Abstract
Growing evidence of epidemiological, clinical and experimental studies has clearly shown a close link between adverse in utero environment and the increased risk of neurological, psychological and psychiatric disorders in later life. Fetal stresses, such as hypoxia, malnutrition, and fetal exposure to nicotine, alcohol, cocaine and glucocorticoids may directly or indirectly act at cellular and molecular levels to alter the brain development and result in programming of heightened brain vulnerability to hypoxic-ischemic encephalopathy and the development of neurological diseases in the postnatal life. The underlying mechanisms are not well understood. However, glucocorticoids may play a crucial role in epigenetic programming of neurological disorders of fetal origins. This review summarizes the recent studies about the effects of fetal stress on the abnormal brain development, focusing on the cellular, molecular and epigenetic mechanisms and highlighting the central effects of glucocorticoids on programming of hypoxicischemic-sensitive phenotype in the neonatal brain, which may enhance the understanding of brain pathophysiology resulting from fetal stress and help explore potential targets of timely diagnosis, prevention and intervention in neonatal hypoxic-ischemic encephalopathy and other for brain disorders.
Keywords: Fetal stress, brain development, reprogramming, hypoxic-ischemic encephalopathy, glucocorticoids, epigenetics
1. Introduction
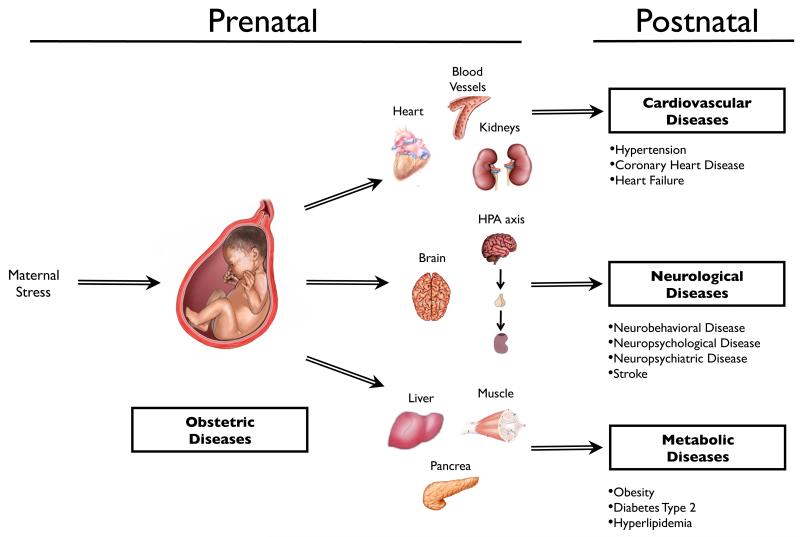
Fetal growth and development are a complex and dynamic process that depends on sophisticated interactions among the mother, placenta and fetus to ensure optimal growth and survival conditions (Warner and Ozanne, 2010). Numerous epidemiological, clinical and experimental studies have shown clearly that a compromised intrauterine environment may have subtle or drastic impact on tissue/organ ontogeny, structure and function, and alter the vulnerability or resiliency to some challenges and diseases in later life (Cottrell and Seckl, 2009; Dudley et al., 2011). Indeed, there is a wealth of evidence indicating that an adverse fetal environment, mostly manifested as intrauterine growth restriction (IUGR), is closely associated with increased risks of development of hypertension, coronary heart disease, insulin resistance, type 2 diabetes, central obesity, hyperlipidaemia, and other neurobehavioral, neuropsychological and neuropsychiatric disorders in adulthood (Barker et al., 1993a; Barker et al., 2009; Dudley et al., 2011; Gluckman and Hanson, 2004; Gluckman et al., 2008; Harris and Seckl, 2011). The hypothesis of “developmental programming of health and disease” or “fetal origins of adult disease” was put forward to elucidate these links between adverse intrauterine environment, fetal growth and development, and disease later in life (Figure 1) (Barker et al., 1993a; de Boo and Harding, 2006; Langley-Evans and McMullen, 2010; Seckl, 1998; Wadhwa et al., 2009; Warner and Ozanne, 2010). As being stated, environmental signals can be transmitted from the mother to the fetus, impacting specific vulnerable tissues in their sensitive developmental stage, modulating normal development trajectory, remodeling their structure and function and reprogramming the resiliency or susceptibility to diseases in postnatal life (Harris and Seckl, 2011). Such programming processes may be determined by multiple factors including gestational age, duration and mode of exposure and nature of the stressor, and these processes are tissue/organ specific (Harris and Seckl, 2011). Genetic traits, epigenetic modifications and central stress mediators such as glucocorticoids may underpin such phenotypic plasticity.
Figure 1. Developmental programming of health and disease.
Maternal stress impacts normal fetal tissues/organs development and increases the risk of development of cardiovascular, metabolic syndrome, stroke and various neurobehavioral, neuropsychological, neuropsychiatric diseases later in life. HPA, hypothalamic-pituitary-adrenal
Brain is one of the critical targets of stressors and is also the central organ responsible for stress responses, determining the adaptive or maladaptive responsiveness to various acute and chronic stressful events via making corresponding alterations in its structure and function (McEwen, 2008). The developing brain in the fetal stage is also highly plastic, flexible, and especially sensitive to numerous adverse environmental factors. Combined with its specific genetic traits, these changes of fetal brain contribute to high incidence of a wide spectrum of neurodevelopmental disorders in the postnatal life. It has been well documented that fetal stresses, such as hypoxia, malnutrition, substances exposure (nicotine, alcohol and cocaine) and excess glucocorticoids (endogenous or exogenous), have long lasting impact on the developing brain; altering brain’s ontogeny, organization, structure and function; remodeling brain’s development trajectory, and reprogramming brain’s vulnerability or resiliency of some neurobehavioral, neuropsychological and neuropsychiatric disorders in later life (Archer, 2011; Chen and Zhang, 2011; Chiriboga, 1998; Harris and Seckl, 2011; Seckl and Meaney, 2004; Zhang et al., 2005).
Neonatal hypoxic ischemic encephalopathy (HIE) is one of major causes of acute mortality as well as chronic neurological disability in newborns (Chen et al., 2009b; Vannucci, 2000). Up to 25% of survivors demonstrate permanent neurological deficits such as cerebral palsy, mental retardation, learning disability and epilepsy (Perlman, 2006; Vannucci, 2000). However, there is no universally accepted therapy available for HIE except that a few studies implied that moderate hypothermia, administered in the early phase for full term neonates with mild or moderate encephalopathy, may reduce mortality and disability at 18 months (Perlman, 2006; Rees et al., 2011). Before the availability of more potent effective therapy emerges, it is essential to explore all potential modifiable risk factors that may provide us with promising targets to prevent or improve the outcome of this encephalopathy. There is strong evidence suggesting that various prenatal stress insults may be the promising candidates meriting exploration.
In this review, we summarize recent studies about the programming effects of prenatal stress on fetal brain development and its associated diseases in later life, especially the programming effects of sensitive phenotype to neonatal hypoxic ischemic encephalopathy, particularly highlighting the cellular and molecular mechanisms and emphasizing the critical roles of glucocorticoids and epigenetic modification, which may enrich us with the knowledge of its underlying pathophysiology and contribute to exploration of some potential preventive and therapeutic interventions for neonatal HIE injury.
2. Fetal stress, abnormal brain development and associated diseases
2.1. Fetal hypoxia
Prenatal hypoxia-ischemia (HI) refers to a reduced level of oxygen (hypoxia) and a decreased blood flow (ischemia) during fetal development, which can cause various complications during pregnancy associated with neurological deficits and long-term neurodevelopmental disabilities in later life. One of these complications is cerebral palsy that occurs in 2 per 1,000 babies (Graham et al., 2008). Of these, 15% - 20% will die during the postnatal period, and another 25% will develop permanent severe neuropsychological conditions.
The hypoxia-inducible transcription factors (HIFs) are one of the adaptive mechanisms activated during the HI insult. Hypoxia stabilizes HIF-1α subunit that binds to HIF-1β subunit and induces target genes transcription to regulate oxygen homeostasis. Some of these genes associated with the HIF-1 regulation include erythropoietin (EPO) that plays an important role in cell survival, vascular endothelial growth factor (VEGF) that activates endothelial cells leading to capillary sprouting (Vazquez-Valls et al., 2011) and glucose transporter-1 (GLUT-1) that affects the cellular glucose metabolism (Wood et al., 2009). HIFs play a crucial role in stimulating vascular development, angiogenesis and metabolic adaptation during brain development, which have been demonstrated in gene knockout experiments (Milosevic et al., 2007; Tomita et al., 2003).
Fetal hypoxia affects normal brain development and induces abnormal behavioral presentations. The cerebral cortex, hippocampus and sub-ventricular zone are the most vulnerable regions to the hypoxic insult (Northington et al., 2001). A mouse model study has indicated that prenatal hypoxia produces a mild neurological deficit in a variety of behavioral tests. For example, the duration in an accelerating rotarod test was shorter for the offspring with prenatal hypoxic exposure compared to the control offspring, and they traveled a shorter distance and spent most of their time stationary compared with the control group (Ireland et al., 2010). Some structural proteins of the white matter were measured in adult offspring with prenatal hypoxic exposure during gestational days 7 to 21. These structural proteins were associated with normal development of myelin and axon, and their expression levels decreased due to maternal hypoxia while the expression of protein related to astroglia increased, predisposing the individual to white matter changes later in life (Wang et al., 2010). Hippocampus is one of the most common targets in the brain during ischemic injury. Phospholipase A2 (PLA2) plays an important role in the underlying mechanism associated with the neuronal degeneration as was found in a study performed with hippocampal slices of Wistar/ST rats. The PLA2 activity was evaluated in an oxygen-glucose deprivation environment in which the most vulnerable sub-region of the hippocampus was CA1 and cytosolic PLA2 (cPLA2) was associated with neuronal death (Arai et al., 2001). Another possible mechanism of fetal brain injury due to prenatal hypoxia could be associated with inflammation. A recent work reported that chronic hypoxia exposure induced an increase in the lactate:pyruvate ratio and a decrease in the GSH:GSSG ratio, a favorable pro-oxidant state, in the brain of Duncan-Harley guinea pigs. Additionally, the expression levels of pro-apoptotic proteins Bax, Bcl-2 and p53 increased as well as the levels of some pro-inflammatory cytokines (Guo et al., 2010).
Prenatal hypoxia exposure also affects other vital organs/tissues in addition to the brain, which may contribute to various pathologies in later life. Studies in rats have shown that gestation hypoxia causes fetal heart remodeling and increases heart susceptibility to ischemia and reperfusion injury in offspring (Tong et al., 2011; Tong and Zhang, 2011; Li et al., 2003; Patterson et al., 2010; Rueda-Clausen et al., 2009). In mice, maternal hypoxia resulted in a significant increase in pulmonary mRNA levels of angiotensin converting enzyme (ACE) 1, 2 and angiotensin II Type 1b (AT-1b) receptors and the protein levels of renin and ACE-2, but a decrease in protein levels of ACE-1 (Goyal et al., 2011). These results demonstrated that prenatal hypoxia affected the expression patterns of pulmonary renin-angiotensin-system (RAS) and suggested a possible mechanism contributing to the pathophysiology of pulmonary hypertension in offspring. Hypoxia may also be associated with fetal inflammatory response syndrome (FIRS). Maternal hypoxia has been shown to increase protein levels of interlukin-6 (IL-6) and tumor necrosis factor alpha (TNF-a) in fetal guinea pig sera and the mRNA expression in the lung, heart and brain (Yafeng et al., 2009). In the placenta, 11-beta-hydroxysteroid dehydrogenase type 2 (11β-HSD2) that catalyzes the conversion of cortisol to inactive cortisone, plays an important role in protecting the fetus from exposure to a high level of maternal glucocorticoids. Studies in human trophoblast cells demonstrated that hypoxia inhibited the activity of 11β-HSD2 and decreased its protein expression (Homan et al., 2006).
2.2. Maternal malnutrition
One of the most common hostile environmental insults for the fetus is maternal malnutrition during the gestational period. Maternal malnutrition includes maternal overnutrition (high-fat, high-energy and high-protein diets) and maternal undernutrition (caloric and protein restriction diets or low-vitamin intake), both of which may contribute to perinatal programming (Elahi, et al., 2009; Erkkola et al., 2011; Gniuli, et al., 2008; Guilloteau et al., 2009; Rasch et al., 2004).
In addition to its well-studied effects on programming of metabolic and cardiovascular disease (Barker and Osmond, 1986; Bateson et al., 2004; Gluckman et al., 2008; McMillen and Robinson, 2005), maternal malnutrition during pregnancy causes permanent brain dysfunction, especially cognitive and behavior deficits accompanied by alterations of neuronal excitability as well as structural changes in the developing and adult brain (Grantham-McGregor and Baker-Henningham, 2005; Levitsky and Strupp, 1995; Morgane et al., 1993; Morley and Lucas, 1997; Olness, 2003; Walker et al., 2007). A recent study evaluated the impact of global 30% maternal nutrient reduction on early fetal baboon brain maturation and found major cerebral developmental disturbances including neurotrophic factor suppression, cell proliferation and cell death imbalance, impaired glial maturation and neuronal process formation, down-regulation of gene ontological pathways and related gene products, and up-regulated transcription of cerebral catabolism without fetal growth restriction or marked maternal weight reduction (Antonow-Schlorke et al., 2010). This finding suggests that moderate nutrient reduction during pregnancy is an important epigenetic factor that provides suboptimal conditions for appropriate fetal brain development with potential life-long consequences.
Maternal undernutrition causes marked epigenetic changes in hypothalamic genes and increases both glucocorticoid receptor (GR) and proopiomelanocortin (POMC) gene expression in the fetal brain, which is likely to contribute to fetal programming of a predisposition to obesity via altered GR regulation of POMC and neuropeptide Y as well as to altered regulation of food intake, energy expenditure, and glucose homeostasis later in life (Stevens et al., 2010). Additionally, maternal undernutrition may affect sensorimotor functions via its action on the CNS (Sanches et al., 2011; Ba, 2005), the growth of major and minor cranial components (Cesani et al., 2006), the structure of the brain (Torres et al., 2010), and brain development itself (Melse-Boonstra et al., 2010; Ohishi et al., 2010; Ranade et al., 2011). Maternal malnutrition may selectively decrease the number of neurons in some regions of the hippocampus, for example, CA2, CA4 and DG but not in CA1 and CA3 (Florian et al., 2010). In addition to global maternal nutrient reduction, maternal choline deficiency during pregnancy alters neurogenesis and angiogenesis in fetal hippocampus (Albright et al., 1999a, b, 2005; Craciunescu et al., 2003; Mehedint et al., 2010; Niculescu et al., 2004, 2006).
Not only does maternal undernutrition have a negative impact on fetal brain development, but maternal overnutrition during gestation has also been shown to permanently alter brain structure and function in the offspring. Studies in pregnant rats fed a high-fat diet showed increased neural progenitor proliferation in the hypothalamus of fetal and neonatal brains (Chang et al., 2008). Additional studies in mice demonstrated that maternal high-fat diet altered fetal hippocampal development as indicated by region-specific changes in proliferation of neural precursors, decreased apoptosis and neuronal differentiation within the dentate gyrus, resulting in the decreased neurogenesis in the dentate gyrus in young adult offspring (Niculescu and Lupu, 2009; Tozuka et al., 2009). Neonates exposed to maternal high-fat diets also showed a negative impact on the brain development (Walker et al., 2008).
2.3. Fetal nicotine exposure
Although the negative effects of cigarette smoking on the development of the fetus and the newborn are well-known, it is estimated approximately 22% of mothers and 45% of fathers continue to smoke during the time of their children’s birth (Nelson and Taylor, 2001). Studies indicate there are about 250 million female smokers around the world and over 700,000 children born with exposure to cigarette smoking each year in the United States (Pauly and Slotkin, 2008). Thus, cigarette smoking may represent the single largest modifiable neuropharmacological exposure for the fetus and newborn (Wickstrom, 2007). Currently, nicotine replacement therapy (NRT) is recommended by some obstetricians to help women quit smoking during pregnancy although there are serious concerns about its effectiveness and safety to the mother and her fetus (Pauly and Slotkin, 2008; Wickstrom, 2007).
There are more than 4,000 chemicals in tobacco including carbon monoxide, cyanide, etc., of which nicotine is the major compound with neurotoxicity (Dwyer et al., 2009). Nicotine can easily cross the placental barrier and concentrate in fetal circulation, brain, amniotic fluid and even breast milk during lactation (Wickstrom, 2007). Directly or indirectly, nicotine can exert a variety of adverse effects on fetal development. Nicotine may induce poor nutritional status of mothers via its anorexigenic effect and compromise blood flow to the placenta through enhanced release of catecholamine from adrenals and sympathetic nerve terminals, which may also contribute to chronic placenta insufficiency. More importantly, nicotine can directly affect fetal developmental patterns through the activation of nicotinic acetylcholine receptors (nAChRs). Ample human studies have revealed nicotine exposure during pregnancy is associated with a spectrum of adverse fetal and obstetrical outcomes: spontaneous abortion, placenta previa, placental abruption, preterm birth, stillbirth, fetal growth restriction, low birth weight, and, more severely, sudden infant death syndrome (SIDS) (Archer, 2011; Bruin et al., 2010; Eppolito and Smith, 2006; Slotkin, 1998).
Epidemiological, clinical and experimental studies indicate that adverse effects of prenatal nicotine exposure are far beyond the pregnancy outcomes and neonatal morbidity or mortality. Long-term adverse neurodevelopmental consequences of perinatal nicotine exposure constitute the greatest impact on society. A large amount of evidence suggests that nicotine plays a key role in mediation of long-term neurological developmental deficits resulting from maternal smoking. As one of the major psychoactive agents, nicotine exerts its effects via interaction with various subtypes of nAChRs localized in specific brain regions with programmed temporal and spatial distribution patterns, affecting a multitude of neurotransmitters’ synthesis, release, reuptake and turnover; modulating neural proliferation, differentiation, migration and apoptosis, etc.; altering brain structure, organization and morphology; disrupting normal brain development, which finally contributes to heightened vulnerability to various neurobehavioral, neuropsychological and neuropsychiatric disorders in postnatal life (Bruin et al., 2010; Dwyer et al., 2008; Dwyer et al., 2009; Ernst et al., 2001; Pauly and Slotkin, 2008; Wickstrom, 2007).
Growing epidemiological studies have revealed that prenatal nicotine exposure is associated with various levels of motor and sensory deficits, high incidence of externalizing behavioral problems (such as oppositional, aggressive, overactive), increased risk of attention-deficit/hyperactivity disorder (ADHD) and conduct disorder (CD), cognitive function impairment in memory, attention and learning, and the risk for developing drug dependence (e.g. nicotine, cocaine) (Eppolito and Smith, 2006; Ernst et al., 2001; Wickstrom, 2007). Consistently, animal studies, mostly in rodents with prenatal nicotine exposure, also demonstrate similar presentations including hyperactivity, cognitive and somatosensory impairment, exaggerated anxiety, neurochemical imbalance, nicotine self-administration, reduction of neural cell survival and aberrant synaptogenesis (Dwyer et al., 2008; Dwyer et al., 2009; Eppolito and Smith, 2006; Levitt, 1998). These detrimental brain effects can be induced without apparent birth weight reduction, a crude marker of poor intrauterine environment, implying the threshold of brain damage by nicotine is much lower than that of inducing IUGR (Slotkin, 1998).
Another problem deserving concern is the NRT during pregnancy. Recently, the NRT is widely accepted and recommended to pregnant smokers although there is a lack of convincing solid evidence for its efficacy and safety. Pharmacologically, the plasma half-life of nicotine is about 2 h (Wickstrom, 2007). However, nicotine is metabolized more quickly during pregnancy, which indicates that higher doses of NRT may be needed to attain an effect for cessation of smoking (Wickstrom, 2007). In addition, most formulations of NRT deliver nicotine continuously compared to episodic smoking in smokers. The total exposure dosage of nicotine by NRT may actually exceed those of pregnant women with mild or moderate cigarette smoking (Wickstrom et al., 2002). Furthermore, given the fact of lower threshold for altering brain development by nicotine, more extensive studies should be conducted to justify the efficacy and safety of NRT to the fetus before its continued application in pregnancy (Pauly and Slotkin, 2008; Wickstrom, 2007).
2.4. Fetal cocaine exposure
Cocaine has been one of the most popular illicit drugs over the past 30 years. It is estimated that approximately 30% of young adults reported having used cocaine at the height of the epidemic years (O’Malley et al., 1991). In some urban areas, about 10-45% of women consume cocaine during pregnancy (Chiriboga, 1998; Gressens et al., 2001). Although cocaine abuse began to decline in recent years, it is still one of the major concerns of public health because of its potential long-term adverse effects, especially for the offspring prenatally exposed to cocaine.
Similar to nicotine, cocaine is a highly psychoactive stimulating agent with various effects but has much shorter half-life compared with nicotine (Slotkin, 1998). The most prominent pharmacological effect of cocaine is the inhibition of synaptic reuptake of monoamines, such as catecholamine, dopamine and serotonin, which may contribute to disturbance of autonomic function and aberrant neurotransmission (Chiriboga, 1998; Seidler and Slotkin, 1992; Slotkin, 1998). Like nicotine, cocaine can also easily cross the placenta and concentrate in the fetus with variable levels (Chiriboga, 1998). In addition to its direct inhibitory effects on cell replication, cocaine can induce intense vasoconstriction and compromise maternal cardiovascular system, leading to significant fetal hypoxia/ischemia and/or malnutrition, exerting great impact on fetal development (Anderson-Brown et al., 1990; Chiriboga, 1998; Seidler and Slotkin, 1992).
Although there are some controversies about its negative effects on fetal development recently, large epidemiological, clinical and animal studies have shown the correlation of prenatal cocaine exposure with numerous obstetrical, pediatric and neurobehavioral abnormalities (Ackerman et al., 2010; Chae and Covington, 2009; Chiriboga, 1998; Gressens et al., 2001). For example, in early epidemic years, the term “crack/cocaine baby” was widely employed to depict the infants born to women with cocaine abuse during pregnancy although such a term lacked solid substantiation and was challenged by careful analysis (Chiriboga, 1998). Intrauterine cocaine exposure has been linked to increased risk of spontaneous abortion, abruption of placenta, stillbirth, fetal stress, meconium staining, and premature delivery (Chiriboga, 1998). More importantly, prenatal cocaine exposure is associated with microcephaly, cerebral malformation, focal hypoxic/ischemic or intraventricular hemorrhage damage and perturbation of cerebral cytoarchitecture (Chiriboga, 1998; Gressens et al., 2001; Kandall et al., 1993; Levitt, 1998). After delivery, the offspring with cocaine exposure demonstrates sleep disturbances, feeding difficulties, hypertonic tetraparesis and in some cases with seizure attacks (Chiriboga, 1998; Gressens et al., 2001). Some studies even suggested a possible correlation of cocaine exposure with SIDS (Durand et al., 1990; Gressens et al., 2001; Kandall et al., 1993). These clinical presentations are usually associated with transiently abnormal neurophysiological tests, which may disappear within the first year (Gressens et al., 2001).
It seems that the effects of cocaine exposure on the developing brain are more subtle than those of nicotine (Slotkin, 1998). However, behavioral studies in animal models and recent clinical findings in humans have revealed that prenatal cocaine exposure may also produce long-term neurodevelopmental consequences although its outcome may be subtle and need more sophisticated or challenging methodologies to revelation. The children with cocaine exposure often demonstrate some moderate but significant neuropsychological deficits at school age including difficulty in concentration, weak resistance to distracters, aggressive behavior and impulsivity (Ackerman et al., 2010; Gressens et al., 2001). However, their IQ score may be within the normal range. In addition, these children are vulnerable to development of anxiety or depression (Gressens et al., 2001). Alteration of anatomical structure and perturbation of neurotransmission induced by prenatal cocaine exposure may contribute to such deficits. In addition to its neurological effects, animal studies showed that fetal cocaine exposure resulted in programming of cardiovascular dysfunction in offspring (Bae and Zhang, 2005; Bae et al., 2005; Xiao et al., 2009a, b).
2.5. Fetal alcohol exposure
As a well-known teratogen, consumption of alcohol (ethanol) at different gestational stages can produce a wide range of adverse effects on the normal growth and development of the fetus. Fetal alcohol spectrum disorders (FASD) is the term used to describe many problems associated with prenatal alcohol exposure (PAE) (Jones et al., 2010). In the USA, 1 in 12 pregnant women drink during the gestational period and each year around 40,000 babies are born with FASD. Fetal alcohol syndrome (FAS) is the most severe form of the alcohol spectrum disorders, associated with pre- and postnatal growth retardation and delayed neurological development, and occurs in 0.3-2.2 per 1,000 babies in the USA for the past half-century (Ripabelli et al., 2006). Ethanol crosses the placenta and circulates in the bloodstream of the fetus, affecting the development of fetal cells and tissues. The effect of ethanol in the fetus depends on a series of factors such as doses, exposure time, gestational age and others (de Licona et al., 2009; Maier and West, 2001).
Neurocognitive deficits have been related constantly to PAE. Epidemiology studies also reported that adults with FAS exhibit various neurobehavioral problems. FAS patients present a lot of social problems and don’t have friends, but on the other hand, they feel vulnerable and need care and assistance. Their academic performance is also very poor, most of them drop out or fail school, while aggressiveness is the most common emotional disorder in them (Freunscht and Feldmann, 2011). Rats in PAE demonstrate anxiety- and depressive-like behaviors similar to FAS, and a mechanism that involved oxidative stress has been suggested to induce this effect (Brocardo et al., 2011). Low levels of human alpha-fetoprotein (HAFP) in pregnant women have been associated with FAS in the offspring (Halmesmaki et al., 1987). Although the physiological role for HAFP is not clear, it is known that this protein binds to some transcription factor initiators (e.g., Retinoic acid and estradiol). Thus it may be associated with gene regulation as part of epigenetic mechanisms (King, 2011).
2.6. Fetal glucocorticoids exposure
Glucocorticoids during development are essential for normal maturation of various vital organs and contribute to immediate survival after birth. However, overexposure of the fetus to glucocorticoids at the critical developmental window of specific organs may alter normal developmental trajectory and lead to permanent reprogramming of structure and function (Cottrell and Seckl, 2009; Harris and Seckl, 2011; Seckl and Meaney, 2004). Glucocorticoids bind to their specific intracellular receptors (glucocorticoid receptor, GR and mineralocorticoid receptor, MR, respectively), acting as nuclear transcription factors to control target gene expression, regulating cell proliferation, differentiation, apoptosis and survival.
Maternal stress, psychosocial or adverse environmental factors can easily trigger the release of various levels of glucocorticoids or disrupt uteroplacental barrier, such as 11β-HSD2 (Mairesse et al., 2007). During glucocorticoids therapy, which is usually employed in conditions such as preterm delivery when immature lung may threaten neonatal survival or antenatal treatment of congenital adrenal hyperplasia (CAH), synthetic glucocorticoids (dexamethasone, betamethasone) may be administered prenatally (Whitelaw and Thoresen, 2000). Although the antenatal glucocorticoids treatment is critical for facilitating the maturation of vital organs and tissues and favoring short-term survival, it may also confer on the fetus adverse levels of glucocorticoids and impair normal fetal development with long-term adverse consequences.
A plethora of evidence has indicated that overexposure to glucocorticoids during vulnerable periods of fetal development correlates with low birth weight, increased risk of premature delivery and adverse outcomes in the offspring. In human studies, there are substantial descriptions of linkage between low birth weight and increased risk of development of cardio-metabolic syndrome, such as hypertension, coronary heart disease, obesity, hyperlipidaemia, insulin resistance, type2 diabetes, stroke, etc., in adulthood (Barker et al., 1993a; Barker et al., 1993b; Cottrell and Seckl, 2009; Fall et al., 1995; Harris and Seckl, 2011; Moore et al., 1996; Rich-Edwards et al., 1997). Such association is independent of other adult life-style risk factors, including smoking, alcohol abuse, lack of exercise, obesity, and poor socioeconomic status (Harris and Seckl, 2011; Leon et al., 1996; Levine et al., 1994; Osmond et al., 1993). Consistently, a wide variety of animal studies also supported these results. For example, enhanced exposure of cortisol from maternal or fetal origins is associated with elevated blood pressure in sheep fetuses (Tangalakis et al., 1992). Dexamethasone treatment during pregnancy in rats demonstrates lower birth weight, increased blood pressure and glucose intolerance in adulthood (Benediktsson et al., 1993). Similar results are found in pregnant baboons treated with repeated doses of betamethasone (Koenen et al., 2002). Lower birth weight may not be the cause of these diseases, which may be considered as a crude indicator of suboptimal intrauterine environment and predictor of increased risk of pathophysiology in later life.
The brain is a major target of glucocorticoids. Both types of glucocorticoid receptors (GR, MR) are widely expressed in the brain, such as in hippocampus, amygdala, lateral septal nuclei and some other cortical areas. Glucocorticoids play a crucial role in normal fetal brain development via initiating terminal maturation, remodeling axon and dendrite growth and affecting cell survival (Harris and Seckl, 2011; Meyer, 1983; Yehuda et al., 1989). Sustained high levels of glucocorticoids in the fetus may exert an adverse impact on brain cell and structure by disturbing the hypothalamic-pituitary-adrenal (HPA) axis, neurotransmitter balance and synaptic plasticity, which may contribute to abnormal neurodevelopment and the heightened brain vulnerability to diseases in the postnatal life (Weinstock, 2008). Studies including a wide range of species demonstrate that prenatal glucocorticoids exposure impairs intrauterine growth, re-sets the HPA axis sensitivity, and increases the risk of cardio-metabolic and affective disorders in later life. The greatest effects of glucocorticoids on birth weight usually occur in late pregnancy when fetal growth is accelerating. Prenatal glucocorticoids exposure may result in programming of heightened HPA axis sensitivity to stressful events in later life, leading to permanently increased levels of cortisol or corticosterone in offspring. Higher HPA axis activity confers enhanced response to stress and challenge, underpinning some neurobehavioral and psychiatric abnormalities (Cottrell and Seckl, 2009; Harris and Seckl, 2011; Seckl and Meaney, 2004).
Numerous studies also revealed the correlation between low birth weight and affective, psychiatric and cognitive disorders, such as schizophrenia, attention deficit/hyperactivity (ADHD), antisocial behavior, increased susceptibility to post-traumatic stress disorder (PTSD), anxiety disorders, lower IQ score or learning disability, and depression-like behaviors (Cannon et al., 2002; Famularo and Fenton, 1994; Harris and Seckl, 2011; Jones et al., 1998; Lahti et al., 2009; Raikkonen et al., 2008; Thompson et al., 2001; Wiles et al., 2005; Wust et al., 2005). These findings in humans have been corroborated in substantial animal model studies. For example, maternal prenatal stress is correlated with attention deficits in offspring of non-human primates. Rodent model studies demonstrated increased anxiety and depressive-like behavior and compromised cognitive capability in adulthood (Meaney and Szyf, 2005). It seems plausible that excess fetal glucocorticoids exposure may at least partly represent one common pathway in which adverse environmental cues transferred from the mother to the fetus, altering brain developmental trajectory, permanently affecting cerebral structure and function, and reprogramming the vulnerability to later challenges and diseases (Cottrell and Seckl, 2009).
3. Mechanisms of fetal stress-mediated programming
3.1. Aberrant cell behavior and structure remodeling in the brain
Brain development consists of a series of progressive and regressive events tightly regulated by the interaction between cellular and environmental factors. Fetal brain development is especially susceptible to environmental perturbations. Prenatal stress exposure affects various neurotransmitters, neuromodulators, neurotrophic factors and cell adhesion molecules, etc., at specifically susceptible stages to alter neuronal development via both acute and chronic effects on cellular behavior and gene expression patterns (Levitt, 1998). Aberrant cellular behavior and gene expression confer permanent structure remodeling and function reprogramming, which may lead the brain to be more vulnerable to later challenges.
Nicotine exerts effects mainly through triggering the release of acetylcholine via stimulation of specific subtypes of nAChRs. The most abundant subtypes of nAChR in vertebrate brain are α4β2 and α7, of which α7 is highly expressed in the immature brain. These are implicated in the response to brain injury and inflammation and participate in regulating the rate of apoptosis, and thus may be a potential candidate mechanism in abnormal fetal brain development caused by nicotine exposure (Pauly et al., 2004; Pauly and Slotkin, 2008; Verbois et al., 2000). Acetylcholine acts as a neurotrophic factor in brain development and is involved in cell proliferation, cell differentiation, survival, apoptosis, neuritic outgrowth, neuronal migration, synaptogenesis, and establishment of neuronal circuitry and modulation of other neurotransmitters releasing. Inappropriate premature stimulation of nAChRs during fetal development may disrupt normal prescheduled program of time and/or intensity of these neurotrophic effects and induce abnormal brain development with long-term consequences.
Human and animal studies have shown that prenatal nicotine exposure can up-regulate nAChRs’ density in specific brain regions. However, such up-regulation may be only a compensatory response accompanied by lower function (Wickstrom, 2007). Fetal nicotine stimulation may cause target cells to prematurely switch from proliferation to differentiation and thus alter synaptogenesis. Additionally, nicotine stimulation can induce inappropriate apoptosis in several cell types, such as undifferentiated hippocampus progenitor neurons, dentate gyrus neurons in rats and murine neurons in olfactory bulb (Pauly and Slotkin, 2008). These cellular damages may be dependent on distribution patterns of nAChRs in the brain, leading to region specific abnormality of cell number and other macromolecular contents. The damages of nicotine to cells in the brain can be indicated by increased levels of cell damage markers (ornithine decarboxylase activity) and reduced DNA levels. In addition, these damages may be accompanied by increased gliogenesis, in which glial cells not neuronal cells replace the missing cells and lead to abnormal function (Pauly and Slotkin, 2008; Slotkin, 1998). Thus, structural alterations such as reduction of brain weight, thinner cortical thickness and smaller cell size ensue.
The adverse effects of prenatal cocaine exposure on brain development are more controversial among major drug abuse. Some clinical data suggest that cocaine might exert minimal effects on body size, brain structure and behavioral abnormality compared with alcohol and nicotine (Levitt, 1998; Richardson, 1998). However, recent animal studies revealed some consistent adverse changes in brain development. Experimental studies in mice and monkeys demonstrated that prenatal cocaine exposure resulted in attenuation of neuronal production, disturbance of neuronal migration and differentiation, aberrant gliogenesis and long-term anatomical, molecular and biochemical changes of aminergic systems (Gressens et al., 1992; Gressens et al., 2001; Kosofsky et al., 1994; Levitt, 1998; Lidow, 1995). Most of studies suggest that these neuroanatomical changes are consistent with the distribution patterns of the dopamine (DA) system in the brain, and DA rich brain regions such as the anterior cingulated cortex (ACC) and medial prefrontal cortex (MPF) are predominantly affected, implying that the DA system is the major target of cocaine and confers its major adverse effects on behavior and emotion programming (Dewar and Reader, 1989; Goldman-Rakic and Brown, 1982; Jones et al., 1996; Levitt, 1998; Levitt et al., 1984). Prenatal glucocorticoids exposure also exerts pronounced effects on brain cellular behavior and structure alteration, which will be discussed in the following part.
Maternal protein restriction (MPR) adversely affects fetal brain development by altering astrocytogenesis, the extracellular matrix, neuronal differentiation and programmed cell death (Gressens et al., 1997). In a recently study, it was reported that maternal protein-restricted diet during pregnancy altered the expression patterns of proteins and mRNA related with brain RAS in the fetus, which could be associated with pathogenesis of hypertension (Goyal et al., 2010). Additionally, maternal low-protein diet decreases the activity of antioxidant enzymes, such as superoxide dismutase (SOD) and catalase (CAT), in fetal rat cerebellums,, which in turn increases the lipid oxidation damage, affecting normal cerebral functions such as coordination control, posture, fine adjustment and cognition process (Bonatto et al., 2006; Torres et al., 2010). It has been further demonstrated that maternal protein-restricted diet decreases lipid levels and DHA in fetal rat brains. These studies suggest that MPR may have a negative impact on fetal brain development. The neuronal metabolic activity is decreased by a low iron diet, and this may affect the recovery of the brain after a brain injury (Rao et al., 1999). MPR also affects the expression of proteins involved in the mitochondrial function, neurogenesis and synaptogenesis. The expression of proteins associated with oxidative pathways in the hippocampus was regulated by MPR (Alexandre-Gouabau et al., 2011). For example, Bcs1L is down-regulated, which is consistent with a decrease in cytochrome c oxidase activity. The expression of NdUf8, a subunit of mitochondrial complex I, is also suppressed by MPR. Another group of proteins involved in the regulation of cellular processes, known as 14-3-3, were also down-regulated as well as PrdX 3, a protein with antioxidant function localized in the mitochondria. It has also been reported that MPR downregulated the expression of Arp3 in the fetal brain, which is part of the Arp2/3 complex involved in the regulation of actin cytoskeleton, as well as Arp1, the fascin and the MAPKK1. Interestingly, MPR upregulated MAP2 and TCP1, playing a significant role in the synaptic activity.
Brain cells consume 20% of the total oxygen available for all the organs, which makes the brain one of the organs with a significant production of reactive oxygen species (ROS) (Sokoloff, 1999). Additionally, the brain tissue is rich in unsaturated fatty acids, which could be the substrate for lipid peroxidation (Halliwell, 1992). Another reason that makes the brain an organ that could produce high levels of ROS is due to the oxidization of neurotransmitters such as DA, serotonin and norepinephrine. In the fetus, brain cells are prone to be affected by neurotoxic effect due to oxidative stress than in adults because the levels of antioxidant agents are lower in fetal brains than those in adult brains (Bergamini et al., 2004; Henderson et al., 1999). Liver is the organ where ethanol is mostly metabolized, and this process can affect the intracellular redox state of the central nervous system due to a direct dysregulation of mitochondrial bioenergetics. Interestingly, mitochondrial dysfunction has been found in cerebral tissues of fetal mice exposed to ethanol (Xu et al., 2005). One of the enzymes that can metabolize ethanol in the liver is cytochrome P450 2E1 (CYP2E1). It was reported that PAE increased the activity of CYP2E1 in the fetal liver and mitochondrial fractions in guinea pigs (Hewitt et al., 2010). Neuronal loss is one of the most prominent alcohol-induced pathological changes. PAE could make the brain more vulnerable by affecting the expression or neuroprotective role of nitric oxide (NO) (Bonthius et al., 2004) associated with the cGMP pathway (Karacay et al., 2007). Studies in mice have shown that deficiency in nNOS may induce neuronal loss and microencephaly (de Licona et al., 2009).
3.2. Dysregulation of the HPA axis and perturbation of neurotransmitters
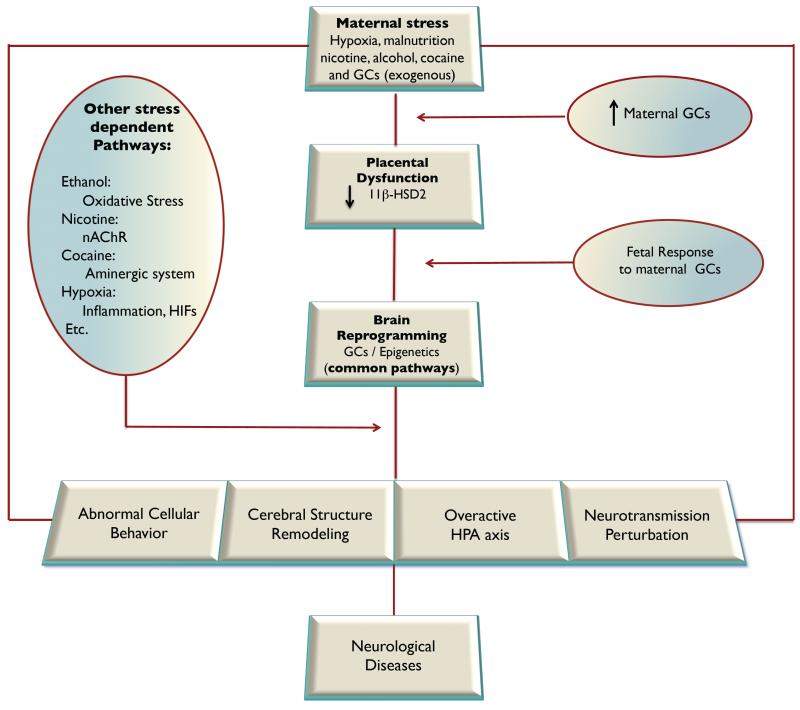
Intrauterine programming of the HPA axis may be one of the key common mechanisms underpinning prenatal stress and increased risk of diseases later in life (Figure 2). Fetal HPA axis is highly susceptible to programming actions during development. Prenatal stress, such as nicotine, cocaine, alcohol, hypoxia, malnutrition and glucocorticoids, can directly or indirectly alter the “set point” of the HPA axis, and enhance the activity of the HPA axis in basal and stressful conditions throughout life. Glucocorticoids play the pivotal role in such programming processes, which may be also associated with other stress mediators, such as catecholamines (Lee et al., 2008). Structures of the limbic system, including hippocampus, hypothalamus, anterior pituitary and amygdala, express high levels of GR, constituting the major target of endogenous or exogenous glucocorticoids in the brain. These GRs, particularly in hippocampus, exert crucial negative feedback regulation on the activity of the HPA axis. Maternal stress or synthetic glucocorticoids administration result in high levels of glucocorticoids exposure to the fetus, leading to down-regulation of GR in hippocampus and attenuation of negative feedback of the HPA axis and enhancement of the HPA axis activity. The overall effects of programming allow body being exposed to sustained elevated endogenous glucocorticoids in both basal and stress conditions, resulting in alteration of behavior, cognition, learning, memory, emotion and predisposing the individual to a variety of cardiovascular and metabolic syndromes in later life (Harris and Seckl, 2011).
Figure 2. Mechanisms of developmental programming of neurological diseases.
Prenatal stress changes normal brain developmental trajectory, alters brain cellular behavior, remodels cerebral structure and morphology, reconstructs the HPA axis activity, disturbs neurotransmission, and reprograms the vulnerability or resiliency to neurological diseases in later life, of which epigenetic modifications of glucocorticoid receptors (GCs) gene expression patterns may represent as a common pathway in response to different adverse intrauterine stimuli. 11β-HSD2, 11-beta-hydroxysteroid dehydrogenase type-2; HIFs, hypoxia inducible factors; HPA, hypothalamic-pituitary-adrenal; nAChR, nicotinic acetylcholine receptor.
The programming effects of HPA function by prenatal stress may be correlated to the exposure’s severity, time, duration, and genetic factors (Harris and Seckl, 2011). Notably, some studies indicate there is a transient period of blunted HPA response during pre-weaning stage in ethanol-exposed offspring followed by a long-term hyper-responsive property (Zhang et al., 2005). Importantly, postnatal events including early handling or maternal behaviors also exert profound effects on programming of HPA function. Weaver (2004) reported the association between early maternal behaviors (licking and grooming, LG and arched back nursing) and alteration of behaviors and HPA responses in adult offspring. The offspring with high LG manipulation demonstrate low levels of fearful behaviors and better-adjusted HPA responses to stress compared with that of offspring with low LG manipulation. Such differences are correlated with altered expression of GR in hippocampus and involve two epigenetic programming mechanisms: demethylation in promoter region of GR gene and hyperacetylation of histones surrounding GR gene. Early maternal behaviors (LG) (within 2 weeks after delivery) can lead to demethylation of the exon 17 promoter region of GR in rat hippocampus, enhance the binding of NGFI-A to exon 17 promoter, up-regulate the expression of GR, strengthen negative feedback regulation of the HPA axis, and result in more optimal glucocorticoids and HPA response to stress challenges in adulthood. These changes between adult offspring with high LG and low LG manipulations can be eliminated by the central infusion of histone deacetylase inhibitor, trichostatic A (TSA), suggesting a reversible potential for genes with long-lasting epigenetic modification (Weaver et al., 2005).
The activity of HPA axis in the fetus can be altered by maternal alcohol exposure. In a study of sheep model, it was reported that PAE during third-trimester-equivalent increased the levels of ACTH and cortisol in the fetus (Cudd et al., 2001). Interestingly, the HPA axis of females is more susceptible to be affected by insults in the fetal environment (McCormick et al., 1995; Szuran et al., 2000). Prenatal alcohol exposure induced sexually dimorphic effects as reported in some studies where females were found more susceptible than males to some effects of maternal exposure (Nelson et al., 1986; Weinberger and Martinez, 1988). One possible mechanism associated with these effects was examined in a study of rat models in which the level of 11β-HSD2 in the placenta of the female pups was found lower than that in the placenta of the male pups (Wilcoxon and Redei, 2004). Additionally, PAE has shown some sexually dimorphic effects on the glucocorticoids-regulated genes where ethanol increases the expression of corticotropin-releasing hormone (CRH) in female but not in male pups, and. interestingly, the mRNA levels of pro-opiomelanocortin were suppressed in male but not in female pups (Aird et al., 1997).
Growing evidence indicates that various neurotransmitters are also profoundly implicated in the programming effects of fetal brain. Appropriate neurotransmitter signals are essential for normal brain development. Perturbation of the time and intensity of neurotransmitter signals at critical developmental stages may result in aberrant cell behavior, changing developmental trajectory and alteration of brain structure and function. Normally, via interaction with their specific receptors, neurotransmitters exert a variety of fundamental effects on brain development through inducing neural cell proliferation, promoting the switch between proliferation and differentiation, modulating axonogenesis and synaptogenesis, triggering or inhibiting apoptosis, initiating appropriate migration and accurate localization of cell groups in specific brain regions (Pauly and Slotkin, 2008). There are a number of neurotransmitters including acetylcholine, dopamine, norepinephrine, serotonin, glutamate and GABA, most of which present in early stages of fetal development. Prenatal stress may directly or indirectly affect the release, synthesis, reuptake and turnover of these neurotransmitters and/or modify their receptors to program fetal brain development. By direct activation of nAChRs, nicotine triggers the release of acetylcholine. Indirectly, nicotine can also regulate other neurotransmitter release by modulation of the presynaptic nAChRs (Wickstrom, 2007). Brain slice preparation studies have shown that nicotine increases presynaptic release of acetylcholine, dopamine, norepinephrine, serotonin, glutamate and GABA (Gauda et al., 2001; Xu et al., 2001). Furthermore, prenatal nicotine exposure can exert more extensive effects through altering some receptor-mediated signaling pathways (Yanai et al., 2002). Compared with nicotine, the major targets of cocaine are the monoaminergic system. Cocaine can act as a competitive antagonist of the transporters of dopamine, serotonin and norepinephrine, resulting in disturbance of dopaminergic circuits in the brain. Chronic prenatal alcohol exposure increases glucocorticoids concentration and induces glutamate release in hippocampus of fetal guinea pigs, presumably as a consequence of enhanced glucocorticoids receptor expression (Iqbal et al., 2006). Additionally, alcohol increases the expression of NMDA receptors, which could be neurotoxic for brain development (Naassila and Daoust, 2002).
3.3. Central effects of stress hormones
3.3.1. Glucocorticoids and fetal programming
Glucocorticoids are extraordinary hormones with numerous effects affecting many vital organs/systems, including the brain, heart and kidney, etc., and may regulate expression patterns of approximately 10% of human genes (Buckingham, 2006). Glucocorticoids are essential for life and play a crucial role in the regulation of growth and development, but also are implicated in various pathogenesis. There are two major endogenous glucocorticoids, cortisol and corticosterone, both synthesized in mammalian species but with different distribution predominance between species. Cortisol is predominant in humans while its counterpart corticosterone is principally produced in rodents. As the key mediators of stress responses, glucocorticoids are mainly synthesized from cholesterol in cells of zona fasciculate of adrenal cortex (Buckingham, 2006). In normal conditions, glucocorticoids levels are strictly regulated by negative feedback of glucocorticoids on the HPA axis. Disturbance of such feedback regulation loop may result in maladaptive impacts on the brain and other organs, contributing to numerous pathophysiological changes throughout life.
The effects of glucocorticoids are mainly mediated via binding to their intracellular receptors. There are two receptors with distinct affinity, glucocorticoid and mineralocorticoid receptor (GR and MR, respectively), both belonging to the nuclear receptor superfamily and modulating target gene expression. Normally, before binding to their specific receptors, about 95% of endogenous glucocorticoids are bound to a carrier protein (corticosteroid-binding globulin, CBG) in circulation, which allows only a small part of free glucocorticoids reaching target cells. Some transporter proteins, belonging to the ATP-binding cassette (ABC) family, also called multidrug-resistant P-glycoproteins (MDR P-glycoproteins), can actively extrude steroids from cells and lower intracellular levels of glucocorticoids (Buckingham, 2006). However, the most important regulation mechanism of glucocorticoids to their receptors is local pre-receptor metabolism within the target cells by 11β-hydroxysteroid dehydrogenase (11β-HSD) that catalyzes the interconversion of cortisol/corticosterone and its inactive metabolites cortisone/11-deoxycorticosterone, respectively. Two isoforms of 11β-HSD have been identified, 11β-HSD1 and 11β-HSD2, showing counteracting effects (Buckingham, 2006). 11β-HSD2, mainly acting to inactivate endogenous glucocorticoids, presents in some tissues such as the placenta and developing brain, which may offer crucial protection for fetus from exposure to excess of glucocorticoids. However, most synthetic glucocorticoids, such as dexamethasone or betamethasone, are not the selective substrates for 11β-HSD2, which may readily cross the utero-placental barrier and add additional detrimental effects on the fetus compared with the same levels of endogenous or other 11β-HSD2 sensitive glucocorticoids (Buckingham, 2006; Holmes et al., 2003; Seckl and Meaney, 2004).
Glucocorticoids are highly lipophilic and easily cross biological barriers. However, in most normal pregnant conditions, glucocorticoids levels in the fetus are much lower than those in the maternal circulation. Such transplacental concentration gradient is principally maintained by placental 11β-HSD2, which actively captures and converts endogenous glucocorticoids into its inactive metabolites and acts as a primary “barrier” to prevent untimely premature and/or inappropriate intensity of glucocorticoids exposure to sensitive tissues during fetal development (Seckl, 2001; Seckl and Meaney, 2004). However, this enzyme is not a perfect barrier, and it varies considerably with progression of gestation in both placenta and brain under physiological conditions and is readily influenced by various environmental factors (Holmes et al., 2003; Seckl and Meaney, 2004; Seckl and Walker, 2001). For example, in vivo and/or in vitro studies have indicated that the level/activity of 11β-HSD2 is downregulated by malnutrition (e.g., maternal protein restriction), hypoxia, catecholamine, pro-inflammatory cytokines and other endocrine factors (Chisaka et al., 2005; Hardy and Yang, 2002; Homan et al., 2006). Given the significant concentration gradient underlying between the mother and her fetus, only a little alteration of 11β-HSD2 in the placenta may result in a great impact of glucocorticoids on the fetal brain development. Indeed, increased fetal glucocorticoids exposure may result from maternal administration (exogenous), increased maternal levels due to prenatal stress, decreased placental 11β-HSD2 level/activity or increased synthesis by fetal adrenal in late gestational stage (Fowden and Forhead, 2004). Inhibition of 11β-HSD2 during pregnancy is closely correlated with reduced birth weight, the increased risk of hypertension and glucose intolerance, elevated HPA axis activity and anxiety-related behaviors, very similar to actions of excess glucocorticoids exposure. Furthermore, the programming effects of 11β-HSD2 inhibition can be reversed by maternal adrenalectomy and metyrapone administration to block glucocorticoids synthesis, implying the critical role of maternal endogenous glucocorticoids in fetal development (Cottrell and Seckl, 2009; Fowden and Forhead, 2004; Seckl and Meaney, 2004).
Glucocorticoids affect fetal brain development mainly via interaction with their receptors, GR and MR. GR and MR are highly expressed in the developing brain with dynamic and complicated ontogeny. During fetal development, GR presents from the early embryonic stage in most tissues, but expression of MR is relatively limited and presents during later stages of development (Holmes et al., 2003). MR is responsible for mediating effects of very low concentrations of glucocorticoids usually in physiological conditions, and GR mediates effects of relatively high levels of glucocorticoids when MR has been saturated, especially in stress response (Buckingham, 2006). Synthetic glucocorticoids are relatively selective for GR. Therefore, GR is likely to be the major player in glucocorticoids overexposure. After processes such as ligand binding, dimerization and phosphorylation, the glucocorticoid receptor-ligand complex translocates into the nucleus and binds to various GREs (glucocorticoids response elements) in the gene promoter region, resulting in activation or repression of target gene expression. These genomic effects usually occur slowly in onset compared with the rapid nongenomic effects possible mediated by novel membrane receptors (Buckingham, 2006).
Genomic studies have identified a large-scale profile of glucocorticoids responsive gene classes in neural tissues that are implicated in diverse functions of neural plasticity and brain development. These genes mediate processes including neurotransmitter release and exocytosis (PCLO, SYT1, SYT4, CLTB, AP2B1, SNAP25); neurotransmitter turnover (MAO-A); neuronal structure, neurite outgrowth, spine formation (GPM6A, LIMK1, TUBB2, MAP1B, NEFL, CHN1); axonal transport; motor activity (DNCLC1, DNCIC1, LIS1, KIF5C, SYT4); and neural cell adhesion molecules (OBCAM, SC1, LAMP, ICAM5, NRXN3, CX3CL1) (Datson et al., 2008; Datson et al., 2001; Morsink et al., 2006). The effects of glucocorticoids on these gene expressions are time, cell, and environmental context dependent, showing significant disparity between specific brain regions under different types of stressors (Datson et al., 2008).
Glucocorticoids exert effects at cellular and molecular levels to affect tissue/organ growth and differentiation. During fetal development, high levels of glucocorticoids exposure change the expression patterns of various receptors, enzymes, ion channels and transporters in most of cell types as well as alteration of various growth factors, cytoarchitecture proteins, binding proteins and other essential components of the intracellular signaling pathways (Fowden and Forhead, 2004). Such changes significantly impact the basal cellular functions and their responses to numerous stimuli, contributing to alteration of cell size, number, proliferation rate and terminal differentiation. Indirectly, glucocorticoids can also affect tissue proliferation and differentiation via altering cellular secretion of proteins, hormones, growth factors and metabolites, which can greatly amplify its programming effects on fetal development (Fowden and Forhead, 2004). At the molecular level, glucocorticoids regulate target gene transcription, mRNA stability, translation/post-translation modifications, etc., which may be mediated by directly controlling via GREs in promoter regions of responsive genes or indirectly via other transcription factors or glucocorticoids dependent hormones (Fowden and Forhead, 2004). All of these changes induced by glucocorticoids will confer an integration of function at the system level, suggesting that glucocorticoids-mediated programming may result in dynamic, multifaceted, co-ordinated and interdependent changes in different tissues (Fowden and Forhead, 2004).
Sufficient glucocorticoids are vital for normal maturation in most regions of the developing CNS. However, during vulnerable stages of development, inappropriate levels of glucocorticoids may remodel developmental trajectories of specific brain structures and alter corresponding functions accompanied by long-lasting adverse consequences, notably disturbance of behavior, cognition and disease susceptibility in later life (Harris and Seckl, 2011; Seckl and Meaney, 2004). For example, prenatal glucocorticoids administration reduces brain weight at birth, delays myelination of the corpus callosum, retards astrocyte and vasculature maturation in sheep, and decreases cortex convolutions index and surface area in humans (Antonow-Schlorke et al., 2009; Modi et al., 2001). Prenatal stress can also diminish dendritic spine density in the anterior cingulated gyrus and orbitofrontal cortex in rats (Murmu et al., 2006). Studies in both humans and animals have revealed that hippocampus is a highly vulnerable structure particularly sensitive to prenatal glucocorticoids exposure, leading to variable memory and behavior deficits. For example, prenatal stress in rats can reduce synaptic spine density in hippocampus, which is associated with impairment of reversal learning (Hayashi et al., 1998). Betamethasone administration in fetal baboons inhibits neurogenesis and impairs neuronal plasticity via downregulation of critical proteins such as cytoskeletal microtubule-associated proteins and synaptophysin, resulting in cognition deficits (Antonow-Schlorke et al., 2003). In addition, antenatal administration of dexamethasone results in neuronal degeneration in the hippocampus subfields and reduces the hippocampus volume in a dose-dependent manner (Uno et al., 1990). It seems that chronic low levels of glucocorticoids exposure may be more deleterious than its short, sharp impact on fetal brain development (Harris and Seckl, 2011). Thus, the alteration of hippocampus structure and function may offer a plausible neuroanatomical basis for the programming effects of glucocorticoids on cognitive ability, behavior and the risk of psychological and psychiatric disorders in later life.
Long-term prenatal glucocorticoids exposure may permanently alter the “set point” and sensitivity of endocrine axis, such as the somatotrophic and hypothalamic-pituitary-adrenal axes (Fowden and Forhead, 2004; Meaney et al., 2007). The HPA axis is an important programming target in the brain. It is strictly controlled by a negative-feedback mechanism in which glucocorticoids from peripheral adrenal cortex interact with GR in hippocampus, hypothalamus and pituitary to modulate its final level and activity of HPA axis in stress. Maternal malnutrition, inhibition of 11β-HSD2 and other prenatal stress may reduce tissue-specific expression of GR, particularly in hippocampus and impair the negative feedback regulation of glucocorticoids, thus altering the “set point” of the HPA axis (Harris and Seckl, 2011; Meaney et al., 2007; Seckl and Meaney, 2004). A large variety of animal studies have shown prenatal glucocorticoids exposure permanently increases basal corticosterone/cortisol levels in plasma and enhances the activity of HPA axis in adult rats, sheep, guinea pigs and primates (Hawkins et al., 2000; Levitt et al., 1996; Seckl and Meaney, 2004; Uno et al., 1994). Such changes are dependent on gestational age of exposure and also show sex-specific features. Prenatal dexamethasone exposure also stimulates CRH expression in the paraventricular nucleus of hypothalamus (PVN) and in central nucleus of the amygdala, increasing corticosterone and ACTH levels in rat offspring (Levitt et al., 1996). Additionally, prenatal stress may heighten the vulnerability of CRH neuron in PVN and also program the development of the HPA axis (Tobe et al., 2005). HPA programming may be a common pathway shared by other prenatal challenges. Furthermore, prenatal glucocorticoids exposure may have effects beyond the CNS and elevate 11β-HSD1 levels in hepatic, visceral adipose tissues, which regenerates more active glucocorticoids from its inactive metabolites and further enhances adverse effects of glucocorticoids on the developing brain (Cleasby et al., 2003; Nyirenda et al., 2009). Given its wide spectrum of physiological and pathophysiological functions, it is predictable that chronic excess of glucocorticoids during fetal development and overactivity of the HPA axis may increase risks of development of hypertension, hyperglycemia, obesity, other metabocardiovascular syndrome, stroke, cognitive impairment, affective and other neuropsychiatric disorders in later life, similar to what is expected in Cushing’s syndrome (Harris and Seckl, 2011).
Excessive glucocorticoids exposure may also lead to reprogramming of offspring behavior in postnatal life. Glucocorticoids can reprogram expression patterns of several key molecules implicated in the regulation of neuronal development, HPA axis and other higher cerebral functions (Drake et al., 2007). For example, prenatal glucocorticoids exposure increases CRH and GR expression in the amygdala, a central structure mediating emotional response such as fear and anxiety (Welberg et al., 2000, 2001). Through elevated CRH and GR, amygdala may positively drive the HPA axis activity, which has been supported by transgenic study in mice (Tronche et al., 1999). Prenatal glucocorticoids exposure can also influence the development of dopaminergic system, contributing to the development of schizo-affective, attention-deficit hyperactivity, extrapyramidal disorders and drug addiction (Drake et al., 2007). Some studies also suggest that prenatal dexamethasone treatment may enhance vulnerability of cholinergic neurons to toxic challenges in later life (Diaz et al., 1995). Human studies have revealed the correlation between stressful events during the second trimester of pregnancy and incidence of schizophrenia. Of importance, such programming effects are also time-dependent (Koenig et al., 2002).
3.3.2. Catecholamines and fetal programming
Given its deep involvement in various acute and chronic stress responses, it is easy to assume the critical position of catecholamines in programming of fetal development by prenatal stress. However, up to now, only a few studies are available to indicate the effects of catecholamines (norepinephrine and epinephrine) in fetal stress-mediated programming of the developing brain. Predictably, prenatal stress can evoke enhanced maternal release of norepinephrine via activation of the sympathetic-adrenal-medullary system, resulting in significant maternal vasoconstriction and/or disturbance of maternal cardiovascular function. This will lead to compromised delivery of oxygen and nutrients to the fetus and exaggerate adverse effects of other stress stimuli on the fetus. More importantly, Sarkar (2001) reported that both norepinephrine and epinephrine rapidly repressed 11β-HSD2 mRNA expression in early and late gestational human trophoblast cell lines, which might increase exposure levels of glucocorticoids to the fetus in uterus. The downregulation of 11β-HSD2 by catecholamines is mainly mediated by activation of α1 and α2 adrenoreceptors and is not dependent on β-adrenergic stimulation. However, no similar studies in vivo have been reported yet. There are studies implying that catecholamines may exert programming effects on the HPA axis in offspring of a fetal ethanol exposure model (Lee et al., 2008). However, most of these studies only confer indirect evidence of programming effects of catecholamines. Notably, some studies have indicated that maternal catecholamines can cross the placenta, and catecholamines are released by the fetus of later stage in stress (Morgan et al., 1972; Thomas et al., 1995), suggesting it is plausible that catecholamines may exert direct programming effects on fetal brain and other organ development via interaction with their specific regionally expressed α and/or β adrenoceptors. Indeed, a recent study in pregnant rats demonstrated a key role of increased norepinephrine in nicotine-mediated promoter methylation and PKCε gene repression in the developing heart and its sustained effect on heightened cardiac vulnerability to ischemic and reperfusion injury in adult offspring (Lawrence et al., 2011).
3.4. Epigenetic mechanisms in fetal programming
One of the important adaptive mechanisms that the human body could be evoked to react to some adverse environments is through epigenetic modification of gene expression patterns. The fetal developmental stage is the most critical period for the human being because in the uterus the fetus could be exposed to inadequate or inappropriate environments that could be chemical/nutritional or non-chemical. These epigenetic changes could be associated with conditions or diseases during adulthood (Joss-Moore et al., 2011; Nistala et al., 2011; Pinney and Simmons, 2010).
The fetus is a critical developmental stage in which different events occur in a way to induce repression or activation of gene transcription via epigenetic mechanisms (Chen and Zhang, 2011). Epigenetic modifications regulate the expression of genes without altering the DNA sequence. The chromatin-based epigenetic is very important to ensure the correct integration of developmental signals at gene regulatory regions in which chromatin modifications play very important roles. Some of these chromatin modifications are mediated by DNA methylation and histone posttranslational modifications (HPTMs), including histone methylation, acetylation, phosphorylation, ubiquitylation, sumoylation and propionylation (Jungel et al., 2010; Ouvry-Patat and Schey, 2007). Other important chromatin modifications and processes are the histone variants, the chromatin remodeling and RNA interference.
Many of these modifications have been associated with disease programming. Studies in mice showed that a low-protein diet in pregnant animals during the second trimester of gestation induced hypomethylation in the CpG islands of the ACE-1 gene promoter in the fetal brain (Goyal et al., 2010). It has also been reported that low-protein diet during pregnancy alters the expression of mmu-mir-27a, mmu-mir27b and mmu-mir-330, which are important miRNAs in the regulation of mRNA stability. These results suggest that the effect of these epigenetic changes may play an important role in the manifestation of brain dysfunction and other disorders later in life. Studies in pregnant rats showed that a low-protein diet during gestation decreased the expression of DNA methyl transferase (DNMT) 1 and the methylation levels of exon 110 at glucocorticoids receptor gene promoter and increased the expression of GR in offspring at postnatal day 34 (Lillycrop et al., 2007).
Other examples of fetal stress-mediated epigenetic modifications of gene expression patterns include the regulation of PKCε gene expression in the developing heart. Several animal models of fetal stress, including hypoxia, cocaine, and nicotine exposure, have demonstrated PKCε gene repression in the developing heart and increased heart vulnerability to ischemia and reperfusion injury in offspring, suggesting a common mechanism of PKCε in fetal programming of heart disease in later adult life (Lawrence et al., 2011; Meyer et al., 2009; Patterson et al., 2010; Zhang et al., 2009). It has been well-documented that PKCε plays a critical role in cardioprotection during cardiac ischemia and reperfusion injury (Heusch et al., 2010; Murriel and Mochly-Rosen, 2003). Fetal stress caused highly specific changes in CpG methylation patterns at PKCε gene promoter and induced subtle epigenetic modifications of PKCε gene repression in the developing heart with pathophysiological consequences in the offspring heart (Lawrence et al., 2011; Meyer et al., 2009; Patterson et al., 2010; Zhang et al., 2009).
Epigenetic regulation is also associated with programming of type 2 diabetes mellitus (T2DM). Chromatin remodeling has been found in cells of IUGR rats. A decrease on histone acetylation in H3 and H4 at the proximal promoter Pdx1, which plays a critical role in the development of endocrine and exocrine pancreas, was observed in the islets isolated from IUGR fetuses (Park et al., 2008). This modification affected the binding of USF1, an activator of Pdx1, and the resulting decrease of Pdx1 transcription causes a significant repercussion in the aberrant development of the pancreas (Li et al., 2006).
Brain-derived neurotrophic factor (BDNF) plays a vital role in the brain development. An epidemiologic study in adolescents whose mothers smoked during pregnancy revealed that prenatal nicotine exposure increases DNA methylation of the BDNF-6 exon, and this may lead to changes in the plasticity and development of the brain (Toledo-Rodriguez et al., 2010). A recent study evaluated the DNA methylation patterns of the genes coding GR, 11β-HSD2, neuronatin and reelin in hippocampus of the offspring rats from pregnant animals that had been treated with a deficient methyl donor diet (MDD). Though the behavior differences were demonstrated between MDD and the control groups, the DNA methylation patterns of these genes were not altered (Konycheva et al., 2011). However, it has been shown that maternal stress of pregnant rats during gestational days 12-16 increases the levels of DNA methylation in frontal cortex and hippocampus in offspring, which is associated with behavioral changes in offspring rats (Mychasiuk et al., 2011). Additionally, it has been found that fetal exposure to bisphenol A (BPA), a xenoestrogen, induces changes in DNA methylation patterns in the 2500 Notl loci, suggesting that the maternal BPA exposure may also exert some programming effects on brain development (Yaoi et al., 2008).
4. Fetal stress reprograms the vulnerability of neonatal hypoxic-ischemic encephalopathy
4.1. Neonatal hypoxic-ischemic encephalopathy
The most common cause of neonatal brain damage is HIE, which is also the most clearly recognized cause of cerebral palsy (Bracci et al., 2006; Perlman, 1997, 2006). Severe HIE disrupts normal brain development, leading to a wide variety of neurodevelopmental deficits presented as various motor and sensory abnormality, learning disability, mental retardation and seizure attacks (Vannucci, 1990; Vexler and Ferriero, 2001). The incidence of asphyxia is approximately 20% in full-term infants and up to 60% in premature infants with low birth weight, of which 20 – 50% asphyxiated infants showing HIE symptoms and signs will die and about 25% of the survivors will be accompanied by permanent severe neuropsychological disability (Vannucci, 2000).
Compromised cerebral blood flow (CBF) is the dominant pathogenetic mechanism for neuropathophysiology due to hypoxia-ischemia, which may arise from acute reduced materno/feto-placental blood flow or from chronically compromised fetal oxygen and energy supply (Perlman, 2006; Terzidou and Bennett, 2001). The resulting patterns of HIE injury consist of periventricular white matter lesions in preterm newborn; cortico-subcortical lesions, particularly in the sensomotor cortex, parasagittal region, and deep gray matter lesions of basal ganglia and thalamus in near-term and term newborns. Such patterns of injury are associated with brain maturation stage and nature of hypoxic-ischemic injury.
The etiology of brain damage secondary to HIE is complicated and multifaceted. It is well documented that energy failure due to reduction of CBF and oxygen delivery initiates the principal pathways contributing to brain cell death. In acute phase, energy depletion (primary energy failure) results in increased neuronal release of glutamate and reduced reuptake of glutamate by astrocyte, lactate acidosis, glutamate receptor (NMDA) activation, intracellular calcium accumulation, generation of ROS, lipid peroxidation, NO formation and neurotoxicity, disruption of cell essential components, and immediate or delayed cell death. Typically, about 6 – 48 h later, a second phase of injury (secondary energy failure) ensues. During this phase, accumulated mitochondrial dysfunction secondary to extended injury from primary insults (calcium influx, excitotoxicity, oxygen free radicals or NO nitrosative stress) leads to release of various cytotoxic enzymes and pro-apoptotic proteins from mitochondria causing delayed cell death (Perlman, 2006; Rees et al., 2008, 2011; Vexler and Ferriero, 2001). Evidence suggests that some circulatory and endogenous inflammatory cells/mediators may also contribute to such ongoing brain injury (Palmer, 1995; Perlman, 2006).
It is also notable that compared with the adult brain, the neonatal brain shows some differences in physiological structure organization, ontogeny, function, cellular composition and signaling pathway related to gene and protein expression, demonstrating more sensitive and plastic features to challenges (Chen et al., 2009b). Such features determine that its response to brain injury is also significantly different from the adult brain, resulting in distinct acute and chronic neurological consequences, which deserves a careful consideration in experimental and clinical studies. For example, the neonatal brain shows more permeable immature blood-brain barrier (BBB) that allows readily cross of various solutes and small insoluble molecules in blood (Chen et al., 2009b). The major responses to injury and cell death mechanisms are different in the neonatal brain, favoring more apoptotic features (Vexler and Ferriero, 2001). Additionally, the response to the treatment in the neonatal brain may be also different from that in the adult brain. In general, compared with the adult brain, the neonatal brain is more resistant to HI damage (Vannucci and Hagberg, 2004).
Up to now, no universally definite effective therapy is available to intervene with this severe neonatal encephalopathy. The only accepted therapy for HIE in clinical practice is moderate hypothermia. A recent meta-analysis of 10 randomized controlled trials confirmed the neuroprotective effects of moderate hypothermia administered within 6 h after birth for full-term newborns with mild or moderate HIE, showing reduced mortality and neurological deficits at 18 months of age (Edwards et al., 2010; Rees et al., 2011). However, it does not improve mortality and neurological outcomes in neonates with severe HI brain injury and is contraindicated in pre-term neonates. Furthermore, the narrow administration time window also greatly restricts its clinical application. Other intervention strategies, such as application of excitatory amino acid antagonists, oxygen free radical inhibitors and scavengers, inhibition of nitric oxide formation, blockade of apoptosis cascades, application of growth factors and neurosteroids, are either still in the experimental stage or in early, ongoing, small scale clinical studies or have already failed in clinical trials, showing the lack of solid evidence to justify their extensive application (Perlman, 2006; Rees et al., 2011).
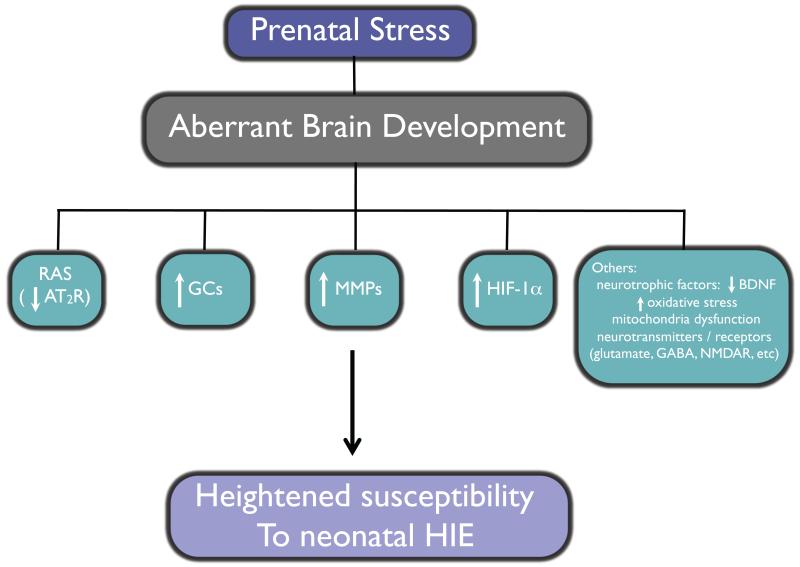
4.2. Fetal stress enhances the vulnerability of neonatal hypoxic-ischemic encephalopathy
A wealth of human and animal studies has indicated the close link between prenatal stress and enhanced risk of development of cardiometabolic syndrome, stroke, neurobehavioural, neuropsychological and neuropsychiatric pathogenesis in adolescence and/or adulthood. However, little research concerns the potential harmful effects of fetal stress on the susceptibility of neonatal HIE. Given the profound impact of prenatal stress on programming of brain structures and functions as discussed above, it is plausible to presume that fetal stress may induce the sensitive phenotype of HIE in the neonatal brain via reprogramming expression patterns of some key functional genes and/or proteins involved in the pathophysiology of HIE. Indeed, recent studies lend necessary supports to such a hypothesis.
Multiple mechanisms may be involved in the fetal stress-mediated increase in the susceptibility of neonatal HIE (Figure 3). Prenatal stress may enhance the vulnerability of neonatal HIE via reprogramming expression patterns of essential components of the reninangiotensin system (RAS) in the brain (Mao et al., 2009a). It is well accepted that RAS is both a circulating and tissue/organ specific hormonal system implicated in various physiological and pathophysiological processes via the major peptide angiotensin II (Ang II) stimulating its specific AT1R and AT2R, which demonstrate opposite effects in many conditions (Sokol et al., 2004; Dasgupta and Zhang, 2011; Shi et al., 2010). Both AT1R and AT2R are present in the brain with different expression patterns and signaling pathways during different developmental stages. Clinical trials and experimental studies indicate that RAS plays an important role in the development and progression of cerebrovascular diseases, but most of these studies were conducted in the mature brains. For example, clinical trials such as LIFE and MOSES have demonstrated that chronic blockade of RAS can offer neuroprotection with the prevention of first or recurrent stroke in high-risk populations, independent of its blood pressure-lowering effects (Lindholm et al., 2002; Schrader et al., 2005). Numerous studies have revealed that AT1R antagonists exhibit anti-apoptotic, anti-inflammatory, anti-oxidant effects and improve cerebral perfusion, demonstrating vascular-dependent and -independent neuroprotection in acute stroke (Ando et al., 2004; Dai et al., 1999; Lou et al., 2004; Zhou et al., 2005). Less is known about the role of AT2R in neurological pathophysiology. Emerging evidence indicates that AT2R also confers beneficial effects in a variety of pathologies including various neurological disorders. Some studies reported that AT2R was up-regulated in stroke, particularly in the ischemic area of the brain, implying its potential role in neuroprotection (Mogi et al., 2006). Increased activation of AT2R may be responsible for some neuroprotective effects of AT1R antagonism (Li et al., 2005). In vitro stimulation of AT2R promotes intense neurite outgrowth, which can be antagonized by PD123319 (Laflamme et al., 1996). Moreover, McCarthy et al. (2009) demonstrated centrally direct stimulation of AT2R with CGP42112 conferred a neuroprotective role in a conscious rat model of stroke, which was beyond blood pressure regulation. The underlying mechanisms of AT2R in neuroprotection remain to be elucidated. Some studies indicated that it may be related to its complicated interaction with AT1R in apoptotic modulation, neuronal regeneration and vasodilation in ischemic regions following stroke (Jones et al., 2008; Saavedra et al., 2006). Stressful stimuli in pre- or perinatal developmental stages may reprogram the expression patterns of AT1R and AT2R in vital organs such as the heart, vasculatures, kidney and brain, contributing to later pathologies. Such programming effects may be glucocorticoids dependent and sometimes with sex diversity and involve complex epigenetic mechanisms. For example, nicotine exposure alters expression patterns of AT1R and AT2R in the kidney and vessels, and enhances vascular response to vasoconstrictors, which maybe contribute to development of hypertension in adulthood (Mao et al., 2009a, b; Xiao et al., 2007, 2008, 2011). In addition, maternal hypoxia during gestation can downregulate glucocorticoid receptors in the heart of fetuses and offspring and decrease GR binding to the GREs at the AT2R promoter region, resulting in increased expression of AT2R and heightened cardiac susceptibility to ischemicreperfusion injury in adult offspring (Xue et al., 2011). Our recent preliminary studies have shown the neuroprotective effects of stimulation of AT2R in the brain by intracerebroventricular injection of its selective agonist and antagonist in postnatal 10 day rat pups with HIE. More importantly, we have revealed that perinatal nicotine exposure significantly affects expression patterns of AT2R in postnatal 10 day neonatal brain in a sex-dependent manner, suppression in male pups and upregulation in female pups, which closely parallels to the exaggerated brain HI-induced infarction size in male pups in nicotine-treated animals. This sex-dependent difference of heightened brain HI injury in neonate rats induced by nicotine can be reversed by intracerebroventricular administration of AT2R agonist or antagonist, which further confirms a key role of AT2R in fetal stress-mediated programming of ischemic-sensitive phenotype in the neonatal brain and suggests a novel mechanism in heightened vulnerability of HIE in neonates. Interestingly, similar findings were obtained in a rat model of fetal hypoxia, showing that prenatal hypoxic stress also enhanced HI-induced infarction size in neonatal pups of HIE model, which was accompanied by significant alteration of expression patterns of AT1R and AT2R in the brain. These studies suggest a common mechanism of Ang II receptors in programming of the vulnerability of neonatal HIE.
Figure 3. Potential mechanisms in programming of neonatal HIE phenotype.
Prenatal stress induces aberrant brain development, reprograms expression patterns of key functional mediators associated with pathophysiology of neonatal HIE, including RAS (AT2R), GCs, MMPs, HIF-1α, BDNF, glutamate, GABA, NMDA-R, etc., which contribute to the heightened susceptibility of neonatal HIE. The response of these mediators may be stress-specific. AT2R, angiotensin II type 2 receptor; BDNF, brain derived neurotrophic factor; GABA, gamma amino butyric acid; GCs, glucocorticoid receptors; HIE, hypoxic-ischemic encephalopathy; HIF-1α, hypoxia inducible factor 1α subunit; MMPs, matrix metalloproteinases; NMDAR, n-methyl-d-aspartic acid receptor; RAS, renin-angiotensin system.
Another promising candidate mediator with potential role in heightened vulnerability of neonatal HIE is glucocorticoids, either cortisol or corticosterone. As discussed above, glucocorticoids exert profound effects on the programming of fetal stress and brain development, particularly their programming effects on the HPA axis activity as well as other important organs and tissues. Sustained overexposure to glucocorticoids down-regulates GR levels in hippocampus, attenuates negative feedback of the HPA axis, permanently resets the activity of HPA axis and enhances basal and stressful glucocorticoids responses in the postnatal life. These will cause the brain to be exposed to chronically high level of glucocorticoids, resulting in aberrant gene regulation and cell behavior and programming of vulnerability of HIE injury. Paradoxically, glucocorticoids show bidirectional effects on the brain, which may be implicated in both neurodegenerative and neuroprotective processes (Abraham et al., 2001). On the one hand, glucocorticoids may inhibit key nutrients such as glucose uptake, modulate both excitatory and inhibitory neurotransmission, increase intracellular calcium concentrations, enhance excitotoxicity and induce perturbation of 11β-HSD, which may retard fetal brain growth, delay myelination, promote synapse degeneration and enhance neuronal vulnerability to hypoxic/ischemic insults (Abraham et al., 1996; Doyle et al., 1993; Joels and de Kloet, 1994; Moghaddam et al., 1994; Seckl and Walker, 2001). On the other hand, some studies indicate via modulating calcium currents, increasing synthesis of neurotrophic factors, such as lipocortin-1, basic fibroblast growth factor (bFGF), nerve growth factor (NGF), and decreasing lipid peroxidation, glucocorticoids may be neuroprotective (Flower and Rothwell, 1994; Joels and de Kloet, 1994; Mocchetti et al., 1996; Young and Flamm, 1982). There are experimental findings suggesting that glucocorticoids affect the vulnerability of fetal and neonatal brain to hypoxiaischemia challenge. However, the results were inconsistent, contradictory, and dependent on experimental protocol, dosage, time, animal age, strains and species (Flavin, 1996; Kauffman et al., 1994; Tombaugh et al., 1992; Tuor, 1995, 1997; Whitelaw and Thoresen, 2000). It appears that the concentration and duration of glucocorticoids treatment are the two key factors determining the detrimental or beneficial effects of glucocorticoids in the brain. Exposure to long-term and high levels of glucocorticoids enhances neurotoxic effects in brain injury, such as in HIE, whereas physiological or slightly higher (slightly supraphysiological elevated levels in a narrow concentration window) levels of glucocorticoids may confer on the brain protective potential to challenges (Abraham et al., 2001). Although there are some controversial reports in the literature, the notion has been widely accepted that overexposure to glucocorticoids enhances neuronal degeneration (Abraham et al., 2001). Given that most prenatal stress increases both basal and stressful glucocorticoids levels in offspring mainly via reprogramming of the HPA axis, which may contribute to enhanced vulnerability of neonatal HIE and other challenges, it is plausible that glucocorticoid itself may be a pivotal mediator in such pathophysiological processes. However, such effects may be variable depending on the duration, timing, severity and types of prenatal stresses.
Fetal stress may also reprogram expression patterns of matrix metalloproteinases (MMPs) in the neonatal brain, which contribute to the enhanced vulnerability of HIE. MMPs belong to a family of zinc-dependent proteases that exert pronounced effects in the ECM turnover. These enzymes remodel almost all components of the matrix and play an essential role in cell signaling regulation, cell survival and cell death. MMPs, especially MMP-2, MMP-3 and MMP-9, may target the extracellular matrix of blood vessels, basal lamina, and tight junctions in endothelial cells, increase the permeability of the blood-brain barrier in neuroinflammation due to hypoxiaischemia, multiple sclerosis and CNS infection, which can result in cytotoxic and vasogenic edema, promote hemorrhagic transformation, induce apoptosis of neurons and oligodendrocytes (Cunningham et al., 2005; Rosenberg, 2009). However, in later stage of such pathology, MMPs play critical roles in tissue repair and remodeling process via inducing angiogenesis and neurogenesis. Growing evidence suggests that overly upregulated activity/expression of MMPs, particularly MMP-2 and MMP-9, are deleterious in the acute phase of stroke. Inhibition of MMPs in the acute phase may reduce the damage to BBB (Gasche et al., 2001). There is a report indicating decreased damage to BBB and reduced infarct size in a focal ischemic MMP-9 knockout model (Asahi et al., 2001). More importantly, a recent study in a neonatal rat HIE model revealed that early inhibition of MMPs conferred acute and long term beneficial effects via reducing tight junction proteins degradation, attenuating the permeability of BBB, improving brain edema, and preventing brain atrophy (Chen et al., 2009a). Fetal hypoxia reprograms expression patterns of MMPs in the heart and brain and increases activities/expressions of both MMP-2 and MMP-9 in the neonatal brain (Tong et al., 2010, 2011; Tong and Zhang, 2011). Considering the evident detrimental effects of MMPs in the acute stroke models and other neurological pathophysiologies, it is plausible that altered expression patterns of MMPs by prenatal stress is another important mediator in programming of ischemic-sensitive phenotype and increased susceptibility of HIE in the neonatal brain.
Hypoxia inducible factor-1 (HIF-1), a key regulator in response to cellular hypoxia and oxygen homeostasis (Wang et al., 1995), may be profoundly involved in the programming effects of prenatal stress on the vulnerability to neonatal HIE. Being a heterodimeric transcription factor, HIF-1 consists of an oxygen-sensitive HIF-1α and a constitutively expressed HIF-1β. The normal oxygen level results in a rapid degradation of HIF-1α, but hypoxia can enhance the stability of HIF-1α and promote the transactivation of its target genes. More than 100 HIF-1α targeted genes have been identified up to now, including erythropoiesis (EPO), angiogenesis (VEGF), cell proliferation (IGF-2), glucose metabolism (Glut-1,3), inflammation (COX-2), cell apoptosis (BNIP3, P53), vascular tone and matrix metabolism, etc. (Ke and Costa, 2006). Based on its regulation of a wide spectrum of genes in diverse contexts, the effects of HIF-1α activation may be very complex and variable, to some extent similar to those of glucocorticoids. During brain challenges, such as in hypoxia-ischemia, HIF-1α may be both anti-apoptotic via enhancing the transcription of EPO, VEGF, IGF-2 and GLUT-1, etc., but it also can be pro-apoptotic by upregulation of factors such as COX-2, BNIP3 and P53 that contribute to cell death (Chen et al., 2009b; Fan et al., 2009). Notably, VEGF promotes the permeability of BBB and enhances brain edema in the acute phase of HI, which is different from its later beneficial effects such as neovascularization (Chen et al., 2009b). The bidirectional effects of HIF-1α in hypoxia may be affected by some factors, such as the duration and severity of hypoxia, and the type of pathological stimuli. Mild hypoxia may predominantly induce anti-apoptotic gene expression, but more sustained and severe hypoxia promotes pro-apoptotic gene expression (Chen et al., 2009b; Fan et al., 2009). In addition, effects of HIF-1α in HI may be cell type specific. In vitro studies suggest that functional loss of HIF-1α may be neuroprotective for astrocyte but enhances neuronal vulnerability to HI injury (Vangeison et al., 2008). Under normal conditions, HIF-1α is essential for normal fetal brain development via the activation of genes such as VEGF because of the relatively lower physiological oxygen level in uterus (Fan et al., 2009; Lee et al., 2001; Trollmann and Gassmann, 2009). In addition to maternal hypoxia, some other prenatal stresses, such as nicotine, cocaine, and ethanol exposure, may also trigger the release of catecholamines, resulting in various degrees of ischemia/hypoxia insult to the fetus and leading to a sustained or episodic upregulation of HIF-1α. Long-term and supraphysiological high levels of HIF-1α in the fetus, combined with its adverse impacts on the developing brain, may persist into the postnatal developmental stage and enhance the vulnerability of neonatal HIE injury.
There are some studies indicating that aberrant development of the monoaminergic system in specific brain regions and/or peripheral organs such as the heart and adrenals also weakens the tolerance to hypoxia/ischemia insults in neonates. It is well recognized that prenatal nicotine exposure is a major risk factor for SIDS in which defective arousal and cardiorespiratory response adjustment are considered to be the potential mechanisms (Milerad and Sundell, 1993; Slotkin, 1998; Wickstrom, 2007). Prenatal nicotine exposure exerts negative effects on the development of central and peripheral catecholaminergic system by decreasing synthesizing enzymes and reducing synthesis and release of catecholamines in brainstem nucleus, adrenals and heart, which may particularly impact the crucial defensive response to acute stress including hypoxia/ischemia and enhance the vulnerability of neonatal HIE (Slotkin et al., 1987; Wickstrom et al., 2002). These detrimental effects in defensive responses appear to correlate with functional loss of some subtypes of nAChRs via activity-dependent desensitization (Cohen et al., 2002). A recent study in rhesus monkey also reported that prenatal nicotine exposure compromises the brainstem serotonergic pathways, another important neural structure implicated in autonomic function, arousal and cardiorespiratory responses to acute hypoxic/ischemic challenge (Slotkin et al., 2011).
Evidently, the impacts of prenatal stress on fetal and neonatal brain development are very complicated, dynamic, variable and multifaceted, which may also be subtle or drastic, and are profoundly affected by exposure age, duration, protocol, severity and nature of stress stimuli, and genetic traits. The underlying mechanisms of neonatal HIE remain to be further elucidated. In addition to the common potential mediators mentioned above, there are other possible factors that may be involved in programming of the vulnerability of neonatal HIE under different types of prenatal stressor. For example, the decreased expression of some neurotrophic factors, such as BDNF; perturbation of neurotransmitters and their receptors, such as glutamate, GABA and NMDA; enhanced oxidative stress; dysfunction of mitochondria; and inflammatory factors, may all act as potential mediators to alter the vulnerability of HIE in the neonatal brain (Archer, 2011; Levitt, 1998; Warner and Ozanne, 2010).
Another factor deserving consideration is the methodologies employed to assess the vulnerability of neonatal HIE. As discussed above, the effects may be varied greatly depending on the methods used. Some changes may be significant enough so that some preliminary methods can reveal the underlying differences, such as quantification of infarction size by TTC staining, brain water content measurement and BBB permeability detection. However, some changes may be so subtle that we may readily deny their existence. In such conditions, more sensitive and challenging methods must be explored and employed, such as histological techniques; neurobehavioral, psychological and psychiatric assessments; and long-term structural and functional evaluations, to reveal or reject the distinction.
5. Potential intervention targets
Trying to avoid potential stress stimuli during pregnancy is essential for effectively preventing or ameliorating the adverse programming effects on fetal development. Quitting use of ethanol, cocaine and nicotine should be encouraged, which can be further supported by behavioral modifications and counseling strategies. Owing to the lack of solid evidence of its efficacy and safety, NRT should not be readily recommended to pregnant women until carefully weighing its potentially adverse effects on the fetus (Pauly and Slotkin, 2008; Slotkin, 1998). For pregnant women who are strongly indicated for glucocorticoids therapy, the selection of glucocorticoids and administration protocol are vital. Normally, 11β-HSD2 sensitive glucocorticoids should be favored and betamethasone may be preferred to dexamethasone, and low dosage administration and fewer times of injection may be more beneficial to the fetus based on available clinical studies (Gulino et al., 2009; Heine and Rowitch, 2009; Whitelaw and Thoresen, 2000). It is also important to treat underlying systemic diseases to prevent or attenuate possible placental insufficiency and fetal ischemia/hypoxia and to improve maternal nutrition status with an optimal balanced diet supplying nutrients including various macro and/or micro nutrients when necessary.
A wide variety of emerging evidence has suggested that epigenetic modifications of gene expression patterns exhibit a central role in fetal stress-mediated programming of neurological and cardiometabolic disorders in later life. Predictably, pharmacological manipulations of epigenetic mechanisms present a promising interventional strategy. Indeed, several experimental studies offered exciting results. As mentioned above, programming of the HPA axis provides an important common pathway for the alteration of vulnerability to various pathophysiologies in later life in which epigenetic modification of GR gene expression patterns in hippocampus plays a critical role. Animal studies conducted in high or low maternal LG offspring have revealed that central infusion of a HDAC inhibitor, trichostatin A (TSA) or methyl donor S-adenosylmethionine (SAM), can reverse the epigenetic modification status in GR promoter region, rescue the binding capacity of NGFI-A to exon 17 region, recover GR expression in hippocampus, restore the HPA axis activity, and reverse the increased vulnerability of neurological dysfunction in later life (Weaver et al., 2004; Weaver et al., 2005). More importantly, these studies imply that it is still reversible for some gene expression controlled by lasting epigenetic modifications, and enriching postnatal environment, or providing pharmacological interventions may restore long-term aberrant programming effects. In addition to HDAC inhibitors and DNA methylation inhibitors, other agents, such as plant-derived isoflavone genistein, leptin, folate, fish oil, omega-3 and vitamin D, can alter the corresponding abnormal epigenetic modification status and improve the adverse programming effects caused by prenatal stress (Gregorio et al., 2008; Hypponen et al., 2007; Torrens et al., 2006; Vickers et al., 2008; Wyrwoll et al., 2007). However, up to now, most of epigenetic therapy compounds exert nonspecific modifications on genes and transposable elements, and thus their adverse effects should not be neglected, including inducing or inhibiting other non-responsible genes expression, the potential tumorigenesis and mutagenesis properties, as well as promoting cell-cycle arrest and apoptosis (Karpf et al., 2001; Laird et al., 1995). In general, current epigenetic therapy is still in its infancy.
For the neonates at risk of HIE or already harmed by HIE, prevention and therapy are complex and somewhat frustrating. Timely diagnosis and therapy are crucial but are also very challenging, which greatly affects the final outcomes. If possible, various available clinical techniques, such as advanced neuroimaging, EEG, some reliable biomarkers of brain damage (e.g., S-100, NSE), should be employed to identify and monitor HIE injury in a timely manner (Perlman, 2006; Rees et al., 2011). Despite apparent limitations, moderate hypothermia is the only currently established available therapy for full-term newborns with mild to moderate HIE. A wealth of animal studies have conferred some promising interventional strategies, such as NMDA receptor blockade, NOS inhibition, prevention of apoptosis and free radical formation, administration of neurotrophic factors and growth factors, AT2 receptor stimulation, as well as early inhibition of MMPs and HIF-1α, all of which should enrich our understanding of HIE pathophysiology and provide us with more potentially promising therapeutic options (Chen et al., 2009a; Chen et al., 2009b; Perlman, 2006; Rees et al., 2011).
6. Conclusions and perspectives
Both human and animal studies have supported the notion of developmental origins of adult health and disease. Prenatal stress including hypoxia, malnutrition, nicotine, cocaine, ethanol and glucocorticords exerts great impacts on the fetus during the vulnerable developmental stage at multifaceted levels, resulting in adverse programming of ischemic-sensitive phenotype in the developing brain and heightened vulnerability of neonatal hypoxicischemic encephalopathy and long-term neurodevelopmental disorders, in addition to cardiovascular and metabolic diseases in later life. All of these fetal stress insults, individually and/or combined, act at cellular and molecular levels to alter normal brain cell behavior and specific cerebral structure, reconstruct the HPA axis, and disturb vital neurotransmissions, which in the end, to various extents, change normal brain development trajectory and enhance the susceptibility of neonatal HIE and long-term neurological disorders. Although there are diverse stress stimuli, strong evidence has suggested that reprogramming of the HPA axis by glucocorticoids may at least in part represent a common underlying pathway, and epigenetic modifications of GR gene expression patterns play a key role in such a process. This provides us with the promising interventional targets although epigenetic pharmacological manipulation is just at the beginning and is mainly tested in animal studies. To protect pregnant mothers from harmful stress exposure is still a critical interventional strategy, which may at least prevent or attenuate the brain injury of HIE in some cases. Because of current deficiency in potent and effective therapy, the prognosis and outcome for most neonatal HIE are less than optimal at the best, which makes further exploration and investigation of pathophysiology and underlying mechanisms for the heightened neonatal HIE in various species of animals and humans particularly urgent. The combination of limited successful diagnostic and therapeutic techniques currently available, plus newly emerged knowledge of fetal programming and potential epigenetic manipulations, as well as improved maternal care and active perinatal intervention strategies, may confer us a further hopeful future in the management of such a catastrophic disease of neonatal HIE.
Highlights.
Fetal stress reprograms vulnerability to disease later in life.
Fetal stress acts at cellular and molecular levels to impact brain development.
Glucocorticoids programming is one of common pathways in such process.
Epigenetic modification plays crucial roles in programming actions.
Fetal stress enhances vulnerability to neonatal hypoxic-ischemic encephalopathy.
Acknowledgements
This work was supported in part by National Institutes of Health Grants HL067745 (LZ), HL082779 (LZ), HL083966 (LZ), HL089012 (LZ), HL110125 (LZ), and HD031226 (LZ). We greatly appreciate Dr. Susan Gardner and Dr. Xiangqun Hu for their valuable suggestions in editing the manuscript. We apologize to those authors whose excellent studies covered by the scope of this review were unable to be cited due to space restriction.
List of Abbreviations
- ABC
ATP-binding cassette
- ACC
anterior cingulated cortex
- ACE
angiotensin-converting enzyme
- ACTH
adrenocorticotropic hormone
- ADHD
attention-deficit/hyperactivity disorder
- Ang II
angiotensin II
- AP-1
activator protein 1
- AP2B1
adaptor-related protein complex 2, beta 1 subunit
- Arp1,2,3
actin-related protein 1,2,3 homolog B
- AT1R
angiotensin II type 1 receptor
- AT2R
angiotensin II type 2 receptor
- Bax
Bcl-2–associated X protein
- BBB
blood brain barrier
- Bcl-2
B-cell lymphoma 2
- Bcs1L
cytochrome b-c1 complex subunit Rieske
- BDNF
brain derived neurotrophic factor
- bFGF
basic fibroblast growth factor
- BNIP3
Bcl-2/adenovirus E1B 19-kDa protein-interacting protein 3
- BPA
Bisphenol A
- CA1,2,3,4
Cornu Ammonis 1,2,3,4
- CAH
congenital adrenal hyperplasia
- CAT
catalase
- CBF
cerebral blood flow
- CBG
corticosteroid-binding globulin
- CD
conduct disorder
- CHN1
chimerin 1
- CLTB
clathrin light polypeptide
- CNS
central nervous system
- COX-2
cyclooxygenase 2
- CpG
cyotsinephosphodiester bond-guanine
- CRH
corticotropin-releasing hormone
- CX3CL1
chemokine (CX3-C motif) ligand 1
- CYP2E1
cytochrome P450 2E1
- DA
dopamine
- DG
dentate gyrus
- DHA
decosahexaenoic acid
- DNCIC1
dynein cytoplasmic intermediate chain 1
- DNCLC1
dynein cytoplasmic light chain 1
- DNMT
DNA methyl transferase
- ECM
extracellular matrix
- ED
embryonic day
- EEG
electroencephalograph
- E2f
E2F transcription factor
- Egr 1
early growth response protein 1
- EPO
erythropoietin
- FAS
fetal alcohol syndrome
- FASD
fetal alcohol spectrum disorders
- FIRS
fetal inflammatory response syndrome
- GABA
gamma amino butyric acid
- Glut1,3
glucose transporter 1, 3
- GPM6A
glycoprotein M6A
- GR
glucocorticoids receptor
- GREs
glucocorticoids response elements
- GSH
glutathione
- GSSG
glutathione disulfide
- HAFP
human alpha-fetoprotein
- HDAC
histone deacetylase
- HIE
hypoxic-ischemic encephalopathy
- HIF-1
hypoxia inducible factor 1
- HIF-1α
hypoxia inducible factor 1 α subunit
- HIF-1β
hypoxia inducible factor 1 β subunit
- HPA
hypothalamic-pituitary-adrenal
- HPTM
histone posttranslational modifications
- 11β-HSD1
11-beta-hydroxysteroid dehydrogenase type-1
- 11β-HSD2
11-beta-hydroxysteroid dehydrogenase type-2
- ICAM5
intercellular adhesion molecule 5
- IGF-2
insulin-like growth factor 2
- IL-6
interlukin-6
- IUGR
intrauterine growth restriction
- KIF5C
kinesin family member 5C
- LAMP
limbic system-associated membrane protein
- LG
licking and grooming
- LIFE
the losartan intervention for endpoint reduction in hypertension study
- LIMK1
LIM domain kinase 1
- LIS1
lissencephaly
- MAO-A
monoamine oxidase A
- MAP1B
microtubule-associated protein 1B
- MAP2
microtubule-associated protein 2
- MAPKK1
mitogen-activated protein kinase kinase 1
- MDD
methyl donor diet
- MDR P-glycoproteins
multidrug-resistant P-glycoproteins
- miRNA
microRNA
- MMPs
matrix metalloproteinases
- MOSES
the morbidity and mortality after stroke, eprosartan compared with nitrendipine for secondary prevention study
- MPF
medial prefrontal cortex
- MPR
Maternal protein restriction
- MR
mineralocorticoid receptor
- nAChR
nicotinic acetylcholine receptor
- Nduf8
NADH dehydrogenase ubiquinol iron-sulphur protein
- NEFL
neurofilament light polypeptide
- NGF
nerve growth factor
- NGFI-A
nerve growth factor-induced protein A
- NMDA
n-methyl-d-aspartic acid
- nNOS
neuronal nitric oxide synthase
- NO
nitric oxide
- NRT
nicotine replacement therapy
- NRXN3
neurexin 3
- NSE
neuron specific enolase
- OBCAM
opioid binding protein/cell adhesion molecule-like
- P53
protein 53 or tumor protein 53
- PAE
prenatal alcohol exposure
- PCLO
piccolo (presynaptic cytomatrix protein)
- Pdx1
pancreatic and duodenal homeobox 1
- PKCε
protein kinase C epsilon
- PLA2
Phospholipase A2
- POMC
proopiomelanocortin
- Prdx3
thioredoxin-dependent peroxide reductase
- PTSD
post-traumatic stress disorder
- PVN
paraventricular nucleus
- RAS
renin-angiotensin system
- ROS
reactive oxygen species
- S-100
s-100 protein
- SAM
S-adenosyl-methionine
- SC1 (=ALCAM)
activated leukocyte cell adhesion molecule
- SIDS
sudden infant death syndrome
- SNAP25
synaptosomal-associated protein 25
- SOD
superoxide dismutase
- SP1
specificity protein 1
- SYT1
synaptotagmin 1
- SYT4
synaptotagmin 4
- TCP1
T-complex protein 1
- T2DM
type 2 diabetes mellitus
- TNF-α
tumor necrosis factor alpha
- TSA
trichostatin A
- TTC
triphenyltetrazolium chloride
- TUBB2
beta-tubulin 2
- USF1
upstream stimulatory factor 1
- VEGF
vascular endothelial growth factor
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of interest The authors declare no conflict of interest.
References
- Abraham I, Juhasz G, Kekesi KA, Kovacs KJ. Effect of intrahippocampal dexamethasone on the levels of amino acid transmitters and neuronal excitability. Brain Res. 1996;733:56–63. doi: 10.1016/0006-8993(96)00538-0. [DOI] [PubMed] [Google Scholar]
- Abraham IM, Harkany T, Horvath KM, Luiten PG. Action of glucocorticoids on survival of nerve cells: promoting neurodegeneration or neuroprotection? J Neuroendocrinol. 2001;13:749–760. doi: 10.1046/j.1365-2826.2001.00705.x. [DOI] [PubMed] [Google Scholar]
- Ackerman JP, Riggins T, Black MM. A review of the effects of prenatal cocaine exposure among school-aged children. Pediatrics. 2010;125:554–565. doi: 10.1542/peds.2009-0637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aird F, Halasz I, Redei E. Ontogeny of hypothalamic corticotropin-releasing factor and anterior pituitary pro-opiomelanocortin expression in male and female offspring of alcohol-exposed and adrenalectomized dams. Alcohol Clin Exp Res. 1997;21:1560–1566. [PubMed] [Google Scholar]
- Albright CD, et al. Regulation of choline deficiency apoptosis by epidermal growth factor in CWSV-1 rat hepatocytes. Cell Physiol Biochem. 2005;15:59–68. doi: 10.1159/000083653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albright CD, Friedrich CB, Brown EC, Mar MH, Zeisel SH. Maternal dietary choline availability alters mitosis, apoptosis and the localization of TOAD-64 protein in the developing fetal rat septum. Brain Res. 1999a;115:123–129. doi: 10.1016/s0165-3806(99)00057-7. [DOI] [PubMed] [Google Scholar]
- Albright CD, Tsai AY, Friedrich CB, Mar MH, Zeisel SH. Choline availability alters embryonic development of the hippocampus and septum in the rat. Brain Res. 1999b;113:13–20. doi: 10.1016/s0165-3806(98)00183-7. [DOI] [PubMed] [Google Scholar]
- Alexandre-Gouabau MC, Bailly E, Moyon TL, Grit IC, Coupe B, Le Drean G, Rogniaux HJ, Parnet P. Postnatal growth velocity modulates alterations of proteins involved in metabolism and neuronal plasticity in neonatal hypothalamus in rats born with intrauterine growth restriction. J Nutr Biochem. 2011 doi: 10.1016/j.jnutbio.2010.11.008. [DOI] [PubMed] [Google Scholar]
- Anderson-Brown T, Slotkin TA, Seidler FJ. Cocaine acutely inhibits DNA synthesis in developing rat brain regions: evidence for direct actions. Brain Res. 1990;537:197–202. doi: 10.1016/0006-8993(90)90358-i. [DOI] [PubMed] [Google Scholar]
- Ando H, Zhou J, Macova M, Imboden H, Saavedra JM. Angiotensin II AT1 receptor blockade reverses pathological hypertrophy and inflammation in brain microvessels of spontaneously hypertensive rats. Stroke. 2004;35:1726–1731. doi: 10.1161/01.STR.0000129788.26346.18. [DOI] [PubMed] [Google Scholar]
- Antonow-Schlorke I, Helgert A, Gey C, Coksaygan T, Schubert H, Nathanielsz PW, Witte OW, Schwab M. Adverse effects of antenatal glucocorticoids on cerebral myelination in sheep. Obstet Gynecol. 2009;113:142–151. doi: 10.1097/AOG.0b013e3181924d3b. [DOI] [PubMed] [Google Scholar]
- Antonow-Schlorke I, Schwab M, Cox LA, Li C, Stuchlik K, Witte OW, Nathanielsz PW, McDonald TJ. Vulnerability of the fetal primate brain to moderate reduction in maternal global nutrient availability. PNAS. 2010;108:3011–3016. doi: 10.1073/pnas.1009838108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antonow-Schlorke I, Schwab M, Li C, Nathanielsz PW. Glucocorticoid exposure at the dose used clinically alters cytoskeletal proteins and presynaptic terminals in the fetal baboon brain. J Physiol. 2003;547:117–123. doi: 10.1113/jphysiol.2002.025700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arai N, Furukawa N, Miyamae T, Goshima Y, Sasaki Y, Ohshima E, Suzuki F, Fujita K, Misu Y. DOPA cyclohexyl ester, a competitive DOPA antagonist, protects glutamate release and resultant delayed neuron death by transient ischemia in hippocampus CA1 of conscious rats. Neurosci Lett. 2001;299:213–216. doi: 10.1016/s0304-3940(01)01520-8. [DOI] [PubMed] [Google Scholar]
- Archer T. Effects of exogenous agents on brain development: stress, abuse and therapeutic compounds. CNS Neurosci Ther. 2011;17:470–489. doi: 10.1111/j.1755-5949.2010.00171.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asahi M, Wang X, Mori T, Sumii T, Jung JC, Moskowitz MA, Fini ME, Lo EH. Effects of matrix metalloproteinase-9 gene knock-out on the proteolysis of blood-brain barrier and white matter components after cerebral ischemia. J Neurosci. 2001;21:7724–7732. doi: 10.1523/JNEUROSCI.21-19-07724.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ba A. Functional vulnerability of developing central nervous system to maternal thiamine deficiencies in the rat. Dev Psychobiol. 2005;47:408–414. doi: 10.1002/dev.20105. [DOI] [PubMed] [Google Scholar]
- Bae S, Gilbert RD, Ducsay CA, Zhang L. Prenatal cocaine exposure increases heart susceptibility to ischemia/reperfusion injury in adult male but not female rats. J Physiol. 2005;565.1:149–158. doi: 10.1113/jphysiol.2005.082701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bae S, Zhang L. Prenatal cocaine exposure increases apoptosis of neonatal rat heart and heart susceptibility to ischemia/reperfusion injury in one-month-old rat. Br J Pharmacol. 2005;144:900–907. doi: 10.1038/sj.bjp.0706129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker DJ, Gluckman PD, Godfrey KM, Harding JE, Owens JA, Robinson JS. Fetal nutrition and cardiovascular disease in adult life. Lancet. 1993a;341:938–941. doi: 10.1016/0140-6736(93)91224-a. [DOI] [PubMed] [Google Scholar]
- Barker DJ, Hales CN, Fall CH, Osmond C, Phipps K, Clark PM. Type 2 (non-insulin-dependent) diabetes mellitus, hypertension and hyperlipidaemia (syndrome X): relation to reduced fetal growth. Diabetologia. 1993b;36:62–67. doi: 10.1007/BF00399095. [DOI] [PubMed] [Google Scholar]
- Barker DJ, Osmond C. Infant mortality, childhood nutrition, and ischaemic heart disease in England and Wales. Lancet. 1986;1:1077–1081. doi: 10.1016/s0140-6736(86)91340-1. [DOI] [PubMed] [Google Scholar]
- Barker DJ, Osmond C, Kajantie E, Eriksson JG. Growth and chronic disease: findings in the Helsinki Birth Cohort. Ann Hum Biol. 2009;36:445–458. doi: 10.1080/03014460902980295. [DOI] [PubMed] [Google Scholar]
- Bateson P, Barker D, Clutton-Brock T, Deb D, D’Udine B, Foley RA, Gluckman P, Godfrey K, Kirkwood T, Lahr MM, McNamara J, Metcalfe NB, Monaghan P, Spencer HG, Sultan SE. Developmental plasticity and human health. Nature. 2004;430:419–421. doi: 10.1038/nature02725. [DOI] [PubMed] [Google Scholar]
- Benediktsson R, Lindsay RS, Noble J, Seckl JR, Edwards CR. Glucocorticoid exposure in utero: new model for adult hypertension. Lancet. 1993;341:339–341. doi: 10.1016/0140-6736(93)90138-7. [DOI] [PubMed] [Google Scholar]
- Bergamini CM, Gambetti S, Dondi A, Cervellati C. Oxygen, reactive oxygen species and tissue damage. Curr Pharm Des. 2004;10:1611–1626. doi: 10.2174/1381612043384664. [DOI] [PubMed] [Google Scholar]
- Bonatto F, Polydoro M, Andrades ME, Conte da Frota ML, Jr., Dal-Pizzol F, Rotta LN, Souza DO, Perry ML, Moreira J. C. Fonseca. Effects of maternal protein malnutrition on oxidative markers in the young rat cortex and cerebellum. Neurosci Lett. 2006;406:281–284. doi: 10.1016/j.neulet.2006.07.052. [DOI] [PubMed] [Google Scholar]
- Bonthius DJ, Karacay B, Dai D, Hutton A, Pantazis NJ. The NO-cGMP-PKG pathway plays an essential role in the acquisition of ethanol resistance by cerebellar granule neurons. Neurotoxicol Teratol. 2004;26:47–57. doi: 10.1016/j.ntt.2003.08.004. [DOI] [PubMed] [Google Scholar]
- Bracci R, Perrone S, Buonocore G. The timing of neonatal brain damage. Biol Neonate. 2006;90:145–155. doi: 10.1159/000092517. [DOI] [PubMed] [Google Scholar]
- Brocardo PS, Boehme F, Patten A, Cox A, Gil-Mohapel J, Christie BR. Anxiety- and depression-like behaviors are accompanied by an increase in oxidative stress in a rat model of fetal alcohol spectrum disorders: Protective effects of voluntary physical exercise. Neuropharmacology. 2011 doi: 10.1016/j.neuropharm.2011.10.006. [DOI] [PubMed] [Google Scholar]
- Bruin JE, Gerstein HC, Holloway AC. Long-term consequences of fetal and neonatal nicotine exposure: a critical review. Toxicol Sci. 2010;116:364–374. doi: 10.1093/toxsci/kfq103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckingham JC. Glucocorticoids: exemplars of multi-tasking. Br J Pharmacol. 2006;147(Suppl 1):S258–268. doi: 10.1038/sj.bjp.0706456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannon M, Jones PB, Murray RM. Obstetric complications and schizophrenia: historical and meta-analytic review. Am J Psychiatry. 2002;159:1080–1092. doi: 10.1176/appi.ajp.159.7.1080. [DOI] [PubMed] [Google Scholar]
- Cesani MF, Orden AB, Oyhenart EE, Zucchi M, Mune MC, Pucciarelli HM. Growth of functional cranial components in rats submitted to intergenerational undernutrition. J Anat. 2006;209:137–147. doi: 10.1111/j.1469-7580.2006.00603.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chae SM, Covington CY. Biobehavioral outcomes in adolescents and young adults prenatally exposed to cocaine: evidence from animal models. Biol Res Nurs. 2009;10:318–330. doi: 10.1177/1099800408330395. [DOI] [PubMed] [Google Scholar]
- Chang GQ, Gaysinskaya V, Karatayev O, Leibowitz SF. Maternal high-fat diet and fetal programming: increased proliferation of hypothalamic peptideproducing neurons that increase risk for overeating and obesity. J Neurosci. 2008;28:12107–12119. doi: 10.1523/JNEUROSCI.2642-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen M, Zhang L. Epigenetic mechanisms in developmental programming of adult disease. Drug Discov Today. 2011;16:1007–1018. doi: 10.1016/j.drudis.2011.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W, Hartman R, Ayer R, Marcantonio S, Kamper J, Tang J, Zhang JH. Matrix metalloproteinases inhibition provides neuroprotection against hypoxia-ischemia in the developing brain. J Neurochem. 2009a;111:726–736. doi: 10.1111/j.1471-4159.2009.06362.x. [DOI] [PubMed] [Google Scholar]
- Chen W, Ostrowski RP, Obenaus A, Zhang JH. Prodeath or prosurvival: two facets of hypoxia inducible factor-1 in perinatal brain injury. Exp Neurol. 2009b;216:7–15. doi: 10.1016/j.expneurol.2008.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiriboga CA. Neurological correlates of fetal cocaine exposure. Ann N Y Acad Sci. 1998;846:109–125. [PubMed] [Google Scholar]
- Chisaka H, Johnstone JF, Premyslova M, Manduch Z, Challis JR. Effect of pro-inflammatory cytokines on expression and activity of 11beta-hydroxysteroid dehydrogenase type 2 in cultured human term placental trophoblast and human choriocarcinoma JEG-3 cells. J Soc Gynecol Investig. 2005;12:303–309. doi: 10.1016/j.jsgi.2005.02.003. [DOI] [PubMed] [Google Scholar]
- Cleasby ME, Kelly PA, Walker BR, Seckl JR. Programming of rat muscle and fat metabolism by in utero overexposure to glucocorticoids. Endocrinology. 2003;144:999–1007. doi: 10.1210/en.2002-220559. [DOI] [PubMed] [Google Scholar]
- Cohen G, Han ZY, Grailhe R, Gallego J, Gaultier C, Changeux JP, Lagercrantz H. beta 2 nicotinic acetylcholine receptor subunit modulates protective responses to stress: A receptor basis for sleep-disordered breathing after nicotine exposure. Proc Natl Acad Sci U S A. 2002;99:13272–13277. doi: 10.1073/pnas.192463599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cottrell EC, Seckl JR. Prenatal stress, glucocorticoids and the programming of adult disease. Front Behav Neurosci. 2009;3:19. doi: 10.3389/neuro.08.019.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craciunescu CN, Albright CD, Mar MH, Song J, Zeisel SH. Choline availability during embryonic development alters progenitor cell mitosis in developing mouse hippocampus. J Nutr. 2003;133:3614–3618. doi: 10.1093/jn/133.11.3614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cudd TA, Chen WJ, West JR. Fetal and maternal sheep hypothalamus pituitary adrenal axis responses to chronic binge ethanol exposure during the third trimester equivalent. Alcohol Clin Exp Res. 2001;25:1065–1071. [PubMed] [Google Scholar]
- Cunningham LA, Wetzel M, Rosenberg GA. Multiple roles for MMPs and TIMPs in cerebral ischemia. Glia. 2005;50:329–339. doi: 10.1002/glia.20169. [DOI] [PubMed] [Google Scholar]
- Dai WJ, Funk A, Herdegen T, Unger T, Culman J. Blockade of central angiotensin AT(1) receptors improves neurological outcome and reduces expression of AP-1 transcription factors after focal brain ischemia in rats. Stroke. 1999;30:2391–2398. doi: 10.1161/01.str.30.11.2391. discussion 2398-2399. [DOI] [PubMed] [Google Scholar]
- Dasgupta C, Zhang L. Angiotensin II receptors and drug discovery in cardiovascular disease. Drug Discovery Today. 2011;16:22–34. doi: 10.1016/j.drudis.2010.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datson NA, Morsink MC, Meijer OC, de Kloet ER. Central corticosteroid actions: Search for gene targets. Eur J Pharmacol. 2008;583:272–289. doi: 10.1016/j.ejphar.2007.11.070. [DOI] [PubMed] [Google Scholar]
- Datson NA, van der Perk J, de Kloet ER, Vreugdenhil E. Identification of corticosteroid-responsive genes in rat hippocampus using serial analysis of gene expression. Eur J Neurosci. 2001;14:675–689. doi: 10.1046/j.0953-816x.2001.01685.x. [DOI] [PubMed] [Google Scholar]
- de Boo HA, Harding JE. The developmental origins of adult disease (Barker) hypothesis. Aust N Z J Obstet Gynaecol. 2006;46:4–14. doi: 10.1111/j.1479-828X.2006.00506.x. [DOI] [PubMed] [Google Scholar]
- de Licona HK, Karacay B, Mahoney J, McDonald E, Luang T, Bonthius DJ. A single exposure to alcohol during brain development induces microencephaly and neuronal losses in genetically susceptible mice, but not in wild type mice. Neurotoxicology. 2009;30:459–470. doi: 10.1016/j.neuro.2009.01.010. [DOI] [PubMed] [Google Scholar]
- Dewar KM, Reader TA. Distribution of dopamine D1 and D2 receptors in rabbit cortical areas, hippocampus, and neostriatum in relation to dopamine contents. Synapse. 1989;4:378–386. doi: 10.1002/syn.890040413. [DOI] [PubMed] [Google Scholar]
- Diaz R, Ogren SO, Blum M, Fuxe K. Prenatal corticosterone increases spontaneous and d-amphetamine induced locomotor activity and brain dopamine metabolism in prepubertal male and female rats. Neuroscience. 1995;66:467–473. doi: 10.1016/0306-4522(94)00605-5. [DOI] [PubMed] [Google Scholar]
- Doyle P, Rohner-Jeanrenaud F, Jeanrenaud B. Local cerebral glucose utilization in brains of lean and genetically obese (fa/fa) rats. Am J Physiol. 1993;264:E29–36. doi: 10.1152/ajpendo.1993.264.1.E29. [DOI] [PubMed] [Google Scholar]
- Drake AJ, Tang JI, Nyirenda MJ. Mechanisms underlying the role of glucocorticoids in the early life programming of adult disease. Clin Sci (Lond) 2007;113:219–232. doi: 10.1042/CS20070107. [DOI] [PubMed] [Google Scholar]
- Dudley KJ, Li X, Kobor MS, Kippin TE, Bredy TW. Epigenetic mechanisms mediating vulnerability and resilience to psychiatric disorders. Neurosci Biobehav Rev. 2011;35:1544–1551. doi: 10.1016/j.neubiorev.2010.12.016. [DOI] [PubMed] [Google Scholar]
- Durand DJ, Espinoza AM, Nickerson BG. Association between prenatal cocaine exposure and sudden infant death syndrome. J Pediatr. 1990;117:909–911. doi: 10.1016/s0022-3476(05)80133-7. [DOI] [PubMed] [Google Scholar]
- Dwyer JB, Broide RS, Leslie FM. Nicotine and brain development. Birth Defects Res C Embryo Today. 2008;84:30–44. doi: 10.1002/bdrc.20118. [DOI] [PubMed] [Google Scholar]
- Dwyer JB, McQuown SC, Leslie FM. The dynamic effects of nicotine on the developing brain. Pharmacol Ther. 2009;122:125–139. doi: 10.1016/j.pharmthera.2009.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards AD, Brocklehurst P, Gunn AJ, Halliday H, Juszczak E, Levene M, Strohm B, Thoresen M, Whitelaw A, Azzopardi D. Neurological outcomes at 18 months of age after moderate hypothermia for perinatal hypoxic ischaemic encephalopathy: synthesis and meta-analysis of trial data. Bmj. 2010;340:c363. doi: 10.1136/bmj.c363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elahi MM, Cagampang FR, Mukhtar D, Anthony FW, Ohri SK, Hanson MA. Long-term maternal high-fat feeding from weaning through pregnancy and lactation predisposes offspring to hypertension, raised plasma lipids and fatty liver in mice. Br J Nutr. 2009;102:514–519. doi: 10.1017/S000711450820749X. [DOI] [PubMed] [Google Scholar]
- Eppolito AK, Smith RF. Long-term behavioral and developmental consequences of pre- and perinatal nicotine. Pharmacol Biochem Behav. 2006;85:835–841. doi: 10.1016/j.pbb.2006.11.020. [DOI] [PubMed] [Google Scholar]
- Erkkola M, Nwaru BI, Viljakainen HT. Maternal vitamin D during pregnancy and its relation to immune-mediated diseases in the offspring. Vitam Horm. 2011;86:239–260. doi: 10.1016/B978-0-12-386960-9.00010-1. [DOI] [PubMed] [Google Scholar]
- Ernst M, Moolchan ET, Robinson ML. Behavioral and neural consequences of prenatal exposure to nicotine. J Am Acad Child Adolesc Psychiatry. 2001;40:630–641. doi: 10.1097/00004583-200106000-00007. [DOI] [PubMed] [Google Scholar]
- Fall CH, Osmond C, Barker DJ, Clark PM, Hales CN, Stirling Y, Meade TW. Fetal and infant growth and cardiovascular risk factors in women. BMJ. 1995;310:428–432. doi: 10.1136/bmj.310.6977.428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Famularo R, Fenton T. Early developmental history and pediatric posttraumatic stress disorder. Arch Pediatr Adolesc Med. 1994;148:1032–1038. doi: 10.1001/archpedi.1994.02170100030007. [DOI] [PubMed] [Google Scholar]
- Fan X, Heijnen CJ, van der Kooij MA, Groenendaal F, van Bel F. The role and regulation of hypoxia-inducible factor-1alpha expression in brain development and neonatal hypoxic-ischemic brain injury. Brain Res Rev. 2009;62:99–108. doi: 10.1016/j.brainresrev.2009.09.006. [DOI] [PubMed] [Google Scholar]
- Flavin MP. Influence of dexamethasone on neurotoxicity caused by oxygen and glucose deprivation in vitro. Exp Neurol. 1996;139:34–38. doi: 10.1006/exnr.1996.0078. [DOI] [PubMed] [Google Scholar]
- Florian ML, Nunes ML. Effects of intra-uterine and early extra-uterine malnutrition on seizure threshold and hippocampal morphometry of pup rats. Nutr Neurosci. 2010;13:265–273. doi: 10.1179/147683010X12611460764804. [DOI] [PubMed] [Google Scholar]
- Flower RJ, Rothwell NJ. Lipocortin-1: cellular mechanisms and clinical relevance. Trends Pharmacol Sci. 1994;15:71–76. doi: 10.1016/0165-6147(94)90281-x. [DOI] [PubMed] [Google Scholar]
- Fowden AL, Forhead AJ. Endocrine mechanisms of intrauterine programming. Reproduction. 2004;127:515–526. doi: 10.1530/rep.1.00033. [DOI] [PubMed] [Google Scholar]
- Freunscht I, Feldmann R. Young adults with Fetal Alcohol Syndrome (FAS): social, emotional and occupational development. Klin Padiatr. 2011;223:33–37. doi: 10.1055/s-0030-1261927. [DOI] [PubMed] [Google Scholar]
- Gasche Y, Copin JC, Sugawara T, Fujimura M, Chan PH. Matrix metalloproteinase inhibition prevents oxidative stress-associated blood-brain barrier disruption after transient focal cerebral ischemia. J Cereb Blood Flow Metab. 2001;21:1393–1400. doi: 10.1097/00004647-200112000-00003. [DOI] [PubMed] [Google Scholar]
- Gauda EB, Cooper R, Akins PK, Wu G. Prenatal nicotine affects catecholamine gene expression in newborn rat carotid body and petrosal ganglion. J Appl Physiol. 2001;91:2157–2165. doi: 10.1152/jappl.2001.91.5.2157. [DOI] [PubMed] [Google Scholar]
- Gluckman PD, Hanson MA. Living with the past: evolution, development, and patterns of disease. Science. 2004;305:1733–1736. doi: 10.1126/science.1095292. [DOI] [PubMed] [Google Scholar]
- Gluckman PD, Hanson MA, Cooper C, Thornburg KL. Effect of in utero and early-life conditions on adult health and disease. N Engl J Med. 2008;359:61–73. doi: 10.1056/NEJMra0708473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gniuli D, Calcagno A, Caristo ME, Mancuso A, Macchi V, Mingrone G, Vettor R. Effects of high-fat diet exposure during fetal life on type 2 diabetes development in the progeny. J Lipid Res. 2008;49:1936–1945. doi: 10.1194/jlr.M800033-JLR200. [DOI] [PubMed] [Google Scholar]
- Goldman-Rakic PS, Brown RM. Postnatal development of monoamine content and synthesis in the cerebral cortex of rhesus monkeys. Brain Res. 1982;256:339–349. doi: 10.1016/0165-3806(82)90146-8. [DOI] [PubMed] [Google Scholar]
- Goyal R, Goyal D, Leitzke A, Gheorghe CP, Longo LD. Brain reninangiotensin system: fetal epigenetic programming by maternal protein restriction during pregnancy. Reprod Sci. 2010;17:227–238. doi: 10.1177/1933719109351935. [DOI] [PubMed] [Google Scholar]
- Goyal R, Papamatheakis DG, Loftin M, Vrancken K, Dawson AS, Osman NJ, Blood AB, Pearce WJ, Longo LD, Wilson SM. Long-term maternal hypoxia: the role of extracellular Ca2+ entry during serotonin-mediated contractility in fetal ovine pulmonary arteries. Reprod Sci. 2011;18:948–962. doi: 10.1177/1933719111401660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham HK, Boyd R, Carlin JB, Dobson F, Lowe K, Nattrass G, Thomason P, Wolfe R, Reddihough D. Does botulinum toxin a combined with bracing prevent hip displacement in children with cerebral palsy and “hips at risk”? A randomized, controlled trial. J Bone Joint Surg Am. 2008;90:23–33. doi: 10.2106/JBJS.F.01416. [DOI] [PubMed] [Google Scholar]
- Grantham-McGregor S, Baker-Henningham H. Review of the evidence linking protein and energy to mental development. Public Health Nutr. 2005;8:1191–1201. doi: 10.1079/phn2005805. [DOI] [PubMed] [Google Scholar]
- Gregorio BM, Souza-Mello V, Mandarim-de-Lacerda CA, Aguila MB. Maternal fish oil supplementation benefits programmed offspring from rat dams fed low-protein diet. Am J Obstet Gynecol. 2008;199:82, e81–87. doi: 10.1016/j.ajog.2007.12.016. [DOI] [PubMed] [Google Scholar]
- Gressens P, Gofflot F, Van Maele-Fabry G, Misson JP, Gadisseux JF, Evrard P, Picard JJ. Early neurogenesis and teratogenesis in whole mouse embryo cultures. Histochemical, immunocytological and ultrastructural study of the premigratory neuronal-glial units in normal mouse embryo and in mouse embryos influenced by cocaine and retinoic acid. J Neuropathol Exp Neurol. 1992;51:206–219. doi: 10.1097/00005072-199203000-00010. [DOI] [PubMed] [Google Scholar]
- Gressens P, Mesples B, Sahir N, Marret S, Sola A. Environmental factors and disturbances of brain development. Semin Neonatol. 2001;6:185–194. doi: 10.1053/siny.2001.0048. [DOI] [PubMed] [Google Scholar]
- Gressens P, Muaku SM, Besse L, Nsegbe E, Gallego J, Delpech B, Gaultier C, Evrard P, Ketelslegers JM, Maiter D. Maternal protein restriction early in rat pregnancy alters brain development in the progeny. Brain Res Dev Brain Res. 1997;103:21–35. doi: 10.1016/s0165-3806(97)00109-0. [DOI] [PubMed] [Google Scholar]
- Guilloteau P, Zabielski R, Hammon HM, Metges CC. Adverse effects of nutritional programming during prenatal and early postnatal life, some aspects of regulation and potential prevention and treatments. J Physiol Pharmacol. 2009;60(Suppl 3):17–35. [PubMed] [Google Scholar]
- Gulino A, De Smaele E, Ferretti E. Glucocorticoids and neonatal brain injury: the hedgehog connection. J Clin Invest. 2009;119:243–246. doi: 10.1172/JCI38387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo R, Hou W, Dong Y, Yu Z, Stites J, Weiner CP. Brain injury caused by chronic fetal hypoxemia is mediated by inflammatory cascade activation. Reprod Sci. 2010;17:540–548. doi: 10.1177/1933719110364061. [DOI] [PubMed] [Google Scholar]
- Halliwell B. Reactive oxygen species and the central nervous system. J Neurochem. 1992;59:1609–1623. doi: 10.1111/j.1471-4159.1992.tb10990.x. [DOI] [PubMed] [Google Scholar]
- Halmesmaki E, Autti I, Granstrom ML, Heikinheimo M, Raivio KO, Ylikorkala O. Prediction of fetal alcohol syndrome by maternal alpha fetoprotein, human placental lactogen and pregnancy specific beta 1-glycoprotein. Alcohol Alcohol Suppl. 1987;1:473–476. [PubMed] [Google Scholar]
- Hardy DB, Yang K. The expression of 11 beta-hydroxysteroid dehydrogenase type 2 is induced during trophoblast differentiation: effects of hypoxia. J Clin Endocrinol Metab. 2002;87:3696–3701. doi: 10.1210/jcem.87.8.8720. [DOI] [PubMed] [Google Scholar]
- Harris A, Seckl J. Glucocorticoids, prenatal stress and the programming of disease. Horm Behav. 2011;59:279–289. doi: 10.1016/j.yhbeh.2010.06.007. [DOI] [PubMed] [Google Scholar]
- Hawkins P, Steyn C, McGarrigle HH, Calder NA, Saito T, Stratford LL, Noakes DE, Hansona MA. Cardiovascular and hypothalamic-pituitary-adrenal axis development in late gestation fetal sheep and young lambs following modest maternal nutrient restriction in early gestation. Reprod Fertil Dev. 2000;12:443–456. doi: 10.1071/rd99071. [DOI] [PubMed] [Google Scholar]
- Hayashi A, Nagaoka M, Yamada K, Ichitani Y, Miake Y, Okado N. Maternal stress induces synaptic loss and developmental disabilities of offspring. Int J Dev Neurosci. 1998;16:209–216. doi: 10.1016/s0736-5748(98)00028-8. [DOI] [PubMed] [Google Scholar]
- Heine VM, Rowitch DH. Hedgehog signaling has a protective effect in glucocorticoid-induced mouse neonatal brain injury through an 11betaHSD2-dependent mechanism. J Clin Invest. 2009;119:267–277. doi: 10.1172/JCI36376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson GI, Chen JJ, Schenker S. Ethanol, oxidative stress, reactive aldehydes, and the fetus. Front Biosci. 1999;4:D541–550. doi: 10.2741/henderson. [DOI] [PubMed] [Google Scholar]
- Heusch P, Canton M, Aker S, van de Sand A, Konietzka I, Rassaf T, Menazza S, Brodde OE, Di Lisa F, Heusch G, Schulz R. The contribution of reactive oxygen species and p38 mitogen-activated protein kinase to myofilament oxidation and progression of heart failure in rabbits. Br J Pharmacol. 2010;160:1408–1416. doi: 10.1111/j.1476-5381.2010.00793.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hewitt AJ, Walker KR, Kobus SM, Poklewska-Koziell M, Reynolds JN, Brien JF. Differential effects of chronic ethanol exposure on cytochrome P450 2E1 and the hypothalamic-pituitary-adrenal axis in the maternal-fetal unit of the guinea pig. Neurotoxicol Teratol. 2010;32:164–170. doi: 10.1016/j.ntt.2009.12.002. [DOI] [PubMed] [Google Scholar]
- Holmes MC, Yau JL, Kotelevtsev Y, Mullins JJ, Seckl JR. 11 Beta-hydroxysteroid dehydrogenases in the brain: two enzymes two roles. Ann N Y Acad Sci. 2003;1007:357–366. doi: 10.1196/annals.1286.035. [DOI] [PubMed] [Google Scholar]
- Homan A, Guan H, Hardy DB, Gratton RJ, Yang K. Hypoxia blocks 11beta-hydroxysteroid dehydrogenase type 2 induction in human trophoblast cells during differentiation by a time-dependent mechanism that involves both translation and transcription. Placenta. 2006;27:832–840. doi: 10.1016/j.placenta.2005.09.006. [DOI] [PubMed] [Google Scholar]
- Hypponen E, Hartikainen AL, Sovio U, Jarvelin MR, Pouta A. Does vitamin D supplementation in infancy reduce the risk of pre-eclampsia? Eur J Clin Nutr. 2007;61:1136–1139. doi: 10.1038/sj.ejcn.1602625. [DOI] [PubMed] [Google Scholar]
- Iqbal U, Brien JF, Kapoor A, Matthews SG, Reynolds JN. Chronic prenatal ethanol exposure increases glucocorticoid-induced glutamate release in the hippocampus of the near-term foetal guinea pig. J Neuroendocrinol. 2006;18:826–834. doi: 10.1111/j.1365-2826.2006.01479.x. [DOI] [PubMed] [Google Scholar]
- Ireland Z, Dickinson H, Fleiss B, Hutton LC, Walker DW. Behavioural effects of near-term acute fetal hypoxia in a small precocial animal, the spiny mouse (Acomys cahirinus) Neonatology. 2010;97:45–51. doi: 10.1159/000227293. [DOI] [PubMed] [Google Scholar]
- Joels M, de Kloet ER. Mineralocorticoid and glucocorticoid receptors in the brain. Implications for ion permeability and transmitter systems. Prog Neurobiol. 1994;43:1–36. doi: 10.1016/0301-0082(94)90014-0. [DOI] [PubMed] [Google Scholar]
- Jones ES, Vinh A, McCarthy CA, Gaspari TA, Widdop RE. AT2 receptors: functional relevance in cardiovascular disease. Pharmacol Ther. 2008;120:292–316. doi: 10.1016/j.pharmthera.2008.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones KL, Hoyme HE, Robinson LK, Del Campo M, Manning MA, Prewitt LM, Chambers CD. Fetal alcohol spectrum disorders: Extending the range of structural defects. Am J Med Genet A. 2010;152A:2731–2735. doi: 10.1002/ajmg.a.33675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones L, Fischer I, Levitt P. Nonuniform alteration of dendritic development in the cerebral cortex following prenatal cocaine exposure. Cereb Cortex. 1996;6:431–445. doi: 10.1093/cercor/6.3.431. [DOI] [PubMed] [Google Scholar]
- Jones PB, Rantakallio P, Hartikainen AL, Isohanni M, Sipila P. Schizophrenia as a long-term outcome of pregnancy, delivery, and perinatal complications: a 28-year follow-up of the 1966 north Finland general population birth cohort. Am J Psychiatry. 1998;155:355–364. doi: 10.1176/ajp.155.3.355. [DOI] [PubMed] [Google Scholar]
- Joss-Moore LA, Albertine KH, Lane RH. Epigenetics and the developmental origins of lung disease. Mol Genet Metab. 2011;104:61–66. doi: 10.1016/j.ymgme.2011.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jungel A, Ospelt C, Gay S. What can we learn from epigenetics in the year 2009? Curr Opin Rheumatol. 2010;22:284–292. doi: 10.1097/BOR.0b013e3283389641. [DOI] [PubMed] [Google Scholar]
- Kandall SR, Gaines J, Habel L, Davidson G, Jessop D. Relationship of maternal substance abuse to subsequent sudden infant death syndrome in offspring. J Pediatr. 1993;123:120–126. doi: 10.1016/s0022-3476(05)81554-9. [DOI] [PubMed] [Google Scholar]
- Karacay B, Li G, Pantazis NJ, Bonthius DJ. Stimulation of the cAMP pathway protects cultured cerebellar granule neurons against alcohol-induced cell death by activating the neuronal nitric oxide synthase (nNOS) gene. Brain Res. 2007;1143:34–45. doi: 10.1016/j.brainres.2007.01.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karpf AR, Moore BC, Ririe TO, Jones DA. Activation of the p53 DNA damage response pathway after inhibition of DNA methyltransferase by 5-aza-2′-deoxycytidine. Mol Pharmacol. 2001;59:751–757. [PubMed] [Google Scholar]
- Kauffman KS, Seidler FJ, Slotkin TA. Prenatal dexamethasone exposure causes loss of neonatal hypoxia tolerance: cellular mechanisms. Pediatr Res. 1994;35:515–522. [PubMed] [Google Scholar]
- Ke Q, Costa M. Hypoxia-inducible factor-1 (HIF-1) Mol Pharmacol. 2006;70:1469–1480. doi: 10.1124/mol.106.027029. [DOI] [PubMed] [Google Scholar]
- King CR. A novel embryological theory of autism causation involving endogenous biochemicals capable of initiating cellular gene transcription: a possible link between twelve autism risk factors and the autism ‘epidemic’. Med Hypotheses. 2011;76:653–660. doi: 10.1016/j.mehy.2011.01.024. [DOI] [PubMed] [Google Scholar]
- Koenen SV, Mecenas CA, Smith GS, Jenkins S, Nathanielsz PW. Effects of maternal betamethasone administration on fetal and maternal blood pressure and heart rate in the baboon at 0.7 of gestation. Am J Obstet Gynecol. 2002;186:812–817. doi: 10.1067/mob.2002.121654. [DOI] [PubMed] [Google Scholar]
- Koenig JI, Kirkpatrick B, Lee P. Glucocorticoid hormones and early brain development in schizophrenia. Neuropsychopharmacology. 2002;27:309–318. doi: 10.1016/S0893-133X(01)00396-7. [DOI] [PubMed] [Google Scholar]
- Konycheva G, Dziadek MA, Ferguson LR, Krageloh CU, Coolen MW, Davison M, Breier BH. Dietary methyl donor deficiency during pregnancy in rats shapes learning and anxiety in offspring. Nutr Res. 2011;31:790–804. doi: 10.1016/j.nutres.2011.09.015. [DOI] [PubMed] [Google Scholar]
- Kosofsky BE, Wilkins AS, Gressens P, Evrard P. Transplacental cocaine exposure: a mouse model demonstrating neuroanatomic and behavioral abnormalities. J Child Neurol. 1994;9:234–241. doi: 10.1177/088307389400900303. [DOI] [PubMed] [Google Scholar]
- Laflamme L, Gasparo M, Gallo JM, Payet MD, Gallo-Payet N. Angiotensin II induction of neurite outgrowth by AT2 receptors in NG108-15 cells. Effect counteracted by the AT1 receptors. J Biol Chem. 1996;271:22729–22735. doi: 10.1074/jbc.271.37.22729. [DOI] [PubMed] [Google Scholar]
- Lahti J, Raikkonen K, Sovio U, Miettunen J, Hartikainen AL, Pouta A, Taanila A, Joukamaa M, Jarvelin MR, Veijola J. Early-life origins of schizotypal traits in adulthood. Br J Psychiatry. 2009;195:132–137. doi: 10.1192/bjp.bp.108.054387. [DOI] [PubMed] [Google Scholar]
- Laird PW, Jackson-Grusby L, Fazeli A, Dickinson SL, Jung WE, Li E, Weinberg RA, Jaenisch R. Suppression of intestinal neoplasia by DNA hypomethylation. Cell. 1995;81:197–205. doi: 10.1016/0092-8674(95)90329-1. [DOI] [PubMed] [Google Scholar]
- Langley-Evans SC, McMullen S. Developmental origins of adult disease. Med Princ Pract. 2010;19:87–98. doi: 10.1159/000273066. [DOI] [PubMed] [Google Scholar]
- Lawrence J, Chen M, Xiong F, Xiao D, Zhang H, Buchholz JN, Zhang L. Fetal nicotine exposure causes PKCε gene repression by promoter methylation in the heart. Cardiovas Res. 2011;89:89–97. doi: 10.1093/cvr/cvq270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S, Choi I, Kang S, Rivier C. Role of various neurotransmitters in mediating the long-term endocrine consequences of prenatal alcohol exposure. Ann N Y Acad Sci. 2008;1144:176–188. doi: 10.1196/annals.1418.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee YM, Jeong CH, Koo SY, Son MJ, Song HS, Bae SK, Raleigh JA, Chung HY, Yoo MA, Kim KW. Determination of hypoxic region by hypoxia marker in developing mouse embryos in vivo: a possible signal for vessel development. Dev Dyn. 2001;220:175–186. doi: 10.1002/1097-0177(20010201)220:2<175::AID-DVDY1101>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- Leon DA, Koupilova I, Lithell HO, Berglund L, Mohsen R, Vagero D, Lithell UB, McKeigue PM. Failure to realise growth potential in utero and adult obesity in relation to blood pressure in 50 year old Swedish men. BMJ. 1996;312:401–406. doi: 10.1136/bmj.312.7028.401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine RS, Hennekens CH, Jesse MJ. Blood pressure in prospective population based cohort of newborn and infant twins. BMJ. 1994;308:298–302. doi: 10.1136/bmj.308.6924.298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levitsky DA, Strupp BJ. Malnutrition and the brain: Changing concepts, changing concerns. J Nutr. 1995;125(Suppl):2212S–2220S. doi: 10.1093/jn/125.suppl_8.2212S. [DOI] [PubMed] [Google Scholar]
- Levitt NS, Lindsay RS, Holmes MC, Seckl JR. Dexamethasone in the last week of pregnancy attenuates hippocampal glucocorticoid receptor gene expression and elevates blood pressure in the adult offspring in the rat. Neuroendocrinology. 1996;64:412–418. doi: 10.1159/000127146. [DOI] [PubMed] [Google Scholar]
- Levitt P. Prenatal effects of drugs of abuse on brain development. Drug Alcohol Depend. 1998;51:109–125. doi: 10.1016/s0376-8716(98)00070-2. [DOI] [PubMed] [Google Scholar]
- Levitt P, Rakic P, Goldman-Rakic P. Region-specific distribution of catecholamine afferents in primate cerebral cortex: a fluorescence histochemical analysis. J Comp Neurol. 1984;227:23–36. doi: 10.1002/cne.902270105. [DOI] [PubMed] [Google Scholar]
- Li G, Xiao Y, Estrella JL, Ducsay CA, Gilbert RD, Zhang L. Effect of fetal hypoxia on heart susceptibility to ischemia and reperfusion injury in the adult rat. J Soc Gynecol Investig. 2003;10:265–274. doi: 10.1016/s1071-5576(03)00074-1. [DOI] [PubMed] [Google Scholar]
- Li H, Rauch T, Chen ZX, Szabo PE, Riggs AD, Pfeifer GP. The histone methyltransferase SETDB1 and the DNA methyltransferase DNMT3A interact directly and localize to promoters silenced in cancer cells. J Biol Chem. 2006;281:19489–19500. doi: 10.1074/jbc.M513249200. [DOI] [PubMed] [Google Scholar]
- Li J, Culman J, Hortnagl H, Zhao Y, Gerova N, Timm M, Blume A, Zimmermann M, Seidel K, Dirnagl U, Unger T. Angiotensin AT2 receptor protects against cerebral ischemia-induced neuronal injury. Faseb J. 2005;19:617–619. doi: 10.1096/fj.04-2960fje. [DOI] [PubMed] [Google Scholar]
- Lidow MS. Prenatal cocaine exposure adversely affects development of the primate cerebral cortex. Synapse. 1995;21:332–341. doi: 10.1002/syn.890210408. [DOI] [PubMed] [Google Scholar]
- Lillycrop KA, Slater-Jefferies JL, Hanson MA, Godfrey KM, Jackson AA, Burdge GC. Induction of altered epigenetic regulation of the hepatic glucocorticoid receptor in the offspring of rats fed a protein-restricted diet during pregnancy suggests that reduced DNA methyltransferase-1 expression is involved in impaired DNA methylation and changes in histone modifications. Br J Nutr. 2007;97:1064–1073. doi: 10.1017/S000711450769196X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindholm LH, Ibsen H, Dahlof B, Devereux RB, Beevers G, de Faire U, Fyhrquist F, Julius S, Kjeldsen SE, Kristiansson K, Lederballe-Pedersen O, Nieminen MS, Omvik P, Oparil S, Wedel H, Aurup P, Edelman J, Snapinn S. Cardiovascular morbidity and mortality in patients with diabetes in the Losartan Intervention For Endpoint reduction in hypertension study (LIFE): a randomised trial against atenolol. Lancet. 2002;359:1004–1010. doi: 10.1016/S0140-6736(02)08090-X. [DOI] [PubMed] [Google Scholar]
- Lou M, Blume A, Zhao Y, Gohlke P, Deuschl G, Herdegen T, Culman J. Sustained blockade of brain AT1 receptors before and after focal cerebral ischemia alleviates neurologic deficits and reduces neuronal injury, apoptosis, and inflammatory responses in the rat. J Cereb Blood Flow Metab. 2004;24:536–547. doi: 10.1097/00004647-200405000-00008. [DOI] [PubMed] [Google Scholar]
- Maier SE, West JR. Regional differences in cell loss associated with binge-like alcohol exposure during the first two trimesters equivalent in the rat. Alcohol. 2001;23:49–57. doi: 10.1016/s0741-8329(00)00133-6. [DOI] [PubMed] [Google Scholar]
- Mairesse J, Lesage J, Breton C, Breant B, Hahn T, Darnaudery M, Dickson SL, Seckl J, Blondeau B, Vieau D, Maccari S, Viltart O. Maternal stress alters endocrine function of the feto-placental unit in rats. Am J Physiol Endocrinol Metab. 2007;292:E1526–1533. doi: 10.1152/ajpendo.00574.2006. [DOI] [PubMed] [Google Scholar]
- Mao C, Shi L, Xu F, Zhang L, Xu Z. Development of fetal brain reninangiotensin system and its influence on programmed hypertension in fetal origins. Prog Neurobiol. 2009a;87:252–263. doi: 10.1016/j.pneurobio.2008.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao C, Wu J, Xiao D, Lv J, Ding Y, Xu Z, Zhang L. The effect of fetal and neonatal nicotine exposure on renal development of AT(1) and AT(2) receptors. Reprod Toxicol. 2009b;27:149–154. doi: 10.1016/j.reprotox.2009.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy CA, Vinh A, Callaway JK, Widdop RE. Angiotensin AT2 receptor stimulation causes neuroprotection in a conscious rat model of stroke. Stroke; a journal of cerebral circulation. 2009;40:1482–1489. doi: 10.1161/STROKEAHA.108.531509. [DOI] [PubMed] [Google Scholar]
- McCormick CM, Smythe JW, Sharma S, Meaney MJ. Sex-specific effects of prenatal stress on hypothalamic-pituitary-adrenal responses to stress and brain glucocorticoid receptor density in adult rats. Brain Res Dev Brain Res. 1995;84:55–61. doi: 10.1016/0165-3806(94)00153-q. [DOI] [PubMed] [Google Scholar]
- McEwen BS. Central effects of stress hormones in health and disease: Understanding the protective and damaging effects of stress and stress mediators. Eur J Pharmacol. 2008;583:174–185. doi: 10.1016/j.ejphar.2007.11.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMillen IC, Robinson JS. Developmental origins of the metabolic syndrome: prediction, plasticity, and programming. Physiol Rev. 2005;85:571–633. doi: 10.1152/physrev.00053.2003. [DOI] [PubMed] [Google Scholar]
- Meaney MJ, Szyf M. Maternal care as a model for experience-dependent chromatin plasticity? Trends Neurosci. 2005;28:456–463. doi: 10.1016/j.tins.2005.07.006. [DOI] [PubMed] [Google Scholar]
- Meaney MJ, Szyf M, Seckl JR. Epigenetic mechanisms of perinatal programming of hypothalamic-pituitary-adrenal function and health. Trends Mol Med. 2007;13:269–277. doi: 10.1016/j.molmed.2007.05.003. [DOI] [PubMed] [Google Scholar]
- Mehedint MG, Craciunescu CN, Zeisel SH. Maternal dietary choline deficiency alters angiogenesis in fetal mouse hippocampus. Proc Natl Acad Sci U S A. 2010;107:12834–12839. doi: 10.1073/pnas.0914328107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melse-Boonstra A, Jaiswal N. Iodine deficiency in pregnancy, infancy and childhood and its consequences for brain development. Best Pract Res Clin Endocrinol Metab. 2010;24:29–38. doi: 10.1016/j.beem.2009.09.002. [DOI] [PubMed] [Google Scholar]
- Meyer JS. Early adrenalectomy stimulates subsequent growth and development of the rat brain. Exp Neurol. 1983;82:432–446. doi: 10.1016/0014-4886(83)90415-6. [DOI] [PubMed] [Google Scholar]
- Meyer K, Zhang H, Zhang L. Direct effect of cocaine on epigenetic regulation of PKCε gene repression in the fetal rat heart. J Mol Cell Cardiol. 2009;47:504–511. doi: 10.1016/j.yjmcc.2009.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milerad J, Sundell H. Nicotine exposure and the risk of SIDS. Acta Paediatr Suppl. 1993;82(Suppl 389):70–72. doi: 10.1111/j.1651-2227.1993.tb12882.x. [DOI] [PubMed] [Google Scholar]
- Milosevic J, Maisel M, Wegner F, Leuchtenberger J, Wenger RH, Gerlach M, Storch A, Schwarz J. Lack of hypoxia-inducible factor-1 alpha impairs midbrain neural precursor cells involving vascular endothelial growth factor signaling. J Neurosci. 2007;27:412–421. doi: 10.1523/JNEUROSCI.2482-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mocchetti I, Spiga G, Hayes VY, Isackson PJ, Colangelo A. Glucocorticoids differentially increase nerve growth factor and basic fibroblast growth factor expression in the rat brain. J Neurosci. 1996;16:2141–2148. doi: 10.1523/JNEUROSCI.16-06-02141.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Modi N, Lewis H, Al-Naqeeb N, Ajayi-Obe M, Dore CJ, Rutherford M. The effects of repeated antenatal glucocorticoid therapy on the developing brain. Pediatr Res. 2001;50:581–585. doi: 10.1203/00006450-200111000-00008. [DOI] [PubMed] [Google Scholar]
- Moghaddam B, Bolinao ML, Stein-Behrens B, Sapolsky R. Glucocorticoids mediate the stress-induced extracellular accumulation of glutamate. Brain Res. 1994;655:251–254. doi: 10.1016/0006-8993(94)91622-5. [DOI] [PubMed] [Google Scholar]
- Mogi M, Li JM, Iwanami J, Min LJ, Tsukuda K, Iwai M, Horiuchi M. Angiotensin II type-2 receptor stimulation prevents neural damage by transcriptional activation of methyl methanesulfonate sensitive 2. Hypertension. 2006;48:141–148. doi: 10.1161/01.HYP.0000229648.67883.f9. [DOI] [PubMed] [Google Scholar]
- Moore VM, Miller AG, Boulton TJ, Cockington RA, Craig IH, Magarey AM, Robinson JS. Placental weight, birth measurements, and blood pressure at age 8 years. Arch Dis Child. 1996;74:538–541. doi: 10.1136/adc.74.6.538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan CD, Sandler M, Panigel M. Placental transfer of catecholamines in vitro and in vivo. Am J Obstet Gynecol. 1972;112:1068–1075. doi: 10.1016/0002-9378(72)90182-2. [DOI] [PubMed] [Google Scholar]
- Morgane PJ, et al. Prenatal malnutrition and development of the brain. Neurosci Biobehav Rev. 1993;17:91–128. doi: 10.1016/s0149-7634(05)80234-9. [DOI] [PubMed] [Google Scholar]
- Morley R, Lucas A. Nutrition and cognitive development. Br Med Bull. 1997;53:123–134. doi: 10.1093/oxfordjournals.bmb.a011595. [DOI] [PubMed] [Google Scholar]
- Morsink MC, Steenbergen PJ, Vos JB, Karst H, Joels M, De Kloet ER, Datson NA. Acute activation of hippocampal glucocorticoid receptors results in different waves of gene expression throughout time. J Neuroendocrinol. 2006;18:239–252. doi: 10.1111/j.1365-2826.2006.01413.x. [DOI] [PubMed] [Google Scholar]
- Murmu MS, Salomon S, Biala Y, Weinstock M, Braun K, Bock J. Changes of spine density and dendritic complexity in the prefrontal cortex in offspring of mothers exposed to stress during pregnancy. Eur J Neurosci. 2006;24:1477–1487. doi: 10.1111/j.1460-9568.2006.05024.x. [DOI] [PubMed] [Google Scholar]
- Murriel CL, Mochly-Rosen D. Opposing roles of delta and epsilonPKC in cardiac ischemia and reperfusion: targeting the apoptotic machinery. Arch Biochem Biophys. 2003;420:246–254. doi: 10.1016/j.abb.2003.08.038. [DOI] [PubMed] [Google Scholar]
- Mychasiuk R, Ilnytskyy S, Kovalchuk O, Kolb B, Gibb R. Intensity matters: brain, behaviour and the epigenome of prenatally stressed rats. Neuroscience. 2011;180:105–110. doi: 10.1016/j.neuroscience.2011.02.026. [DOI] [PubMed] [Google Scholar]
- Naassila M, Daoust M. Effect of prenatal and postnatal ethanol exposure on the developmental profile of mRNAs encoding NMDA receptor subunits in rat hippocampus. J Neurochem. 2002;80:850–860. doi: 10.1046/j.0022-3042.2002.00755.x. [DOI] [PubMed] [Google Scholar]
- Nelson EA, Taylor BJ. International Child Care Practices Study: infant sleep position and parental smoking. Early Hum Dev. 2001;64:7–20. doi: 10.1016/s0378-3782(01)00165-7. [DOI] [PubMed] [Google Scholar]
- Nelson LR, Taylor AN, Lewis JW, Poland RE, Redei E, Branch BJ. Pituitary-adrenal responses to morphine and footshock stress are enhanced following prenatal alcohol exposure. Alcohol Clin Exp Res. 1986;10:397–402. doi: 10.1111/j.1530-0277.1986.tb05112.x. [DOI] [PubMed] [Google Scholar]
- Niculescu MD, Craciunescu CN, Zeisel SH. Dietary choline deficiency alters global and gene-specific DNA methylation in the developing hippocampus of mouse fetal brains. Faseb J. 2006;20:43–49. doi: 10.1096/fj.05-4707com. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niculescu MD, Lupu DS. High fat diet-induced maternal obesity alters fetal hippocampal development. Int J Dev Neurosci. 2009;27:627–633. doi: 10.1016/j.ijdevneu.2009.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niculescu MD, Yamamuro Y, Zeisel SH. Choline availability modulates human neuroblastoma cell proliferation and alters the methylation of the promoter region of the cyclin-dependent kinase inhibitor 3 gene. J Neurochem. 2004;89:1252–1259. doi: 10.1111/j.1471-4159.2004.02414.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nistala R, Hayden MR, Demarco VG, Henriksen EJ, Lackland DT, Sowers JR. Prenatal Programming and Epigenetics in the Genesis of the Cardiorenal Syndrome. Cardiorenal Med. 2011;1:243–254. doi: 10.1159/000332756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Northington FJ, Ferriero DM, Graham EM, Traystman RJ, Martin LJ. Early Neurodegeneration after Hypoxia-Ischemia in Neonatal Rat Is Necrosis while Delayed Neuronal Death Is Apoptosis. Neurobiol Dis. 2001;8:207–219. doi: 10.1006/nbdi.2000.0371. [DOI] [PubMed] [Google Scholar]
- Nyirenda MJ, Carter R, Tang JI, de Vries A, Schlumbohm C, Hillier SG, Streit F, Oellerich M, Armstrong VW, Fuchs E, Seckl JR. Prenatal programming of metabolic syndrome in the common marmoset is associated with increased expression of 11beta-hydroxysteroid dehydrogenase type 1. Diabetes. 2009;58:2873–2879. doi: 10.2337/db09-0873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohishi T, Wang L, Ogawa B, Fujisawa K, Taniai E, Hayashi H, Mitsumori K, Shibutani M. No effect of sustained systemic growth retardation on the distribution of Reelin-expressing interneurons in the neuron-producing hippocampal dentate gyrus in rats. Reprod Toxicol. 2010;30:591–599. doi: 10.1016/j.reprotox.2010.08.009. [DOI] [PubMed] [Google Scholar]
- Olness K. Effects on brain development leading to cognitive impairment: A worldwide epidemic. J Dev Behav Pediatr. 2003;24:120–130. doi: 10.1097/00004703-200304000-00009. [DOI] [PubMed] [Google Scholar]
- O’Malley PM, Johnston LD, Bachman JG. Quantitative and qualitative changes in cocaine use among American high school seniors, college students, and young adults. NIDA Res Monogr. 1991;110:19–43. [PubMed] [Google Scholar]
- Osmond C, Barker DJ, Winter PD, Fall CH, Simmonds SJ. Early growth and death from cardiovascular disease in women. BMJ. 1993;307:1519–1524. doi: 10.1136/bmj.307.6918.1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouvry-Patat SA, Schey KL. Characterization of antimicrobial histone sequences and posttranslational modifications by mass spectrometry. J Mass Spectrom. 2007;42:664–674. doi: 10.1002/jms.1200. [DOI] [PubMed] [Google Scholar]
- Ozanne SE, Dorling MW, Wang CL, Nave BT. Impaired PI 3-kinase activation in adipocytes from early growth-restricted male rats. Am J Physiol Endocrinol Metab. 2001;280:E534–539. doi: 10.1152/ajpendo.2001.280.3.E534. [DOI] [PubMed] [Google Scholar]
- Palmer C. Hypoxic-ischemic encephalopathy. Therapeutic approaches against microvascular injury, and role of neutrophils, PAF, and free radicals. Clin Perinatol. 1995;22:481–517. [PubMed] [Google Scholar]
- Park SW, Davison JM, Rhee J, Hruban RH, Maitra A, Leach SD. Oncogenic KRAS induces progenitor cell expansion and malignant transformation in zebrafish exocrine pancreas. Gastroenterology. 2008;134:2080–2090. doi: 10.1053/j.gastro.2008.02.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson AJ, Chen M, Xue Q, Xiao D, Zhang L. Chronic prenatal hypoxia induces epigenetic programming of PKCε gene repression in rat hearts. Circ Res. 2010;107:365–373. doi: 10.1161/CIRCRESAHA.110.221259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pauly JR, Charriez CM, Guseva MV, Scheff SW. Nicotinic receptor modulation for neuroprotection and enhancement of functional recovery following brain injury or disease. Ann N Y Acad Sci. 2004;1035:316–334. doi: 10.1196/annals.1332.019. [DOI] [PubMed] [Google Scholar]
- Pauly JR, Slotkin TA. Maternal tobacco smoking, nicotine replacement and neurobehavioural development. Acta Paediatr. 2008;97:1331–1337. doi: 10.1111/j.1651-2227.2008.00852.x. [DOI] [PubMed] [Google Scholar]
- Perlman JM. Intrapartum hypoxic-ischemic cerebral injury and subsequent cerebral palsy: medicolegal issues. Pediatrics. 1997;99:851–859. doi: 10.1542/peds.99.6.851. [DOI] [PubMed] [Google Scholar]
- Perlman JM. Intervention strategies for neonatal hypoxic-ischemic cerebral injury. Clin Ther. 2006;28:1353–1365. doi: 10.1016/j.clinthera.2006.09.005. [DOI] [PubMed] [Google Scholar]
- Pinney SE, Simmons RA. Epigenetic mechanisms in the development of type 2 diabetes. Trends Endocrinol Metab. 2010;21:223–229. doi: 10.1016/j.tem.2009.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raikkonen K, Pesonen AK, Heinonen K, Kajantie E, Hovi P, Jarvenpaa AL, Eriksson JG, Andersson S. Depression in young adults with very low birth weight: the Helsinki study of very low-birth-weight adults. Arch Gen Psychiatry. 2008;65:290–296. doi: 10.1001/archgenpsychiatry.2007.40. [DOI] [PubMed] [Google Scholar]
- Ranade SC, Nawaz M. Sarfaraz, Rambtla P. Kumar, Rose AJ, Gressens P, Mani S. Early protein malnutrition disrupts cerebellar development and impairs motor coordination. Br J Nutr. 2011 Nov 4;:1–9. doi: 10.1017/S0007114511004119. 2011. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Rao R, de Ungria M, Sullivan D, Wu P, Wobken JD, Nelson CA, Georgieff MK. Perinatal brain iron deficiency increases the vulnerability of rat hippocampus to hypoxic ischemic insult. J Nutr. 1999;129:199–206. doi: 10.1093/jn/129.1.199. [DOI] [PubMed] [Google Scholar]
- Rasch R, Skriver E, Woods LL. The role of the RAS in programming of adult hypertension. Acta Physiol Scand. 2004;181:537–542. doi: 10.1111/j.1365-201X.2004.01328.x. [DOI] [PubMed] [Google Scholar]
- Rees S, Harding R, Walker D. An adverse intrauterine environment: implications for injury and altered development of the brain. Int J Dev Neurosci. 2008;26:3–11. doi: 10.1016/j.ijdevneu.2007.08.020. [DOI] [PubMed] [Google Scholar]
- Rees S, Harding R, Walker D. The biological basis of injury and neuroprotection in the fetal and neonatal brain. Int J Dev Neurosci. 2011;29:551–563. doi: 10.1016/j.ijdevneu.2011.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rich-Edwards JW, Stampfer MJ, Manson JE, Rosner B, Hankinson SE, Colditz GA, Willett WC, Hennekens CH. Birth weight and risk of cardiovascular disease in a cohort of women followed up since 1976. BMJ. 1997;315:396–400. doi: 10.1136/bmj.315.7105.396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson GA. Prenatal cocaine exposure. A longitudinal study of development. Ann N Y Acad Sci. 1998;846:144–152. [PubMed] [Google Scholar]
- Ripabelli G, Cimmino L, Grasso GM. [Alcohol consumption, pregnancy and fetal alcohol syndrome: implications in public health and preventive strategies] Ann Ig. 2006;18:391–406. [PubMed] [Google Scholar]
- Rosenberg GA. Matrix metalloproteinases and their multiple roles in neurodegenerative diseases. Lancet Neurol. 2009;8:205–216. doi: 10.1016/S1474-4422(09)70016-X. [DOI] [PubMed] [Google Scholar]
- Rueda-Clausen CF, Morton JS, Davidge ST. Effects of hypoxia-induced intrauterine growth restriction on cardiopulmonary structure and function during adulthood. Cardiovasc Res. 2009;81:713–722. doi: 10.1093/cvr/cvn341. [DOI] [PubMed] [Google Scholar]
- Saavedra JM, Benicky J, Zhou J. Mechanisms of the Anti-Ischemic Effect of Angiotensin II AT( 1 ) Receptor Antagonists in the Brain. Cell Mol Neurobiol. 2006;26:1099–1111. doi: 10.1007/s10571-006-9009-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanches EF, Arteni NS, Spindler C, Moyses F, Siqueira IR, Perry ML, Netto CA. Effects of pre- and postnatal protein malnutrition in hypoxic-ischemic rats. Brain Res. 2011 Dec 20; doi: 10.1016/j.brainres.2011.12.024. 2011. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Sarkar S, Tsai SW, Nguyen TT, Plevyak M, Padbury JF, Rubin LP. Inhibition of placental 11beta-hydroxysteroid dehydrogenase type 2 by catecholamines via alpha-adrenergic signaling. Am J Physiol Regul Integr Comp Physiol. 2001;281:R1966–1974. doi: 10.1152/ajpregu.2001.281.6.R1966. [DOI] [PubMed] [Google Scholar]
- Schrader J, Luders S, Kulschewski A, Hammersen F, Plate K, Berger J, Zidek W, Dominiak P, Diener HC. Morbidity and Mortality After Stroke, Eprosartan Compared with Nitrendipine for Secondary Prevention: principal results of a prospective randomized controlled study (MOSES) Stroke. 2005;36:1218–1226. doi: 10.1161/01.STR.0000166048.35740.a9. [DOI] [PubMed] [Google Scholar]
- Seckl JR. Physiologic programming of the fetus. Clin Perinatol. 1998;25:939–962. vii. [PubMed] [Google Scholar]
- Seckl JR. Glucocorticoid programming of the fetus; adult phenotypes and molecular mechanisms. Mol Cell Endocrinol. 2001;185:61–71. doi: 10.1016/s0303-7207(01)00633-5. [DOI] [PubMed] [Google Scholar]
- Seckl JR, Meaney MJ. Glucocorticoid programming. Ann N Y Acad Sci. 2004;1032:63–84. doi: 10.1196/annals.1314.006. [DOI] [PubMed] [Google Scholar]
- Seckl JR, Walker BR. Minireview: 11beta-hydroxysteroid dehydrogenase type 1-a tissue-specific amplifier of glucocorticoid action. Endocrinology. 2001;142:1371–1376. doi: 10.1210/endo.142.4.8114. [DOI] [PubMed] [Google Scholar]
- Seidler FJ, Slotkin TA. Fetal cocaine exposure causes persistent noradrenergic hyperactivity in rat brain regions: effects on neurotransmitter turnover and receptors. J Pharmacol Exp Ther. 1992;263:413–421. [PubMed] [Google Scholar]
- Shi L, Mao C, Xu Z, Zhang L. Angiotensin converting enzymes and drug discovery in cardiovascular diseases. Drug Discovery Today. 2010;15:332–341. doi: 10.1016/j.drudis.2010.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slotkin TA. Fetal nicotine or cocaine exposure: which one is worse? J Pharmacol Exp Ther. 1998;285:931–945. [PubMed] [Google Scholar]
- Slotkin TA, Cho H, Whitmore WL. Effects of prenatal nicotine exposure on neuronal development: selective actions on central and peripheral catecholaminergic pathways. Brain Res Bull. 1987;18:601–611. doi: 10.1016/0361-9230(87)90130-4. [DOI] [PubMed] [Google Scholar]
- Slotkin TA, Seidler FJ, Spindel ER. Prenatal nicotine exposure in rhesus monkeys compromises development of brainstem and cardiac monoamine pathways involved in perinatal adaptation and sudden infant death syndrome: amelioration by vitamin C. Neurotoxicol Teratol. 2011;33:431–434. doi: 10.1016/j.ntt.2011.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokol SI, Portnay EL, Curtis JP, Nelson MA, Hebert PR, Setaro JF, Foody JM. Modulation of the renin-angiotensin-aldosterone system for the secondary prevention of stroke. Neurology. 2004;63:208–213. doi: 10.1212/01.wnl.0000130360.21618.d0. [DOI] [PubMed] [Google Scholar]
- Sokoloff L. Energetics of functional activation in neural tissues. Neurochem Res. 1999;24:321–329. doi: 10.1023/a:1022534709672. [DOI] [PubMed] [Google Scholar]
- Stevens A, Begum G, Cook A, Connor K, Rumball C, Oliver M, Challis J, Bloomfield F, White A. Epigenetic changes in the hypothalamic proopiomelanocortin and glucocorticoid receptor genes in the ovine fetus after periconceptional undernutrition. Endocrinology. 2010;151:3652–3664. doi: 10.1210/en.2010-0094. [DOI] [PubMed] [Google Scholar]
- Srinivasan M, Katewa SD, Palaniyappan A, Pandya JD, Patel MS. Maternal high-fat diet consumption results in fetal malprogramming predisposing to the onset of metabolic syndrome-like phenotype in adulthood. Am J Physiol Endocrinol Metab. 2006;291:E792–799. doi: 10.1152/ajpendo.00078.2006. [DOI] [PubMed] [Google Scholar]
- Szuran TF, Pliska V, Pokorny J, Welzl H. Prenatal stress in rats: effects on plasma corticosterone, hippocampal glucocorticoid receptors, and maze performance. Physiol Behav. 2000;71:353–362. doi: 10.1016/s0031-9384(00)00351-6. [DOI] [PubMed] [Google Scholar]
- Tangalakis K, Lumbers ER, Moritz KM, Towstoless MK, Wintour EM. Effect of cortisol on blood pressure and vascular reactivity in the ovine fetus. Exp Physiol. 1992;77:709–717. doi: 10.1113/expphysiol.1992.sp003637. [DOI] [PubMed] [Google Scholar]
- Terzidou V, Bennett P. Maternal risk factors for fetal and neonatal brain damage. Biol Neonate. 2001;79:157–162. doi: 10.1159/000047084. [DOI] [PubMed] [Google Scholar]
- Thomas SA, Matsumoto AM, Palmiter RD. Noradrenaline is essential for mouse fetal development. Nature. 1995;374:643–646. doi: 10.1038/374643a0. [DOI] [PubMed] [Google Scholar]
- Thompson C, Syddall H, Rodin I, Osmond C, Barker DJ. Birth weight and the risk of depressive disorder in late life. Br J Psychiatry. 2001;179:450–455. doi: 10.1192/bjp.179.5.450. [DOI] [PubMed] [Google Scholar]
- Tobe I, Ishida Y, Tanaka M, Endoh H, Fujioka T, Nakamura S. Effects of repeated maternal stress on FOS expression in the hypothalamic paraventricular nucleus of fetal rats. Neuroscience. 2005;134:387–395. doi: 10.1016/j.neuroscience.2005.04.023. [DOI] [PubMed] [Google Scholar]
- Toledo-Rodriguez M, Lotfipour S, Leonard G, Perron M, Richer L, Veillette S, Pausova Z, Paus T. Maternal smoking during pregnancy is associated with epigenetic modifications of the brain-derived neurotrophic factor-6 exon in adolescent offspring. Am J Med Genet B Neuropsychiatr Genet. 2010;153B:1350–1354. doi: 10.1002/ajmg.b.31109. [DOI] [PubMed] [Google Scholar]
- Tombaugh GC, Yang SH, Swanson RA, Sapolsky RM. Glucocorticoids exacerbate hypoxic and hypoglycemic hippocampal injury in vitro: biochemical correlates and a role for astrocytes. J Neurochem. 1992;59:137–146. doi: 10.1111/j.1471-4159.1992.tb08884.x. [DOI] [PubMed] [Google Scholar]
- Tomita S, Ueno M, Sakamoto M, Kitahama Y, Ueki M, Maekawa N, Sakamoto H, Gassmann M, Kageyama R, Ueda N, Gonzalez FJ, Takahama Y. Defective brain development in mice lacking the Hif-1alpha gene in neural cells. Mol Cell Biol. 2003;23:6739–6749. doi: 10.1128/MCB.23.19.6739-6749.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong W, Chen W, Ostrowski RP, Ma Q, Souvenir R, Zhang L, Zhang JH, Tang J. Maternal hypoxia increases the activity of MMPs and decreases the expression of TIMPs in the brain of neonatal rats. Dev Neurobiol. 2010;70:182–194. doi: 10.1002/dneu.20770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong W, Xue Q, Li Y, Zhang L. Maternal hypoxia alters matrix metalloproteinase expression patterns and causes cardiac remodeling in fetal and neonatal rats. Am J Physiol Heart Circ Physiol. 2011;301:H2113–2121. doi: 10.1152/ajpheart.00356.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong W, Zhang L. Fetal hypoxia and programming of matrix metalloproteinases. Drug Discov Today. 2011 Sep 18; doi: 10.1016/j.drudis.2011.09.011. 2011. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torrens C, Brawley L, Anthony FW, Dance CS, Dunn R, Jackson AA, Poston L, Hanson MA. Folate supplementation during pregnancy improves offspring cardiovascular dysfunction induced by protein restriction. Hypertension. 2006;47:982–987. doi: 10.1161/01.HYP.0000215580.43711.d1. [DOI] [PubMed] [Google Scholar]
- Torres N, Bautista CJ, Tovar AR, Ordaz G, Rodriguez-Cruz M, Ortiz V, Granados O, Nathanielsz PW, Larrea F, Zambrano E. Protein restriction during pregnancy affects maternal liver lipid metabolism and fetal brain lipid composition in the rat. Am J Physiol Endocrinol Metab. 2010;298:E270–277. doi: 10.1152/ajpendo.00437.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tozuka Y, Wada E, Wada K. Diet-induced obesity in female mice leads to peroxidized lipid accumulations and impairment of hippocampal neurogenesisduring the early life of their offspring. FASEB J. 2009;23:1920–1934. doi: 10.1096/fj.08-124784. [DOI] [PubMed] [Google Scholar]
- Trollmann R, Gassmann M. The role of hypoxia-inducible transcription factors in the hypoxic neonatal brain. Brain Dev. 2009;31:503–509. doi: 10.1016/j.braindev.2009.03.007. [DOI] [PubMed] [Google Scholar]
- Tronche F, Kellendonk C, Kretz O, Gass P, Anlag K, Orban PC, Bock R, Klein R, Schutz G. Disruption of the glucocorticoid receptor gene in the nervous system results in reduced anxiety. Nat Genet. 1999;23:99–103. doi: 10.1038/12703. [DOI] [PubMed] [Google Scholar]
- Tuor UI. Dexamethasone and the prevention of neonatal hypoxic-ischemic brain damage. Ann N Y Acad Sci. 1995;765:179–195. doi: 10.1111/j.1749-6632.1995.tb16574.x. discussion 196-177. [DOI] [PubMed] [Google Scholar]
- Tuor UI. Glucocorticoids and the prevention of hypoxic-ischemic brain damage. Neurosci Biobehav Rev. 1997;21:175–179. doi: 10.1016/s0149-7634(96)00007-3. [DOI] [PubMed] [Google Scholar]
- Uno H, Eisele S, Sakai A, Shelton S, Baker E, DeJesus O, Holden J. Neurotoxicity of glucocorticoids in the primate brain. Horm Behav. 1994;28:336–348. doi: 10.1006/hbeh.1994.1030. [DOI] [PubMed] [Google Scholar]
- Uno H, Lohmiller L, Thieme C, Kemnitz JW, Engle MJ, Roecker EB, Farrell PM. Brain damage induced by prenatal exposure to dexamethasone in fetal rhesus macaques. I. Hippocampus. Brain Res Dev Brain Res. 1990;53:157–167. doi: 10.1016/0165-3806(90)90002-g. [DOI] [PubMed] [Google Scholar]
- Vangeison G, Carr D, Federoff HJ, Rempe DA. The good, the bad, and the cell type-specific roles of hypoxia inducible factor-1 alpha in neurons and astrocytes. J Neurosci. 2008;28:1988–1993. doi: 10.1523/JNEUROSCI.5323-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vannucci RC. Experimental biology of cerebral hypoxia-ischemia: relation to perinatal brain damage. Pediatr Res. 1990;27:317–326. doi: 10.1203/00006450-199004000-00001. [DOI] [PubMed] [Google Scholar]
- Vannucci RC. Hypoxic-ischemic encephalopathy. Am J Perinatol. 2000;17:113–120. doi: 10.1055/s-2000-9293. [DOI] [PubMed] [Google Scholar]
- Vannucci SJ, Hagberg H. Hypoxia-ischemia in the immature brain. J Exp Biol. 2004;207:3149–3154. doi: 10.1242/jeb.01064. [DOI] [PubMed] [Google Scholar]
- Vazquez-Valls E, Flores-Soto ME, Chaparro-Huerta V, Torres-Mendoza BM, Gudino-Cabrera G, Rivera-Cervantes MC, Pallas M, Camins A, Armendariz-Borunda J, Beas-Zarate C. HIF-1alpha expression in the hippocampus and peripheral macrophages after glutamate-induced excitotoxicity. J Neuroimmunol. 2011;238:12–18. doi: 10.1016/j.jneuroim.2011.06.001. [DOI] [PubMed] [Google Scholar]
- Verbois SL, Sullivan PG, Scheff SW, Pauly JR. Traumatic brain injury reduces hippocampal alpha7 nicotinic cholinergic receptor binding. J Neurotrauma. 2000;17:1001–1011. doi: 10.1089/neu.2000.17.1001. [DOI] [PubMed] [Google Scholar]
- Vexler ZS, Ferriero DM. Molecular and biochemical mechanisms of perinatal brain injury. Semin Neonatol. 2001;6:99–108. doi: 10.1053/siny.2001.0041. [DOI] [PubMed] [Google Scholar]
- Vickers MH, Gluckman PD, Coveny AH, Hofman PL, Cutfield WS, Gertler A, Breier BH, Harris M. The effect of neonatal leptin treatment on postnatal weight gain in male rats is dependent on maternal nutritional status during pregnancy. Endocrinology. 2008;149:1906–1913. doi: 10.1210/en.2007-0981. [DOI] [PubMed] [Google Scholar]
- Wadhwa PD, Buss C, Entringer S, Swanson JM. Developmental origins of health and disease: brief history of the approach and current focus on epigenetic mechanisms. Semin Reprod Med. 2009;27:358–368. doi: 10.1055/s-0029-1237424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker CD, Naef L, D’asti E, Long H, Xu Z, Moreau A, Azeddine B. Perinatal maternal fat intake affects metabolism and hippocampal function in the offspring: a potential role for leptin. Ann NY Acad Sci. 2008;1144:189–202. doi: 10.1196/annals.1418.023. [DOI] [PubMed] [Google Scholar]
- Walker SP, et al. International Child Development Steering Group (2007) Child development: Risk factors for adverse outcomes in developing countries. Lancet. 2007;369:145–157. doi: 10.1016/S0140-6736(07)60076-2. [DOI] [PubMed] [Google Scholar]
- Wang GL, Jiang BH, Rue EA, Semenza GL. Hypoxia-inducible factor 1 is a basic-helix-loop-helix-PAS heterodimer regulated by cellular O2 tension. Proc Natl Acad Sci U S A. 1995;92:5510–5514. doi: 10.1073/pnas.92.12.5510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Cai R, Lv G, Huang Z, Wang Z. Hypoxia during pregnancy in rats leads to the changes of the cerebral white matter in adult offspring. Biochem Biophys Res Commun. 2010;396:445–450. doi: 10.1016/j.bbrc.2010.04.114. [DOI] [PubMed] [Google Scholar]
- Warner MJ, Ozanne SE. Mechanisms involved in the developmental programming of adulthood disease. Biochem J. 2010;427:333–347. doi: 10.1042/BJ20091861. [DOI] [PubMed] [Google Scholar]
- Weaver IC, Cervoni N, Champagne FA, D’Alessio AC, Sharma S, Seckl JR, Dymov S, Szyf M, Meaney MJ. Epigenetic programming by maternal behavior. Nat Neurosci. 2004;7:847–854. doi: 10.1038/nn1276. [DOI] [PubMed] [Google Scholar]
- Weaver IC, Champagne FA, Brown SE, Dymov S, Sharma S, Meaney MJ, Szyf M. Reversal of maternal programming of stress responses in adult offspring through methyl supplementation: altering epigenetic marking later in life. J Neurosci. 2005;25:11045–11054. doi: 10.1523/JNEUROSCI.3652-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberger SB, Martinez JL., Jr. Characterization of hydrolysis of [leu]enkephalin and D-ala2-[L-leu]enkephalin in rat plasma. J Pharmacol Exp Ther. 1988;247:129–135. [PubMed] [Google Scholar]
- Weinstock M. The long-term behavioural consequences of prenatal stress. Neurosci Biobehav Rev. 2008;32:1073–1086. doi: 10.1016/j.neubiorev.2008.03.002. [DOI] [PubMed] [Google Scholar]
- Welberg LA, Seckl JR, Holmes MC. Inhibition of 11beta-hydroxysteroid dehydrogenase, the foeto-placental barrier to maternal glucocorticoids, permanently programs amygdala GR mRNA expression and anxiety-like behaviour in the offspring. Eur J Neurosci. 2000;12:1047–1054. doi: 10.1046/j.1460-9568.2000.00958.x. [DOI] [PubMed] [Google Scholar]
- Welberg LA, Seckl JR, Holmes MC. Prenatal glucocorticoid programming of brain corticosteroid receptors and corticotrophin-releasing hormone: possible implications for behaviour. Neuroscience. 2001;104:71–79. doi: 10.1016/s0306-4522(01)00065-3. [DOI] [PubMed] [Google Scholar]
- Whitelaw A, Thoresen M. Antenatal steroids and the developing brain. Arch Dis Child Fetal Neonatal Ed. 2000;83:F154–157. doi: 10.1136/fn.83.2.F154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickstrom HR, Mas C, Simonneau M, Holgert H, Hokfelt T, Lagercrantz H. Perinatal nicotine attenuates the hypoxia-induced up-regulation of tyrosine hydroxylase and galanin mRNA in locus ceruleus of the newborn mouse. Pediatr Res. 2002;52:763–769. doi: 10.1203/00006450-200211000-00025. [DOI] [PubMed] [Google Scholar]
- Wickstrom R. Effects of nicotine during pregnancy: human and experimental evidence. Curr Neuropharmacol. 2007;5:213–222. doi: 10.2174/157015907781695955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilcoxon JS, Redei EE. Prenatal programming of adult thyroid function by alcohol and thyroid hormones. Am J Physiol Endocrinol Metab. 2004;287:E318–326. doi: 10.1152/ajpendo.00022.2004. [DOI] [PubMed] [Google Scholar]
- Wiles NJ, Peters TJ, Leon DA, Lewis G. Birth weight and psychological distress at age 45-51 years: results from the Aberdeen Children of the 1950s cohort study. Br J Psychiatry. 2005;187:21–28. doi: 10.1192/bjp.187.1.21. [DOI] [PubMed] [Google Scholar]
- Wood IS, de Heredia FP, Wang B, Trayhurn P. Cellular hypoxia and adipose tissue dysfunction in obesity. Proc Nutr Soc. 2009;68:370–377. doi: 10.1017/S0029665109990206. [DOI] [PubMed] [Google Scholar]
- Woods LL, Morgan TK, Resko JA. Castration fails to prevent prenatally programmed hypertension in male rats. Am J Physiol Regul Integr Comp Physiol. 2010;298:R1111–1116. doi: 10.1152/ajpregu.00803.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wust S, Entringer S, Federenko IS, Schlotz W, Hellhammer DH. Birth weight is associated with salivary cortisol responses to psychosocial stress in adult life. Psychoneuroendocrinology. 2005;30:591–598. doi: 10.1016/j.psyneuen.2005.01.008. [DOI] [PubMed] [Google Scholar]
- Wyrwoll CS, Mark PJ, Waddell BJ. Developmental programming of renal glucocorticoid sensitivity and the renin-angiotensin system. Hypertension. 2007;50:579–584. doi: 10.1161/HYPERTENSIONAHA.107.091603. [DOI] [PubMed] [Google Scholar]
- Xiao D, Huang X, Xu Z, Yang S, Zhang L. Prenatal cocaine exposure differentially causes vascular dysfunction in adult offspring. Hypertension. 2009a;53:937–943. doi: 10.1161/HYPERTENSIONAHA.108.121830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao D, Huang X, Yang S, Zhang L. Direct effects of nicotine on contractility of the uterine artery in pregnancy. J Pharmacol Exp Ther. 2007;322:180–185. doi: 10.1124/jpet.107.119354. [DOI] [PubMed] [Google Scholar]
- Xiao D, Huang X, Yang S, Zhang L. Antenatal nicotine induces heightened oxidative stress and vascular dysfunction in rat offspring. Br J Pharmacol. 2011;164:1400–1409. doi: 10.1111/j.1476-5381.2011.01437.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao D, Xu Z, Huang X, Longo LD, Yang S, Zhang L. Prenatal gender-related nicotine exposure increases blood pressure response to angiotensin II in adult offspring. Hypertension. 2008;51:1239–1247. doi: 10.1161/HYPERTENSIONAHA.107.106203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao D, Yang S, Zhang L. Prenatal cocaine exposure causes sex-dependent impairment in the myogenic reactivity of coronary arteries in adult offspring. Hypertension. 2009b;54:1123–1128. doi: 10.1161/HYPERTENSIONAHA.109.138024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y, Liu P, Li Y. Impaired development of mitochondria plays a role in the central nervous system defects of fetal alcohol syndrome. Birth Defects Res A Clin Mol Teratol. 2005;73:83–91. doi: 10.1002/bdra.20110. [DOI] [PubMed] [Google Scholar]
- Xu Z, Seidler FJ, Ali SF, Slikker W, Jr., Slotkin TA. Fetal and adolescent nicotine administration: effects on CNS serotonergic systems. Brain Res. 2001;914:166–178. doi: 10.1016/s0006-8993(01)02797-4. [DOI] [PubMed] [Google Scholar]
- Xue Q, Dasgupta C, Chen M, Zhang L. Foetal hypoxia increases cardiac AT(2)R expression and subsequent vulnerability to adult ischaemic injury. Cardiovasc Res. 2011;89:300–308. doi: 10.1093/cvr/cvq303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yafeng D, Weijian H, Jiaxue W, Weiner CP. Chronic hypoxemia absent bacterial infection is one cause of the fetal inflammatory response syndrome (FIRS) Reprod Sci. 2009;16:650–656. doi: 10.1177/1933719109333662. [DOI] [PubMed] [Google Scholar]
- Yanai J, Vatury O, Slotkin TA. Cell signaling as a target and underlying mechanism for neurobehavioral teratogenesis. Ann N Y Acad Sci. 2002;965:473–478. doi: 10.1111/j.1749-6632.2002.tb04188.x. [DOI] [PubMed] [Google Scholar]
- Yaoi T, Itoh K, Nakamura K, Ogi H, Fujiwara Y, Fushiki S. Genome-wide analysis of epigenomic alterations in fetal mouse forebrain after exposure to low doses of bisphenol A. Biochem Biophys Res Commun. 2008;376:563–567. doi: 10.1016/j.bbrc.2008.09.028. [DOI] [PubMed] [Google Scholar]
- Yehuda R, Fairman KR, Meyer JS. Enhanced brain cell proliferation following early adrenalectomy in rats. J Neurochem. 1989;53:241–248. doi: 10.1111/j.1471-4159.1989.tb07320.x. [DOI] [PubMed] [Google Scholar]
- Young W, Flamm ES. Effect of high-dose corticosteroid therapy on blood flow, evoked potentials, and extracellular calcium in experimental spinal injury. J Neurosurg. 1982;57:667–673. doi: 10.3171/jns.1982.57.5.0667. [DOI] [PubMed] [Google Scholar]
- Zhang H, Meyer KD, Zhang L. Fetal exposure to cocaine causes programming of Prkce gene repression in the left ventricle of adult rat offspring. Biol Reprod. 2009;80:440–448. doi: 10.1095/biolreprod.108.072983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Sliwowska JH, Weinberg J. Prenatal alcohol exposure and fetal programming: effects on neuroendocrine and immune function. Exp Biol Med (Maywood) 2005;230:376–388. doi: 10.1177/15353702-0323006-05. [DOI] [PubMed] [Google Scholar]
- Zhou J, Ando H, Macova M, Dou J, Saavedra JM. Angiotensin II AT1 receptor blockade abolishes brain microvascular inflammation and heat shock protein responses in hypertensive rats. J Cereb Blood Flow Metab. 2005;25:878–886. doi: 10.1038/sj.jcbfm.9600082. [DOI] [PubMed] [Google Scholar]