Abstract
Background & Aims
Microparticles released into the bloodstream upon activation or apoptosis of CD4+ and CD8+ T cells correlate with inflammation, determined by histologic analysis, in patients with chronic hepatitis C (CHC). Patients with nonalcoholic fatter liver (NAFL) or nonalcoholic steatohepatitis (NASH) can be differentiated from those with CHC based on activation of distinct sets of immune cells in the liver.
Methods
We compared profiles of circulating microparticles from patients with NAFL and NASH (n=67) to those with CHC (n=42), compared with healthy individuals (controls) using flow cytometry; the profiles were correlated with inflammation grade and fibrosis stage, based on histologic analyses. We assessed the ability of the profiles determine the severity of inflammation and fibrosis, based on serologic and histologic analyses.
Results
Patients with CHC had increased levels of microparticles from CD4+ and CD8+ T cells; the levels correlated with disease severity, based on histologic analysis and levels of alanine aminotransferase (ALT). Patients with NAFL or NASH had significant increases in numbers of microparticles from invariant natural killer T (iNKT) cells and macrophages/monocytes (CD14+), which mediate pathogenesis of NASH. Microparticles from CD14+ and iNKT cells correlated with levels of ALT and severity of NASH (based on histology). Levels of microparticles could differentiate between patients with NAFL or NASH and those with CHC, or either group of patients and controls (area under the receiver operating characteristic curves ranging from 0.56 to 0.99).
Conclusions
Quantification of immune cell microparticles from serum samples can be used to assess the extent and characteristics of hepatic inflammation in patients with chronic liver disease.
Keywords: Biomarker, CD1c, CD4+, CD8+, CD14+, CD15+, CD41+, CD16+, ectosome, fibrosis, HCV, inflammation, iNKT, NKT, liver, macrophage, microparticle, monocyte, NASH, plasma, serum, T cell, non-invasive assay, lymphocyte, serum assay, biomarker assay
Introduction
Cell membrane-derived microparticles (MP) represent a novel route of horizontal communication between different cells. These MP are generated through a process of cell membrane blebbing (ectosome shedding) during cellular activation or early apoptosis in vitro and in vivo1. Of note, MP resemble their cell of origin on a smaller scale, with many parental cell characteristics, such as surface receptors, integral membrane and certain cytosolic proteins, some mRNAs and even miRNAs1. Thus MP may transfer complete cell surface receptor signaling pathways into the recipient cell that are specific for the MP releasing cell, exchange genetic information1, or transfer antigen via MHC class II molecules2. We have shown that T cell-derived MP could transfer CD147 (the matrix metalloproteinase inducer EMMPRIN) to hepatic stellate cells (HSC) leading to upregulation of fibrolytic matrix metalloproteinases (MMPs) in HSC, and that the process of MP fusion with HSC membranes was ICAM-1 dependent3. Others have demonstrated successful rescue of CXCR4 and CD81 deficient cells by transfer of MP carrying these surface receptors4, 5. Furthermore, biliary MP were shown to contain biologically active Hedgehog Ligand6.
However, data on the presence of inflammatory cell MP and their role in vivo are scarce. Thus, only the role of platelet (CD41+) derived MP was explored in some detail in vitro and in vivo, such as an elevation of CD41+ MP in synovial fluid and blood plasma of patients with rheumatoid arthritis7, HIV infection8, or severe malaria9.
We demonstrated that CD4+ and CD8+ T cell derived MP are elevated in the plasma of patients with chronic hepatitis C (CHC), a disease which is dominated by CD8+>CD4+ hepatic T cell infiltration, and that levels of these MP correlated with histological severity (grade and stage)3. Based on these data we reasoned that other liver diseases might have a disease specific MP signature. We therefore focused on patients with non-alcoholic fatty liver (NAFL) and included patients with simple steatosis (NAS score <3) or with NASH (NAS score >4). To this aim we studied an extended panel of MP surface markers, including CD14+ (monocytes/macrophages, and myeloid dendritic cells), CD15+ (neutrophils), CD41+ (platelets and endothelial cells) and Valpha24/Vbeta11 (iNKT cells). We found that indeed, MP derived from CD14+ and iNKT cells, two cell populations that have been implicated as being central to adipose tissue inflammation10, 11, were uniquely and characteristically elevated in patients with NAFL and further increased in patients with histologically severe NASH, but not in CHC. We conclude that circulating MP may qualify as a novel tool to quantify the underlying type and extent of inflammation in NAFL/NASH.
Material and Methods
Human study cohort
The Committee for Clinical Investigations (CCI) at the Beth Israel Deaconess Medical Center (BIDMC) approved the study and all patients gave their informed consent prior to participation. 42 patients with CHC, 67 patients with histologically proven NAFL or NASH and 44 healthy controls were enrolled. 10 of the CHC patients from our earlier study were included to increase the power of this group3. All patients were followed up in the BIDMC Liver Center and received physical exams, regular blood draws and a diagnostic liver biopsy as part of their standard care. Both patient cohorts had comparable age (55 and 49 years, respectively; p>0.05) and gender (Tab.1). Patients with a major second known comorbidity that could affect immune cell activation, such as HIV infection, autoimmune diseases or another hepatitis virus infection, were excluded. Patients with CHC were characterized as HCV antibody and RNA positive for more than 6 months, and patients with NAFL/NASH by standard clinical criteria confirmed by liver biopsy with the absence of other liver diseases12. The used criteria for assessing the health of the each participant were summarized in Suppl. Tab.1.
Isolation of T cell microparticles from human serum and plasma
From controls both plasma and serum was drawn at the same time point, in order to compare results for MP isolation between the two methods. Additionally, to assess changes in MP profiles in short-term follow up, healthy controls were subjected to serial blood sampling 7 days apart. For plasma, blood was collected in citrate containing tubes and for serum, blood was collected in standard vacutainers (both BD Vacutainer, BD Franklin Lakes, NJ, USA) and left for 1 hour at 37°C to allow to clot followed by centrifugation at 4000 rpm for 20 min at 4°C. Clot palettes were carefully separated and plasma or serum supernatans were stored at −80°C for further MP isolation. MP were isolated by differential centrifugation between 10,000 and 100,000 g as described3, and S100-MP sedimenting at 100,000 g were characterized by FACS using staining for Annexin V, CD1c, CD3, CD4, CD8, CD14, CD16, CD15, CD41, CD147, Valpha24/Vbeta11 (eBioscience™, San Diego, CA; BioLegend; Becton Dickinson, San Jose, CA). Notably, these surface markers were not described on exosomes13, another class of membrane coated vesicles. All antibodies were titrated against the matching isotype control prior use on patient's samples, as shown in detail in Suppl. Fig.1. MP preparations were characterized on a LSR2 FACS sorter (Becton Dickinson, San Jose, CA) and cytometric data was analyzed with FlowJo 8.8.6 software for MAC OSX (Tree Star, Inc., Ashland, Oregon). MP were gated on forward and sideward scatter (FSC and SSC). A detailed overview of our gating strategy is shown in Suppl. Fig.2. To avoid non-specific antibody binding, Fc receptors on MP and target cells were blocked with FcR Blocking Reagent (Miltenyi Biotec, Auburn, CA). Antibody solutions were centrifuged prior to FACS to avoid artifacts due to aggregation.
Liver histology
Liver biopsies were performed with an 18 gauge Menghini needle for clinical indications and encompassed at least 8 portal tracts. Biopsy specimens were formalin-fixed, paraffin-embedded, sectioned, and stained with hematoxylin and eosin, Masson's trichome, reticulin, and periodic acid-Schiff (PAS) stains. Two experienced histopathologists graded and staged the liver samples according to Metavir (CHC and NAFL/NASH)14 and the NAS score (NAFL/NASH)15. Only specimens predating the MP isolation from the patients' serum by no more than 12 months were used for comparisons.
Statistical analysis
All data are arithmetic means with SD. Differences between independent experimental groups (NAFL/NASH, CHC and controls) were characterized using the Kruskal Wallis test with subsequent pairwise comparisons and adjustment for multiple comparisons using Dunn's Multiple Comparison Test. Differences between plasma and serum MP levels were analyzed using the Mann Whitney test. A pairwise Pearson algorithm was used for correlation analysis of MP levels with blood cells, ALT, histological grade and stage, and the NAS score. Scatterplots of the pairwise data are presented with corresponding linear regression lines. To assess the predictive ability of the 6 MP populations (CD4+, CD8+, CD14+, CD15+, CD41+ and iNKT) for discriminating between individuals with CHC and NAFL/NASH, we calculated sensitivity, specificity and areas under the receiver operating characteristics (AUROC) curve. All calculations were done with Prism 5 (GraphPad Software, Inc., USA). An error level p<0.05 was considered significant.
Results
MP isolated from plasma and serum yield comparable results
We previously described MP from plasma3. In order to test if (stored) serum could be used for MP quantification as well, we compared matched plasma-serum pairs from a subset of patients and healthy controls. When comparing relative levels of S100-MP (in the following simply termed MP) populations in serum vs. plasma, both serum CD15+ (neutrophil) and CD41+ (platelet-derived) MP were reduced, the latter reported previously by others16, CD4+ MP were slightly decreased and CD8+ MP were significantly increased, whereas CD14+ and iNKT S100-MP remained unchanged. At present, we cannot explain these minor to moderate differences of certain MP populations in serum vs. plasma, but the profiling of those MP turned out not to be relevant for the present study on patients with NAFL and NASH, or their differentiation from CHC patients. We also found some variation in percentages of MP in some individuals, but overall this was not significant (Suppl. Fig.3A). Such changes may result from slight alterations of immune activation in individual patients, e.g., due to minor (sub-clinical) infection or by physical activity1, 17–19.
Additionally, we did not observe a significant correlation between blood cell counts and corresponding MP percentages, as demonstrated for monocytes (CD14+), neutrophils (CD15+) or platelets (CD41+) (see Suppl. Fig.3B). These results are in line with our previous findings in patients with CHC and support the hypothesis that it is activated cells within affected organs (such as the liver) that are the source of plasma membrane MP and not the majority of non-activated circulating blood cells3, 18
Patients with CHC and NAFL/NASH show characteristic MP profiles
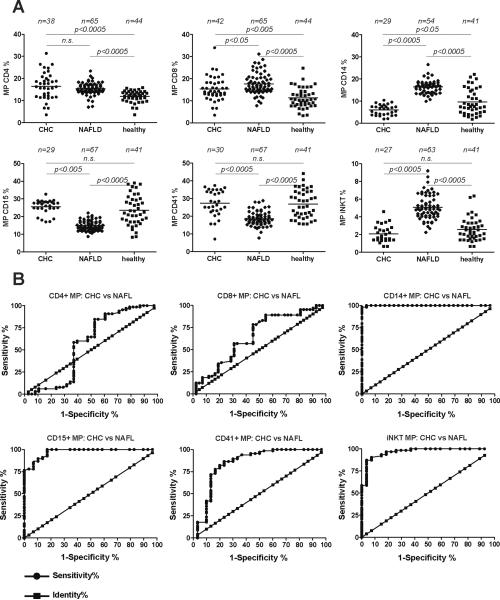
67 and 42 patients with NASH/NAFL and CHC, respectively, were included in the study (Tab.1). Microparticle results are summarized in Fig.2A and Suppl. Tab.2, and resp. AUROC curves are shown in Fig.2B and Suppl. Fig.4A and 4B. CHC and NAFL/NASH were associated with increased percentages of CD4+ MP compared to healthy controls (40% and 29%, respectively). Similarly, levels of CD8+ MP were significantly higher in NAFL/NASH (56%) and in CHC (26%) compared to healthy controls. However, CD4+ and CD8+ S100-MP did not discriminate between CHC and NAFL/NASH, with low AUROC values of 0.71 and 0.59, respectively (Suppl.Tabl.2). In contrast, the exclusive elevation of CD14+ and iNKT MP in NAFL/NASH compared to CHC led to AUROC values of >0.99 and 0.97, respectively. At cutoffs of 9.7% and 3.6%, resp., we observed sensitivity and specificity values >87% for differentiation from CHC. Of, note these two cell populations have recently been implicated as being central to NAFL/NASH pathogenesis11, 20. In NAFL/NASH CD15+ and CD41+ MP levels were reduced significantly by 42% and 32%, respectively, compared to CHC. This reduction for CD15+ and CD41+ MP percentages was also associated with a high specificity score >96%, but with lower sensitivity scores of 78% and 18%, resp.
Figure 2. Gross overview/profile of the percentages of different S100-MP populations in patients with non-alcoholic fatty liver (NAFL), chronic hepatitis C (CHC) and in healthy controls.
(A) MP were isolated by differential centrifugation and analyzed by FACS as described in Materials and Methods. The overall p-value for each MP population for the Kruskal-Wallis test was set at p<0.0001 before assessing pairwise relationships by the post-hoc Dunn's multiple comparisons approach to compare the 3 study cohorts (CHC, NAFL/NASH and healthy controls). (B) AUROC curves were created using those cut-off values that yielded the highest likelihood to differentiate between CHC, NAFL and healthy controls (see also Suppl. Tab.1). n, number of patients in each MP analysis. Occasional missing data points are due to limitation of serum. 10 additional patients with CHC for whom only CD4+ and CD8+ MP were available were included (see Table 1).
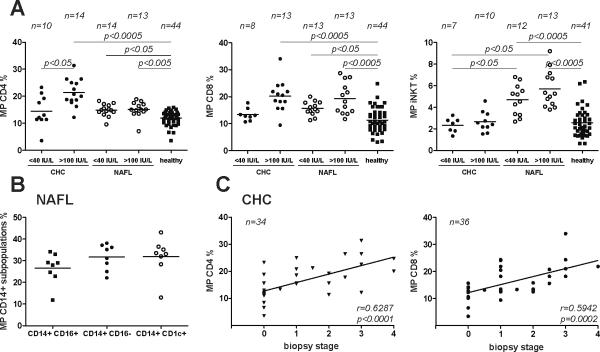
Fig.3A shows a MP analysis for the extremes of ALT values of the two liver disease cohorts focusing on T cell and iNKT MP. Here, we confirmed our earlier finding that patients with CHC and an ALT >100 IU/L (termed active) had significantly elevated levels of circulating CD4+ MP as compared to CHC patients with ALT <40 IU/L (termed mild) and to healthy controls. In contrast, NAFL/NASH patients with both low and high ALT levels were characterized by only a minor elevation of CD4+ MP (28%) compared to healthy controls, though this was statistically significant. CD8+ MP were also increased in NAFL/NASH, especially in the high ALT group, similar to serologically active vs. inactive CHC.
Figure 3. MP levels in relation to normal and high ALT values and biopsy stage, and analysis CD14+ MP subsets.
(A) MP levels in CHC or NAFL/NASH patients with normal ALT values (<40 IU/mL, numbers as indicated) or high ALT values (>100 IU/mL, numbers as indicated) as a surrogate of hepatic inflammation, and in comparison to S100-MP populations from healthy controls. The overall p-value for each MP population for the Kruskal-Wallis test was set at p<0.0001 before assessing pairwise relationships by the post-hoc Dunn's multiple comparisons approach to compare the 3 study cohorts (CHC, NAFL/NASH and healthy controls). (B) Analysis of CD14+ S100-MP subpopulations in a representative cohort of NAFL/NASH patients. CD14+ CD16−: “classically activated” monocytes; CD14+CD16+: “non classically activated, inflammatory” monocytes, CD14+CD1c+: myeloid DCs. (C) In CHC CD4+ and CD8+ MP correlated well with fibrosis stage as described by us before in a smaller cohort3 (r=0.63, p<0.0001; r=0.59,p=0.0002, respectively). However, neither CD4+, nor CD8+, CD14+ or iNKT MP levels correlated with stage in NAFL/NASH (data not shown). Variations in numbers are due to limitation of serum.
There was only a non-significant (p>0.05) increase of iNKT MP in CHC, either with low or with high ALT values, vs. healthy controls. However, in all patients with NAFL/NASH iNKT MP were strikingly elevated (NAFL/NASH with high ALT (>100 IU/L): by 124% vs. normal controls; NAFL/NASH with ALT <100 IU/L): by 114% vs. CHC with high ALT).
CD14+ MP subgroup analysis in NASH
Recently, a link between chronic liver disease progression and CD14+ CD16− and CD14+CD16+ cells was reported21. In a representative cohort of NAFL/NASH patients (ALT range 31–109 IU/L) we found comparable percentages of MP from CD14+ CD16− (classical), CD14+CD16+ (non-classical, proinflammatory) monocytes and CD14+CD1c+ myeloid dendritic cells (Fig.3B).
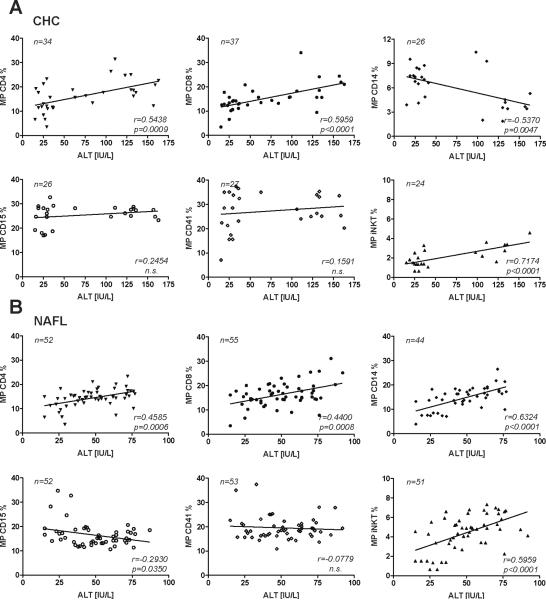
Correlation between ALT and MP in patients with NAFL/NASH and CHC
Correlations are shown in Fig.4. As expected, CD4+ and CD8+ MP correlated slightly better with ALT levels in patients with CHC (Fig.4A) as compared to NAFL/NASH (Fig.4B). However, the good correlations for all MP subpopulations with ALT in NAFL/NASH was lost when patients with ALT>80 IU/ml were included (data not shown) and improved with ALT<80 IU/L. The best correlations were found between ALT levels of patients with NAFL/NASH and their circulating CD14+ and iNKT MP (r=0.63, p<0.0001; r=0.59, p=0.0001). Interestingly, for CD8+, CD15+ and CD41+ MP no clinically relevant correlations were found (r<0.5), although the correlations for CD8+ and CD15+ MP were statistically significant. Subanalysis ruled out a gender effect for all correlations (data not shown).
Figure 4. Correlations of circulating S100-MP with ALT.
Correlations of CD4+, CD8+, CD14+ and iNKT S100-MP with patients' ALT values from blood samples used simultaneously for S100-MP isolation and ALT determination. (A) CHC, (B) NAFL/NASH. Correlations were calculated using the Pearson algorithm, with r-values and p-values shown in the lower right corner of each graph. Variations in numbers are due to limitation of serum.
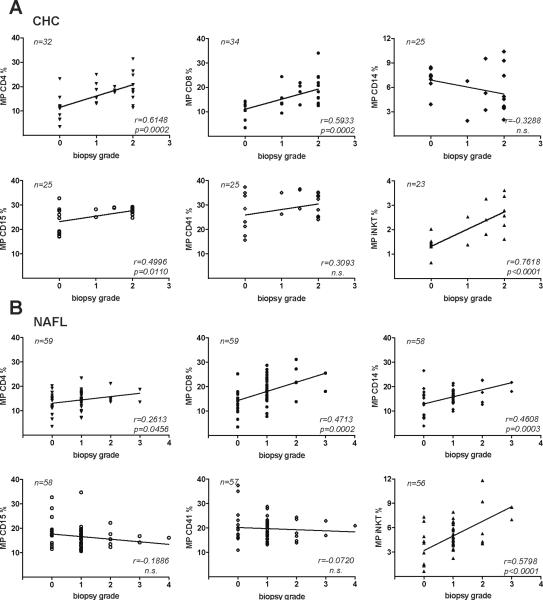
Circulating MP as predictors of histological grade in CHC and NAFL/NASH
We confirmed our prior findings of a good correlation between circulating CD4+ and CD8+ MP and histological inflammation grade in CHC3. Moreover, iNKT MP correlated even better with histological grade in patients with CHC than CD4+ and CD8+ MP (r=0.76, p<0.0001, Fig.5A). Though CD14+, CD15+ and CD41+ MP did statistically correlate with grade in CHC, this was considered clinically irrelevant (r<0.5). In NAFL/NASH, iNKT MP correlated well with histological grade (r=0.58, p<0.0001), and this correlation must be considered as clinically relevant (Fig.5B). Other correlations, even when statistically significant, turned out to be clinically irrelevant (r<0.5).
Figure 5. Correlations of circulating S100-MP with histological grade.
S100-MP populations were isolated from the serum of CHC (A) or NAFL/NASH patients (B) with paired biopsies. Grading was done as detailed in Material and Methods. Correlations were calculated using Pearson's algorithm. Variations in numbers are due to limitation of serum.
Correlation between biopsy stage and MP in patients with NAFL/NASH and CHC
In CHC CD4+ and CD8+ MP correlated well with fibrosis stage as described by us before in a smaller cohort3 (r=0.63, p<0.0001; r=0.59,p=0.0002, respectively, Fig.3C). However, neither CD4+, nor CD8+, CD14+ or iNKT MP levels correlated well with stage in NAFL/NASH (data not shown). Moreover, no correlations, neither for CHC nor for NAFL/NASH, were found with circulating CD15+ and CD41+ MP (data not shown).
Circulating MP as predictors of the histological severity in NAFL/NASH
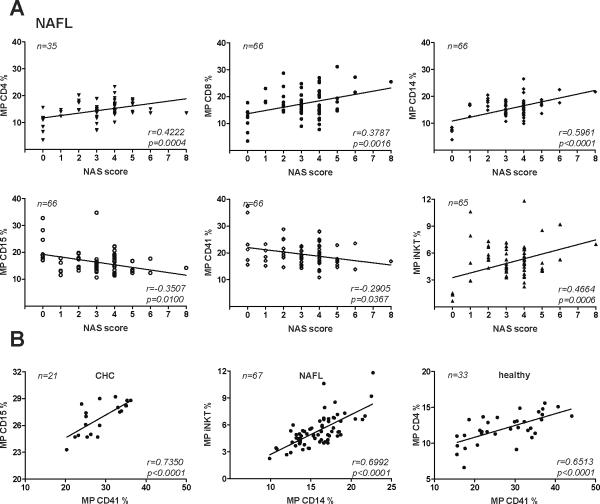
MP were correlated with the NAS score, currently considered the gold standard for the assessment of the severity of inflammation and apoptosis in NAFL/NASH (Fig.6A). Though statistically significant, CD4+ and CD8+ MP correlated only weakly (therefore lacking clinical relevance) with the NAS score (r=0.42, p=0.0004; r=0.38, p=0.0016, resp.), as did iNKT MP (r=0.47, p=0.0006). As previously noted, CD14+ MP, derived from cells that to play a particular role in NASH pathogenesis correlated best with the NAS score (r=0.60, p<0.0001;).
Figure 6. Correlations of circulating S100-MP populations with the NAS score and between MP populations.
(A) Correlations of CD4+, CD8+, CD14+, CD15+, CD41+ and iNKT (Valpha24/Vbeta11 double positive) S100-MP with the NAS score using the Pearson algorithm. (B) The strongest correlations between S100-MP subsets for each liver disease are shown. The strong correlation between CD14+ and iNKT S100-MP supports a tight functional link between these inflammatory cell types in NAFL/NASH. Variations in numbers are due to limitation of serum.
Notably, CD14+ S100-MP correlated strongly with iNKT MP in all patients with NAFL/NASH (r=0.7, p<0.0001) (Fig.6B), far exceeding correlations between all other combinations, thus supporting the hypothesis that these two MP populations are not only uniquely released in NAFL, but also linked in pathogenesis.
Discussion
Cell-derived MP are recently discovered vehicles of intercellular communication and emerging tools to quantitate cell-specific pathological processes. Several publications describe MP shedding in inflammatory conditions, such as malaria (platelet, red blood cell and monocyte derived MP)9, heart failure (endothelium derived MP)22, arthritis7, HIV infection8, end stage renal failure23, in coagulation disorders (platelet-derived MP)24, and even after moderate exercise19. We recently showed that T cell S100-MP, which are found at increased levels in the plasma of patients with CHC, can fuse with HSC plasma membranes via ICAM-1 and consequently upregulate HSC fibrolytic gene expression by transfer of CD147, suggesting a possible functional role of inflammatory cell MP in chronic liver diseases3.
The aim of this study was to explore if two prevalent, but mechanistically different chronic liver diseases, CHC and NAFL/NASH, can be distinguished by their S100-MP profiles, if these profiles are biologically plausible and if they could serve as novel plasma or serum biomarkers of disease activity.
Typically hepatic fibrosis progression and acute hepatitis requires activation of various immunological competent cells, such as T cells, NKT cells, DCs, macrophages (Kupffer cells)25. Since activation or early apoptosis of cells can result in shedding of MP, levels of certain MP originating from different cell types can be measured by FACS in healthy subjects and in patients with different diseases1, 3, 18.
By analyzing serum MP for 6 cell surface markers representing major immune cell populations that are involved in hepatic inflammation and fibrogenesis (CD4+/8+ T cells, CD14+ monocytes/macrophages/DCs, CD15+ neutrophils, CD41+ platelets and Valpha24/Vbeta11 positive iNKT cells3, 7, 9, 18), we could identify S100-MP profiles that are highly characteristic for either NAFL/NASH or CHC. Thus CHC was dominated by CD8+ and CD4+ MP, whereas NAFL/NASH patients showed a unique elevation of CD14+ and iNKT MP and a decrease in CD15+ and CD41+ S100-MP, irrespective of ALT levels or histological markers of disease activity. The S100-MP patterns allowed an almost complete separation of patients with CHC or NASH, and healthy controls.
These findings are not only in excellent agreement with the pathophysiology of both diseases, but also reveal novel insights into disease pathogenesis, as published in recent studies. First, the homing of circulating immune cells to the liver and their turnover increases during hepatic inflammation, likely increasing circulating MP released during their activation or apoptosis1. Second, CD8+>CD4+ Tells26, as well as NKT cell populations27, including iNKT cells28 are major immune effectors in CHC, although histological inflammation of the liver appears to be reflected better by iNKT MP than by blood iNKT cells27, 28. Third, iNKT cells have been implicated as major drivers of inflammation and fibrosis progression in NASH20, 29. Fourth, CD14+ macrophages>monocytes appear to play a prominent role in peripheral adipose tissue inflammation, the associated metabolic syndrome30, 31, and hepatic necroinflammation32. In the latter context, “inflammatory” CD14+ CD16+ monocytes are linked to disease progression and fibrogenic activation of HSCs21, as has been shown for a variety of inflammatory diseases including rheumatoid arthritis, diabetes, atherosclerosis and bacterial infections33. Our CD14+ S100-MP subgroup analysis revealed that CD14+ S100-MP in NAFL/NASH originated in equal proportions from CD16- (classical monocytes), CD1c+ (myeloid DCs), and CD16+ (inflammatory monocytes). Since the latter cell population is lower in normal subjects (7.5%), the elevated count of CD14+CD16+ S100-MP in patients with NAFL/NASH indicates activation of inflammatory monocytes/macrophages21. Furthermore, there was an excellent correlation between CD14+ and iNKT MP in patients with NAFL/NASH, which underscores a functional link between activation of these immune cells in patients with fatty liver and NASH.
While inflammatory cell S100-MP correlated with ALT levels and histological staging/grading, these correlations were limited. This is expected, since ALT is a suboptimal surrogate of hepatic inflammation, apoptosis or necrosis34, and biopsy is a tarnished gold standard due to high sampling variability35–37. In CHC, the disease with the lowest expected sampling variability (~25 and 33% for 1 Metavir grade and stage difference, respectively37) there was a good correlation of CD4+ or CD8+ MP with ALT and biopsy grade and stage. In NAFL/NASH, which incurs a higher biopsy sampling variability (~40% for 1 Metavir grade or stage,38), CD14+ and iNKT MP only correlated well with ALT up to 80 IU/L, but poorly with fibrosis stage. The histological NAS score, considered the gold standard for the diagnosis of NASH, showed only a modest correlation with CD14+ and iNKT MP. While a positive NAS score >4 renders the best evidence for NASH, a borderline or negative score does not exclude its presence, since this score that consists of three subscores for hepatic steatosis, lobular inflammation, and ballooning, is affected by significant sampling variability, especially for detection of hepatocellular ballooning39, which is missed in at least 50% of biopsies38. Additionally, due to its multicomponent character, the NAS score is influenced by the skill and experience of the reading pathologist, more than other histological scores, and thus prone to intra- and inter-observer variability15, 40. In contrast, a quantitative diagnostic test that can measure overall activation of a certain immune cell subset, such as circulating MP, may circumvent biopsy sampling and observer variabilities. Furthermore, it may rather reflect current disease activity compared to a more static picture as reflected by biopsy assessment, permitting e.g. the “real time” monitoring of disease specific anti-inflammatory therapies. However, at present we cannot present evidence that S100-MP quantification is superior to state of the art diagnostics for NAFL/NASH, which will require future large prospective and follow up studies. It will be particularly interesting to follow up MP profiles in patients with NASH who undergo treatment with e.g. insulin sensitizers, antioxidants, or who begin to favorably change their life style41–43.
In conclusion, 1) by analyzing circulating S100-MP a systemic profile of immune cell subsets that are prominently involved in CHC or NAFL/NASH can be obtained; 2) MP profiling corroborates the in vivo significance of pathophysiological hypotheses on immune mediated liver diseases; 3) as shown here for CHC and NAFL/NASH, S100-MP appear to represent a novel diagnostic tool to assess overall disease severity and especially activity, with the advantage of being specific, noninvasive and quantitative.
Supplementary Material
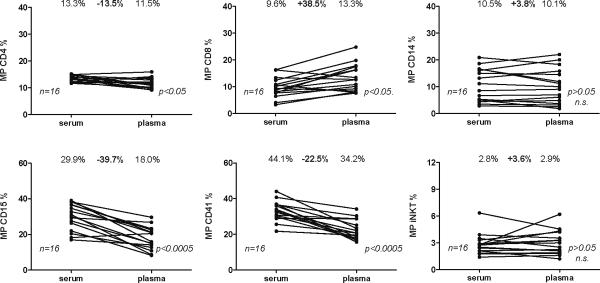
Figure 1. Comparison of S100-MP determinations from serum and plasma.
FACS analysis revealed that both fresh plasma and stored serum samples can be used reliably to determine the levels of CD4+, CD14+ and iNKT MP. In agreement with previous reports, levels of platelet-derived MP (CD41+) were significantly decreased in serum16. n indicates the number of serum/plasma pairs, the bold number the difference (in percent) between the means (not bold) of the measured MP population in serum vs. plasma. Differences (percent in bold) between serum and plasma were calculated using the following formula: (mean plasma MP – mean serum MP)/ mean serum MP.
Table 1.
Summary of demographic, biochemical and histological parameters of patients with CHC and NAFL/NASH.
| Column1 | CHC | NAFL total | NAS score 0–3 | NAS score 4–8 |
|---|---|---|---|---|
| patients [#] | 42 | 67 | 33 | 34 |
| female [%] | 31% (13) | 38.8% (26) | 36.4% (12) | 41.2% (14) |
| male [%] | 69% (29) | 61.2% (41) | 64.6% (21) | 58.8% (20) |
| age [years] | 55.1 (30–81) | 48.7 (28–73) | 47.9 (28–73) | 49.4 (31–68) |
| ALT [IU/L] | 89.7 (16–291) | 70.1 (13–201) | 64.3 (13–201) | 75.8 (36–165) |
| biopsy grade | 1.63 (0–3) | 48 (0–4) | 26 (0–2) | 22 (1–4) |
| biopsy stage | 1.61 (0–4) | 1.07 (0–4) | 0.39 (0–4) | 1.76 (0–4) |
| NAS score | n.a. | 3.46 (0–8) | 2.45 (0–8) | 4.5 (0–8) |
| african-american [%] | 4.8 | 3.1 | 6.5 | 0 |
| asian [%] | 4.8 | 10.8 | 12.9 | 8.8 |
| caucasion [%] | 83.2 | 69.2 | 61.2 | 76.5 |
| hispanic [%] | 4.8 | 10.8 | 6.5 | 14.7 |
| others [%] | 2.4 | 6.2 | 12.9 | 0 |
#, total number of patients in each cohort; %, percentage of gender distribution (absolute numbers in parenthesis); age is given as means (plus range) and ALT as means in IU/L (plus range); Bx (biopsy) grade and stage, as well as NAS score are shown as means (and their respective ranges). NAFL/NASH patients were subdivided according to their NAS score as indicated.
Acknowledgements
This work was supported by NIDDK grant 1 R21 DK075857-01A2 to D.S., CA143748 to M.E., and DFG fellowship grant KO4103/1-1 to M.K.. Part of this work was presented as oral presentation by MK during the EASL annual meeting 2011 in Berlin, Germany, and as poster at DDW2011, Chicago, USA.
Abbreviations
- ALT
alanine aminotransferase
- CHC
chronic hepatitis C
- MP
microparticle
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Authorship Contributions M.K. designed and did all experiments, analyzed and interpreted data and contributed to the writing of the manuscript; M.Ly., S.M., M.La., M.E. and N.H.A. contributed tools, discussed and interpreted results and helped to edit the manuscript; M.K. and D.S. developed the concept of the study and obtained research funding. D.S. supervised the project, designed and interpreted experiments and wrote the manuscript.
Conflict of Interest There are no conflicts of interest.
REFERENCES
- 1.Beyer C, Pisetsky DS. The role of microparticles in the pathogenesis of rheumatic diseases. Nat Rev Rheumatol. 2010;6:21–9. doi: 10.1038/nrrheum.2009.229. [DOI] [PubMed] [Google Scholar]
- 2.Thery C, Ostrowski M, Segura E. Membrane vesicles as conveyors of immune responses. Nat Rev Immunol. 2009;9:581–93. doi: 10.1038/nri2567. [DOI] [PubMed] [Google Scholar]
- 3.Kornek M, Popov Y, Libermann TA, Afdhal NH, Schuppan D. Human T cell microparticles circulate in blood of hepatitis patients and induce fibrolytic activation of hepatic stellate cells. Hepatology. 2010 doi: 10.1002/hep.23999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fritzsching B, Schwer B, Kartenbeck J, Pedal A, Horejsi V, Ott M. Release and intercellular transfer of cell surface CD81 via microparticles. J Immunol. 2002;169:5531–7. doi: 10.4049/jimmunol.169.10.5531. [DOI] [PubMed] [Google Scholar]
- 5.Rozmyslowicz T, Majka M, Kijowski J, Murphy SL, Conover DO, Poncz M, Ratajczak J, Gaulton GN, Ratajczak MZ. Platelet- and megakaryocyte-derived microparticles transfer CXCR4 receptor to CXCR4-null cells and make them susceptible to infection by X4-HIV. AIDS. 2003;17:33–42. doi: 10.1097/00002030-200301030-00006. [DOI] [PubMed] [Google Scholar]
- 6.Witek RP, Yang L, Liu R, Jung Y, Omenetti A, Syn WK, Choi SS, Cheong Y, Fearing CM, Agboola KM, Chen W, Diehl AM. Liver cell-derived microparticles activate hedgehog signaling and alter gene expression in hepatic endothelial cells. Gastroenterology. 2009;136:320–330. e2. doi: 10.1053/j.gastro.2008.09.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boilard E, Nigrovic PA, Larabee K, Watts GF, Coblyn JS, Weinblatt ME, Massarotti EM, Remold-O'Donnell E, Farndale RW, Ware J, Lee DM. Platelets amplify inflammation in arthritis via collagen-dependent microparticle production. Science. 2010;327:580–3. doi: 10.1126/science.1181928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Corrales-Medina VF, Simkins J, Chirinos JA, Serpa JA, Horstman LL, Jy W, Ahn YS. Increased levels of platelet microparticles in HIV-infected patients with good response to highly active antiretroviral therapy. J Acquir Immune Defic Syndr. 2010;54:217–8. doi: 10.1097/QAI.0b013e3181c8f4c9. [DOI] [PubMed] [Google Scholar]
- 9.Pankoui Mfonkeu JB, Gouado I, Fotso Kuate H, Zambou O, Amvam Zollo PH, Grau GE, Combes V. Elevated cell-specific microparticles are a biological marker for cerebral dysfunctions in human severe malaria. PLoS One. 2010;5:e13415. doi: 10.1371/journal.pone.0013415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Day CP. From fat to inflammation. Gastroenterology. 2006;130:207–10. doi: 10.1053/j.gastro.2005.11.017. [DOI] [PubMed] [Google Scholar]
- 11.Karlmark KR, Weiskirchen R, Zimmermann HW, Gassler N, Ginhoux F, Weber C, Merad M, Luedde T, Trautwein C, Tacke F. Hepatic recruitment of the inflammatory Gr1+ monocyte subset upon liver injury promotes hepatic fibrosis. Hepatology. 2009;50:261–74. doi: 10.1002/hep.22950. [DOI] [PubMed] [Google Scholar]
- 12.Vuppalanchi R, Chalasani N. Nonalcoholic fatty liver disease and nonalcoholic steatohepatitis: Selected practical issues in their evaluation and management. Hepatology. 2009;49:306–17. doi: 10.1002/hep.22603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mathivanan S, Simpson RJ. ExoCarta: A compendium of exosomal proteins and RNA. Proteomics. 2009;9:4997–5000. doi: 10.1002/pmic.200900351. [DOI] [PubMed] [Google Scholar]
- 14.Poynard T, Bedossa P, Opolon P. Natural history of liver fibrosis progression in patients with chronic hepatitis C. The OBSVIRC, METAVIR, CLINIVIR, and DOSVIRC groups. Lancet. 1997;349:825–32. doi: 10.1016/s0140-6736(96)07642-8. [DOI] [PubMed] [Google Scholar]
- 15.Kleiner DE, Brunt EM, Van Natta M, Behling C, Contos MJ, Cummings OW, Ferrell LD, Liu YC, Torbenson MS, Unalp-Arida A, Yeh M, McCullough AJ, Sanyal AJ, Nonalcoholic Steatohepatitis Clinical Research N Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology. 2005;41:1313–21. doi: 10.1002/hep.20701. [DOI] [PubMed] [Google Scholar]
- 16.George JN, Thoi LL, McManus LM, Reimann TA. Isolation of human platelet membrane microparticles from plasma and serum. Blood. 1982;60:834–40. [PubMed] [Google Scholar]
- 17.Burnier L, Fontana P, Kwak BR, Angelillo-Scherrer A. Cell-derived microparticles in haemostasis and vascular medicine. Thromb Haemost. 2009;101:439–51. [PubMed] [Google Scholar]
- 18.Roos MA, Gennero L, Denysenko T, Reguzzi S, Cavallo G, Pescarmona GP, Ponzetto A. Microparticles in physiological and in pathological conditions. Cell Biochem Funct. 2010;28:539–48. doi: 10.1002/cbf.1695. [DOI] [PubMed] [Google Scholar]
- 19.Sossdorf M, Otto GP, Claus RA, Gabriel HH, Losche W. Cell-derived microparticles promote coagulation after moderate exercise. Med Sci Sports Exerc. 2011;43:1169–76. doi: 10.1249/MSS.0b013e3182068645. [DOI] [PubMed] [Google Scholar]
- 20.Syn WK, Oo YH, Pereira TA, Karaca GF, Jung Y, Omenetti A, Witek RP, Choi SS, Guy CD, Fearing CM, Teaberry V, Pereira FE, Adams DH, Diehl AM. Accumulation of natural killer T cells in progressive nonalcoholic fatty liver disease. Hepatology. 2010;51:1998–2007. doi: 10.1002/hep.23599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zimmermann HW, Seidler S, Nattermann J, Gassler N, Hellerbrand C, Zernecke A, Tischendorf JJ, Luedde T, Weiskirchen R, Trautwein C, Tacke F. Functional contribution of elevated circulating and hepatic non-classical CD14CD16 monocytes to inflammation and human liver fibrosis. PLoS One. 2010;5:e11049. doi: 10.1371/journal.pone.0011049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nozaki T, Sugiyama S, Sugamura K, Ohba K, Matsuzawa Y, Konishi M, Matsubara J, Akiyama E, Sumida H, Matsui K, Jinnouchi H, Ogawa H. Prognostic value of endothelial microparticles in patients with heart failure. Eur J Heart Fail. 2010;12:1223–8. doi: 10.1093/eurjhf/hfq145. [DOI] [PubMed] [Google Scholar]
- 23.Amabile N, Guerin AP, Leroyer A, Mallat Z, Nguyen C, Boddaert J, London GM, Tedgui A, Boulanger CM. Circulating endothelial microparticles are associated with vascular dysfunction in patients with end-stage renal failure. J Am Soc Nephrol. 2005;16:3381–8. doi: 10.1681/ASN.2005050535. [DOI] [PubMed] [Google Scholar]
- 24.Falati S, Liu Q, Gross P, Merrill-Skoloff G, Chou J, Vandendries E, Celi A, Croce K, Furie BC, Furie B. Accumulation of tissue factor into developing thrombi in vivo is dependent upon microparticle P-selectin glycoprotein ligand 1 and platelet P-selectin. J Exp Med. 2003;197:1585–98. doi: 10.1084/jem.20021868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hernandez-Gea V, Friedman SL. Pathogenesis of Liver Fibrosis. Annu Rev Pathol. 2010 doi: 10.1146/annurev-pathol-011110-130246. [DOI] [PubMed] [Google Scholar]
- 26.Spengler U, Nattermann J. Immunopathogenesis in hepatitis C virus cirrhosis. Clin Sci (Lond) 2007;112:141–55. doi: 10.1042/CS20060171. [DOI] [PubMed] [Google Scholar]
- 27.Durante-Mangoni E, Wang R, Shaulov A, He Q, Nasser I, Afdhal N, Koziel MJ, Exley MA. Hepatic CD1d expression in hepatitis C virus infection and recognition by resident proinflammatory CD1d-reactive T cells. J Immunol. 2004;173:2159–66. doi: 10.4049/jimmunol.173.3.2159. [DOI] [PubMed] [Google Scholar]
- 28.Lucas M, Gadola S, Meier U, Young NT, Harcourt G, Karadimitris A, Coumi N, Brown D, Dusheiko G, Cerundolo V, Klenerman P. Frequency and phenotype of circulating Valpha24/Vbeta11 double-positive natural killer T cells during hepatitis C virus infection. J Virol. 2003;77:2251–7. doi: 10.1128/JVI.77.3.2251-2257.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Park O, Jeong WI, Wang L, Wang H, Lian ZX, Gershwin ME, Gao B. Diverse roles of invariant natural killer T cells in liver injury and fibrosis induced by carbon tetrachloride. Hepatology. 2009;49:1683–94. doi: 10.1002/hep.22813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Odegaard JI, Chawla A. Mechanisms of macrophage activation in obesity-induced insulin resistance. Nat Clin Pract Endocrinol Metab. 2008;4:619–26. doi: 10.1038/ncpendmet0976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bouloumie A, Curat CA, Sengenes C, Lolmede K, Miranville A, Busse R. Role of macrophage tissue infiltration in metabolic diseases. Curr Opin Clin Nutr Metab Care. 2005;8:347–54. doi: 10.1097/01.mco.0000172571.41149.52. [DOI] [PubMed] [Google Scholar]
- 32.Xu H, Barnes GT, Yang Q, Tan G, Yang D, Chou CJ, Sole J, Nichols A, Ross JS, Tartaglia LA, Chen H. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J Clin Invest. 2003;112:1821–30. doi: 10.1172/JCI19451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ziegler-Heitbrock L. The CD14+ CD16+ blood monocytes: their role in infection and inflammation. J Leukoc Biol. 2007;81:584–92. doi: 10.1189/jlb.0806510. [DOI] [PubMed] [Google Scholar]
- 34.Green RM, Flamm S. AGA technical review on the evaluation of liver chemistry tests. Gastroenterology. 2002;123:1367–84. doi: 10.1053/gast.2002.36061. [DOI] [PubMed] [Google Scholar]
- 35.Ratziu V, Charlotte F, Heurtier A, Gombert S, Giral P, Bruckert E, Grimaldi A, Capron F, Poynard T. Sampling variability of liver biopsy in nonalcoholic fatty liver disease. Gastroenterology. 2005;128:1898–906. doi: 10.1053/j.gastro.2005.03.084. [DOI] [PubMed] [Google Scholar]
- 36.Schuppan D, Afdhal NH. Liver cirrhosis. Lancet. 2008;371:838–51. doi: 10.1016/S0140-6736(08)60383-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Regev A, Berho M, Jeffers LJ, Milikowski C, Molina EG, Pyrsopoulos NT, Feng ZZ, Reddy KR, Schiff ER. Sampling error and intraobserver variation in liver biopsy in patients with chronic HCV infection. Am J Gastroenterol. 2002;97:2614–8. doi: 10.1111/j.1572-0241.2002.06038.x. [DOI] [PubMed] [Google Scholar]
- 38.Ratziu V, Charlotte F, Heurtier A, Gombert S, Giral P, Bruckert E, Grimaldi A, Capron F, Poynard T, Group LS. Sampling variability of liver biopsy in nonalcoholic fatty liver disease. Gastroenterology. 2005;128:1898–906. doi: 10.1053/j.gastro.2005.03.084. [DOI] [PubMed] [Google Scholar]
- 39.Wieckowska A, Feldstein AE. Diagnosis of nonalcoholic fatty liver disease: invasive versus noninvasive. Semin Liver Dis. 2008;28:386–95. doi: 10.1055/s-0028-1091983. [DOI] [PubMed] [Google Scholar]
- 40.Younossi ZM, Gramlich T, Liu YC, Matteoni C, Petrelli M, Goldblum J, Rybicki L, McCullough AJ. Nonalcoholic fatty liver disease: assessment of variability in pathologic interpretations. Mod Pathol. 1998;11:560–5. [PubMed] [Google Scholar]
- 41.Dowman JK, Armstrong MJ, Tomlinson JW, Newsome PN. Current therapeutic strategies in non-alcoholic fatty liver disease. Diabetes Obes Metab. 2011 doi: 10.1111/j.1463-1326.2011.01403.x. [DOI] [PubMed] [Google Scholar]
- 42.Sanyal AJ, Chalasani N, Kowdley KV, McCullough A, Diehl AM, Bass NM, Neuschwander-Tetri BA, Lavine JE, Tonascia J, Unalp A, Van Natta M, Clark J, Brunt EM, Kleiner DE, Hoofnagle JH, Robuck PR, Nash CRN. Pioglitazone, vitamin E, or placebo for nonalcoholic steatohepatitis. N Engl J Med. 2010;362:1675–85. doi: 10.1056/NEJMoa0907929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schuppan D, Gorrell MD, Klein T, Mark M, Afdhal NH. The challenge of developing novel pharmacological therapies for non-alcoholic steatohepatitis. Liver Int. 2010;30:795–808. doi: 10.1111/j.1478-3231.2010.02264.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.