treatment | |
|
| |||||||||
| F987 | 0 | A | 5,668 | 22,888 | 605,979 | None | None | None | |
| 3 | A | <100 | 141 | 36,182 | Not tested | Not tested | YI81C | Adherence 28% month 3 | |
| 6 | A | 758 | 14,422 | 2,237,334 | M184I, Y181C* |
M184I, Y181C | M184IMV, Y181C |
Adherence 84% month 4 | |
| 12 | A | <100 | <100 | 2,814 | Not tested | Not tested | M184V, Y181C |
Adherence 57% month 9, 77% month 10, 59% month 11, late refill month 11 (19-day interruption) |
|
|
| |||||||||
| H368 | 0 | A | 38,394 | <100 | 7,743,412 | None | Not tested | None | |
| 3 | <100 | <100 | 498 | Not tested | Not tested | None | |||
| 6 | A | <100 | <100 | 54,318 | Not tested | Not tested | M184V, G190A |
Reported adherence >95% throughout | |
| 12 | A | 378 | 1,133 | 63,553 | Not amplified | M184V, G190A |
M184V, G190A |
Adherence 91%, late refill month 11 (3- day interruption); adherence 94%, late refill month 12 (2-day interruption) |
|
Based on only one PCR that amplified.
ART = antiretroviral therapy, c/mL = copies/milliliter, c/swab = copies/swab, CVL = cervical viral load, PVL = plasma viral load, Rec = recombinant, TB = tuberculosis, VVL = vaginal viral load
N.B. Where the result for resistance is “None,” this refers to a sample that amplified and for which the product demonstrated no resistance.
Phylogenetic Sequences
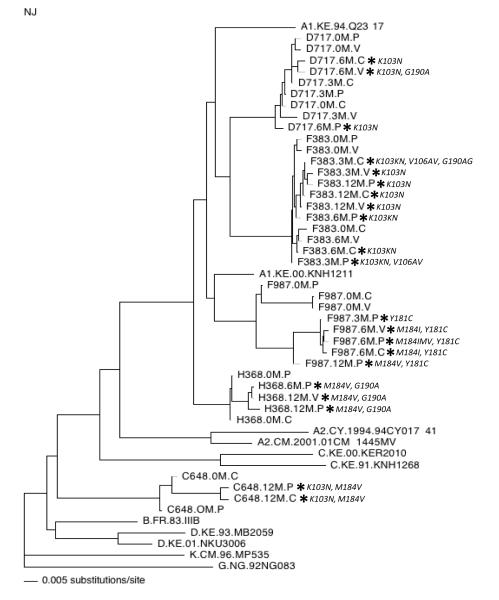
Figure 2 presents the results of phylogenetic sequence analysis for the five women in whom genital tract resistance emerged over the course of therapy, and the resistance mutations detected in each isolate. The polymerase sequences from four women clustered with subtype A reference sequences, while sequences from a fifth woman (C648) did not clearly group with any one subtype. In two cases (H368, C648), resistant isolates from different compartments clustered closely together on the tree. The most significant divergence in plasma versus genital tract drug-resistant variants was in F987, the individual who harbored the greatest overall sequence diversity. However, the resistant viruses were more related to each other than to the wild-type virus present at baseline. Because only two on-treatment genital samples from this participant had HIV-1 RNA levels high enough for resistance testing, it is difficult to draw conclusions about the origin of resistant variants.
Figure 2. Phylogenetic tree of HIV-1 polymerase sequences from plasma, cervical secretions, and vaginal secretions collected from five women with incident antiretroviral resistance detected in genital tract secretions.
The tree includes a 648-base-pair region of polymerase. Relevant reference sequences representing different subtypes are included and are identified by their subtype designation (e.g., A1) followed by the sequence name. Sequences from the current study are identified by participant identification number (e.g., D717), time of collection (e.g., 0M = Baseline, 3M = Month 3 from ART initiation), and compartment (P = plasma, C = cervical, V = vaginal). An asterisk (*) indicates sequences in which antiretroviral resistance mutations were detected and is followed by the specific mutations detected (see also table 2.)
For participants D717 and F383, although data were inadequate for robust bootstrap support, sequences were available at multiple time-points from all three sites. Some interesting suggestions emerged from a combination of the phylogeny, different resistance patterns, and specific mutations that led to evolution of K103N. In the case of D717, the month 6 plasma sample harboring K103N was more distant from the month 6 cervical and vaginal samples (Figure 2). In addition, the mutation that conferred K103N in the plasma was AAA to AAT, whereas the codon change leading to K103N in cervical virus was AAA to AAC (see Table, Supplemental Digital Content 1 for full sequence data). Both changes (AAC and AAT) were detected in the vaginal viral sequences, as well as a second resistance mutation (G190A), suggesting the possibility that resistance emerged independently in different compartments. Similarly, at the first time point when resistance was detected in participant F383 (month 3), resistant virus in plasma was genetically distinct from resistant viruses in genital secretion samples (Figure 2). In addition, a unique resistance pattern was observed in each compartment at month 3 in F383 (Table 2), also suggesting the possibility that resistance emerged independently in different compartments. Of note, both D717 and F383 had early treatment interruptions.
Predictors of Genital Tract Resistance
Table 3 presents bivariate and multivariate analyses of factors associated with the detection of resistant HIV-1 RNA in genital tract secretions after ART initiation. On bivariate analysis, any treatment interruption >48 hours, adherence by pill count or VAS, and baseline log10 plasma viral load were all significantly associated with the incidence of detectable genital tract resistance. In multivariate analysis, the model included treatment interruption, pill count adherence, and baseline log10 plasma viral load. In this model, only treatment interruption was associated with incident genital tract resistance (adjusted hazard ratio [aHR] 14.19, 95% CI 1.27 – 158.41). Results were similar if VAS adherence was substituted for pill count adherence. All incident cases of detectable genital tract resistance occurred in the setting of plasma resistance, detectable plasma and genital viral load, and adherence <95% in the preceding period. Therefore, these factors could not be statistically evaluated as predictors. Of note, all these factors were strongly associated with treatment interruption (data not shown).
Table 3.
Factors Associated with Genital Tract Resistance
| Bivariate Analysis | Multivariate Analysis | |||
|---|---|---|---|---|
| Factor | HR (95% CI) | P value | aHR (95% CI) | P value |
| Treatment interruption* | 23.10 (2.49 – 214.62) | 0.006 | 14.19 (1.27 – 158.41) | 0.03 |
| Pill count adherence* | 0.95 (0.92 – 0.98) | 0.001 | 0.98 (0.95 – 1.02) | 0.41 |
| VAS adherence* | 0.96 (0.94 – 0.99) | 0.002 | ||
| DMPA use* | 1.23 (0.14 – 11.05) | 0.85 | ||
| Bacterial vaginosis* | 0.84 (0.14 – 5.10) | 0.85 | ||
| Vaginal candidiasis* | 0.80 (0.09 – 7.29) | 0.84 | ||
| Baseline log10 plasma viral load | 5.80 (1.05 – 31.92) | 0.04 | 2.98 (0.59 – 14.96) | 0.19 |
| Baseline log10 cervical viral load | 1.54 (0.54 – 4.36) | 0.42 | ||
| Baseline log10 vaginal viral load | 1.07 (0.48 – 2.38) | 0.86 | ||
| Baseline CD4 count | 0.87 (0.71 – 1.07) | 0.19 | ||
aHR = adjusted hazard ratio, DMPA = depot medroxyprogesterone acetate, HR = hazard ratio, VAS = visual analog scale
Time-dependent covariate.
N.B. No incident genital tract resistance occurred in the setting of trichomoniasis, cervicitis, genital ulcer disease, syphilis, chancroid, Norplant use, or oral contraceptive pill use.
Discussion
To our knowledge, this is the first study to investigate the relationship between antiretroviral adherence and development of genotypic resistance to antiretrovirals in the genital tract. After ART initiation, the incidence of detectable antiretroviral resistance in HIV-1 RNA from genital tract secretions was estimated at 5.5 per 100 pyo. We found that ART adherence was a key determinant of genital tract resistance and that treatment interruption of whatever cause led to a substantial increase in the hazard of detecting genotypic resistance to antiretrovirals in female genital tract secretions. All cases of incident genital tract resistance occurred among women with detectable resistance in plasma, in accordance with previous evidence suggesting that isolated female genital tract resistance is rare.[13] One woman had detectable genital tract resistance before she died, and it seems plausible that others who did not complete this 12-month study due to death, withdrawal, or loss to follow-up also developed antiretroviral resistance in genital tract HIV-1 RNA. Therefore our reported incidence of genital tract resistance after ART initiation is probably an underestimate.
We have previously shown that adherence, measured by pill count or VAS, is an important predictor of female genital HIV-1 shedding after ART initiation.[14] Although adherence to ART has been established as a key predictor of antiretroviral resistance in plasma,[15, 16] evaluation of the relationship between adherence and genital tract resistance is also important. The risk of resistance varies according to antiretroviral class used in the regimen,[4, 5, 17] and the highest risk of developing plasma resistance during NNRTI-based treatment occurs at low levels of adherence (i.e., <50%).[4] The risk of plasma resistance is elevated early in treatment when plasma HIV-1 RNA levels are highest, then decreases with increasing time on treatment.[18, 19] In this respect, it is noteworthy that two of five women with incident genital tract resistance documented in this study had treatment interruptions due to adverse events (nevirapine-related hepatitis and tuberculosis-related immune reconstitution inflammatory syndrome) during the first 6 months of treatment.
Among patients taking NNRTI-based regimens, there is an increasing risk of virologic rebound in plasma for each consecutive day off therapy.[20] In particular, treatment interruptions of at least 48 hours have been associated with the development of resistance mutations in plasma.[20, 21] We have found that treatment interruptions of 48 hours or longer were associated with an increased risk of detecting resistant genital HIV-1 RNA. The reasons for treatment interruption in this study included both unavoidable discontinuations due to drug toxicity or systemic illness and avoidable interruptions due to late refills, when it is likely that consecutive doses were missed. Despite a comprehensive program of adherence support including pre-ART counseling, directly administered therapy during the first month of treatment, a support group, pillboxes, and transportation reimbursements, we were unable to prevent these events.
Structural barriers to adherence, such as transportation difficulties and pharmacy stock-outs, have emerged as important barriers to ART adherence in resource-limited settings.[21, 22] Our results suggest that such barriers may lead to the development of genital tract resistance due to treatment interruptions, suggesting an increased risk for transmission of drug-resistant virus. Transmitted drug resistance has been increasing in sub-Saharan Africa since ART became widely available.[23, 24] Importantly, clinical outcomes are compromised for those affected.[25] In a recent review of Early Warning Indicators for HIV drug resistance conducted by the WHO, only 41% of clinics in Africa met the target of on-time drug pick up by ≥90% of patients, and only 47% met the target of 100% drug supply continuity.[26] Our findings suggest that improvement in these indicators will be important for preventing the emergence of genital tract resistance to antiretrovirals and limiting the spread of transmitted drug resistance.
This study had a number of strengths, including prospective sampling of plasma and genital virus for all women, high retention, and testing of all samples with HIV-1 RNA levels that could be amplified for genotypic resistance to first-line antiretroviral agents. In addition, because ART only became widely available in Kenya in 2005, transmitted drug resistance would have been uncommon during the time of our study; indeed, only one woman had a resistance mutation detected in a baseline sample. There were also a number of limitations. First, we had relatively few cases of incident genital tract resistance. As a result, we had limited power to detect associations between cofactors and genital tract resistance. Nonetheless, we detected a strong association between treatment interruption and detection of genital tract resistance at a subsequent visit. Second, the sensitivity of the resistance assay used is limited for HIV-1 RNA levels at or below 1,000 copies/mL or copies/swab, and we may have failed to detect some resistance mutations in this range when samples failed to amplify. In addition, it is possible that samples with HIV-1 RNA levels <1,000 copies/mL or copies/swab at month 6 or month 12 harbored resistance mutations that we failed to detect. Third, our population consisted of Kenyan women attending a research clinic and as such, is not representative of all African women. While this may limit the generalizability of some findings (i.e., virologic failure rates), it is not likely to have affected the internal validity of this study. Fourth, because the relationship between antiretroviral adherence and the development resistance is likely different for protease inhibitors (PI),[4, 5, 17] we cannot infer that these results apply to the development of PI resistance. Finally, the precise relationship between genital shedding of drug-resistant HIV-1 isolates and transmitted drug resistance is unknown and requires further study. However, heterosexual transmission has been associated with genital HIV-1 RNA levels in the range detected in this study,[27] and sexual transmission of drug-resistant HIV-1 variants is likely related to their presence in the genital tract.
In conclusion, the development of genital tract resistance to antiretroviral agents is an important problem, as it is likely to reflect the risk of transmitting drug-resistant HIV-1. We have found that treatment interruptions >48 hours were associated with a substantial increase in the risk of genital tract resistance after treatment initiation. Efforts to prevent treatment interruptions by improving program effectiveness, promoting adherence and timely refills, and avoiding the use of more toxic antiretroviral agents could therefore play an important role in reducing transmitted drug resistance. ART not only improves clinically outcomes but also greatly reduces transmission risk from HIV-1-infected persons to their sexual partners.[28] As access to ART expands, transmission of antiretroviral-resistant HIV-1 will be one of the most important threats to the population-level prevention and treatment benefits of ART in resource-limited settings.
Supplementary Material
Acknowledgements
We thank the research staff for their contributions, Mombasa Municipal Council for clinical space, Coast Provincial General Hospital for laboratory space, Dara Lehman for advice on drug resistance assays, Stephanie Rainwater for assistance with preparing the phylogenetic tree, and the Kenya Medical Research Institute Director for permission to publish. Special thanks go to our participants.
This work was supported by the National Institutes of Health (NIH) [AI-58698, AI-38518, and AI-069990 to SMG]. Additional support for the Mombasa Field Site was provided by the University of Washington Center for AIDS Research, an NIH-funded program [P30 AI027757]. JS was a scholar in the International AIDS Research and Training Program, supported by the Fogarty International Center [D43 TW000007].
Footnotes
Conflict of Interest and Source of Funding. All authors report no conflict of interest.
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Antiretroviral therapy for HIV infection in adults and adolescents: recommendations for a public health approach. World Health Organization; Geneva: 2010. [PubMed] [Google Scholar]
- 2.Paterson DL, Swindells S, Mohr J, et al. Adherence to protease inhibitor therapy and outcomes in patients with HIV infection. Ann Intern Med. 2000;133:21–30. doi: 10.7326/0003-4819-133-1-200007040-00004. [DOI] [PubMed] [Google Scholar]
- 3.Bangsberg DR, Hecht FM, Charlebois ED, et al. Adherence to protease inhibitors, HIV-1 viral load, and development of drug resistance in an indigent population. AIDS. 2000;14:357–366. doi: 10.1097/00002030-200003100-00008. [DOI] [PubMed] [Google Scholar]
- 4.Bangsberg DR, Acosta EP, Gupta R, et al. Adherence-resistance relationships for protease and non-nucleoside reverse transcriptase inhibitors explained by virological fitness. AIDS. 2006;20:223–231. doi: 10.1097/01.aids.0000199825.34241.49. [DOI] [PubMed] [Google Scholar]
- 5.Maggiolo F, Airoldi M, Kleinloog HD, et al. Effect of adherence to HAART on virologic outcome and on the selection of resistance-conferring mutations in NNRTI- or PI-treated patients. HIV Clin Trials. 2007;8:282–292. doi: 10.1310/hct0805-282. [DOI] [PubMed] [Google Scholar]
- 6.Eron JJ, Vernazza PL, Johnston DM, et al. Resistance of HIV-1 to antiretroviral agents in blood and seminal plasma: implications for transmission. AIDS. 1998;12:F181–F189. doi: 10.1097/00002030-199815000-00003. [DOI] [PubMed] [Google Scholar]
- 7.Hecht FM, Grant RM, Petropoulos CJ, et al. Sexual transmission of an HIV-1 variant resistant to multiple reverse-transcriptase and protease inhibitors. N Engl J Med. 1998;339:307–311. doi: 10.1056/NEJM199807303390504. [DOI] [PubMed] [Google Scholar]
- 8.Guidelines to antiretroviral drug therapy in Kenya. Kenya Ministry of Health; Nairobi: 2001. [Google Scholar]
- 9.Oyugi JH, Byakika-Tusiime J, Charlebois ED, et al. Multiple validated measures of adherence indicate high levels of adherence to generic HIV antiretroviral therapy in a resource-limited setting. J Acquir Immune Defic Syndr. 2004;36:1100–1102. doi: 10.1097/00126334-200408150-00014. [DOI] [PubMed] [Google Scholar]
- 10.John GC, Sheppard H, Mbori-Ngacha D, et al. Comparison of techniques for HIV-1 RNA detection and quantitation in cervicovaginal secretions. J Acquir Immune Defic Syndr. 2001;26:170–175. doi: 10.1097/00042560-200102010-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Panteleeff D DeVange, Emery S, Richardson BA, et al. Validation of performance of the gen-probe human immunodeficiency virus type 1 viral load assay with genital swabs and breast milk samples. J Clin Microbiol. 2002;40:3929–3937. doi: 10.1128/JCM.40.11.3929-3937.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lehman DA, Chung MH, Mabuka JM, et al. Lower risk of resistance after short-course HAART compared with zidovudine/single-dose nevirapine used for prevention of HIV-1 mother-to-child transmission. J Acquir Immune Defic Syndr. 2009;51:522–529. doi: 10.1097/QAI.0b013e3181aa8a22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Frenkel LM, McKernan J, Dinh PV, et al. HIV type 1 zidovudine (ZDV) resistance in blood and uterine cervical secretions of pregnant women. AIDS Res Hum Retroviruses. 2006;22:870–873. doi: 10.1089/aid.2006.22.870. [DOI] [PubMed] [Google Scholar]
- 14.Graham SM, Masese L, Gitau R, et al. Antiretroviral adherence and development of drug resistance are the strongest predictors of genital HIV-1 shedding among women initiating treatment. J Infect Dis. 2010;202:1538–1542. doi: 10.1086/656790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mannheimer S, Friedland G, Matts J, et al. The consistency of adherence to antiretroviral therapy predicts biologic outcomes for human immunodeficiency virus-infected persons in clinical trials. Clin Infect Dis. 2002;34:1115–1121. doi: 10.1086/339074. [DOI] [PubMed] [Google Scholar]
- 16.Sethi AK, Celentano DD, Gange SJ, et al. Association between adherence to antiretroviral therapy and human immunodeficiency virus drug resistance. Clin Infect Dis. 2003;37:1112–1118. doi: 10.1086/378301. [DOI] [PubMed] [Google Scholar]
- 17.von Wyl V, Yerly S, Böni J, et al. Incidence of HIV-1 drug resistance among antiretroviral treatment-naïve individuals starting modern therapy combinations. Clin Infect Dis. 2012;54:131–140. doi: 10.1093/cid/cir728. [DOI] [PubMed] [Google Scholar]
- 18.Rosenblum M, Deeks SG, van der Laan M, et al. The risk of virologic failure decreases with duration of HIV suppression, at greater than 50% adherence to antiretroviral therapy. PLoS One. 2009;4:e7196. doi: 10.1371/journal.pone.0007196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lima VD, Bangsberg DR, Harrigan PR, et al. Risk of viral failure declines with duration of suppression on highly active antiretroviral therapy irrespective of adherence level. J Acquir Immune Defic Syndr. 2010;55:460–465. doi: 10.1097/QAI.0b013e3181f2ac87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Parienti JJ, Das-Douglas M, Massari V, et al. Not all missed doses are the same: sustained NNRTI treatment interruptions predict HIV rebound at low-to-moderate adherence levels. PLoS One. 2008;3:e2783. doi: 10.1371/journal.pone.0002783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Oyugi JH, Byakika-Tusiime J, Ragland K, et al. Treatment interruptions predict resistance in HIV-positive individuals purchasing fixed-dose combination antiretroviral therapy in Kampala, Uganda. AIDS. 2007;21:965–971. doi: 10.1097/QAD.0b013e32802e6bfa. [DOI] [PubMed] [Google Scholar]
- 22.Nachega JB, Mills EJ, Schechter M. Antiretroviral therapy adherence and retention in care in middle-income and low-income countries: current status of knowledge and research priorities. Curr Opin HIV AIDS. 2010;5:70–77. doi: 10.1097/COH.0b013e328333ad61. [DOI] [PubMed] [Google Scholar]
- 23.Price MA, Wallis CL, Lakhi S, et al. Transmitted HIV type 1 drug resistance among individuals with recent HIV infection in East and Southern Africa. AIDS Res Hum Retroviruses. 2011;27:5–12. doi: 10.1089/aid.2010.0030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hamers RL, Wallis CL, Kityo C, et al. HIV-1 drug resistance in antiretroviral-naive individuals in sub-Saharan Africa after rollout of antiretroviral therapy: a multicentre observational study. Lancet Infect Dis. 2011;11:750–759. doi: 10.1016/S1473-3099(11)70149-9. [DOI] [PubMed] [Google Scholar]
- 25.Hamers RL, Schuurman R, Sigaloff KC, et al. Effect of pretreatment HIV-1 drug resistance on immunological, virological, and drug-resistance outcomes of first-line antiretroviral treatment in sub-Saharan Africa: a multicentre cohort study. Lancet Infect Dis. 2011 doi: 10.1016/S1473-3099(11)70255-9. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 26.Jordan M, Kelley K, Hassani A Saadani, et al. Monitoring ART: Clinic and program performance using WHO HIV drug resistance early warning indicators in 21 countries. Presented at the 18th Conference on Retroviruses and Opportunistic Infections (Boston); 2011. [paper 626] [Google Scholar]
- 27.Baeten JM, Kahle E, Lingappa JR, et al. Genital HIV-1 RNA predicts risk of heterosexual HIV-1 transmission. Sci Transl Med. 2011;3:77ra29. doi: 10.1126/scitranslmed.3001888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cohen MS, Chen YQ, McCauley M, et al. Prevention of HIV-1 infection with early antiretroviral therapy. New Engl J Med. 2011;365:493–505. doi: 10.1056/NEJMoa1105243. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.