Introduction
It is now appreciated that emerging therapeutic strategies for recovery must include the cerebral vasculature and that induction of angiogenesis will stimulate endogenous recovery mechanisms including neurogenesis, synaptogenesis, and neuronal and synaptic plasticity. These events are all involved in the long term repair and restoration process of the brain after an ischemic event. Several recent excellent reviews provided detailed information on the mechanisms and molecular targets for angiogenesis after stroke. 1, 2 The purpose of this review is to evaluate the evidence that angiogenesis is a target for recovery after an ischemic stroke.
Angiogenic response to ischemic brain injury: a multipurpose pathway
Early reports of increased angiogenesis in the ischemic border zone of human brain autopsy sections,3 which was decreased in patients of advanced age,4 led to interest in the time course and impact of this phenomenon on functional recovery. It is clear that angiogenesis genes are upregulated within minutes of the onset of cerebral ischemia in rodents5 and angiogenic proteins remain increased in the area of ischemia for days to weeks.6 It is unclear, however, whether the angiogenic response leads to the development of functional new blood vessels that improve brain function after stroke. Clinical and experimental studies in other vascular beds have emphasized the potential for adverse consequences related to neovascularization.7, 8 In the diabetic retina, for example, pathologic angiogenesis results in hemorrhage, edema and ultimately, blindness.9 In the brain, pathologic angiogenesis is implicated in the development of hereditary hemorrhagic telangiectasia (HHT)10.
The correlation between angiogenesis and improved functional outcome after ischemic stroke remains and is seen in both animal models and in human stroke patients.5, 11–13 It is likely that the “proangiogenic state”, induced in response to an ischemic insult, has multiple purposes in the hours to weeks after the injury (Figure). First, the growth factors expressed may promote survival of the endothelial, glial and neuronal cell types in the penumbral area.14–18 Second, the neovascularization may act to remove damaged tissue. In experimental stroke, it was demonstrated that angiogenesis only occurred transiently in the cortex of the ischemic hemisphere, implying that the new vessels were merely part of the “clean up” after stroke, rather than a contribution to neurorestoration.19 Lastly, the proangiogenic state may create a “vascular niche” in which neural stem cells are generated 20 and allowed to migrate.21
Figure 1. Interventions to improve recovery after stroke.
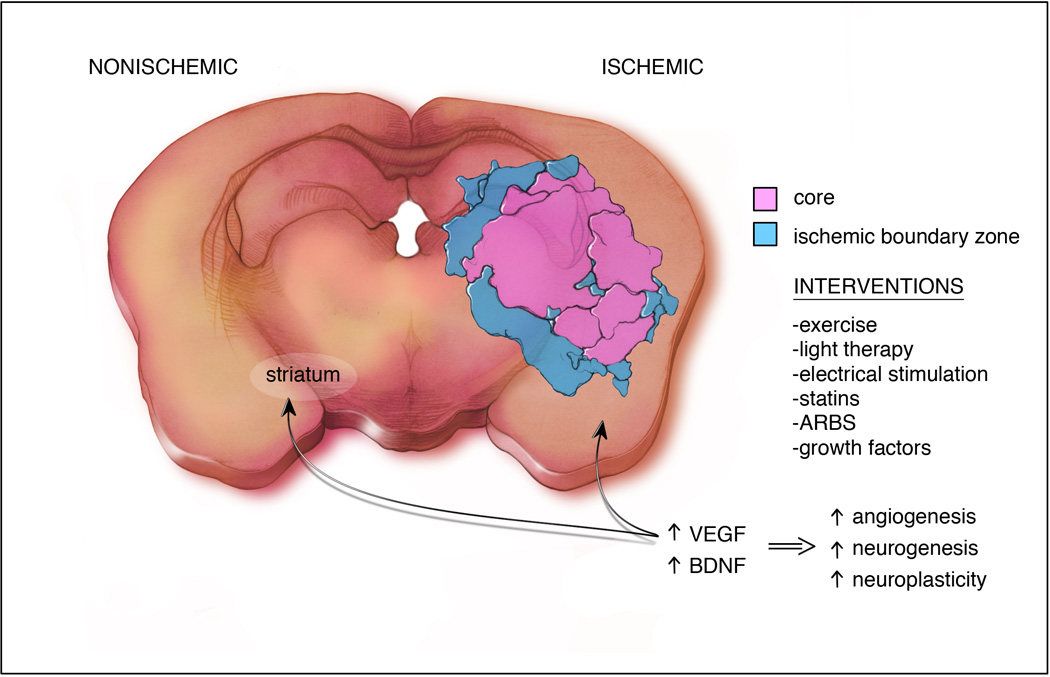
All interventions increase VEGF and BDNF which will:1) promote survival of cells of the neurovascular unit in the ischemic boundary zone, 2) remove damaged tissue in the core, 3) create a vascular niche in the subventricular zone for neurogenesis and allow neuroblast migration to site of recovery.
Growth Factors
Numerous growth factors have been implicated in the recovery process after ischemic stroke and include basic fibroblastic growth factor (FGF2), brain derived neurotrophic factor (BDNF), vascular endothelial growth factor (VEGF), granulocyte colony stimulating factor (G-CSF), and other neurotrophins.22, 23 Neurotrophins promote recovery by enhancing angiogenesis,24 neurogenesis,22 synaptogenesis, and neuronal plasticity.25 The most commonly studied proangiogenic molecule in stroke is VEGF. Although touted for its neuroprotective and neurogenic properties,26 VEGF can worsen edema and hemorrhage after stroke through its enhancement of vascular permeability27 and this effect is dose-related28. However, a great deal of evidence exists that the proangiogenic and vascular permeability effects of VEGF can be “uncoupled”.29–31 When this occurs, the protective effects predominate and recovery is promoted. Differential expression of the various isoforms of VEGF may be important in this process after stroke, where VEGFB promotes cell survival without the predominant vascular permeability and angiogenic effects demonstrated with the better studied VEGFA isoform32.
BDNF is a member of the neurotrophin family that is widely expressed and has many functions extending beyond its neuronal effects.33 Following experimental ischemia, higher levels of BDNF in the brain improved functional recovery 34, 35 whereas BDNF knock down worsened functional outcome.25 Mice expressing a mutant variant of BDNF (Met BDNF) had a worse outcome in comparison with wild type mice after cerebral ischemia and this worsened outcome was attributed to an impaired angiogenic response in animals expressing mutant BDNF.36 A unique aspect of BDNF biology was the identification of its involvement in the crosstalk between the constituents of the neurovascular unit to promote recovery after CNS ischemic insults.20, 37 Additionally, BDNF has been reported to regulate the expression of VEGF.33, 38 BDNF has been implicated in cardiovascular development33 and found to induce a robust angiogenic effect in endothelial cells comparable to that of VEGF.20
Nonpharmacologic interventions that improve recovery: Effect on angiogenesis
Exercise/Physical Therapy
It is clear that exercise preconditioning induces tolerance to brain ischemia through many proposed mechanisms, including induction of VEGF and stimulation of angiogenesis and neurogenesis. 39 Physical activity after stroke has also been shown to induce angiogenesis and improve long term outcome as well. 40 The most important finding in this study, however, was that inhibition of endothelial nitric oxide synthase or angiogenesis (with endostatin) abolished the beneficial effects of exercise on outcome.40
Peripheral / Transcranial Magnetic Stimulation (TMS)
Noninvasive brain stimulation has been employed extensively in rehabilitation research and the promise in promoting recovery after brain injury has been reviewed recently.41 The mechanism of the persistent benefit of this intervention, when combined with physical therapy, is not entirely understood. In an experimental study of middle cerebral artery occlusion (MCAO) in a rat model, electrical stimulation resulted in decreased apoptosis and inflammation and increased angiogenesis in the ischemic cortex42 These effects were accompanied by a robust increase in neurotrophic factors, including VEGF and BDNF and the antiapoptotic effect was abolished by inhibiting Akt activation.42 The importance of angiogenesis was further implicated in the positive response to peripheral stimulation, when mice treated with a specific inhibitor of VEGFR2 had decreased neuroblast migration toward the injured area.43
Low Level Laser Therapy
Light therapy has been shown to reduce infarct size and improve outcome when administered at least 6 hours after experimental stroke onset.44, 45 Experts in the technique have agreed on a critical role of VEGF and angiogenesis to the protection afforded by light therapy.46, 47 Laser therapy is currently in clinical trials for promotion of recovery after ischemic stroke.48
Repurposed pharmacologic interventions that improve stroke recovery
Statins
Statins have been shown to limit the ischemic insult and improve functional outcome and recovery following experimental ischemia.15, 49 The observed improved outcome has been attributed to the statins ability to induce neurogenesis, neuroprotection, neuroplasticity and angiogenesis in the ischemic border zone.14, 15 Data from in vitro and ex vivo angiogenesis research support a biphasic dose dependent effect of statins in angiogenesis where lower concentrations have a proangiogenic effect and higher statin concentrations produce an antiangiogenic response in endothelial cells.14, 15 In a striking contrast to the proangiogenic effect of statins in models of ischemia, statins have been shown to have an antiangiogenic effect in both cancer50 and inflammation settings.51, 52 It can be concluded that statins have a dose and context -dependent modulatory effect on angiogenesis as has been previously reviewed.53
Angiotensin II Receptor Type 1 (AT1) antagonists
Similar to the statins, reports of the effects of the AT1 antagonists on angiogenesis have been conflicting. A meta-analysis of clinical trials using these agents in various disease states pointed to a small, but significant, increase in the incidence of lung cancer in treated patients.54 However, AT1 antagonists have been shown to be both pro- and antiangiogenic, depending on the situation (ischemia or not) and tissue involved.55 In a recent set of experiments done in a rat model of ischemic stroke, treatment with candesartan after 3 hours of temporary MCAO resulted in improved long term functional outcomes and a proangiogenic state.56 This acute treatment was subsequently shown to be associated with increased VEGF expression in both the ischemic and nonischemic hemispheres at 24 hours after treatment and increased vascular density in both hemispheres at 7 days.32 Most believe that angiogenesis only occurs in the ipsilateral hemisphere after stroke57 and experimental studies compare only the cortical tissue in the two sides for evidence to support this claim. It is well known, however, that ischemic injury in the brain can result in diaschisis (distant areas of decreased flow and metabolism)58 and functional MRI studies in human stroke victims have consistently shown involvement of the contralesional hemisphere in recovery.59 It is possible that induction of angiogenesis in the striatum of the contralesional hemisphere is important to functional recovery after ischemic stroke, by improving recruitment of neuroblasts to the area of recovery.
PhosphodiesteraseType 5 (PDE5) inhibitors
Recent evidence shows that PDE5 expression varies among vascular beds and is an important determinant of angiogenic potential,60 with endothelium expressing the lowest levels of PDE5 being the most angiogenic. The PDE5 inhibitors, sildenafil and tadalafil, have both been studied in rodent models of ischemic stroke and found to promote functional recovery when initiated at 24 hours after the onset of ischemia61, 62 Sensitive imaging techniques revealed that rodents treated with sildenafil after embolic stroke experienced increased angiogenesis and axonal remodeling in the ischemic boundary zone compared to saline treated controls, beginning at one week after injury.63 The authors concluded that the temporal and spatial co-localization of the two processes supported the notion that angiogenesis promoted axonal remodeling and therefore, recovery.
Growth Factor Treatment
Many growth factors, including granulocyte colony stimulating factor (G-CSF) and erythropoietin (EPO) have been studied for their neuroregenerative effects64 but dose-limiting toxicity and drug delivery barriers have tempered their development as stroke therapies.65 A small clinical trial of 328 stroke patients treated with G-CSF or placebo failed to show a benefit of treatment at 90 days but it may have been underpowered to do so66. EPO has been the most studied and was found to enhance angiogenesis, neurogenesis and functional recovery after stroke in rats and this was associated with an increase in both VEGF and BDNF expression.67 Clinical trials with EPO, at first promising, were discontinued when increased hemorrhage and mortality occurred in stroke patients receiving the combination of EPO (within 6 hours of onset) and tissue plasminogen activator (tPA).68 Subsequently it was shown that EPO increases matrix metalloproteases (MMPs), which may have explained this interaction.69 When administered beyond the first 24 hours after ischemia, however, the data on EPO continues to be encouraging. In a recent study, EPO administered 3 days after focal ischemia, increased recovery in affected mice and was associated with increased remodeling of the ischemic boundary zone and contralesional axonal sprouting.70 The increased expression of proangiogenic genes and proteins in the nonischemic hemisphere is an exciting area for future study.
Which came first?
Spatial and temporal co-localization of angiogenesis and neurogenesis does not prove that angiogenesis is necessary for the neuronal recovery after treatment with the above interventions. The best evidence supporting causation comes from a classic set of experiments in the female canary. When testosterone was administered to these songbirds, an abrupt increase in VEGF expression in the vocal control nucleus led to increased angiogenesis and this was followed by a significant increase in BDNF two weeks later 71. The increase in BDNF, primarily from endothelial cells, was shown to significantly increase neuronal migration to the nucleus. When VEGF signaling was blocked with a receptor inhibitor, recruitment of new neurons was impaired 71. In stroke, although angiogenesis may not be necessary for the neuroproliferative response to ischemia 26, long-term migration of neuroblasts to the site of recoveryy has been shown to occur only in areas of newly increased vascularity 72.
Consequences of impaired angiogenesis
As commented in a recent review,1 diabetic and/or hypertensive patients may develop dysfunctional vasculature after stimulation of angiogenesis in the recovering penumbra following stroke, and this can be detrimental. There is strong evidence that hyperglycemia-mediated oxidative damage to microvascular endothelial cells triggers a cascade of events that cause excessive angiogenesis and result in vascular proliferative retinopathy.73–75 These immature vessels then break and leak worsening vascular and neuronal damage.33, 73, 76 The effect of hyperglycemia in the cerebral vasculature, especially after an ischemic event, is less clear. We reported extensive vascular remodeling and arteriogenesis in the pial vessels in Goto-Kakizaki (GK) rats, a lean and mild model of Type 2 diabetes.51, 77 Also, cerebrovascular permeability, VEGF A and BDNF levels are increased in this model. After stroke, these diabetic animals bleed into the brain and perform poorly on neurobehavioral tests.77 Prevention of pial remodeling by glycemic control or inhibition of matrix metalloproteinases (MMPs) reduces vascular damage and improves neurologic outcome.78 Although antiangiogenic therapy has been successful in reducing damage when applied locally to the retina 79, systemic use is associated with increased blood pressure, a perilous pursuit in patients at risk for stroke.
In hypertension, it is well established that cerebrovascular remodeling occurs80–83 but our knowledge of how hypertension affects angiogenesis is limited. Increased angiogenesis occurs in the mesenteric microvascular network of spontaneously hypertensive rats, but it is followed by a phase of increased pruning(ref 86). The time course and significance of this phenomenon in the cerebral vasculature has yet to be determined.
Conclusions
Angiogenesis occurs after stroke and can be augmented by pharmacologic and nonpharmacologic means. Although new blood vessel growth may be transient in the ischemic boundary zone after injury, the association of angiogenesis with neuronal plasticity, especially in the contralesional hemisphere, could result in enduring recovery. The beneficial effects of angiogenesis may be negatively impacted by premorbid disease, as is seen in diabetes and hypertension, and this area requires further investigation. Progress in this area has been limited by reliance on the nonischemic hemisphere as a convenient control, focus on the ischemic boundary zone, and failure to include an assessment of preexisting vascular remodeling. Harnessing the restorative power of the vasculature in the entire brain, in the face of vascular diseases, should be a high priority for future study.
Acknowledgments
Funding: Support was received from RO1-NS063965 (SCF), Veterans Affairs Merit Review (SCF and AE), American Heart Association Established Investigator 0740002N (AE), NS054688 (AE), Jordan University of Science and Technology (fellowship to AA).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: Dr. Fagan is a consultant for Pfizer, Inc and Genentech, Inc and has received donated drug (candesartan) from Astra-Zeneca. Illustration by Colby Polansky.
References
- 1.Navaratna D, Guo S, Arai K, Lo EH. Mechanisms and targets for angiogenic therapy after stroke. Cell Adh Migr. 2009;3:216–223. doi: 10.4161/cam.3.2.8396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Xiong Y, Mahmood A, Chopp M. Angiogenesis, neurogenesis and brain recovery of function following injury. Curr Opin Investig Drugs. 2010;11:298–308. [PMC free article] [PubMed] [Google Scholar]
- 3.Krupinski J, Kaluza J, Kumar P, Wang M, Kumar S. Prognostic value of blood vessel density in ischaemic stroke. Lancet. 1993;342:742. doi: 10.1016/0140-6736(93)91734-4. [DOI] [PubMed] [Google Scholar]
- 4.Szpak GM, Lechowicz W, Lewandowska E, Bertrand E, Wierzba-Bobrowicz T, Dymecki J. Border zone neovascularization in cerebral ischemic infarct. Folia Neuropathol. 1999;37:264–268. [PubMed] [Google Scholar]
- 5.Hayashi T, Noshita N, Sugawara T, Chan PH. Temporal profile of angiogenesis and expression of related genes in the brain after ischemia. J Cereb Blood Flow Metab. 2003;23:166–180. doi: 10.1097/01.WCB.0000041283.53351.CB. [DOI] [PubMed] [Google Scholar]
- 6.Krupinski J, Issa R, Bujny T, Slevin M, Kumar P, Kumar S, et al. A putative role for platelet-derived growth factor in angiogenesis and neuroprotection after ischemic stroke in humans. Stroke. 1997;28:564–573. doi: 10.1161/01.str.28.3.564. [DOI] [PubMed] [Google Scholar]
- 7.Makrilia N, Lappa T, Xyla V, Nikolaidis I, Syrigos K. The role of angiogenesis in solid tumours: An overview. Eur J Intern Med. 2009;20:663–671. doi: 10.1016/j.ejim.2009.07.009. [DOI] [PubMed] [Google Scholar]
- 8.Virmani R, Kolodgie FD, Burke AP, Finn AV, Gold HK, Tulenko TN, et al. Atherosclerotic plaque progression and vulnerability to rupture: Angiogenesis as a source of intraplaque hemorrhage. Arterioscler Thromb Vasc Biol. 2005;25:2054–2061. doi: 10.1161/01.ATV.0000178991.71605.18. [DOI] [PubMed] [Google Scholar]
- 9.Durham JT, Herman IM. Microvascular modifications in diabetic retinopathy. Curr Diab Rep. 2011;11:253–264. doi: 10.1007/s11892-011-0204-0. [DOI] [PubMed] [Google Scholar]
- 10.Seki T, Yun J, Oh SP. Arterial endothelium-specific activin receptor-like kinase 1 expression suggests its role in arterialization and vascular remodeling. Circ Res. 2003;93:682–689. doi: 10.1161/01.RES.0000095246.40391.3B. [DOI] [PubMed] [Google Scholar]
- 11.Liu XS, Zhang ZG, Zhang RL, Gregg S, Morris DC, Wang Y, et al. Stroke induces gene profile changes associated with neurogenesis and angiogenesis in adult subventricular zone progenitor cells. J Cereb Blood Flow Metab. 2007;27:564–574. doi: 10.1038/sj.jcbfm.9600371. [DOI] [PubMed] [Google Scholar]
- 12.Krupinski J, Kaluza J, Kumar P, Kumar S, Wang JM. Role of angiogenesis in patients with cerebral ischemic stroke. Stroke. 1994;25:1794–1798. doi: 10.1161/01.str.25.9.1794. [DOI] [PubMed] [Google Scholar]
- 13.Henderson RD, Eliasziw M, Fox AJ, Rothwell PM, Barnett HJ. Angiographically defined collateral circulation and risk of stroke in patients with severe carotid artery stenosis. North american symptomatic carotid endarterectomy trial (nascet) group. Stroke. 2000;31:128–132. doi: 10.1161/01.str.31.1.128. [DOI] [PubMed] [Google Scholar]
- 14.Chen J, Cui X, Zacharek A, Chopp M. Increasing ang1/tie2 expression by simvastatin treatment induces vascular stabilization and neuroblast migration after stroke. J Cell Mol Med. 2009;13:1348–1357. doi: 10.1111/j.1582-4934.2008.00380.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen J, Zhang C, Jiang H, Li Y, Zhang L, Robin A, et al. Atorvastatin induction of vegf and bdnf promotes brain plasticity after stroke in mice. J Cereb Blood Flow Metab. 2005;25:281–290. doi: 10.1038/sj.jcbfm.9600034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ding J, Cheng Y, Gao S, Chen J. Effects of nerve growth factor and noggin-modified bone marrow stromal cells on stroke in rats. J Neurosci Res. 2011;89:222–230. doi: 10.1002/jnr.22535. [DOI] [PubMed] [Google Scholar]
- 17.Jiang WL, Zhang SP, Zhu HB, Hou J. Effect of 8-o-acetyl shanzhiside methylester increases angiogenesis and improves functional recovery after stroke. Basic Clin Pharmacol Toxicol. 2011;108:21–27. doi: 10.1111/j.1742-7843.2010.00620.x. [DOI] [PubMed] [Google Scholar]
- 18.Qu WS, Wang YH, Wang JP, Tang YX, Zhang Q, Tian DS, et al. Galectin-1 enhances astrocytic bdnf production and improves functional outcome in rats following ischemia. Neurochem Res. 2010;35:1716–1724. doi: 10.1007/s11064-010-0234-z. [DOI] [PubMed] [Google Scholar]
- 19.Manoonkitiwongsa PS, Jackson-Friedman C, McMillan PJ, Schultz RL, Lyden PD. Angiogenesis after stroke is correlated with increased numbers of macrophages: The clean-up hypothesis. J Cereb Blood Flow Metab. 2001;21:1223–1231. doi: 10.1097/00004647-200110000-00011. [DOI] [PubMed] [Google Scholar]
- 20.Li Q, Ford MC, Lavik EB, Madri JA. Modeling the neurovascular niche: Vegf- and bdnf-mediated cross-talk between neural stem cells and endothelial cells: An in vitro study. J Neurosci Res. 2006;84:1656–1668. doi: 10.1002/jnr.21087. [DOI] [PubMed] [Google Scholar]
- 21.Petraglia AL, Marky AH, Walker C, Thiyagarajan M, Zlokovic BV. Activated protein c is neuroprotective and mediates new blood vessel formation and neurogenesis after controlled cortical impact. Neurosurgery. 2010;66:165–171. doi: 10.1227/01.NEU.0000363148.49779.68. discussion 171-162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Leker RR, Lasri V, Chernoguz D. Growth factors improve neurogenesis and outcome after focal cerebral ischemia. J Neural Transm. 2009;116:1397–1402. doi: 10.1007/s00702-009-0329-3. [DOI] [PubMed] [Google Scholar]
- 23.Hermann DM, Zechariah A. Implications of vascular endothelial growth factor for postischemic neurovascular remodeling. J Cereb Blood Flow Metab. 2009;29:1620–1643. doi: 10.1038/jcbfm.2009.100. [DOI] [PubMed] [Google Scholar]
- 24.Hoang S, Liauw J, Choi M, Guzman RG, Steinberg GK. Netrin-4 enhances angiogenesis and neurologic outcome after cerebral ischemia. J Cereb Blood Flow Metab. 2009;29:385–397. doi: 10.1038/jcbfm.2008.128. [DOI] [PubMed] [Google Scholar]
- 25.Ploughman M, Windle V, MacLellan CL, White N, Dore JJ, Corbett D. Brain-derived neurotrophic factor contributes to recovery of skilled reaching after focal ischemia in rats. Stroke. 2009;40:1490–1495. doi: 10.1161/STROKEAHA.108.531806. [DOI] [PubMed] [Google Scholar]
- 26.Sun Y, Jin K, Xie L, Childs J, Mao XO, Logvinova A, et al. Vegf-induced neuroprotection, neurogenesis, and angiogenesis after focal cerebral ischemia. J Clin Invest. 2003;111:1843–1851. doi: 10.1172/JCI17977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang ZG, Zhang L, Jiang Q, Zhang R, Davies K, Powers C, et al. Vegf enhances angiogenesis and promotes blood-brain barrier leakage in the ischemic brain. J Clin Invest. 2000;106:829–838. doi: 10.1172/JCI9369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Manoonkitiwongsa PS, Schultz RL, McCreery DB, Whitter EF, Lyden PD. Neuroprotection of ischemic brain by vascular endothelial growth factor is critically dependent on proper dosage and may be compromised by angiogenesis. J Cereb Blood Flow Metab. 2004;24:693–702. doi: 10.1097/01.WCB.0000126236.54306.21. [DOI] [PubMed] [Google Scholar]
- 29.Valable S, Montaner J, Bellail A, Berezowski V, Brillault J, Cecchelli R, et al. Vegf-induced bbb permeability is associated with an mmp-9 activity increase in cerebral ischemia: Both effects decreased by ang-1. J Cereb Blood Flow Metab. 2005;25:1491–1504. doi: 10.1038/sj.jcbfm.9600148. [DOI] [PubMed] [Google Scholar]
- 30.Zheng Y, Murakami M, Takahashi H, Yamauchi M, Kiba A, Yamaguchi S, et al. Chimeric vegf-e(nz7)/plgf promotes angiogenesis via vegfr-2 without significant enhancement of vascular permeability and inflammation. Arterioscler Thromb Vasc Biol. 2006;26:2019–2026. doi: 10.1161/01.ATV.0000233336.53574.a1. [DOI] [PubMed] [Google Scholar]
- 31.Gomez R, Gonzalez-Izquierdo M, Zimmermann RC, Novella-Maestre E, Alonso-Muriel I, Sanchez-Criado J, et al. Low-dose dopamine agonist administration blocks vascular endothelial growth factor (vegf)-mediated vascular hyperpermeability without altering vegf receptor 2-dependent luteal angiogenesis in a rat ovarian hyperstimulation model. Endocrinology. 2006;147:5400–5411. doi: 10.1210/en.2006-0657. [DOI] [PubMed] [Google Scholar]
- 32.Guan W, Somanath PR, Kozak A, Goc A, El-Remessy AB, Ergul A, et al. Vascular protection by angiotensin receptor antagonism involves differential vegf expression in both hemispheres after experimental stroke. PLoS One. 2011;6:e24551. doi: 10.1371/journal.pone.0024551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Caporali A, Emanueli C. Cardiovascular actions of neurotrophins. Physiol Rev. 2009;89:279–308. doi: 10.1152/physrev.00007.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kurozumi K, Nakamura K, Tamiya T, Kawano Y, Kobune M, Hirai S, et al. Bdnf gene-modified mesenchymal stem cells promote functional recovery and reduce infarct size in the rat middle cerebral artery occlusion model. Mol Ther. 2004;9:189–197. doi: 10.1016/j.ymthe.2003.10.012. [DOI] [PubMed] [Google Scholar]
- 35.Schabitz WR, Steigleder T, Cooper-Kuhn CM, Schwab S, Sommer C, Schneider A, et al. Intravenous brain-derived neurotrophic factor enhances poststroke sensorimotor recovery and stimulates neurogenesis. Stroke. 2007;38:2165–2172. doi: 10.1161/STROKEAHA.106.477331. [DOI] [PubMed] [Google Scholar]
- 36.Qin L, Kim E, Ratan R, Lee FS, Cho S. Genetic variant of bdnf (val66met) polymorphism attenuates stroke-induced angiogenic responses by enhancing anti-angiogenic mediator cd36 expression. J Neurosci. 2011;31:775–783. doi: 10.1523/JNEUROSCI.4547-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Guo S, Kim WJ, Lok J, Lee SR, Besancon E, Luo BH, et al. Neuroprotection via matrix-trophic coupling between cerebral endothelial cells and neurons. Proc Natl Acad Sci U S A. 2008;105:7582–7587. doi: 10.1073/pnas.0801105105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nakamura K, Martin KC, Jackson JK, Beppu K, Woo CW, Thiele CJ. Brain-derived neurotrophic factor activation of trkb induces vascular endothelial growth factor expression via hypoxia-inducible factor-1alpha in neuroblastoma cells. Cancer Res. 2006;66:4249–4255. doi: 10.1158/0008-5472.CAN-05-2789. [DOI] [PubMed] [Google Scholar]
- 39.Zhang F, Wu Y, Jia J. Exercise preconditioning and brain ischemic tolerance. Neuroscience. 2011;177:170–176. doi: 10.1016/j.neuroscience.2011.01.018. [DOI] [PubMed] [Google Scholar]
- 40.Gertz K, Priller J, Kronenberg G, Fink KB, Winter B, Schrock H, et al. Physical activity improves long-term stroke outcome via endothelial nitric oxide synthase-dependent augmentation of neovascularization and cerebral blood flow. Circ Res. 2006;99:1132–1140. doi: 10.1161/01.RES.0000250175.14861.77. [DOI] [PubMed] [Google Scholar]
- 41.Bolognini N, Pascual-Leone A, Fregni F. Using non-invasive brain stimulation to augment motor training-induced plasticity. J Neuroeng Rehabil. 2009;6:8. doi: 10.1186/1743-0003-6-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Baba T, Kameda M, Yasuhara T, Morimoto T, Kondo A, Shingo T, et al. Electrical stimulation of the cerebral cortex exerts antiapoptotic, angiogenic, and anti-inflammatory effects in ischemic stroke rats through phosphoinositide 3-kinase/akt signaling pathway. Stroke. 2009;40:e598–e605. doi: 10.1161/STROKEAHA.109.563627. [DOI] [PubMed] [Google Scholar]
- 43.Li WL, Fraser JL, Yu SP, Zhu J, Jiang YJ, Wei L. The role of vegf/vegfr2 signaling in peripheral stimulation-induced cerebral neurovascular regeneration after ischemic stroke in mice. Exp Brain Res. 2011;214:503–513. doi: 10.1007/s00221-011-2849-y. [DOI] [PubMed] [Google Scholar]
- 44.Oron A, Oron U, Chen J, Eilam A, Zhang C, Sadeh M, et al. Low-level laser therapy applied transcranially to rats after induction of stroke significantly reduces long-term neurological deficits. Stroke. 2006;37:2620–2624. doi: 10.1161/01.STR.0000242775.14642.b8. [DOI] [PubMed] [Google Scholar]
- 45.Lapchak PA, Salgado KF, Chao CH, Zivin JA. Transcranial near-infrared light therapy improves motor function following embolic strokes in rabbits: An extended therapeutic window study using continuous and pulse frequency delivery modes. Neuroscience. 2007;148:907–914. doi: 10.1016/j.neuroscience.2007.07.002. [DOI] [PubMed] [Google Scholar]
- 46.Streeter J, De Taboada L, Oron U. Mechanisms of action of light therapy for stroke and acute myocardial infarction. Mitochondrion. 2004;4:569–576. doi: 10.1016/j.mito.2004.07.037. [DOI] [PubMed] [Google Scholar]
- 47.Tuby H, Maltz L, Oron U. Modulations of vegf and inos in the rat heart by low level laser therapy are associated with cardioprotection and enhanced angiogenesis. Lasers Surg Med. 2006;38:682–688. doi: 10.1002/lsm.20377. [DOI] [PubMed] [Google Scholar]
- 48.Lampl Y, Zivin JA, Fisher M, Lew R, Welin L, Dahlof B, et al. Infrared laser therapy for ischemic stroke: A new treatment strategy: Results of the neurothera effectiveness and safety trial-1 (nest-1) Stroke. 2007;38:1843–1849. doi: 10.1161/STROKEAHA.106.478230. [DOI] [PubMed] [Google Scholar]
- 49.Elewa HF, Kozak A, El-Remessy AB, Frye RF, Johnson MH, Ergul A, et al. Early atorvastatin reduces hemorrhage after acute cerebral ischemia in diabetic rats. J Pharmacol Exp Ther. 2009;330:532–540. doi: 10.1124/jpet.108.146951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhao TT, Trinh D, Addison CL, Dimitroulakos J. Lovastatin inhibits vegfr and akt activation: Synergistic cytotoxicity in combination with vegfr inhibitors. PLoS One. 2010;5:e12563. doi: 10.1371/journal.pone.0012563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Araujo FA, Rocha MA, Mendes JB, Andrade SP. Atorvastatin inhibits inflammatory angiogenesis in mice through down regulation of vegf, tnf-alpha and tgf-beta1. Biomed Pharmacother. 2010;64:29–34. doi: 10.1016/j.biopha.2009.03.003. [DOI] [PubMed] [Google Scholar]
- 52.Massaro M, Zampolli A, Scoditti E, Carluccio MA, Storelli C, Distante A, et al. Statins inhibit cyclooxygenase-2 and matrix metalloproteinase-9 in human endothelial cells: Anti-angiogenic actions possibly contributing to plaque stability. Cardiovasc Res. 2010;86:311–320. doi: 10.1093/cvr/cvp375. [DOI] [PubMed] [Google Scholar]
- 53.Elewa HF, El-Remessy AB, Somanath PR, Fagan SC. Diverse effects of statins on angiogenesis: New therapeutic avenues. Pharmacotherapy. 2010;30:169–176. doi: 10.1592/phco.30.2.169. [DOI] [PubMed] [Google Scholar]
- 54.Sipahi I, Debanne SM, Rowland DY, Simon DI, Fang JC. Angiotensin-receptor blockade and risk of cancer: Meta-analysis of randomised controlled trials. Lancet Oncol. 2010;11:627–636. doi: 10.1016/S1470-2045(10)70106-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Willis LM, El-Remessy AB, Somanath PR, Deremer DL, Fagan SC. Angiotensin receptor blockers and angiogenesis: Clinical and experimental evidence. Clin Sci (Lond) 2011;120:307–319. doi: 10.1042/cs20100389. [DOI] [PubMed] [Google Scholar]
- 56.Kozak A, Ergul A, El-Remessy AB, Johnson MH, Machado LS, Elewa HF, et al. Candesartan augments ischemia-induced proangiogenic state and results in sustained improvement after stroke. Stroke. 2009;40:1870–1876. doi: 10.1161/STROKEAHA.108.537225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Font MA, Arboix A, Krupinski J. Angiogenesis, neurogenesis and neuroplasticity in ischemic stroke. Curr Cardiol Rev. 2010;6:238–244. doi: 10.2174/157340310791658802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Carmichael ST, Tatsukawa K, Katsman D, Tsuyuguchi N, Kornblum HI. Evolution of diaschisis in a focal stroke model. Stroke. 2004;35:758–763. doi: 10.1161/01.STR.0000117235.11156.55. [DOI] [PubMed] [Google Scholar]
- 59.Carey JR, Kimberley TJ, Lewis SM, Auerbach EJ, Dorsey L, Rundquist P, et al. Analysis of fmri and finger tracking training in subjects with chronic stroke. Brain. 2002;125:773–788. doi: 10.1093/brain/awf091. [DOI] [PubMed] [Google Scholar]
- 60.Zhu B, Zhang L, Alexeyev M, Alvarez DF, Strada SJ, Stevens T. Type 5 phosphodiesterase expression is a critical determinant of the endothelial cell angiogenic phenotype. Am J Physiol Lung Cell Mol Physiol. 2009;296:L220–L228. doi: 10.1152/ajplung.90474.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Li L, Jiang Q, Zhang L, Ding G, Gang Zhang Z, Li Q, et al. Angiogenesis and improved cerebral blood flow in the ischemic boundary area detected by mri after administration of sildenafil to rats with embolic stroke. Brain Res. 2007;1132:185–192. doi: 10.1016/j.brainres.2006.10.098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhang L, Zhang Z, Zhang RL, Cui Y, LaPointe MC, Silver B, et al. Tadalafil, a long-acting type 5 phosphodiesterase isoenzyme inhibitor, improves neurological functional recovery in a rat model of embolic stroke. Brain Res. 2006;1118:192–198. doi: 10.1016/j.brainres.2006.08.028. [DOI] [PubMed] [Google Scholar]
- 63.Ding G, Jiang Q, Li L, Zhang L, Zhang ZG, Ledbetter KA, et al. Magnetic resonance imaging investigation of axonal remodeling and angiogenesis after embolic stroke in sildenafil-treated rats. J Cereb Blood Flow Metab. 2008;28:1440–1448. doi: 10.1038/jcbfm.2008.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Maurer MH, Schabitz WR, Schneider A. Old friends in new constellations--the hematopoetic growth factors g-csf, gm-csf, and epo for the treatment of neurological diseases. Curr Med Chem. 2008;15:1407–1411. doi: 10.2174/092986708784567671. [DOI] [PubMed] [Google Scholar]
- 65.Ren JM, Finklestein SP. Growth factor treatment of stroke. Curr Drug Targets CNS Neurol Disord. 2005;4:121–125. doi: 10.2174/1568007053544101. [DOI] [PubMed] [Google Scholar]
- 66.Sygnis announces key results of its phase ii trial of ax200 in acute ischemic stroke. 2012;2012 [Google Scholar]
- 67.Wang L, Zhang Z, Wang Y, Zhang R, Chopp M. Treatment of stroke with erythropoietin enhances neurogenesis and angiogenesis and improves neurological function in rats. Stroke. 2004;35:1732–1737. doi: 10.1161/01.STR.0000132196.49028.a4. [DOI] [PubMed] [Google Scholar]
- 68.Ehrenreich H, Weissenborn K, Prange H, Schneider D, Weimar C, Wartenberg K, et al. Recombinant human erythropoietin in the treatment of acute ischemic stroke. Stroke. 2009;40:e647–e656. doi: 10.1161/STROKEAHA.109.564872. [DOI] [PubMed] [Google Scholar]
- 69.Zechariah A, ElAli A, Hermann DM. Combination of tissue-plasminogen activator with erythropoietin induces blood-brain barrier permeability, extracellular matrix disaggregation, and DNA fragmentation after focal cerebral ischemia in mice. Stroke. 2010;41:1008–1012. doi: 10.1161/STROKEAHA.109.574418. [DOI] [PubMed] [Google Scholar]
- 70.Reitmeir R, Kilic E, Kilic U, Bacigaluppi M, ElAli A, Salani G, et al. Post-acute delivery of erythropoietin induces stroke recovery by promoting perilesional tissue remodelling and contralesional pyramidal tract plasticity. Brain. 2011;134:84–99. doi: 10.1093/brain/awq344. [DOI] [PubMed] [Google Scholar]
- 71.Louissaint A, Jr, Rao S, Leventhal C, Goldman SA. Coordinated interaction of neurogenesis and angiogenesis in the adult songbird brain. Neuron. 2002;34:945–960. doi: 10.1016/s0896-6273(02)00722-5. [DOI] [PubMed] [Google Scholar]
- 72.Thored P, Wood J, Arvidsson A, Cammenga J, Kokaia Z, Lindvall O. Long-term neuroblast migration along blood vessels in an area with transient angiogenesis and increased vascularization after stroke. Stroke. 2007;38:3032–3039. doi: 10.1161/STROKEAHA.107.488445. [DOI] [PubMed] [Google Scholar]
- 73.Caldwell RB, Bartoli M, Behzadian MA, El-Remessy AE, Al-Shabrawey M, Platt DH, et al. Vascular endothelial growth factor and diabetic retinopathy: Pathophysiological mechanisms and treatment perspectives. Diabetes Metab Res Rev. 2003;19:442–455. doi: 10.1002/dmrr.415. [DOI] [PubMed] [Google Scholar]
- 74.Silvestre JS, Levy BI. Molecular basis of angiopathy in diabetes mellitus. Circ Res. 2006;98:4–6. doi: 10.1161/01.RES.0000200396.90220.41. [DOI] [PubMed] [Google Scholar]
- 75.Silvestre JS. Vascular progenitor cells and diabetes: Role in postischemic neovascularisation. Diabetes Metab. 2008;34(Suppl 1):S33–S36. doi: 10.1016/S1262-3636(08)70101-0. [DOI] [PubMed] [Google Scholar]
- 76.Caldwell RB, Bartoli M, Behzadian MA, El-Remessy AE, Al-Shabrawey M, Platt DH, et al. Vascular endothelial growth factor and diabetic retinopathy: Role of oxidative stress. Curr Drug Targets. 2005;6:511–524. doi: 10.2174/1389450054021981. [DOI] [PubMed] [Google Scholar]
- 77.Ergul A, Elgebaly MM, Middlemore ML, Li W, Elewa H, Switzer JA, et al. Increased hemorrhagic transformation and altered infarct size and localization after experimental stroke in a rat model type 2 diabetes. BMC Neurol. 2007;7:33. doi: 10.1186/1471-2377-7-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Elgebaly MM, Prakash R, Li W, Ogbi S, Johnson MH, Mezzetti EM, et al. Vascular protection in diabetic stroke: Role of matrix metalloprotease-dependent vascular remodeling. J Cereb Blood Flow Metab. 2010;30:1928–1938. doi: 10.1038/jcbfm.2010.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Semeraro F, Morescalchi F, Parmeggiani F, Arcidiacono B, Costagliola C. Systemic adverse drug reactions secondary to anti-vegf intravitreal injection in patients with neovascular age-related macular degeneration. Curr Vasc Pharmacol. 2011;9:629–646. doi: 10.2174/157016111796642670. [DOI] [PubMed] [Google Scholar]
- 80.Duan DD. Volume matters: Novel roles of the volume-regulated clc-3 channels in hypertension-induced cerebrovascular remodeling. Hypertension. 2010;56:346–348. doi: 10.1161/HYPERTENSIONAHA.110.155770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Dupuis F, Vincent JM, Liminana P, Chillon JM, Capdeville-Atkinson C, Atkinson J. Effects of suboptimal doses of the at1 receptor blocker, telmisartan, with the angiotensin-converting enzyme inhibitor, ramipril, on cerebral arterioles in spontaneously hypertensive rat. J Hypertens. 2010;28:1566–1573. doi: 10.1097/hjh.0b013e328339f1f3. [DOI] [PubMed] [Google Scholar]
- 82.Faraci FM, Baumbach GL, Heistad DD. Cerebral circulation: Humoral regulation and effects of chronic hypertension. J Am Soc Nephrol. 1990;1:53–57. doi: 10.1681/ASN.V1153. [DOI] [PubMed] [Google Scholar]
- 83.Heistad DD, Baumbach GL. Cerebral vascular changes during chronic hypertension: Good guys and bad guys. J Hypertens Suppl. 1992;10:S71–S75. [PubMed] [Google Scholar]


