Abstract
Background & Aims
Invariant natural killer T (iNKT) cells undergo canonical, Vα14–Jα18 rearrangement of the T-cell receptor (TCR) in mice; this form of the TCR recognizes glycolipids presented by CD1d. iNKT cells mediate many different immune reactions. Their constitutive activated and memory phenotype and rapid initiation of effector functions after stimulation indicate previous antigen-specific stimulation. However, little is known about this process. We investigated whether symbiotic microbes can determine the activated phenotype and function of iNKT cells.
Methods
We analyzed the numbers, phenotypes, and functions of iNKT cells in germ-free mice, germ-free mice reconstituted with specified bacteria, and mice housed in specific pathogen-free (SPF) environments.
Results
SPF mice, obtained from different vendors, have different intestinal microbiota. iNKT cells isolated from these mice differed in TCR Vβ7 frequency and cytokine response to antigen, which depended on the environment. iNKT cells isolated from germ-free mice had a less mature phenotype and were hypo-responsive to activation with the antigen α-galactosylceramide. Intra-gastric exposure of germ-free mice to Sphingomonas bacteria, which carry iNKT cell antigens, fully established phenotypic maturity of iNKT cells. In contrast, reconstitution with Escherichia coli, which lack specific antigens for iNKT cells, did not affect the phenotype of iNKT cells. The effects of intestinal microbes on iNKT cell responsiveness did not require toll-like receptor signals, which can activate iNKT cells independently of TCR stimulation.
Conclusions
Intestinal microbes can affect iNKT cell phenotypes and functions in mice.
Keywords: αGalCer, T-cell activation, mucosa, TLR
Introduction
Invariant natural killer T (iNKT) cells are a unique subset of T lymphocytes characterized by the expression of an invariant TCR rearrangement, Vα14-Jα18 in mice (Vα14i NKT cells) and an orthologous Vα24-Jα18 (Vα24i) in humans, and the recognition of antigens presented by CD1d, a non-polymorphic MHC class I-like antigen-presenting molecule1–4. CD1d binds lipid structures, and one of the best-studied iNKT cell antigens is α-galactosylceramide (αGalCer), a synthetic version of a glycolipid originally isolated from a marine sponge1.
iNKT cells express surface molecules characteristic of antigen-experienced lymphocytes, and antigenic stimulation leads to the rapid induction of effector functions by iNKT cells such as the production of Th1- and Th2 cytokines and potent cytotoxicity1–4. As a consequence of their vigorous early response, iNKT cells have been implicated in diverse immune reactions, including the pathogenesis of inflammatory diseases of the liver, pancreas and intestine. Similar data in human patients are relatively sparse, still they suggest comparable roles for iNKT cells in different contexts. In the case of inflammatory bowel disease, most of the findings are consistent with a protective role for iNKT cells during Th1 mediated diseases and a deleterious one in Th2 diseases5,6. The fact that iNKT cells can cause either beneficial or detrimental effects in different disease models illustrates their dichotomous function and their ability to polarize the ensuing immune response in either a Th1- or Th2 - direction7. In contrast to this diversity in the functional outcome, a protective role of iNKT cells has almost uniformly been reported both in animal models and in human patients with type I diabetes8,9.
In addition to αGalCer, glycolipid antigens known to stimulate the majority of iNKT cell have been reported in two types of bacteria. One type is Sphingomonas/Sphingobium species, which have glycosphingolipids similar to the original sponge antigen10,11. The second type, Borrelia burgdorferi, is the causative agent of Lyme disease12. Several additional pathogens have been reported to have glycolipid antigens that activate iNKT cells, include Leishmania donovani and Helicobacter pylori13–15, but in such cases it may be only a subset of the cells that are stimulated. More generally, the distribution and prevalence of iNKT cell antigens in the microbiota and in the wider environment, as well as their role in iNKT cell function under non-inflammatory conditions remain to be determined.
The constitutively activated phenotype of iNKT cells has been attributed to the presence of self-agonist glycolipid ligands that drive the selection of these cells and stimulate them continually in the periphery. While there is some evidence for this, we set out to determine the role of intestinal bacteria in shaping the phenotype and function of iNKT cells. Our hypothesis was substantiated by the previous finding that ribosomal DNA sequences from Sphingobium yanoikuyae and related species are found in the mouse intestine16,17, suggesting these could be commensal organisms. Furthermore, sequences from the related bacteria Novosphingobium aromaticivorans have been found in the human intestine18. In addition, we showed previously that intragastric challenge with S. yanoikuyae stimulated peripheral iNKT cells16. This indicated that gut-derived iNKT cell antigens are capable of activating peripheral iNKT cells. Here we show that intestinal bacteria can modulate the phenotype, TCR Vβ-usage and the immune responses of iNKT cells, and that antigens from commensals that engage the semi-invariant TCR are a likely contributing factor.
Material and Methods
Mice and cell lines
Mice were housed under SPF conditions at the animal facilities of the La Jolla Institute for Allergy and Immunology (La Jolla, CA), the The Scripps Research Institute (La Jolla, CA) and the Department of Pathology and Laboratory Medicine (Los Angeles, CA) or housed under germ-free conditions at the California Institute of Technology (Pasadena, CA) in accordance with the Institutional Animal Care Committee guidelines. C57BL/6 mice were purchased from the Jackson Laboratory (Bar Harbor, ME) or from Taconic Farms (Hudson, NY); Swiss Webster germ-free and SPF housed animals from Taconic Farms; and B6.129S1-Il12btm1Jm/J (IL-12p40−/−) from Jackson Laboratory. MyD88 and TRIF (Lps2) double deficient mice19 and restricted flora (RF) mice have been described previously20,21. Sphingobium yanoikuyae and Escherichia coli were purchased from American Type Culture Collection (Manassas, VA). The T cell lymphoma RMA was virally transfected to stably express CD1d as previously described22, resulting in the line RMA-CD1d.
Reagents and monoclonal antibodies
α-galactosylceramide (αGalCer) was obtained from the Kirin Pharmaceutical Research Corporation (Gunma, Japan). CFDA-SE was obtained from Invitrogen (Carlsbad, CA). Monoclonal antibodies (mAbs) against the following mouse antigens were used in this study: β7-integrin (M293), CCR9 (9B1, eBioCW-1.2), CD1d (1B1), CD3ε (145.2C11, 17A2), CD4 (GK1.5, RM4-5), CD5 (53-7.3), CD8α (53-6.7, 5H10), CD19 (1D3, 6D5), CD25 (PC61.5), CD44 (IM7), CD45R (B220, RA3-6B2), CD69 (H1.2F3), CD103 (2E7), CD122 (TM-b1), CD127 (A7R34), TCRβ (H57-597), NK1.1 (PK136), Vβ2 (B20.6), Vβ7 (TR310), GM-CSF (MP1-22E9), IL-2 (JES6-5H4), IL-4 (11B11), IL-13 (eBio13A), IFNγ (XMG1.2) and TNFα (MP6-XT22). Antibodies were purchased from BD Biosciences (San Diego, CA), BioLegend (San Diego, CA), eBioscience (San Diego, CA) or Invitrogen. αGalCer-loaded CD1d tetramers were produced as described23.
Cell Preparation, in vivo challenge and flow cytometry
Single-cell suspensions from liver, spleen, thymus and intestine were prepared as described24,25. In vivo cytotoxicity assays and cell staining for flow cytometry were performed as reported previously24. iNKT cells were activated in vivo by i.v. injection of 1μg αGalCer and analyzed 90min later. Bacterial suspensions were gavaged using a 20Gx1.5 feeding needles.
Statistical analysis
Results are expressed as mean ± standard error of the mean (SEM). Comparisons were drawn using a two-tailed Student t-test or ANOVA test. p-values <0.05 were considered significant and are indicated with *p<0.05, **p≤0.01 and ***p≤0.001. Each experiment was repeated at least twice, and background values were subtracted.
Results
Distribution and phenotype of intestinal iNKT cells
We examined the frequency of iNKT cells in different sites in the intestine, because there have been conflicting data on the frequency and distribution of these cells in the gut mucosa5. We analyzed iNKT cells in the LPL and IEL compartments of the small (SI) and large intestines (LI). iNKT cells could readily be detected in LPL and IEL from both, small and large intestine of specific-pathogen-free (SPF) C57BL/6J mice (Figure 1A). The signal was specific for iNKT cells, as indicated by the low background when utilizing unloaded CD1d tetramers (Figure 1A). We consistently observed a higher frequency of iNKT cells in the small intestine than in the large intestine; and a higher frequency in the LPL than in IEL (Figure 1B). It is notable that the frequency of iNKT cells in the SI-LPL was comparable to the value in the spleen. The majority of intestinal iNKT cells were CD4+ and NK1.1+, with the exception of decreased NK1.1 expression by LI-IEL iNKT cells (Figure 1C and Supplemental figure 1A). Most intestinal iNKT cells were CD69+, CD44+ and CD122+ similar to their splenic counterparts (data not shown). Furthermore, only a small fraction of intestinal iNKT cells expressed CD103 (Supplemental figure 1B, 1C), and intestinal iNKT cells also were mostly negative for the β7-integrin (data not shown). In contrast, we detected expression of CCR9 on iNKT cells derived from the LPL (Figure 1D).
Figure 1. Distribution and phenotype of intestinal iNKT cells.
(A) Lymphocytes from the indicated sites were incubated either with αGalCer loaded or unloaded CD1d-tetramers, analyzed by flow cytometry and the frequencies of tetramer-positive cells within live TCRβ+CD44+CD8α−CD19− cells are shown. (B, C) Relative percentage of iNKT cells within total live lymphocytes (B) and their expression of CD4 and NK1.1 (C), from indicated sites. The graphs summarize data from 3–5 independent experiments, with 6–9 samples per group. (D) Representative expression of CCR9 on iNKT cells derived from the spleen (tinted, in both panels), IEL (dashed) or LPL (black line) from the small or large intestine. The numbers in histograms denote the geometric mean values for CCR9 on iNKT cells.
Environmental influences on the responsiveness and Vβ-usage of iNKT cells
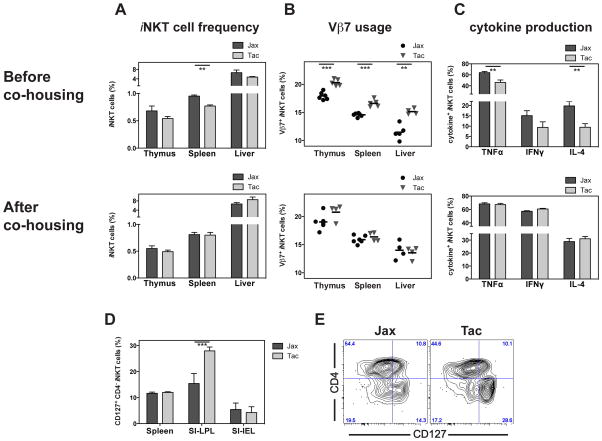
It has been reported that the housing conditions provided by the commercial vendors at Taconic Farms (Tac) and the Jackson Laboratory (Jax), and the consequent difference in the intestinal microbiota, can impact the composition and function of conventional, CD4+ T lymphocytes in the intestine17. To test if such differences could influence the responsiveness of peripheral iNKT cells, we directly compared their phenotype and function in SPF C57BL/6 animals from both vendors. The percentage of iNKT cells in the thymus, spleen and liver of Tac mice tended to be lower than in Jax mice, a difference which was statistically significant in all experiments however only in the spleen (Figure 2A). Primary Vα14i NKT cells use commonly three Vβ chains paired with the invariant TCR α-chain. Vβ8.1/2 is most abundant, comprising approximately 55% of the total, with the other principal ones being Vβ7 (14%) and Vβ2 (7%)1,26. The analysis of the Vβ-usage of the iNKT cells from Tac and Jax C57BL/6 mice revealed a significantly higher frequency of Vβ7+ iNKT cells in the thymus, spleen and liver of Tac mice (Figure 2B). No difference, however, was observed for Vβ7 usage of iNKT cells in either SI-IEL or SI-LPL (Supplemental figure 2A), or for the frequency of Vβ2+ iNKT cells in any of the organs analyzed (Supplemental figure 2A, 2B). The decrease of Vβ7+ iNKT cells in Jax mice was balanced out by a correlative increase of iNKT cells expressing Vβ8 (data not shown). Furthermore, we observed no significant differences between Tac and Jax iNKT cells in the surface expression of NK1.1, CD4, CD25, CD44, CD69 and CD122 in any tissue analyzed (data not shown). However, we noted in the SI-LPL, but not in any other organ analyzed, a significantly increased frequency of CD127+CD4− iNKT cells in the Tac compared to the Jax derived mice (Figure 2D, 2E and data not shown). We also assessed the effector functions of the iNKT cells following activation with αGalCer. The frequency of cytokine-producing iNKT cells tended to be lower in Tac mice (Figure 2C). This difference, however, was statistically significant only for TNFα in all four experiments, while significance for differences in IL-4 and IFNγ was not consistently observed. The lower TNFα production of Tac iNKT cells could not be explained by the difference in the Vβ-usage, as the cytokine production in the Tac iNKT cells was reduced irrespective of the Vβ expressed (Supplemental figure 2C).
Figure 2. Environmental influences on the responsiveness and Vβ-usage of iNKT cells.
(A-C) C57BL/6 animals, purchased from either Taconic Farms (Tac) or Jackson Laboratory (Jax), were either analyzed within one week after delivery (top panels). Or alternatively, new-born offspring from Tac or Jax mice were co-housed from 2–5 days after birth until analysis 8–10 weeks later (lower panels). Relative frequency of iNKT cells (A) and their Vβ7-usage (B) in indicated organs is shown. Production of indicated cytokines by splenic iNKT cells 90min after i.v. injection of αGalCer was analyzed by intracellular staining (C). Representative data from four (top panels) or three (lower panels) independent experiments are shown. (D, E) Frequency of CD127+CD4− iNKT cells in indicated organs (D) or from SI-LPL (E) from indicated mice. Representative data from three independent experiments are shown.
To determine if the observed differences were acquired and did not stem from minor variations due to genetic drift, we co-housed newborn offsprings of Tac and Jax mice, to allow the environmental factors, including the intestinal microbiota, to equalize. When analyzed side-by-side after eight to ten weeks later, we did not find difference in iNKT cell frequency, nor in Vβ-usage and function (Figure 2A–C). Therefore, these data clearly demonstrate that differences in the environment of Tac- and Jax-derived animals can modulate the frequency, Vβ-usage and cytokine production of iNKT cells.
iNKT cells from germ-free mice are hyporesponsive
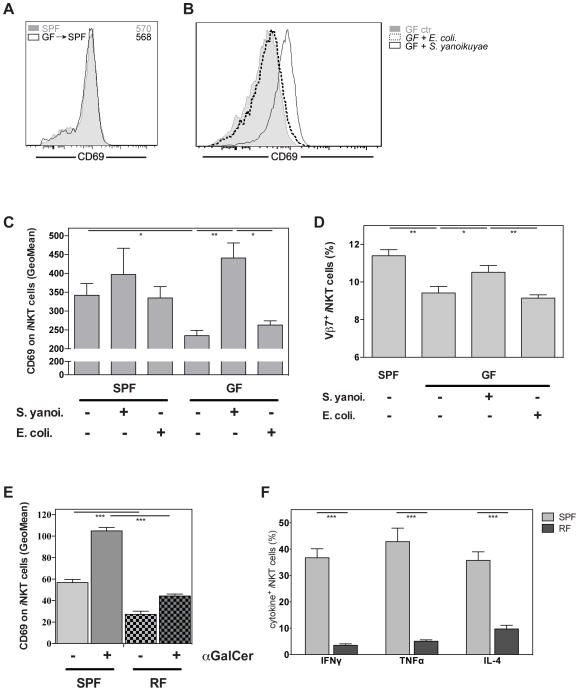
To determine more directly if the normal gut microbiota affects the development and function of peripheral iNKT cells, we compared iNKT cells derived from Swiss Webster animals raised in germ-free (GF) conditions with those from mice raised in SPF conditions. Although relative iNKT cell numbers recovered from GF and SPF animals did not differ significantly (data not shown), we observed that unstimulated iNKT cells from the spleen, liver and thymus of GF mice uniformly expressed lower levels of the activation markers CD69, CD25 and CD5 (Figure 3A and supplemental figure 3A). When GF and SPF animals were challenged with the potent iNKT cell antigen αGalCer, the difference in CD69 expression between GF and SPF mice was even more pronounced (Figure 3B), suggesting that iNKT cells from GF animals respond less vigorously. Importantly, cytokine production by iNKT cells from GF animals, as measured by intracellular cytokine staining, was significantly lower compared to their SPF counterparts (Figure 3B). We also observed a similar difference after stimulating splenocytes from GF and SPF mice with αGalCer in vitro (Supplemental figure 3B). iNKT cells are not highly dependent on co-stimulation for activation27 and the expression level of CD1d on antigen presenting cells (APCs) was comparable in SPF and GF animals (data not shown). Nonetheless, it was possible that differences in the maturation state of APC caused the reduced responses of iNKT cells from GF mice. To avoid the influence of endogenous APCs, we stimulated splenocytes from GF- and SPF-raised animals with αGalCer-loaded, CD1d transfected RMA lymphoma cells in vitro. In this experimental set-up and similar to the previous results, iNKT cells derived from GF animals produced significantly less cytokines than cells from SPF animals (Figure 3C). These data demonstrate that, independently of any putative effect on APCs, iNKT cells from GF mice respond less vigorously to antigen stimulation than iNKT cells from SPF animals.
Figure 3. iNKT cells from germ-free animals are hyporesponsive.
(A) Expression of CD69, CD25 and CD5 by iNKT cells from indicated organs derived from germ-free (GF) or specific-pathogen-free (SPF) housed Swiss Webster mice. (B) Expression of CD69 (left panel) and indicated cytokines (right panel) by splenic iNKT cells from GF or SPF housed Swiss Webster mice with or without αGalCer challenge in vivo (90min). The expression of CD69 following αGalCer increased on SPF derived iNKT cells 1.9fold (MFI), whereas the increase on GF derived iNKT cells was lower at 1.75fold (p(SPF +/- αGalCer vs GF +/- αGalCer)= 0.004). (C) Splenocytes from GF and SPF Swiss Webster mice were co-cultured with αGalCer loaded RMA-CD1d cells for 4h and cytokine production by iNKT cells was analyzed by intracellular staining. (D) GF or SPF housed animals on the C57BL/6 background were injected with αGalCer and the cytokine production by splenic iNKT cells was analyzed 90min later. The graph summarizes data from two independent experiments, with 4–5 mice per group. (E) αGalCer-specific in vivo cytotoxicity in spleen 4h after injection of B cell targets into GF or SPF housed Swiss Webster mice. Representative data from two independent experiments are shown. (F) Relative percentage of iNKT cells within TCRβ+ live lymphocytes (left) and of CD127+CD4− iNKT cells (right) from indicated organs of GF or SPF housed Swiss Webster animals. The graphs summarize data from three independent experiments, with 5–8 mice per group.
As Swiss Webster mice are not fully inbred, we aimed to confirm that iNKT cells from GF mice are hyporesponsive by testing GF animals on the C57BL/6 background. Similar to their Swiss Webster counterparts, splenic iNKT cells from C57BL/6 GF animals showed a significant impairment in antigen-stimulated cytokine production (Figure 3D) and up-regulation of CD69 expression (Supplemental figure 3C).
Apart from cytokine production, activated iNKT cells display potent cytotoxic activity. To test if the presence of the intestinal microbiota affects the cytotoxic potential of iNKT cells, we injected GF- and SPF-housed Swiss Webster animals with CFSE-labeled B cells loaded in vitro with αGalCer, and measured cytotoxicity in vivo four hours later24. The αGalCer specific in vivo cytotoxicity in GF mice was significantly lower than that observed in SPF animals (Figure 3E), indicating that the microbiota is also important for the development and/or maintenance of the cytotoxic capability of iNKT cells. Altogether these data demonstrate that iNKT cells from GF animals are hypo-responsive to antigen stimulation in a cell-intrinsic fashion.
Furthermore, we found a significantly higher frequency of intestinal iNKT cells in GF than in SPF Swiss Webster mice in all four intestinal compartments (Figure 3F), suggesting that the homing/expansion of iNKT cells to the intestine does not require the gut microbiota to the same extent as for αβT cells28. The analysis of the Vβ-usage of the iNKT cells from GF and SPF C57BL/6 mice revealed a significantly lower frequency of Vβ7+ iNKT cells in the thymus and spleen of GF mice (Supplemental figure 3D). Similar to other organs analyzed (Figure 3A), in GF mice the expression of CD69 was lower on intestinal iNKT cells than in SPF mice (Supplemental figure 3E and data not shown). Furthermore, while no differences for the expression of CD103, β7-integrin and CCR9 on intestinal iNKT cells from GF compared to SPF mice were observed (Supplemental figure 3F and data not shown), the frequency of CD127+CD4− iNKT cells in the small intestine was lower in the GF animals (Figure 3F).
Bacterial products promote iNKT cell responsiveness in a TLR independent fashion
Bacterial products, via TLR signaling and induction of IL-12 and other cytokines by APCs, can activate iNKT cells even in the absence of a microbial antigen that engages their TCR. In order to establish if this alternative route of stimulation plays a role in shaping the iNKT cell antigen responsiveness to intestinal microbiota, we utilized MyD88 and TRIF double-deficient animals, which cannot respond to TLR ligands29. We did not detect any phenotypic differences between C57BL/6 control and MyD88−/−TrifLps2/Lps2 mice (Figure 4A). Activation of iNKT cells from MyD88−/−TrifLps2/Lps2 animals with αGalCer caused phenotypic changes that were also indistinguishable from the controls (Figure 4A). Furthermore, we did not observe differences in αGalCer-induced cytokine production by iNKT cells (Figure 4B). Similarly, analysis of IL-12−/− animals showed no phenotypic or functional differences with iNKT cells from wild-type animals (Figure 4C, 4D). Consistent with these data, the frequency and phenotype of intestinal iNKT cells in the IL-12−/− animals were similar to C57BL/6 control mice (Supplemental figure 4). These data suggest that the pathways of iNKT cell stimulation that depend on TLR stimulation of APCs cannot account for the hyporesponsive phenotype and function of iNKT cells in GF mice.
Figure 4. Bacterial products promote iNKT cell responsiveness in a TLR independent fashion.
(A, B) C57BL/6J wild-type and MyD88−/−TrifLps2/Lps2 animals were either mock treated or injected with αGalCer and 90min later the expression of indicated surface markers (A) and cytokines (B) by splenic iNKT cells was analyzed. (C, D) C57BL/6J wild-type and IL-12−/− animals were either mock treated or injected with αGalCer and 90min later the expression of indicated surface markers (C) and cytokines (D) by splenic iNKT cells was analyzed. Representative data from two independent experiments are shown.
Bacterial reconstitution corrects the hyporesponsive phenotype of iNKT cells
We then tested if the hyporesponsive phenotype of iNKT cells in GF animals could be reversed. To this end, we co-housed GF with SPF animals for four weeks under SPF conditions. After this time, we found that the phenotype of iNKT cells from SPF mice was indistinguishable from the ones of previously GF mice (Figure 5A).
Figure 5. Bacterial exposure corrects the hyporesponsive phenotype of iNKT cells.
(A) GF and SPF housed Swiss Webster mice were co-housed for four weeks and expression of CD69 in splenic iNKT cells was analyzed. The numbers in histograms denote the geometric mean values for CD69 on iNKT cells. (B–D) GF and SPF housed Swiss Webster mice were mock treated or intra-gastrically challenged with either S. yanoikuyae or E. coli bacteria as indicated. Four to five days later the expression of CD69 in splenic iNKT cells was analyzed and is represented as example histogram (B) or as summary (C). Furthermore, the relative frequency of Vβ7+ iNKT cells is shown (D). (E, F) Expression of CD69 (E) and indicated cytokines (F) by splenic iNKT cells from restricted-flora (RF) and SPF housed C57BL/6 mice with or without αGalCer challenge in vivo (90min). Representative data from two (A, E, F), three (D) or four (B, C) independent experiments are shown.
Next we reconstituted GF animals by gavage with live bacteria, either with the Sphingomonas/Sphingobium species S. yanoikuyae, which have iNKT cell antigens10, or with E. coli, which are devoid of such antigens (data not shown). Analysis of CD69 expression on iNKT cells showed that reconstitution with S. yanoikuyae was sufficient to normalize the hypo-responsive phenotype of iNKT cells from GF mice (Figure 5B, 5C). In contrast, reconstitution of the GF animals with E. coli bacteria did not cause such a change in the iNKT cell phenotype (Figure 5B, 5C). These data suggest that intestinal-derived iNKT cell specific antigens from microbes are necessary to render peripheral iNKT cells fully mature and ready to respond. Similar to C57BL/6 GF (Supplemental figure 3D) iNKT cells from SW-GF animals displayed a lower frequency of Vβ7+ cells and this frequency normalized following reconstitution with S. yanoikuyae, but not with E. coli (Figure 5D), suggesting antigen driven proliferation of iNKT cells.
To analyze the effect of a limited set of intestinal organisms on the responsiveness of iNKT cells we also tested mice bearing a restricted flora (RF)16. RF mice carry an altered and reduced microbiota, including different fungal and bacterial species as compared to SPF mice20,21. The bacterial microbioata of RF mice is enriched for Firmicutes spp and devoid of Sphingomonas/Sphingobium spp16. Although iNKT cell numbers are reduced in RF mice16, their response to αGalCer can be measured. iNKT cells derived from RF mice displayed a higher frequency of Vβ7+ iNKT cells in the spleen (Supplemental figure 5). Under resting conditions splenic iNKT cells from RF mice expressed lower CD69 levels and displayed a lower up-regulation of this marker following αGalCer stimulation (Figure 5E). Furthermore, fewer iNKT cells produced cytokines in the RF mice compared to the SPF controls (Figure 5F), recapitulating the data we obtained in the GF animals.
Discussion
Here we report a detailed record of the distribution and phenotype of iNKT cells in the intestine. Furthermore, we show that bacterial products from the intestinal microbiota contribute to the full responsiveness of peripheral Vα14i NKT cells and can modulate their phenotype and TCR Vβ-usage. iNKT cells from specific-pathogen-free (SPF) mice derived from different vendors differed in the frequency of iNKT cells, the proportion that expressed Vβ7, and in their cytokine response after antigen stimulation. Additionally, iNKT cells derived from GF animals displayed a less mature phenotype and were hypo-responsive to antigen-specific activation, as measured by up-regulation of activation markers and the production of cytokines. These effects on the acute, antigen-specific response of iNKT cells in GF mice could be reversed days after oral exposure to bacteria expressing iNKT cell antigens. Furthermore, full iNKT cell maturation and the constitutive activation state of these cells did not require TLR-mediated signals. Together these findings suggest that antigens from the microbiota that engage the semi-invariant TCR likely are responsible for the effects observed.
In light of these findings, we were surprised that iNKT cells were increased in GF mice in the lamina propria and epithelium of the small and large intestines, although like their counterparts in the spleen and liver, the intestinal iNKT cells from GF mice expressed lower amounts of the activation antigen CD69. These data are consistent with a recent report showing that iNKT cells are increased in the colon of GF mice due to increased production of the chemokine CXCL16 (Olszak et al., Science in press, DOI: 10.1126/science.1219328). Interestingly, colonization of neonatal mice with intestinal flora prevented both the increased accumulation of iNKT cells in the intestine and the contribution of these cells to inflammation in the intestine and the lung (Olszak et al., Science in press, DOI: 10.1126/science.1219328), providing additional evidence for the modulation of iNKT cell function as well as number by intestinal microbes.
The frequency and distribution of iNKT cells in intestinal tissue has not been fully characterized, despite the role of iNKT cells in several models of inflammatory bowel disease and intestinal infections5,6. Many of the studies reporting the presence of NKT cells in the intestine relied on the co-expression of the TCR/CD3ε complex and NK cell receptors5,6, which does not allow for the unequivocal identification of iNKT cells. Using CD1d tetramers loaded with αGalCer, however, iNKT cells have been reported in LPL30,31 and in SI-IEL, where 80% of them were NK1.1neg26. A later report, however, did not detect iNKT cells in SI-IEL30. Here we report on the presence of iNKT cells in IEL and LPL of both small and large intestine. We detected a higher relative percentage of iNKT cells in the small rather than the large intestine, and also generally more in the LPL than in the IEL compartments. The frequencies in LI-LPL were comparable to those in the lymph node, and for the SI-LPL to those in the spleen. These data demonstrate that iNKT cells constitute a significant lymphocyte population within the lamina propria.
It has been reported that iNKT cells can influence the microbial colonization and the composition of intestinal bacteria32. Here we provide evidence that this influence is mutual. iNKT cells derived from SPF animals from two different vendors, Taconic Farms and Jackson Laboratory, showed differences in the frequency of iNKT cells, Vβ7-usage and cytokine production. These differences were dependent on the environment, as CD1d expression was not different between the two strains and co-housing of the offspring diminished them. Interestingly, although iNKT cells expressing Vβ7 have a lower avidity for αGalCer33, it has been inferred that they have a higher avidity for the endogenous selecting antigen(s)34. The environment-dependent increase in Vβ7+ iNKT cells in the Tac C57BL/6 mice could therefore be due to differences in intestinal iNKT cell antigens. However, in preliminary experiments we could not recover detectable antigenic iNKT cell activity in the intestinal contents from SPF mice (data not shown). While a difference in the intestinal microbiota is likely responsible, especially considering the known differences between Jax and Tac mice17, further experiments are required to determine the parameters in the environment that are responsible for the differences in iNKT cells between mice from the two vendors.
The finding that iNKT cells from GF Swiss Webster mice were hypo-responsive are in contrast to those by Park et al.35, where no impairment of the iNKT cell response of GF animals was detected. Several technical differences, however, set our study apart from the previous one, including: (a) the use of CD1d/αGalCer-tetramers to unequivocally detect Vα14i NKT cells, in contrast to measuring NK1.1+TCRβ+ cells, the only tools available at that time; (b) the quantitative analysis of activation marker expression levels by determining the mean fluorescence intensity, rather than expression by NKT cells per se; and finally (c) the analysis of the iNKT cell cytokine response on a single cell level, rather than analysis of cytokine mRNA from total splenocytes. However, despite the marked differences we observed in iNKT cells from GF mice, we should not overlook the significant phenotypic and functional overlap they have with iNKT cells from SPF mice, including an expanded population, increased activation marker expression compared to naïve T lymphocytes, and the ability of some of the cells, albeit a reduced percentage, to produce effector cytokines rapidly.
Our data obtained from MyD88−/−TrifLps2/Lps2 and IL-12−/− mice indicated that TLR-ligands from the intestinal contents are not required for the full maturation of peripheral iNKT cells. These data do not exclude a potential role for other sensing molecules, like RIG-I-like receptors (RLRs), and NOD-like receptors (NLRs)36, but their role in iNKT cell activation are currently unknown. Therefore, the indirect or cytokine-mediated pathway for iNKT cell activation is likely not responsible for the homeostatic maintenance of the highly activated and responsive state of these cells in SPF mice. Furthermore, reconstitution of GF mice by oral administration of S. yanoikuyae, which contain relatively high affinity glycosphingolipid antigens for the iNKT cell TCR, could recover the full phenotypic maturity of these cells. In the absence of an isogenic S. yanoikuyae strain, we carried out reconstitution with E. coli, a bacterium believed to lack antigens for the iNKT cell TCR. Administration of E. coli did not normalize the phenotype of iNKT cells. These data demonstrate the importance of intestinal bacterial products for facilitating the full degree of iNKT cell responsiveness, and they suggest that antigens for the semi-invariant TCR are responsible.
As iNKT cell antigens have so far only been identified from a few bacterial sources3,4, the distribution of iNKT cell antigens in the microbiota, and more generally in the environment, remains incompletely characterized. We previously demonstrated specific iNKT cell antigens in Sphingobium yanoikuyae10. Such Sphingomonas/Sphingobium species are ubiquitously present in water and soil15 and are commensal species in the gut16,17. Therefore we cannot exclude that a similar bacteria is a likely source of the intestinal iNKT cell antigens. Still, the Sphingomonas yanoikuyae species were not reported to substantially differ between Tac and Jax C57BL/6 animals17. In this context the observation is of interest that mice bearing a restricted flora (RF) were not able to support full reactivity of iNKT cells. RF mice lack Sphingomonas/Sphingobium species16, but also numerous other bacteria species normally present in SPF mice20,21. We expect, however, that additional bacteria, many of them non-infectious, contain iNKT cell antigens. For example, patients with primary biliary cirrhosis (PBC) expressed antibodies against enzymes from the Sphingomonas/Sphingobium species Novosphingobium aromaticivorans18. N. aromaticivorans was detected in the gut of PBC patients18 and the activation of iNKT cells by N. aromaticivorans-derived antigens was linked to disease progression37,38. These data demonstrated that commensal bacteria expressing iNKT cell antigens can contribute to iNKT cell-mediated inflammation. Together with our data, these findings suggest that the composition of the intestinal microbiota may be an important exacerbating or causative factor in other autoimmune diseases, with a possible contribution of iNKT cells.
The body exchanges substances with the environment via the mucosal surfaces of the lung and the intestine. We recently demonstrated that iNKT cell antigens are present in house dust and that the adjuvant effect they exerted during airway inflammation is dependent on iNKT cells19. Here we show that materials from the intestinal microbiota, likely iNKT cell antigens, modulate the phenotype and function of peripheral iNKT cells. Together these reports demonstrate that iNKT cells are sensitive in responding to the environment and that antigens recognized by these cells are far more prevalent than previously anticipated.
Importantly, our findings indicate that the composition of the intestinal microbiota influences the cytokine responsiveness of iNKT cells. It is thus conceivable that such modulation not only could pertain to the magnitude of the antigen-induced cytokine response, but also its polarization. Given the important role iNKT cells play in numerous infectious and autoimmune diseases, our findings imply that the intestinal microbiota-mediated modulation of iNKT cells could significantly affect the outcome of these diseases.
Supplementary Material
Acknowledgments
This work was funded by NIH grants RO1 AI45053 and R37 AI71922 (M.K.), DK46763 (J.B., M.K.), DK078938 (S.K.M.) and an Outgoing International Fellowship by the Marie Curie Actions (G.W.). The authors wish to thank Olga Turovskaya, Archana Khurana, Christopher Lena and the Department of Laboratory Animal Care at the La Jolla Institute for Allergy & Immunology for excellent technical assistance. We are grateful to the scientific contributions of Hilde Cheroutre, Maureen Bower, Mushtaq Husain, Yunji Park, Niranjana Nagarajan, Anup Datta and Dirk Warnecke.
Abbreviations used in this paper
- αGalCer
α-galactosylceramide
- APC
antigen presenting cell
- GF
germ-free
- i
invariant
- IEL
intraepithelial lymphocytes
- LI
large intestine
- LPL
lamina propria lymphocytes
- mAb
monoclonal antibody
- NKT
Natural Killer T
- RF
restricted flora
- SI
small intestine
- SPF
specific pathogen free
- Th1
T helper type 1
- Th2
T helper type 2
- TLR
toll like receptor
- Vα14i
invariant Vα14 to Jα18 TCR rearrangement
Footnotes
Competing Interest
The authors have no competing interests regarding this work.
Author contributions
G.W. and M.K. designed research, G.W., D.S., P.K., L.L. B.W. and S.M. conducted experiments and acquired data, G.W. and M.K wrote the manuscript, S.K.M., J.B. and M.K. obtained funding. All authors approved the manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bendelac A, Rivera MN, Park SH, et al. Mouse CD1-specific NK1 T cells: development, specificity, and function. Annu Rev Immunol. 1997;15:535–62. doi: 10.1146/annurev.immunol.15.1.535. [DOI] [PubMed] [Google Scholar]
- 2.Taniguchi M, Harada M, Kojo S, et al. The regulatory role of Valpha14 NKT cells in innate and acquired immune response. Annu Rev Immunol. 2003;21:483–513. doi: 10.1146/annurev.immunol.21.120601.141057. [DOI] [PubMed] [Google Scholar]
- 3.Bendelac A, Savage PB, Teyton L. The Biology of NKT Cells. Annu Rev Immunol. 2007;25:297–336. doi: 10.1146/annurev.immunol.25.022106.141711. [DOI] [PubMed] [Google Scholar]
- 4.Kronenberg M. Toward an understanding of NKT cell biology: progress and paradoxes. Annu Rev Immunol. 2005;23:877–900. doi: 10.1146/annurev.immunol.23.021704.115742. [DOI] [PubMed] [Google Scholar]
- 5.Wingender G, Kronenberg M. Role of NKT cells in the digestive system. IV. The role of canonical natural killer T cells in mucosal immunity and inflammation. Am J Physiol Gastrointest Liver Physiol. 2008;294:G1–8. doi: 10.1152/ajpgi.00437.2007. [DOI] [PubMed] [Google Scholar]
- 6.Zeissig S, Kaser A, Dougan SK, et al. Role of NKT cells in the digestive system. III. Role of NKT cells in intestinal immunity. Am J Physiol Gastrointest Liver Physiol. 2007;293:G1101–5. doi: 10.1152/ajpgi.00342.2007. [DOI] [PubMed] [Google Scholar]
- 7.Godfrey DI, Kronenberg M. Going both ways: immune regulation via CD1d-dependent NKT cells. J Clin Invest. 2004;114:1379–88. doi: 10.1172/JCI23594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wu L, Van Kaer L. Role of NKT cells in the digestive system. II. NKT cells and diabetes. Am J Physiol Gastrointest Liver Physiol. 2007;293:G919–22. doi: 10.1152/ajpgi.00242.2007. [DOI] [PubMed] [Google Scholar]
- 9.Fletcher MT, Baxter AG. Clinical application of NKT cell biology in type I (autoimmune) diabetes mellitus. Immunol Cell Biol. 2009 doi: 10.1038/icb.2009.5. [DOI] [PubMed] [Google Scholar]
- 10.Kinjo Y, Wu D, Kim G, et al. Recognition of bacterial glycosphingolipids by natural killer T cells. Nature. 2005;434:520–5. doi: 10.1038/nature03407. [DOI] [PubMed] [Google Scholar]
- 11.Mattner J, Debord KL, Ismail N, et al. Exogenous and endogenous glycolipid antigens activate NKT cells during microbial infections. Nature. 2005;434:525–9. doi: 10.1038/nature03408. [DOI] [PubMed] [Google Scholar]
- 12.Kinjo Y, Tupin E, Wu D, et al. Natural killer T cells recognize diacylglycerol antigens from pathogenic bacteria. Nat Immunol. 2006;7:978–86. doi: 10.1038/ni1380. [DOI] [PubMed] [Google Scholar]
- 13.Chang YJ, Kim HY, Albacker LA, et al. Influenza infection in suckling mice expands an NKT cell subset that protects against airway hyperreactivity. J Clin Invest. 2011;121:57–69. doi: 10.1172/JCI44845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Amprey JL, Im JS, Turco SJ, et al. A subset of liver NK T cells is activated during Leishmania donovani infection by CD1d-bound lipophosphoglycan. J Exp Med. 2004;200:895–904. doi: 10.1084/jem.20040704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wingender G, Kronenberg M. Invariant natural killer cells in the response to bacteria: the advent of specific antigens. Future Microbiol. 2006;1:325–40. doi: 10.2217/17460913.1.3.325. [DOI] [PubMed] [Google Scholar]
- 16.Wei B, Wingender G, Fujiwara D, et al. Commensal microbiota and CD8+ T cells shape the formation of invariant NKT cells. J Immunol. 2010;184:1218–26. doi: 10.4049/jimmunol.0902620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ivanov II, Atarashi K, Manel N, et al. Induction of intestinal Th17 cells by segmented filamentous bacteria. Cell. 2009;139:485–98. doi: 10.1016/j.cell.2009.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Selmi C, Balkwill DL, Invernizzi P, et al. Patients with primary biliary cirrhosis react against a ubiquitous xenobiotic-metabolizing bacterium. Hepatology. 2003;38:1250–7. doi: 10.1053/jhep.2003.50446. [DOI] [PubMed] [Google Scholar]
- 19.Wingender G, Rogers P, Batzer G, et al. Invariant NKT cells are required for airway inflammation induced by environmental antigens. J Exp Med. 2011;208:1151–62. doi: 10.1084/jem.20102229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Scupham AJ, Presley LL, Wei B, et al. Abundant and diverse fungal microbiota in the murine intestine. Appl Environ Microbiol. 2006;72:793–801. doi: 10.1128/AEM.72.1.793-801.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fujiwara D, Wei B, Presley LL, et al. Systemic control of plasmacytoid dendritic cells by CD8+ T cells and commensal microbiota. J Immunol. 2008;180:5843–52. doi: 10.4049/jimmunol.180.9.5843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brossay L, Chioda M, Burdin N, et al. CD1d-mediated recognition of an alpha-galactosylceramide by natural killer T cells is highly conserved through mammalian evolution. J Exp Med. 1998;188:1521–8. doi: 10.1084/jem.188.8.1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sidobre S, Kronenberg M. CD1 tetramers: a powerful tool for the analysis of glycolipid-reactive T cells. J Immunol Methods. 2002;268:107–21. doi: 10.1016/s0022-1759(02)00204-1. [DOI] [PubMed] [Google Scholar]
- 24.Wingender G, Krebs P, Beutler B, et al. Antigen-specific cytotoxicity by invariant NKT cells in vivo is CD95/CD178-dependent and is correlated with antigenic potency. J Immunol. 2010;185:2721–9. doi: 10.4049/jimmunol.1001018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Aranda R, Sydora BC, McAllister PL, et al. Analysis of intestinal lymphocytes in mouse colitis mediated by transfer of CD4+, CD45RBhigh T cells to SCID recipients. J Immunol. 1997;158:3464–73. [PubMed] [Google Scholar]
- 26.Matsuda JL, Naidenko OV, Gapin L, et al. Tracking the response of natural killer T cells to a glycolipid antigen using CD1d tetramers. J Exp Med. 2000;192:741–54. doi: 10.1084/jem.192.5.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Matsuda JL, Gapin L, Baron JL, et al. Mouse V alpha 14i natural killer T cells are resistant to cytokine polarization in vivo. Proc Natl Acad Sci U S A. 2003;100:8395–400. doi: 10.1073/pnas.1332805100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mazmanian SK, Liu CH, Tzianabos AO, et al. An immunomodulatory molecule of symbiotic bacteria directs maturation of the host immune system. Cell. 2005;122:107–18. doi: 10.1016/j.cell.2005.05.007. [DOI] [PubMed] [Google Scholar]
- 29.Hoebe K, Du X, Georgel P, et al. Identification of Lps2 as a key transducer of MyD88-independent TIR signalling. Nature. 2003;424:743–8. doi: 10.1038/nature01889. [DOI] [PubMed] [Google Scholar]
- 30.Ronet C, Darche S, Leite de Moraes M, et al. NKT cells are critical for the initiation of an inflammatory bowel response against Toxoplasma gondii. J Immunol. 2005;175:899–908. doi: 10.4049/jimmunol.175.2.899. [DOI] [PubMed] [Google Scholar]
- 31.Chang JH, Lee JM, Youn HJ, et al. Functional maturation of lamina propria dendritic cells by activation of NKT cells mediates the abrogation of oral tolerance. Eur J Immunol. 2008;38:2727–39. doi: 10.1002/eji.200838159. [DOI] [PubMed] [Google Scholar]
- 32.Nieuwenhuis EE, Matsumoto T, Lindenbergh D, et al. Cd1d-dependent regulation of bacterial colonization in the intestine of mice. J Clin Invest. 2009;119:1241–50. doi: 10.1172/JCI36509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schumann J, Voyle RB, Wei BY, et al. Cutting edge: influence of the TCR V beta domain on the avidity of CD1d:alpha-galactosylceramide binding by invariant V alpha 14 NKT cells. J Immunol. 2003;170:5815–9. doi: 10.4049/jimmunol.170.12.5815. [DOI] [PubMed] [Google Scholar]
- 34.Schumann J, Mycko MP, Dellabona P, et al. Cutting edge: influence of the TCR Vbeta domain on the selection of semi-invariant NKT cells by endogenous ligands. J Immunol. 2006;176:2064–8. doi: 10.4049/jimmunol.176.4.2064. [DOI] [PubMed] [Google Scholar]
- 35.Park SH, Benlagha K, Lee D, et al. Unaltered phenotype, tissue distribution and function of Valpha14(+) NKT cells in germ-free mice. Eur J Immunol. 2000;30:620–5. doi: 10.1002/1521-4141(200002)30:2<620::AID-IMMU620>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 36.Yokota S, Okabayashi T, Fujii N. The battle between virus and host: modulation of Toll-like receptor signaling pathways by virus infection. Mediators Inflamm. 2010;2010:184328. doi: 10.1155/2010/184328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Selmi C, Gershwin ME. Bacteria and human autoimmunity: the case of primary biliary cirrhosis. Curr Opin Rheumatol. 2004;16:406–10. doi: 10.1097/01.bor.0000130538.76808.c2. [DOI] [PubMed] [Google Scholar]
- 38.Mattner J, Savage PB, Leung P, et al. Liver autoimmunity triggered by microbial activation of natural killer T cells. Cell Host Microbe. 2008;3:304–15. doi: 10.1016/j.chom.2008.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.