Abstract
Background
The renin–angiotensin system (RAS) plays an important role in normal homeostasis, carcinogenesis-related angiogenesis, and cell proliferation. Helicobacter pylori infection causes infiltration of inflammatory cells into the gastric mucosa and is considered the major cause of gastric cancer. Whether RAS plays a role in H. pylori infection-related gastric diseases remains unclear. We investigated the changes in gastric mucosal angiotensin II type 1 receptor (AT1R) and type 2 receptor (AT2R) mRNA levels throughout the time course of H. pylori infection in Mongolian gerbils.
Methods
Mongolian gerbils were infected with wild-type H. pylori (for 12 months) or with its isogenic oipA mutant (for 3 months). Gastric mucosal AT1R and AT2R mRNA levels were assessed using real-time reverse transcription-polymerase chain reaction.
Results
The gastric mucosal AT1R mRNA level was significantly associated with the severity of inflammatory cell infiltration into the gastric mucosa that reached maximal levels at 12 months after infection in both the antrum and body. Inflammatory cell infiltration scores and AT1R and AT2R mRNA levels were significantly lower in oipA mutant than wild-type infections. Mucosal AT1R and AT2R mRNA expressions in wild-type H. pylori-infected gerbils with gastric ulcers were significantly higher than in those without ulcers (P <0.01).
Conclusions
Gastric mucosal ATR expression gradually increases during the course of H. pylori infection. Up-regulation of the RAS in association with progressive gastric inflammation suggests a potential role of the RAS in gastric carcinogenesis. OipA appears to play a role in AT1R and AT2R expression and the resulting inflammation.
Keywords: H. pylori, Angiotensin II type 1 receptor, Mongolian gerbil, OipA, Renin–angiotensin system
Introduction
In 1994, the World Health Organization/International Agency for Research on Cancer (WHO/IARC) designated Helicobacter pylori as a definite biological group I carcinogen of gastric cancer, and many lines of additional evidence now support the relationship between H. pylori infection and gastric carcinogenesis [1–4]. For example, gastric cancer developed in 2.9% of H. pylori-positive patients followed up for about 8 years, whereas no gastric cancer was reported among uninfected patients [1]. In patients with peptic ulcers, successful eradication of the H. pylori infection reduced the risk of peptic ulcer recurrence as well as the risk of gastric cancer [2]. In a recent study, patients with early gastric cancer who received both endoscopic mucosal resection and H. pylori eradication therapy experienced a significant reduction in the risk of subsequent gastric cancer relative to patients who did not receive eradication therapy [4]. Overall, an H. pylori infection is now considered the most important cause of gastric cancer, and eradication therapy has been recommended as a strategy for gastric cancer prevention [1–4].
The pathology of H. pylori infections is characterized by a marked infiltration of neutrophils, lymphocytes, monocytes, and plasma cells into the gastric mucosa. Migration and activation of these cells results in mucosal inflammation in response to the local H. pylori-induced production of pro-inflammatory cytokines [5–8]. H. pylori infections initially present as an antral-predominant gastritis followed by advancing inflammation into the gastric body, eventually leading to atrophic gastritis with metaplasia, often with gastric ulcers [9–13] and less often with gastric cancer [14, 15]. The pathogenesis and progression to gastric cancer involves a variety of processes, including cell proliferation, cell differentiation, angiogenesis, and degradation of the extracellular matrix.
The renin–angiotensin system (RAS) consists of angiotensinogen, angiotensin I, angiotensin II, renin, angiotensin-I converting enzyme (ACE), and chymase and plays a key role in blood pressure regulation. Recent evidence indicates that angiotensin II is also involved in the regulation of cell proliferation, angiogenesis, inflammation, and tissue remodeling via angiotensin II type 1 receptors (AT1Rs) [16–18]. An epidemiological study of an elderly cohort in a southern California community demonstrated that over a 9-year period, the systolic blood pressure associated with enhanced RAS was a significant predictor of subsequent cancer mortality [19]. Moreover, ACE inhibitors and AT1R blockers (ARBs) have been reported to reduce tumor progression, vascularization, and metastasis [20]. These data highlight the relationship between the angiotensin II/AT1R pathway and carcinogenesis, suggesting that this pathway may be a target for chemoprevention for several neoplastic lesions [20, 21].
Angiotensin II, chymase, and ACE have been reported to be more highly expressed in the gastric mucosa of patients with peptic ulcer and gastric cancer than in the gastric mucosa of H. pylori-negative patients [22–25]. Recently, in a Mongolian gerbil model of H. pylori infection, it was demonstrated that AT1R and AT2R were highly expressed in a subpopulation of endocrine and inflammatory cells in the gastric wall [26]. Infected gerbils showed significantly increased AT1R protein production and an increased number of infiltrating polymorphonuclear cells (PMNs) in the gastric mucosa at 12 months after infection [26].
Of interest, RAS genes (e.g., for ACE, chymase, and angiotensinogen) have been found to have point mutations which influence the production of RAS proteins; individuals with high-producer genotypes of RAS genes have been found to be at increased risk of developing gastric cancer and peptic ulcer disease [27, 28]. Although alterations in the RAS genes are clearly associated with the pathogenesis of H. pylori-related disorders and gastric carcinogenesis, the nature of the changes in gastric mucosal AT1R and AT2R expression in the different phases of H. pylori infection remains to be elucidated. Therefore, we investigated the AT1R and AT2R profiles in the acute and chronic phases of H. pylori infection in Mongolian gerbils, using real-time reverse transcription-polymerase chain reaction (RT-PCR).
A number of outer membrane proteins in H. pylori are adhesins (e.g., outer inflammatory protein [OipA]) [29–31]. The expression of OipA has been reported to be associated with severe inflammation and an increased risk of gastrointestinal diseases, suggesting that OipA may be involved in the pathogenesis of mucosal injury [29–31]. However, it is not known whether the oipA gene status affects AT1R and AT2R levels. Therefore, the present study also investigated the relationship between oipA gene status and gastric mucosal AT1R and AT2R production.
Methods
Experimental design
H. pylori strain 7.13 (kindly provided by Dr. Richard M. Peek) was chosen for this study because it has been shown to cause reproducible mucosal damage in the Mongolian gerbil model, and some 7.13 H. pylori-infected gerbils have been reported to develop gastric cancer [32, 33]. Infections with wild-type H. pylori were carried out as follows. Mongolian gerbils were randomly divided into 2 groups. Group A was uninfected (negative control) and Group B was infected with H. pylori 7.13. At 1, 3, 6, and 12 months after inoculation, 5–10 gerbils were killed. To investigate the effect of the oipA gene on ATR expression, gerbils were inoculated with an isogenic oipA mutant of H. pylori 7.13 constructed as previously described [29]. This group was designated Group C, and 5–7 gerbils were killed at 1 and 3 months after inoculation.
After the animals were killed, the stomachs were removed and opened along the greater curvature. The longitudinal half of the stomach was fixed in 10% buffered formalin for histological examination. The other half was further divided into the pyloric gland mucosa (antrum) and fundic mucosa (body) for real-time RT-PCR analysis. The presence of gastric ulcers in the antrum and body was assessed macroscopically.
The experimental protocol was approved by the Animal Care Committee of Baylor College of Medicine and Michael E. DeBakey Veterans Affairs Medical Center, Houston, Texas.
Animals and bacterial inoculation
We purchased 6-week-old male specific-pathogen-free Mongolian gerbils (MGS/Sea) with an average weight of approximately 50 g from Charles River Laboratories (Wilmington, MA, USA). The gerbils were housed in groups of 5 per cage with hard wood chip bedding, under a 12-h light/12-h dark cycle. The gerbils were cared for in accordance with our institutional guidelines. The gerbils had free access to food and drinking water throughout the experimental periods.
H. pylori cultures were grown with shaking at 200 rpm in brain heart infusion (BHI) (BD, Sparks, MD, USA) broth containing 15% fetal bovine serum (FBS) for 20 h at 37°C under microaerobic conditions (12% CO2) and saturated humidity (95%). Gerbils were inoculated with either 1 mL of H. pylori (109 CFU/mL) or 1 mL of BHI medium without FBS (control group), using a metal stomach catheter, 3 days in a row. The H. pylori-infection status was assessed by culture and histopathology [hematoxylin/eosin (H&E) stain and Genta stain] after the animals were killed.
Histological examination
Tissues were sectioned (4 μm) and routinely stained with H&E and Genta stain. The presence of inflammatory mononuclear cells (MNCs), PMNs, and intestinal metaplasia was microscopically graded from 0 (absent/normal) to 5 (maximal intensity), using a visual analogue scale [34]. The Sydney system “mild” corresponds to grades 1 and 2 in this system [34], “moderate” corresponds to grade 3, and “severe” is equivalent to grades 4 and 5. Histological evaluations from the antral and body mucosa (3 different points each) were graded and the results were averaged to obtain the final score for each animal. The results for each group are presented as medians and ranges of the gastric mucosal PMN/MNC score.
Real-time RT-PCR
Total RNA was isolated from homogenized antral and body mucosa using RNeasy Mini Kits (Qiagen, German-town, MD, USA) and 0.5 μg of RNA from each sample was reverse-transcribed using 50 units of SuperScript III RT (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s protocol. One microliter of cDNA was amplified by PCR using the qPCR MasterMix Plus for SYBR Green I Kit (Eurogentec, San Diego, CA, USA) and specific primers (0.1 nM) for AT1R, AT2R, and β-actin in the Applied Biosystems 7300 real-time RT-PCR system (Life Technologies, Carlsbad, CA, USA) (Table 1).
Table 1.
Primer pairs
| Gene | Sequence (5′–3′) | |
|---|---|---|
| AT1R | Forward | GAA CAT CCT GGG CTT TGT GT |
| Reverse | CTG AAT GAG CAC GTC CAG AA | |
| AT2R | Forward | AAA TAT GCC CAG TGG TCT GC |
| Reverse | CAG GAC TTG GTC CCT GGT TA | |
| β-actin | Forward | TCC TCC CTG GAG AAG AGC TA |
| Reverse | CCA GAC AGC ACT GTG TTG GC |
AT1R angiotensin II type 1 receptor, AT2R angiotensin II type 2 receptor
Data analysis
Data are presented as medians and ranges (histological evaluation scores) or as means and standard error of the mean (SEM) (gastric mucosal AT1R and AT2R mRNA levels). The Mann–Whitney U-test was used to determine whether the histological scores differed in relation to the different observation periods or H. pylori strains. Statistically significant differences in the gastric mucosal AT1R and AT2R mRNA levels among the different observation periods or among the different H. pylori strains were determined by using the Student’s t-test. Statistically significant associations between AT1R or AT2R mRNA levels and the median gastric mucosal PMN/MNC score and between the ratios of AT1R/β-actin mRNA and AT2R/β-actin mRNA were assessed by calculating Spearman’s correlation coefficients. A P value of <0.05 was considered to indicate statistical significance. Calculations were carried out using the statistical software StatView 5.0 (SAS Institute, Cary, NC, USA).
Results
Development of gastric ulcers after H. pylori infection
All gerbils inoculated with wild-type H. pylori and oipA mutant were successfully infected. For gerbils infected with wild-type H. pylori, macroscopic gastric ulcers were observed in the antrum in 4 of 9 gerbils (44%) at 3 months, 3 of 10 gerbils (30%) at 6 months, and 3 of 5 gerbils (60%) at 12 months (Table 2). Gastric ulcers were observed in the body in 5 of 9 gerbils (56%) at 3 months and 1 of 10 gerbils (10%) at 6 months (Table 2). No gastric ulcers or erosions were seen in gerbils infected with the oipA mutant at 3 months after inoculation (Table 2). In addition, no ulcers were seen in the negative control group.
Table 2.
Incidence of gastric ulcer after inoculation with Helico-bacter pylori
| Strain | Period (months) | Number | Peptic ulcer
|
|
|---|---|---|---|---|
| Antrum | Body | |||
| Wild-type | 1 | 6 | 0 (0%) | 0 (0%) |
| Wild-type | 3 | 9 | 4 (44%) | 5 (56%) |
| Wild-type | 6 | 10 | 3 (30%) | 1 (10%) |
| Wild-type | 12 | 5 | 3 (60%) | 0 (0%) |
| oipA mutant | 1 | 6 | 0 (0%) | 0 (0%) |
| oipA mutant | 3 | 5 | 0 (0%) | 0 (0%) |
Histopathological findings after inoculation with wild-type H. pylori and oipA mutant
At 6 and 12 months, all gerbils infected with wild-type H. pylori had intestinal metaplasia (data not shown), which is thought to increase the risk of developing gastric cancer. However, in contrast to previous studies using H. pylori strain 7.13 [32, 33], no infected gerbils developed gastric cancer. Intestinal metaplasia or gastric cancer was not seen in gerbils infected with the oipA mutant or in the uninfected control group.
Mucosal infiltration of MNCs and PMNs was rare or absent in control animals. A significant increase in the MNC and PMN scores in the antrum was observed at 3–12 months compared with those in the pre-infection period or at 1 month (Table 3). Median MNC and PMN scores and the thickness of the gastric antral and body wall in gerbils infected with wild-type H. pylori were always greater than these parameters in uninfected controls at all time points. In the antrum and body of the gastric ulcer group, the median levels of MNC and PMN infiltration were also significantly higher than those in the non-ulcer groups in each area (Table 4).
Table 3.
Infiltration of mononuclear and polymorphonuclear cells in the gastric antral and body mucosa of Mongolian gerbils infected with wild-type Helicobacter pylori or the oipA knockout strain
| Period | Antrum
|
Body
|
||||
|---|---|---|---|---|---|---|
| Uninfected control | Wild-type | oipA mutant | Uninfected control | Wild-type | oipA mutant | |
| MNC (months) | ||||||
| 0 | 0 (0–0) | 0 (0–0) | 0 (0–0) | 0 (0–0) | 0 (0–0) | 0 (0–0) |
| 1 | 0 (0–0) | 1 (0–3) | 0 (0–0) | 0 (0–0) | 0 (0–1) | 0 (0–0) |
| 3 | 0 (0–0) | 4 (0–5)*,**,# | 3 (0–4)*,** | 0 (0–0) | 2 (0–4)*,**,# | 0 (0–1) |
| 6 | 0 (0–0) | 5 (4–5)*,** | NE | 0 (0–0) | 3 (3–4)*,**,*** | NE |
| 12 | 0 (0–0) | 4 (2–4)*,** | NE | 0 (0–0) | 4 (2–4)*,**,*** | NE |
| PMN (months) | ||||||
| 0 | 0 (0–0) | 0 (0–0) | 0 (0–0) | 0 (0–0) | 0 (0–0) | 0 (0–0) |
| 1 | 0 (0–0) | 0 (0–2) | 0 (0–0) | 0 (0–0) | 0 (0–1) | 0 (0–0) |
| 3 | 0 (0–0) | 3 (0–4)*,**,# | 1 (0–2) | 0 (0–0) | 1 (0–3)*,**,# | 0 (0–0) |
| 6 | 0 (0–0) | 3 (2–4)*,** | NE | 0 (0–0) | 2 (1–3)*,** | NE |
| 12 | 0 (0–0) | 3 (0–3)*,** | NE | 0 (0–0) | 2 (0–3)*,** | NE |
MNC and PMN infiltration levels are expressed as medians (ranges)
MNC mononuclear cells, PMN polymorphonuclear cells, NE not examined
P <0.05 compared with 0 month
P <0.05 compared with the wild-type infected group at 1 month
P <0.05 compared with the wild-type infected group at 3 months
P <0.05 compared with the oipA mutant-infected group at the same time point after infection
Table 4.
Infiltration of mononuclear and polymorphonuclear cells in the gastric antral and body mucosa of Mongolian gerbils with/without gastric ulcer
| Period (months) | Antrum
|
Body
|
||
|---|---|---|---|---|
| GU(+) | GU(−) | GU(+) | GU(−) | |
| MNC | 3 (1–4)* | 1 (0–4) | 5 (4–5)* | 3 (0–5) |
| 0 | No case | 0 (0–0) | No case | 0 (0–0) |
| 1 | No case | 0 (0–1) | No case | 0 (0–3) |
| 3 | 2 (1–4)* | 0 (0–4) | 4.5 (4–5)* | 3 (0–5) |
| 6 | 4 (4–4) | 3 (3–4) | 5 (5–5) | 4.5 (4–5) |
| 12 | 4 (3–4)* | 2 (2–2) | No case | 3.5 (2–4) |
| PMN | 2 (0–3)* | 0 (0–3) | 2 (0–4)* | 2 (0–4) |
| 0 | No case | 0 (0–0) | No case | 0 (0–0) |
| 1 | No case | 0 (0–1) | No case | 0 (0–2) |
| 3 | 1 (0–3) | 0 (0–3) | 3.5 (3–4)* | 1 (0–4) |
| 6 | 2 (2–2) | 1.5 (1–3) | 4 (2–4) | 3 (3–3) |
| 12 | 3 (2–3)* | 0 (0–0) | No case | 2.5 (0–3) |
MNC and PMN infiltration levels are shown as medians (ranges)
Gastric ulcer (GU) (+) and GU (−); only wild-type Helicobacter pylori-infected animals with and without gastric ulcers, respectively
MNC mononuclear cell, PMN polymorphonuclear cell
P <0.05 compared with the GU (−) group
The oipA mutant induced significantly milder inflammation compared with the wild-type H. pylori strain in both the antrum and body at 3 months after inoculation (P <0.05, for both antrum and body) (Table 3). However, the median MNC scores in the antrum were significantly higher in gerbils infected with the oipA mutant at 3 months after inoculation compared to uninfected controls at 0 or 3 months (Table 3).
Gastric mucosal AT1R and AT2R mRNA levels in Mongolian gerbils infected with H. pylori
Gastric mucosal AT1R and AT2R/β-actin mRNA ratios were approximately zero in the gastric mucosa of uninfected animals throughout the 12-month observation period in both the antral and body mucosa. In animals infected with wild-type H. pylori, both the AT1R and AT2R/β-actin mRNA ratios gradually increased during the time course in both the antrum and body, with the exception of the AT2R/β-actin mRNA ratio in the antrum (the ratio was lower at 12 months than at 6 months) (Fig. 1a, b). At 1 and 6 months after the inoculation of wild-type H. pylori, the AT1R/β-actin mRNA ratio in the antrum was significantly higher than that in the body (Fig. 1a). At 12 months, the AT2R/β-actin mRNA ratio in the body was significantly higher than that in the antrum (Fig. 1b). The AT1R and AT2R/β-actin mRNA ratios in the antrum and body in the wild-type inoculated group were significantly greater than the values in the controls at all time points (data not shown).
Fig. 1.
Gastric antrum (white circles) and body (black squares) mucosal AT1R and AT2R mRNA levels in Mongolian gerbils infected with Helicobacter pylori strain 7.13 (wild-type) over 12 months. The maximum levels of AT1R and AT2R mRNA were seen 12 months after inoculation in both the antrum and body, except for AT2R in the antrum. Data are shown as means ± SEM. *P <0.05 compared with the antrum. AT1R angiotensin II type 1 receptor, AT2R angiotensin II type 2 receptor
In gerbils infected with the oipA mutant, the AT1R and AT2R/β-actin mRNA ratios in the antrum and the AT2R/β-actin mRNA ratio in the body were significantly lower than the values in gerbils infected with wild-type H. pylori at 3 months (Fig. 2a, b). However, the AT1R/β-actin mRNA ratio in the body mucosa in gerbils infected with the oipA mutant was similar to that in wild-type infected gerbils (Fig. 2c). The AT1R and AT2R/β-actin mRNA ratios in the gastric antrum and body in gerbils infected with the oipA mutant were significantly greater than the values in uninfected controls at 1 month, but not at 3 months (Fig. 2).
Fig. 2.
AT1R and AT2R mRNA levels in the gastric antral (a, b) and body (c, d) mucosa of Mongolian gerbils infected with Helicobacter pylori strain 7.13 (wild-type) (white circles), Mongolian gerbils infected with oipA mutant (black triangles), and negative controls (white squares) over 3 months. Data are shown as means ± SEM. *P <0.05; compared with wild-type. AT1R angiotensin II type 1 receptor, AT2R angiotensin II type 2 receptor
Gastric mucosal AT1R and AT2R mRNA levels, MNC infiltration, and gastric ulcers in gerbils
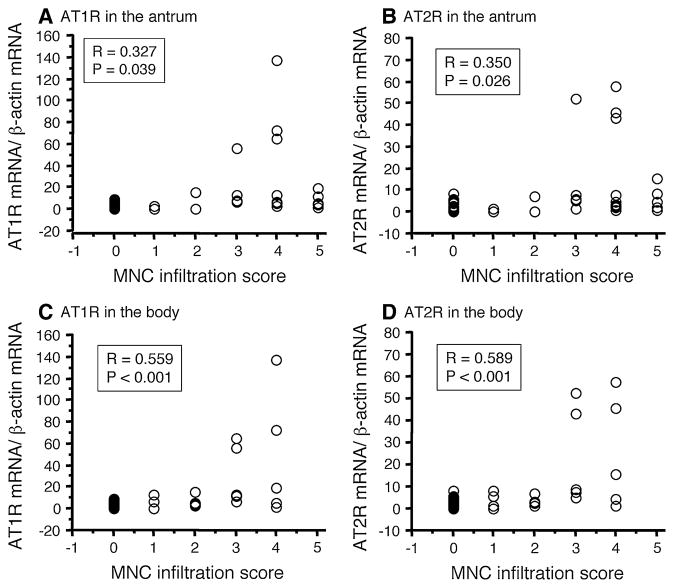
The median MNC infiltration score was significantly correlated with the AT1R levels in the antrum (r = 0.327, P = 0.039) and the body (r = 0.559, P <0.001), and with the AT2R levels in the antrum (r = 0.350, P = 0.026) and the body (r = 0.589, P <0.001) (Fig. 3). Moreover, AT1R mRNA levels were strongly correlated with AT2R levels (r = 0.887, P <0.001; Fig. 4). Interestingly, antral AT1R and AT2R mRNA levels were significantly higher in gerbils that developed gastric ulcers than in gerbils without gastric ulcer following wild-type H. pylori infection (P <0.001 for each) (Fig. 5).
Fig. 3.
Correlations between AT1R and AT2R mRNA levels and MNC infiltration scores in the antrum (a, b) and body (c, d). AT1R and AT2R mRNA levels were significantly correlated with the MNC infiltration score. AT1R angiotensin II type 1 receptor, AT2R angiotensin II type 2 receptor, MNC mononuclear cell
Fig. 4.
Correlation between AT1R and AT2R mRNA levels. AT1R mRNA levels were strongly correlated with AT2R mRNA levels. AT1R angiotensin II type 1 receptor, AT2R angiotensin II type 2 receptor
Fig. 5.
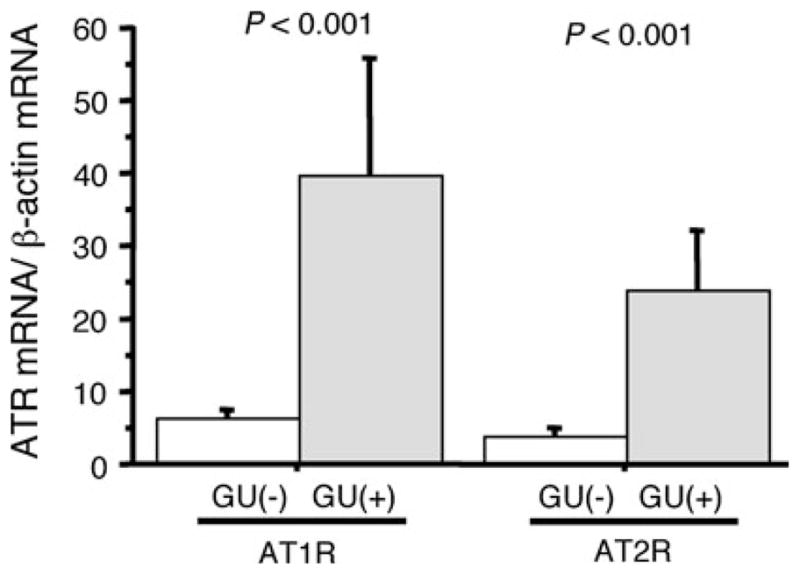
AT1R and AT2R levels in the antral gastric mucosa of the Mongolian gerbil ulcer group (GU+) and non-ulcer group (GU−). *P <0.05 (vs. non-ulcer group). AT1R angiotensin II type 1 receptor, AT2R angiotensin II type 2 receptor, GU gastric ulcer
Discussion
Up-regulation of the RAS pathway leads to increased angiotensin II, ACE, chymase, and AT1R levels, as well as increased infiltration of inflammatory cells, such as MNCs, PMNs, and mast cells [35–38]. RAS components were not expressed in the gastric mucosa of gerbils unless they were infected with H. pylori [22–25, 39]. AT1R, AT2R, and other RAS components (e.g., angiotensinogen, renin, ACE, and neprilysin) are highly expressed in a subpopulation of endocrine cells, vascular endothelial cells, and inflammatory cells in the gastric wall of H. pylori-infected gerbils [26] and humans [39]. In addition, AT1R protein expression has been found to be 3–4 times higher in the gastric mucosa of H. pylori-positive subjects compared to the gastric mucosa of H. pylori-negative subjects [39]. The biological roles of AT1R include contraction of blood vessels, enhancement of sympathetic nerves, and increases in blood pressure and cell proliferation. Over-expression of AT1R may be associated with enhanced gastric mucosal inflammation, atrophy, and carcinogenesis. The roles of AT2R are opposite to those of AT1R.
A recent study in humans suggested that the administration of ACE inhibitors or ARBs, which are inhibitors/blockers of the RAS signaling system, was associated with a reduction in the risk of peptic ulcers among patients taking low-dose aspirin [40] In the present study, we demonstrated that in the Mongolian gerbil model, the gastric AT1R mRNA levels gradually increased in both the antral and body mucosa during H. pylori infection. These levels were not increased in the gastric antral and body mucosa of uninfected animals. The increase in AT1R mRNA levels also correlated with the severity of gastric mucosal MNC infiltration assessed 3–12 months after inoculation. Moreover, 41.7% of gerbils infected with wild-type H. pylori developed gastric ulcers, which was associated with high levels of inflammatory cell infiltration and AT1R mRNA. These results are consistent with the notion that the increased expression of AT1R and the subsequent increase in inflammatory cell infiltration are related to an increased incidence of gastric ulcers. In addition, these results are in agreement with data obtained in humans showing that inhibition of the gastric mucosal RAS level was associated with a decreased risk of gastric ulcers.
The Mongolian gerbil model has also been used as an animal model of gastric cancer, in which between 30 and 50% of animals develop gastric cancer 12–18 months after H. pylori infection and co-administration of a chemical carcinogen [41, 42]. In the present study, no carcinogen was administered and no gastric cancers developed during the observation period; however, most infected gerbils developed intestinal metaplasia, which is considered a precursor of gastric cancer. Gastric ulcers are also related to atrophic gastritis, and individuals with gastric ulcer are at increased risk of gastric cancer. Overall, these data suggest that the up-regulation of the RAS is related to the severity of gastric mucosal inflammation and the development of atrophy/intestinal metaplasia; thus, the RAS likely plays a role in the development of gastric cancer. This suggestion is also supported by recent epidemiological and animal model studies showing chemoprevention by ACE inhibitors and ARBs in relation to the development of gastric cancer [20, 43, 44].
Although angiotensin II-AT1R signaling pathways are generally thought to be associated with cell proliferation, angiogenesis, and inflammation [16–18, 45], the details of the effect of RAS signaling on gastric mucosal atrophy, intestinal metaplasia, and gastric cancer development remain unknown. It is known that activation of AT1R enhances the transcription of several pro-inflammatory cytokines [e.g., interleukin (IL)-1, IL-6, IL-12, and tumor necrosis factor (TNF)-α] and chemokines via nuclear factor kappa B and activator protein-1 [16]. For example, Carl-McGrath et al. [46] reported that exposure to H. pylori or inflammatory cytokines, e.g., IL-1β, IL-6, IL-8, TNF-α, and transforming growth factor (TGF)-β1 resulted in increased ACE levels in the gastric cancer cell lines MKN28, N87, and MKN45. Moreover, Bregonzio et al. [47] reported that the administration of ARBs led to a 70–80% reduction in gastric ulcer formation in spontaneously hypertensive rats treated with cold-restraint stress, and ARB administration was also associated with decreases in the stress-induced expression of TNF-α in the antrum and neutrophil infiltration into the gastric mucosa. We previously reported that inoculation of Mongolian gerbils with H. pylori resulted in acute inflammation that was paralleled by an increase in mucosal cytokine expression, especially IL-1β. In contrast, increases in chronic gastric inflammation tended to parallel interferon (IFN)-γ and IL-17 expression [8, 48]. In preliminary experiments, we measured gastric mucosal IL-17 mRNA levels in the tissue samples analyzed for ATR mRNA. IL-17 is known to play an important role in the inflammatory response to H. pylori infection and to ultimately influence the outcome of H. pylori-associated diseases [49]. We found that the gastric mucosal IL-17 mRNA levels were also correlated with AT1R and AT2R levels (data not shown). On the basis of the results reported here, we propose that H. pylori infection-induced inflammatory cell infiltration into the gastric mucosa results in enhanced RAS signaling followed by the enhanced transcription of inflammatory cytokines, which subsequently promotes increased inflammation, thus representing an increased risk of gastric mucosal atrophy, intestinal metaplasia, and gastric cancer.
H. pylori-induced gastric inflammation, atrophy, and malignancy have been linked to the presence of H. pylori virulence factors [29–31]. OipA is a pro-inflammatory response-inducing protein associated with high H. pylori density and severe PMN and MNC infiltration [30], as confirmed in the present study. OipA also functions as an adhesin and is thought to contribute to the pathogenesis of peptic ulcer and gastric cancer [29]. In the present study no gastric ulcers developed in oipA mutant-infected gerbils. Strains constitutively expressing high levels of OipA attach more tightly to the gastric mucosa and enhance gastric inflammation more effectively than strains in which OipA is poorly expressed [30, 50, 51]. We found that gastric mucosal AT1R and AT2R expression in gastric inflammatory cells was significantly lower in gerbils infected with the oipA knockout strain, which is consistent with previous findings showing that IL-1, IL-17, IL-18, and TNF-α levels were significantly lower in oipA-knockout strain-infected Mongolian gerbils compared to gerbils infected with OipA-expressing strains [8, 48]. These data are consistent with the hypothesis that OipA has an important role in modulating gastric ATR levels and contributes to the development of gastric ulcer and gastric cancer [30, 50, 51].
In previous reports, patients with a high-producer allele/genotype of RAS component genes, e.g., chymase, angiotensinogen, and ACE, were at increased risk of developing gastric cancer and peptic ulcer compared with non-carriers [27, 28]. Patients with higher RAS activity, such as those with hypertension and those receiving chronic hemodialysis, have also been reported to have an increased risk of both gastric cancer and peptic ulcer [19], suggesting that increased expression of gastric mucosal ATR mRNAs is related to the pathogenesis of ulcer and is not simply the result of gastric ulcer formation. In our animal study, the conditions (e.g., food, age, H. pylori strain, other bacterial infections, and genetic factors, such as polymorphisms) did not differ between animals that did and did not develop gastric ulcers. Future studies in which these possibly important parameters can be experimentally varied are necessary to evaluate their roles in the different clinical outcomes observed in experimental animals.
In conclusion, we have demonstrated that AT1R and AT2R play important roles in the maintenance and regulation of chronic gastric inflammation in H. pylori-infected Mongolian gerbils. Although we did not show a direct role of AT1R and AT2R in gastric carcinogenesis, the results suggest that the RAS plays an important role in gastric carcinogenesis through its effects on gastric inflammation. Moreover, modulation of the RAS may prove to be a useful approach to prevent and/or treat gastric and other cancers. We plan to use this model to clarify the role of the RAS; for example, by using ACE inhibitors and ARBs to block some functions of the RAS pathway, and to determine the effects on H. pylori-induced gastric carcinogenesis.
Acknowledgments
We acknowledge receipt of the Office of Research and Development Medical Research Service Department of Veterans Affairs, Public Health Service grant DK56338, which funds the Texas Medical Center Digestive Diseases Center, and the National Institutes of Health (NIH) grant DK 62813.
Abbreviations
- ACE
Angiotensin-I converting enzyme
- ARB
AT1R blocker
- ATR
Angiotensin II receptor
- AT1R
Angiotensin II type 1 receptor
- AT2R
Angiotensin II type 2 receptor
- CMA
Chymase
- COX
Cyclooxygenase
- FBS
Fetal bovine serum
- MNC
Mononuclear cell
- OipA
Outer inflammatory protein
- PMN
Polymorphonuclear cell
- RAS
Renin–angiotensin system
- RT-PCR
Real-time reverse transcription-polymerase chain reaction
Footnotes
Conflict of interest The authors declare no conflicts of interest.
Contributor Information
Mitsushige Sugimoto, Department of Medicine-Gastroenterology, Michael E. DeBakey Veterans Affairs Medical Center and Baylor College of Medicine, Houston, TX, USA.
Tomoyuki Ohno, Department of Medicine-Gastroenterology, Michael E. DeBakey Veterans Affairs Medical Center and Baylor College of Medicine, Houston, TX, USA.
Yoshio Yamaoka, Email: yyamaoka@oita-u.ac.jp, yyamaoka@bcm.edu, Department of Medicine-Gastroenterology, Michael E. DeBakey Veterans Affairs Medical Center and Baylor College of Medicine, Houston, TX, USA. Department of Environmental and Preventive Medicine, Oita University Faculty of Medicine, 1-1 Idaigaoka, Hasama-machi, Yufu, Oita 879-5593, Japan.
References
- 1.Uemura N, Okamoto S, Yamamoto S, Matsumura N, Yamaguchi S, Yamakido M, et al. Helicobacter pylori infection and the development of gastric cancer. N Engl J Med. 2001;345:784–9. doi: 10.1056/NEJMoa001999. [DOI] [PubMed] [Google Scholar]
- 2.Take S, Mizuno M, Ishiki K, Nagahara Y, Yoshida T, Yokota K, et al. The effect of eradicating Helicobacter pylori on the development of gastric cancer in patients with peptic ulcer disease. Am J Gastroenterol. 2005;100:1037–42. doi: 10.1111/j.1572-0241.2005.41384.x. [DOI] [PubMed] [Google Scholar]
- 3.Wong BC, Lam SK, Wong WM, Chen JS, Zheng TT, Feng RE, et al. Helicobacter pylori eradication to prevent gastric cancer in a high-risk region of China: a randomized controlled trial. JAMA. 2004;291:187–94. doi: 10.1001/jama.291.2.187. [DOI] [PubMed] [Google Scholar]
- 4.Fukase K, Kato M, Kikuchi S, Inoue K, Uemura N, Okamoto S, et al. Effect of eradication of Helicobacter pylori on incidence of metachronous gastric carcinoma after endoscopic resection of early gastric cancer: an open-label, randomised controlled trial. Lancet. 2008;372:392–7. doi: 10.1016/S0140-6736(08)61159-9. [DOI] [PubMed] [Google Scholar]
- 5.Bamford KB, Fan X, Crowe SE, Leary JF, Gourley WK, Luthra GK, et al. Lymphocytes in the human gastric mucosa during Helicobacter pylori have a T helper cell 1 phenotype. Gastroenterology. 1998;114:482–92. doi: 10.1016/s0016-5085(98)70531-1. [DOI] [PubMed] [Google Scholar]
- 6.Yamaoka Y, Kita M, Kodama T, Sawai N, Kashima K, Imanishi J. Expression of cytokine mRNA in gastric mucosa with Helicobacter pylori infection. Scand J Gastroenterol. 1995;30:1153–9. doi: 10.3109/00365529509101624. [DOI] [PubMed] [Google Scholar]
- 7.Yamaoka Y, Kita M, Kodama T, Sawai N, Kashima K, Imanishi J. Induction of various cytokines and development of severe mucosal inflammation by cagA gene positive Helicobacter pylori strains. Gut. 1997;41:442–51. doi: 10.1136/gut.41.4.442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yamaoka Y, Yamauchi K, Ota H, Sugiyama A, Ishizone S, Graham DY, et al. Natural history of gastric mucosal cytokine expression in Helicobacter pylori gastritis in Mongolian gerbils. Infect Immun. 2005;73:2205–12. doi: 10.1128/IAI.73.4.2205-2212.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hirayama F, Takagi S, Kusuhara H, Iwao E, Yokoyama Y, Ikeda Y. Induction of gastric ulcer and intestinal metaplasia in Mongolian gerbils infected with Helicobacter pylori. J Gastroenterol. 1996;31:755–7. doi: 10.1007/BF02347631. [DOI] [PubMed] [Google Scholar]
- 10.Ikeno T, Ota H, Sugiyama A, Ishida K, Katsuyama T, Genta RM, et al. Helicobacter pylori-induced chronic active gastritis, intestinal metaplasia, and gastric ulcer in Mongolian gerbils. Am J Pathol. 1999;154:951–60. doi: 10.1016/S0002-9440(10)65343-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kumagai T, Yan J, Graham DY, Tozuka M, Okimura Y, Ikeno T, et al. Serum immunoglobulin G immune response to Helico-bacter pylori antigens in Mongolian gerbils. J Clin Microbiol. 2001;39:1283–8. doi: 10.1128/JCM.39.4.1283-1288.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sakai T, Fukui H, Franceschi F, Penland R, Sepulveda AR, Fujimori T, et al. Cyclooxygenase expression during Helicobacter pylori infection in Mongolian gerbils. Dig Dis Sci. 2003;48:2139–46. doi: 10.1023/b:ddas.0000004517.83166.26. [DOI] [PubMed] [Google Scholar]
- 13.Takashima M, Furuta T, Hanai H, Sugimura H, Kaneko E. Effects of Helicobacter pylori infection on gastric acid secretion and serum gastrin levels in Mongolian gerbils. Gut. 2001;48:765–73. doi: 10.1136/gut.48.6.765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ogura K, Maeda S, Nakao M, Watanabe T, Tada M, Kyutoku T, et al. Virulence factors of Helicobacter pylori responsible for gastric diseases in Mongolian gerbil. J Exp Med. 2000;192:1601–10. doi: 10.1084/jem.192.11.1601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Watanabe T, Tada M, Nagai H, Sasaki S, Nakao M. Helicobacter pylori infection induces gastric cancer in Mongolian gerbils. Gastroenterology. 1998;115:642–8. doi: 10.1016/s0016-5085(98)70143-x. [DOI] [PubMed] [Google Scholar]
- 16.Suzuki Y, Ruiz-Ortega M, Lorenzo O, Ruperez M, Esteban V, Egido J. Inflammation and angiotensin II. Int J Biochem Cell Biol. 2003;35:881–900. doi: 10.1016/s1357-2725(02)00271-6. [DOI] [PubMed] [Google Scholar]
- 17.Le Noble FA, Hekking JW, Van Straaten HW, Slaaf DW, Struyker Boudier HA. Angiotensin II stimulates angiogenesis in the chorio-allantoic membrane of the chick embryo. Eur J Pharmacol. 1991;195:305–6. doi: 10.1016/0014-2999(91)90552-2. [DOI] [PubMed] [Google Scholar]
- 18.Muramatsu M, Yamada M, Takai S, Miyazaki M. Suppression of basic fibroblast growth factor-induced angiogenesis by a specific chymase inhibitor, BCEAB, through the chymase-angiotensin-dependent pathway in hamster sponge granulomas. Br J Pharmacol. 2002;137:554–60. doi: 10.1038/sj.bjp.0704893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Khaw KT, Barrett-Connor E. Systolic blood pressure and cancer mortality in an elderly population. Am J Epidemiol. 1984;120:550–8. doi: 10.1093/oxfordjournals.aje.a113916. [DOI] [PubMed] [Google Scholar]
- 20.Lever AF, Hole DJ, Gillis CR, McCallum IR, McInnes GT, MacKinnon PL, et al. Do inhibitors of angiotensin-I-converting enzyme protect against risk of cancer? Lancet. 1998;352:179–84. doi: 10.1016/S0140-6736(98)03228-0. [DOI] [PubMed] [Google Scholar]
- 21.Yasumaru M, Tsuji S, Tsujii M, Irie T, Komori M, Kimura A, et al. Inhibition of angiotensin II activity enhanced the antitumor effect of cyclooxygenase-2 inhibitors via insulin-like growth factor I receptor pathway. Cancer Res. 2003;63:6726–34. [PubMed] [Google Scholar]
- 22.Kondo K, Muramatsu M, Okamoto Y, Jin D, Takai S, Tanigawa N, et al. Expression of chymase-positive cells in gastric cancer and its correlation with the angiogenesis. J Surg Oncol. 2006;93:36–42. doi: 10.1002/jso.20394. [DOI] [PubMed] [Google Scholar]
- 23.Rocken C, Lendeckel U, Dierkes J, Westphal S, Carl-McGrath S, Peters B, et al. The numberoflymph node metastasesin gastric cancer correlates with the angiotensin I-converting enzyme gene insertion/deletion polymorphism. Clin Cancer Res. 2005;11:2526–30. doi: 10.1158/1078-0432.CCR-04-1922. [DOI] [PubMed] [Google Scholar]
- 24.Matsuo T, Ikura Y, Ohsawa M, Ogami M, Kayo S, Yoshimi N, et al. Mast cell chymase expression in Helicobacter pylori-associated gastritis. Histopathology. 2003;43:538–49. doi: 10.1111/j.1365-2559.2003.01731.x. [DOI] [PubMed] [Google Scholar]
- 25.Bregonzio C, Armando I, Ando H, Jezova M, Baiardi G, Saavedra JM. Anti-inflammatory effects of angiotensin II AT1 receptor antagonism prevent stress-induced gastric injury. Am J Physiol Gastrointest Liver Physiol. 2003;285:G414–23. doi: 10.1152/ajpgi.00058.2003. [DOI] [PubMed] [Google Scholar]
- 26.Hallersund P, Helander HF, Casselbrant A, Edebo A, Fandriks L, Elfvin A. Angiotensin II receptor expression and relation to Helicobacter pylori-infection in the stomach of the Mongolian gerbil. BMC Gastroenterol. 2010;10:3. doi: 10.1186/1471-230X-10-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sugimoto M, Furuta T, Shirai N, Ikuma M, Sugimura H, Hishida A. Influences of chymase and angiotensin I-converting enzyme gene polymorphisms on gastric cancer risks in Japan. Cancer Epidemiol Biomarkers Prev. 2006;15:1929–34. doi: 10.1158/1055-9965.EPI-06-0339. [DOI] [PubMed] [Google Scholar]
- 28.Sugimoto M, Furuta T, Shirai N, Kodaira C, Nishino M, Ikuma M, et al. Role of angiotensinogen gene polymorphism on Helicobacter pylori infection-related gastric cancer risk in Japanese. Carcinogenesis. 2007;28:2036–40. doi: 10.1093/carcin/bgm074. [DOI] [PubMed] [Google Scholar]
- 29.Yamaoka Y, Kwon DH, Graham DY. A M(r) 34,000 proinflammatory outer membrane protein (oipA) of Helicobacter pylori. Proc Natl Acad Sci USA. 2000;97:7533–8. doi: 10.1073/pnas.130079797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yamaoka Y, Kikuchi S, el-Zimaity HM, Gutierrez O, Osato MS, Graham DY. Importance of Helicobacter pylori oipA in clinical presentation, gastric inflammation, and mucosal interleukin 8 production. Gastroenterology. 2002;123:414–24. doi: 10.1053/gast.2002.34781. [DOI] [PubMed] [Google Scholar]
- 31.Kudo T, Nurgalieva ZZ, Conner ME, Crawford S, Odenbreit S, Haas R, et al. Correlation between Helicobacter pylori OipA protein expression and oipA gene switch status. J Clin Microbiol. 2004;42:2279–81. doi: 10.1128/JCM.42.5.2279-2281.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Franco AT, Israel DA, Washington MK, Krishna U, Fox JG, Rogers AB, et al. Activation of beta-catenin by carcinogenic Helicobacter pylori. Proc Natl Acad Sci USA. 2005;102:10646–51. doi: 10.1073/pnas.0504927102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Franco AT, Johnston E, Krishna U, Yamaoka Y, Israel DA, Nagy TA, et al. Regulation of gastric carcinogenesis by Helicobacter pylori virulence factors. Cancer Res. 2008;68:379–87. doi: 10.1158/0008-5472.CAN-07-0824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.el-Zimaity HM, Graham DY, al-Assi MT, Malaty H, Karttunen TJ, Graham DP, et al. Interobserver variation in the histopathological assessment of Helicobacter pylori gastritis. Hum Pathol. 1996;27:35–41. doi: 10.1016/s0046-8177(96)90135-5. [DOI] [PubMed] [Google Scholar]
- 35.Rigat B, Hubert C, Alhenc-Gelas F, Cambien F, Corvol P, Soubrier F. An insertion/deletion polymorphism in the angiotensin I-converting enzyme gene accounting for half the variance of serum enzyme levels. J Clin Invest. 1990;86:1343–6. doi: 10.1172/JCI114844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Grzeszczak W, Zychma MJ, Lacka B, Zukowska-Szczechowska E. Angiotensin I-converting enzyme gene polymorphisms: relationship to nephropathy in patients with non-insulin dependent diabetes mellitus. J Am Soc Nephrol. 1998;9:1664–9. doi: 10.1681/ASN.V991664. [DOI] [PubMed] [Google Scholar]
- 37.Villard E, Tiret L, Visvikis S, Rakotovao R, Cambien F, Soubrier F. Identification of new polymorphisms of the angiotensin I-converting enzyme (ACE) gene, and study of their relationship to plasma ACE levels by two-QTL segregation-linkage analysis. Am J Hum Genet. 1996;58:1268–78. [PMC free article] [PubMed] [Google Scholar]
- 38.Nakajima S, Krishnan B, Ota H, Segura AM, Hattori T, Graham DY, et al. Mast cell involvement in gastritis with or without Helicobacter pylori infection. Gastroenterology. 1997;113:746–54. doi: 10.1016/s0016-5085(97)70167-7. [DOI] [PubMed] [Google Scholar]
- 39.Hallersund P, Elfvin A, Helander HF, Fandriks L. The expression of renin–angiotensin system components in the human gastric mucosa. J Renin Angiotensin Aldosterone Syst. 2011;12:54–64. doi: 10.1177/1470320310379066. [DOI] [PubMed] [Google Scholar]
- 40.Shiotani A, Sakakibara T, Yamanaka Y, Imamura H, Tarumi K, Manabe N, et al. Upper gastrointestinal ulcer in Japanese patients taking low-dose aspirin. J Gastroenterol. 2009;44:126–31. doi: 10.1007/s00535-008-2290-6. [DOI] [PubMed] [Google Scholar]
- 41.Sugiyama A, Maruta F, Ikeno T, Ishida K, Kawasaki S, Katsuyama T, et al. Helicobacter pylori infection enhances N-methyl-N-nitrosourea-induced stomach carcinogenesis in the Mongolian gerbil. Cancer Res. 1998;58:2067–9. [PubMed] [Google Scholar]
- 42.Tatematsu M, Tsukamoto T, Mizoshita T. Role of Helicobacter pylori in gastric carcinogenesis: the origin of gastric cancers and heterotopic proliferative glands in Mongolian gerbils. Helicobacter. 2005;10:97–106. doi: 10.1111/j.1523-5378.2005.00305.x. [DOI] [PubMed] [Google Scholar]
- 43.Fujita M, Hayashi I, Yamashina S, Itoman M, Majima M. Blockade of angiotensin AT1a receptor signaling reduces tumor growth, angiogenesis, and metastasis. Biochem Biophys Res Commun. 2002;294:441–7. doi: 10.1016/S0006-291X(02)00496-5. [DOI] [PubMed] [Google Scholar]
- 44.Egami K, Murohara T, Shimada T, Sasaki K, Shintani S, Sugaya T, et al. Role of host angiotensin II type 1 receptor in tumor angiogenesis and growth. J Clin Invest. 2003;112:67–75. doi: 10.1172/JCI16645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lee JM, Lo AC, Yang SY, Tsau HS, Chen RJ, Lee YC. Association of angiotensin-converting enzyme insertion/deletion polymorphism with serum level and development of pulmonary complications following esophagectomy. Ann Surg. 2005;241:659–65. doi: 10.1097/01.sla.0000157132.08833.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Carl-McGrath S, Grantzdorffer I, Lendeckel U, Ebert MP, Rocken C. Angiotensin II-generating enzymes, angiotensin-converting enzyme (ACE) and mast cell chymase (CMA1), in gastric inflammation may be regulated by H. pylori and associated cytokines. Pathology. 2009;41:419–27. doi: 10.1080/00313020902885037. [DOI] [PubMed] [Google Scholar]
- 47.Bregonzio C, Armando I, Ando H, Jezova M, Baiardi G, Saavedra JM. Angiotensin II AT1 receptor blockade prevents gastric ulcers during cold-restraint stress. Ann N Y Acad Sci. 2004;1018:351–5. doi: 10.1196/annals.1296.044. [DOI] [PubMed] [Google Scholar]
- 48.Sugimoto M, Ohno T, Graham DY, Yamaoka Y. Gastric mucosal interleukin-17 and -18 mRNA expression in Helicobacter pylori-induced Mongolian gerbils. Cancer Sci. 2009;100:2152–9. doi: 10.1111/j.1349-7006.2009.01291.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shiomi S, Toriie A, Imamura S, Konishi H, Mitsufuji S, Iwakura Y, et al. IL-17 is involved in Helicobacter pylori-induced gastric inflammatory responses in a mouse model. Helicobacter. 2008;13:518–24. doi: 10.1111/j.1523-5378.2008.00629.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yamaoka Y, Kudo T, Lu H, Casola A, Brasier AR, Graham DY. Role of interferon-stimulated responsive element-like element in interleukin-8 promoter in Helicobacter pylori infection. Gastroenterology. 2004;126:1030–43. doi: 10.1053/j.gastro.2003.12.048. [DOI] [PubMed] [Google Scholar]
- 51.Dossumbekova A, Prinz C, Mages J, Lang R, Kusters JG, van Vliet AH, et al. Helicobacter pylori HopH (OipA) and bacterial pathogenicity: genetic and functional genomic analysis of hopH gene polymorphisms. J Infect Dis. 2006;194:1346–55. doi: 10.1086/508426. [DOI] [PubMed] [Google Scholar]