Abstract
In altricial species, like the human, caregiver presence is necessary for typical emotional development. Children who have been raised in institutional care early in life experience caregiver deprivation and are at significantly elevated risk for emotional difficulties. The current manuscript examines the non-human and human literatures on amygdala development following caregiver deprivation and presents an argument that in the absence of the species-expected caregiver presence, human amygdala development exhibits rapid development and perhaps premature engagement that results in some of the emotional phenotypes observed following early institutional care.
Keywords: human, amygdala, caregiver, deprivation, cortisol, HPA axis
The parent-child dyad is a very special and close relationship that is critical for normal human development. Attachment theory emphasizes the role of caregivers in providing stability, security, and safety, which greatly influences the emotional health of the offspring (Bowlby, 1963). Hofer’s (1994b) work has shown that the mere physical presence of the parent may act to regulate aspects of the infant rat pup’s behavior and development. That it is an external agent to the infant, yet so critical to the progression of normal development, qualifies caregiver presence as a species-expected environmental influence. Greenough et al (1987) have defined experience-expected developmental processes as those which “utilize the sort of environmental information that is ubiquitous and has been so throughout much of evolutionary history of the species” (p.540). Because of this ubiquity of the caregiving stimulus, postnatal maternal deprivation should present a serious challenge to the developing human. When these types of experiences are absent, the effects on the targeted system can be severe and long-lasting.
In the absence of this expected environmental influence, the developing system must adapt to the unexpected environment to promote survival independently. These adaptations may promote survival in the short term, but severely impair the individual’s ability to optimally cope with changing environmental demands that accompany changes in age. While caregiver-deprivation is rare in human populations, children in orphanages are raised en masse by staff members in an institutional setting, which does not provide a stable caregiver. Thus, in these environments, infants experience a species-atypical care environment. There are commonly-observed behavioral consequences of such caregiver-deprivation. Orphanage environments increase a child’s tendency to approach unfamiliar adults (Chisholm, 1998; Zeanah, Smyke, & Dumitrescu, 2002), decrease a child’s threshold for responding to sensory stimulation (Wilbarger, Gunnar, Schneider, & Pollak, 2010), and increase a child’s reactivity and sensitivity to emotional information (Fries & Pollak, 2004; Tottenham, et al., 2010). While these behaviors may be seen as examples of adaptation to orphanage life, children raised in this situation are also at elevated risk for externalizing and internalizing problems, such as anxiety and inattention (Casey, et al., 2009; Juffer & van Ijzendoorn, 2005; Kreppner, O’Connor, & Rutter, 2001; Zeanah, et al., 2009). In these circumstances, the developmental process is severely altered to meet the demands of the highly atypical early environment. The current paper discusses human brain development in the absence of caregiving, focusing on the amygdala, a neural region that is highly susceptible to early adversity and whose activity can greatly influence emotional behaviors. The hypothesis motivating this paper is that the absence of early caregiving prematurely engages the amygdala in learning about the environment, and in the long term, these activations result in hypervigilance and altered emotional responding.
This hypothesis is not a new one and has been proposed by several researchers with regard to rodent pup development (e.g., Jones, et al., 2009; Moriceau & Sullivan, 2005; Ono, et al., 2008). The current paper will examine this hypothesis with regard to human development. We reference the rodent literature heavily to discuss potential developmental mechanisms in the human for the reason that, although the relationship is ultimately more complex in the human, both (human and rodent) species are altricial, relying on the caregiver for survival, nutrients, autonomic regulation (Hofer, 1994a; Stone, Bonnet, & Hofer, 1976) and for learning about the mother to form attachments during the infant period (Moriceau, Roth, & Sullivan, 2010). This learning forms the basis for the relationship that ensures food, protection, and physiological regulation. It is possible therefore, that the ontogeny of the neural circuitry that guide proximity seeking remained phylogenetically intact during the postnatal period. Indeed, infants, whether human or rodents, can physically survive with some nominal degree of caregiving, as was seen in Spitz’s studies of children in institutional care (Spitz, 1945) and in Hofer’s work examining rat pup survival with access to discrete aspects of the mother (Hofer, 1994a), but as we will discuss, the consequences of caregiver deprivation on brain development can be significant.
The Amygdala
The amygdala has been implicated in learning about the emotional significance of stimuli (Davis & Whalen, 2001). Through a process of classical conditioning, that is pairing an initially neutral stimulus with an emotionally significant stimulus until the conditioned stimulus itself elicits the emotional response, an individual learns about the relative safety or danger of environmental stimuli. In adults, this process depends on the amygdala. Neuroimaging studies have confirmed that, like in rodents and non-human primates, the human amygdala responds to both negatively and positively valenced stimuli (Breiter, et al., 1996; Hennenlotter, et al., 2005; Somerville, Kim, Johnstone, Alexander, & Whalen, 2004), suggesting it supports learning about the emotional significance of the environment in general. Studies of fear conditioning confirm the role of the human amygdala in emotional learning, where forming an association between an emotional stimulus (e.g., shock) and a shock recruits amygdala activity (Delgado, Olsson, & Phelps, 2006; LaBar, Gatenby, Gore, LeDoux, & Phelps, 1998). As will be discussed, the process of emotional learning during early development may be fundamentally different.
Rodent Studies
Much of what is known about early postnatal amygdala development comes from rodent models. Studies of developing rat pups show that the amygdala is capable of demonstrating adult-like plasticity early in life (in that the adult, the amygdala becomes engaged during learning and thus, the animal shows avoidance learning), although under typical conditions the developing amygdala will not (Moriceau, Roth, Okotoghaide, & Sullivan, 2004). That is, early in life, the amygdala is fairly uninvolved in emotional learning (Moriceau, Wilson, Levine, & Sullivan, 2006), despite being anatomically developed, and hence avoidance learning is not typically observed in rodent pups. More commonly, preference learning is observed, where the rat pup will show a preference for stimuli that had been paired with an aversive stimulus (e.g., shock). This drastic difference between the behavior of the adult animal and the infant pup emphasizes the developmental differences in amygdala functional phenotype across this period.
There is an intimate association between the ontogenetic engagement of the amygdala and the activity of the HPA axis. A stressor sufficiently strong will elicit a full stress response (Kemeny, 2009), which includes activation of the hypothalamic-pituitary-adrenocortical (HPA) axis, a primary mammalian stress axis. The HPA axis response leads to peripheral (systemic) glucocorticoid (CORT) increases via hypothalamus, pituitary, and adrenal gland activity and increases in corticotropin releasing hormone in the brain (CRH, Makino, Gold, & Schulkin, 1994). Because CORT can pass the blood-brain barrier, it feeds back onto receptors in the brain including the amygdala. Numerous studies have demonstrated that stressors increase activity of the amygdala via elevations in CORT. For example, CORT administration acts to augment the CRH-enhanced startle response in rats (Lee, Schulkin, & Davis, 1994), a process dependent on the amygdala (Lang, Bradley, & Cuthbert, 1990). Cell bodies of the amygdala are rich with CORT receptors (Honkaniemi, et al., 1992), and stressors (Bonaz & Rivest, 1998), as well as direct administration of CORT, result in increased activity of the amygdala including new synthesis CRH receptors (Makino et al., 1994). The likelihood of stress impacting amygdala activity in a similar manner early in life is high given that stress hormones and CRH mRNA production in amygdala have a developmentally early onset, appearing as early as postnatal day 2 in the rat (Avishai-Eliner, Yi, & Baram, 1996; Vazquez, et al., 2006).
Despite its early structural development, rodent work suggests that under normal circumstances, that is when the mother is present, the amygdala remains functionally dormant until the pup begins to independently explore its environment. Pups stay in the nest with the mother for the first 10 days of life, and this period is characterized by very low levels of CORT production (Moriceau & Sullivan, 2006). This stress hyporesponsive period is a time in pup development when emotional learning differs dramatically from adult learning. In contrast to the adult animal, where pairing an aversive stimulus with a conditioned stimulus (CS) results in an aversion of the CS, these pairings result in a preference for the paired stimulus in the rat pup (Camp & Rudy, 1988; Moriceau, et al. 2010). The reason pups do not exhibit aversion learning lies in the fact that the amygdala plays relatively little role in mediating the association between the aversive stimulus and the CS, and in the absence of amygdala-mediated learning, other learning circuits (e.g., olfactory bulb, piriform cortex) are free to mediate the learning (which results in preference learning at this point in development) (Moriceau & Sullivan, 2004). The amygdala is not involved in learning yet because the low CORT levels keep dopamine levels at a minimum (Barr, et al., 2009), and dopamine increases are necessary for amygdala plasticity. Once the pup starts leaving the nest and the stress hyporesponsive period comes to an end, CORT production increases, learning becomes amygdala-mediated, and the animal begins to exhibit aversion learning rather than preference learning.
Critical to this whole process is the presence of the mother. The presence of the mother maintains low CORT levels (Stanton & Levine, 1988, 1990), and thus the amygdala does not participate in the emotional learning process (Moriceau & Sullivan, 2006). However, removal of the mother results in CORT increases, and amygdala-mediated aversion learning ensues. This same effect of premature amygdala involvement can be replicated by direct administration of CORT to the amygdala. It has been suggested that this pattern of emotional learning early in life facilitates caregiver identification and preference (Moriceau & Sullivan, 2005). Indeed in expected caregiving environments, the absence of amygdala-mediated learning may serve as a means of ensuring that infant pups form preferential attachments to caregivers. However, in conditions where the mother is absent and CORT significantly increases, a neural circuitry involving the amygdala becomes engaged prematurely, switching the animal’s state of one from preference learning to one where they display avoidance related behaviors towards learned stimuli.
Human studies
Structurally, the human amygdala seems to undergo rapid development early in life (Tottenham, Hare, & Casey, 2009). The basic neuroanatomical architecture of the human amygdala is present by birth (Humphrey, 1968; Ulfig, Setzer, & Bohl, 2003), and in girls, amygdala structural growth is complete by four years old (Giedd, et al., 1996). Longitudinal non-human research supports the notion that the primate amygdala is an early developing structure; based on repeated structural images, the most rapid rate of amygdala development occurs within the first 2 postnatal weeks, and this rate stabilizes early - around 8 months old (Payne, Machado, Bliwise, & Bachevalier, 2009). This rapid rate of change early in life may heighten the vulnerability of the amygdala to environmental influence early in life. It has been argued (Lupien, McEwen, Gunnar, & Heim, 2009) that the sensitive period of development for neural regions will center around its peak period of development (i.e., fastest rate of change), making periods of rapid development those times when that system is most vulnerable to environmental manipulation. For some regions, like the prefrontal cortex, this period will be quite late and extended; for the amygdala, it will be early and rapid. Given the early peak in amygdala development, we might predict that early-life is a sensitive period for the human amygdala, much in the same way that has been demonstrated in the rodent.
In humans, it is not yet known whether the mother exerts a similar buffering effect over amygdala reactivity, yet we may surmise that given the phylogenetic conservation of the amygdala and the similar need to form an attachment to a caregiver, that there exists at least a consistent process in the postnatal period of the human. Certainly, a mother’s presence buffers against elevations in CORT, particularly for highly fearful children (Nachmias, Gunnar, Mangelsdorf, Parritz, & Buss, 1996). Like the rat pup, a similar stress hyporesponsive period has been identified in human infants, where physical exams and inoculations do not result in CORT elevations during infancy (despite outward displays of distress and protest, Gunnar & Donzella, 2002). Basal levels are also typically low at birth and seem to reach a production low during the preschool period (Grunau, Weinberg, & Whitfield, 2004; Watamura, Donzella, Kertes, & Gunnar, 2004). Given the extended period of caregiver-dependence in the human species, we might expect this period of amygdala development to extend throughout childhood, which would afford the system several opportunities to learn about highly complex environments including caregiver preference.
Evidence from human neuroimaging with children and adolescents provides evidence of developmental change during childhood. The amygdala shows evidence of functionality in childhood that is followed by change across development (Baird, et al., 1999; Thomas, et al., 2001; Tottenham, et al., 2009), that continues until a reactivity peak in adolescence (Hare, et al., 2008). Thomas and colleagues (2001) showed that the amygdala response in children was lower for fear faces than it was for neutral faces, the opposite pattern that has been repeatedly identified in adults (reviewed in Davis & Whalen, 2001). While these data have been interpreted as indexing the ambiguity associated with neutral faces in childhood, these data might also be interpreted as indicating that the amygdala does not respond to negatively valenced faces, like fear, in the same fashion as adults. Indeed, several studies have shown that amygdala signal to emotional faces increases through childhood, reaching a peak in adolescence (Guyer, et al., 2008; Hare, et al., 2008; Killgore & Yurgelun-Todd, 2007; Monk, McClure, et al., 2003; Somerville, Hare, & Casey, in press). Thus, throughout childhood, when less is known about the relative safety or danger of different cues, the amygdala may to be playing an increasing role in assigning valence to stimuli through learning processes like those observed in fear conditioning paradigms. To date, little is known about amygdala involvement in fear conditioning during typical development. One study using an airpuff to the larynx showed adolescents recruited amygdala to cues associated with the unconditioned stimulus (Monk, Grillon, et al., 2003), suggesting that at least by adolescence, the amygdala is engaged by emotional learning in a similar fashion to adults.
Although neuroimaging based fear-conditioning studies have not yet been performed in children prior to adolescence, we may make some inferences about the normal ontogenetic engagement of the amygdala during emotional learning based on behavioral findings. In terms of general fear experience, according to self-reports, fears, nightmares, and worrying increase from the preschool period to middle childhood (Muris, Merckelbach, Gadet, & Moulaert, 2000; Muris, Merckelbach, Ollendick, King, & Bogie, 2001). Some naturalistic studies examining the development of learned fears showed that trauma experienced prior to age 7 years old (needing to be rescued while swimming) was not associated with fear (of swimming) when measured at age 18 (Poulton, Menzies, Craske, Langley, & Silva, 1999), and only late (after 18 years old)-onset dental fear, but not early-onset dental fear, was associated with aversive conditioning experiences at the dentist office (Poulton, Waldie, Thomson, & Locker, 2001). While these naturalistic studies are few in number, in conjunction with the neuroimaging studies discussed above, showing a developmental increase in amygdala recruitment to emotional stimuli, the findings are consistent with the hypothesis that in typical development, the amygdala is less engaged during emotional learning that during adulthood. Consistent with finding from these naturalistic studies, laboratory-based fear conditioning studies that have measured heart rate and galvanic skin responses with young children show that fear conditioning increases from the preschool period to middle childhood (Block, Sersen, & Wortis, 1970; Gao, Raine, Venables, Dawson, & Mednick, 2010). It will be important to further examine fear conditioning processes in the preschool and childhood period, particularly in the context of neuroimaging paradigms, to determine whether the hyporesponsivity of the stress system results in preference learning in humans as it does in the rodent. While this prolonged period of amygdala development allows the individual to learn about the environment, the changes in the amygdala prior to adolescence leaves it vulnerable to adverse early experiences that can have long lasting effects. We will present findings from human imaging and animal models of amygdala development under conditions of expected rearing environments and those when the expected parental input is absent.
Adversity and the Amygdala
Adverse events do not impact the whole brain in a uniform fashion, but instead the effects are region specific, exhibiting some of the largest effects in the amygdala. In adult animals, stress or administration of stress hormones increases the growth and activity of amygdala neurons (Armony, Corbo, Clement, & Brunet, 2005; Liberzon, et al., 1999; Rauch, et al., 2000). Cells in the amygdala participate in the earliest reaction to environmental stressors, often initiating the HPA cascade. They are quickly activated by stress and express immediate-early genes (Honkaniemi, et al., 1992). Stressful events produce elevations in CRH that occur first in the amygdala and are observed only afterwards in other neural regions like the hippocampus (reviewed in Baram & Hatalski, 1998). Indeed, both psychosocial stress and administration of CORT enhance expression of CRH in the amygdala (reviewed in McEwen, 2003; Schulkin, Gold, & McEwen, 1998). Numerous neuroimaging studies have demonstrated that the amygdala is altered structurally and functionally by psychosocial stress in humans. Studies that have examined the amygdala in individuals who experienced traumatic events (e.g., combat, physical assault) show that in adults the amygdala is both smaller (Driessen, et al., 2000; Schmahl, Vermetten, Elzinga, & Bremner, 2003) and more reactive to emotional stimuli (Armony, et al., 2005; Liberzon, et al., 1999; Rauch, et al., 2000; Shin, et al., 2004; reviewed in Shin, Rauch, & Pitman, 2006).
In the developing animal, adversity can also impact amygdala activity, perhaps even more so than adversity that occurs later in life (reviewed in Tottenham & Sheridan, 2010). This position is supported by rat models of early stress, which find that the amount of CRH required to produce amygdala-originating seizures in developing animals is 200 times smaller than required for adult animals (reviewed in Baram & Hatalski, 1998). Neonatal separation stress resulted in an increase in CRF containing neurons in the amygdala in juvenile rodents (Becker, Abraham, Kindler, Helmeke, & Braun, 2007). Adverse and/or absent caregiving is a stressor for the developing animal, and studies that have examined the timing of exposure suggest that early adverse caregiving has effects on brain development that last throughout the lifespan (Matsumoto, Yoshioka, & Togashi, 2009; McEwen, 2008). For example, poor caregiving in rodents results in increased anxiety- and aggressive-behaviors in adulthood, which is associated with accelerated amygdala development (Kikusui & Mori, 2009), early myelination (Ono, et al., 2008), more CRH-containing neurons (Becker, et al., 2007), and sensitization of the adult amygdala (Salzberg, et al., 2007). A linear dose-response association has been identified for the quality of maternal care (increased arched-back nursing and licking/grooming) and amygdala phenotype (increased benzodiazepine receptor binding)(Caldji, et al., 1998). Although the amygdala is functionally dormant in the rat neonatal period, significantly stressful events can precociously activate the amygdala (Moriceau, et al., 2004), perhaps because of the early presence of CRH mRNA (present in postnatal day 2 )(Avishai-Eliner, et al., 1996; Fenoglio, Brunson, Avishai-Eliner, Chen, & Baram, 2004; Vazquez, et al., 2006), and these effects of stress on the amygdala can be observed in adulthood, as has been shown by alterations at the level of the receptor (benzodiazepine and CRH receptors), facilitated amygdala kindling, impaired emotional learning, and emotional responses to future stressors (reviewed in Caldji, Francis, Sharma, Plotsky, & Meaney, 2000; Caldji, et al., 1998; Jones, et al., 2009; Kosten, Lee, & Kim, 2006; Plotsky, et al., 2005; Sevelinges, et al., 2007; Sevelinges, Sullivan, Messaoudi, & Mouly, 2008). Non-human primate work shows that maternal deprivation stress also influences primate amygdala development, including changes in amygdala gene expression and associated emotional behaviors (Sabatini, et al., 2007) and has more devastating effects the earlier in life it occurs. This long-lasting effect may in part be related to the resistance amygdalar cells show to recovery, unlike stress-induced changes in other regions of the brain, like the hippocampus (Vyas, Pillai, & Chattarji, 2004).
Caregiving adversity and the HPA Axis
Poor or absent caregiving has similar effects on the HPA axis of developing humans. Although we have been focusing on caregiver deprivation, which might impact an experience-expected developmental processes, there may be some important insights from examining the literature on differing qualities of attachments, which may instead influence experience-dependent processes (Smyke, Zeanah, Fox, Nelson, & Guthrie, 2010). In the sustained presence of a caregiver, an attachment bond typically forms and infant behavior is organized to maintain proximity with the attachment figure (Ainsworth, 1969; Cairns, 1966), regardless of the quality of care provided by the caregiver (Kovach & Hess, 1963; Raineki, Moriceau, & Sullivan; Reichmann-Decker, DePrince, & McIntosh, 2009). In contrast to low quality caregiving, orphanage care is institutional care, which is characterized by staff members who rotate shifts and are responsible for the survival of an extraordinarily high number of infants. Not surprisingly, children developing in these environments may show highly atypical attachment behaviors (O’Connor, Marvin, Rutter, Olrick, & Britner, 2003; Smyke, et al., 2010) or what some have termed reactive attachments (Zeanah, Smyke, Koga, & Carlson, 2005). That a bond forms when a sustained caregiver is present may signify important differences in terms of stress physiology between children who receive poor quality caregiving and those who experience maternal deprivation. In the first case, an attachment system becomes engaged and in the second, we hypothesize that in place of the typical attachment system, a stress-mediating system, including the amygdala, becomes prematurely engaged. We present first a discussion of poor quality caregiving and the HPA axis, followed by a discussion of the role of caregiver deprivation on HPA axis function.
Low quality caregiving and the HPA axis
Relative to children in secure attachment relationships, children classified as having insecure attachment relationships (that is, those relationships typically characterized by low sensitive caregiving) in infancy are at greater risk for atypically high elevations in cortisol, especially in combination with fearful temperaments (Nachmias, et al., 1996). Maternal stress and/or maternal depression early in life has been associated with elevated cortisol production in preschool (Essex, Klein, & Kalin, 2002) as well as in 8-year old children (Ashman, Dawson, Panagiotides, Yamada, & Wilkinson, 2002). CORT alterations may not always be evidenced as elevations, as Bruce and colleagues (2009) found that children in the United States foster care system showed significantly decreased morning cortisol production and these decreases were more likely in children who experienced caregiver neglect, as opposed to emotional maltreatment. Similarly, CORT baseline and reactivity levels were atypically low in preschool children whose mothers were clinically depressed (Fernald, Burke, & Gunnar, 2008) and in children who had experienced maltreatment from caregivers (Cicchetti, Rogosch, Gunnar, & Toth; Hart, Gunnar, & Cicchetti, 2008). While high levels of CORT production are typically associated with HPA dysfunction, lower CORT levels are not uncommon in studies of samples with histories of adversity (Gunnar & Vazquez, 2001). Because of the complex dynamics of the HPA axis, atypically low CORT levels are usually interpreted as signs of dysregulation of the axis following chronic elevations in CORT and may in part reflect the nature of the caregiving adversity (Cicchetti & Rogosch, 2001).
Caregiver deprivation and the HPA axis
Unlike children receiving low quality caregiving, children raised in institutional care experience a postnatal environment that is void of a sustained caregiver presence. Gunnar and colleagues (Gunnar, Morison, Chisholm, & Schuder, 2001; Kertes, Gunnar, Madsen, & Long, 2008) found in a sample of previously-institutionalized children adopted from Romania, that those who spent most of their first year of life in an orphanage showed cortisol production that was at levels 2 standard deviations above typically reared children. Fries and colleagues (2008) found that following interaction with the adoptive parent, previously-institutionalized children exhibited significant CORT elevations as compared to children without early caregiver deprivation.
Early alterations to HPA activity provide a cellular environment that can prematurely activate the amygdala, which is rich with stress related receptors. As animal models have shown, this early sensitivity of the amygdala to adversity makes it vulnerable to being prematurely activated under conditions when species-expected environments are not available. We have been studying a group of children who were raised in orphanages during infancy and then were subsequently removed via international adoption in order to test whether similar mechanisms operate in the developing human. Orphanage care is sparse and unstable (Gunnar, Bruce, & Grotevant, 2000) and fails to mimic the caregiving that most children receive during infancy. Because children adopted out of orphanages are placed into stable caregiving arrangements, the timing of caregiver absence is restricted to the early postnatal period. We found that amygdala volumes were enlarged in this group of children even though measurements were taken years after the initial adversity (Figure 1, Tottenham, et al., 2010). Notably, the older children were when adopted, the larger the amygdala volumes. This dose-response association suggests that longer stays in institutional care are associated with severity of impact on amygdala development. Although these data contrast with studies of older individuals who have experienced stress, who often exhibit smaller amygdala volumes (Driessen, et al., 2000; Schmahl, et al., 2003), the developmental data have been independently replicated with a separate sample of children adopted from orphanage care (Mehta, et al., 2009), and are consistent with studies finding amygdala growth at the cellular level following chronic stress administration (Vyas, Mitra, Shankaranarayana Rao, & Chattarji, 2002; Vyas, et al., 2004). The contrast between the human adult data and the developmental data provides a suggestion that the timing of adverse experiences and the timing of amygdala measurement are important factors in assessing the impact of environmental exposure on amygdala phenotype (McEwen, 2003; Tottenham & Sheridan, 2010). As a group, previously-institutionalized children are more likely to exhibit behavioral phenotypes that are typically associated with increased vigilance, such as internalizing problems and heightened anxiety (Figure 2)(Casey, et al., 2009), and these anxious phenotypes are positively associated with amygdala volume (Tottenham, et al., 2010). These volumetric data are consistent with the hypothesis that the absence of early caregiving alters emotional behavior by acting on amygdala development.
Figure 1.
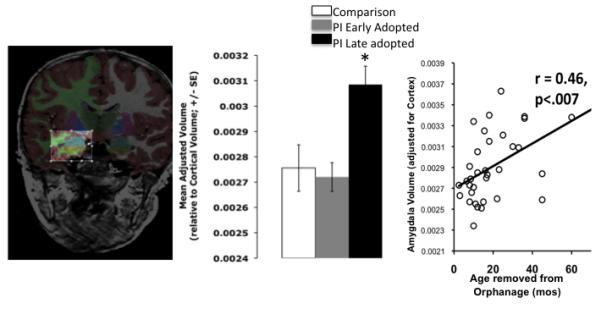
Amygdala Volume following Early Caregiver Deprivation. (Left) Amygdala segmentation highlighted in blue. (Middle) Post-institutionalized (PI) children who had been adopted later in life showed significant increases in amygdala volume. (Right) Longer periods of orphanage care were associated with larger amygdala volumes. PI = previously-institutionalized. Adapted from Tottenham et al., 2010.
Fig 2.
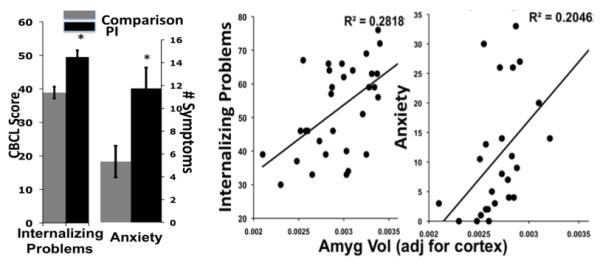
(left) PI children show more internalizing problems as measured by the Child Behavior Checklist (CBCL) & anxiety symptoms as measured by the SCARED-(Right 2 panels) Amygdala volume is positively associated with internalizing problems and continuous anxiety symptoms. PI = previously-institutionalized. Adapted from Casey et al., 2009 & Tottenham et al., 2010.
Along with amygdala enlargement, previously-institutionalized children show evidence of greater sensitivity to negatively valenced faces. We tested children on the Emotional Face Go/Nogo, which assesses the degree to which emotional faces interfere with the successful execution of a behavioral response. We showed that early caregiver deprivation was associated with a greater number of errors during conditions when negative faces were present (Figure 3, left), but no effect for positively valenced faces (Tottenham, et al., 2010). These data show that the behavior of previously-institutionalized children was more affected by negative emotional information. Amygdala activity was also elevated for previously-institutionalized children for negatively valenced faces (i.e., fear) relative to the typically-reared children (Figure 3, right, Tottenham, et al., in press). The pattern of amygdala response to fearful versus neutral faces more closely resembles that of adult subjects than typically-raised children of the same age, consistent with the hypothesis that early caregiver deprivation results in premature functional developmental of the amygdala. There was no between-group difference in amygdala response to neutral faces, and similar to the findings from the Thomas et al. (2001) study, the comparison group of children showed significantly greater amygdala activation to neutral faces than for fear faces. Taken together, these behavioral and neuroimaging data support the theoretical framework that children who lack stable caregiving early in life are at risk for atypical - perhaps earlier - development of amygdala and associated function. This early engagement of the amygdala may, in part, be evidence of the system recruiting stress circuitry for short-term survival, with the system adapting to the constraints afforded by the environment. These adaptations may serve as an immediate resiliance factor for the child (Masten & Obradovic, 2008), but in the long-term may have detrimental consequences for mental health (e.g., anxiety).
Fig 3.
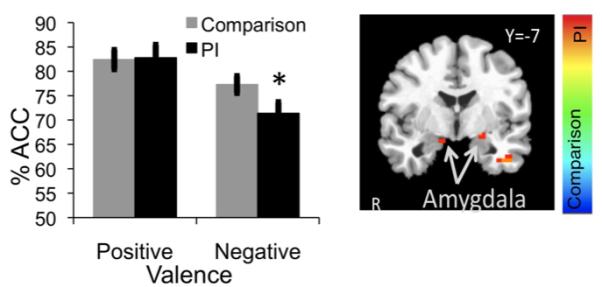
(Left) During performance on the Emotional Face Go/Nogo task, behavioral performance is more impaired by negatively valenced faces in the PI group. (Right) Direct group contrast show that PI children (orange) show heightened amygdala activity in response to fear faces. PI = previously-institutionalized. Adopted from Tottenham et al., 2010 and Tottenham et al., in press
While elevated amygdala during childhood seems to confer hyper-reponsivity to emotional information and anxiety-like symptoms, as children typically become more independent from caregivers, it is advantageous for the amygdala to become engaged in the learning process. In the rat pup, amygdala-mediated learning coincides with the end of the stress hyporesponsive period, when rat pups begin leaving the nest and exploring independently (Moriceau & Sullivan, 2005). Although amygdala-mediating learning is advantageous for navigating emotional environments independently (Delgado, Jou, Ledoux, & Phelps, 2009), the system must guard against overactivity of the amygdala. In mature animals, the activity of the amygdala is regulated by its connections with regions within the prefrontal cortex (PFC). Regulation of the amygdala via PFC-amygdala connections may be especially important for predicting outcome in children who experience early-adversity. Thus, individual differences in long-term resiliance may in part depend on the development of regulatory processes (Masten & Obradovic, 2008) emerging from functional development of the PFC.
Regulation of the amygdala
A growing human neuroimaging literature combined with a large non-human animal literature implicates the amygdala and its connections with the ventral PFC (which includes the orbitofrontal cortex and ventral anterior cingulate cortex) as biological substrates that underlie difficulties in emotional reactivity and regulation (reviewed in Monk, 2008). This is important because a failure to effectively recruit this circuitry has been implicated in a wide range of mental illnesses (e.g., anxiety, depression, schizophrenia, bipolar disorder, sociopathy, personality disorder). The amygdala has direct structural and functional connections with the ventral PFC (Amaral & Carmichael, 1992; Ghashghaei, Hilgetag, & Barbas, 2007; Milad & Quirk, 2002). Studies of structural and functional connectivity in humans have shown that greater coupling between the two regions is associated with better regulation of emotion (Banks, Eddy, Angstadt, Nathan, & Phan, 2007). The activity of these structures represents the simplest form of emotional regulation, which is extinction learning. Extinction studies in adult animals support this position and show that inhibition of the fear response to a conditioned stimulus requires the ventral PFC (Phelps, Delgado, Nearing, & LeDoux, 2004; Quirk, Russo, Barron, & Lebron, 2000). The ventral PFC can modulate emotional responses through GABAergic inhibitory projections to the amygdala, perhaps via afferents to the intercalated cells that inhibit amygdala activity (Akirav, Raizel, & Maroun, 2006; Harris & Westbrook, 1998). Lesioning or inactivation of the ventral PFC in adults with protein synthesis blockers (e.g., anisomycin) interferes with extinction learning (reviewed in Quirk & Mueller, 2008) - the animal will continue to exhibit fear responses when they are no longer needed. Consistent with a theorized regulatory role of the ventral PFC over the amygdala (reviewed in Quirk & Beer, 2006), the human amygdala response to emotional stimuli, such as faces, habituates over the course of the fMRI scanning session (Hare, et al., 2008), presumably because the emotional face is a conditioned stimulus that, in the context of the experimental session, is not predictive of any threat. This habituation has been likened to an extinction process, whereby ventral PFC engagement results in a progressive reduction amygdala activity over the course of the session. Amygdala habituation is associated with stronger negative connectivity between amygdala and ventral PFC, and adolescents and adults who show more amygdala habituation are less anxious (Hare, et al., 2008). The habituation seems to be supported by strong connectivity between amygdala and ventral PFC (Hare, et al., 2008; Pezawas, et al., 2005).
The development of amygdala-ventral PFC connectivity is not well characterized. Animal models suggest that emotional processes involving the amygdala and ventral PFC operate differently across development (Kim, Hamlin, & Richardson, 2009). In the rodent, the PFC develops late in life, not showing its volumetric peak until the adolescent period (van Eden & Uylings, 1985). Connectivity between the PFC and the amygdala does not emerge until the adolescent period (Cunningham, Bhattacharyya, & Benes, 2002), when amygdala projections to PFC first emerge (Verwer, Van Vulpen, & Van Uum, 1996). Consistent with the notion of a late developing ventral PFC, rodent studies show that, unlike in adulthood, pre-adolescent extinction learning does not rely on the PFC, where PFC inactivation does not impair extinction learning. Consequently, pre-adolescent extinction differs in behavioral phenotype (e.g., renewal and reinstatement) from the extinction observed in mature animals (Kim, et al., 2009; Kim & Richardson, 2009). Currently, much less is known about human amygdala-PFC connectivity.
Even less is known about the development of the PFC following early adversity, although some rodent studies show that early life adversity alters PFC development both structurally (higher synaptic densities compared to controls - Ovtscharoff & Braun, 2001) and functionally (reduced tyrosine hydroxylase-positive fiber innervation - Braun, Lange, Metzger, & Poeggel, 1999). How early adversity alters amygdala-PFC connections is unknown at this point, but we can speculate that the stress-induced changes in the amygdala may over the course of development, weaken amygdala-PFC connections and communication. Our findings with previously-institutionalized children showed group differences in ventral PFC activity in response to fearful faces during the Emotional Face Go/Nogo (Tottenham, et al., in press). Specifically, typically-reared children showed modulation of the ventral PFC in response to fearful faces by decreasing activity, whereas the fearful faces did not alter PFC activity in previously-institutionalized children. Decreased coupling between the amygdala and PFC has routinely been observed in individuals with poor emotion regulation (Hare, et al., 2008; Marsh, et al., 2008; Shin, et al., 2006). These data with previously-institutionalized children are consistent with poor communication between amygdala and PFC in the previously-institutionalized group, and diffusion tensor imaging has identified reduced white matter between amygdala and prefrontal cortex in previously-institutionalized children (Govindan, Behen, Helder, Makki, & Chugani, 2009). If this interpretation of the functional imaging data is correct, then it suggests that there may be developmental/consequential effects of early caregiver deprivation on the amygdala’s emerging connectivity with those regions that regulate its activity.
Much behavioral work has demonstrated that early caregiver deprivation results in significant emotional difficulty later in life. The replicability of these findings strongly suggests that the presence of caregivers is a species-expected environment, as described by Greenough et al. (1987), whose absence can cause significant detriment to the typical developmental process. Studies that use non-human animals have provided important clues regarding the potential neurobiological mechanisms that might mediate the associations between caregiver deprivation and altered emotional behavior. In the current manuscript, we used these findings to generate hypotheses about the neurobiological development of humans following caregiver deprivation. Future studies examining the development of human prefrontal connectivity with the amygdala will provide insight into individual differences in resilience (Masten & Obradovic, 2008), particularly during adolescence - a time when we expect prefrontal cortex connections to be rapidly forming and therefore, highly amenable to environmental-resilience factors (e.g., positive family environments). While the human neural data are currently sparse, they are mounting and support the hypothesis that caregiver deprivation alters emotional behavior by taking advantage of the readiness of the developing amygdala to respond to environmental adversity.
Acknowledgements
The project described was supported by grant number R01MH091864 from the National Institute of Mental Health. The content is solely the responsibility of the author and does not necessarily represent the official views of the National Institute of Mental Health or the National Institutes of Health.
References
- Ainsworth MD. Object relations, dependency, and attachment: a theoretical review of the infant-mother relationship. Child Dev. 1969;40(4):969–1025. [PubMed] [Google Scholar]
- Akirav I, Raizel H, Maroun M. Enhancement of conditioned fear extinction by infusion of the GABA(A) agonist muscimol into the rat prefrontal cortex and amygdala. Eur J Neurosci. 2006;23(3):758–764. doi: 10.1111/j.1460-9568.2006.04603.x. [DOI] [PubMed] [Google Scholar]
- Amaral DG, J.L. P, A P, Carmichael ST. Anatomical organization of the primate amygdaloid complex. In: Aggleton JP, editor. The Amygdala: Neurobiological Aspects of Emotion, Memory, and Mental Dysfunction. Wiley; New York: 1992. pp. 1–66. [Google Scholar]
- Armony JL, Corbo V, Clement MH, Brunet A. Amygdala response in patients with acute PTSD to masked and unmasked emotional facial expressions. Am J Psychiatry. 2005;162(10):1961–1963. doi: 10.1176/appi.ajp.162.10.1961. [DOI] [PubMed] [Google Scholar]
- Ashman SB, Dawson G, Panagiotides H, Yamada E, Wilkinson CW. Stress hormone levels of children of depressed mothers. Dev Psychopathol. 2002;14(2):333–349. doi: 10.1017/s0954579402002080. [DOI] [PubMed] [Google Scholar]
- Avishai-Eliner S, Yi SJ, Baram TZ. Developmental profile of messenger RNA for the corticotropin-releasing hormone receptor in the rat limbic system. Developmental Brain Research. 1996;91(2):159–163. doi: 10.1016/0165-3806(95)00158-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baird AA, Gruber SA, Fein DA, Maas LC, Steingard RJ, Renshaw PF, et al. Functional magnetic resonance imaging of facial affect recognition in children and adolescents. J Am Acad Child Adolesc Psychiatry. 1999;38(2):195–199. doi: 10.1097/00004583-199902000-00019. [DOI] [PubMed] [Google Scholar]
- Banks SJ, Eddy KT, Angstadt M, Nathan PJ, Phan KL. Amygdala-frontal connectivity during emotion regulation. Soc Cogn Affect Neurosci. 2007;2(4):303–312. doi: 10.1093/scan/nsm029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baram TZ, Hatalski CG. Neuropeptide-mediated excitability: a key triggering mechanism for seizure generation in the developing brain. Trends Neurosci. 1998;21(11):471–476. doi: 10.1016/s0166-2236(98)01275-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barr GA, Moriceau S, Shionoya K, Muzny K, Gao P, Wang S, et al. Transitions in infant learning are modulated by dopamine in the amygdala. Nat Neurosci. 2009;12(11):1367–1369. doi: 10.1038/nn.2403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker K, Abraham A, Kindler J, Helmeke C, Braun K. Exposure to neonatal separation stress alters exploratory behavior and corticotropin releasing factor expression in neurons in the amygdala and hippocampus. Developmental Neurobiology. 2007;67(5):617–629. doi: 10.1002/dneu.20372. [DOI] [PubMed] [Google Scholar]
- Block JD, Sersen EA, Wortis J. Cardiac classical conditioning and reversal in the mongoloid, encephalopathic, and normal child. Child Development. 1970;41:771–785. [Google Scholar]
- Bonaz B, Rivest S. Effect of a chronic stress on CRF neuronal activity and expression of its type 1 receptor in the rat brain. Am J Physiol. 1998;275(5 Pt 2):R1438–1449. doi: 10.1152/ajpregu.1998.275.5.R1438. [DOI] [PubMed] [Google Scholar]
- Bowlby J. Pathological Mourning and Childhood Mourning. Journal of the American Psychoanalytic Association. 1963;11(3):500–541. doi: 10.1177/000306516301100303. [DOI] [PubMed] [Google Scholar]
- Braun K, Lange E, Metzger M, Poeggel G. Maternal separation followed by early social deprivation affects the development of monoaminergic fiber systems in the medial prefrontal cortex of Octodon degus. Neuroscience. 1999;95(1):309–318. doi: 10.1016/s0306-4522(99)00420-0. [DOI] [PubMed] [Google Scholar]
- Breiter H, Etcoff NL, Whalen PJ, Kennedy WA, Rauch S, Buckner RL, et al. Response and habituation of the human amygdala during visual processing of facial expression. Neuron. 1996;17:875–887. doi: 10.1016/s0896-6273(00)80219-6. [DOI] [PubMed] [Google Scholar]
- Bruce J, Fisher PA, Pears KC, Levine S. Morning cortisol Levels in preschool-aged foster children: differential effects of maltreatment type. Developmental Psychobiology. 2009;51(1):14–23. doi: 10.1002/dev.20333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cairns RB. Attachment behavior of mammals. Psychol Rev. 1966;73(5):409–426. doi: 10.1037/h0023691. [DOI] [PubMed] [Google Scholar]
- Caldji C, Francis D, Sharma S, Plotsky PM, Meaney MJ. The Effects of Early Rearing Environment on the Development of GABA-A and Central Benzodiazepine Receptor Levels and Novelty-Induced Fearfulness in the Rat. Neuropsychopharmacology. 2000;22(3):219–229. doi: 10.1016/S0893-133X(99)00110-4. [DOI] [PubMed] [Google Scholar]
- Caldji C, Tannenbaum B, Sharma S, Francis D, Plotsky PM, Meaney MJ. Maternal care during infancy regulates the development of neural systems mediating the expression of fearfulness in the rat. Proc Natl Acad Sci U S A. 1998;95(9):5335–5340. doi: 10.1073/pnas.95.9.5335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camp LL, Rudy JW. Changes in the categorization of appetitive and aversive events during postnatal development of the rat. Dev Psychobiol. 1988;21(1):25–42. doi: 10.1002/dev.420210103. [DOI] [PubMed] [Google Scholar]
- Casey BJ, Glatt CE, Tottenham N, Soliman F, Bath K, Amso D, et al. Brain-derived neurotrophic factor as a model system for examining gene by environment interactions across development. Neuroscience. 2009;164(1):108–120. doi: 10.1016/j.neuroscience.2009.03.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chisholm K. A three year follow-up of attachment and indiscriminate friendliness in children adopted from Romanian orphanages. Child Dev. 1998;69(4):1092–1106. [PubMed] [Google Scholar]
- Cicchetti D, Rogosch FA. Diverse patterns of neuroendocrine activity in maltreated children. Dev Psychopathol. 2001;13(3):677–693. doi: 10.1017/s0954579401003145. [DOI] [PubMed] [Google Scholar]
- Cicchetti D, Rogosch FA, Gunnar MR, Toth SL. The differential impacts of early physical and sexual abuse and internalizing problems on daytime cortisol rhythm in school-aged children. Child Dev. 81(1):252–269. doi: 10.1111/j.1467-8624.2009.01393.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham MG, Bhattacharyya S, Benes FM. Amygdalo-cortical sprouting continues into early adulthood: implications for the development of normal and abnormal function during adolescence. J Comp Neurol. 2002;453(2):116–130. doi: 10.1002/cne.10376. [DOI] [PubMed] [Google Scholar]
- Davis M, Whalen PJ. The amygdala: vigilance and emotion. Molecular Psychiatry. 2001;6(1):13–34. doi: 10.1038/sj.mp.4000812. [DOI] [PubMed] [Google Scholar]
- Delgado MR, Jou RL, Ledoux JE, Phelps EA. Avoiding negative outcomes: tracking the mechanisms of avoidance learning in humans during fear conditioning. Front Behav Neurosci. 2009;3:33. doi: 10.3389/neuro.08.033.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delgado MR, Olsson A, Phelps EA. Extending animal models of fear conditioning to humans. Biol Psychol. 2006;73(1):39–48. doi: 10.1016/j.biopsycho.2006.01.006. [DOI] [PubMed] [Google Scholar]
- Driessen M, Herrmann J, Stahl K, Zwaan M, Meier S, Hill A, et al. Magnetic Resonance Imaging Volumes of the Hippocampus and the Amygdala in Women With Borderline Personality Disorder and Early Traumatization. Archives of General Psychiatry. 2000;57:1115–1122. doi: 10.1001/archpsyc.57.12.1115. [DOI] [PubMed] [Google Scholar]
- Essex MJ, Klein MH, Kalin N. Maternal stress beginning in infancy may sensitize children to later stress exposure: effects on cortisol and behavior. Biological Psychiatry. 2002;62(8):776–784. doi: 10.1016/s0006-3223(02)01553-6. [DOI] [PubMed] [Google Scholar]
- Fenoglio KA, Brunson KL, Avishai-Eliner S, Chen Y, Baram TZ. Region-specific onset of handling-induced changes in corticotropin-releasing factor and glucocorticoid receptor expression. Endocrinology. 2004;145(6):2702–2706. doi: 10.1210/en.2004-0111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernald LC, Burke HM, Gunnar MR. Salivary cortisol levels in children of low-income women with high depressive symptomatology. Dev Psychopathol. 2008;20(2):423–436. doi: 10.1017/S0954579408000205. [DOI] [PubMed] [Google Scholar]
- Fries AB, Pollak SD. Emotion understanding in postinstitutionalized Eastern European children. Dev Psychopathol. 2004;16(2):355–369. doi: 10.1017/S0954579404044554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fries AB, Shirtcliff EA, Pollak SD. Neuroendocrine dysregulation following early social deprivation in children. Dev Psychobiol. 2008;50(6):588–599. doi: 10.1002/dev.20319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Y, Raine A, Venables PH, Dawson ME, Mednick SA. The development of skin conductance fear conditioning in children from ages 3 to 8 years. Dev Sci. 2010;13(1):201–212. doi: 10.1111/j.1467-7687.2009.00874.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghashghaei HT, Hilgetag CC, Barbas H. Sequence of information processing for emotions based on the anatomic dialogue between prefrontal cortex and amygdala. Neuroimage. 2007;34(3):905–923. doi: 10.1016/j.neuroimage.2006.09.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giedd JN, Vaituzis AC, Hamburger SD, Lange N, Rajapakse JC, Kaysen D, et al. Quantitative MRI of the temporal lobe, amygdala, and hippocampus in normal human development: ages 4-18 years. Journal of Comparative Neurology. 1996;366(2):223–230. doi: 10.1002/(SICI)1096-9861(19960304)366:2<223::AID-CNE3>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Govindan RM, Behen ME, Helder E, Makki MI, Chugani HT. Altered Water Diffusivity in Cortical Association Tracts in Children with Early Deprivation Identified with Tract-Based Spatial Statistics (TBSS) Cereb Cortex. 2009 doi: 10.1093/cercor/bhp122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenough WT, Black JE, Wallace CS. Experience and brain development. Child Dev. 1987;58(3):539–559. [PubMed] [Google Scholar]
- Grunau RE, Weinberg J, Whitfield MF. Neonatal procedural pain and preterm infant cortisol response to novelty at 8 months. Pediatrics. 2004;114(1):e77–84. doi: 10.1542/peds.114.1.e77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunnar MR, Bruce J, Grotevant HD. International adoption of institutionally reared children: research and policy. Dev Psychopathol. 2000;12(4):677–693. doi: 10.1017/s0954579400004077. [DOI] [PubMed] [Google Scholar]
- Gunnar MR, Donzella B. Social regulation of the cortisol levels in early human development. Psychoneuroendocrinology. 2002;27(1-2):199–220. doi: 10.1016/s0306-4530(01)00045-2. [DOI] [PubMed] [Google Scholar]
- Gunnar MR, Morison SJ, Chisholm K, Schuder M. Salivary Cortisol Levels in Children Adopted from Romanian Orphanages. Development and Psychopathology. 2001;13(3):611–628. doi: 10.1017/s095457940100311x. [DOI] [PubMed] [Google Scholar]
- Gunnar MR, Vazquez DM. Low cortisol and a flattening of expected daytime rhythm: potential indices of risk in human development. Dev Psychopathol. 2001;13(3):515–538. doi: 10.1017/s0954579401003066. [DOI] [PubMed] [Google Scholar]
- Guyer AE, Monk CS, McClure-Tone EB, Nelson EE, Roberson-Nay R, Adler AD, et al. A developmental examination of amygdala response to facial expressions. J Cogn Neurosci. 2008;20(9):1565–1582. doi: 10.1162/jocn.2008.20114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hare TA, Tottenham N, Galvan A, Voss HU, Glover GH, Casey BJ. Biological substrates of emotional reactivity and regulation in adolescence during an emotional go-nogo task. Biol Psychiatry. 2008;63(10):927–934. doi: 10.1016/j.biopsych.2008.03.015015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris JA, Westbrook RF. Benzodiazepine-induced amnesia in rats: reinstatement of conditioned performance by noxious stimulation on test. Behav Neurosci. 1998;112(1):183–192. doi: 10.1037//0735-7044.112.1.183. [DOI] [PubMed] [Google Scholar]
- Hart J, Gunnar M, Cicchetti D. Salivary cortisol in maltreated children: Evidence of relations between neuroendocrine activity and social competence. Development and Psychopathology. 2008;7:11–26. [Google Scholar]
- Hennenlotter A, Schroeder U, Erhard P, Castrop F, Haslinger B, Stoecker D, et al. A common neural basis for receptive and expressive communication of pleasant facial affect. Neuroimage. 2005;26(2):581–591. doi: 10.1016/j.neuroimage.2005.01.057. [DOI] [PubMed] [Google Scholar]
- Hofer MA. Early relationships as regulators of infant physiology and behavior. Acta Pediatrica. 1994a;83(s397):9–18. doi: 10.1111/j.1651-2227.1994.tb13260.x. [DOI] [PubMed] [Google Scholar]
- Hofer MA. Hidden regulators in attachment, separation, and loss. Monogr Soc Res Child Dev. 1994b;59(2-3):192–207. [PubMed] [Google Scholar]
- Honkaniemi J, Kainu T, Ceccatelli S, Rechardt L, Hokfelt T, Pelto-Huikko M. Fos and jun in rat central amygdaloid nucleus and paraventricular nucleus after stress. NeuroReport. 1992;3(10):849–852. doi: 10.1097/00001756-199210000-00007. [DOI] [PubMed] [Google Scholar]
- Humphrey T. The development of the human amygdala during early embryonic life. The Journal of Comparative Neurology. 1968;132(1):135–165. doi: 10.1002/cne.901320108. [DOI] [PubMed] [Google Scholar]
- Jones NC, Kumar G, O’Brien TJ, Morris MJ, Rees SM, Salzberg MR. Anxiolytic effects of rapid amygdala kindling, and the influence of early life experience in rats. Behav Brain Res. 2009;203(1):81–87. doi: 10.1016/j.bbr.2009.04.023. [DOI] [PubMed] [Google Scholar]
- Juffer F, van Ijzendoorn MH. Behavior problems and mental health referrals of international adoptees: a meta-analysis. Journal of the American Medical Association. 2005;293(20):2501–2515. doi: 10.1001/jama.293.20.2501. [DOI] [PubMed] [Google Scholar]
- Kemeny ME. Psychobiological responses to social threat: evolution of a psychological model in psychoneuroimmunology. Brain Behav Immun. 2009;23(1):1–9. doi: 10.1016/j.bbi.2008.08.008. [DOI] [PubMed] [Google Scholar]
- Kertes DA, Gunnar MR, Madsen NJ, Long JD. Early deprivation and home basal cortisol levels: a study of internationally adopted children. Development and Psychopathology. 2008;20(2):473–491. doi: 10.1017/S0954579408000230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kikusui T, Mori Y. Behavioural and neurochemical consequences of early weaning in rodents. Journal of Neuroendocinrology. 2009;21(4):427–431. doi: 10.1111/j.1365-2826.2009.01837.x. [DOI] [PubMed] [Google Scholar]
- Killgore WD, Yurgelun-Todd DA. Unconscious processing of facial affect in children and adolescents. Soc Neurosci. 2007;2(1):28–47. doi: 10.1080/17470910701214186. [DOI] [PubMed] [Google Scholar]
- Kim JH, Hamlin AS, Richardson R. Fear extinction across development: the involvement of the medial prefrontal cortex as assessed by temporary inactivation and immunohistochemistry. J Neurosci. 2009;29(35):10802–10808. doi: 10.1523/JNEUROSCI.0596-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JH, Richardson R. New Findings on Extinction of Conditioned Fear Early in Development: Theoretical and Clinical Implications. Biol Psychiatry. 2009 doi: 10.1016/j.biopsych.2009.09.003. [DOI] [PubMed] [Google Scholar]
- Kosten TA, Lee HJ, Kim JJ. Early life stress impairs fear conditioning in adult male and female rats. Brain Res. 2006;1087(1):142–150. doi: 10.1016/j.brainres.2006.03.009. [DOI] [PubMed] [Google Scholar]
- Kovach JK, Hess EH. Imprinting: effects of painful stimulation upon the following response. J Comp Physiol Psychol. 1963;56:461–464. doi: 10.1037/h0047033. [DOI] [PubMed] [Google Scholar]
- Kreppner JM, O’Connor TG, Rutter M. Can inattention/overactivity be an institutional deprivation syndrome? J Abnorm Child Psychol. 2001;29(6):513–528. doi: 10.1023/a:1012229209190. [DOI] [PubMed] [Google Scholar]
- LaBar KS, Gatenby JC, Gore JC, LeDoux JE, Phelps EA. Human amygdala activation during conditioned fear acquisition and extinction: A Mixed-trial fMRI study. Neuron. 1998;20:937–945. doi: 10.1016/s0896-6273(00)80475-4. [DOI] [PubMed] [Google Scholar]
- Lang PJ, Bradley MM, Cuthbert BN. Emotion, attention, and the startle reflex. Psychol Rev. 1990;97(3):377–395. [PubMed] [Google Scholar]
- Lee Y, Schulkin J, Davis M. Effect of corticosterone on the enhancement of the acoustic startle reflex by corticotropin releasing factor (CRF) Brain Res. 1994;666(1):93–98. doi: 10.1016/0006-8993(94)90286-0. [DOI] [PubMed] [Google Scholar]
- Liberzon I, Taylor SF, Amdur R, Jung TD, Chamberlain KR, Minoshima S, et al. Brain activation in PTSD in response to trauma-related stimuli. Biol Psychiatry. 1999;45(7):817–826. doi: 10.1016/s0006-3223(98)00246-7. [DOI] [PubMed] [Google Scholar]
- Lupien SJ, McEwen BS, Gunnar MR, Heim C. Effects of stress throughout the lifespan on the brain, behaviour and cognition. Nature Reviews Neuroscience. 2009;10(6):434–445. doi: 10.1038/nrn2639. [DOI] [PubMed] [Google Scholar]
- Makino S, Gold PW, Schulkin J. Corticosterone effects on corticotropin-releasing hormone mRNA in the central nucleus of the amygdala and the parvocellular region of the paraventricular nucleus of the hypothalamus. Brain Res. 1994;640(1-2):105–112. doi: 10.1016/0006-8993(94)91862-7. [DOI] [PubMed] [Google Scholar]
- Marsh AA, Finger EC, Mitchell DG, Reid ME, Sims C, Kosson DS, et al. Reduced amygdala response to fearful expressions in children and adolescents with callous-unemotional traits and disruptive behavior disorders. American Journal of Psychiatry. 2008;165(6):712–720. doi: 10.1176/appi.ajp.2007.07071145. [DOI] [PubMed] [Google Scholar]
- Masten A, Obradovic J. Disaster preparation and recovery: Lessons from research on resilience in human development. Ecology and Society. 2008;13(1):9. [Google Scholar]
- Matsumoto M, Yoshioka M, Togashi H. Early postnatal stress and neural circuit underlying emotional regulation. Int Rev Neurobiol. 2009;85:95–107. doi: 10.1016/S0074-7742(09)85007-1. [DOI] [PubMed] [Google Scholar]
- McEwen BS. Early life influences on life-long patterns of behavior and health. Ment Retard Dev Disabil Res Rev. 2003;9(3):149–154. doi: 10.1002/mrdd.10074. [DOI] [PubMed] [Google Scholar]
- McEwen BS. Understanding the potency of stressful early life experiences on brain and body function. Metabolism. 2008;57(Suppl 2):S11–15. doi: 10.1016/j.metabol.2008.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta MA, Golembo NI, Nosarti C, Colvert E, Mota A, Williams SC, et al. Amygdala, hippocampal and corpus callosum size following severe early institutional deprivation: the English and Romanian Adoptees study pilot. Journal of Child Psychology & Psychiatry. 2009;50(8):943–951. doi: 10.1111/j.1469-7610.2009.02084.x. [DOI] [PubMed] [Google Scholar]
- Milad MR, Quirk GJ. Neurons in medial prefrontal cortex signal memory for fear extinction. Nature. 2002;420(6911):70–74. doi: 10.1038/nature01138. [DOI] [PubMed] [Google Scholar]
- Monk CS. The development of emotion-related neural circuitry in health and psychopathology. Dev Psychopathol. 2008;20(4):1231–1250. doi: 10.1017/S095457940800059X. [DOI] [PubMed] [Google Scholar]
- Monk CS, Grillon C, Baas JM, McClure EB, Nelson EE, Zarahn E, et al. A neuroimaging method for the study of threat in adolescents. Dev Psychobiol. 2003;43(4):359–366. doi: 10.1002/dev.10146. [DOI] [PubMed] [Google Scholar]
- Monk CS, McClure EB, Nelson EE, Zarahn E, Bilder RM, Leibenluft E, et al. Adolescent immaturity in attention-related brain engagement to emotional facial expressions. Neuroimage. 2003;20(1):420–428. doi: 10.1016/s1053-8119(03)00355-0. [DOI] [PubMed] [Google Scholar]
- Moriceau S, Roth TL, Okotoghaide T, Sullivan RM. Corticosterone controls the developmental emergence of fear and amygdala function to predator odors in infant rat pups. Int J Dev Neurosci. 2004;22(5-6):415–422. doi: 10.1016/j.ijdevneu.2004.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriceau S, Roth TL, Sullivan RM. Rodent model of infant attachment learning and stress. Dev Psychobiol. 2010;52(7):651–660. doi: 10.1002/dev.20482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriceau S, Sullivan RM. Corticosterone influences on Mammalian neonatal sensitive-period learning. Behav Neurosci. 2004;118(2):274–281. doi: 10.1037/0735-7044.118.2.274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriceau S, Sullivan RM. Neurobiology of infant attachment. Dev Psychobiol. 2005;47(3):230–242. doi: 10.1002/dev.20093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriceau S, Sullivan RM. Maternal presence serves as a switch between learning fear and attraction in infancy. Nat Neurosci. 2006;9(8):1004–1006. doi: 10.1038/nn1733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriceau S, Wilson DA, Levine S, Sullivan RM. Dual circuitry for odor-shock conditioning during infancy: corticosterone switches between fear and attraction via amygdala. J Neurosci. 2006;26(25):6737–6748. doi: 10.1523/JNEUROSCI.0499-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muris P, Merckelbach H, Gadet B, Moulaert V. Fears, worries, and scary dreams in 4- to 12-year-old children: their content, developmental pattern, and origins. J Clin Child Psychol. 2000;29(1):43–52. doi: 10.1207/S15374424jccp2901_5. [DOI] [PubMed] [Google Scholar]
- Muris P, Merckelbach H, Ollendick TH, King NJ, Bogie N. Children’s nighttime fears: parent-child ratings of frequency, content, origins, coping behaviors and severity. Behav Res Ther. 2001;39(1):13–28. doi: 10.1016/s0005-7967(99)00155-2. [DOI] [PubMed] [Google Scholar]
- Nachmias M, Gunnar M, Mangelsdorf S, Parritz RH, Buss K. Behavioral inhibition and stress reactivity: the moderating role of attachment security. Child Dev. 1996;67(2):508–522. [PubMed] [Google Scholar]
- O’Connor TG, Marvin RS, Rutter M, Olrick JT, Britner PA. Child-parent attachment following early institutional deprivation. Development and Psychopathology. 2003;15(1):19–38. doi: 10.1017/s0954579403000026. [DOI] [PubMed] [Google Scholar]
- Ono M, Kikusui T, Sasaki N, Ichikawa M, Mori Y, Murakami-Murofushi K. Early weaning induces anxiety and precocious myelination in the anterior part of the basolateral amygdala of male Balb/c mice. Neuroscience. 2008;156(4):1103–1110. doi: 10.1016/j.neuroscience.2008.07.078. [DOI] [PubMed] [Google Scholar]
- Ovtscharoff W, Jr., Braun K. Maternal separation and social isolation modulate the postnatal development of synaptic composition in the infralimbic cortex of Octodon degus. Neuroscience. 2001;104(1):33–40. doi: 10.1016/s0306-4522(01)00059-8. [DOI] [PubMed] [Google Scholar]
- Payne C, Machado CJ, Bliwise NG, Bachevalier J. Maturation of the hippocampal formation and amygdala in Macaca mulatta: A volumetric magnetic resonance imaging study. Hippocampus. 2009 doi: 10.1002/hipo.20688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pezawas L, Meyer-Lindenberg A, Drabant EM, Verchinski BA, Munoz KE, Kolachana BS, et al. 5-HTTLPR polymorphism impacts human cingulateamygdala interactions: a genetic susceptibility mechanism for depression. Nat Neurosci. 2005;8(6):828–834. doi: 10.1038/nn1463. [DOI] [PubMed] [Google Scholar]
- Phelps EA, Delgado MR, Nearing KI, LeDoux JE. Extinction learning in humans: role of the amygdala and vmPFC. Neuron. 2004;43(6):897–905. doi: 10.1016/j.neuron.2004.08.042. [DOI] [PubMed] [Google Scholar]
- Plotsky PM, Thrivikraman KV, Nemeroff CB, Caldji C, Sharma S, Meaney MJ. Long-term consequences of neonatal rearing on central corticotropin-releasing factor systems in adult male rat offspring. Neuropsychopharmacology. 2005;30(12):2192–2204. doi: 10.1038/sj.npp.1300769. [DOI] [PubMed] [Google Scholar]
- Poulton R, Menzies RG, Craske MG, Langley JD, Silva PA. Water trauma and swimming experiences up to age 9 and fear of water at age 18: a longitudinal study. Behav Res Ther. 1999;37(1):39–48. doi: 10.1016/s0005-7967(98)00103-x. [DOI] [PubMed] [Google Scholar]
- Poulton R, Waldie KE, Thomson WM, Locker D. Determinants of early-vs late-onset dental fear in a longitudinal-epidemiological study. Behav Res Ther. 2001;39(7):777–785. doi: 10.1016/s0005-7967(00)00060-7. [DOI] [PubMed] [Google Scholar]
- Quirk GJ, Beer JS. Prefrontal involvement in the regulation of emotion: convergence of rat and human studies. Current Opinion in Neurobiology. 2006;16(6):723–727. doi: 10.1016/j.conb.2006.07.004. [DOI] [PubMed] [Google Scholar]
- Quirk GJ, Mueller D. Neural mechanisms of extinction learning and retrieval. Neuropsychopharmacology. 2008;33(1):56–72. doi: 10.1038/sj.npp.1301555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quirk GJ, Russo GK, Barron JL, Lebron K. The role of ventromedial prefrontal cortex in the recovery of extinguished fear. J Neurosci. 2000;20(16):6225–6231. doi: 10.1523/JNEUROSCI.20-16-06225.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raineki C, Moriceau S, Sullivan RM. Developing a neurobehavioral animal model of infant attachment to an abusive caregiver. Biol Psychiatry. 67(12):1137–1145. doi: 10.1016/j.biopsych.2009.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauch S, Whalen P, Shin L, McInerney S, Macklin M, Lasko N, et al. Exaggerated amygdala response to masked facial stimuli in posttraumatic stress disorder: a functional MRI study. Biological Psychiatry. 2000;47(9):769–776. doi: 10.1016/s0006-3223(00)00828-3. [DOI] [PubMed] [Google Scholar]
- Rauch SL, Whalen PJ, Shin LM, McInerney SC, Macklin ML, Lasko NB, et al. Exaggerated amygdala response to masked facial stimuli in posttraumatic stress disorder: a functional MRI study. Biol Psychiatry. 2000;47(9):769–776. doi: 10.1016/s0006-3223(00)00828-3. [DOI] [PubMed] [Google Scholar]
- Reichmann-Decker A, DePrince AP, McIntosh DN. Affective responsiveness, betrayal, and childhood abuse. J Trauma Dissociation. 2009;10(3):276–296. doi: 10.1080/15299730902956788. [DOI] [PubMed] [Google Scholar]
- Sabatini MJ, Ebert P, Lewis DA, Levitt P, Cameron JL, Mirnics K. Amygdala gene expression correlates of social behavior in monkeys experienceing maternal separation. Journal of Neuroscience. 2007;27(12):3295–3304. doi: 10.1523/JNEUROSCI.4765-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salzberg M, Kumar G, Supit L, Jones NC, Morris MJ, Rees S, et al. Early postnatal stress confers enduring vulnerability to limbic epileptogenesis. Epilepsia. 2007;48(11):2079–2085. doi: 10.1111/j.1528-1167.2007.01246.x. [DOI] [PubMed] [Google Scholar]
- Schmahl CG, Vermetten E, Elzinga BM, Bremner DJ. Magnetic resonance imaging of hippocampal and amygdala volume in women with childhood abuse and borderline personality disorder. Psychiatry Res. 2003;122(3):193–198. doi: 10.1016/s0925-4927(03)00023-4. [DOI] [PubMed] [Google Scholar]
- Schulkin J, Gold PW, McEwen BS. Induction of corticotropin-releasing hormone gene expression by glucocorticoids: implication for understanding the states of fear and anxiety and allostatic load. Psychoneuroendocrinology. 1998;23(3):219–243. doi: 10.1016/s0306-4530(97)00099-1. [DOI] [PubMed] [Google Scholar]
- Sevelinges Y, Moriceau S, Holman P, Miner C, Muzny K, Gervais R, et al. Enduring effects of infant memories: infant odor-shock conditioning attenuates amygdala activity and adult fear conditioning. Biol Psychiatry. 2007;62(10):1070–1079. doi: 10.1016/j.biopsych.2007.04.025. [DOI] [PubMed] [Google Scholar]
- Sevelinges Y, Sullivan RM, Messaoudi B, Mouly AM. Neonatal odor-shock conditioning alters the neural network involved in odor fear learning at adulthood. Learning and Memory. 2008;15(9):649–656. doi: 10.1101/lm.998508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin LM, Orr SP, Carson MA, Rauch SL, Macklin ML, Lasko NB, et al. Regional cerebral blood flow in the amygdala and medial prefrontal cortex during traumatic imagery in male and female Vietnam veterans with PTSD. Arch Gen Psychiatry. 2004;61(2):168–176. doi: 10.1001/archpsyc.61.2.168. [DOI] [PubMed] [Google Scholar]
- Shin LM, Rauch SL, Pitman RK. Amygdala, medial prefrontal cortex, and hippocampal function in PTSD. Annals of the New York Academy of Sciences. 2006;1071:67–79. doi: 10.1196/annals.1364.007. [DOI] [PubMed] [Google Scholar]
- Smyke AT, Zeanah CH, Fox NA, Nelson CA, Guthrie D. Placement in foster care enhances quality of attachment among young institutionalized children. Child Dev. 2010;81(1):212–223. doi: 10.1111/j.1467-8624.2009.01390.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somerville LH, Hare TA, Casey BJ. Frontostriatal maturation predicts behavioral regulation failures to appetitive cues in adolescence. Journal of Cognitive Neuroscience. doi: 10.1162/jocn.2010.21572. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somerville LH, Kim H, Johnstone T, Alexander AL, Whalen PJ. Human amygdala responses during presentation of happy and neutral faces: correlations with state anxiety. Biol Psychiatry. 2004;55(9):897–903. doi: 10.1016/j.biopsych.2004.01.007. [DOI] [PubMed] [Google Scholar]
- Spitz RA. Hospitalism: An inquiry into the genesis of psychiatric conditions in early childhood. Psychoanalytic Study of the Child. 1945;1:53–74. [PubMed] [Google Scholar]
- Stanton ME, Levine S. Maternal modulation of infant glucocorticoid stress responses: Role of age and maternal deprivation. Psychobiology. 1988;16(3):223–228. [Google Scholar]
- Stanton ME, Levine S. Inhibition of infant glucocorticoid stress response: specific role of maternal cues. Dev Psychobiol. 1990;23(5):411–426. doi: 10.1002/dev.420230504. [DOI] [PubMed] [Google Scholar]
- Stone EA, Bonnet KA, Hofer MA. Survival and development of maternally deprived rats: role of body temperature. Psychosom Med. 1976;38(4):242–249. doi: 10.1097/00006842-197607000-00002. [DOI] [PubMed] [Google Scholar]
- Thomas KM, Drevets WC, Whalen PJ, Eccard CH, Dahl RE, Ryan ND, et al. Amygdala response to facial expressions in children and adults. Biological Psychiatry. 2001;49(4):309–316. doi: 10.1016/s0006-3223(00)01066-0. [DOI] [PubMed] [Google Scholar]
- Tottenham N, Hare TA, Casey BJ. A Developmental Perspective on Human Amygdala Function. In: Phelps E, Whalen P, editors. The Human Amygdala. Guilford Press; New York: 2009. pp. 107–117. [Google Scholar]
- Tottenham N, Hare TA, Millner A, Gilhooly T, Zevin JD, Casey BJ. Elevated Amygdala Response to Faces Following Early Deprivation. Developmental Science. doi: 10.1111/j.1467-7687.2010.00971.x. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tottenham N, Hare TA, Quinn BT, McCarry T, Nurse M, Gilhooly T, et al. Prolonged institutional rearing is associated with atypically large amygdala volume and emotion regulation difficulties. Developmental Science. 2010;13(1):46–61. doi: 10.1111/j.1467-7687.2009.00852.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tottenham N, Sheridan M. A Review of adversity, the amygdala and the hippocampus: A Consideration of developmental timing. Frontiers in Human Neuroscience. 2010;3:1–18. doi: 10.3389/neuro.09.068.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulfig N, Setzer M, Bohl J. Ontogeny of the human amygdala. Annals of the New York Academy of Sciences. 2003;985:22–33. doi: 10.1111/j.1749-6632.2003.tb07068.x. [DOI] [PubMed] [Google Scholar]
- van Eden CG, Uylings HBM. Postnatal volumetric development of the prefrontal cortex in the rat. Journal of Comparative Neurology. 1985;241(3):268–274. doi: 10.1002/cne.902410303. [DOI] [PubMed] [Google Scholar]
- Vazquez DM, Bailey C, Dent GW, Okimoto DK, Steffek A, Lopez JF, et al. Brain corticotropin-releasing hormone (CRH) circuits in the developing rat: effect of maternal deprivation. Brain Res. 2006;1121(1):83–94. doi: 10.1016/j.brainres.2006.08.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verwer RW, Van Vulpen EH, Van Uum JF. Postnatal development of amygdaloid projections to the prefrontal cortex in the rat studied with retrograde and anterograde tracers. J Comp Neurol. 1996;376(1):75–96. doi: 10.1002/(SICI)1096-9861(19961202)376:1<75::AID-CNE5>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- Vyas A, Mitra R, Shankaranarayana Rao BS, Chattarji S. Chronic stress induces contrasting patterns of dendritic remodeling in hippocampal and amygdaloid neurons. J Neurosci. 2002;22(15):6810–6818. doi: 10.1523/JNEUROSCI.22-15-06810.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vyas A, Pillai AG, Chattarji S. Recovery after chronic stress fails to reverse amygdaloid neuronal hypertrophy and enhanced anxiety-like behavior. Neuroscience. 2004;128(4):667–673. doi: 10.1016/j.neuroscience.2004.07.013. [DOI] [PubMed] [Google Scholar]
- Watamura SE, Donzella B, Kertes DA, Gunnar MR. Developmental changes in baseline cortisol activity in early childhood: relations with napping and effortful control. Dev Psychobiol. 2004;45(3):125–133. doi: 10.1002/dev.20026. [DOI] [PubMed] [Google Scholar]
- Wilbarger J, Gunnar M, Schneider M, Pollak S. Sensory processing in internationally adopted, post-institutionalized children. J Child Psychol Psychiatry. 2010;51(10):1105–1114. doi: 10.1111/j.1469-7610.2010.02255.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeanah CH, Egger HL, Smyke AT, Nelson CA, Fox NA, Marshall PJ, et al. Institutional rearing and psychiatric disorders in Romanian preschool children. Am J Psychiatry. 2009;166(7):777–785. doi: 10.1176/appi.ajp.2009.08091438. [DOI] [PubMed] [Google Scholar]
- Zeanah CH, Smyke AT, Dumitrescu A. Attachment disturbances in young children. II: Indiscriminate behavior and institutional care. J Am Acad Child Adolesc Psychiatry. 2002;41(8):983–989. doi: 10.1097/00004583-200208000-00017. [DOI] [PubMed] [Google Scholar]
- Zeanah CH, Smyke AT, Koga SF, Carlson E. Attachment in institutionalized and community children in Romania. Child Dev. 2005;76(5):1015–1028. doi: 10.1111/j.1467-8624.2005.00894.x. [DOI] [PubMed] [Google Scholar]


