Abstract
BACKGROUND
The incidence of pediatric ulcerative colitis (UC), a chronic autoinflammatory disease of the colon, is on the rise. Although an increased infiltration of B cells from the peripheral blood into the colon occurs in UC, B cell trafficking is understudied. We hypothesized that the frequency of circulating plasmablasts (PBs) and their trafficking receptor (TR) expression may be indicative of the location and degree of pathology in pediatric UC.
METHODS
We conducted multicolor flow cytometry analyses of circulating IgA+/− PBs and IgA+ memory B cells (MBCs) in pediatric UC patients with remission, mild, moderate and severe state of disease (n=12), and healthy pediatric (n=2) and adult donors (n=11).
RESULTS
Compared to healthy donors the average frequency of PBs among total peripheral blood lymphocytes is increased 30-fold during severe UC activity, and positively correlates with Pediatric Ulcerative Colitis Activity Index score, C-reactive protein level and erythrocyte sedimentation rate. A greater percent of PBs in severe patients express the gut-homing receptors α4β7 and CCR10, and the inflammatory homing molecule P-selectin ligand (P-sel lig). The percent of IgA+ MBCs expressing α4β7, however, is reduced. Furthermore, expression of the small intestine TR CCR9 is decreased on α4β7high PBs, and on α4β7high/CCR10high PBs and MBCs in these patients, consistent with preferential cell targeting to the colon.
CONCLUSIONS
Peripheral blood PBs with a colon-homing phenotype (α4β7/CCR10/P-sel lig) are elevated in children with severe UC. Screening this B cell subset may provide a complementary approach in monitoring disease activity or therapeutic efficacy in pediatric UC.
Keywords: Inflammatory Bowel Disease, memory B cell, plasmablast, trafficking receptor, ulcerative colitis
Introduction
Inflammatory Bowel Disease (IBD) is a group of chronic autoinflammatory disorders of the gastrointestinal tract, characterized by lesions in the intestinal mucosa. While the precise etiology of IBD remains unclear, it is apparent that a genetic predisposition coupled with environmental triggers leads to an abnormal immunologic response in the gut1. Ulcerative colitis (UC) is an IBD typified by diffuse lesions spread throughout the colon2. Although the onset of UC may occur in either childhood or adulthood, pediatric patients often present with a more extensive disease at diagnosis3. Furthermore, the incidence of pediatric UC has risen globally4, as well as in Northern California5, the location of our study.
Intestinal homeostatic conditions are altered in UC, and antibody-secreting cells (ASCs) are implicated in this immunopathology6,7. There is an increased infiltration of immature proliferative plasma cells in ulcer bases8, and an increased level of IgA and IgG ASCs in the colonic mucosa of active UC patients9,10. Furthermore, autoantibody production is detected in UC; IgG ASCs from the peripheral blood and colon of UC patients secrete anti-colon antibodies11. In patients with inactive disease, increased numbers of ASCs in the colonic lamina propria is a predictive factor of relapse12.
While it is clear that B cells contribute to UC, the mechanism controlling their migration to the affected tissue is not fully understood, and is especially understudied in pediatric UC. Under homeostatic conditions B cells circulate through lymphoid tissues to develop and sustain an immune defense. Their recirculation is tightly controlled by the sequential association of adhesion molecules with integrin receptors and chemo-attractant ‘trafficking receptors’ (TRs) expressed on the B cell surface13. Unique combinations of TRs direct circulating B cells to specific tissues14. For instance many IgA+ ASCs express TRs that can direct their homing to the intestines, including α4β7, an integrin receptor for the mucosal vascular addressin MAdCAM-115 and CCR9 and/or CCR10, chemoattractant receptors for the small intestine chemokine, CCL25 and the common mucosal chemokine CCL28, which is highly expressed in the colon14,16,17
Peripheral blood plasmablasts are proliferating, immature precursors of tissue. plasma cells, and thus may be indicative of disease activity in the colon of UC patients. While healthy adults and children maintain low levels of PBs in the peripheral blood18,19, these levels rise during natural infection20, after vaccination21, in bacterial septicemia and liver cirrhosis22, in children and adults with active systemic lupus erythematosus (SLE)19,22, and in adults with active IBD23. In this study we hypothesize that the percentage and TR phenotype of peripheral blood PBs will reflect the disease severity and location of pathology in pediatric UC. Thus, we compared the frequency of peripheral blood B cell subsets and their associated TR expression in UC patients with different disease activity and healthy donors. Through multicolor flow cytometry analysis we show an increased percent of IgA+/− PBs expressing α4β7, CCR10, and P-selectin binding epitopes in the peripheral blood of children with severe UC. This TR phenotype may be indicative of the mechanism of migration of these cells to the inflamed colon.
Ethical Considerations
This study was performed with IRB approval from Kaiser Permanente (IRB# CN-09AWong-01-H) and San Jose State University (IRB# S0902237). All blood samples used in this study were obtained with informed consent.
Materials and Methods
Experimental design and subjects
Pediatric UC patients were enrolled in this study by the Pediatric Gastroenterologists at Kaiser Permanente in Northern California, use clinical lab tests, endoscopy/colonoscopy, histopathology and radiology findings to establish a diagnosis of UC. Disease activity was assessed using the Pediatric Ulcerative Colitis Activity Index (PUCAI)24, and patients were grouped according to disease severity for analysis (Table 1). Peripheral blood samples were obtained from the patients during routine blood-work, or prior to anti-TNF-α infusion therapy. A total of twelve pediatric UC patients participated in this study. A second sample was obtained from five patients during different disease severity and/or at a different time point from their first donation, for a total of seventeen peripheral blood samples processed for this study. There were six female and six male patients, ranging from 8-16 years of age (mean of 13). Samples were grouped for analysis according to the patient’s disease severity at the time of the blood draw, as outlined in Table 1.
Table 1.
List of pediatric UC patients and sample information
| Sample | PUCAI score (0-85) |
£PUCAI disease activity group |
Age (years) | Gender (M/F) |
ESR | CRP | Treatment |
|---|---|---|---|---|---|---|---|
| 1 | 0 | ¥Remission | 12 | M | 9 | 0.2 | **Methotrexate |
|
| |||||||
| £2 | 10 | Mild | 16 | M | 3 | 0.1 | ***Remicade |
| 3 | 10 | Mild | 9 | F | 33 | 0.1 | **Imuran, *5ASA |
| 4 | 15 | Mild | 16 | M | 51 | 1.6 | ***Humira |
| §5 | 20 | Mild | 8 | M | 13 | 0 | *Steroids |
| ξ6 | 20 | Mild | 16 | M | 8 | 0.1 | ***Remicade |
| 7 | 25 | Mild | 15 | F | 35 | 0 | ***Remicade, **Imuran |
| 8 | 30 | Mild | 15 | F | 24 | 0.1 | *Steroids |
|
| |||||||
| 9 | 35 | Moderate | 15 | F | 31 | 0 |
*5ASA *5ASA, *Steroids, and **6MP before |
| 10 | 50 | Moderate | 15 | F | 13 | 0.2 | ***Remicade |
| †11 | 60 | Moderate | 9 | M | 40 | 0.8 | ***Humira |
|
| |||||||
| †12 | 65 | Severe | 9 | M | 39 | 0.8 | ***Humira |
| δ13 | 65 | Severe | 15 | M | 29 | 3.7 | ***Remicade |
| §14 | 75 | Severe | 8 | M | 87 | 2.3 | *Steroids |
| ‡15 | 75 | Severe | 15 | F | 48 | 4.2 | ***Remicade |
| δ16 | 75 | Severe | 15 | M | 85 | 6.9 | ***Remicade |
| ‡17 | 75 | Severe | 15 | F | 71 | 3.9 | ***Remicade |
Activity group is based on PUCAI user guide (score range from 0-85): Remission, <10; Mild, 10-34; Moderate, 35-64; Severe, ≥65.
The remission group was excluded from most analyses due to the small sample size, which precluded the use of statistical analyses on this patient as a group.
Anti-inflammatory agents: 5ASA and steroids.
Immunomodulators: Imuran, Methotrexate, and 6MP.
Biological therapies: anti-TNF-α monoclonal antibodies, Humira and Remicade.
Samples with the same symbol were obtained from the same patient. M male, F female, ESR erythrocyte sedimentation rate, CRP C-reactive protein, 5ASA 5-aminosalicylic acid, 6MP 6-mercaptopurine.
Samples with the same symbol were obtained from the same patient. M male, F female, ESR erythrocyte sedimentation rate, CRP C-reactive protein, 5ASA 5-aminosalicylic acid, 6MP 6-mercaptopurine.
Samples with the same symbol were obtained from the same patient. M male, F female, ESR erythrocyte sedimentation rate, CRP C-reactive protein, 5ASA 5-aminosalicylic acid, 6MP 6-mercaptopurine.
Samples with the same symbol were obtained from the same patient. M male, F female, ESR erythrocyte sedimentation rate, CRP C-reactive protein, 5ASA 5-aminosalicylic acid, 6MP 6-mercaptopurine.
Samples with the same symbol were obtained from the same patient. M male, F female, ESR erythrocyte sedimentation rate, CRP C-reactive protein, 5ASA 5-aminosalicylic acid, 6MP 6-mercaptopurine.
Healthy donors were selected for this study based on lack of IBD disease, as well as any other illness (gastrointestinal, respiratory, etc.) for at least one month prior to blood collection. A total of thirteen different healthy donors (two pediatric and eleven adult) participated in this study—ten female and three male—ranging from 15-47 years of age (mean of 32). One sample per healthy donor was used.
Lymphocyte separation from whole blood
Heparinized peripheral blood samples were obtained via venipuncture from the different donor populations: 10 ml from pediatric UC patients (n=17) and 20 ml from healthy donors (n=13). Blood samples were processed using Ficoll (Histopaque-1077, Sigma-Aldrich, St. Louis, MO) density gradient centrifugation, and the peripheral blood mononuclear cell (PBMC) layer was extracted. PBMCs were washed twice in HBSS without Ca++/Mg++, and were resuspended in staining buffer (1X PBS with 2% FCS and 0.1% sodium azide). Cells were blocked overnight with normal human serum (H4522, Sigma-Aldrich) and normal goat serum (Gibco, Invitrogen by Life Technologies, Carlsbad, CA), and cells were then stained and acquired.
Monoclonal antibodies for flow cytometry phenotypic analysis
Primary conjugated antibodies
mouse anti-human CD38 PE-Cy7 (Clone HB7, BD Biosciences, San Jose, CA), goat F(ab’)2 anti-human IgA FITC (Invitrogen), mouse anti-human CD19 Alexa Fluor 700 (Clone HIB19, BD Biosciences), rat anti-human CD49f PE-Cy5 (Clone GoH3, BD Biosciences), and mouse anti-human CD4 V500 (Clone RPA-T4, BD Biosciences).
Primary unconjugated antibodies
mouse anti-human CCR9 (ATCC, Manassas, VA) and anti-human CCR10 (courtesy Dr. Dulce Soler-Ferran, Millennium Pharmaceuticals, Cambridge, MA) were labeled in house using goat F(ab’)2 anti-mouse Pacific Blue (Invitrogen) and goat F(ab’)2 anti-mouse Qdot655 (Invitrogen) respectively. Mouse anti-human α4β7 (Act-1, ATCC) was PE conjugated by Chromoprobe (St. Louis, MO). Recombinant human P-selectin Fc chimera (R&D Systems, Minneapolis, MN) was labeled in house with Zenon Alexa Fluor 647 human IgG labeling kit (Invitrogen) as directed. Biotin rat anti-human CLA (cutaneous lymphocyte antigen; Clone HECA-452, BD Biosciences) was labeled in house with Streptavidin Qdot565 (Invitrogen).
Cell staining and flow cytometry analysis
3×10^6 PBMCs per donor sample were stained with the previously mentioned antibodies. First, cells were stained with primary anti-CCR9 antibody, washed, followed by secondary anti-mouse Pacific Blue labeling. After a second wash, cells were blocked with normal mouse serum (NMS, Santa Cruz Biotechnology, Santa Cruz, CA). Next, primary anti-CCR10 antibody was labeled with secondary anti-mouse Qdot655, and was then added to the cells. A bench-top conjugation with P-selectin and Zenon AF647 was performed, then was added to the cells to stain P-selectin ligand (P-sel lig). A cocktail of the primary conjugated antibodies was then applied to the cells. Subsequently, cells were stained with primary anti-CLA Biotin antibody, washed, and then labeled with Streptavidin Qdot565. Then a final wash was performed.
Alongside the donor sample, an isotype control with 3×10^6 PBMCs was prepared for the four primary antibodies labeled in house, using the second stage alone of n-1 (addition of Pacific Blue without CCR9, Qdot655 without CCR10, and Qdot565 without CLA), and an incubation of the cells with 0.5M EDTA (to interrupt the binding of P-sel with P-sel lig). In addition, 10% NMS was added in place of the primary antibodies CCR9, CCR10, and CLA. Apart from these modifications, the isotype control was prepared in the same manner as the donor sample. All samples were acquired on a BD Biosciences LSRII flow cytometer (Butcher lab at Stanford University) using FACSDiva software.
Flow cytometry data was analyzed using FlowJo software (Tree Star Inc., Ashland, OR). TR gates were placed based on the isotype controls, or a bimodal comparison of the positive and negative staining populations. During data analysis, positive staining in the isotype control for CCR9, CCR10, P-sel lig, and CLA were subtracted from the donor sample value to account for non-specific binding.
Statistical analyses
Differences in the percent of IgA+/− PBs and IgA+ MBCs between the pediatric (n=2) and adult (n=11) healthy donors were analyzed using the Wilcoxon test. In addition, relationships between the demographic variables of the UC patients (age, gender, and drug treatment) and the response variables IgA+ PBs, IgA− PBs, and IgA+ MBCs were investigated. To determine whether there is a relationship between the age of the patient and the response levels, we regressed the responses individually on age and tested for zero slope in regression with a regression F-test. To test whether the gender of the patients has an influence on the responses, we used Wilcoxon’s test. To test whether the mean responses of the patients vary by the treatment regimen they are subjected to, we performed an analysis of variance (ANOVA F-test). For this analysis the patients were placed into three groups based on treatment: anti-inflammatory agents, immunomodulators, and biological therapies (therapies detailed in Table 1). If the patient was treated with multiple therapies the patient was placed in a treatment group based on the strongest therapy received (anti-inflammatory agents<immunomodulators<biological therapies). All other statistical analyses were performed using Student’s t-test or linear regression analysis, as identified in the Results section. A p<0.05 value was considered statistically significant.
Results
Peripheral blood B cell subsets in pediatric UC patients
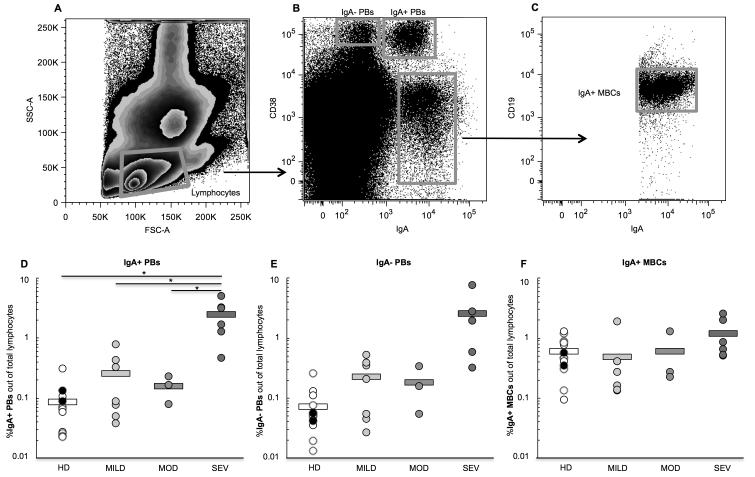
Circulating plasmablasts (PBs) are in transit to the tissue, where they mature into antibody-secreting cells (ASCs); thus, an increased quantity in the peripheral blood may indicate disease activity in the colon of UC patients. Memory B cells (MBCs) are an immunologic surveillance population that reflect past antigen encounter, and comprise a pool of cells from which PBs specific to previously encountered antigens may be derived. We monitored the percent of IgA+ PBs, IgA− PBs, and IgA+ MBCs in the peripheral blood, to determine whether these B cell subsets were increased in pediatric UC.
Three B cell populations were gated from the PBMCs (Figure 1A-C): CD38high/IgA− cells (IgA− PBs), CD38high/IgA+ cells (IgA+ PBs), and CD38med/low/IgA+ cells (IgA+ MBCs)20. IgA+ MBCs were further gated to select for CD19 expression. CD4+ cells were gated out to exclude them from analysis. There was no difference in the frequency of IgA+ PBs between pediatric (0.11%) and adult (0.08%) healthy donors (Wilcoxon test, p=0.23), or in the frequency of IgA− PBs (0.05 and 0.08% respectively, p=0.92) or IgA+MBCs (0.47 and 0.62%, p=0.77) (data not shown). Therefore all healthy donors were combined for statistical comparisons.
Figure 1.
Representative flow cytometry plots of B cell subset gating strategy. From left to right: (A) lymphocytes are gated, (B) three B cell subsets are identified— the IgA−plasmablasts (PBs), the IgA+ PBs, and the IgA+ memory B cells (MBCs), then (C) the IgA+ MBCs are further gated for CD19 expression. The percent of (D) IgA+ PBs, (E) IgA− PBs, and (F) IgA+ MBCs out of total peripheral blood lymphocytes was determined for all healthy donor and pediatric UC patient blood samples. Samples were grouped according to type of donor; groups here are healthy donors (HD) (black dots are pediatric HD, and white dots are adult HD), and pediatric patients with mild UC (MILD), moderate UC (MOD), and severe UC (SEV) disease activity. Circles represent individual samples and bars represent the group’s average. Student’s t-test was performed between all UC patient groups vs. healthy donors, and between the severe group vs. the mild and moderate UC groups. *p<0.05; no asterisk indicates p>0.05. Significance (p<0.05) is present in (E) when the severe patient with the highest percent of IgA− PBs (a high outlier) is removed from the analysis.
Pediatric UC patients with severe disease had a greater percent of IgA+ PBs among total peripheral blood lymphocytes (2.48%) than healthy controls (0.09%, p=0.02), as well as patients with mild (0.26%, p=0.02) and moderate (0.16%, p=0.02) disease activity (Figure 1D). While the percent of IgA− PBs was greater in severe UC patients (2.54%) compared to other groups, this difference was not statistically significant (Figure 1E). However, when a severe patient who had 7.6% IgA− PBs (a high outlier) was removed from the analysis, the percent of IgA− PBs in severe patients (1.5%) was greater than that in healthy donors (0.07%, p=0.03), mild (0.23%, p=0.048) and moderate (0.18%, p=0.04) patients. In contrast to the PBs, the percent of IgA+ MBCs was similar between healthy and UC patient groups (average range from 0.5-1.2%, p>0.05 for all comparisons) (Figure 1F).
Within the UC patients, there was no correlation between age of the patient and percent of IgA+ PBs (regression F-test, p=0.93), IgA− PBs (p=0.67), or IgA+ MBCs (p=0.31), or between the gender of the patient and the percent of IgA+ PBs (Wilcoxon test, p=0.35), IgA− PBs (p=0.92), or IgA+ MBCs (p=0.92) (data not shown). Additionally, no significant difference was observed in the mean percent of IgA+ PBs between the patients being treated with anti-inflammatory agents, immunomodulators, or biological therapies (Anova F-test, p=0.62), or in the percent of IgA− PBs (p=0.72) and IgA+ MBCs (p=0.56) (data not shown).
Correlation between circulating plasmablasts and clinical parameters in pediatric UC patients
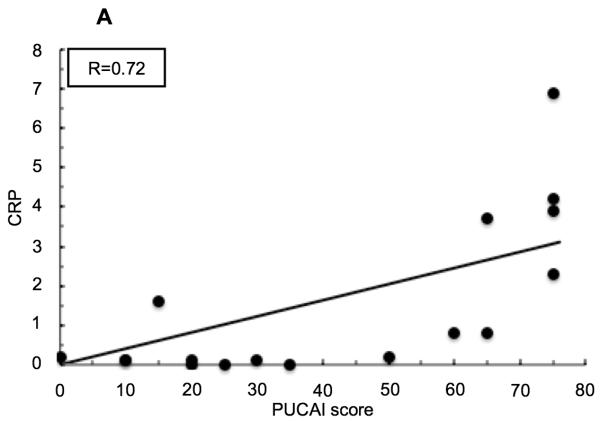
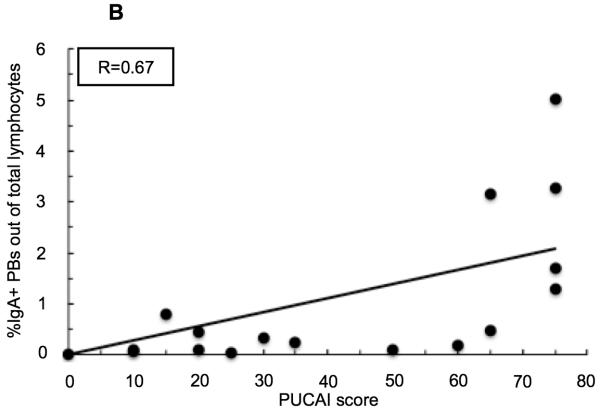
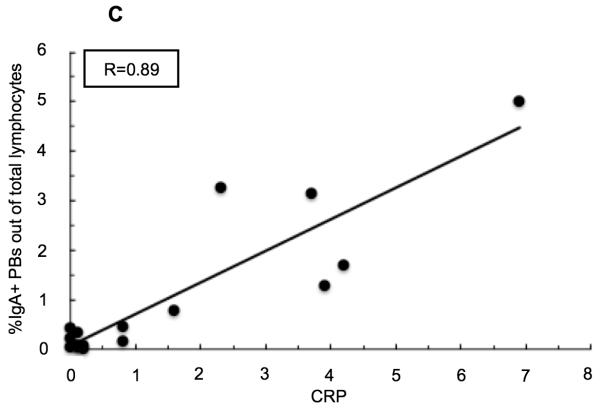
Inflammatory activity in pediatric UC patients is closely monitored using clinical lab tests such as C-reactive protein (CRP) and erythrocyte sedimentation rate (ESR). In addition, the PUCAI scoring system is an assessment tool that provides a way to quantify UC disease severity based on reported symptoms.
Both CRP and ESR values positively correlate with PUCAI score in pediatric UC patients (Figure 2) (R=0.72 and R=0.71 respectively). The percent of peripheral blood IgA+ PBs, and to a lesser degree IgA− PBs, correlate with PUCAI score at a comparable level to these established clinical tests (R=0.67 and R=0.54 respectively). In addition, the percent of IgA+ PBs in circulation correlates very well with CRP (R=0.89) and with ESR (R=0.74), while the percent of IgA− PBs correlates well, but to a lesser extent (with CRP R=0.87, and with ESR R=0.62). Overall, the frequency of IgA+ PBs in the peripheral blood correlated well, and better, than the IgA− PBs with each of the tested parameters (data not shown for IgA− PBs or ESR).
Figure 2.
Linear regression analyses in pediatric patients with UC. (A) Correlation between serum C-reactive protein (CRP) levels and PUCAI score. (B) Correlation between percent of IgA+ plasmablasts (PBs) out of total peripheral blood lymphocytes and Pediatric Ulcerative Colitis Activity Index (PUCAI) score. (C) Correlation between percent of IgA+ PBs out of total peripheral blood lymphocytes and CRP.
Single trafficking receptor analysis of plasmablasts
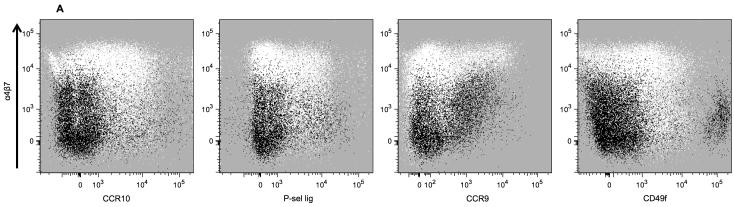
Previous studies indicate that expression of the TR ligands MAdCAM-1 and CCL28, and the vascular receptor P-selectin, is increased in the inflamed colon tissue of UC patients25,26,27. Thus, we determined whether circulating PBs in UC patients demonstrate an altered expression of the corresponding TRs, α4β7 and CCR10, and P-sel lig respectively. In addition CCR9 (a TR with particular relevance to small intestine homing but not colon homing16,28) was examined. Since extraintestinal manifestations may occur in patients with IBD, we also measured the expression of the skin homing receptor CLA29, a carbohydrate ligand for vascular E-selectin, and the integrin α6 chain (i.e. CD49f), whose expression is associated with homing to the skin, lung, and other extraintestinal sites30,31, IgA+/− PBs were identified as shown in Figure 1, then TR expression was examined using the gating strategy in Figure 3A.
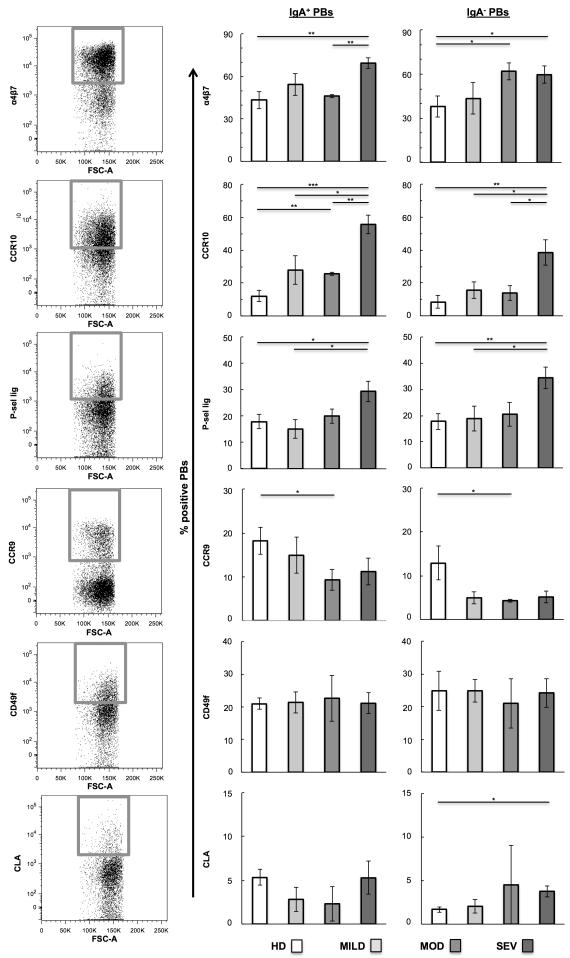
Figure 3.
Single trafficking receptor (TR) analysis on IgA+/− plasmablasts (PBs). (A) Representative flow cytometry plots of individual TR expression on PBs. Gates represent high-TR-expressing cells. Samples were grouped according to type of donor: healthy donors (HD) and pediatric patients with mild UC (MILD), moderate UC (MOD), and severe UC (SEV) disease activity. Average percent of (B) IgA+ PBs and (C) IgA− PBs with TRhigh expression of α4β7, CCR10, P-selectin ligand (P-sel lig), CCR9, CD49f, or CLA. Student’s t-test was performed between all UC patient groups vs. healthy, and between the severe group vs. the mild and moderate UC groups. *p<0.05, **p<0.01, ***p<0.001; no asterisk indicates p>0.05.
Severe UC patients displayed a greater percent of α4β7high IgA+ PBs (69% of IgA+ PBs) in circulation than healthy donors (43%, p=0.002) and moderate patients (46%, p=0.001) (Figure 3B). Similarly, the percent of CCR10high IgA+ PBs was increased in severe UC patients (56% of IgA+ PBs) compared to healthy donors (12%, p=0.0001), and patients with mild (28%, p=0.02) and moderate (26%, p=0.003) disease. Furthermore, P-sel lighigh IgA+ PBs were more frequent in severe UC patients (29% of IgA+ PBs) compared to healthy donors (18%, p=0.03) and patients with mild disease (15%, p=0.02). The IgA− PBs demonstrated similar trends to the IgA+ PBs for all three TRs (Figure 3C).
On average UC patients had fewer CCR9high PBs than HDs, and UC patients with moderate disease demonstrated significantly less CCR9high IgA+ and IgA− PBs (9 and 4% respectively) than healthy donors (18 and 13%, p=0.04 and p=0.048 respectively). The percent of CD49fhigh and CLAhigh PBs was mainly similar between groups.
Trafficking receptor expression on mucosal and non-mucosal plasmablasts
The combinatorial expression of TRs drives cell homing to the intestines and other sites13. Since we observed an increase in PBs that express CCR10 and P-sel lig in severe UC, we further investigated whether this correlated with α4β7 expression, and whether this TR expression occurred primarily on the mucosal (α4β7high) or non-mucosal (α4β7low) homing cells. IgA+ and IgA− PBs from Figure 1 were segregated into α4β7high and α4β7low populations, then analyzed for the expression of other TRs as shown in Figure 4A.
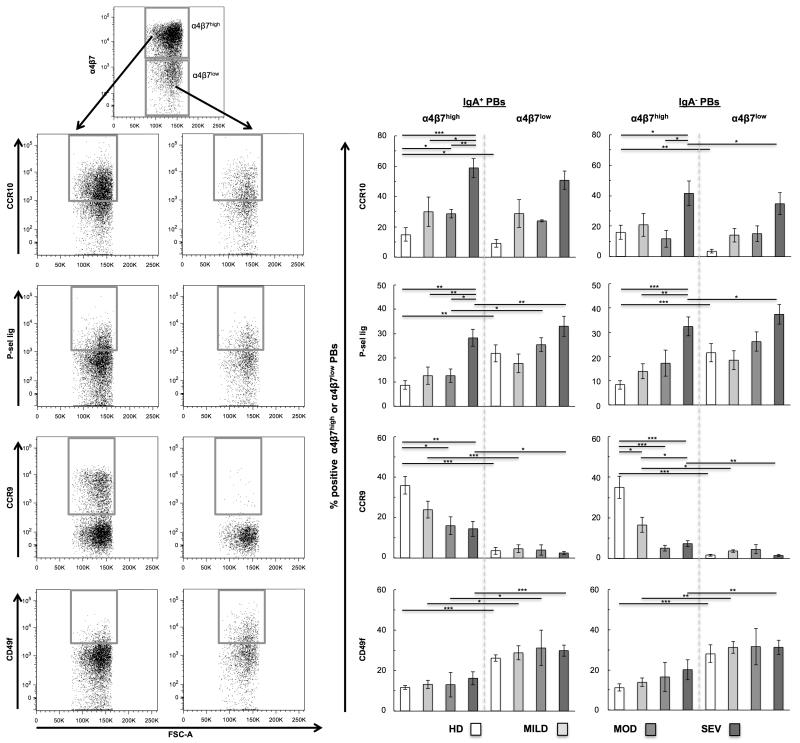
Figure 4.
Trafficking receptor (TR) analysis on mucosal (α4β7high) and non-mucosal (α4β7low) IgA+/− plasmablasts (PBs). (A) Representative flow cytometry plots for TR gating strategy on PBs. From top to bottom: IgA+/− PBs are gated for α4β7high expression (upper gate) and α4β7low expression (lower gate), then individual TR expression is examined on each of these subsets. Gates represent cells with TRhigh expression of CCR10, P-selectin ligand, CCR9, or CD49f. Bar graphs display the average percent of α4β7high and α4β7low (B) IgA+ PBs and (C) IgA− PBs with TRhigh expression. Samples were grouped according to type of donor: healthy donors (HD) and pediatric patients with mild UC (MILD), moderate UC (MOD), and severe UC (SEV) disease activity. Statistical analyses for α4β7high cells were performed between all UC patient groups vs. healthy, and between the severe group vs. the mild and moderate UC groups. In addition, statistical analyses were performed between α4β7high and α4β7low cells within groups using Student’s t-test. *p<0.05, **p<0.01, ***p<0.001; no asterisk indicates p>0.05.
The percent of mucosal IgA+ PBs with CCR10high and P-sel lighigh expression was greater in severe UC patients (59% and 28% respectively) than in healthy controls (15%, p=0.0002, and 9%, p=0.001 respectively), and patients with mild (30%, p=0.03, and 13%, p=0.0099) and moderate disease (29%, p=0.004, and 13%, p=0.01) (Figure 4B). Although the percent of CCR10high cells was similar in the α4β7high and α4β7low IgA+ PB subsets, P-sel lig had a greater percentage of expression in the non-mucosal cells. Similar expression patterns for CCR10 and P-sel lig were observed in the mucosal and non-mucosal IgA− PBs (Figure 4C).
The frequency of mucosal IgA+ PBs with CCR9high expression was decreased in moderate (16%, p=0.02) and severe (15%, p=0.002) UC patients compared to healthy donors (36%); similar trends were observed in the IgA− PBs. The percent of CCR9high non-mucosal PBs was low in all groups, and on average less than 5% of non-mucosal PBs demonstrated CCR9high expression. The frequency of mucosal IgA+/− PBs with CD49f high expression was similar for healthy and UC patient groups (average range between 12-20%). However, α4β7low non-mucosal PBs with CD49f high expression were consistently increased (average range between 27-32%) compared to their α4β7high mucosal homing counterparts.
The cellular expression of α4β7 and CLA are usually mutually exclusive, as these TRs mediate lymphocyte homing to different sites. Therefore the percent of mucosalPBs that were CLAhigh was very low—on average less than 6% of cells (CLA data not shown). The frequency of CLAhigh cells among non-mucosal PBs was also low—on average less than 10% of cells.
Trafficking receptor expression on memory B cells in pediatric UC patients
MBCs act as an immunological surveillance population, and their presence reflects past antigen encounter. Since UC is a chronic disease, we predicted that TR expression on peripheral blood MBCs may be indicative of the location of past disease activity in UC patients. We compared the TR expression of IgA+ MBCs to their activated counterparts, the IgA+ PBs, and also compared IgA+ MBC TR expression between donor groups.
The differential TR expression on IgA+ PBs and MBCs is visually demonstrated in overlay dot plots of the two B cell populations from all severe UC patients (Figure 5A). Co-expression of α4β7 and CCR10 or P-sel lig was consistently higher on PBs than MBCs. However, the co-expression of α4β7 and CCR9 or CD49f was more similar between the cell subsets.
Figure 5.
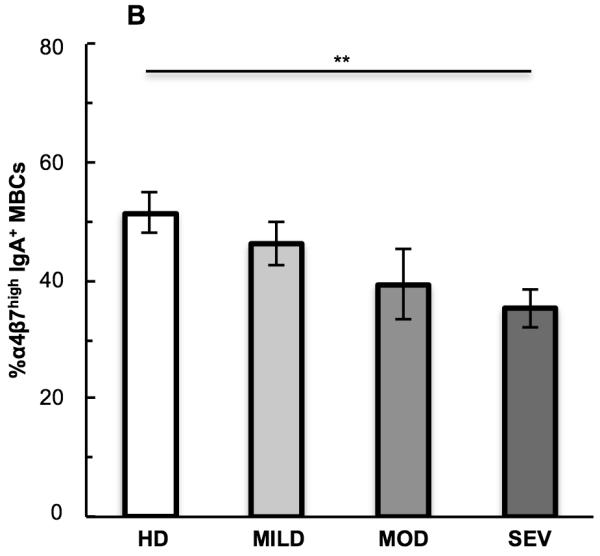
Comparison of IgA+ plasmablasts (PBs) and IgA+ memory B cells (MBCs). (A) Flow cytometry dot plot overlays of trafficking receptor (TR) co-expression of α4β7 with CCR10, P-sel lig, CCR9, or CD49f on IgA+ PBs (white dots) and IgA+ MBCs (black dots) from all severe UC patients combined. (B) Single TR expression on IgA+ MBCs, showing the average percent of MBCs with α4β7high expression in each donor group. Student’s t-test was performed between all UC patient groups vs. healthy, and between the severe group vs. the mild and moderate UC groups. **p<0.01; no asterisk indicates p>0.05.
Unlike the PBs, the frequency of CCR10high, P-sel lighigh, CCR9high, and CD49fhigh IgA+ MBCs was similar in healthy donors and UC patients (p>0.05 for all comparisons; data not shown). However in contrast to the α4β7high PBs, the frequency of α4β7high IgA+ MBCs was decreased in severe UC patients (35%) compared to healthy donors (51%, p=0.004) (Figure 5B).
CCR9 expression on B cell subsets co-expressing α4β7 and CCR10
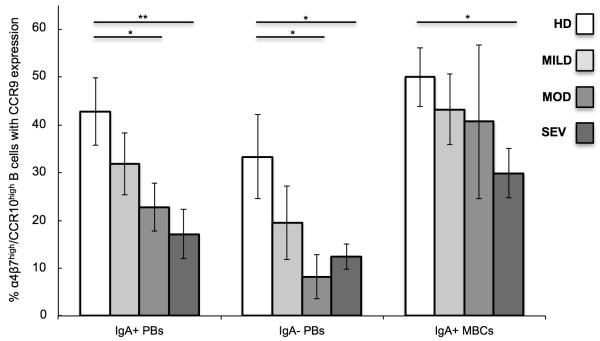
α4β7 and CCR10 expression without CCR9 is expected to favor cell homing to the colon, as MAdCAM-1 and CCL28 are highly expressed in the colon, whereas CCR9 and its ligand CCL25 are major determinants of lymphocyte recruitment to the small intestine14. Therefore we examined α4β7high/CCR10high B cell subsets for CCR9 expression.
In general the proportion of α4β7high/CCR10high PBs and MBCs expressing CCR9 decreased as the severity of UC disease intensified (Figure 6). The frequency of α4β7high/CCR10high IgA+ and IgA− PBs with CCR9 expression was decreased in the moderate (23%, p=0.04, and 8%, p=0.03 respectively) and severe (17%, p=0.009, and 12%, p=0.04) UC disease activity groups compared to healthy donors (43 and 33%). In addition, the percent of α4β7high/CCR10high IgA+ MBCs expressing CCR9 in severe UC (30%) was decreased compared to healthy controls (50%, p=0.02).
Figure 6.
CCR9 expression on α4β7high/CCR10high IgA+/− plasmablasts (PBs), and IgA+ memory B cells (MBCs). Samples were grouped according to type of donor: healthy donors (HD) and pediatric patients with mild UC (MILD), moderate UC (MOD), and severe UC (SEV) disease activity. Statistical analyses within each B cell subset were performed between healthy donors and UC patient disease activity groups using Student’s t-test. *p<0.05; no asterisk indicates p>0.05.
Discussion
B lymphocytes contribute to the immunopathology of UC6,7, however the mechanisms controlling migration to the affected tissue in this disease is understudied. We examined peripheral blood B cell subsets in UC patients using the rationale that their frequency and migratory potential, reflected by their TR expression, would be altered and therefore indicative of B cell activity in the colon. The purpose of this study was to characterize TR expression on B cell subsets, specifically PBs, elicited in UC. We focused our study on pediatric patients with recent onset of illness, and hence with fewer confounding effects of comorbidities and long-term medication.
Here we show that the average frequency of both IgA+ and IgA− PBs are elevated in children with severe UC as compared to healthy donors and patients with mild and moderate disease activity. Similar findings have recently been reported by Hosomi et. al23 in adult UC patients with active disease. In contrast, the percent of peripheral blood IgA+ MBCs was unaltered in patients at all disease activity levels compared to healthy individuals; a feature that has been demonstrated in other chronic autoimmune disorders, such as pediatric SLE19. Therefore the frequency of peripheral blood PBs, but not MBCs, is a good indicator of disease severity and inflammation in UC.
As the UC disease activity increased in severity, we observed a dramatic increase in the percent of IgA+ and IgA− PBs with α4β7high and CCR10high or P-sel lighigh co-expression, supporting the hypothesis that these TRs play a role in PB migration to the colon in UC. Of note, Hosomi et al.23 did not observe an increase in CCR10 expressing PBs (defined as CD19+/CD20− cells) in adult UC. Since all activity levels were combined in their study, perhaps a rise in CCR10 expressing PBs was masked by UC patients with milder disease. Another possibility is that the relevance of CCR10 may be different in adult UC vs. pediatric UC, and this TR may have greater significance during the initial onset or early in disease.
P-sel lig, along with its corresponding receptor P-selectin, is upregulated in immune cells and on the vascular endothelium in response to inflammation32. Here we observed an increased frequency of both α4β7low and α4β7high PBs expressing P-sel lig in severe UC, further indicating the high levels of colon inflammation at this stage of disease, and perhaps reflecting the massive migration of cells expressing P-sel lig into the inflamed colon.
There was a trend in the mild and moderate activity groups for an increased percent of α4β7high PBs but not CCR10high and P-sel lighigh cells; only patients with severe disease activity displayed a higher frequency of CCR10high and P-sel lighigh PBs. It is possible that UC therapeutics decrease certain TR responses, i.e. CCR10 and P-sel lig upregulation on cells. Alternatively, high CCR10 and high P-sel lig expression may be imprinted on PBs only at advanced stages of disease, where intensified exposure to colon antigens has occurred due to extensive inflammation and tissue damage. Thus, a rise in the frequency of peripheral blood PBs imprinted with high CCR10 and high P-sel lig expression may reflect a more acute manifestation of UC, than does a rise in PBs with high α4β7 only.
The frequency of IgA+ MBCs expressing α4β7 at high levels decreased as UC disease activity intensified, and severe patients had a lower proportion of these cells compared to healthy donors. α4β7high MBCs may redistribute from the blood to the intestines in response to inflammation, or may be re-activated in patients during a flare in disease activity, and thus become depleted from the circulating α4β7 MBC pool.
We found a reduced frequency of PBs with CCR9high expression in pediatric UC, and moderate and severe patients had fewer PBs co-expressing α4β7 and CCR9. There is no small intestine involvement in UC, a disease that solely affects the colon. Therefore no upregulation in CCR9, which preferentially targets lymphocytes to the small intestine, was expected. In severe UC patients there was also a decrease in the percent of α4β7high/CCR10high PBs and MBCs that express CCR9, which indicates these patients have increased levels of PBs that may preferentially migrate to the colon vs. the small intestine33.
Extraintestinal manifestations may occur in pediatric IBD, and some patients develop pathologies of the skin, liver and other complications34,35. The proportion of cells that expressed CLA, a trafficking molecule associated with skin homing, was low on both PBs and MBCs. However, only one of the pediatric UC patients in this study developed a skin pathology, which may explain the low frequency of cells expressing this molecule.
While the role of the integrin receptor CD49f (aka α6) in UC is not well documented, B cells in the colon of UC patients, but not in the colon of Crohn’s or non-IBD patients, were found to express this receptor36. In our study the frequency of B cells expressing CD49f was unaltered in UC, and the expression of the receptor was more prominent on α4β7low cells, supporting the idea that CD49f is not associated with gut-homing lymphocytes30. Our results indicate that CD49f plays a minimal role in B cell trafficking in pediatric UC.
In conclusion, our study demonstrates an increase in the frequency of IgA+ and IgA− PBs in the peripheral blood of severe UC patients. PB frequency positively correlates with UC disease severity and inflammation, indicating circulating PB subsets as a good indicator of disease activity in pediatric UC. In addition, circulating IgA+/− PBs with α4β7high/CCR10high/P-sel lighigh expression are elevated in pediatric UC patients with severe disease. In combination these trafficking receptors likely facilitate PB homing to the inflamed colonic tissue. Thus, this cell subset may provide a therapeutic target for the treatment of UC, and the surveillance of circulating PBs and their associated TR expression may potentially be used as a non-invasive, complementary approach to monitor disease activity or therapeutic efficacy in children with this disease.
Acknowledgements
We are very thankful to both the patients and healthy donors who participated in this study, without whose contribution this study would not have been possible. We would like to thank Dr. Kenneth Youngman (San Jose State University) for critical editing of this manuscript.
Funding: This work was supported by funds from CSUPERB, from NIH grants R37 AI047822, U19 AI090019, and RC1 AI087257, and funds from the Gates Foundation.
Abbreviations used in this manuscript
- ASC
antibody secreting cell
- CRP
C-reactive protein
- ESR
erythrocyte sedimentation rate
- HD
healthy donor
- IBD
Inflammatory Bowel Disease
- MBC
memory B cell
- PB
plasmablast
- PBMC
peripheral blood mononuclear cell
- P-sel lig
P-selectin ligand
- PUCAI
Pediatric Ulcerative Colitis Activity Index
- SLE
systemic lupus erythematosus
- TR
trafficking receptor
- UC
ulcerative colitis
Footnotes
All authors have nothing to disclose.
References
- 1.Loftus EV, Jr., Sandborn WJ. Epidemiology of inflammatory bowel disease. Gastroenterol Clin North Am. 2002;31:1–20. doi: 10.1016/s0889-8553(01)00002-4. [DOI] [PubMed] [Google Scholar]
- 2.Podolsky DK. Inflammatory bowel disease. N Engl J Med. 2002;347:417–29. doi: 10.1056/NEJMra020831. [DOI] [PubMed] [Google Scholar]
- 3.Langholz E, Munkholm P, Krasilnikoff PA, et al. Inflammatory bowel diseases with onset in childhood. Clinical features, morbidity, and mortality in a regional cohort. Scand J Gastroenterol. 1997;32:139–47. doi: 10.3109/00365529709000184. [DOI] [PubMed] [Google Scholar]
- 4.Benchimol EI, Fortinsky KJ, Gozdyra P, et al. Epidemiology of pediatric inflammatory bowel disease: a systematic review of international trends. Inflamm Bowel Dis. 17:423–39. doi: 10.1002/ibd.21349. [DOI] [PubMed] [Google Scholar]
- 5.Abramson O, Durant M, Mow W, et al. Incidence, prevalence, and time trends of pediatric inflammatory bowel disease in Northern California, 1996 to 2006. J Pediatr. 157:233–239. e1. doi: 10.1016/j.jpeds.2010.02.024. [DOI] [PubMed] [Google Scholar]
- 6.Brandtzaeg P, Haraldsen G, Rugtveit J. Immunopathology of human inflammatory bowel disease. Springer Semin Immunopathol. 1997;18:555–89. doi: 10.1007/BF00824058. [DOI] [PubMed] [Google Scholar]
- 7.Brandtzaeg P, Carlsen HS, Halstensen TS. The B-cell system in inflammatory bowel disease. Adv Exp Med Biol. 2006;579:149–67. doi: 10.1007/0-387-33778-4_10. [DOI] [PubMed] [Google Scholar]
- 8.Jinno Y, Ohtani H, Nakamura S, et al. Infiltration of CD19+ plasma cells with frequent labeling of Ki-67 in corticosteroid-resistant active ulcerative colitis. Virchows Arch. 2006;448:412–21. doi: 10.1007/s00428-005-0136-7. [DOI] [PubMed] [Google Scholar]
- 9.Keren DF. Autoreactivity and altered immune responses in inflammatory bowel disease. Clin Lab Med. 1988;8:325–36. [PubMed] [Google Scholar]
- 10.Yacyshyn BR. Activated CD19+ B cell lamina propria lymphocytes in ulcerative colitis. Immunol Cell Biol. 1993;71(Pt 4):265–74. doi: 10.1038/icb.1993.31. [DOI] [PubMed] [Google Scholar]
- 11.Hibi T, Ohara M, Toda K, et al. In vitro anticolon antibody production by mucosal or peripheral blood lymphocytes from patients with ulcerative colitis. Gut. 1990;31:1371–6. doi: 10.1136/gut.31.12.1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bitton A, Peppercorn MA, Antonioli DA, et al. Clinical, biological, and histologic parameters as predictors of relapse in ulcerative colitis. Gastroenterology. 2001;120:13–20. doi: 10.1053/gast.2001.20912. [DOI] [PubMed] [Google Scholar]
- 13.Butcher EC, Picker LJ. Lymphocyte homing and homeostasis. Science. 1996;272:60–6. doi: 10.1126/science.272.5258.60. [DOI] [PubMed] [Google Scholar]
- 14.Kunkel EJ, Butcher EC. Plasma-cell homing. Nat Rev Immunol. 2003;3:822–9. doi: 10.1038/nri1203. [DOI] [PubMed] [Google Scholar]
- 15.Berlin C, Berg EL, Briskin MJ, et al. Alpha 4 beta 7 integrin mediates lymphocyte binding to the mucosal vascular addressin MAdCAM-1. Cell. 1993;74:185–95. doi: 10.1016/0092-8674(93)90305-a. [DOI] [PubMed] [Google Scholar]
- 16.Hieshima K, Kawasaki Y, Hanamoto H, et al. CC chemokine ligands 25 and 28 play essential roles in intestinal extravasation of IgA antibody-secreting cells. J Immunol. 2004;173:3668–75. doi: 10.4049/jimmunol.173.6.3668. [DOI] [PubMed] [Google Scholar]
- 17.Lazarus NH, Kunkel EJ, Johnston B, et al. A common mucosal chemokine (mucosae-associated epithelial chemokine/CCL28) selectively attracts IgA plasmablasts. J Immunol. 2003;170:3799–805. doi: 10.4049/jimmunol.170.7.3799. [DOI] [PubMed] [Google Scholar]
- 18.Fernandes JR, Snider DP. Polymeric IgA-secreting and mucosal homing pre-plasma cells in normal human peripheral blood. Int Immunol. 22:527–40. doi: 10.1093/intimm/dxq037. [DOI] [PubMed] [Google Scholar]
- 19.Odendahl M, Keitzer R, Wahn U, et al. Perturbations of peripheral B lymphocyte homoeostasis in children with systemic lupus erythematosus. Ann Rheum Dis. 2003;62:851–8. doi: 10.1136/ard.62.9.851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jaimes MC, Rojas OL, Kunkel EJ, et al. Maturation and trafficking markers on rotavirus-specific B cells during acute infection and convalescence in children. J Virol. 2004;78:10967–76. doi: 10.1128/JVI.78.20.10967-10976.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mei HE, Yoshida T, Sime W, et al. Blood-borne human plasma cells in steady state are derived from mucosal immune responses. Blood. 2009;113:2461–9. doi: 10.1182/blood-2008-04-153544. [DOI] [PubMed] [Google Scholar]
- 22.Harada Y, Kawano MM, Huang N, et al. Identification of early plasma cells in peripheral blood and their clinical significance. Br J Haematol. 1996;92:184–91. doi: 10.1046/j.1365-2141.1996.300835.x. [DOI] [PubMed] [Google Scholar]
- 23.Hosomi S, Oshitani N, Kamata N, et al. Increased numbers of immature plasma cells in peripheral blood specifically overexpress chemokine receptor CXCR3 and CXCR4 in patients with ulcerative colitis. Clin Exp Immunol. 163:215–24. doi: 10.1111/j.1365-2249.2010.04290.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Turner D, Otley AR, Mack D, et al. Development, validation, and evaluation of a pediatric ulcerative colitis activity index: a prospective multicenter study. Gastroenterology. 2007;133:423–32. doi: 10.1053/j.gastro.2007.05.029. [DOI] [PubMed] [Google Scholar]
- 25.Souza HS, Elia CC, Spencer J, et al. Expression of lymphocyte-endothelial receptor-ligand pairs, alpha4beta7/MAdCAM-1 and OX40/OX40 ligand in the colon and jejunum of patients with inflammatory bowel disease. Gut. 1999;45:856–63. doi: 10.1136/gut.45.6.856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ogawa H, Iimura M, Eckmann L, et al. Regulated production of the chemokine CCL28 in human colon epithelium. Am J Physiol Gastrointest Liver Physiol. 2004;287:G1062–9. doi: 10.1152/ajpgi.00162.2004. [DOI] [PubMed] [Google Scholar]
- 27.Schurmann GM, Bishop AE, Facer P, et al. Increased expression of cell adhesion molecule P-selectin in active inflammatory bowel disease. Gut. 1995;36:411–8. doi: 10.1136/gut.36.3.411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Papadakis KA, Prehn J, Nelson V, et al. The role of thymus-expressed chemokine and its receptor CCR9 on lymphocytes in the regional specialization of the mucosal immune system. J Immunol. 2000;165:5069–76. doi: 10.4049/jimmunol.165.9.5069. [DOI] [PubMed] [Google Scholar]
- 29.Kantele A, Savilahti E, Tiimonen H, et al. Cutaneous lymphocyte antigen expression on human effector B cells depends on the site and on the nature of antigen encounter. Eur J Immunol. 2003;33:3275–83. doi: 10.1002/eji.200324311. [DOI] [PubMed] [Google Scholar]
- 30.Mackay CR, Marston WL, Dudler L, et al. Tissue-specific migration pathways by phenotypically distinct subpopulations of memory T cells. Eur J Immunol. 1992;22:887–95. doi: 10.1002/eji.1830220402. [DOI] [PubMed] [Google Scholar]
- 31.Abitorabi MA, Mackay CR, Jerome EH, et al. Differential expression of homing molecules on recirculating lymphocytes from sheep gut, peripheral, and lung lymph. J Immunol. 1996;156:3111–7. [PubMed] [Google Scholar]
- 32.Kansas GS. Selectins and their ligands: current concepts and controversies. Blood. 1996;88:3259–87. [PubMed] [Google Scholar]
- 33.Kunkel EJ, Campbell DJ, Butcher EC. Chemokines in lymphocyte trafficking and intestinal immunity. Microcirculation. 2003;10:313–23. doi: 10.1038/sj.mn.7800196. [DOI] [PubMed] [Google Scholar]
- 34.Dotson JL, Hyams JS, Markowitz J, et al. Extraintestinal manifestations of pediatric inflammatory bowel disease and their relation to disease type and severity. J Pediatr Gastroenterol Nutr. 51:140–5. doi: 10.1097/MPG.0b013e3181ca4db4. [DOI] [PubMed] [Google Scholar]
- 35.Jose FA, Garnett EA, Vittinghoff E, et al. Development of extraintestinal manifestations in pediatric patients with inflammatory bowel disease. Inflamm Bowel Dis. 2009;15:63–8. doi: 10.1002/ibd.20604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yacyshyn BR, Lazarovits A, Tsai V, et al. Crohn’s disease, ulcerative colitis, and normal intestinal lymphocytes express integrins in dissimilar patterns. Gastroenterology. 1994;107:1364–71. doi: 10.1016/0016-5085(94)90538-x. [DOI] [PubMed] [Google Scholar]