Abstract
Gold nanocages have recently emerged as a novel class of photothermal transducers and drug carriers for cancer treatment. However, their pharmacokinetics and tumor targeting capability remain to be largely unexplored due to the lack of an imaging modality for quick and reliable mapping of their distributions in vivo. Herein, Au nanocages were prepared with controlled physicochemical properties and radiolabeled with 64Cu in high specific activities for in vivo evaluation using positron emission tomography (PET). Our pharmacokinetic studies with femtomolar administrations suggest that nanocages of 30 nm in size had a greatly improved biodistribution profile than nanocages of 55 nm in size, together with higher blood retention and lower hepatic and splenic uptakes. In a murine EMT-6 breast cancer model, the small cages also showed a significantly higher level of tumor uptake and a greater tumor-to-muscle ratio than the large cages. Quantitative PET imaging confirmed rapid accumulation and retention of Au nanocages inside the tumors. The ability to directly and quickly image the distribution of Au nanocages in vivo allows us to further optimize their physicochemical properties for a range of theranostic applications.
Keywords: gold nanocage, radiolabeling, positron emission tomography (PET), biodistribution, cancer targeting
Nanomedicine has drawn much attention in recent years because of its great potential in early detection, accurate diagnosis, and personalized therapy of various diseases, especially cancer.1,2 A rich variety of different nanomaterials such as polymer nanoparticles, liposomes, and metal nanostructures have been demonstrated as the platform for a range of applications related to nanomedicine.3–6 Among them, metal nanostructures, including those based on Au, have received great attention for cancer diagnosis and treatment owing to their bio-inertness, easiness of surface modification, and unique optical properties such as localized surface plasmon resonance (LSPR) and efficient photothermal conversion.7–9
As a novel class of nanomaterials, Au nanocages (AuNCs) have recently been explored for cancer imaging and treatment.10–12 The AuNCs can be easily prepared in large quantities with tunable wall thickness in the range of 2–10 nm through a straightforward, reliable galvanic replacement procedure that involves Ag nanocubes and HAuCl4 in an aqueous solution.13 By controlling the stoichiometry in a fashion similar to titration, their LSPR peaks can be precisely and reproducibly positioned anywhere in the range of 600–1200 nm, making them ideal candidates as contrast agents for a number of optical imaging modalities.10,12 Their intrinsic hollow and porous structures can also be used to encapsulate therapeutic payloads for applications related to controlled release or drug delivery.14,15 Additionally, AuNCs are effective photothermal transducers, capable of converting light into heat and causing the local temperature to rise substantially.16,17 All these attributes make AuNCs attractive for an array of theranostic applications.
Despite the successful use of AuNCs in a number of early studies, the pharmacokinetics and in vivo tumor targeting capability of AuNCs remain largely unexplored due to the lack of an appropriate imaging modality for quick, quantitative, and reliable evaluation of their biodistribution. Positron emission tomography (PET), because of its noninvasive, highly sensitive nature and high patient compliance, has emerged as one of the most frequently used techniques for early-stage diagnosis and staging of cancer and other diseases.18–21 In the last decade, PET imaging in conjunction with well-defined nanostructures have become an increasingly popular tool in various biomedical studies because of the enhanced specificity, sensitivity, and targeting efficiency.22–26 Here we address the pharmacokinetic and in vivo cancer targeting issues of AuNCs by functionalizing their surfaces with radioactive 64Cu2+ ions for PET imaging. Specifically, AuNCs of two different sizes were prepared, PEGylated, and radiolabeled with 64Cu2+ ions. We then evaluated the radiolabeling efficiency and in vivo pharmacokinetics in normal rodents. In addition, we examined the passive targeting capability of AuNCs via the enhanced permeability and retention (EPR) effect in an EMT-6 mouse mammary tumor model by directly imaging with small animal PET/CT.27 The intratumoral distribution of 64Cu-labeled AuNCs was also examined by autoradiography. This work provides a new platform for further optimization of the physicochemical properties of AuNCs to target a range of theranostic applications.
RESULTS AND DISCUSSION
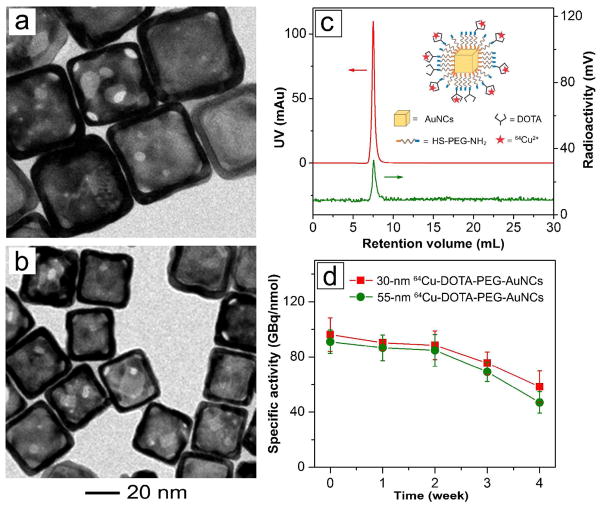
The main objective of this study is to evaluate the in vivo pharmacokinetics and PET imaging capacity of AuNCs with 64Cu radiolabeling so they can be better used in cancer diagnosis and therapy. Nanoparticles with sizes of 10–100 nm are desirable since they may escape from the renal filtering elimination and accumulate at the tumors after prolonged circulation.28 Moreover, nanoparticles smaller than 60 nm are expected to have better tumor penetration away from blood vessels.29 Therefore, we prepared AuNCs of 55 and 30 nm in edge length via the galvanic replacement reaction between Ag nanocubes of 47 and 25 nm, respectively, in size and aqueous HAuCl4 solution. As shown by the UV-vis spectra in Figure S1, the LSPR peaks of the 55- and 30-nm AuNCs were located at 805 and 760 nm, respectively. Heterofunctional poly(ethylene glycol) (PEG) was then conjugated to the surface of AuNCs through an Au-S linkage to generate PEGylated AuNCs with amine (–NH2) groups on the outer surface. The –NH2 groups were then coupled with 1,4,7,10-tetraazacyclododecane-1,4,7,10-tetraacetic acid mono(N-hydroxysuccinimide ester) (DOTA-NHS-ester) through an amide reaction via NHS-activated ester, followed by chelating with 64Cu2+ ions. As shown by the TEM images in Figure 1, a and b, the large and small 64Cu-DOTA-PEG-AuNCs had edge lengths of 54.5±4.4 and 30.3±4.2 nm, respectively. Their hydrodynamic diameters were measured to be 96.0±12.0 and 63.7±7.3 nm, respectively, by dynamic light scattering (DLS), together with zeta potentials (ζ) of 18.7±6.5 and 10.2±1.1 mV (see Table 1). The polydispersity indexes were less than 0.2 for both samples.
Figure 1.
Typical transmission electron microscopy images of DOTA-PEG-AuNCs with an edge length of (a) 54.5±4.4 nm and (b) 30.3±4.2 nm; (c) fast protein liquid chromatography analysis of the 30-nm 64Cu-DOTA-PEG-AuNCs; and (d) longitudinal specific activities of the 64Cu-DOTA-PEG-AuNCs after incubation at 4 ºC for different periods of time.
Table 1.
Summary of the diameters and zeta-potentials of the 64Cu2+-labeled PEGylated AuNCs
| Parameters | Large 64Cu-DOTA-PEG-AuNCs |
Small 64Cu-DOTA-PEG-AuNCs |
|---|---|---|
| Edge length (nm)a | 54.5±4.4 | 30.3±4.2 |
| Diameter (nm)b | 96.0±12.0 | 63.7±7.3 |
| Zeta potential (mV)b | 18.7±6.5 | 10.2±1.1 |
: Determined using transmission electron microscopy (TEM)
: Determined using dynamic light scattering (DLS)
To quantify the coverage densities of PEG chains on AuNCs, a fluorescein-tagged PEG-thiol (FITC-PEG-SH, Mw ≈ 5,000) was mixed with NH2-PEG-SH (Mw ≈ 5,000) at a molar ratio of 1:100 for surface functionalization.30 After conjugation, AuNCs were completely dissolved in 0.1 M potassium cyanide (KCN) to release the –S-PEG-FITC chains from the metal surface, which would dimerize to form disulfide compounds. The coverage densities of the PEG chains were then quantified using fluorescence spectroscopy with an established calibration curve for FITC-PEG-SH. The average number of PEG chains on each AuNC was found to be approximately 45,000 and 17,000 for the 55- and 30-nm AuNCs, respectively. Therefore, the PEG coverage densities on the surfaces of AuNCs were 1.43 and 1.86 per nm2 for the 55- and 30-nm AuNCs, respectively. Here the surface area of a AuNC was calculated by including both the outer and inner surfaces, with contributions from the pores being excluded.30
The AuNCs were radiolabeled with 64Cu2+ ions using a procedure previously reported for radiolabeling nanoparticles.25 Figure 1c shows the typical fast protein liquid chromatography (FPLC) profile of the 30-nm 64Cu-DOTA-PEG-AuNCs traced by both radioactivity and UV detectors. Clearly, the 64Cu-DOTA-PEG-AuNCs could be purified with no aggregation by using centrifugation to remove the remaining free 64Cu2+ ions.
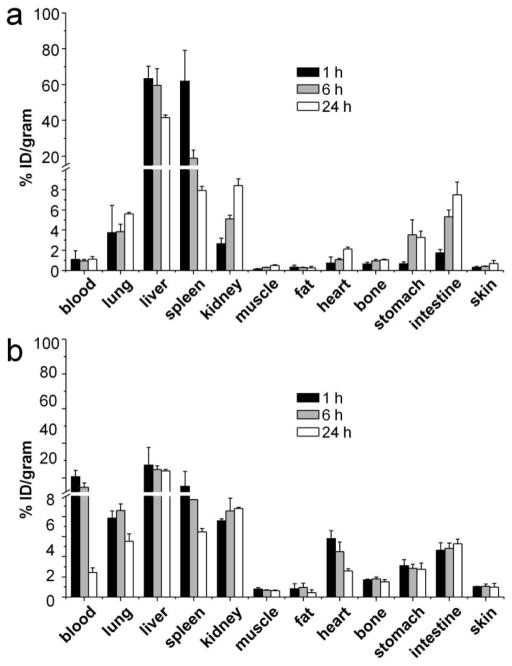
During the initial exploration of 64Cu radiolabeling of DOTA-PEG-AuNCs, high specific activity could be obtained using the freshly prepared DOTA-PEG-AuNCs. However, during the storage of DOTA-PEG-AuNCs at 4 °C in water, Ag rapidly leached out from the Ag/Au alloy walls of DOTA-PEG-AuNCs and the Ag+ ion could compete for the DOTA chelator,31 leading to a significant decrease in the available DOTA for 64Cu radiolabeling and rapid reduction of specific activity in a week. This issue greatly limits the use of AuNCs for PET imaging. To solve this problem, hydrogen peroxide (H2O2) was employed as an effective etchant to remove Ag from the surface of AuNCs,32 which led to a 6.2±0.8% reduction of Ag in the AuNCs as measured by inductively coupled plasma mass spectrometry (ICP-MS, data not shown). As shown in Figure 1d, after treatment with H2O2, high specific activity (81.4–107.3 GBq/nmol) of 64Cu radiolabeling was readily achieved for both large and small DOTA-PEG-AuNCs. More importantly, the DOTA-PEG-AuNCs could be stored at 4 °C for a period of time relevant for biological applications while retaining high radiolabeling specific activity, due to the removal of Ag from the surface. Furthermore, our serum stability studies of 64Cu-DOTA-PEG-AuNCs showed that their radiochemical purity only dropped from the original value of >97% to 90.2±0.3% at 4 h and 81.5±1.4% at 24 h, respectively, after incubation with mouse serum (10% in PBS) at 37 °C, indicating good stability for the 64Cu-labeled AuNCs. Our previous study demonstrated that AuNCs of 55 nm in size and coated with methoxy-PEG-thiol (Mw ≈ 5,000) could serve as photothermal transducers for effective cancer treatment.17 However, like other Au nanostructures reported in literature,33,34 the poor pharmacokinetics resulted in low blood retention and low tumor-to-muscle ratio for the AuNCs,17 limiting their application as a cancer treatment strategy in translational research. In this study, in vivo biodistribution studies of the 55-nm 64Cu-DOTA-PEG-AuNCs showed fast systemic clearance due to high uptakes by the reticuloendothelial system (RES) (liver and spleen) and low blood retention in C57BL/6 mice throughout the study. Liver and spleen uptakes were dominant among all the organs with more than 60 %ID/g at 1 h post injection. However, the accumulation in liver gradually decreased to 41.6± 1.5 %ID/g at 24 h while the uptake by spleen quickly dropped to 7.9±0.4 %ID/g (Figure 2a).
Figure 2.
Biodistribution of (a) the large 64Cu-DOTA-PEG-AuNCs (55 nm) and (b) the small 64Cu-DOTA-PEG-AuNCs (30 nm) in C57BL/6 mice (0.37 MBq injection/mouse, n = 4 per group).
For the 30-nm AuNCs, although their photothermal effect was comparable to the 55-nm AuNCs when normalized to the number of Au atoms (Figure S2), their biodistributions were found to be considerably different. At 1 h post injection, the blood retention of the 30-nm 64Cu-DOTA-PEG-AuNCs was 20.6±3.6 %ID/g, six times greater than what was obtained with the 55-nm 64Cu-DOTA-PEG-AuNCs, and then gradually decreased to 14.6±2.4 %ID/g at 4 h, and less than 3 %ID/g at 24 h. As expected, the liver uptake was the greatest among all the organs, which remained essentially constant throughout the 24 h period. Similar to the 55-nm AuNCs, the spleen uptake of the 30-nm AuNCs also displayed a fast clearance profile with less than 50% of the initial accumulation remained at 24 h. Interestingly, kidney levels of the small AuNCs were constant (Figure 2b) during the 24 h of study while the large AuNCs showed a 3-fold increase of kidney accumulation from 1 h to 24 h (Figure 2a). Compared to the 55-nm AuNCs, the 30-nm AuNCs showed much improved in vivo pharmacokinetics with high retention in blood pool (blood, lung, and heart) and decreased RES uptake, likely due to the reduction in size and surface charge. This trend was consistent with previous reports about the effects of size and surface charge of nanostructures on in vivo biodistribution.34,35
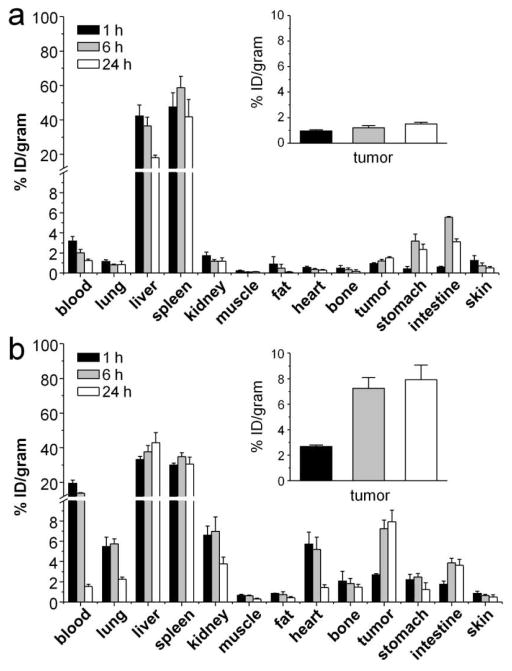
We next studied the biodistribution of 64Cu-DOTA-PEG-AuNCs in EMT-6 tumor bearing mice. The accumulation level in tumors depends on factors such as the size of nanoparticles, the blood circulation half-life (a longer half-life leads to higher accumulation), the degree of tumor vascularization (higher accumulation in more vascularized tumors), and the degree of angiogenesis (high accumulation in tumors of rapid and defected angiogenesis). The EMT-6 tumor model was selected for this study because it is known to grow rapidly and featured with tumor angiogenesis, as well as high vessel permeability.36,37 For the EMT-6 tumor bearing BALB/c mice, the 55-nm 64Cu-DOTA-PEG-AuNCs showed a distribution pattern similar to what was obtained in normal mice with high hepatic and splenic uptakes. Interestingly, unlike the rapid drop of spleen accumulation observed in C57BL/6 mice, the spleen accumulation of 55-nm 64Cu-DOTA-PEG-AuNCs in EMT 6 tumor bearing mice was constant up to 24 h, probably due to the difference in animal species. However, tumor uptake of the 55-nm 64Cu-DOTA-PEG-AuNCs was low (together with a slight increase during the 24 h of study) due to their poor retention in blood (Figure 3a). With decrease in muscle uptake, the tumor-to-muscle ratio increased dramatically from 4.13±0.96 at 1 h to 11.9±1.72 at 4 h, and remained constant afterwards (12.8±4.17 at 24 h). The tumor-to-blood ratio also increased from 0.30±0.05 at 1 h to 1.20±0.18 at 24 h.
Figure 3.
Biodistribution of (a) the large 64Cu-DOTA-PEG-AuNCs (55 nm) and (b) the small 64Cu-DOTA-PEG-AuNCs (30 nm) in EMT-6 tumor bearing mice (0.37 MBq injection/mouse, n = 4 per group).
The 30-nm 64Cu-DOTA-PEG-AuNCs in tumor bearing mice had a biodistribution profile similar to what was acquired for normal animals with high RES system uptakes. The blood retentions were all significantly (p<0.05, n = 4) higher than those obtained with the 55-nm 64Cu-DOTA-PEG-AuNCs, with about 20 %ID/g at beginning and then rapidly cleared. The initial tumor uptake was 2.68±0.12 %ID/g at 1 h, rapidly increased to 7.2±0.9 %ID/g at 4 h, and then gradually increased to 7.9±1.1 %ID/g at 24 h (Figure 3b), which was more than 4 times higher than what was obtained with the 55-nm 64Cu-DOTA-PEG-AuNCs, likely due to a longer blood circulation time and a better EPR effect. Further, these small AuNCs exhibited a rapid increase and then sustained retention profile for tumor uptake, which is important for a longitudinal and repeated photothermal treatment of cancer. Additionally, owing to the rapid blood clearance, the tumor-to-muscle ratios also rapidly increased to 25.7±6.9 at 24 h. For tumor-to-blood ratio, it also increased from 0.14±0.01 at 1 h to 5.15±1.05 at 24 h. Although the large AuNCs showed comparable contrast ratios at the beginning, the contrast ratio of the small AuNCs at 24 h was twice as much as that of the large AuNCs owning to the higher tumor uptake. It is worth mentioning that extending the measurement time would increase error due to the low counts since only a trace amount 64Cu2+ was administrated in vivo (73% of the 64Cu2+ ions had decayed at 24 h), as well as the fact that more 64Cu2+ could be dissociated from the 64Cu-DOTA-PEG-AuNCs over time (radiochemical purity dropped to 81.5±1.4% at 24 h). In contrast to the in vivo applications of other radiolabeled Au nanostructures (e.g., nanoshells and nanorods) in tumor models, the small 64Cu-DOTA-PEG-AuNCs displayed a comparable level of tumor uptake and tumor-to-muscle ratio.34
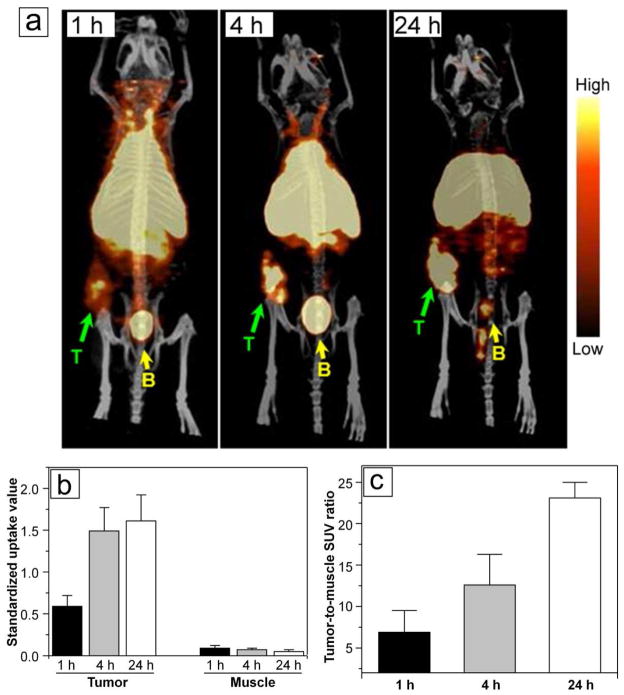
As indicated in Figure 4a, PET/CT images clearly showed rapid localization of the 30-nm 64Cu-DOTA-PEG-AuNCs in tumors at 1 h post injection even only with the administration of a trace amount (23.8 fmol). A quantitative analysis of the region of interest (ROI) drawn around the tumor showed increased standardized uptake values (SUVs) from 1 h to 24 h, consistent with the biodistribution data (Figure 4b). The increase of tumor-to-muscle ratios was calculated to be consistent with the biodistribution data shown in Figure 4c. Interestingly, compared to the other Au nanostructures, the uptake of the small 64Cu-DOTA-PEG-AuNCs was localized at the center of the tumor as shown by the PET/CT image obtained at 1 h post injection (Figure 4a). The fast increase of SUVs extracted from the centric tumor suggested the potential to quickly concentrate the AuNCs at the center of a tumor for effective photothermal therapy (Figure 4b).
Figure 4.
(a) PET/CT images of the 30-nm 64Cu-DOTA-PEG-AuNCs in a mouse bearing an EMT-6 tumor at 1 h, 4 h, and 24 h, respectively, post injection (3.7 MBq injection/mouse). T: tumor, B: bladder. (b) Standardized uptake values (SUV) in tumor and muscle regions obtained from PET/CT images taken at different times. (c) Tumor-to-muscle SUV ratios obtained from PET/CT images taken at different time points.
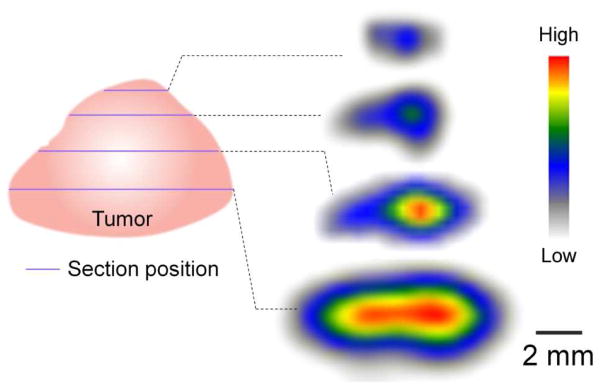
To confirm the intratumoral distribution profile, the EMT-6 tumor was sectioned into 40 μm slices at 24 h post injection for autoradiographic analysis. The collected tumor images, depicted in Figure 5, clearly showed accumulation of the small 64Cu-DOTA-PEG-AuNCs in the central region of the tumor. For anti-cancer therapeutic intratumoral delivery, a major challenge is that the drug is limited to the periphery of the tumor mass close to the vasculature due to the physiological barriers presented by the abnormal tumor vasculature and interstitial matrix.38–40 As such, the central region of a tumor might remain untouched, becoming a potential source for cancer relapse or metastasis.41 Besides the enhanced EPR effect in tumor, the main mechanism for transporting nanoparticles intratumorally is diffusion, which is largely affected by the size, surface charge, and morphology, as well as the physicochemical properties of the interstitial matrix.42 Previously we found that the 55-nm AuNCs, following tail vein injection, were more abundant at the host interface rather than the central region. Here, owning to the small size and nearly neutral charge of the 30-nm 64Cu-DOTA-PEG-AuNCs,43 a uniform blood flow of the EMT-6 tumor,40 and possibly a low interstitial pressure in the tumor,44 the small AuNCs displayed a centralized localization in the EMT-6 tumor, which may reduce its extravasation into the surrounding tissue during photothermal treatment and improve the therapeutic efficacy.45 Further, the quantitative SUV analysis showed high contrast ratios of tumor uptakes to the surrounding tissue, consistent with the bio-distribution results. Combined with their good biocompatibility (Figure S3), especially upon PEGylation, the 64Cu-labeled AuNCs show great promise for tracking their in vivo fates by PET imaging.
Figure 5.
Tumor autoradiography revealing centralized intratumoral accumulation of the 30-nm 64Cu-DOTA-PEG-AuNCs (3.7 MBq injection/mouse) in a mouse bearing an EMT-6 tumor. The tumor was resected at different depth from surface to core region and sectioned into slices of 40 μm thick at 24 h post injection.
CONCLUSION
We have demonstrated the modular construction of differently sized AuNCs (55 and 30 nm) with tailored physiochemical properties, in vivo pharmacokinetics evaluation, and PET imaging of 64Cu-labeled DOTA-PEG-AuNCs in an EMT-6 murine mammary carcinoma model. Both the large and small 64Cu-DOTA-PEG-AuNCs had high radiolabeling specific activities and stabilities. The 30-nm 64Cu-DOTA-PEG-AuNCs showed much improved in vivo pharmacokinetics with decreased RES system uptake and enhanced blood circulation compared to the 55-nm samples. The PET/CT imaging demonstrated rapid accumulation and centralized distribution of the 30-nm 64Cu-DOTA-PEG-AuNCs in tumors, and more importantly high tumor-to-muscle ratios. These results suggest the use of PET imaging as a powerful tool for optimizing the great potential of AuNCs as a theranostic agent for cancer research.
MATERIALS AND METHODS
Chemicals
Amine polyethylene glycol thiol (NH2-PEG-SH, Mw ≈ 5,000) was purchased from Laysan (Arab, AL). Fluorescein tagged polyethylene glycol thiol (FITC-PEG-SH, Mw ≈ 5,000) was obtained from Nanocs (Boston, MA). 1,4,7,10-tetraazacyclododecane-1,4,7,10-tetraacetic acid mono (N-hydroxysuccinimide ester) (DOTA-NHS-ester) was purchased from Macrocyclics (Dallas, TX) and used as received. All other chemicals were obtained from Sigma-Aldrich (St. Louis, MO).
Preparation, Functionalization, and Characterization of AuNCs
AuNCs with two different sizes (55 and 30 nm in edge length) covered with poly(vinyl pyrrolidone) were prepared using a galvanic replacement reaction between Ag nanocubes (47 and 25 nm in edge length, respectively) and chloroauric acid following the previously reported protocol.46 The reaction was monitored by measuring the localized surface plasmon resonance (LSPR) peaks with UV-vis spectroscopy (Cary 50 spectrometer, Varian, Palo Alto, CA) during the reaction. The LSPR was tuned to ~800 nm for 55-nm AuNCs while 30-nm AuNCs were obtained at ~760 nm.
Both AuNCs were first functionalized with NH2-PEG-SH and then conjugated with DOTA-NHS-ester for 64Cu radiolabeling. Briefly, 5.0 mL of 2 nM AuNCs in ultrapurified water (Millipre, Billerica, MA) was added to 5.0 mg of NH2-PEG-SH (PEG:AuNC = 105:1) and reacted overnight at 4 ºC under stirring. The excess NH2-PEG-SH was removed by centrifugation at 12,000 rpm for 8 min and washed five times with ultrapurified water to obtain PEGylated AuNCs. The PEGylated AuNCs were reconstituted in 0.1 M (pH 7.4) phosphate buffer pre-chelexed to remove any trace metal. The solution was mixed with 9.5 mg of DOTA-NHS-ester (DOTA:AuNC = 106:1) and reacted at 20 ºC for 1h followed by thorough centrifugation at 12,000 rpm for 8 min and washing with ultrapurified water to remove the unconjugated DOTA-NHS-ester and obtain DOTA-PEG-AuNCs.
The samples were examined using a Tecnai G2 Spirit transmission electron microscope (TEM) operated at 120 kV (FEI, Hillsboro, OR). Dynamic light scattering (NanoZS, Malvern, Worcestershire, UK) was performed to measure the hydrodynamic diameter, zeta potential, and polydispersity index of these samples.
Quantification of PEGylation Density
To measure the PEGylation density, FITC-PEG-SH (Mw ≈ 5,000) was mixed with NH2-PEG-SH (Mw ≈ 5,000) at a 1:100 molar ratio for surface conjugation. After reaction overnight at 4 °C, the excess NH2-PEG-SH was removed by centrifugation at 12,000 rpm for 8 min and washed five times with ultrapurified water. The FITC labeled PEGylated AuNCs were completely dissolved in 0.1 M potassium cyanide (KCN). The fluorescence signal was measured with excitation at 488 nm and emission collection at 520 nm. The quantification of AuNCs PEGylation density was calculated from a calibration curve established from FITC-PEG-SH.
64Cu Radiolabeling and Stability
Copper-64 (t1/2 = 12.7h, β+ = 17%, β− = 40%) was produced on the Washington University Medical School CS-15 cyclotron by the 64Ni (p,n) 64Cu nuclear reaction at a specific activity of 1.85–7.40 GBq/\g at the end of bombardment.47 DOTA-PEG-AuNCs (about 0.1 pmol) was incubated with 185 MBq 64Cu in 0.1M sodium acetate buffer (pH 7.0) at 55 ºC for 1h to get the maximal labeling specific activity. After ethylene diamine tetraacetic acid (EDTA, 10 mM in 50 mM pH 7.4 phosphate buffer) challenge, the 64Cu labeled DOTA-PEG-AuNCs was purified by ultracentrifugation at 12,000 rpm for 8 min for five times. The radiochemical purity of 64Cu-DOTA-PEG-AuNCs was monitored by radio instant thin layer chromatography (radio-ITLC, Bioscan, Washington, DC). DOTA-PEG-AuNCs were labeled with non-radioactive CuCl2 following the same labeling and purification procedures as 64Cu.
To minimize the interference of Ag ions leached from the AuNCs during 64Cu radiolabeling, hydrogen peroxide was used to pretreat the AuNCs prior to PEGylation and DOTA functionalization.32 The concentrations of Au and Ag in AuNCs were measured by Elan DRC II inductively coupled plasma mass spectrometry (ICP-MS, Perkin Elmer, Waltham, MA). Briefly, 10 μL of AuNCs aqueous suspensions were completely digested with 8 mL of aqua regia (HCl:HNO3 = 3:1 (v:v)) in a 100 mL beaker at 130 ºC. The solution was evaporated to 5 mL and subsequently diluted to 12 mL using 0.5% HCl and 2% HNO3. Quantification was carried out by external five-point calibration with internal standard correction.
The long-term radiolabeling stability of 64Cu-DOTA-PEG-AuNCs was tested in ultrapurified water at 4 ºC over a one month period. The serum stability of 64Cu-DOTA-PEG-AuNCs was also assessed by incubation with mouse serum at 37 ºC over a 24 h period. The radiochemical purity and chemical purity of the 64Cu radiolabeled AuNC was measured by ÄKTA fast protein liquid chromatography system (FPLC) equipped with both radioisotope detector (Beckman 170, Beckman, Brea, CA) and UV detectors (GE Healthcare, Bucks, UK). The AuNC separation was performed on a Superpose 12 10/300 GL size exclusion column (GE Healthcare, Bucks, UK) eluted with 20 mM N-(2-hydroxyethyl)piperazine-N9 -(2-ethanesulfonic acid) (HEPES) and 150 mM NaCl (pH 7.3) with a flow rate of 0.8 mL/min. The UV detector was set at 254 nm.
Animal Biodistribution Studies
All animal studies were performed in compliance with guidelines set forth by the NIH Office of Laboratory Animal Welfare and approved by the Washington University Animal Studies Committee. Biodistribution studies were performed in male C57BL/6 mice weighing 20–25 g (n = 4, Charles River Laboratory, Wilmington, MA) and about 0.37 MBq (2.38 fmol) of 64Cu-DOTA-PEG-AuNCs in 100 μL saline (APP pharmaceuticals, Schaumburg, IL) was injected via the tail vein. The mice were anesthetized with inhaled isoflurane and re-anesthetized before euthanizing them by cervical dislocation at each time point (1 h, 6 h, and 24 h post injection). Organs of interest were collected, weighed, and counted in a well Beckman 8000 gamma counter (Beckman, Brea, CA). Standards were prepared and measured along with the samples to calculate the percentage of the injected dose per gram of tissue (%ID/g).48
The EMT-6 murine mammary carcinoma cells were cultured in Waymouth’s MB 752/1 medium with 85% 2 mM L-glutamine and 15% fetal bovine serum at 37 ºC with 5% CO2. BALB/c mice nude mice weighing 20–30 g (Charles River Laboratory, Wilmington, MA) were subcutaneously implanted with 6×105 EMT-6 cells into the hind flank. The tumors were allowed 10 days (tumor approximately 0.3–0.4 g) of growth before beginning the biodistribution study. The biodistribution studies of 64Cu-DOTA-PEG-AuNCs in EMT-6 tumor bearing mice (n = 4, 0.37 MBq injection/mouse) were carried out following the same procedures with that in C57BL/6 mice.
PET/CT Imaging
Ten days after the EMT-6 murine mammary carcinoma cells were implanted, mice were anesthetized with isoflurane and injected with 3.7 MBq (23.8 fmol) of 64Cu-DOTA-PEG-AuNCs in 100 μL saline via the tail vein. MicroPET scans were performed on either microPET Focus 220 (Siemens, Malvern, PA) or Inveon PET/CT system (Siemens, Malvern, PA) at 1 h (15 min frame), 4 h (20 min frame), and 24 h post injection (30 min frame). The microPET images were corrected for attenuation, scatter, normalization, and camera dead time, and co-registered with microCT images. All the PET scanners were cross-calibrated periodically. The microPET images were reconstructed with the maximum a posteriori (MAP) algorithm and analyzed by ASIPro.49 The tumor uptake of 64Cu-DOTA-PEG-AuNCs was calculated in terms of the standardized uptake value (SUV) in three-dimensional regions of interest (ROIs). In general, SUV is defined as the tissue concentration of radiotracer divided by the activity injected per body weight and is calculated according to the following equation. All the SUV data was not corrected for partial volume effect.50
Autoradiography Studies
Digital autoradiographs defining the intratumoral distribution of 64Cu-DOTA-PEG-AuNCs were collected with Canberra Packard Instant Imager (Canberra, Meriden, CT) in a 60-min acquisition period shortly after the tumor was sectioned at 24 h post injection.
Supplementary Material
Acknowledgments
We thank T. Sharp, N. Fettig, M. Morris, A. Roth, L. Strong, and A. Stroncek for their assistance with animal and imaging studies; T. Voller, E. Madrid, P. Eisenbies, and C. Carey for 64Cu production. This work was supported in part by National Cancer Institute (1R01CA138527-01A1) and start-up funds from Washington University in St. Louis.
Footnotes
Supporting Information Available: UV-vis spectra of AuNCs aqueous suspensions; in vitro photothermal study; in vitro cell growth inhibition study. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.Petros RA, DeSimone JM. Strategies in the Design of Nanoparticles for Therapeutic Applications. Nat Rev Drug Discov. 2010;9:615–627. doi: 10.1038/nrd2591. [DOI] [PubMed] [Google Scholar]
- 2.Xie J, Lee S, Chen XY. Nanoparticle-Based Theranostic Agents. Adv Drug Deliver Rev. 2010;62:1064–1079. doi: 10.1016/j.addr.2010.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ng KK, Lovell JF, Zheng G. Lipoprotein-Inspired Nanoparticles for Cancer Theranostics. Acc Chem Res. 2011;44:1105–1113. doi: 10.1021/ar200017e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yoo D, Lee JH, Shin TH, Cheon J. Theranostic Magnetic Nanoparticles. Acc Chem Res. 2011;44:863–874. doi: 10.1021/ar200085c. [DOI] [PubMed] [Google Scholar]
- 5.Zrazhevskiy P, Gao XH. Multifunctional Quantum Dots for Personalized Medicine. Nano Today. 2009;4:414–428. doi: 10.1016/j.nantod.2009.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lammers T, Aime S, Hennink WE, Storm G, Kiessling F. Theranostic Nanomedicine. Acc Chem Res. 2011;44:1029–1038. doi: 10.1021/ar200019c. [DOI] [PubMed] [Google Scholar]
- 7.Eustis S, El-Sayed MA. Why Gold Nanoparticles Are More Precious Than Pretty Gold: Noble Metal Surface Plasmon Resonance and Its Enhancement of the Radiative and Nonradiative Properties of Nanocrystals of Different Shapes. Chem Soc Rev. 2006;35:209–217. doi: 10.1039/b514191e. [DOI] [PubMed] [Google Scholar]
- 8.Erathodiyil N, Ying JY. Functionalization of Inorganic Nanoparticles for Bioimaging Applications. Acc Chem Res. 2011;44:925–935. doi: 10.1021/ar2000327. [DOI] [PubMed] [Google Scholar]
- 9.Giljohann DA, Seferos DS, Daniel WL, Massich MD, Patel PC, Mirkin CA. Gold Nanoparticles for Biology and Medicine. Angew Chem, Int Ed. 2010;49:3280–3294. doi: 10.1002/anie.200904359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xia Y, Li W, Cobley CM, Chen J, Xia X, Zhang Q, Yang M, Cho EC, Brown PK. Gold Nanocages: from Synthesis to Theranostic Applications. Acc Chem Res. 2011;44:914–924. doi: 10.1021/ar200061q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim C, Cho EC, Chen J, Song KH, Au L, Favazza C, Zhang Q, Cobley CM, Gao F, Xia Y, et al. In Vivo Molecular Photoacoustic Tomography of Melanomas Targeted by Bioconjugated Gold Nanocages. ACS Nano. 2010;4:4559–4564. doi: 10.1021/nn100736c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cai X, Li W, Kim CH, Yuan Y, Wang LV, Xia Y. In Vivo Quantitative Evaluation of the Transport Kinetics of Gold Nanocages in a Lymphatic System by Noninvasive Photoacoustic Tomography. ACS Nano. 2011;5:9658–9667. doi: 10.1021/nn203124x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sun Y, Xia Y. Mechanistic Study on the Replacement Reaction Between Silver Nanostructures and Chloroauric Acid in Aqueous Medium. J Am Chem Soc. 2004;126:3892–3901. doi: 10.1021/ja039734c. [DOI] [PubMed] [Google Scholar]
- 14.Moon GD, Choi SW, Cai X, Li W, Cho EC, Jeong U, Wang LV, Xia Y. A New Theranostic System Based on Gold Nanocages and Phase-Change Materials with Unique Features for Photoacoustic Imaging and Controlled Release. J Am Chem Soc. 2011;133:4762–4765. doi: 10.1021/ja200894u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yavuz MS, Cheng Y, Chen J, Cobley CM, Zhang Q, Rycenga M, Xie J, Kim C, Song KH, Schwartz AG, et al. Gold Nanocages Covered by Smart Polymers for Controlled Release with Near-Infrared Light. Nature Mater. 2009;8:935–939. doi: 10.1038/nmat2564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen J, Wang D, Xi J, Au L, Siekkinen A, Warsen A, Li ZY, Zhang H, Xia Y, Li X. Immuno Gold Nanocages with Tailored Optical Properties for Targeted Photothermal Destruction of Cancer Cells. Nano Lett. 2007;7:1318–1322. doi: 10.1021/nl070345g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen J, Glaus C, Laforest R, Zhang Q, Yang M, Gidding M, Welch MJ, Xia Y. Gold Nanocages as Photothermal Transducers for Cancer Treatment. Small. 2010;6:811–817. doi: 10.1002/smll.200902216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Morrison AR, Sinusas AJ. Advances in Radionuclide Molecular Imaging in Myocardial Biology. J Nucl Cardiol. 2010;17:116–134. doi: 10.1007/s12350-009-9167-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ametamey SM, Honer M, Schubiger PA. Molecular Imaging with PET. Chem Rev. 2008;108:1501–1516. doi: 10.1021/cr0782426. [DOI] [PubMed] [Google Scholar]
- 20.Blodgett TM, Meltzer CC, Townsend DW. PET/CT: Form and Function. Radiology. 2007;242:360–385. doi: 10.1148/radiol.2422051113. [DOI] [PubMed] [Google Scholar]
- 21.Nielsen CH, Kimura RH, Withofs N, Tran PT, Miao Z, Cochran JR, Cheng Z, Felsher D, Kjaer A, Willmann JK, et al. PET Imaging of Tumor Neovascularization in a Transgenic Mouse Model with a Novel 64Cu-DOTA-Knottin Peptide. Cancer Res. 2010;70:9022–9030. doi: 10.1158/0008-5472.CAN-10-1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shokeen M, Pressly ED, Hagooly A, Zheleznyak A, Ramos N, Fiamengo AL, Welch MJ, Hawker CJ, Anderson CJ. Evaluation of Multivalent, Functional Polymeric Nanoparticles for Imaging Applications. ACS Nano. 2011;5:738–747. doi: 10.1021/nn102278w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cai WB, Chen K, Li ZB, Gambhir SS, Chen XY. Dual-Function Probe for PET and Near-Infrared Fluorescence Imaging of Tumor Vasculature. J Nucl Med. 2007;48:1862–1870. doi: 10.2967/jnumed.107.043216. [DOI] [PubMed] [Google Scholar]
- 24.Welch MJ, Hawker CJ, Wooley KL. The Advantages of Nanoparticles for PET. J Nucl Med. 2009;50:1743–1746. doi: 10.2967/jnumed.109.061846. [DOI] [PubMed] [Google Scholar]
- 25.Liu Y, Pressly ED, Abendschein DR, Hawker CJ, Woodard GE, Woodard PK, Welch MJ. Targeting Angiogenesis Using a C-Type Atrial Natriuretic Factor-Conjugated Nanoprobe and PET. J Nucl Med. 2011;52:1956–1963. doi: 10.2967/jnumed.111.089581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cheon J, Lee JH. Synergistically Integrated Nanoparticles as Multimodal Probes for Nanobiotechnology. Acc Chem Res. 2008;41:1630–1640. doi: 10.1021/ar800045c. [DOI] [PubMed] [Google Scholar]
- 27.Vavere AL, Welch MJ. Preparation, Biodistribution, and Small Animal PET of 45 Ti-Transferrin. J Nucl Med. 2005;46:683–690. [PubMed] [Google Scholar]
- 28.Rao JH. Shedding Light on Tumors Using Nanoparticles. ACS Nano. 2008;2:1984–1986. doi: 10.1021/nn800669n. [DOI] [PubMed] [Google Scholar]
- 29.Popovic Z, Liu WH, Chauhan VP, Lee J, Wong C, Greytak AB, Insin N, Nocera DG, Fukumura D, Jain RK, et al. A Nanoparticle Size Series for in Vivo Fluorescence Imaging. Angew Chem, Int Ed. 2010;49:8649–8652. doi: 10.1002/anie.201003142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xia X, Yang M, Wang Y, Zheng Y, Li Q, Chen J, Xia Y. Quantifying the Coverage Density of Poly(ethylene glycol) Chains on the Surface of Gold Nanostructures. ACS Nano. 2012;6:512–522. doi: 10.1021/nn2038516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kodama M, Mahatma AB, Koike T, Kimura E. The Electrochemical Study of Ag(II) Complexes of Polyaza Macrocycles and Their Acetic Acid Derivatives. Bull Chem Soc Jpn. 1990;63:2803–2808. [Google Scholar]
- 32.Zhang Q, Cobley CM, Zeng J, Wen LP, Chen J, Xia Y. Dissolving Ag from Au-Ag Alloy Nanoboxes with H2O2: a Method for Both Tailoring the Optical Properties and Measuring the H2O2 Concentration. J Phys Chem C. 2010;114:6396–6400. doi: 10.1021/jp100354z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lipka J, Semmler-Behnke M, Sperling RA, Wenk A, Takenaka S, Schleh C, Kissel T, Parak WJ, Kreyling WG. Biodistribution of PEG-Modified Gold Nanoparticles Following Intratracheal Instillation and Intravenous Injection. Biomaterials. 2010;31:6574–6581. doi: 10.1016/j.biomaterials.2010.05.009. [DOI] [PubMed] [Google Scholar]
- 34.Xie H, Wang ZJ, Bao A, Goins B, Phillips WT. In Vivo PET Imaging and Biodistribution of Radiolabeled Gold Nanoshells in Rats with Tumor Xenografts. Int J Pharm. 2010;395:324–330. doi: 10.1016/j.ijpharm.2010.06.005. [DOI] [PubMed] [Google Scholar]
- 35.Schipper ML, Iyer G, Koh AL, Cheng Z, Ebenstein Y, Aharoni A, Keren S, Bentolila LA, Li J, Rao J, et al. Particle Size, Surface Coating, and PEGylation Influence the Biodistribution of Quantum Dots in Living Mice. Small. 2009;5:126–134. doi: 10.1002/smll.200800003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McQuade P, Knight LC, Welch MJ. Evaluation of 64Cu- and 125I-radiolabeled Bitistatin as Potential Agents for Targeting αvβ3 Integrins in Tumor Angiogenesis. Bioconjugate Chem. 2004;15:988–996. doi: 10.1021/bc049961j. [DOI] [PubMed] [Google Scholar]
- 37.Roberts WG, Hasan T. Tumor-Secreted Vascular-Permeability Factor Vascular Endothelial Growth-Factor Influences Photosensitizer Uptake. Cancer Res. 1993;53:153–157. [PubMed] [Google Scholar]
- 38.Obata A, Yoshimoto M, Kasamatsu S, Naiki H, Takamatsu S, Kashikura K, Furukawa T, Lewis JS, Welch MJ, Saji H, et al. Intra-Tumoral Distribution of 64Cu-ATSM: a Comparison Study with FDG. Nucl Med Biol. 2003;30:529–534. doi: 10.1016/s0969-8051(03)00047-7. [DOI] [PubMed] [Google Scholar]
- 39.Minchinton AI, Tannock IF. Drug Penetration in Solid Tumours. Nat Rev Cancer. 2006;6:583–592. doi: 10.1038/nrc1893. [DOI] [PubMed] [Google Scholar]
- 40.Lewis JS, McCarthy DW, McCarthy TJ, Fujibayashi Y, Welch MJ. Evaluation of 64Cu-ATSM in Vitro and in Vivo in a Hypoxic Tumor Model. J Nucl Med. 1999;40:177–183. [PubMed] [Google Scholar]
- 41.Holback H, Yeo Y. Intratumoral Drug Delivery with Nanoparticulate Carriers. Pharm Res. 2011;28:1819–1830. doi: 10.1007/s11095-010-0360-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jain RK. Transport of Molecules, Particles, and Cells in Solid Tumors. Annu Rev Biomed Eng. 1999;1:241–263. doi: 10.1146/annurev.bioeng.1.1.241. [DOI] [PubMed] [Google Scholar]
- 43.Pluen A, Boucher Y, Ramanujan S, McKee TD, Gohongi T, di Tomaso E, Brown EB, Izumi Y, Campbell RB, Berk DA, et al. Role of Tumor-Host Interactions in Interstitial Diffusion of Macromolecules: Cranial vs. Subcutaneous Tumors. Proc Natl Acad Sci US A. 2001;98:4628–4633. doi: 10.1073/pnas.081626898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schadlich A, Caysa H, Mueller T, Tenambergen F, Rose C, Gopferich A, Kuntsche J, Mader K. Tumor Accumulation of NIR Fluorescent PEG-PLA Nanoparticles: Impact of Particle Size and Human Xenograft Tumor Model. ACS Nano. 2011;5:8710–8720. doi: 10.1021/nn2026353. [DOI] [PubMed] [Google Scholar]
- 45.Kong G, Braun RD, Dewhirst MW. Characterization of the Effect of Hyperthermia on Nanoparticle Extravasation from Tumor Vasculature. Cancer Res. 2001;61:3027–3032. [PubMed] [Google Scholar]
- 46.Skrabalak SE, Au L, Li X, Xia Y. Facile Synthesis of Ag Nanocubes and Au Nanocages. Nat Protoc. 2007;2:2182–2190. doi: 10.1038/nprot.2007.326. [DOI] [PubMed] [Google Scholar]
- 47.McCarthy DW, Shefer RE, Klinkowstein RE, Bass LA, Margeneau WH, Cutler CS, Anderson CJ, Welch MJ. Efficient Production of High Specific Activity 64Cu using a Biomedical Cyclotron. Nucl Med Biol. 1997;24:35–43. doi: 10.1016/s0969-8051(96)00157-6. [DOI] [PubMed] [Google Scholar]
- 48.Liu Y, Ibricevic A, Cohen JA, Cohen JL, Gunsten SP, Frechet JM, Walter MJ, Welch MJ, Brody SL. Impact of Hydrogel Nanoparticle Size and Functionalization on in Vivo Behavior for Lung Imaging and Therapeutics. Mol Pharm. 2009;6:1891–1902. doi: 10.1021/mp900215p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Almutairi A, Rossin R, Shokeen M, Hagooly A, Ananth A, Capoccia B, Guillaudeu S, Abendschein D, Anderson CJ, Welch MJ, et al. Biodegradable Dendritic Positron-Emitting Nanoprobes for the Noninvasive Imaging of Angiogenesis. Proc Natl Acad Sci US A. 2009;106:685–690. doi: 10.1073/pnas.0811757106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Liu Y, Abendschein D, Woodard GE, Rossin R, McCommis K, Zheng J, Welch MJ, Woodard PK. Molecular Imaging of Atherosclerotic Plaque with 64Cu-Labeled Natriuretic Peptide and PET. J Nucl Med. 2010;51:85–91. doi: 10.2967/jnumed.109.066977. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.