Abstract
Androgen receptor (AR) signaling exerts an antiestrogenic, growth-inhibitory influence in normal breast tissue, and this role may be sustained in estrogen receptor α (ERα)-positive luminal breast cancers. Conversely, AR signaling may promote growth of a subset of ERα-negative, AR-positive breast cancers with a molecular apocrine phenotype. Understanding the molecular mechanisms whereby androgens can elicit distinct gene expression programs and opposing proliferative responses in these two breast cancer phenotypes is critical to the development of new therapeutic strategies to target the AR in breast cancer.
Antagonism between estrogen and androgen hormone action is elegantly exemplified by the sexual dimorphism of breast development; full mammary gland growth and maturation occurs in females due to the dominance of estrogen action and does not occur in males due to the dominance of androgen action. Perturbations of sex-specific hormone levels can cause abnormal breast development in males and females. This also occurs at the receptor level, whereby the mammary gland fails to develop in females lacking a functional estrogen receptor α (ERα) and fully develops in males lacking a functional androgen receptor (AR). Antagonistic cross talk between these sex hormone signaling pathways has ramifications for breast carcinogenesis and the management of breast cancer. The oncogenic role of ERα signaling in breast cancer is recognized, extensively characterized, and has been therapeutically exploited for nearly half a century (1–3). Although the presence of AR in malignant breast tissues was discovered decades ago (4–6), and androgen treatment was historically and successfully used to treat breast cancer (7–10), scientific investigation of the role and workings of AR in breast carcinogenesis lagged significantly behind that of ERα. However, clinical and molecular studies involving AR in breast cancer over the past decade, and in particular over the last year, have reinvigorated interest, and the AR has emerged as a prime new candidate for therapeutic targeting in women with this disease. From a molecular biology perspective, the recent findings gleaned from transcriptome and cistrome profiling studies are particularly fascinating and raise two provocative questions. 1) Does sex hormone antagonism involve AR and ERα competing for the same DNA binding sites in a cell? 2) In the absence of ERα, can AR adopt an ERα-like oncogenic role? This review will describe the ability of AR action to inhibit normal breast tissue growth, examine the prevalence and prognostic value of AR in breast cancer, and critically appraise the evidence that AR has dichotomous roles in breast carcinogenesis that depend, at least in part, on whether it can duel with ERα or not.
Sex Hormone Antagonism within Normal Breast Tissue
The rise and fall of circulating hormones
It is often not appreciated or acknowledged that many female tissues, including the breast, possess a functional AR signaling axis. Androgenic hormones are the major circulating sex hormones in females as well as males (11), and although most can be metabolized into estrogenic hormones, circulating androgens can also act directly as, or be metabolized into, AR agonists. Endogenous AR ligands include testosterone and 5α-dihydrotestosterone (DHT), the former representing a major circulating hormone and the latter representing a more potent, locally produced and used metabolite (see below). Testosterone is mainly synthesized and secreted by the ovaries and adrenal glands in women, with additional biosynthesis occurring within peripheral tissues via metabolic conversion of circulating adrenal proandrogens (12). In premenopausal women, circulating testosterone and estradiol levels peak at midcycle, but in transition to the luteal phase, testosterone levels go down and estradiol levels increase further (13). Breast epithelial cell proliferation is highest during the luteal phase of the menstrual cycle in premenopausal women (14). During the follicular phase, circulating estradiol levels reach a nadir, but testosterone levels stay constant, a period that corresponds with the highest rate of apoptosis in the breast epithelium. After menopause, circulating estradiol levels decrease by 10-fold due to cessation of ovulation, but testosterone levels decrease by only 1.5-fold (13). In the absence of estrogenic hormone stimulation, postmenopausal breast tissue undergoes a slow, progressive involution characterized by increasing atrophy of glandular tissue and fibrosis of the stromal tissues. These morphological changes may be due to unopposed androgen action, a concept supported by the fact that dramatically accelerated involution occurs when pharmacological doses of androgen are given to women undergoing a female-to-male sex reversal (15).
Although the relative levels of circulating sex hormones clearly influence the proliferative capacity of breast epithelial cells, the breast as an organ has a complex intracrinology due to the ability of various tissue compartments to metabolize circulating steroids (11, 16). Testosterone has the potential to be metabolized within breast tissue to 17β-estradiol (E2), the most potent natural ERα ligand, or DHT, the most potent natural AR ligand, via the activity of aromatase and 5α-reductase enzymes, respectively (12). Therefore, the influence of circulating testosterone on the proliferative capacity of breast epithelial cells is in part dependent upon the relative expression and activity of aromatase and 5α-reductase that occur within the breast tissues. In studies of transgenic mice that overexpress the aromatase gene (AROM+), males undergo abnormal breast development due to excess exposure to estradiol (17). Administration of an aromatase inhibitor to AROM+ transgenic male mice causes involution of the breast tissue, an event that is associated with increased exposure to testosterone and a marked induction of AR expression in the breast epithelium (18). Aromatase expression within the breast tissue is particularly important in women after menopause, when local synthesis becomes the primary source of E2 and abnormally elevated aromatase activity can contribute to breast carcinogenesis (19). Menopause can be simulated in animals via removal of the ovaries, thereby eliminating cyclic fluctuations of E2 and testosterone. In such models, administration of testosterone or E2 alone can stimulate proliferation of breast epithelial cells, suggesting that in the absence of estrogen, testosterone is preferentially converted to E2 within the breast. In contrast, when testosterone and E2 are administered conjointly, the stimulatory effects of E2 alone are abrogated, suggesting that testosterone is acting directly or being preferentially converted to DHT, to exert antiestrogenic, antiproliferative effects (20, 21). Under normal physiological conditions, this adaptive intracrinology ensures that breast epithelial cells are stimulated to proliferate, enter cell cycle arrest, or die in a controlled manner via a balance of stimulatory and inhibitory sex hormone influences.
Cellular mediators of the sex hormone duel
At the cellular level, estrogen and androgen hormone signals are transduced via the action of specific members of a superfamily of nuclear receptors with lipophilic ligands, including steroid hormones. Receptors within this superfamily are classified into groups based on evolutionary conservation of their DNA and ligand-binding domains; ERα and AR belong to the third subclass, comprised of steroid hormone receptors, and are identified by the symbols NR3A1 and NR3C4, respectively. ERα is the ancestral prototype from which all of the other class 3 nuclear receptors have evolved (22). There are two major forms of the estrogen receptor (ERα and ERβ) that are transcribed from independent somatic genes (23, 24), but one major form of the AR that arises from a single gene on the X-chromosome (25, 26). In general, steroid hormone receptors are ligand-activated transcription factors that have four main functional domains: the N-terminal transactivation domain, the DNA-binding domain, a hinge region, and a ligand-binding domain. Steroid hormone receptors interact with a host of different coregulatory proteins and the transcriptional machinery to regulate gene transcription. As there is a dedicated website with comprehensive information on nuclear receptors, their coregulators, and their transcriptional activities in relation to various diseases (www.nursa.org) (27), this will not be further detailed herein. Studies over the recent past that have profiled global patterns of nuclear receptor DNA binding in various cell lines using methodologies such as chromatin immunoprecipitation followed by massively parallel DNA sequencing (ChIP-seq) suggest that receptors such as ERα and AR act more as long-range enhancers instead of direct promoters of gene transcription (28, 29). More recent genomic interrogation using global run-on and sequencing (GRO-seq) has revealed that ERα and AR regulate the expression of noncoding RNA that arise from intergenic regions of the genome proximal to AR or ERα DNA docking sites in breast and prostate cancer cells, respectively (30, 31). Differential and temporal activation of these enhancer-templated noncoding RNA may represent an important means by which steroid receptors act as master regulators of gene transcription during normal development and disease progression (31), but this has yet to be extensively investigated. Cell-type specificity of steroid receptor action appears to be mediated via epigenetic mechanisms that govern the binding of specific pioneer factors that in turn make chromatin more accessible to nuclear receptors to bind DNA. A forkhead box (FOX) family member (FOXA1) has been shown to be a critical pioneer factor that facilitates ERα and AR to bind DNA in breast and prostate cancer model systems, respectively (32).
Androgen and estrogen action during mammary gland development
At birth, the mammary gland is a rudimentary ductal structure that around the time of puberty is stimulated to extend, invade into the surrounding connective tissue, and develop terminal structures destined to be secretory glands during lactation. In most species, expression of ERα is restricted to luminal epithelial cells that line the mammary ducts and glandular alveoli and is critical for postpubertal mammary morphogenesis (33). Although estrogen hormones can also stimulate ERβ, and this isoform is more abundantly expressed in breast tissue than ERα, female mice lacking ERβ have mammary glands that are structurally and functionally similar to wild-type glands (34). As such, the primary ER isoform involved in mammary gland morphogenesis is considered to be ERα. Expression of FOXA1 is required for mammary epithelial cells to acquire ERα expression and for ERα to subsequently stimulate mammary gland development, because FOXA1-null female mice have a breast phenotype nearly identical to that exhibited by ERα-null females (35). However, FOXA1-null animals develop end buds at the termini of stunted ductal structures that are capable of some terminal differentiation, which does not occur in ERα-null animals (35). Like ERα, the AR is also present in luminal mammary epithelial cells, but immunoreactive AR is additionally evident in other cell types within the breast including stromal fibroblasts and adipocytes (Fig. 1). Whereas AR is not critical for breast morphogenesis in transgenic mouse models, the peripubertal phenotype is influenced in a manner dependent on which transgenic model of AR deficiency is being used. One model involves a global ablative strategy that results in expression of a severely truncated AR protein, and null females exhibit mildly stunted mammary gland development characterized by reduced pre- and postpubertal ductal branching and reduced glandular development during adolescence, pregnancy, and lactation (36). The second model involves a global ablative strategy that results in expression of a mutated AR protein that cannot bind DNA and is therefore functionally deficient (37). AR-deficient females generated by the latter study display a trend toward enhanced mammary ductal branching and exhibit increased breast epithelial cell proliferation and increased sensitivity to 7,12-dimethylbenz(a)anthracine (DMBA) induced mammary tumor formation. Both models of AR-null female mice have ovarian defects that reduce the levels of circulating ovarian hormones, but this phenotype is less severe in the functionally deficient AR model (38). Collectively, the data from these two studies indicate that the influence of AR on the breast epithelium is dependent on the relative exposure to ovarian hormones. In nontransgenic female mice, the level of AR protein in the breast epithelium doubles during the pubertal growth phase, and antagonizing AR action at this time increases the number of proliferating cells approximately 8-fold (39). Likewise, AR antagonism in adult female monkeys significantly enhances estrogen-stimulated breast epithelial cell proliferation, with demonstrated effects on cell cycle and survival genes (20). The effect of this AR-mediated growth restriction in breast tissue is perhaps most dramatically illustrated by the full breast development that occurs in genetically male (XY) individuals with complete androgen insensitivity syndrome due to loss of a functional AR (40). Hence, although AR signaling may not be required for breast development to occur in females, it is clearly an important inhibitor of ERα-driven breast growth at puberty in both sexes and an inhibitor of estrogen-stimulated proliferation in the adult female. Disruption of this inhibitory influence at either life stage is likely to have implications for breast carcinogenesis.
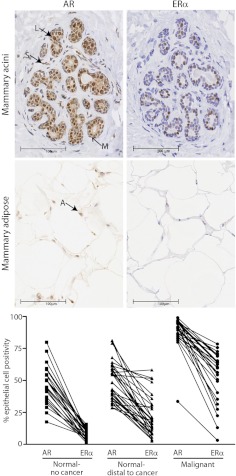
Fig. 1.
AR and ERα in human breast tissues. Panel A, Representative images of immunostaining for AR (left panel) and ERα (right panel) in acini of human breast tissue. Luminal epithelial (L), myoepithelial (M), and stromal (S) cells are indicated. Panel B, Representative images of immunostaining for AR (left panel) and ERα (right panel) in breast adipose tissue. An AR-positive adipose (A) nucleus is indicated by the arrow. Panel C, Percentage of AR- and ERα-positive epithelial cells in prospectively collected breast tissues representing normal breast tissues from women without breast cancer (n = 23), normal breast tissues distal to a malignancy (n = 38), and malignant breast tumor tissues (n = 27).
AR and ERα: sometimes partners, sometimes not
The molecular biology that underlies androgen and estrogen sex hormone antagonism is being elucidated in malignant breast epithelial cells, but very little is known about the mechanics of this antagonism in normal breast epithelial cells, in part due to loss of sex steroid receptor expression in transformed, nonmalignant breast epithelial cell lines (41). AR protein levels are high (59–75% AR-positive epithelial cells) in ducts and lobules of the adult primate mammary gland and remain stable across the menstrual cycle and early stages of pregnancy, only exhibiting a dramatic reduction during late stages of pregnancy and during lactation (42). In contrast, ERα levels are lower (0–12.5% ERα-positive epithelial cells) and change markedly during different menstrual cycle and gestational stages (42). Similar patterns of AR and ERα expression are evident in the breast epithelia of women without breast cancer (Fig. 1C). At the cellular level, ERα and AR have been shown to colocalize in normal and ERα-positive malignant human breast epithelial cells within whole tissue sections (43), providing evidence that interaction between these two signaling pathways can occur in the same cell. However, because the number of AR-positive epithelial cells normally exceed the number of ERα-positive cells in normal and malignant human breast tissues (Fig. 1C), it is not surprising that many AR-positive, ERα-negative cells exist (43). Therefore, AR can operate together with or in isolation from ERα at the cellular level. It is important to note that estrogen-stimulated proliferation in the normal breast epithelium occurs largely in a paracrine manner, because ERα rarely colocalizes with markers of proliferation (42, 44–46). This relationship becomes perturbed in breast cancer cells, in which ERα and Ki67, a clinical marker of proliferation, colocalize and estrogen stimulates proliferation in an autocrine manner. To date, studies examining the colocalization of AR with markers of proliferation in the normal breast epithelium have not been published, so it is unknown whether an AR-positive, ERα-negative luminal breast epithelial cell can proliferate or not. However, preliminary data from our laboratory suggests that AR and Ki67 do not colocalize in normal human breast epithelia (Fig. 2). Therefore, AR signaling may exert antiproliferative effects that are not dependent on direct interaction with ERα in the same cell.
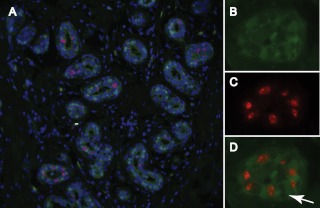
Fig. 2.
Colocalization of AR and Ki67 in normal human breast tissue. A, Representative image of florescent immunostaining for AR (green), the proliferation marker Ki67 (red), and 4′,6-diamidino-2-phenylindole (DAPI) (blue) in normal human breast tissue; B–D, individual staining patterns for AR (B), Ki67 (C), and both antigens (merged image) (D) in a breast acinus with a high proliferative index. Note the lack of colocalization of AR and Ki67. Arrow points to an AR-negative, Ki67-negative cell.
AR in Clinical Breast Cancers
The AR is the most prevalent sex steroid receptor in in situ, invasive, and metastatic breast cancers, occurring in up to 90% of primary tumors and 75% of metastases (47–51). Additionally, AR is the sole sex steroid receptor in 25% of metastases (47). More recently, a strong correlation between AR expression in the primary tumor and metastatic lesions resected at the same time has been demonstrated (52). Although loss of AR was evident with metastatic progression, AR was still present in approximately 50% of metastatic lesions resected at autopsy (52). Therefore, AR is a highly prevalent target in breast cancer, with potential significance for therapeutic management of both primary and advanced disease. However, AR activity may play different roles at different stages of disease or in different subtypes of breast cancer and thereby necessitate diverse AR targeting strategies.
AR in unselected or ERα-positive breast cancer cohorts
In unselected breast cancer cohorts, expression of AR is positively correlated with ERα and PR expression as well as markers of low grade disease and advanced differentiation (48, 49, 53–56). Several studies in recent years show AR to be an independent prognostic factor for breast cancer outcomes, particularly in selected cohorts of ERα-positive disease (43, 57–60), which represents the large majority (at least 70%, depending on how ERα status is defined) of breast cancers. In prognostic studies, higher levels of AR confer a survival advantage, which strongly suggests that AR action may have a tumor-suppressive effect in malignant breast epithelial cells. Based on our retrospective study, AR levels greater than or equal to the median AR percent positivity (75%) independently predicted breast cancer-associated survival in women with ERα-positive disease (43). Since then, we have observed that AR positivity was over 75% in the large majority of prospectively collected cases of ERα-positive breast cancers (Fig. 1). This degree of AR positivity was higher than that observed in normal breast tissues from women with or without breast cancer (Fig. 1). These findings are consistent with a PCR-based study showing that AR mRNA levels remain the same or are significantly increased in malignant compared with normal breast tissues and that there was a negative correlation between high AR levels and overexpression of the c-myc oncogene (61). Moreover, testosterone inhibited estradiol-induced expression of c-myc in the breast tissues of surgically menopausal female monkeys (20). Based on these collective observations, we speculate that AR levels increase with the progression of an ERα-positive malignancy in a homeostatic attempt to restore the balance of sex hormone activity that normally regulates mammary epithelial cell proliferation. This increase may reflect increased availability of androgenic ligands (62–64). Failure to up-regulate AR signaling may result in insufficient androgenic antagonism, thereby providing a growth advantage that contributes to disease progression.
AR in ERα-negative breast cancers
Up to 50% of ERα-negative breast cancers are reported to be positive for AR, and these tend to be enriched for overexpression of human epidermal growth factor receptor 2 (designated HER2+) (53, 56, 65, 66). In contrast to ERα-positive breast cancer, the prognostic value of AR as an independent predictor of relapse-free or overall survival in ERα-negative disease is less clear and variably reported to have no influence or for AR-positive tumors to have better outcomes (43, 53, 60, 67–69). Major confounding factors among studies are the lower frequency and greater phenotypic heterogeneity of ERα-negative compared with ERα-positive breast cancers, leaving many studies underpowered to make definitive conclusions. Molecular profiling has established five general subtypes of breast cancer that are associated with specific breast cancer outcomes: luminal A, luminal B, HER2+, basal, and normal-like (70–72). The two luminal subtypes clearly represent ERα-positive disease and are associated with better outcomes than the other three groups. HER2+ breast cancers identified by molecular profiling, immunohistochemistry, or gene amplification are a clinically more diverse mixture of both ERα-positive (in particular, luminal B) and ERα-negative breast cancers (73). In contrast, the basal subgroup is entirely composed of ERα-negative cases. Recent data suggest that the normal-like subgroup does not represent a distinct subtype of breast cancer but was derived from tissue samples that lacked a sufficient epithelial component to cluster with the other subtypes (74). Farmer et al. (75) redefined the five breast cancer subtypes into three based on ERα and AR status: luminal disease is positive for both receptors (ERα+AR+), basal disease is negative for both receptors (ERα−AR−), and a newly described group named molecular apocrine is negative for ERα and positive for AR (ERα−AR+). The molecular apocrine appellation arose because these cancers tended to be enriched for apocrine-like differentiation, in which tumor cells have a large cytoplasmic area that is granular and strongly eosinophilic. Normal apocrine glands, apocrine metaplasias, and pure apocrine carcinomas have the same steroid receptor profile (ERα−AR+), and AR signaling has been shown to regulate a number of genes in normal and malignant apocrine cells, including gross cystic disease fluid protein-15 (GCDF-15), a biomarker of apocrine carcinoma (76–78). Accordingly, molecular apocrine cancers cluster, at least in part, due to transcriptomic profiles that are enriched for AR signaling pathways. Interestingly, these profiles are similar to those stimulated by AR signaling in prostate cancer cells (75, 79). Molecular apocrine cancers also cluster more closely to luminal breast cancers than the other two ERα-negative breast cancer subgroups, because they sustain a luminal gene signature despite the lack of ERα expression. A genomic meta-analysis confirmed that AR and HER2 signaling pathways play a significant role in molecular apocrine carcinomas (80). Other studies that have performed more extensive molecular clustering of ERα-negative breast cancers in general (81), or triple-negative breast cancers (TNBC) (defined as tumors that lack ERα, PR, and HER2+) in particular (82), identified molecular subtypes associated with steroid receptor (SR) response genes or luminal AR (LAR) for having a luminal-like gene profile. These clearly represent molecular subtypes of breast cancer similar to those identified as being molecular apocrine by other studies. Importantly, the SR and LAR subtypes were associated with poorer outcomes of disease than other molecular subtypes of ERα-negative breast cancers (81, 82). So far, only one immunohistochemical study has examined the prognostic significance of AR in breast cancers specifically classified as molecular apocrine based on an ERα−AR+HER2+ profile and report a trend toward worse outcomes in the group with greater AR expression (59). Collectively, these findings suggest that AR may exert an oncogenic influence in the context of clinical ERα-negative disease, particularly when HER2 is overexpressed.
AR as a Potential Tumor Suppressor of ERα-Positive Breast Cancer
There are several comprehensive reviews examining the influence of androgens and AR in breast cancer (12, 83–85). Herein, we will specifically focus on AR signaling in relation to ERα signaling capacity and include more recent data concerning mechanistic cross talk between these two steroid receptor signaling pathways. The most widely employed cell line models of luminal breast cancer, defined by positive expression for ERα and AR as well as a molecular profile that clusters with primary luminal tumors (86, 87), are MCF-7, T47D, and ZR-75-1. In terms of AR levels, ZR-75-1cells have the highest and MCF-7 cells the lowest (88). The reproducibility among independent studies to demonstrate an AR-mediated, antiproliferative effect in these cell lines appears to be influenced by the relative levels of endogenous AR and ERα. Basal and estrogen-stimulated proliferation of ZR-75-1 and T47D cells is typically inhibited by AR signaling via mechanisms that involve regulation of cell cycle and survival pathways, resulting in a reversible cell cycle arrest (88–92). AR-induced apoptosis has also been reported in these cell lines (93). In contrast, AR-mediated proliferative effects are highly variable in MCF-7 cells, and studies report both stimulatory and inhibitory influences (89, 94–102). In part, steroid metabolism influences the androgenic influence on proliferation in this cell line. For example, DHT is metabolized to estrogenic metabolites in MCF-7 cells under conditions of estrogen starvation (103), and estrogen can stimulate expression of metabolic enzymes that inactivate DHT (104–106). In addition, DHT can directly bind to ERα to stimulate proliferation and ERα-mediated transcriptional events when present at supraphysiological doses (95). However, the relative levels of AR and ERα in MCF-7 cells also have a strong influence on proliferative responses. MCF-7 cells engineered to transiently or stably overexpress the full-length wild-type AR (MCF-AR) were potently growth inhibited by androgen treatment (97, 98). This antiproliferative effect was dependent on the level of AR in the stably transfected cells, whereby clones with the highest level of AR had the greatest ability to inhibit proliferation, induce cell cycle arrest, and interfere with ERα-mediated transactivation (97). Similar AR dose-dependent, antiproliferative, and antiestrogenic effects were observed in MCF-7 and T47D cells transfected with a ligand-binding domain-truncated, constitutively active AR (43, 99). In the parental MCF-7 cell line, variation in the original source of cells (5), changes associated with extensive serial passaging, relative cell density, and differential media conditions can all feasibly influence the levels of AR and thereby determine whether androgens stimulate or inhibit proliferation. Interestingly, De Amicis et al. (107) demonstrated that tamoxifen stimulates colony formation and xenograft growth of an AR-overexpressing derivative of MCF-7 cells (MCF7-AR11) in an AR-dependent manner under conditions of estrogen deprivation. Collectively, these in vitro studies, together with the clinical studies discussed in the previous section, indicate that the role of AR in breast epithelial cells may shift from an antiproliferative to a proliferative stimulus depending on the relative status of AR and ERα and the availability of their cognate ligands.
Several mechanisms have been identified that enable AR activity to inhibit ERα activity. Androgens induce a regression of DMBA- induced rat mammary carcinomas that is associated with marked depletion of ERα (108). AR-mediated down-regulation of ERα has also been demonstrated in the breast tissues of testosterone-treated female monkeys and in the ZR-75-1 and MCF-7 breast cancer cell lines (20, 91, 99). Additionally, AR has been shown to inhibit ERα-mediated transcriptional activity by directly binding to ERα in a manner that was dependent upon the AR N-terminal transactivation domain or full-length AR and maximally stimulated when agonists for both receptors were present (109). In the latter study, AR did not bind to ERβ, suggesting that this oppositional mechanism is specific for the ERα-isoform. Nuclear receptors interact with a broad array of coregulatory molecules to regulate transcriptional events, and many of these interact with both the AR and the ERα. Indeed, cofactors that facilitate the oncogenic activity of ERα in breast cancer cells also facilitate the oncogenic activity of AR in prostate cancer cells (110). Therefore, in a cellular context in which both of these sex steroid receptors are present, competition for coregulatory molecules can occur, with concomitant effects on proliferative capacity. An excellent example of this is provided by Lanzino et al. (99) who demonstrated in MCF-7 cells that AR and ERα can both bind to the coregulatory protein ARA70 and that this interaction can coactivate either steroid receptor, but with divergent effects on proliferation. Therefore, in parental MCF-7 cells, ARA70 predominantly bound to ERα and augmented ERα-induced proliferation, but in MCF7-AR cells, it bound to AR and augmented its antiproliferative effects. Another study by this group has shown that direct down-regulation of the cyclin D1 gene is involved in the antiproliferative effect of androgen signaling in MCF-7 cells, mediated via interaction with an orphan nuclear receptor called DAX1 (111). Although cofactor competition studies have not been performed with other common coregulatory proteins of AR and ERα in breast cancer cells, candidates include known breast cancer oncogenes such as BRCA1 and BRCA2, p160 coactivators (SRC-1, -2, and -3), and E6-associated protein (E6AP or UBE3A) (110).
Genomic studies add additional insight into AR and ERα cross talk in breast cancer cells and generally support the concept that the ability of AR to antagonize ERα signaling is partly dependent upon the relative levels of these two steroid receptors. Using techniques that sustain expression of ERα at a constant level while inducing incremental levels of a constitutively active AR, Peters et al. (43) demonstrated AR dose-dependent inhibition of ERα in steroid receptor-deficient and nondeficient breast cancer cell lines. The latter study also demonstrated the ability of AR to bind to a synthetic consensus estrogen response element (ERE) using EMSA as well as an endogenous ERE in the PR gene using chromatin immunoprecipitation-PCR. Molecular modeling supported these findings, and collectively, these data led to the hypothesis that AR could antagonize ERα signaling in breast cancer cells by competing with ERα for binding to EREs in the genome. Although definitive evidence for this hypothesis is still forthcoming, we have observed that sites bound by AR in prostate cancer cells can directly overlap with sites bound by ERα in breast cancer cells (Fig. 3).
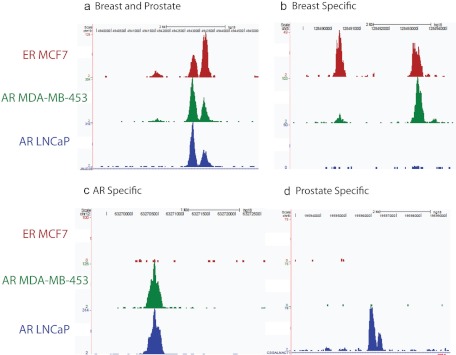
Fig. 3.
AR and ERα DNA-binding events in breast and prostate cancer cells. Examples of aligned DNA-binding sites (peaks) representing ERα binding in MCF-7 breast cancer cells, AR binding in MDA-MB-453 breast cancer cells, and AR binding in LNCaP prostate cancer cells. Binding sites occupied by ERα in breast and AR in both breast and prostate cancer cells (A) ERα and AR in breast but not by AR in prostate cancer cells, (B), AR in breast and prostate but not ERα in breast cancer cells (C), and AR in prostate cancer cells only (D).
AR as a Potential Oncogene in ERα-Negative Breast Cancers
Identification of the molecular apocrine subtype of breast cancer, and the recognition that AR represented its most dominant steroid receptor signaling pathway, reinvigorated interest in the role of AR in breast cancer and its potential as a therapeutic target (75, 79). Studies that predate characterization of the molecular apocrine subtype had shown that AR signaling stimulates proliferation of MDA-MB-453 cells (88, 113), but the clinical significance of this was unknown until it was shown to cluster with primary molecular apocrine breast cancers (79). Subsequently, Naderi and Hughes-Davies (114) demonstrated functional cross talk between the AR and HER2 signaling pathways in MDA-MB-453 and SUM-190 breast cancer cell lines that mimicked interactions observed in the LNCaP prostate cancer cell line, while at the same time stimulating expression of select genes characteristic of luminal breast cancers. The similarity between molecular apocrine breast cancer and prostate cancer is further substantiated by recent genomic studies discussed in detail below.
Primary breast cancers that cluster in the molecular apocrine group are close relatives of luminal breast cancers despite their lack of ERα expression. An intriguing explanation for this conundrum was provided in our study (115) that demonstrated that AR was required for MDA-MB-453 cells to sustain a luminal gene signature and to form colonies in soft agar. Moreover, this study showed a remarkable 98% overlap between the AR- and FOXA1-associated cistromes. Loss of FOXA1 in this cell line abrogated the luminal gene signature and abolished colony formation. Binding of ERα to DNA in cell line models of luminal breast cancer is exquisitely dependent upon FOXA1 (116). Indeed, AR-binding sites in the MDA-MB-453 cells showed greater overlap with ERα-binding sites in MCF-7 cells than AR-binding sites in LNCaP prostate cancer cells (115). The unexpected conclusion of the latter study was that AR adopts an oncogenic role in MDA-MB-453 cells by behaving like a surrogate ERα. Although these data do not demonstrate that AR can compete with ERα for binding to specific ERE in the context of a luminal breast cancer cell, as suggested by studies in ZR-75-1 cells, the MDA-MB-453 data do show that AR can bind to sites normally occupied by ERα in a breast cancer context (Fig. 3). Additionally, AR binds to sites in MDA-MB-453 cells that are AR-binding sites in LNCaP cells but not ERα-binding sites in MCF-7 cells (Fig. 3). These intriguing data not only have implications for different subtypes of breast cancer but also shed light on the potential similarities between ERα and AR when behaving like oncogenes to drive hormonal carcinogenesis.
A contemporary study by Ni et al. (117) furthers mechanistic understanding of AR signaling in molecular apocrine breast cancers. In the latter study, MDA-MB-453 breast cancer cells were also used as the primary experimental model and a significant overlap between AR and FOXA1 DNA-binding sites was observed, albeit to a lesser degree than observed in the study by Robinson et al. (115). Another difference between these two studies was that Ni and colleagues found a greater overlap between the AR cistrome of MDA-MB-453 cells and the AR cistrome of LNCaP cells than the ERα cistrome of MCF-7 cells. These differences between studies could be attributed to differences in experimental methodology, lab-to-lab variation in cell line phenotypes, and different sequencing platforms. In particular, the two AR cistromes were generated under different conditions; one study used asynchronous cells cultured under normal serum conditions, and the other used cells cultured under steroid-deplete conditions followed by a period (4 or 16 h) of exposure to DHT. Nevertheless, data from the two studies concur that the oncogenic nature of AR in this cell line involves AR acting like ERα in a breast cancer context but also acting like AR in a prostate cancer context. Indeed, MDA-MB-453 breast cancer cells have levels of AR tantamount to those in LNCaP prostate cancer cells, and the two cell lines demonstrate many similar features, including the oncogenic role of AR, the necessity for FOXA1 as a pioneer factor to facilitate AR binding to DNA, and functional cross talk between the AR and HER2 signaling pathways.
The study by Ni and colleagues (117) also provided new insight into cross talk between AR and HER2 signaling pathways in molecular apocrine cells. A sequential AR-mediated, FOXA1-dependent up-regulation of the wnt/β-catenin signaling pathway was demonstrated, with a subsequent up-regulation of HER3, an activating binding partner of HER2, that was dependent upon cooperative interaction between AR, FOXA1, and β-catenin. Additional analyses demonstrated a DHT-stimulated increase in HER2 and HER3 phosphorylation, with subsequent activation of the phosphatidylinositol-3 kinase pathway. Inhibition of AR, wnt/β-catenin, or HER2 signaling pathways attenuated growth of MDA-MB-453 cells and another model of molecular apocrine breast cancer, SUM185PE cells. Importantly, the HER2 inhibitors were effective only when cells were simultaneously stimulated with DHT. These findings were further supported by in vivo data showing that treatment with the nonsteroidal AR antagonist, bicalutamide, inhibited DHT-stimulated growth of MDA-MB-453 xenografts, diminished activation of HER2, and induced apoptosis in the tumor cells. A contemporary study by Naderi and colleagues (118) added to this mechanistic insight by demonstrating direct up-regulation of the HER2 gene by AR as well as up-regulation of the AR gene by cAMP response element binding protein 1 as a result of HER2-stimulated ERK activity. The latter research group has also demonstrated that combinatorial inhibition of AR and downstream signaling cascades of HER2 results in synergistic antiproliferative effects in the MDA-MB-453 cell line both in vitro and in vivo (114, 118–120). Collectively, these studies suggest that AR signaling represents a major mechanism that dysregulates HER2 expression and activation in molecular apocrine breast cancers, with hyperactivation of HER2 enhancing AR action in a positive feedback loop. These observations may explain the positive correlation between AR and HER2 overexpression that is evident in clinical breast cancers (53, 59, 65, 75) and provide evidence that there may be clinical benefit in combining anti-HER2 and antiandrogen strategies for molecular apocrine disease. Interestingly, although MDA-MB-453 cells have a HER2 gene amplification, HER2 is not overexpressed in these cells under basal conditions. The studies discussed above indicate that excessive AR activation may be pivotal to unleashing the oncogenic potential of HER2 in this breast cancer subtype.
The MDA-MB-453 cell line has been the most studied model of molecular apocrine breast cancer so far. Although most studies involving MDA-MB-453 cells report that AR activity stimulates proliferation, Wang et al. (121) report that AR directly up-regulates the tumor suppressor gene PTEN and thereby inhibits proliferation and survival of this and other breast cancer cell lines with a luminal molecular profile. Therefore, AR signaling may have dichotomous effects on proliferation of MDA-MB-453 cells, possibly dependent upon differing experimental conditions that impact on AR expression or action. Similarly, there are inconsistencies in the literature concerning the proliferative capacity of AR signaling in other breast cancer cell lines with a molecular apocrine phenotype or sex steroid receptor profile. For example, the MFM-223 cell line was shown by Lehmann et al. (82) to cluster into the LAR molecular subgroup of TNBC, along with MDA-MB-453 cells, and be growth inhibited by AR silencing or treatment with the AR antagonist bicalutamide. However, MFM-223 cells were originally propagated and characterized by Hackenberg and colleagues (122) as being strongly growth inhibited by AR signaling, and we have observed antiproliferative effects of DHT in this cell line (Fig. 4). Interestingly, the parental cells that gave rise to the MFM-223 cell line possessed a small degree of ERα expression, which was rapidly lost with passage in culture. This suggests that tumors with a molecular apocrine phenotype may have evolved from tumors with a luminal phenotype that subsequently lost ERα expression, hence their close association in molecular clustering analyses. Indeed, in a study of HER2+ breast cancers, AR was strongly correlated to HER2 regardless of ERα expression, and the authors hypothesized that HER2 overexpression arises when AR levels are high and ERα levels are low or absent (123). The fact that AR signaling can inhibit growth of MDA-MB-453 and MFM-223 cells under some circumstances and not others suggests that the oncogenic potential of AR remains plastic in these cell lines. Interestingly, of the five cell lines representative of LAR in the study of TNBC, MDA-MB-453, and MFM-223 cells were least sensitive to inhibition by bicalutamide (82). The latter study also shows that AR is expressed in some TNBC cell lines that did not cluster in the LAR subgroup. Although having the same sex steroid receptor profile as molecular apocrine cancers, these cell lines have a phenotype more like basal breast cancers. Although treatment with bicalutamide strongly inhibited the AR-positive basal-like breast cancer cell lines, others have demonstrated AR-mediated antiproliferative effects that were dependent upon relative exposure to steroid stimuli in some of the same cell lines (124, 125). Collectively, these data suggest that the oncogenic potential of AR is influenced by multiple factors, including the steroid milieu and levels of AR, ERα, and HER2.
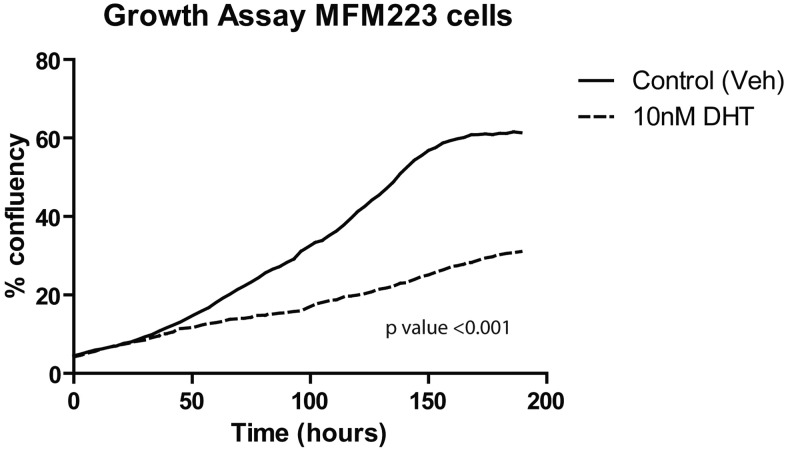
Fig. 4.
Antiproliferative effect of DHT in MFM-223 breast cancer cells. The proliferation of MFM-223 cells, a model of molecular apocrine breast cancer, is significantly inhibited by treatment with the potent androgen receptor agonist DHT (10 nm). Cell numbers were assessed via continuous live cell imaging (Incucyte). Veh, Vehicle.
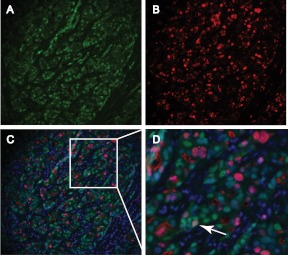
The clinical relevance of data garnered from study of AR-positive, ERα-negative cell lines to clinical breast cancers with the same steroid receptor profile remains to be determined. The MDA-MB-453 cell line harbors genetic and intracellular signaling aberrations not present in apocrine breast cancers, which challenges the clinical relevance of this model (126). Therefore, it is premature to consider the role of AR in ERα-negative disease as being unequivocally oncogenic. However, our preliminary investigations indicate that AR and Ki67 can colocalize in a primary ERα-negative breast cancer (Fig. 5), which does not occur in normal breast tissues (Fig. 2). This adds support to the view that AR can become an oncogene similar to the way that ERα becomes an oncogene during breast carcinogenesis. It will be important in future studies to determine what other cell characteristics influence the ability of AR to inhibit or stimulate proliferation of breast cancer cells other than the presence or absence of ERα. Two possibilities are the relative expression of ERα variants that are not detected by commercial antibodies for full-length ERα (127) and expression of ERβ1, which is positively correlated with AR expression in apocrine carcinomas (128) and can bind to the same ERE as ERα when the latter is not present (129, 130). It is feasible that cross talk occurs between AR and these other ER isoforms in ERα-negative breast cancers in a manner similar to that proposed for ERα-positive breast cancers.
Fig. 5.
Colocalization of AR and Ki67 in an ERα-negative breast carcinoma. A–C, Representative images of fluorescent immunostaining for AR (green) (A), Ki67 (red) (B), and both antigens merged with DAPI (blue) (C) in an ERα-negative breast carcinoma; D, higher-power image of boxed area in C showing an AR-positive, Ki67-positive tumor cell (white arrow).
AR as a Therapeutic Target for Breast Cancer
Historically, systemic androgen treatment was employed in the clinical management of breast cancer and was associated with a 15–30% incidence of disease regression, particularly when the tumor expressed ERα (7–9, 131). Additionally, treatment with fluoxymesterone, an androgen resistant to metabolism, was associated with a tumor-suppressive effect similar to the selective estrogen receptor modulator tamoxifen, with some evidence for beneficial combinatorial treatment effects (131–135). Fluoxymesterone was also reported to improve the therapeutic efficacy of chemotherapy (136). Because androgen treatment could induce undesirable side effects (e.g. hirsutism) and a combination of tamoxifen plus androgen therapy had equivocal additive therapeutic benefit, androgen treatment ceased to be clinically employed. Presumably, the effectiveness of androgen treatment was in part due to its antiproliferative, antiestrogenic actions in the breast tumor cells, particularly those of a luminal phenotype. As mentioned above, multiple studies show that AR levels predict positive outcomes for women with luminal breast cancers, and one of these by Castellano et al. (57) shows that AR is independently prognostic in the luminal B subtype, which is a more aggressive form of luminal disease. The latter study also shows that AR does not predict outcome of hormone therapy alone but does predict outcome of endocrine-chemotherapy. However, to date, studies that have examined the ability of AR to predict outcome of hormone therapy generally include all forms (selective estrogen receptor modulators and aromatase inhibitors) in a single group. Because these two hormonal treatment strategies employ different mechanisms to inhibit ERα action, AR may influence their efficacy in differential ways. For example, tamoxifen-bound ERα binds to DNA at similar sites to the estrogen-bound receptor but preferentially recruits corepressors rather than coactivators, with subsequent blockade of gene transcription. It is possible that if this blockade was maximally effective, AR action would not have any further tumor-suppressive effect, as indicated by the tamoxifen plus androgen clinical trials mentioned above. Moveover, considering the study by De Amicis et al. (107), it is provocative to speculate that in some situations, tamoxifen-bound ERα competes with AR for corespressors, thus allowing AR to recruit coactivators and stimulate an ERα-like gene program that results in tamoxifen resistance. In contrast to tamoxifen, aromatase inhibition blocks conversion of androgens to estrogens and thus potentially increases levels of available testosterone and or DHT. Indeed, studies have implicated activation of AR as a partial mediator of aromatase inhibitor therapeutic efficacy (63, 100). Given that approximately one third of women with ERα-positive disease either do not respond to hormone therapy or become resistant to it over time, there may be scope for revisiting androgen treatment for hormone therapy in such cases (138), with concomitant trials to titrate the hormone dose or to test currently available or newly developed selective androgen receptor modulators that are potent but tissue-selective AR agonists (139) to maximize the tumor-suppressive effect and reduce potential systemic side effects. So far, AR has not been implicated as playing a role in breast cancer resistance to aromatase inhibition. However, if AR is indeed coopted into playing a procarcinogenic role in tamoxifen-resistant disease (107), it would be important to develop biomarkers that could identify such cases for exclusion from AR agonist therapy but possible inclusion for AR antagonist therapy. In addition, approximately 20–30% of luminal breast cancers lose ERα expression when they become metastases (140–142) but may sustain expression of AR (47). Given recent data that over 90% of metastases from luminal tumors retain FOXA1 expression (143), it is feasible that coexpression of AR and FOXA1 in metastases may mark a luminal to molecular apocrine transition and necessitate an antiandrogenic rather than an antiestrogenic or proandrogenic target strategy.
There is a desperate need for new therapies to clinically manage women with ERα-negative breast cancer, particularly those with TNBC, because there are few treatment options outside of chemotherapy or anti-HER2 agents for women with HER2+ disease. Therefore, the finding that AR may be oncogenic in a subgroup of women with these types of breast cancer provided strong impetus to therapeutically target AR, especially because many antiandrogenic therapies are available and approved for clinical use in treating prostate cancer. Indeed, a phase II clinical trial is underway in the United States to examine the ability of bicalutamide to impede disease progression in women with advanced ERα and PR-negative, AR-positive breast cancer (www.clinicaltrials.gov; identifier NCT00468715). In addition, a clinical trial in the United Kingdom aims to test the efficacy of abiraterone, a drug that blocks androgen synthesis by inhibiting the cytochrome p-450 (CYP17) steroidogenic enzyme, in patients with advanced ERα-positive or -negative disease (identifiers include NCT00755885, CDR0000614059, CRUK-CR9304-21, and EUDRACT-2007-003240-30). Although the results of these trials will be instructive, better strategies to select women whose tumors are amenable to AR targeting therapies are required. As for patients with luminal disease, there are likely to be cases of molecular apocrine breast cancer that should not be treated with antiandrogenic therapies, as suggested by in vitro studies of breast cancer cell lines with this phenotype that are inhibited rather than stimulated by AR signaling. Again, it will be important to develop biomarkers specific for ERα-negative AR-positive disease that will identify appropriate cases for inclusion or exclusion.
Conclusions
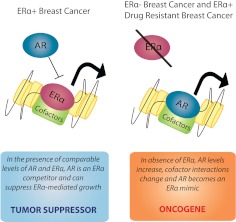
In prostate cancer cells, there is a strong biological drive for AR-dependent growth stimulation, and multiple mechanisms have been identified that enable malignant cells to sustain AR activity in antiandrogenic environments, including the evolution of mutations, amplification, and alternative splicing of the AR gene, increased intratumoral androgen biosynthesis, and aberrant coregulator expression (112, 144). Likewise, most breast cancer cells have a similar dependency on ERα-driven growth, and disease progression involves adaptive mechanisms to sustain this dependency. Recent data suggest that mutations or alternative splicing of the ERα gene may also play a role in breast cancer progression (137). Herein, we reviewed data that describe an alternative adaptive strategy, schematically shown in Fig. 6. We propose that there exists an optimal range of relative AR and ERα expression that allows ERα signaling to stimulate, and AR signaling to suppress, normal and malignant breast epithelial cell proliferation. In part, this involves competition at several levels: 1) receptor binding at specific ERE or other sites in the genome; 2) interaction with common coregulatory molecules, and 3) interaction with pioneering factors such as FOXA1. Oppositional sex hormone effects could also be mediated via signaling events involving cytoplasmic or membrane-associated AR and ERα, or intracellular signaling events mediated via androgen or estrogen interaction with SHBG, but it was out of the scope of this review to include a detailed discussion of these mechanisms. In the event that estrogen action is reduced or nullified, either via loss of ERα or under conditions of long-term estrogen deprivation, AR levels increase, coregulatory interactions change, and AR becomes a surrogate ERα to sustain tumor growth. At a certain level of AR expression, breast cancer cells may adopt a phenotype similar to prostate cancer cells in which AR exerts oncogenic rather than tumor suppressor activity. These insights not only have implications for novel, more personalized ways to approach hormone therapy for different subtypes of breast cancer, but also open the door to investigation of ER and AR interaction in the pathogenesis of other types of hormone-driven cancers such as those that occur in the prostate, ovary, and endometrium.
Fig. 6.
Proposed model of the tumor suppressor vs. oncogenic roles of AR in breast cancer.
Acknowledgments
We thank Dr. Shalini Jindal for fluorescent images and Ms. Natalie Ryan for preparation of figures.
T.E.H. holds a Postdoctoral Fellowship Award from the U.S. Department of Defense Breast Cancer Research Program (W81XWH-11-1-0592). This work was funded by grants from the National Health and Medical Research Council (NHMRC) of Australia (to W.D.T., ID:1008349), the National Breast Cancer Foundation (to T.E.H., IDNC-08-12), the University of Cambridge, Cancer Research UK (to J.S.C.), the European Research Council (to J.S.C.), and the European Molecular Biology Organization (to J.S.C.). The contents of this published material are solely the responsibility of the authors and do not reflect the views of the NHMRC.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- AR
- androgen receptor
- DHT
- 5α-dihydrotestosterone
- E2
- 17β-estradiol
- ERα
- estrogen receptor α
- ERE
- estrogen response element
- FOX
- forkhead box
- LAR
- luminal AR
- SR
- steroid receptor
- TNBC
- triple-negative breast cancer.
References
- 1. Jordan VC. 2009. A century of deciphering the control mechanisms of sex steroid action in breast and prostate cancer: the origins of targeted therapy and chemoprevention. Cancer Res 69:1243–1254 [DOI] [PubMed] [Google Scholar]
- 2. Jordan VC, Obiorah I, Fan P, Kim HR, Ariazi E, Cunliffe H, Brauch H. 2011. The St. Gallen Prize Lecture 2011: evolution of long-term adjuvant anti-hormone therapy: consequences and opportunities. Breast 20(Suppl 3):S1–S11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Yager JD, Davidson NE. 2006. Estrogen carcinogenesis in breast cancer. N Engl J Med 354:270–282 [DOI] [PubMed] [Google Scholar]
- 4. Wagner RK, Gorlich L, Jungblut PW. 1973. Dihydrotestosterone receptor in human mammary cancer. Acta Endocrinol Suppl (Copenh) 173:65. [PubMed] [Google Scholar]
- 5. Horwitz KB, Zava DT, Thilagar AK, Jensen EM, McGuire WL. 1978. Steroid receptor analyses of nine human breast cancer cell lines. Cancer Res 38:2434–2437 [PubMed] [Google Scholar]
- 6. Allegra JC, Lippman ME, Thompson EB, Simon R, Barlock A, Green L, Huff KK, Do HM, Aitken SC. 1979. Distribution, frequency, and quantitative analysis of estrogen, progesterone, androgen, and glucocorticoid receptors in human breast cancer. Cancer Res 39:1447–1454 [PubMed] [Google Scholar]
- 7. Goldenberg IS. 1964. Testosterone propionate therapy in breast cancer. JAMA 188:1069–1072 [DOI] [PubMed] [Google Scholar]
- 8. Goldenberg IS, Hayes MA. 1961. Hormonal therapy of metastatic female breast carcinoma. II. 2α-Methyl dihydrotestosterone propionate. Cancer 14:705–706 [DOI] [PubMed] [Google Scholar]
- 9. Goldenberg IS, Sedransk N, Volk H, Segaloff A, Kelley RM, Haines CR. 1975. Combined androgen and antimetabolite therapy of advanced female breast cancer. A report of the cooperative breast cancer group. Cancer 36:308–310 [DOI] [PubMed] [Google Scholar]
- 10. Talley RW, Haines CR, Waters MN, Goldenberg IS, Olson KB, Bisel HF. 1973. A dose-response evaluation of androgens in the treatment of metastatic breast cancer. Cancer 32:315–320 [DOI] [PubMed] [Google Scholar]
- 11. Burger HG. 2002. Androgen production in women. Fertil Steril 77(Suppl 4):S3–S5 [DOI] [PubMed] [Google Scholar]
- 12. Labrie F, Luu-The V, Labrie C, Bélanger A, Simard J, Lin SX, Pelletier G. 2003. Endocrine and intracrine sources of androgens in women: inhibition of breast cancer and other roles of androgens and their precursor dehydroepiandrosterone. Endocr Rev 24:152–182 [DOI] [PubMed] [Google Scholar]
- 13. Rothman MS, Carlson NE, Xu M, Wang C, Swerdloff R, Lee P, Goh VH, Ridgway EC, Wierman ME. 2011. Reexamination of testosterone, dihydrotestosterone, estradiol and estrone levels across the menstrual cycle and in postmenopausal women measured by liquid chromatography-tandem mass spectrometry. Steroids 76:177–182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Pike MC, Spicer DV, Dahmoush L, Press MF. 1993. Estrogens, progestogens, normal breast cell proliferation, and breast cancer risk. Epidemiol Rev 15:17–35 [DOI] [PubMed] [Google Scholar]
- 15. Grynberg M, Fanchin R, Dubost G, Colau JC, Brémont-Weil C, Frydman R, Ayoubi JM. 2010. Histology of genital tract and breast tissue after long-term testosterone administration in a female-to-male transsexual population. Reprod Biomed Online 20:553–558 [DOI] [PubMed] [Google Scholar]
- 16. McNamara KM, Harwood DT, Simanainen U, Walters KA, Jimenez M, Handelsman DJ. 2010. Measurement of sex steroids in murine blood and reproductive tissues by liquid chromatography-tandem mass spectrometry. J Steroid Biochem Mol Biol 121:611–618 [DOI] [PubMed] [Google Scholar]
- 17. Li X. 2010. Aromatase over expression transgenic murine models for aromatase inhibitor studies. Mol Hum Reprod 16:80–86 [DOI] [PubMed] [Google Scholar]
- 18. Li X, Strauss L, Mäkelä S, Streng T, Huhtaniemi I, Santti R, Poutanen M. 2004. Multiple structural and functional abnormalities in the p450 aromatase expressing transgenic male mice are ameliorated by a p450 aromatase inhibitor. Am J Pathol 164:1039–1048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Santen RJ, Brodie H, Simpson ER, Siiteri PK, Brodie A. 2009. History of aromatase: saga of an important biological mediator and therapeutic target. Endocr Rev 30:343–375 [DOI] [PubMed] [Google Scholar]
- 20. Dimitrakakis C, Zhou J, Wang J, Belanger A, LaBrie F, Cheng C, Powell D, Bondy C. 2003. A physiologic role for testosterone in limiting estrogenic stimulation of the breast. Menopause 10:292–298 [DOI] [PubMed] [Google Scholar]
- 21. Zhou J, Ng S, Adesanya-Famuiya O, Anderson K, Bondy CA. 2000. Testosterone inhibits estrogen-induced mammary epithelial proliferation and suppresses estrogen receptor expression. FASEB J 14:1725–1730 [DOI] [PubMed] [Google Scholar]
- 22. Eick GN, Thornton JW. 2011. Evolution of steroid receptors from an estrogen-sensitive ancestral receptor. Mol Cell Endocrinol 334:31–38 [DOI] [PubMed] [Google Scholar]
- 23. Greene GL, Gilna P, Waterfield M, Baker A, Hort Y, Shine J. 1986. Sequence and expression of human estrogen receptor complementary DNA. Science 231:1150–1154 [DOI] [PubMed] [Google Scholar]
- 24. Enmark E, Pelto-Huikko M, Grandien K, Lagercrantz S, Lagercrantz J, Fried G, Nordenskjöld M, Gustafsson JA. 1997. Human estrogen receptor β-gene structure, chromosomal localization, and expression pattern. J Clin Endocrinol Metab 82:4258–4265 [DOI] [PubMed] [Google Scholar]
- 25. Tilley WD, Marcelli M, Wilson JD, McPhaul MJ. 1989. Characterization and expression of a cDNA encoding the human androgen receptor. Proc Natl Acad Sci USA 86:327–331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lubahn DB, Joseph DR, Sullivan PM, Willard HF, French FS, Wilson EM. 1988. Cloning of human androgen receptor complementary DNA and localization to the X chromosome. Science 240:327–330 [DOI] [PubMed] [Google Scholar]
- 27. Margolis RN, Evans RM, O'Malley BW. 2005. The nuclear receptor signaling atlas: development of a functional atlas of nuclear receptors. Mol Endocrinol 19:2433–2436 [DOI] [PubMed] [Google Scholar]
- 28. Biddie SC, John S, Hager GL. 2010. Genome-wide mechanisms of nuclear receptor action. Trends Endocrinol Metab 21:3–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Tang Q, Chen Y, Meyer C, Geistlinger T, Lupien M, Wang Q, Liu T, Zhang Y, Brown M, Liu XS. 2011. A comprehensive view of nuclear receptor cancer cistromes. Cancer Res 71:6940–6947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hah N, Danko CG, Core L, Waterfall JJ, Siepel A, Lis JT, Kraus WL. 2011. A rapid, extensive, and transient transcriptional response to estrogen signaling in breast cancer cells. Cell 145:622–634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Wang D, Garcia-Bassets I, Benner C, Li W, Su X, Zhou Y, Qiu J, Liu W, Kaikkonen MU, Ohgi KA, Glass CK, Rosenfeld MG, Fu XD. 2011. Reprogramming transcription by distinct classes of enhancers functionally defined by eRNA. Nature 474:390–394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Augello MA, Hickey TE, Knudsen KE. 2011. FOXA1: master of steroid receptor function in cancer. EMBO J 30:3885–3894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Anderson E, Clarke RB. 2004. Steroid receptors and cell cycle in normal mammary epithelium. J Mammary Gland Biol Neoplasia 9:3–13 [DOI] [PubMed] [Google Scholar]
- 34. Curtis Hewitt S, Couse JF, Korach KS. 2000. Estrogen receptor transcription and transactivation: Estrogen receptor knockout mice: what their phenotypes reveal about mechanisms of estrogen action. Breast Cancer Res 2:345–352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Bernardo GM, Lozada KL, Miedler JD, Harburg G, Hewitt SC, Mosley JD, Godwin AK, Korach KS, Visvader JE, Kaestner KH, Abdul-Karim FW, Montano MM, Keri RA. 2010. FOXA1 is an essential determinant of ERα expression and mammary ductal morphogenesis. Development 137:2045–2054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Yeh S, Hu YC, Wang PH, Xie C, Xu Q, Tsai MY, Dong Z, Wang RS, Lee TH, Chang C. 2003. Abnormal mammary gland development and growth retardation in female mice and MCF7 breast cancer cells lacking androgen receptor. J Exp Med 198:1899–1908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Simanainen U, Gao YR, Walters KA, Watson G, Desai R, Jimenez M, Handelsman DJ. 2012. Androgen resistance in female mice increases susceptibility to DMBA-induced mammary tumors. Horm Cancer 3:113–124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Walters KA, Simanainen U, Handelsman DJ. 2010. Molecular insights into androgen actions in male and female reproductive function from androgen receptor knockout models. Hum Reprod Update 16:543–558 [DOI] [PubMed] [Google Scholar]
- 39. Peters AA, Ingman WV, Tilley WD, Butler LM. 2011. Differential effects of exogenous androgen and an androgen receptor antagonist in the peri- and postpubertal murine mammary gland. Endocrinology 152:3728–3737 [DOI] [PubMed] [Google Scholar]
- 40. Jääskeläinen J. 2012. Molecular biology of androgen insensitivity. Mol Cell Endocrinol 352:4–12 [DOI] [PubMed] [Google Scholar]
- 41. Anderson E. 2001. Ovarian steroids and control of proliferation in the normal human breast. Breast 10:273–278 [DOI] [PubMed] [Google Scholar]
- 42. Cheng G, Li Y, Omoto Y, Wang Y, Berg T, Nord M, Vihko P, Warner M, Piao YS, Gustafsson JA. 2005. Differential regulation of estrogen receptor (ER)α and ERβ in primate mammary gland. J Clin Endocrinol Metab 90:435–444 [DOI] [PubMed] [Google Scholar]
- 43. Peters AA, Buchanan G, Ricciardelli C, Bianco-Miotto T, Centenera MM, Harris JM, Jindal S, Segara D, Jia L, Moore NL, Henshall SM, Birrell SN, Coetzee GA, Sutherland RL, Butler LM, Tilley WD. 2009. Androgen receptor inhibits estrogen receptor-α activity and is prognostic in breast cancer. Cancer Res 69:6131–6140 [DOI] [PubMed] [Google Scholar]
- 44. Cheng G, Weihua Z, Warner M, Gustafsson JA. 2004. Estrogen receptors ERα and ERβ in proliferation in the rodent mammary gland. Proc Natl Acad Sci USA 101:3739–3746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Clarke RB, Howell A, Potten CS, Anderson E. 1997. Dissociation between steroid receptor expression and cell proliferation in the human breast. Cancer Res 57:4987–4991 [PubMed] [Google Scholar]
- 46. Russo J, Ao X, Grill C, Russo IH. 1999. Pattern of distribution of cells positive for estrogen receptor α and progesterone receptor in relation to proliferating cells in the mammary gland. Breast Cancer Res Treat 53:217–227 [DOI] [PubMed] [Google Scholar]
- 47. Lea OA, Kvinnsland S, Thorsen T. 1989. Improved measurement of androgen receptors in human breast cancer. Cancer Res 49:7162–7167 [PubMed] [Google Scholar]
- 48. Kuenen-Boumeester V, Van der Kwast TH, van Putten WL, Claassen C, van Ooijen B, Henzen-Logmans SC. 1992. Immunohistochemical determination of androgen receptors in relation to oestrogen and progesterone receptors in female breast cancer. Int J Cancer 52:581–584 [DOI] [PubMed] [Google Scholar]
- 49. Isola JJ. 1993. Immunohistochemical demonstration of androgen receptor in breast cancer and its relationship to other prognostic factors. J Pathol 170:31–35 [DOI] [PubMed] [Google Scholar]
- 50. Hall RE, Aspinall JO, Horsfall DJ, Birrell SN, Bentel JM, Sutherland RL, Tilley WD. 1996. Expression of the androgen receptor and an androgen-responsive protein, apolipoprotein D, in human breast cancer. Br J Cancer 74:1175–1180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Moinfar F, Okcu M, Tsybrovskyy O, Regitnig P, Lax SF, Weybora W, Ratschek M, Tavassoli FA, Denk H. 2003. Androgen receptors frequently are expressed in breast carcinomas: potential relevance to new therapeutic strategies. Cancer 98:703–711 [DOI] [PubMed] [Google Scholar]
- 52. Cimino-Mathews A, Hicks JL, Illei PB, Halushka MK, Fetting JH, De Marzo AM, Park BH, Argani P. 9 December 2011. Androgen receptor expression is usually maintained in initial surgically resected breast cancer metastases but is often lost in end-stage metastases found at autopsy. Hum Pathol org/10.1016/j.humpath.2011.08.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Agoff SN, Swanson PE, Linden H, Hawes SE, Lawton TJ. 2003. Androgen receptor expression in estrogen receptor-negative breast cancer. Immunohistochemical, clinical, and prognostic associations. Am J Clin Pathol 120:725–731 [DOI] [PubMed] [Google Scholar]
- 54. Gonzalez LO, Corte MD, Vazquez J, Junquera S, Sanchez R, Alvarez AC, Rodriguez JC, Lamelas ML, Vizoso FJ. 2008. Androgen receptor expression in breast cancer: relationship with clinicopathological characteristics of the tumors, prognosis, and expression of metalloproteases and their inhibitors. BMC Cancer 8:149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Riva C, Dainese E, Caprara G, Rocca PC, Massarelli G, Tot T, Capella C, Eusebi V. 2005. Immunohistochemical study of androgen receptors in breast carcinoma. Evidence of their frequent expression in lobular carcinoma. Virchows Arch 447:695–700 [DOI] [PubMed] [Google Scholar]
- 56. Park S, Koo J, Park HS, Kim JH, Choi SY, Lee JH, Park BW, Lee KS. 2010. Expression of androgen receptors in primary breast cancer. Ann Oncol 21:488–492 [DOI] [PubMed] [Google Scholar]
- 57. Castellano I, Allia E, Accortanzo V, Vandone AM, Chiusa L, Arisio R, Durando A, Donadio M, Bussolati G, Coates AS, Viale G, Sapino A. 2010. Androgen receptor expression is a significant prognostic factor in estrogen receptor positive breast cancers. Breast Cancer Res Treat 124:607–617 [DOI] [PubMed] [Google Scholar]
- 58. Gonzalez-Angulo AM, Stemke-Hale K, Palla SL, Carey M, Agarwal R, Meric-Berstam F, Traina TA, Hudis C, Hortobagyi GN, Gerald WL, Mills GB, Hennessy BT. 2009. Androgen receptor levels and association with PIK3CA mutations and prognosis in breast cancer. Clin Cancer Res 15:2472–2478 [DOI] [PubMed] [Google Scholar]
- 59. Park S, Koo JS, Kim MS, Park HS, Lee JS, Lee JS, Kim SI, Park BW, Lee KS. 2011. Androgen receptor expression is significantly associated with better outcomes in estrogen receptor-positive breast cancers. Ann Oncol 22:1755–1762 [DOI] [PubMed] [Google Scholar]
- 60. Hu R, Dawood S, Holmes MD, Collins LC, Schnitt SJ, Cole K, Marotti JD, Hankinson SE, Colditz GA, Tamimi RM. 2011. Androgen receptor expression and breast cancer survival in postmenopausal women. Clin Cancer Res 17:1867–1874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Bièche I, Parfait B, Tozlu S, Lidereau R, Vidaud M. 2001. Quantitation of androgen receptor gene expression in sporadic breast tumors by real-time RT-PCR: evidence that MYC is an AR-regulated gene. Carcinogenesis 22:1521–1526 [DOI] [PubMed] [Google Scholar]
- 62. Suzuki T, Darnel AD, Akahira JI, Ariga N, Ogawa S, Kaneko C, Takeyama J, Moriya T, Sasano H. 2001. 5α-reductases in human breast carcinoma: possible modulator of in situ androgenic actions. J Clin Endocrinol Metab 86:2250–2257 [DOI] [PubMed] [Google Scholar]
- 63. Chanplakorn N, Chanplakorn P, Suzuki T, Ono K, Wang L, Chan MS, Wing L, Yiu CC, Chow LW, Sasano H. 2011. Increased 5α-reductase type 2 expression in human breast carcinoma following aromatase inhibitor therapy: the correlation with decreased tumor cell proliferation. Horm Cancer 2:73–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Suzuki T, Miki Y, Moriya T, Akahira J, Ishida T, Hirakawa H, Yamaguchi Y, Hayashi S, Sasano H. 2007. 5α-Reductase type 1 and aromatase in breast carcinoma as regulators of in situ androgen production. Int J Cancer 120:285–291 [DOI] [PubMed] [Google Scholar]
- 65. Niemeier LA, Dabbs DJ, Beriwal S, Striebel JM, Bhargava R. 2010. Androgen receptor in breast cancer: expression in estrogen receptor-positive tumors and in estrogen receptor-negative tumors with apocrine differentiation. Mod Pathol 23:205–212 [DOI] [PubMed] [Google Scholar]
- 66. Vranic S, Tawfik O, Palazzo J, Bilalovic N, Eyzaguirre E, Lee LM, Adegboyega P, Hagenkord J, Gatalica Z. 2010. EGFR and HER-2/neu expression in invasive apocrine carcinoma of the breast. Mod Pathol 23:644–653 [DOI] [PubMed] [Google Scholar]
- 67. He J, Peng R, Yuan Z, Wang S, Peng J, Lin G, Jiang X, Qin T. 2012. Prognostic value of androgen receptor expression in operable triple-negative breast cancer: a retrospective analysis based on a tissue microarray. Med Oncol 29:406–410 [DOI] [PubMed] [Google Scholar]
- 68. Micello D, Marando A, Sahnane N, Riva C, Capella C, Sessa F. 2010. Androgen receptor is frequently expressed in HER2-positive, ER/PR-negative breast cancers. Virchows Arch 457:467–476 [DOI] [PubMed] [Google Scholar]
- 69. Tang D, Xu S, Zhang Q, Zhao W. 2012. The expression and clinical significance of the androgen receptor and E-cadherin in triple-negative breast cancer. Med Oncol 29:526–533 [DOI] [PubMed] [Google Scholar]
- 70. Perou CM, Sørlie T, Eisen MB, van de Rijn M, Jeffrey SS, Rees CA, Pollack JR, Ross DT, Johnsen H, Akslen LA, Fluge O, Pergamenschikov A, Williams C, Zhu SX, Lønning PE, Børresen-Dale AL, Brown PO, Botstein D. 2000. Molecular portraits of human breast tumours. Nature 406:747–752 [DOI] [PubMed] [Google Scholar]
- 71. Sørlie T, Perou CM, Tibshirani R, Aas T, Geisler S, Johnsen H, Hastie T, Eisen MB, van de Rijn M, Jeffrey SS, Thorsen T, Quist H, Matese JC, Brown PO, Botstein D, Lønning PE, Børresen-Dale AL. 2001. Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc Natl Acad Sci USA 98:10869–10874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Sorlie T, Tibshirani R, Parker J, Hastie T, Marron JS, Nobel A, Deng S, Johnsen H, Pesich R, Geisler S, Demeter J, Perou CM, Lønning PE, Brown PO, Børresen-Dale AL, Botstein D. 2003. Repeated observation of breast tumor subtypes in independent gene expression data sets. Proc Natl Acad Sci USA 100:8418–8423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Rouzier R, Perou CM, Symmans WF, Ibrahim N, Cristofanilli M, Anderson K, Hess KR, Stec J, Ayers M, Wagner P, Morandi P, Fan C, Rabiul I, Ross JS, Hortobagyi GN, Pusztai L. 2005. Breast cancer molecular subtypes respond differently to preoperative chemotherapy. Clin Cancer Res 11:5678–5685 [DOI] [PubMed] [Google Scholar]
- 74. Haibe-Kains B, Desmedt C, Loi S, Culhane AC, Bontempi G, Quackenbush J, Sotiriou C. 2012. A three-gene model to robustly identify breast cancer molecular subtypes. J Natl Cancer Inst 104:311–325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Farmer P, Bonnefoi H, Becette V, Tubiana-Hulin M, Fumoleau P, Larsimont D, Macgrogan G, Bergh J, Cameron D, Goldstein D, Duss S, Nicoulaz AL, Brisken C, Fiche M, Delorenzi M, Iggo R. 2005. Identification of molecular apocrine breast tumours by microarray analysis. Oncogene 24:4660–4671 [DOI] [PubMed] [Google Scholar]
- 76. O'Malley FP, Bane A. 2008. An update on apocrine lesions of the breast. Histopathology 52:3–10 [DOI] [PubMed] [Google Scholar]
- 77. Honma N, Takubo K, Akiyama F, Sawabe M, Arai T, Younes M, Kasumi F, Sakamoto G. 2005. Expression of GCDFP-15 and AR decreases in larger or node-positive apocrine carcinomas of the breast. Histopathology 47:195–201 [DOI] [PubMed] [Google Scholar]
- 78. Hall RE, Clements JA, Birrell SN, Tilley WD. 1998. Prostate-specific antigen and gross cystic disease fluid protein-15 are co-expressed in androgen receptor-positive breast tumours. Br J Cancer 78:360–365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Doane AS, Danso M, Lal P, Donaton M, Zhang L, Hudis C, Gerald WL. 2006. An estrogen receptor-negative breast cancer subset characterized by a hormonally regulated transcriptional program and response to androgen. Oncogene 25:3994–4008 [DOI] [PubMed] [Google Scholar]
- 80. Sanga S, Broom BM, Cristini V, Edgerton ME. 2009. Gene expression meta-analysis supports existence of molecular apocrine breast cancer with a role for androgen receptor and implies interactions with ErbB family. BMC Med Genomics 2:59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Teschendorff AE, Miremadi A, Pinder SE, Ellis IO, Caldas C. 2007. An immune response gene expression module identifies a good prognosis subtype in estrogen receptor negative breast cancer. Genome Biol 8:R157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Lehmann BD, Bauer JA, Chen X, Sanders ME, Chakravarthy AB, Shyr Y, Pietenpol JA. 2011. Identification of human triple-negative breast cancer subtypes and preclinical models for selection of targeted therapies. J Clin Invest 121:2750–2767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Birrell SN, Butler LM, Harris JM, Buchanan G, Tilley WD. 2007. Disruption of androgen receptor signaling by synthetic progestins may increase risk of developing breast cancer. FASEB J 21:2285–2293 [DOI] [PubMed] [Google Scholar]
- 84. Dimitrakakis C, Bondy C. 2009. Androgens and the breast. Breast Cancer Res 11:212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Somboonporn W, Davis SR. 2004. Testosterone effects on the breast: implications for testosterone therapy for women. Endocr Rev 25:374–388 [DOI] [PubMed] [Google Scholar]
- 86. Kao J, Salari K, Bocanegra M, Choi YL, Girard L, Gandhi J, Kwei KA, Hernandez-Boussard T, Wang P, Gazdar AF, Minna JD, Pollack JR. 2009. Molecular profiling of breast cancer cell lines defines relevant tumor models and provides a resource for cancer gene discovery. PLoS One 4:e6146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Neve RM, Chin K, Fridlyand J, Yeh J, Baehner FL, Fevr T, Clark L, Bayani N, Coppe JP, Tong F, Speed T, Spellman PT, DeVries S, Lapuk A, Wang NJ, Kuo WL, Stilwell JL, Pinkel D, Albertson DG, Waldman FM, McCormick F, Dickson RB, Johnson MD, Lippman M, Ethier S, Gazdar A, Gray JW. 2006. A collection of breast cancer cell lines for the study of functionally distinct cancer subtypes. Cancer Cell 10:515–527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Birrell SN, Bentel JM, Hickey TE, Ricciardelli C, Weger MA, Horsfall DJ, Tilley WD. 1995. Androgens induce divergent proliferative responses in human breast cancer cell lines. J Steroid Biochem Mol Biol 52:459–467 [DOI] [PubMed] [Google Scholar]
- 89. Cops EJ, Bianco-Miotto T, Moore NL, Clarke CL, Birrell SN, Butler LM, Tilley WD. 2008. Antiproliferative actions of the synthetic androgen, mibolerone, in breast cancer cells are mediated by both androgen and progesterone receptors. J Steroid Biochem Mol Biol 110:236–243 [DOI] [PubMed] [Google Scholar]
- 90. Lapointe J, Fournier A, Richard V, Labrie C. 1999. Androgens down-regulate bcl-2 protooncogene expression in ZR-75-1 human breast cancer cells. Endocrinology 140:416–421 [DOI] [PubMed] [Google Scholar]
- 91. Poulin R, Baker D, Labrie F. 1988. Androgens inhibit basal and estrogen-induced cell proliferation in the ZR-75-1 human breast cancer cell line. Breast Cancer Res Treat 12:213–225 [DOI] [PubMed] [Google Scholar]
- 92. Ortmann J, Prifti S, Bohlmann MK, Rehberger-Schneider S, Strowitzki T, Rabe T. 2002. Testosterone and 5α-dihydrotestosterone inhibit in vitro growth of human breast cancer cell lines. Gynecol Endocrinol 16:113–120 [PubMed] [Google Scholar]
- 93. Kandouz M, Lombet A, Perrot JY, Jacob D, Carvajal S, Kazem A, Rostene W, Therwath A, Gompel A. 1999. Proapoptotic effects of antiestrogens, progestins and androgen in breast cancer cells. J Steroid Biochem Mol Biol 69:463–471 [DOI] [PubMed] [Google Scholar]
- 94. Lippman M, Bolan G, Huff K. 1976. The effects of androgens and antiandrogens on hormone-responsive human breast cancer in long-term tissue culture. Cancer Res 36:4610–4618 [PubMed] [Google Scholar]
- 95. Zava DT, McGuire WL. 1978. Human breast cancer: androgen action mediated by estrogen receptor. Science 199:787–788 [DOI] [PubMed] [Google Scholar]
- 96. MacIndoe JH, Etre LA. 1980. Androgens inhibit estrogen action in MCF-7 human breast cancer cells. Life Sci 27:1643–1648 [DOI] [PubMed] [Google Scholar]
- 97. Szelei J, Jimenez J, Soto AM, Luizzi MF, Sonnenschein C. 1997. Androgen-induced inhibition of proliferation in human breast cancer MCF7 cells transfected with androgen receptor. Endocrinology 138:1406–1412 [DOI] [PubMed] [Google Scholar]
- 98. Andò S, De Amicis F, Rago V, Carpino A, Maggiolini M, Panno ML, Lanzino M. 2002. Breast cancer: from estrogen to androgen receptor. Mol Cell Endocrinol 193:121–128 [DOI] [PubMed] [Google Scholar]
- 99. Lanzino M, De Amicis F, McPhaul MJ, Marsico S, Panno ML, Andò S. 2005. Endogenous coactivator ARA70 interacts with estrogen receptor α (ERα) and modulates the functional ERα/androgen receptor interplay in MCF-7 cells. J Biol Chem 280:20421–20430 [DOI] [PubMed] [Google Scholar]
- 100. Macedo LF, Guo Z, Tilghman SL, Sabnis GJ, Qiu Y, Brodie A. 2006. Role of androgens on MCF-7 breast cancer cell growth and on the inhibitory effect of letrozole. Cancer Res 66:7775–7782 [DOI] [PubMed] [Google Scholar]
- 101. Hackenberg R, Hofmann J, Hölzel F, Schulz KD. 1988. Stimulatory effects of androgen and antiandrogen on the in vitro proliferation of human mammary carcinoma cells. J Cancer Res Clin Oncol 114:593–601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Greeve MA, Allan RK, Harvey JM, Bentel JM. 2004. Inhibition of MCF-7 breast cancer cell proliferation by 5α-dihydrotestosterone; a role for p21(Cip1/Waf1). J Mol Endocrinol 32:793–810 [DOI] [PubMed] [Google Scholar]
- 103. Sikora MJ, Cordero KE, Larios JM, Johnson MD, Lippman ME, Rae JM. 2009. The androgen metabolite 5α-androstane-3β,17β-diol (3βAdiol) induces breast cancer growth via estrogen receptor: implications for aromatase inhibitor resistance. Breast Cancer Res Treat 115:289–296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Roy R, Dauvois S, Labrie F, Belanger A. 1992. Estrogen-stimulated glucuronidation of dihydrotestosterone in MCF-7 human breast cancer cells. J Steroid Biochem Mol Biol 41:579–582 [DOI] [PubMed] [Google Scholar]
- 105. Hu DG, Mackenzie PI. 2009. Estrogen receptor α, fos-related antigen-2, and c-Jun coordinately regulate human UDP glucuronosyltransferase 2B15 and 2B17 expression in response to 17β-estradiol in MCF-7 cells. Mol Pharmacol 76:425–439 [DOI] [PubMed] [Google Scholar]
- 106. Harrington WR, Sengupta S, Katzenellenbogen BS. 2006. Estrogen regulation of the glucuronidation enzyme UGT2B15 in estrogen receptor-positive breast cancer cells. Endocrinology 147:3843–3850 [DOI] [PubMed] [Google Scholar]
- 107. De Amicis F, Thirugnansampanthan J, Cui Y, Selever J, Beyer A, Parra I, Weigel NL, Herynk MH, Tsimelzon A, Lewis MT, Chamness GC, Hilsenbeck SG, Andò S, Fuqua SA. 2010. Androgen receptor overexpression induces tamoxifen resistance in human breast cancer cells. Breast Cancer Res Treat 121:1–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Zava DT, McGuire WL. 1977. Estrogen receptors in androgen-induced breast tumor regression. Cancer Res 37:1608–1610 [PubMed] [Google Scholar]
- 109. Panet-Raymond V, Gottlieb B, Beitel LK, Pinsky L, Trifiro MA. 2000. Interactions between androgen and estrogen receptors and the effects on their transactivational properties. Mol Cell Endocrinol 167:139–150 [DOI] [PubMed] [Google Scholar]
- 110. Risbridger GP, Davis ID, Birrell SN, Tilley WD. 2010. Breast and prostate cancer: more similar than different. Nat Rev Cancer 10:205–212 [DOI] [PubMed] [Google Scholar]
- 111. Lanzino M, Sisci D, Morelli C, Garofalo C, Catalano S, Casaburi I, Capparelli C, Giordano C, Giordano F, Maggiolini M, Andò S. 2010. Inhibition of cyclin D1 expression by androgen receptor in breast cancer cells–identification of a novel androgen response element. Nucleic Acids Res 38:5351–5365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Scher HI, Buchanan G, Gerald W, Butler LM, Tilley WD. 2004. Targeting the androgen receptor: improving outcomes for castration-resistant prostate cancer. Endocr Relat Cancer 11:459–476 [DOI] [PubMed] [Google Scholar]
- 113. Hall RE, Birrell SN, Tilley WD, Sutherland RL. 1994. MDA-MB-453, an androgen-responsive human breast carcinoma cell line with high level androgen receptor expression. Eur J Cancer 30A:484–490 [DOI] [PubMed] [Google Scholar]
- 114. Naderi A, Hughes-Davies L. 2008. A functionally significant cross talk between androgen receptor and ErbB2 pathways in estrogen receptor negative breast cancer. Neoplasia 10:542–548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Robinson JL, Macarthur S, Ross-Innes CS, Tilley WD, Neal DE, Mills IG, Carroll JS. 2011. Androgen receptor driven transcription in molecular apocrine breast cancer is mediated by FoxA1. EMBO J 30:3019–3027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Hurtado A, Holmes KA, Ross-Innes CS, Schmidt D, Carroll JS. 2011. FOXA1 is a key determinant of estrogen receptor function and endocrine response. Nat Genet 43:27–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Ni M, Chen Y, Lim E, Wimberly H, Bailey ST, Imai Y, Rimm DL, Liu XS, Brown M. 2011. Targeting androgen receptor in estrogen receptor-negative breast cancer. Cancer Cell 20:119–131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Chia KM, Liu J, Francis GD, Naderi A. 2011. A feedback loop between androgen receptor and ERK signaling in estrogen receptor-negative breast cancer. Neoplasia 13:154–166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Naderi A, Chia KM, Liu J. 2011. Synergy between inhibitors of androgen receptor and MEK has therapeutic implications in estrogen receptor-negative breast cancer. Breast Cancer Res 13:R36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Naderi A, Liu J. 2010. Inhibition of androgen receptor and Cdc25A phosphatase as a combination targeted therapy in molecular apocrine breast cancer. Cancer Lett 298:74–87 [DOI] [PubMed] [Google Scholar]
- 121. Wang Y, Romigh T, He X, Tan MH, Orloff MS, Silverman RH, Heston WD, Eng C. 2011. Differential regulation of PTEN expression by androgen receptor in prostate and breast cancers. Oncogene 30:4327–4338 [DOI] [PubMed] [Google Scholar]
- 122. Hackenberg R, Lüttchens S, Hofmann J, Kunzmann R, Hölzel F, Schulz KD. 1991. Androgen sensitivity of the new human breast cancer cell line MFM-223. Cancer Res 51:5722–5727 [PubMed] [Google Scholar]
- 123. Lin Fde M, Pincerato KM, Bacchi CE, Baracat EC, Carvalho FM. 2012. Coordinated expression of oestrogen and androgen receptors in HER2-positive breast carcinomas: impact on proliferative activity. J Clin Pathol 65:64–68 [DOI] [PubMed] [Google Scholar]
- 124. Toth-Fejel S, Cheek J, Calhoun K, Muller P, Pommier RF. 2004. Estrogen and androgen receptors as comediators of breast cancer cell proliferation: providing a new therapeutic tool. Arch Surg 139:50–54 [DOI] [PubMed] [Google Scholar]
- 125. Hardin C, Pommier R, Calhoun K, Muller P, Jackson T, Pommier S. 2007. A new hormonal therapy for estrogen receptor-negative breast cancer. World J Surg 31:1041–1046 [DOI] [PubMed] [Google Scholar]
- 126. Vranic S, Gatalica Z, Wang ZY. 2011. Update on the molecular profile of the MDA-MB-453 cell line as a model for apocrine breast carcinoma studies. Oncol Lett 2:1131–1137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Vranic S, Gatalica Z, Deng H, Frkovic-Grazio S, Lee LM, Gurjeva O, Wang ZY. 2011. ER-α36, a novel isoform of ER-α66, is commonly over-expressed in apocrine and adenoid cystic carcinomas of the breast. J Clin Pathol 64:54–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Honma N, Saji S, Kurabayashi R, Aida J, Arai T, Horii R, Akiyama F, Iwase T, Harada N, Younes M, Toi M, Takubo K, Sakamoto G. 2008. Oestrogen receptor-β1 but not oestrogen receptor-βcx is of prognostic value in apocrine carcinoma of the breast. APMIS 116:923–930 [DOI] [PubMed] [Google Scholar]
- 129. Charn TH, Liu ET, Chang EC, Lee YK, Katzenellenbogen JA, Katzenellenbogen BS. 2010. Genome-wide dynamics of chromatin binding of estrogen receptors α and β: mutual restriction and competitive site selection. Mol Endocrinol 24:47–59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Grober OM, Mutarelli M, Giurato G, Ravo M, Cicatiello L, De Filippo MR, Ferraro L, Nassa G, Papa MF, Paris O, Tarallo R, Luo S, Schroth GP, Benes V, Weisz A. 2011. A Global analysis of estrogen receptor β binding to breast cancer cell genome reveals an extensive interplay with estrogen receptor α for target gene regulation. BMC Genomics 12:36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Manni A, Arafah BM, Pearson OH. 1981. Androgen-induced remissions after antiestrogen and hypophysectomy in stage IV breast cancer. Cancer 48:2507–2509 [DOI] [PubMed] [Google Scholar]
- 132. Ingle JN, Suman VJ, Mailliard JA, Kugler JW, Krook JE, Michalak JC, Pisansky TM, Wold LE, Donohue JH, Goetz MP, Perez EA. 2006. Randomized trial of tamoxifen alone or combined with fluoxymesterone as adjuvant therapy in postmenopausal women with resected estrogen receptor positive breast cancer. North Central Cancer Treatment Group Trial 89-30-52. Breast Cancer Res Treat 98:217–222 [DOI] [PubMed] [Google Scholar]
- 133. Rose C, Kamby C, Mouridsen HT, Andersson M, Bastholt L, Møller KA, Andersen J, Munkholm P, Dombernowsky P, Christensen IJ. 2000. Combined endocrine treatment of elderly postmenopausal patients with metastatic breast cancer. A randomized trial of tamoxifen vs. tamoxifen + aminoglutethimide and hydrocortisone and tamoxifen + fluoxymesterone in women above 65 years of age. Breast Cancer Res Treat 61:103–110 [DOI] [PubMed] [Google Scholar]
- 134. Tormey DC, Simon RM, Lippman ME, Bull JM, Myers CE. 1976. Evaluation of tamoxifen dose in advanced breast cancer: a progress report. Cancer Treat Rep 60:1451–1459 [PubMed] [Google Scholar]
- 135. Tormey DC, Lippman ME, Edwards BK, Cassidy JG. 1983. Evaluation of tamoxifen doses with and without fluoxymesterone in advanced breast cancer. Ann Intern Med 98:139–144 [DOI] [PubMed] [Google Scholar]
- 136. Tormey DC, Gelman R, Band PR, Sears M, Bauer M, Arseneau JC, Falkson G. 1981. A prospective evaluation of chemohormonal therapy remission maintenance in advanced breast cancer. Breast Cancer Res Treat 1:111–119 [DOI] [PubMed] [Google Scholar]
- 137. Barone I, Brusco L, Fuqua SA. 2010. Estrogen receptor mutations and changes in downstream gene expression and signaling. Clin Cancer Res 16:2702–2708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Lønning PE. 2009. Additive endocrine therapy for advanced breast cancer: back to the future. Acta Oncol 48:1092–1101 [DOI] [PubMed] [Google Scholar]
- 139. Tiefenbacher K, Daxenbichler G. 2008. The role of androgens in normal and malignant breast tissue. Breast Care (Basel) 3:325–331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Harrell JC, Dye WW, Allred DC, Jedlicka P, Spoelstra NS, Sartorius CA, Horwitz KB. 2006. Estrogen receptor positive breast cancer metastasis: altered hormonal sensitivity and tumor aggressiveness in lymphatic vessels and lymph nodes. Cancer Res 66:9308–9315 [DOI] [PubMed] [Google Scholar]
- 141. Zheng WQ, Lu J, Zheng JM, Hu FX, Ni CR. 2001. Variation of ER status between primary and metastatic breast cancer and relationship to p53 expression*. Steroids 66:905–910 [DOI] [PubMed] [Google Scholar]
- 142. Butler JA, Trezona T, Vargas H, State D. 1989. Value of measuring hormone receptor levels of regional metastatic carcinoma of the breast. Arch Surg 124:1131–1134; discussion 1134–1135 [DOI] [PubMed] [Google Scholar]
- 143. Ross-Innes CS, Stark R, Teschendorff AE, Holmes KA, Ali HR, Dunning MJ, Brown GD, Gojis O, Ellis IO, Green AR, Ali S, Chin SF, Palmieri C, Caldas C, Carroll JS. 2012. Differential oestrogen receptor binding is associated with clinical outcome in breast cancer. Nature 481:389–393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Knudsen KE, Penning TM. 2010. Partners in crime: deregulation of AR activity and androgen synthesis in prostate cancer. Trends Endocrinol Metab 21:315–324 [DOI] [PMC free article] [PubMed] [Google Scholar]