Abstract
Background
Cervical tissue based organ cultures have been used successfully to evaluate microbicides for toxicity and antiviral activity. The antimicrobial peptide retrocyclin RC-101 has been shown to have potent anti-HIV activity in cell culture.
Objective
To evaluate RC-101 in organ culture for toxicity and its ability to block HIV-1 transmission across cervical mucosa.
Methods
A Cervical tissue based organ culture was used to measure antiviral activity of RC101. Cytotoxicity in tissues was determined by immunostaining of cellular proteins and by measuring inflammatory cytokines using realtime RTPCR and luminex technology.
Results
RC-101 blocked transmission of both R5 and X4 HIV-1 across cervical mucosa in this organ culture model. Furthermore, film-formulated RC-101 exhibited potent antiviral activity in organ culture. Such antiviral activity of RC-101 was retained in the presence of semen and vaginal fluid. RC-101 showed no cytotoxicity in cervical tissue. Furthermore, RC-101 did not induce proinflammatory cytokine response in tissues. RC-101 also did not have any effect on NK activity, cell proliferation of CD4 and CD8 cells, and did not show chemotactic activity.
Conclusion
Therefore, because of strong antiviral activity and low cytotoxicity in cervical tissues, RC-101 should be considered as an excellent microbicide candidate against HIV-1.
Keywords: retrocyclin, HIV, organ culture, microbicide
Introduction
Despite the decline in HIV-1-related disease and death in the United States over the past 8 years, due to the availability of antiretroviral therapies, there are major gaps in our current HIV-1 prevention strategies. Although an HIV-1 vaccine will be important to the control of the global epidemic, no effective vaccine against HIV-1 is available thus far. In the absence of an effective vaccine against HIV-1 there is an increasing recognition that topical microbicides for vaginal use that can prevent HIV-1 transmission during sexual intercourse may be the most viable current option in controlling the epidemic 1–3. Most of the antiviral properties of microbicides have been evaluated by examining their effect on the replication of virus in human primary T lymphocytes transformed T cell lines and epithelial cells 4–9. However, there are concerns that these assays may have less relevance when applied to sexual transmission of HIV-1 across epithelial cells of vaginal and cervical origin.
To address these concerns a number of investigators have used primary cervical/vaginal tissue based organ culture and organotypic cervicovaginal tissues to evaluate toxicity and antiviral activity of microbicides 10–18. We have previously established a quantitative high-throughput tissue transmission assay using the organ culture model to evaluate potential topical microbicides for their ability to block HIV-1 transmission across the epithelial mucosal barrier 11, 19, 20. Compounds evaluated in this system include RT inhibitors (PMPA and UC781), various HIV entry inhibiting antimicrobial peptides (WLBU-2, LL37, and UC781), and membrane cholesterol dissolving drugs such as beta-cyclodextrin 19, 20. In addition, the organ culture assay has allowed us to directly test the potential cellular toxicity of microbicide candidates by measuring immune and non-immune cellular markers, and pro-inflammatory cytokine responses. Our organ culture assay thus provides an ideal system for evaluating potential topical microbicides in their ability to block HIV-1 transmission across the epithelial mucosa and directly test the potential toxicity of microbicide candidates in cervical tissue.
A limited number of anti-HIV-1 microbicides that can be applied topically in the vagina have been developed. First generation of microbicides consisting of surfactants and polyanionic polymers that are supposed to inactivate HIV-1 upon contact, were found to be either toxic or ineffective in blocking HIV-1 transmission in clinical trials 21–23. A second generation of HIV-1 RT inhibiting compounds with potent antiviral activity in vitro has been recently described. Gel formulations of both nucleoside analogue PMPA (Tenofovir) and non-nucleoside analogs UC781 and TMC120 are currently in clinical trials 24–27. Vaginal application of 1% Tenofovir gel has recently been shown to reduce HIV-1 transmission by 40% 28. Another class of microbicides in development blocks viral attachment to CD4, co-receptor (CCR5 and CXCR4) interactions, or gp41-mediated fusion. Such compounds may provide a highly effective strategy for preventing localized mucosal infection as they prevent the initial stages of the infectious life cycle 29, 30. Unlike surfactant-based microbicides, such an approach is highly unlikely to perturb the protective effects of resident microflora, or have contraceptive potential. Cellular co-receptor antagonists, such as CMPD167 and AOP-RANTES (CCR5 inhibitors), and AMD3465 (X4 inhibitor) have been tested in monkeys 30, 31 and are being considered potential microbicides in humans.
The cyclic antimicrobial peptide Retrocyclin RC-101 is a cationic β-sheet, 18-residue peptide which interacts with gp41 and prevents fusion. It is considered a potential microbicide candidate due to its anti-HIV activity against a wide variety of HIV with no toxicity in cell culture 32–34. In this report we have evaluated the antiviral and cytotoxic profile of RC-101 in our cervical tissue based organ culture model. Our data indicate that RC-101 blocks HIV-1 transmission in the cervical tissue organ culture. Furthermore, RC-101 showed no cytotoxicity as measured by a variety of immune and non-immune functions.
Methods
RC-101 formulation as intravaginal films
The 18 amino acid RC-101 peptide was prepared on a 0.25 mmol scale with an ABI 431A peptide synthesizer using FastMocTM chemistry. The purified RC-101 was subsequently oxidized and cyclized as described elsewhere 32. The peptides were analyzed by MALDI-TOF mass spectrometry to confirm homogeneity and that the measured mass agrees well with its expected mass. RC-101 was formulated by solvent casting technique as a quick dissolving polymeric 27.5mm × 33.5mm vaginal film, composed of 2.0mg RC-101, 6% polyvinyl alcohol (Kuraray America Inc., New York, NY), 0.12% hydroxypropyl methylcellulose 6 cps (HPMC) (Sigma, St. Louis, MO), and 3% glycerin (Dow Chemical Co., Midland, MI) as described by Sassi et al 35 All films were stored in PET/Aluminum foil pouches (Amcor Flexibles Healthcare, Inc., Mundelein, IL) until used.
Virus and cell culture
Cell-free HIV-1 BAL was grown in phytohemagglutinin (PHA)-stimulated CD8-depleted PBMCs from seronegative persons and titered in the same cells. CD8-depleted PBMCs were prepared from blood bank donors by use of anti-CD8 monoclonal antibody-coated immunomagnetic beads (Dynal, Oslo, Norway) as described previously 36.
Testing antiviral activity of RC-101 in organ culture
Cervical tissues were obtained from seronegative premenopausal women aged 50 years old or less undergoing hysterectomy or anterior/posterior repair procedures at the Magee Women’s Hospital under an IRB approved protocol of the University of Pittsburgh. The organ culture was set up with CD8-depleted PBMCs (500,000) from a seronegative normal donor as indicator cells in the bottom chamber of the Transwell system as previously described 11, 20. A transwell with agarose only in the top chamber served as a negative control, while transwells with the membrane only served as a positive control. To measure antiviral activity cell-free HIV-1 BAL or IIIB (1× 105 TCID50) were preincubated with RC-101(10–40ug/ml) for 1 hr. RC-101 and virus mixture were added to the top chambers of the tissue. As a control, HIV-1 incubated with media for 1 hr was added to another tissue well, and agarose control and membrane control wells, and incubated at 37°C for 3–4 days. After incubation, the top chamber of the well was removed and culture of CD8-depleted cells in the bottom chamber was continued for an additional 10 days. Viral growth was monitored by measuring HIV-1 p24 antigen levels in the culture supernatant in the bottom chamber of the tissue well. A 3-fold or greater increase in HIV-1 p24 during the 2-week period was taken as positive virus infection of the CD8-depleted cells. On the last day of culture, leakiness of the organ culture system was routinely monitored by examining transmission of blue dextran, a 2 ×106 MW polysaccharide, through the tissue-containing Transwell and the agarose control into the bottom chamber as described previously 19. The amount of blue dextran transmitted was less than 1% in agarose control well and tissue sample wells routinely showed less blue dextran transmission than did the agarose control wells. Most of the experiments were done with tissues from 2–3 subjects in duplicate or triplicates depending on the availability of tissues.
Measurement on Intracellular Ki67 and Cytokeratin protein
The level of intracellular ki67 and Cytokeratin ( AE1/AE3) was determined by quantitative immunostaining as described previously 11.
Measurement of Proinflammatory Cytokines response to RC-101
The level of proinflammatory cytokines, IL-1β, IL-6, IL-8 and TNFα messages were measured in the tissues by real time RT PCR. Total RNA from tissues was isolated with RNA-Bee™ (TEL-TEST, INC, Friendswood, TX) and followed by reverse transcription with TaqMan® Reverse Transcription Reagents (Applied Biosystems) according to manufacturer’s protocols. A 30ul PCR mixture consists of 3 ul cDNA (12ng total RNA equivalent), 2×TaqMan® Universal PCR Master Mix and 20× TaqMan® Pre-Developed Assay Reagents (Applied Biosystems). ABI Prism 7000 Sequence Detection System was used to carry out Real-Time PCR under the following cycling condition: 50 °C for 2 min, 95 °C for 10 min, 40 cycles of 95 °C for 15 sec and 60 °C for 1 min. We selected Human β-2M primers and probe labeled with VIC / TAMRA for endogenous control. Human TNFα, IL1β, IL6 and IL8 primers and probes labeled with FAM / MGB were used for proinflammatory cytokine measurements. Assays were performed under manufacture suggested conditions which were designed to exclude the detection of genomic DNA. Each sample was run in duplicate. No template control was applied in each assay to ensure no cross contamination. Relative gene expression data were analyzed with Relative Quantification (((Ct) method with 7000 System SDS software (Applied Biosystems). Our preliminary data showed that the amplification efficiency of β2M and other cytokines are approximately equal; therefore using the ((Ct calculation was valid in our assay system (User bulletin #2, Applied Biosystems). The cytokine gene expression results were reported as relative fold change.
Secreted cytokines in culture supernatant were measured by Luminex technology according to manufacturer’s (Bio-Rad Laboratory, Hercules, CA) instruction.
NK cell-mediated target cell lysis assay
PBMC (5×106/mL) from normal donor were stimulated with PHA (1µg/mL) and IL-2 (200U/mL) and exposed to the indicated doses of RC-101. Three days post-treatment cells were washed and degranulation of NK cells within PBMCs was measured following co-incubation of total PBMCs with K562 (PBMC:K562=1:1 in a total volume of 1mL) for 2 hours including last 1 hour with Golgy stop. Surface staining was performed using CD3-ECD, CD56-PE and CD107a-FITC.Expression of CD107-a in CD3-and C56+ gated NK cells were determined by FACS. Vehicle 20 µL 0.1% acetic acid /mL of culture. Number in quadrant represents percentage of CD3− CD56+ cells expressing CD107a 37.
Cell proliferation assay
Cell proliferation assay was done as described previously 38. Briefly, 1× 105 PBMC were incubated with CFSE dye (10 µg CFSE in 1 ml of 0.2% bovine serum albumin-phosphate-buffered saline; Molecular Probes, Eugene, OR) at 37°C for 10 min. After incubation, 5 ml of cold complete medium was added and incubated on ice for 5 min. Cells were washed with cold RPMI 1640 medium containing 10% heat-inactivated human AB+ serum (Sigma), 1% l-glutamine, 1% HEPES buffer, and 1% penicillin-streptomycin and cultured for 6 days with various concentration of RC-101 (10–40ug/ml) at 37°C. After 6 days of incubation, the cells were harvested, washed, and stained for surface markers by using anti-CD8-peridinin chlorophyll protein and anti-CD4-phycoerythrin (PE) MAb (BD Biosciences). Following staining, the cells were washed, fixed, and analyzed in a FACS Canto II flow cytometer (BD Immunocytometry Systems, San Diego, CA). Negative controls (medium only) and positive controls (phytohemagglutinin, 5µg/ml; Sigma) were included in each assay. The results are expressed as net % CFSE-positive T cells (% positive peptide-stimulated T cells − % medium control).
Chemotaxis assay
Chemotaxis assays were performed as described previously 39. Briefly, PBMC were loaded onto top well of a 96-well ChemoTx Chemotaxis System (5-_m pore; NeuroProbe). RC-101 or the control chemokines were added in the bottom chamber. Cells were incubated for 5 h at 37°C in 5% CO2, the cells on top of the membrane were removed with a scraper and the migrated cells in the bottom wells were counted using a hemocytometer.
Results
Antiviral activity of unformulated and formulated RC-101 in organ culture
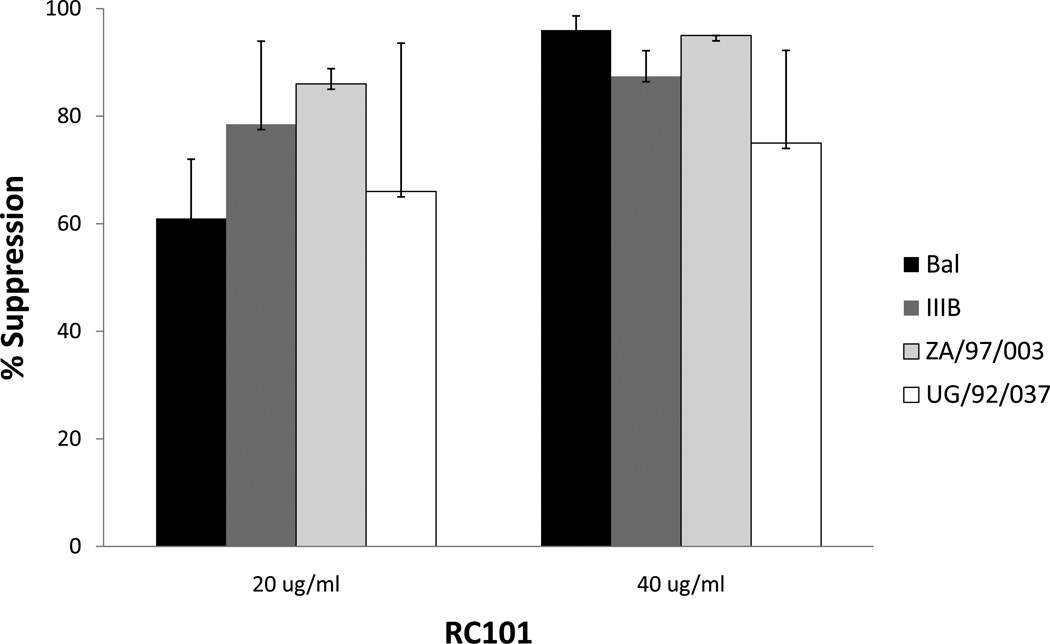
RC-101 was solubilized in water and tested in organ culture for its ability to block HIV-1 transmission across cervical mucosa. As shown in Figure 1, RC-101 blocked transmission of HIV-1 in a dose dependent manner. Antiviral activity was demonstrated against both R5 HIV-1 BAL and X4 HIV-1 IIIB. More than 90% inhibition was obtained at 40ug/ml of RC-101. Antiviral activity was similar to that observed with an RT inhibiting microbicide UC781 19, 20. We also examined the antiviral activity of RC-101 against two other clades of HIV-1 of African origin in organ culture. They were: ZA/97/003) clade A, R5 HIV-1 and UG/92/037, clade A, X4 HIV-1. As shown in Figure 1, RC-101 effectively blocked transmission of both African strains across cervical mucosa, although it showed higher antiviral activity against African isolate ZA/97/003.
Figure 1. Antiviral activities of RC-101 in an organ culture on the transmission of cell-free HIV-1 BAL, HIV-1 ZA/97/003 and UG/92/037 across the cervical mucosa.
The percentage of HIV-1 suppression was calculated by dividing the amount of HIV-1 p24 produced on day 14 in the bottom well in the presence of RC-101 by the amount produced in the absence of RC-101. The data shown represent the mean +/− 3 standard deviation of 3 independent experiments performed in triplicate
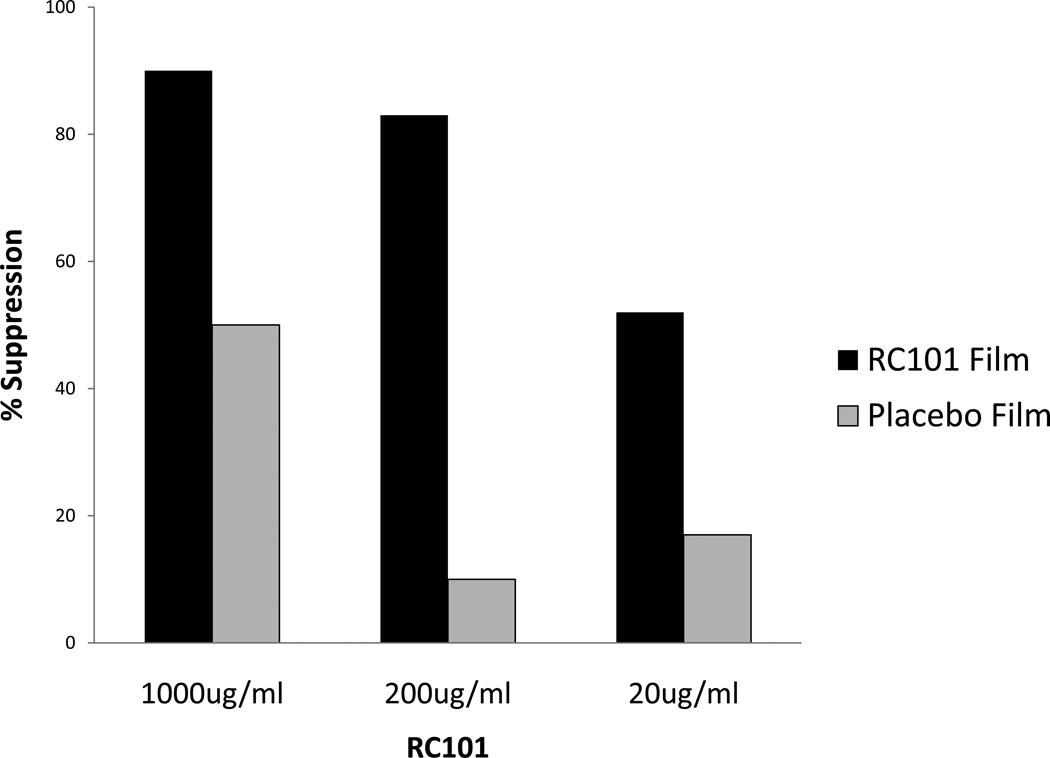
Next, we measured antiviral activity of film formulated RC-101. For this purpose one film containing 2mg RC-101 was dissolved quickly in 1 ml culture medium and tested at various dilutions in duplicate for antiviral activity in cervical organ culture. As shown in Figure 2, film formulated RC-101 blocked HIV-1 transmission in a dose dependent manner with more than 80% inhibition at 200ug/ml. interestingly; the placebo film also exhibited some antiviral activity.
Figure 2. Antiviral activity of film formulated RC-101 in an organ culture.
RC-101 formulated and placebo formulated films were compared for their ability to block transmission of HIV-1 BAL. The percentage of HIV-1 suppression was calculated as described in legend to figure 1. Results shown here represent average of triplicate tissue wells.
Evaluation of cytotoxicity of RC-101 in organ culture
Subtle microbicide-induced cytotoxicity may not be fully ascertained by physical examination or histological examination of the vaginal/cervical tissues. However, measurements of the level of inflammatory cytokines and cellular markers cytokeratin and Ki67 in response to microbicides in tissues in an organ culture model are excellent approaches to monitor cellular injury and cytotoxicity caused by microbicides. Therefore, we determined the effect of RC-101 on the level of intracellular Ki67 protein, a cell division marker, and cytokeratin, an epithelial cell differentiation marker. Tissues in the organ culture format with no indicator cells in the bottom well were exposed to unformulated RC-101 (40ug/ml) for 24 – 72 hr at 37°C. Following incubation tissues were harvested from top well, fixed and analyzed for Ki67 and Cytokeratin (AE1/AE3) by quantitative immunostaining as described previously 11. As shown in Table 1, the relative % Ki67 and Cytokeratin in RC-101-exposed cervical tissues ranged between 20.4 and 26.1 for ki67 and between 36.9 and 44.5 for cytokeratin. This was similar to levels recovered from controls (17.5–24.7% for cytokeratin and 38–41% for cytokeratin). In contrast, treatment with N9, a known cytotoxic microbicide, resulted in approximately 3-fold reduction of both Ki67 (7.3%) and 2-fold reduction in cytokeratin (19.7%) markers.
Table 1.
Measurement of the Intracellular Expression of Ki67 and Cytokeratin in Cervical Tissues in Presence of RC101 by Quantitative Immunohistochemistry
| Percentage of total cells staineda | ||
|---|---|---|
| Application | Ki67 (%) | AE1/A3 Cytokeratin (%) |
| RC101 (24 hr) RC101 (48 hr) RC101 (72 hr) |
22.5±6.2 26.1±4.9 20.4±9.2 |
42.7±10.3 44.9±7.9 36.9±8.1 |
| Media (24 hr) Media (48 hr) |
17.5±5.6 24.7±8.4 |
38.9±6.1 41.0±12.0 |
| 4% N-9 | 7.3±3.4 | 19.7±6.8 |
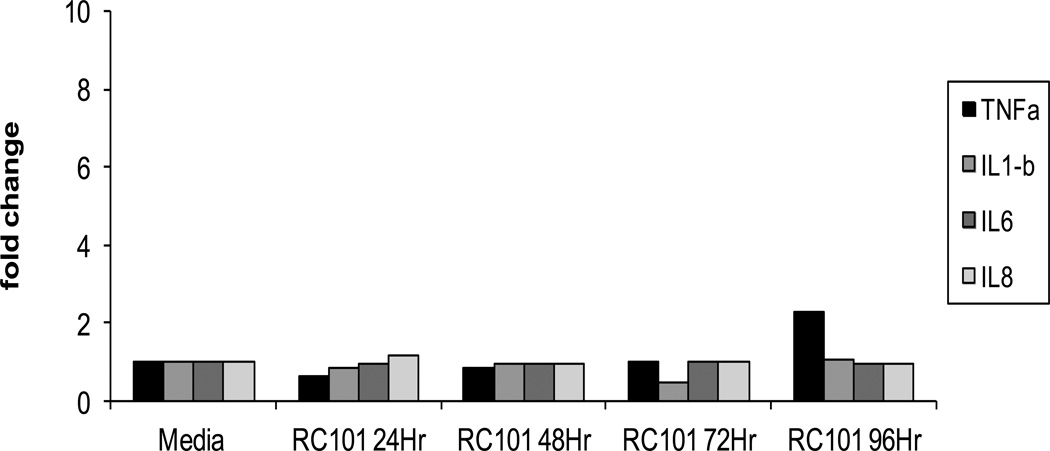
Next, we measured in our organ culture the inflammatory cytokine response to unformulated RC-101. Our standard organ culture was set up as described above with no indicator cells in the bottom. Cervical tissues were exposed to RC-101 (40ug/ml) for various length of time. Tissues were then harvested and examined for IL-1β, IL-6, IL-8 and TNFα cytokine messages by real time RT-PCR and secreted cytokines in the supernatant by the Bio-Rad Bio-Plex System using the Luminex technology according to manufacturer’s (Bio-Rad Laboratory, Hercules, CA) instruction. The RT-PCR assay conditions were designed to exclude the detection of genomic DNA. Each sample was run in duplicate and average CT values were used for gene expression calculation. Results are expressed as the fold increase in cytokine expression as compared to tissue incubated in media alone. Tissues treated with RC 101 for 24–48hr exhibited level of cytokine messages (supplement Figure 1) and secreted cytokine proteins (Figure 3) similar to controls. Longer incubation resulted in slightly higher levels of TNFα protein (Figure 3). In contrast, N9, a microbicide known to cause epithelial irritation in women 40–42, elicited more than 5 fold of TNFα messages within 24 hr of exposure of tissue with 4% N9, reaching a maximum level of more than 30 fold with 1% and 4% N9 after 48 hr. A 3-fold increase in IL-1β messages was also noted after 48 hr culture following exposure to 1% N9 for 1 hr (data not shown). There was no change in the level of expression of IL-6 and IL-8. Loss of inflammatory response at 72 hr in 4% N9 was due to loss of cell viability at 72 hr at that concentration. Since there were no indicator cells in the bottom well, we conclude that cytokine response was elicited by the tissues.
Figure 3. Kinetics of expression of secreted cytokines following exposure of cervical tissues to RC-101.
In tissues with media alone control, the levels of IL1β and TNFα were negligible (<0.05ng/ml) and the levels of IL-6 and IL-8 at 24, 48, and 72 hr were: 13, 173, 156 ng/ml (IL-6) and 1.2,515,564 ng/ml (IL-8), respectively. The data shown represent the mean +/− 2 standard deviation of 3 independent experiments performed in triplicate
Effect of RC-101 on Immune functions
Cervical tissues exposed to HIV-1 induce a number of immune activators to control viral infection. We evaluated the effect of unformulated RC-101 on three common immune parameters: NK cell activity, chemotactic activity and cell proliferation. Since these experiments are difficult to perform in tissues, we evaluated effects of RC-101 in lymphocyte cell cultures. It is noteworthy that RC-101 also exhibits profound antiviral activity in PBMC (Gupta, P manuscript in preparation). As shown in Supplement Figure 2, RC-101 up to 20ug/ml had no significant effect on NK activity, although it showed some inhibition at 40ug/ml. Similarly RC-101 up to a concentration of 40ug/ml had no significant chemotactic activity of lymphocytes, compared to positive control chemokines CXCL11 and CCL21 (Supplement Figure 3). RC-101 up to a concentration of 40ug/ml also showed little effect on proliferation of CD4 and CD8 lymphocytes. As a positive control PHA showed high degree proliferation (Supplement Figure 4).
Effect of seminal and vaginal fluids on the antiviral activity of RC-101 in cervical organ culture
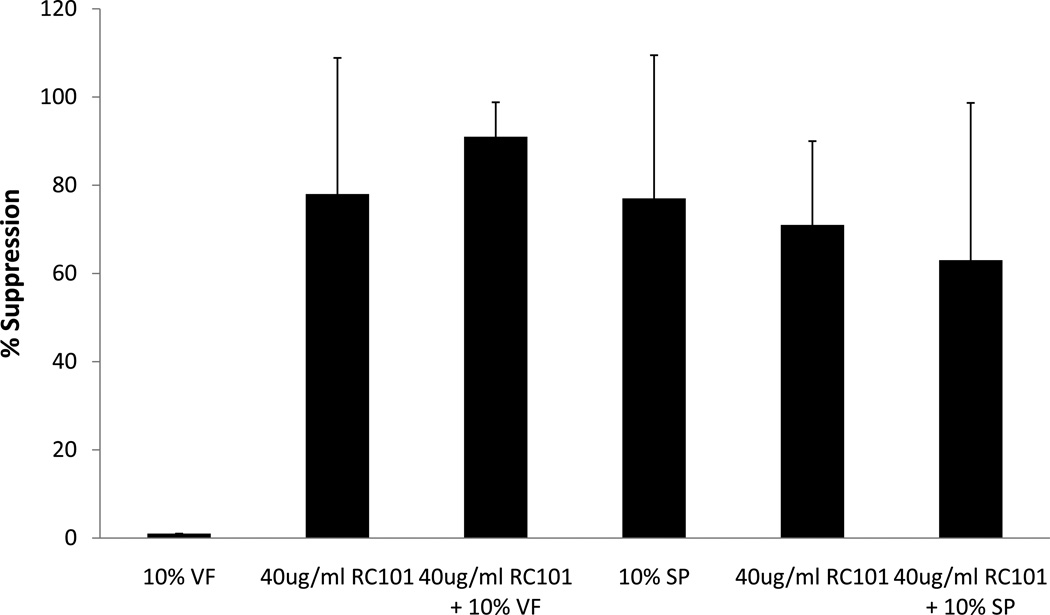
The antiviral activity of RC-101 was evaluated in the presence of 10% human seminal and vaginal fluids. For this purpose unformulated RC-101 was incubated for 1 hr at 37°C with HIV-1 BAL in the presence and absence of 10% seminal fluid or vaginal fluid obtained from seronegative control men and women, respectively. Because of the reported toxicity of seminal fluid on cells/tissues, we have recovered HIV-1 following incubation from seminal fluid/by high speed centrifugation of the semen/microbicide mixture. Pelleted virus was resuspended in small volume of medium and then added onto the tissue and cultured for 3–4 days. Transmitted virus was measured by its growth in the indicator cells in the bottom well. Since vaginal fluid is not toxic for cells, after incubation we added vaginal fluid/HIV-1/RC-101 mixture directly into cervical tissue in the organ culture. As shown in Figure 4, seminal fluid or vaginal fluid did not significantly affect antiviral activity of RC-101 in cervical tissues.
Figure 4. Effect of human seminal plasma (SP) and vaginal Fluid (VF) on antiviral activity of RC-101 in the organ culture.
Experiment was conducted in the presence of 10% seminal plasma or vaginal fluid as described in the text. Percentage of suppression was calculated as described in the legend to Figure 1. The data shown represent the mean +/− 3 standard deviation of 3 independent experiments performed in triplicate for seminal plasma and in 2 independent experiments performed in triplicate for vaginal fluid.
Discussion
Retrocyclins are members of a class of antimicrobial peptides called θ-defensins. While monkeys produce θ-defensin peptides, humans cannot produce these peptides because the gene contains a premature stop codon in the peptide’s signal sequence 43. Previous studies have demonstrated that retrocyclins, such as RC-101 can protect primary T cells from in vitro infection by both X4 and R5 strains of HIV-1, and are much more active in vitro than other closely related defensin molecules 32–34. However, the relevance of these assays is uncertain when considered in the context of sexual transmission of HIV across epithelial cells of vaginal and cervical origin. Therefore, in this report we evaluated RC-101 in a cervical tissue matrix in an organ culture that closely mimics in vivo conditions. We have shown that RC-101 blocks stransmission of both R5 and X4 HIV-1 across cervical mucosa in this organ culture model. Furthermore, film formulated RC-101 retained antiviral activity in organ culture. However, it needed higher concentration of RC101 when film formulated to achieve same level of suppression by the unformulated RC101. This is not unexpected, because similar situation was reported for other mcrobicides, For example, 1% RC101 produces same amount of antiviral activity as 0.01% RC101 in aqueous form (35). Both vaginal fluid and seminal fluid had no deleterious effect on the antiviral activity of RC101 in organ culture, although seminal plasma itself has some antiviral activity which is not due toxic effect. It is possible that semen has innate immunity against HIV-1. Regardless, these data further illustrates the importance of RC-101 as microbicide by demonstration of its antiviral activity in the presence of semen and vaginal fluid.
Since microbicides will be topically applied to the vagina, it is important to determine their cytotoxicity in vaginal/cervical tissues. The failure of N9 and cellulose phosphate in phase III clinical trial 21, 22, 44 warned us that safety evaluation of a microbicide candidate should be performed as early as possible. Although monitoring for lesions in the cervix/vagina has been used to test for cytotoxicity in Phase 1 clinical trials for microbicides 21, 22, 45, it cannot detect subtle changes in the mucosal barrier, such as induction of clinically inapparent inflammation. Although proinflammatory responses are beneficial to control vaginal bacterial infection, inflammatory cytokines may enhance HIV-1 transcription in infected cells and increase HIV-1 transmission 46, 47. The rabbit vaginal irritation model has also been utilized to study the inflammation and toxicity of the drug formulation to the genital mucosa. Its usage is limited due to anatomical differences of the vaginal and cervical mucosa between human and rabbit. The cervical tissues in our organ culture conditions have been shown to maintain histological and cellular (immunological and non-immunological) markers during the six days incubation period 11 and therefore provide a relevant and convenient model to evaluate microbicides for potential cytotoxicity in the tissue matrix. Using this organ culture we have demonstrated that RC-101 had no cytotoxic effect as evidenced by lack of any inhibition on two key cellular proteins Ki67, a cell proliferation marker and cytokeratin, an epithelial cell marker. Furthermore, RC-101 did not induce proinflammatory response in tissues up to 72 hr. after exposure. RC-101 also did not alter key immune functions such as NK activity, cell proliferation of CD4 and CD8 cells, and did not show chemotactic activity of lymphocytes. These results together with previously reported in vitro data showing its non- hemagglutinating properties 32strongly suggest that RC-101 would be a safe microbicide for application in human. This suggestion gained support from our recent collaborative study in the non-human primate model showing no vaginal cytotoxicity 48
Currently microbicides based on NNRTI’s (UC781 and TMC120) as well as one with a nucleoside RT inhibitor (tenofovir) are in Phase 1Iand III clinical trials. However, there is a definite need to identify new antiviral compounds as backup microbicidal agents as many drugs with promising preclinical properties fail during advanced clinical evaluation as they may be ineffective in preventing sexual transmission of resistant variants. In that regard RC-101 induces very low level of resistance even after 28 passages in cell culture 49 and that can be overcome with slight increases in peptide concentration (A.M.C. unpublished data). Retrocyclin being an evolutionary conserved host protein 32, 50 may be responsible for induction of low resistance. An HIV-1 entry-inhibiting microbicide, such as RC-101 has distinct advantage over RT inhibiting microbicides is that it blocks HIV-1 transmission before the virus can infect a target cell. Therefore, probability of developing resistance is lower because of limited viral replication at the beginning of infection.
In summary, RC-101 possesses many of the properties of an ideal microbicide candidate with strong antiviral activity and low cytotoxicity in cell culture and tissues. For these reasons combined with recent safety demonstrated in the macaque model 48 RC-101 should be considered an excellent candidate microbicide for clinical trials.
Supplementary Material
Cytokines messages were determined by using realtime RTPCR as described in Methods.
Three days post-treatment with RC-101 degranulation of NK cells in PBMCs was determined by measuring expression of CD107-a in CD3-and C56+ gated NK cells by flow cytometric analysis. Percentage of CD3− CD56+ cells expressing CD107a in the presence 10, 20 and 40ug/ml of RC-101 were 49, 42 and 35 compared 45 with vehicle control.
The number of cell migrated to the bottom well in the presence of different concentration of RC-101 and media control are shown. Chemokine’s CXCL11 and CCL21 were included as a positive control.
Percentage of CFSE+ CD4+ and CFSE+CD8+ was determined after PBMC were exposed to various concentration of RC-101. PHA treatment was included as a positive control.
Acknowledgement
We thank Ms. Varsha Sridhar and Dr. Yue Chen for editorial assistance and Dr. Yongjun Sui for technical assistance. This work was funded by the grant NIH U19 AI65430.
Source of Funding: This work was supported by a grant R01 HD052436 from the National Institute of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest: None of the authors received honoraria from any Company or is on the speaker’s bureau or CME organizers for any organization.
References
- 1.Darroch JE, Frost JJ. Women's interest in vaginal microbicides. Fam Plann Perspect. 1999;31:16–23. [PubMed] [Google Scholar]
- 2.Klasse PJ, Shattock RJ, Moore JP. Which topical microbicides for blocking HIV-1 transmission will work in the real world? PLoS Med. 2006;3:e351. doi: 10.1371/journal.pmed.0030351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Abdool Karim Q, Abdool Karim SS, Frohlich JA, et al. Effectiveness and safety of tenofovir gel an antiretroviral microbicide, for the prevention of HIV infection in women. Science. 329:1168–1174. doi: 10.1126/science.1193748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baba M, Schols D, Pauwels R, Nakashima H, De Clercq E. Sulfated polysaccharides as potent inhibitors of HIV-induced syncytium formation: a new strategy towards AIDS chemotherapy. J Acquir Immune Defic Syndr. 1990;3:493–499. [PubMed] [Google Scholar]
- 5.Balzarini J, Naesens L, Verbeken E, et al. Preclinical studies on thiocarboxanilide UC-781 as a virucidal agent. AIDS. 1998;12:1129–1138. doi: 10.1097/00002030-199810000-00004. [DOI] [PubMed] [Google Scholar]
- 6.Borkow G, Barnard J, Nguyen TM, Belmonte A, Wainberg MA, Parniak MA. Chemical barriers to human immunodeficiency virus type 1 (HIV-1) infection: retrovirucidal activity of UC781, a thiocarboxanilide nonnucleoside inhibitor of HIV-1 reverse transcriptase. J Virol. 1997;71:3023–3030. doi: 10.1128/jvi.71.4.3023-3030.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ito M, Baba M, Sato A, Pauwels R, De Clercq E, Shigeta S. Inhibitory effect of dextran sulfate and heparin on the replication of human immunodeficiency virus (HIV) in vitro. Antiviral Res. 1987;7:361–367. doi: 10.1016/0166-3542(87)90018-0. [DOI] [PubMed] [Google Scholar]
- 8.Srinivas RV, Fridland A. Antiviral activities of 9-R-2-phosphonomethoxypropyl adenine (PMPA) and bis(isopropyloxymethylcarbonyl)PMPA against various drug-resistant human immunodeficiency virus strains. Antimicrob Agents Chemother. 1998;42:1484–1487. doi: 10.1128/aac.42.6.1484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Roth S, Monsour M, Dowland A, et al. Effect of topical microbicides on infectious human immunodeficiency virus type 1 binding to epithelial cells. Antimicrob Agents Chemother. 2007;51:1972–1978. doi: 10.1128/AAC.01358-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Abner SR, Guenthner PC, Guarner J, et al. A human colorectal explant culture to evaluate topical microbicides for the prevention of HIV infection. J Infect Dis. 2005;192:1545–1556. doi: 10.1086/462424. [DOI] [PubMed] [Google Scholar]
- 11.Collins KB, Patterson BK, Naus GJ, Landers DV, Gupta P. Development of an in vitro organ culture model to study transmission of HIV-1 in the female genital tract. Nat Med. 2000;6:475–479. doi: 10.1038/74743. [DOI] [PubMed] [Google Scholar]
- 12.Corpa JM, Peris B, Ribes V, Palacio J, Liste F. Hydrocephalus in a newborn bottlenosed dolphin (Tursiops truncatus) Vet Rec. 2004;155:208–210. doi: 10.1136/vr.155.7.208. [DOI] [PubMed] [Google Scholar]
- 13.Cummins JE, Christensen L, Lennox JL, et al. Mucosal innate immune factors in the female genital tract are associated with vaginal HIV-1 shedding independent of plasma viral load. AIDS Res Hum Retroviruses. 2006;22:788–795. doi: 10.1089/aid.2006.22.788. [DOI] [PubMed] [Google Scholar]
- 14.Cummins JE, Jr, Guarner J, Flowers L, et al. Preclinical testing of candidate topical microbicides for anti-human immunodeficiency virus type 1 activity and tissue toxicity in a human cervical explant culture. Antimicrob Agents Chemother. 2007;51:1770–1779. doi: 10.1128/AAC.01129-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fletcher PS, Elliott J, Grivel JC, et al. Ex vivo culture of human colorectal tissue for the evaluation of candidate microbicides. AIDS. 2006;20:1237–1245. doi: 10.1097/01.aids.0000232230.96134.80. [DOI] [PubMed] [Google Scholar]
- 16.Greenhead P, Hayes P, Watts PS, Laing KG, Griffin GE, Shattock RJ. Parameters of human immunodeficiency virus infection of human cervical tissue and inhibition by vaginal virucides. J Virol. 2000;74:5577–5586. doi: 10.1128/jvi.74.12.5577-5586.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Palacio J, Souberbielle BE, Shattock RJ, Robinson G, Manyonda I, Griffin GE. In vitro HIV1 infection of human cervical tissue. Res Virol. 1994;145:155–161. doi: 10.1016/s0923-2516(07)80017-3. [DOI] [PubMed] [Google Scholar]
- 18.Zussman A, Lara L, Lara HH, Bentwich Z, Borkow G. Blocking of cell-free and cell-associated HIV-1 transmission through human cervix organ culture with UC781. AIDS. 2003;17:653–661. doi: 10.1097/00002030-200303280-00002. [DOI] [PubMed] [Google Scholar]
- 19.Gupta P, Collins KB, Ratner D, et al. Memory CD4(+) T cells are the earliest detectable human immunodeficiency virus type 1 (HIV-1)-infected cells in the female genital mucosal tissue during HIV-1 transmission in an organ culture system. J Virol. 2002;76:9868–9876. doi: 10.1128/JVI.76.19.9868-9876.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gupta P, Ratner D, Patterson BK, et al. Use of frozen-thawed cervical tissues in the organ culture system to measure anti-HIV activities of candidate microbicides. AIDS Res Hum Retroviruses. 2006;22:419–424. doi: 10.1089/aid.2006.22.419. [DOI] [PubMed] [Google Scholar]
- 21.Hira SK, Feldblum PJ, Kamanga J, Mukelabai G, Weir SS, Thomas JC. Condom and nonoxynol-9 use and the incidence of HIV infection in serodiscordant couples in Zambia. Int J STD AIDS. 1997;8:243–250. doi: 10.1258/0956462971919994. [DOI] [PubMed] [Google Scholar]
- 22.Roddy RE, Zekeng L, Ryan KA, Tamoufe U, Weir SS, Wong EL. A controlled trial of nonoxynol 9 film to reduce male-to-female transmission of sexually transmitted diseases. N Engl J Med. 1998;339:504–510. doi: 10.1056/NEJM199808203390803. [DOI] [PubMed] [Google Scholar]
- 23.van De Wijgert J, Fullem A, Kelly C, et al. Phase 1 trial of the topical microbicide BufferGel: safety results from four international sites. J Acquir Immune Defic Syndr. 2001;26:21–27. doi: 10.1097/00126334-200101010-00003. [DOI] [PubMed] [Google Scholar]
- 24.Garcia-Lerma JG, Otten RA, Qari SH, et al. Prevention of rectal SHIV transmission in macaques by daily or intermittent prophylaxis with emtricitabine and tenofovir. PLoS Med. 2008;5:e28. doi: 10.1371/journal.pmed.0050028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jespers VA, Van Roey JM, Beets GI, Buve AM. Dose-ranging phase 1 study of TMC120, a promising vaginal microbicide, in HIV-negative and HIV-positive female volunteers. J Acquir Immune Defic Syndr. 2007;44:154–158. doi: 10.1097/QAI.0b013e31802bb35f. [DOI] [PubMed] [Google Scholar]
- 26.Rosen RK, Morrow KM, Carballo-Dieguez A, et al. Acceptability of tenofovir gel as a vaginal microbicide among women in a phase I trial: a mixed-methods study. J Womens Health (Larchmt) 2008;17:383–392. doi: 10.1089/jwh.2006.0325. [DOI] [PubMed] [Google Scholar]
- 27.Woolfson AD, Malcolm RK, Morrow RJ, Toner CF, McCullagh SD. Intravaginal ring delivery of the reverse transcriptase inhibitor TMC 120 as an HIV microbicide. Int J Pharm. 2006;325:82–89. doi: 10.1016/j.ijpharm.2006.06.026. [DOI] [PubMed] [Google Scholar]
- 28.Karim QA. Quarraisha Abdool Karim: investigating HIV/AIDS in South Africa. Interview by Priva Shetty. Lancet. 2009;374:871. doi: 10.1016/S0140-6736(09)61615-9. [DOI] [PubMed] [Google Scholar]
- 29.Veazey RS, Klasse PJ, Ketas TJ, et al. Use of a small molecule CCR5 inhibitor in macaques to treat simian immunodeficiency virus infection or prevent simian-human immunodeficiency virus infection. J Exp Med. 2003;198:1551–1562. doi: 10.1084/jem.20031266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Veazey RS, Klasse PJ, Schader SM, et al. Protection of macaques from vaginal SHIV challenge by vaginally delivered inhibitors of virus-cell fusion. Nature. 2005;438:99–102. doi: 10.1038/nature04055. [DOI] [PubMed] [Google Scholar]
- 31.Ketas TJ, Schader SM, Zurita J, et al. Entry inhibitor-based microbicides are active in vitro against HIV-1 isolates from multiple genetic subtypes. Virology. 2007;364:431–440. doi: 10.1016/j.virol.2007.03.001. [DOI] [PubMed] [Google Scholar]
- 32.Cole AM, Hong T, Boo LM, et al. Retrocyclin: a primate peptide that protects cells from infection by T- and M-tropic strains of HIV-1. Proc Natl Acad Sci U S A. 2002;99:1813–1818. doi: 10.1073/pnas.052706399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Munk C, Wei G, Yang OO, et al. The theta-defensin, retrocyclin, inhibits HIV-1 entry. AIDS Res Hum Retroviruses. 2003;19:875–881. doi: 10.1089/088922203322493049. [DOI] [PubMed] [Google Scholar]
- 34.Owen SM, Rudolph DL, Wang W, et al. RC-101, a retrocyclin-1 analogue with enhanced activity against primary HIV type 1 isolates. AIDS Res Hum Retroviruses. 2004;20:1157–1165. doi: 10.1089/aid.2004.20.1157. [DOI] [PubMed] [Google Scholar]
- 35.Sassi AB, Cost MR, Cole AL, et al. Formulation Development of Retrocyclin 1 Analog RC-101 as an Anti-HIV Vaginal Microbicide Product. Antimicrob Agents Chemother. 2011;55:2282–2289. doi: 10.1128/AAC.01190-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chen Y, Rinaldo C, Gupta P. A semiquantitative assay for CD8+ T-cell-mediated suppression of human immunodeficiency virus type 1 infection. Clin Diagn Lab Immunol. 1997;4:4–10. doi: 10.1128/cdli.4.1.4-10.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Majumder B, Venkatachari NJ, O'Leary S, Ayyavoo V. Infection with Vpr-positive human immunodeficiency virus type 1 impairs NK cell function indirectly through cytokine dysregulation of infected target cells. J Virol. 2008;82:7189–7200. doi: 10.1128/JVI.01979-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Huang XL, Fan Z, Borowski L, Rinaldo CR. Multiple T-cell responses to human immunodeficiency virus type 1 are enhanced by dendritic cells. Clin Vaccine Immunol. 2009;16:1504–1516. doi: 10.1128/CVI.00104-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Qin S, Sui Y, Soloff AC, et al. Chemokine and cytokine mediated loss of regulatory T cells in lymph nodes during pathogenic simian immunodeficiency virus infection. J Immunol. 2008;180:5530–5536. doi: 10.4049/jimmunol.180.8.5530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Niruthisard S, Roddy RE, Chutivongse S. The effects of frequent nonoxynol-9 use on the vaginal and cervical mucosa. Sex Transm Dis. 1991;18:176–179. doi: 10.1097/00007435-199107000-00010. [DOI] [PubMed] [Google Scholar]
- 41.Patton DL, Cosgrove Sweeney YT, Rabe LK, Hillier SL. Rectal applications of nonoxynol-9 cause tissue disruption in a monkey model. Sex Transm Dis. 2002;29:581–587. doi: 10.1097/00007435-200210000-00004. [DOI] [PubMed] [Google Scholar]
- 42.Roddy RE, Cordero M, Cordero C, Fortney JA. A dosing study of nonoxynol-9 and genital irritation. Int J STD AIDS. 1993;4:165–170. doi: 10.1177/095646249300400308. [DOI] [PubMed] [Google Scholar]
- 43.Venkataraman N, Cole AL, Ruchala P, et al. Reawakening retrocyclins: ancestral human defensins active against HIV-1. PLoS Biol. 2009;7:e95. doi: 10.1371/journal.pbio.1000095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Van Damme L, Govinden R, Mirembe FM, et al. Lack of effectiveness of cellulose sulfate gel for the prevention of vaginal HIV transmission. N Engl J Med. 2008;359:463–472. doi: 10.1056/NEJMoa0707957. [DOI] [PubMed] [Google Scholar]
- 45.Patton DL, Sweeney YT, Balkus JE, et al. Preclinical safety assessments of UC781 anti-human immunodeficiency virus topical microbicide formulations. Antimicrob Agents Chemother. 2007;51:1608–1615. doi: 10.1128/AAC.00984-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pantaleo G, Graziosi C, Fauci AS. New concepts in the immunopathogenesis of human immunodeficiency virus infection. N Engl J Med. 1993;328:327–335. doi: 10.1056/NEJM199302043280508. [DOI] [PubMed] [Google Scholar]
- 47.Poli G, Fauci AS. The effect of cytokines and pharmacologic agents on chronic HIV infection. AIDS Res Hum Retroviruses. 1992;8:191–197. doi: 10.1089/aid.1992.8.191. [DOI] [PubMed] [Google Scholar]
- 48.Cole AM, Patton DL, Rohan LC, et al. The formulated microbicide RC-101 was safe and antivirally active following intravaginal application in pigtailed macaques. PLoS One. 5:e15111. doi: 10.1371/journal.pone.0015111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cole AL, Yang OO, Warren AD, Waring AJ, Lehrer RI, Cole AM. HIV-1 adapts to a retrocyclin with cationic amino acid substitutions that reduce fusion efficiency of gp41. J Immunol. 2006;176:6900–6905. doi: 10.4049/jimmunol.176.11.6900. [DOI] [PubMed] [Google Scholar]
- 50.Nguyen TX, Cole AM, Lehrer RI. Evolution of primate theta-defensins: a serpentine path to a sweet tooth. Peptides. 2003;24:1647–1654. doi: 10.1016/j.peptides.2003.07.023. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Cytokines messages were determined by using realtime RTPCR as described in Methods.
Three days post-treatment with RC-101 degranulation of NK cells in PBMCs was determined by measuring expression of CD107-a in CD3-and C56+ gated NK cells by flow cytometric analysis. Percentage of CD3− CD56+ cells expressing CD107a in the presence 10, 20 and 40ug/ml of RC-101 were 49, 42 and 35 compared 45 with vehicle control.
The number of cell migrated to the bottom well in the presence of different concentration of RC-101 and media control are shown. Chemokine’s CXCL11 and CCL21 were included as a positive control.
Percentage of CFSE+ CD4+ and CFSE+CD8+ was determined after PBMC were exposed to various concentration of RC-101. PHA treatment was included as a positive control.