Abstract
We aimed to assess associations between clinical, imaging, pathological and genetic features and frontal lobe asymmetry in behavioral variant frontotemporal dementia (bvFTD). Volumes of the left and right dorsolateral, medial and orbital frontal lobes were measured in 80 bvFTD subjects and subjects were classified into three groups according to the degree of asymmetry (asymmetric left, asymmetric right, symmetric) using cluster analysis. The majority of subjects were symmetric (65%), with 20% asymmetric left and 15% asymmetric right. There were no clinical differences across groups, although there was a trend for greater behavioral dyscontrol in right asymmetric compared to left asymmetric subjects. More widespread atrophy involving the parietal lobe was observed in the symmetric group. Genetic features differed across groups with symmetric frontal lobes associated with C9ORF72 and tau mutations, while asymmetric frontal lobes were associated with progranulin mutations. These findings therefore suggest that neuroanatomical patterns of frontal lobe atrophy in bvFTD are influenced by specific gene mutations.
Keywords: Frontotemporal dementia, frontal lobes, MRI, asymmetry, microtubule associated protein tau, progranulin, C9ORF72, pathology
1. Introduction
Behavioral variant frontotemporal dementia (bvFTD) is a progressive neurodegenerative disorder characterized by behavioral and personality change (Neary et al., 1998). It is often associated with atrophy of the frontal lobes, although neuroanatomical subtypes have been identified with differing patterns of frontal, temporal, and parietal involvement (Whitwell et al., 2009). Frontal atrophy is generally regarded as being symmetric, or right-side predominant (Boccardi et al., 2002), yet a systematic assessment of asymmetry in bvFTD is lacking. The aims of this study therefore were to determine whether clinical, neuroanatomical, pathological and genetic features differ between 1) subjects with asymmetric left versus asymmetric right frontal lobe atrophy, and 2) subjects with asymmetric frontal lobe atrophy (right or left) versus those with symmetric frontal lobe atrophy.
2. Methods
We identified 97 subjects from the Mayo Clinic Alzheimer’s Disease Research Center (ADRC) with a clinical diagnosis of bvFTD (Neary et al., 1998) and MRI. These subjects were age and gender-matched to 30 healthy controls (mean ± standard deviation age at MRI=61.2±12.3 years, 63% female; compared to 60.6±11.8 years, 56% female in bvFTD cohort).
All subjects had a T1-weighted volumetric MRI performed with a standardized protocol (Whitwell et al., 2009). All images underwent correction for gradient non linearity and intensity non-uniformity. The first MRI after presentation was used in all cases. An atlas-based parcellation technique was employed using SPM5 and the automated anatomic labeling atlas in order to generate grey matter volumes for left and right medial frontal, dorsolateral frontal and orbitofrontal lobe (Whitwell et al., 2009). Z scores were calculated using total intracranial volume-corrected frontal lobe volumes for each bvFTD subject compared to controls. In order to ensure that all bvFTD subjects had atrophy of the frontal lobes, all cases with Z scores less than -1 (n=17) were excluded from the study. For the remaining 80 cases, hierarchical cluster analysis using average linkage method was performed using three variables of interest (difference between the left and right Z scores for medial frontal, dorsolateral frontal and orbitofrontal lobes) to classify subjects in an unbiased manner into groups according to the degree of frontal lobe asymmetry. Voxel-based morphometry (VBM) (Ashburner and Friston, 2000) using SPM5 was utilized to assess patterns of grey matter atrophy across clusters, using standard processing (Whitwell et al., 2009).
All subjects with a positive family history that had available DNA were screened for mutations in the microtubule-associated protein tau (MAPT) and progranulin (GRN) gene (Gass et al., 2006; Hutton et al., 1998). In addition, subjects were screened for the GGGGCC hexanucleotide repeat in C9ORF72 (Dejesus-Hernandez et al., 2011; Renton et al., 2011). Neuropathological examinations were performed according to standard protocol. All cases were reclassified based on recent consensus recommendations (Mackenzie et al., 2009).
3. Results
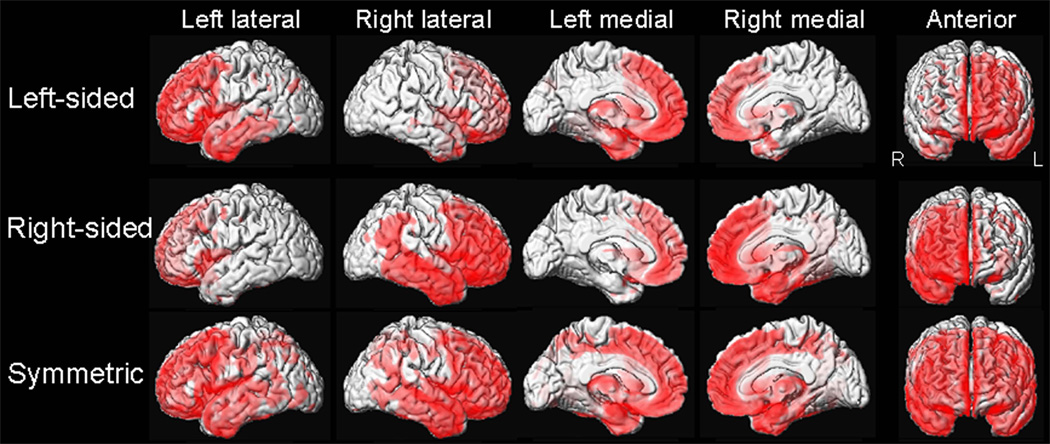
The cluster analysis divided the cohort into three groups: 20% were classified by greater frontal atrophy in the left hemisphere (asymmetric left), 15% by greater frontal atrophy in the right hemisphere (asymmetric right), and 65% by symmetric atrophy (Supplemental Table). VBM patterns of frontal atrophy matched the cluster classifications (Figure). However, the proportion of anatomical subtypes differed between asymmetric (asymmetric right and left groups combined) and symmetric bvFTD (p=0.001) (Table). The temporofrontoparietal subtype was most common in symmetric bvFTD, whereas frontotemporal and frontal dominant subtypes were most common in asymmetric bvFTD.
Figure.
Voxel-based morphometry patterns of grey matter loss in asymmetric left, asymmetric right and symmetric bvFTD vs. controls (family wise error corrected, p<0.05).
Table.
Demographic, clinical, imaging, pathological and genetic differences across groups
| Asymmetric left n=16 |
Asymmetric right n=12 |
Asymmetric n=28 |
Symmetric n=52 |
||
|---|---|---|---|---|---|
| Gender (% female) | 11 (69%) | 8 (67%) | 19 (68%) | 27 (52%) | |
| Age at MRI, yrs | 59.44 (13.00) | 56.83 (12.30) | 58.32 (10.48) | 61.87 (12.30) | |
| Age at onset, yrs | 55.88 (14.39) | 53.58 (12.73) | 54.89 (11.34) | 56.77 (12.73) | |
| Onset-MRI, yrs | 3.56 (5.10) | 3.25 (4.78) | 3.43 (2.97) | 5.10 (4.78) | |
| Education | 14.38 (2.87) | 14.33 (3.13) | 14.36 (2.83) | 15.24 (3.13) | |
| Handedness (Right: left: ambidextrous: unknown) | 15:0:0:1 | 11:0:1:0 | 26:0:1:1 | 47:2:0:3 | |
| Apolipoprotein e4 (%) | 3 (19%) | 2 (17%) | 5 (18%) | 15 (29%) | |
| CDR-SB (/18) | 5.73 (2.72) | 7.00 (4.10) | 6.30 (3.98) | 6.25 (4.10) | |
| Mini-mental State Exam (/30) | 22.64 (5.80) | 22.45 (6.20) | 22.56 (6.12) | 23.64 (6.20) | |
| NPI-Q total score | 7.80 (6.01) | 12.00 (6.25) | 9.67 (6.37) | 9.61 (4.73) | |
| Anatomical subtype‡ | |||||
| Frontal dominant (%) | 7 (44%) | 3 (25%) | 10 (36%) | 15 (29%) | |
| Frontotemporal (%) | 4 (25%) | 7 (58%) | 11 (39%) | 6 (11%) | |
| Temporal dominant (%) | 3 (19%) | 1 (8%) | 4 (14%) | 5 (10%) | |
| Temporofrontoparietal (%) | 2 (12%) | 1 (8%) | 3 (11%) | 26 (50%) | |
| Biochemical classification | |||||
| FTLD-Tau | 3 | 2 | 5 | 3 | |
| FTLD-TDP | 4 | 5 | 9 | 9 | |
| FTLD-FUS | 1 | 0 | 1 | 1 | |
| Alzheimer’s disease | 1 | 0 | 1 | 1 | |
| Genetic mutations*‡ | |||||
| C90RF72 | 1 | 0 | 1 | 10 | |
| MAPT | 5 | 1 | 6 | 10 | |
| Progranulin | 1 | 4 | 5 | 1 | |
Data shown as mean (standard deviation). CDR-SB = Clinical Dementia Rating sum of boxes; FTLD = frontotemporal lobar degeneration; TDP = TAR DNA binding protein 43; FUS = fused in sarcoma; MAPT = microtubule associated protein tau.
Significant difference between asymmetric and symmetric bvFTD at p=0.001.
Significant difference between asymmetric left and asymmetric right at p<0.05. Statistical comparisons were performed using Kruskall Wallis test for continuous variables and Chi-Squared/ Fishers exact test for categorical variables.
No demographic, clinical or pathological differences were observed across groups (Table). There was a trend for higher NPI-Q scores in asymmetric right compared to asymmetric left bvFTD (p=0.07). There were striking differences across groups in genetic associations. The majority of subjects with C9ORF72 mutations (10/11, 91%) and MAPT mutations (10/16, 63%) had symmetric frontal atrophy, while the majority of subjects with GRN mutations had asymmetric frontal atrophy (5/6, 83%). Of those MAPT and GRN subjects that were asymmetric, a right-sided pattern was more common in GRN while a left-sided pattern was more common in MAPT.
4. Discussion
This study assessed frontal lobe asymmetry in bvFTD subjects and found that the majority showed symmetric frontal lobe volumes. There were significant associations between genetic phenotypes and patterns of atrophy beyond the frontal lobes across asymmetric left, asymmetric right, and symmetric groups.
The finding that the majority of MAPT and C9ORF72 subjects had symmetric frontal atrophy, while the majority of GRN subjects had asymmetric atrophy, concurs with a recent study that has similarly observed symmetric patterns of atrophy in MAPT and C9ORF72, and more asymmetric patterns in GRN (Whitwell et al., 2012). It is unclear however why asymmetry is particularly associated with GRN mutations. Interestingly, whenever MAPT subjects were asymmetric they were most likely to be associated with left-sided asymmetry, while GRN was associated with right-sided asymmetry. These results suggest that specific gene mutations may target one hemisphere over the other. It is important to recognize however that this study only assessed subjects with bvFTD; clinical syndromes such as aphasia which target the left hemisphere have also been associated with GRN mutations (Pickering-Brown et al., 2008).
The proportion of different anatomical subtypes (Whitwell et al., 2009) also significantly differed across asymmetric and symmetric bvFTD, with symmetric subjects more likely to have the temporofrontoparietal subtype, and asymmetric subjects more likely to have frontotemporal or frontal dominant subtypes. This was also evident in the VBM findings which showed more parietal atrophy in symmetric bvFTD. There was no difference across groups in time from onset to MRI, and the symmetric subjects with the temporofrontoparietal subtype did not have a longer time from onset to MRI than symmetric subjects with the other anatomical subtypes; hence the greater extent of non-frontal atrophy in the symmetric subjects was not due to longer disease duration. Similarly, disease severity was no different across groups. Given the presence of parietal lobe involvement in symmetric bvFTD, one could postulate that the underlying pathology is Alzheimer’s disease or symmetric corticobasal degeneration (Hassan et al., 2010) in some subjects.
It was surprising that demographic and clinical measures did not differ between groups. This suggests that asymmetry does not greatly influence these features. There was a trend for greater behavioral severity in asymmetric right compared to asymmetric left bvFTD, suggesting an association between behavioral change and the right hemisphere. In fact, behavioral dyscontrol has been linked to right frontal (Rosen et al., 2005) and temporal (Thompson et al., 2003) atrophy.
The findings from this study better our understanding of the anatomical, clinical and genetic heterogeneity of bvFTD and demonstrate that C9ORF72 is associated with symmetric atrophy.
Supplementary Material
Acknowledgments
NIH grants (P50-AG16574, R01-AG11378, R01-AG037491, R01-NS65782). We acknowledge Matthew Baker, Mariely DeJesus-Hernandez and Nicola J. Rutherford for genetic sequencing.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure statement: The authors do not have any conflicts of interest.
References
- Ashburner J, Friston KJ. Voxel-based morphometry--the methods. Neuroimage. 2000;11:805–821. doi: 10.1006/nimg.2000.0582. [DOI] [PubMed] [Google Scholar]
- Boccardi M, Laakso MP, Bresciani L, Geroldi C, Beltramello A, Frisoni GB. Clinical characteristics of frontotemporal patients with symmetric brain atrophy. Eur Arch Psychiatry Clin Neurosci. 2002;252:235–239. doi: 10.1007/s00406-002-0388-z. [DOI] [PubMed] [Google Scholar]
- Dejesus-Hernandez M, Mackenzie IR, Boeve BF, Boxer AL, Baker M, Rutherford NJ, et al. Expanded GGGGCC Hexanucleotide Repeat in Noncoding Region of C9ORF72 Causes Chromosome 9p-Linked FTD and ALS. Neuron. 2011 doi: 10.1016/j.neuron.2011.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gass J, Cannon A, Mackenzie IR, Boeve B, Baker M, Adamson J, et al. Mutations in progranulin are a major cause of ubiquitin-positive frontotemporal lobar degeneration. Hum Mol Genet. 2006;15:2988–3001. doi: 10.1093/hmg/ddl241. [DOI] [PubMed] [Google Scholar]
- Hassan A, Whitwell JL, Boeve BF, Jack CR, Jr., Parisi JE, Dickson DW, et al. Symmetric corticobasal degeneration (S-CBD) Parkinsonism Relat Disord. 2010;16:208–214. doi: 10.1016/j.parkreldis.2009.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutton M, Lendon CL, Rizzu P, Baker M, Froelich S, Houlden H, et al. Association of missense and 5'-splice-site mutations in tau with the inherited dementia FTDP-17. Nature. 1998;393:702–705. doi: 10.1038/31508. [DOI] [PubMed] [Google Scholar]
- Mackenzie IR, Neumann M, Bigio EH, Cairns NJ, Alafuzoff I, Kril J, et al. Nomenclature for neuropathologic subtypes of frontotemporal lobar degeneration: consensus recommendations. Acta Neuropathol. 2009;117:15–18. doi: 10.1007/s00401-008-0460-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neary D, Snowden JS, Gustafson L, Passant U, Stuss D, Black S, et al. Frontotemporal lobar degeneration: a consensus on clinical diagnostic criteria. Neurology. 1998;51:1546–1554. doi: 10.1212/wnl.51.6.1546. [DOI] [PubMed] [Google Scholar]
- Pickering-Brown SM, Rollinson S, Du Plessis D, Morrison KE, Varma A, Richardson AM, et al. Frequency and clinical characteristics of progranulin mutation carriers in the Manchester frontotemporal lobar degeneration cohort: comparison with patients with MAPT and no known mutations. Brain. 2008;131:721–731. doi: 10.1093/brain/awm331. [DOI] [PubMed] [Google Scholar]
- Renton AE, Majounie E, Waite A, Simon-Sanchez J, Rollinson S, Gibbs JR, et al. A Hexanucleotide Repeat Expansion in C9ORF72 Is the Cause of Chromosome 9p21-Linked ALS-FTD. Neuron. 2011 doi: 10.1016/j.neuron.2011.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen HJ, Allison SC, Schauer GF, Gorno-Tempini ML, Weiner MW, Miller BL. Neuroanatomical correlates of behavioural disorders in dementia. Brain. 2005;128:2612–2625. doi: 10.1093/brain/awh628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson SA, Patterson K, Hodges JR. Left/right asymmetry of atrophy in semantic dementia: behavioral-cognitive implications. Neurology. 2003;61:1196–1203. doi: 10.1212/01.wnl.0000091868.28557.b8. [DOI] [PubMed] [Google Scholar]
- Whitwell JL, Przybelski SA, Weigand SD, Ivnik RJ, Vemuri P, Gunter JL, et al. Distinct anatomical subtypes of the behavioural variant of frontotemporal dementia: a cluster analysis study. Brain. 2009;132:2932–2946. doi: 10.1093/brain/awp232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitwell JL, Weigand SD, Boeve BF, Senjem ML, Gunter JL, Dejesus-Hernandez M, et al. Neuroanatomical signatures of frontotemporal dementia genetics: C9ORF72, tau, progranulin and sporadics. Brain. 2012 doi: 10.1093/brain/aws001. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.