Abstract
The ERK and p38 MAPK pathways are well-known transducers of signals that regulate proliferation and differentiation, but precisely how these pathways control growth plate chondrocyte development is unclear. For example, the ERK pathway has been reported to be required by some investigators but inhibitory to chondrocyte development by others. Moreover, how these two pathways interact to regulate chondrocyte development is even less clear. Using primary bovine growth plate chondrocytes and murine ATDC5 cells, we demonstrate that the ERK and p38 pathways have opposing effects on proliferation but are both absolutely required for differentiation. Two factors that promote chondrocyte differentiation, brain-derived neurotrophic factor (BDNF) and C-type natriuretic peptide, increase p38 activity while decreasing, but not completely inhibiting, ERK activity. The attenuation of ERK activity by BDNF occurs via p38-dependent raf-1 inhibition. The inhibition of raf-1 by p38 is direct, because purified p38 protein inhibits the kinase activity of purified active raf-1 as well as raf-1 immunoprecipitated from chondrocyte lysates. Moreover, IGF-I, which stimulates proliferation, suppresses p38 activation. This work describes a model wherein unopposed IGF-I promotes high ERK/p38 activity ratios favoring proliferation, whereas BDNF signals a transition to differentiation by decreasing the ERK/p38 activity ratio without completely inhibiting ERK, which involves the direct inhibition of raf-1 by p38.
Growth of long bones of the vertebrate skeleton occurs at a layer of specialized cartilage between the epiphysis and the metaphysis called the growth plate (1, 2). Within the growth plate, chondrocytes undergo a tightly regulated developmental program such that cells are continually recruited from the reserve zone to proliferate along the long axis of the bone. Early in the differentiation program, the proliferating chondrocytes express collagens II, IX, and XI and other matrix components, such as aggrecan. As development within the growth plate proceeds, the collagen synthesized is primarily type X collagen (COLX), and cells hypertrophy by accumulating intracellular glycogen and begin to mineralize the surrounding extracellular matrix. Eventually, these hypertrophic chondrocytes give way to bone forming cells that convert the COLX-containing extracellular matrix of the growth plate to trabecular bone. Although chondrocyte proliferation in large mammals is regulated primarily by IGF, the transition from the proliferative to prehypertrophic stage is controlled by many factors, such as thyroid hormone, TGF, bone morphogenic proteins, PTH-related peptide, indian hedgehog, and fibroblast growth factors, to name only a few. More recently, C-type natriuretic peptide (CNP) has been shown to be an important regulator of growth plate chondrocyte development.
These extracellular factors regulate chondrocyte development by interacting with cell surface receptors that then activate a wide array of intracellular signaling cascades. The signaling pathways that figure most prominently are the MAPK cascades (3–8). The MAPKs are activated by stimuli as varied as peptide hormones and cellular stress; the receptor families activating one or more MAPK cascades include receptor tyrosine kinases, cytokine receptors, G protein-coupled receptors, and serine-threonine kinase receptors. The three major MAPK cascades consist of the classic ERK1/2 cascade, which is responsive to mitogens such as IGF-I, and the c-Jun N-terminal kinase (JNK) and p38 MAPK cascades, which respond to various cellular stresses and cytokines. Each of the MAPK cascades is organized into modules, which contain protein kinases working in series. In the case of ERK1/2, the module consists of the MAPK p44 ERK1 and p42 ERK2, the immediate upstream kinases MAPK kinase (MEK)1 and MEK2 that activate ERK1/2 by phosphorylating the dual TEY motif in the ERK kinase domain, and the kinase that activates MEK1/2, raf-1; raf-1, in turn, is activated by the recruitment of the small G protein Ras by Grb/SOS complex formation after receptor tyrosine kinase activation. In the p38 MAPK module, the MAPK kinases 3/6 phosphorylate the TGY motif in the kinase domain of p38, and are themselves activated by upstream kinases, such as MEK kinases 2 and 3. Activation of the p38 pathway often follows cellular stress, such as that caused by UV light or osmotic changes, but the pathway is also activated by a variety of extracellular stimuli, such as cytokines and growth factors.
We recently identified brain-derived neurotrophic factor (BDNF), stem cell factor, and growth arrest-specific gene 6 as factors that block IGF-I-stimulated proliferation but enhance differentiation in growth plate chondrocytes and showed that ERK activity is required for chondrocyte proliferation (9). The three factors, as well as CNP, inhibit chondrocyte proliferation by reducing ERK activity but do not affect IGF-I-dependent phosphorylation of the IGF-I receptor or the immediate downstream signaling molecules Shc1 and Grb2, suggesting that these factors act on the ERK pathway downstream of the Shc1/Grb2 complex.
In the present article, we further define the roles of and interactions between the ERK and p38 pathways in growth plate chondrocyte development. We demonstrate that the two MAPK pathways interact to control the transition from proliferation to differentiation, ERK is required for both proliferation and differentiation, and that BDNF attenuates ERK activity by stimulating p38 to directly inhibit the activity of raf-1 in growth plate chondrocytes.
Materials and Methods
Bovine chondrocyte isolation and cell proliferation assay
Growth plate chondrocytes from the metacarpals of male cattle aged 6–10 months were removed and fractionated by continuous density gradient centrifugation as previously described (10). Cells from the highest density fraction corresponding to the proliferative zone were removed and rinsed. The cells were cultured on 60-mm plastic plates at a density of 1 × 105 cells per well in DMEM:F12 medium (Invitrogen, Carlsbad, CA) plus 1 mg/ml BSA, 25 μg/ml ascorbic acid, and penicillin/streptomycin/amphotericin B (Invitrogen) and were maintained in a humidified incubator at 37 C and 5% CO2. At 12 h, various factors were added at the following concentrations: IGF-I (100 ng/ml; Sigma, St. Louis, MO), the MEK inhibitor U0126 (10 μm; Promega, Madison, WI), p38 inhibitor SB203580 (10 μm; Calbiochem, San Diego, CA), JNK inhibitor SP600125 (10 μm; BIOMOL International, Farmingdale, NY), or the phosphatidylinositol 3 kinase (PI3K) inhibitor Wortmannin (100 nm; Sigma). Eight hours later, [3H]thymidine (95 Ci/mmol; Amersham, Piscataway, NJ) was added at 1 μCi per well. Sixteen hours later, the cells were scraped into 5% trichloroacetic acid, transferred to 24-mm Whatman glass filters, and rinsed three times with cold 5% trichloroacetic acid on a vacuum manifold, after which they were dried completely. Incorporated radioactivity was measured on a scintillation counter.
Note that 15 min after IGF-I addition, AKT (the kinase downstream of PI3K) phosphorylation was approximately 50-fold over baseline in ATDC5 cells; cells treated with 100 nm Wortmannin were indistinguishable from untreated cells with respect to AKT activation. At 15 min after IGF-I addition, JNK phosphorylation was not significantly above baseline. However, we were able to detect JNK phosphorylation approximately 8-fold over baseline at 5 min that was completely abrogated by the addition of 10 μm SP600125.
ATDC5 cell culture and transfections
ATDC5 cells from the RIKEN BioResource Center (Tsukuba, Japan) were maintained in DMEM:F12 with 10% fetal bovine serum (Invitrogen) plus penicillin/streptomycin/amphotericin B. To induce differentiation, cells were plated at 1 × 105 cells per well and grown to confluence, at which time the medium was changed to DMEM:F12 with 5% fetal bovine serum plus 10 μg/ml insulin, 10 μg/ml human transferrin, and 10 ng/ml sodium selenite (ITS). The medium was changed every other day. For cell transfection experiments, cells at 80% confluence were treated with FuGENE 6 (Roche, Indianapolis, IN) and 1 μg of each DNA plasmid for 24 h according to the manufacturer's instructions, before the cells were switched to medium containing ITS. DNA constructs encoding the MEK mutant (R4F), wild-type ERK2, and ERK2 mutant (K52R) were generous gifts from Melanie H. Cobb (University of Texas Southwestern Medical Center). R4F, the constitutively active form of MEK (ΔN3-S218/222E), has two amino acid substitutions and a gain-of-function amino-terminal truncation that significantly increases its activity toward ERK1/2. The dominant negative form of ERK2 (K52R) is catalytically inactive and, when overexpressed, blocks ERK activation of downstream targets.
For proliferation experiments using ATDC5 cells, confluent cells were incubated for 24 h in ITS as above, at which time the cells were trypsinized and replated at 1 × 105 cells per well in DMEM:F12 medium (Invitrogen) plus 1 mg/ml BSA, 25 μg/ml ascorbic acid, and penicillin/streptomycin/amphotericin B as described above. Although fully confluent cells exposed to insulin did not tolerate serum deprivation, the cells at lower density showed more than 95% viability after 12 h as determined by trypan blue staining. After 12 h of serum deprivation, various factors were added and [3H]thymidine uptake determined as described above.
Alcian blue uptake
ATDC5 cells were induced to differentiate with ITS as above. Additionally, the cells received the factors CNP (200 ng/ml) or BDNF (100 ng/ml) or various kinase inhibitors: 10 μm U0126, 10 μm SB203580, 100 nm Wortmannin, 10 μm SP600125, or the neurotrophin kinase receptor B (TrkB) inhibitors K-252a (3 nm; Calbiochem) or AG879 (10 μm; Calbiochem). The medium and inhibitors were changed every other day for 14 d. At the time points indicated, cells were fixed in 100% methanol at −20 C for 5 min, then stained overnight in 0.1% Alcian blue in 0.1 m HCl. After three washes with cold PBS, cells were solubilized in 6 m guanidine HCl overnight at room temperature. The optical density of each sample was measured at 650 nm. Each time point was assayed in triplicate.
RNA isolation, cDNA synthesis, and real-time RT-PCR
ATDC5 cells were induced to differentiate with ITS; indicated kinase inhibitors were added simultaneously. The medium was changed on d 2, and the cells were harvested into RNA STAT-60 (TelTest, Inc., Friendswood, TX) on d 3. RNA was extracted per the manufacturer's instructions. Genomic DNA was removed from each sample using DNA-Free (Ambion, Austin, TX), and 5 μg of RNA were reverse transcribed with the High-Capacity cDNA Archive kit (Applied Biosystems, Foster City, CA). Real-time RT-PCR was performed using the Roche LightCycler 480 following the protocol supplied by the manufacturer. 18S detection was done using TaqMan with the LightCycler 480 Probes Master Mix (Roche) and 100 nm each primer and probe. Mouse Col2a1 (collagen II alpha 1), Agc1 (aggrecan 1), and ColX targets were analyzed using double-stranded DNA dye SYBR Green with the LightCycler 480 SYBR Green 1 Master Mix (Roche) and 200 nm each primer. Wherever possible, the primers spanned an intron/exon boundary, and all RT-PCR were confirmed to produce only a single PCR product by comparison of the melt curves at the completion of each PCR. The primer sets for the mouse targets have been previously described (9). Relative gene expression for each mRNA was calculated by the ΔΔCT method using the “insulin only” sample as calibrator.
Western blot analysis
Isolated bovine proliferative zone chondrocytes were plated at 1 × 106 cells per 60-mm plate in serum-free DMEM:F12 plus 1 mg/ml BSA and allowed to rest overnight. Cells were pretreated with either 10 μm SB203580 or vehicle only for 30 min, after which they received either 100 ng/ml BDNF, 200 ng/ml CNP, or no treatment for an additional 30 min. At this point, the cells received either 100 ng/ml IGF-I or no further treatment and were incubated another 30 min. Whole-cell lysates were prepared in lysis buffer plus protease inhibitors and phosphatase inhibitors (1 mm NaF and 1 mm Na3VO4). Equal amounts of total protein were separated on 10% sodium dodecyl sulfate (SDS)-polyacrylamide gels, and transferred to Hybond-P membranes (Amersham). Membranes were blocked in, and all primary and secondary antibody incubations were performed in, StartingBlock Blocking buffer (Thermo Scientific, Rockford, IL) diluted 1:4 in Tris-buffered saline plus 0.05% Tween 20. The primary antibodies used were: antiphospho-ERK1/2 (1:5000; Invitrogen); antitotal-ERK1/2 (1:20,000; Sigma); antiphospho-p38 (1:1000; Cell Signaling, Beverly, MA); antitotal-p38 (1:2000; Sigma); antiphospho-MEK1/2 (1:1000; Cell Signaling); antitotal-MEK1/2 (1:1000; Cell Signaling); antiphospho-SAPK and antitotal-SAPK (1:1000; Cell Signaling); and antiphospho-AKT Ser473 and antitotal-AKT (1:1000; Cell Signaling). After washing with Tris-buffered saline plus 0.05% Tween 20, membranes were incubated with a horseradish peroxidase-labeled secondary antibody at 1:5000, and bands were visualized by chemiluminescence and Hyperfilm (Amersham). Relative band intensity was determined using ImageJ software analysis of scanned films, taking care to select exposures within the linear range of the film. The ratio of phosphorylated protein over corresponding total protein calculated from a minimum of four separate experiments was used to determine relative kinase activation; mean ± se is shown.
raf-1 kinase assays
Isolated proliferative bovine chondrocytes were treated with SB203580, BDNF, CNP, and IGF-I as described above. Whole-cell lysates were prepared in lysis buffer (50 mm Tris 7.4, 150 mm NaCl, 0.1% SDS, 0.01% Triton-X 100, and 1 mm phenylmethylsulfonylfluoride) plus phosphatase inhibitors (100 mm Na3VO4, 20 mm NaF, and 25 mm β-glycerophosphate). Lysates normalized for total protein content were precleared with 30 μl of protein-A agarose (Invitrogen) for 30 min at 4 C. Anti-raf-1 antisera (Santa Cruz Biotechnology, Inc., Santa Cruz, CA) was added at 1:500 plus 30 μl of protein-A agarose, and lysates incubated for 2 h with rocking at 4 C. Pellets were washed three times with lysis buffer and then washed three times with kinase buffer (50 mm Tris 7.4, 50 mm NaCl, 5 mm MnCl2, 2 mm dithiothreitol, and 1 mm phenylmethylsulfonylfluoride) plus phosphatase inhibitors as above. Kinase assays were performed in a total reaction volume of 25 μl containing 10 μm nonradiolabeled ATP, 5 μCi [γ-32P]ATP (3000 Ci/mmol; Amersham), and 0.5 μg of recombinant kinase-inactive glutathione S-transferase (GSK)-MEK K97M, a kind gift from Melanie H. Cobb. Samples were incubated for 30 min at 30 C, reactions were stopped by the addition of 2× SDS sample buffer, and proteins resolved by SDS-PAGE. Gels were stained with Coomassie blue, dried onto Whatman paper, and bands corresponding to GST-MEK were excised. Incorporated radioactivity was measured by scintillation counting. All reactions were performed in triplicate.
For the in vitro kinase assays, 10 ng of recombinant (His)6-tagged p38 protein (expressed in bacteria, a gift of Melanie H. Cobb) was incubated with either 10 ng of recombinant human constitutively active raf-1 EE (Y340E Y341E, 306-648) (purchased from R&D Systems, Minneapolis, MN) or with raf-1 immunoprecipitates from ATDC5 cell lysates as above, in 25 μl of kinase buffer plus 1 mg/ml BSA; after 30 min at 30 C, 0.5 μg of recombinant kinase-inactive GST-MEK and 5 μCi [γ-32P]ATP were added to these reactions, and the reactions were incubated for another 30 min at 30 C. Phosphate incorporation into MEK was assessed as above.
For the in vitro inhibition of p38, 50 ng of (His)6-tagged p38 protein was incubated with or without 10 μm SB203580 in the presence of 1:1000 anti-p38 antibody (Sigma-Aldrich, St. Louis, MO) and 20 μl of protein-A agarose; a parallel incubation was done with SB203580, antibody, and protein-A agarose but no p38 protein. After 20 min at room temperature, the beads were washed three times with 1 ml of kinase buffer plus 1 mg/ml BSA, and 10 ng of raf-1 EE were incubated directly with the beads for 30 min at 30 C, followed by assessment of phosphate incorporation into MEK as above. We found that SB203580 appeared to adhere to nickel resin (data not shown), making its use impractical for this experiment.
Statistical analyses
Except where noted, significance between groups was determined by one-way ANOVA (SigmaPlot 11.0; SyStat Software, Inc., San Jose, CA). Differences were considered significant at P < 0.05. Where noted, the post hoc pair-wise analyses were done by Student's t test and were considered significant at P < 0.05.
Results
ERK and p38 have opposing effects on proliferation
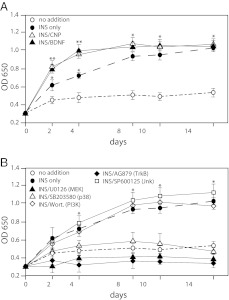
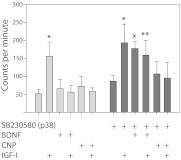
To examine the roles of the major intracellular kinase pathways in growth plate chondrocyte development, we exposed isolated primary bovine growth plate reserve/proliferative zone chondrocytes to various protein kinase inhibitors and measured the proliferative response to IGF-I at 24 h. U0126, which inhibits MEK, the kinase immediately upstream of ERK1/2(10), completely inhibited IGF-I-stimulated 3H-thymidine uptake (Fig. 1A). In contrast, the p38 inhibitor SB203580 (11, 12) doubled the amount of 3H-thymidine incorporation over that of IGF-I alone, suggesting that in the unstimulated state tonic levels of p38 activity negatively regulate chondrocyte proliferation. When ATDC5 cells were used, almost identical results were obtained (Fig. 1B). The PI3K inhibitor Wortmannin and the JNK inhibitor SP600125 had no effect on proliferation in either cell type. The positive control for activity of Wortmannin was inhibition of AKT phosphorylation 15 min after IGF-I addition in ATDC5 cells, and control for activity of SP600125 was inhibition of JNK phosphorylation 5 min after IGF-I addition (data not shown).
Fig. 1.
Kinase pathways involved in the proliferative response of chondrocytes to IGF-I. Isolated bovine reserve/proliferative zone cells (A) or ATDC5 cells after 24 h in the presence of insulin (ITS), which represent the very earliest stage of chondrocyte development (B), were cultured with IGF-I (100 ng/ml) and various protein kinase inhibitors for 24 h. Proliferation was assessed by 3H-thymidine uptake. *, P < 0.001 as compared with no addition control. Data points represent the mean ± sd of 12 samples.
ERK and p38, but not PI3K and JNK, are required for chondrocyte differentiation
We then examined the roles of these kinase pathways on chondrocyte differentiation. Although certain mesenchymal cells can be made to differentiate in micromass culture (13), we find that primary bovine chondrocytes dedifferentiate after several days in monolayer, as has been described (14, 15). Therefore, we used the mouse chondrogenic cell line ATDC5, which, in the presence of insulin, recapitulates many features of the growth plate chondrocyte differentiation program (16). ATDC5 cells synthesize and accumulate extracellular matrix proteins and proteoglycans during the course of differentiation; Alcian blue, a proteoglycan stain, is taken up by the cells in greater quantities as the cells mature and can be used to quantitate differentiation. Both CNP and BDNF enhanced Alcian blue uptake in the presence of insulin (Fig. 2A). Over a 14-d period, Alcian blue staining in the presence of either the PI3K inhibitor or the JNK inhibitor was similar to that seen with insulin alone (i.e. the inhibitors had little effect) (Fig. 2B). However, Alcian blue uptake in the presence of either the p38 inhibitor SB203589 or MEK inhibitor U0126 was no greater than that seen when the cells were cultured without insulin (i.e. marked inhibition of differentiation). Because we had previously shown that the neurotrophin BDNF promotes the differentiation of ATDC5 cells, we cultured the cells in the presence of two inhibitors of the Trk family of neurotrophin receptors, AG879 (Fig. 2B) and K-252a (data not shown), which also completely suppressed Alcian blue uptake. Cell viability for all time points and treatments was uniformly greater than 95%, and total cell number was comparable except for U0126-treated samples, which had a somewhat reduced cell number at each time point.
Fig. 2.
Kinase pathways involved in the differentiation of ATDC5 cells. Once at confluence, ATDC5 cells were made to differentiate by the addition of insulin (ITS) (10 μg/ml), and the medium was changed every other day for 14 d to recapitulate the entire chondrocyte developmental program. CNP (200 ng/ml) or BDNF (100 ng/ml) plus insulin were compared with insulin alone or no addition (A), or various protein kinase inhibitors were added simultaneously (B). Proteoglycan accumulation was assessed by Alcian blue uptake as measured by absorbance at OD650 of whole-cell lysates. Cell number and viability were similar across all time points, with the exception of the U0126-treated samples, which had a slightly lower cell number at each time point. *, P < 0.001 by Student's t test as compared with same time point for no addition control; **, P < 0.001 as compared with same time point for insulin only control. One-way ANOVA, P < 0.05. Data points represent the mean ± sd of six samples.
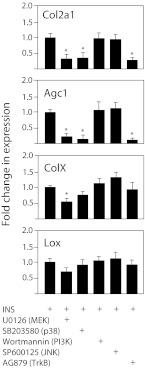
To confirm the results of the Alcian blue uptake assay, we used RT-PCR to assess the expression of two markers of early chondrocyte differentiation, Col2a1 and Agc1, and two markers of late-stage differentiation, ColX and lysyl oxidase (Lox), in ATDC5 cells cultured with insulin plus kinase inhibitors for 3 d, a duration representing the early phase of chondrocyte differentiation. Note that during this early phase of ATDC5 cell differentiation, Col2a1 and Agc1 mRNA levels are increased, but late stage markers LOX and COLX are not (9). As seen for proliferation, the PI3K and JNK inhibitors had no effect on insulin-stimulated chondrocyte differentiation (Fig. 3). Complete inhibition of the ERK pathway with the MEK inhibitor U0126 suppressed mRNA expression of the early phase markers by 70–80%; the late-phase marker ColX was decreased by 50%, and the change for Lox did not reach significance. Inhibition of p38 with SB203589 suppressed only the early markers Col2a1 and Agc1, in a pattern almost identical to that seen with the Trk neurotrophin receptor kinase inhibitor AG879 (Fig. 3).
Fig. 3.
Kinase pathways involved in the expression of markers of differentiation in ATDC5 cells. Once at confluence, ATDC5 cells were made to differentiate by the addition of insulin (ITS). Various protein kinase inhibitors were added simultaneously, and the media changed every other day for a total of 3 d, which corresponds to the early phase of differentiation. Differentiation was assessed by RT-PCR for the markers Col2a1, Agc1, ColX, and Lox. Data points represent the mean ± sd of six samples, expressed as fold difference from insulin alone (calibrator); *, P < 0.001.
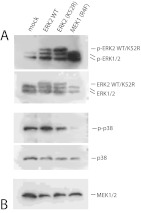
Although the small molecule kinase inhibitors used in these experiments are considered to be specific for their respective targets, we sought to confirm these findings with use of an alternate approach to alter ERK activity during ATDC5 differentiation. Cells were transfected with either wild-type ERK2 protein, a kinase-dead mutant ERK2-K52R that does not affect ERK kinase activity directly but instead blocks downstream activation of ERK targets, or a constitutively active mutant of MEK1, R4F. Mock-transfected cells received only transfection reagent. ATDC5 cells were then made to differentiate for 3 d in insulin (again, representing the early phase of development), and Col2a1, Agc1, ColX, and Lox expression was assessed with RT-PCR as above. Interference with pathways downstream of ERK1/2 with the dominant negative ERK2 mutant inhibited the expression of the early phase markers Col2a1 and Agc1, similar to the MEK inhibitor U0126, but had no significant affect on ColX or Lox expression during the early phase of ATDC5 cell differentiation (Fig. 4A). An increase in ERK activity, either by overexpression of ERK2 protein or by expression of the constitutively active MEK1 mutant, MEK-R4F, resulted in a similar pattern of inhibition of suppression of early markers of differentiation. Figure 4B shows that expression of MEK-R4F increased endogenous ERK1/2 phosphorylation and decreased p38 phosphorylation. However, overexpression of kinase-inactive ERK2 did not alter p38 activity.
Fig. 4.
Alteration of ERK activity inhibits differentiation of ATDC5 cells. ATDC5 cells at 80% confluence were transfected with constructs expressing the following proteins: ERK2-WT (wild-type ERK2), ERK2–K52R (a kinase dead mutant), and MEK-R4F (constitutively active MEK1). Mock cells received only transfection reagent. After 24 h, cells were switched to medium containing insulin, and at d 3 after insulin addition, differentiation was assessed by RT-PCR for the markers Col2a1, Agc1,ColX, and Lox, with mock samples as calibrator; *, P < 0.001 (A). Simultaneous samples were collected and assessed for expression and activity status of ERK, MEK, and p38 by Western blotting (B). Note that exogenous ERK2 protein migrates above endogenous ERK2 because it contains a 3xFLAG peptide tag.
BDNF and IGF-I in combination activate p38, but IGF-I alone suppresses p38 activity
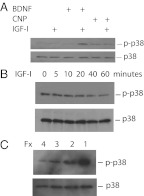
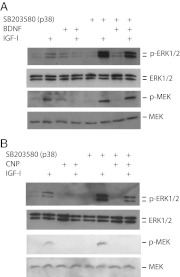
We have previously shown that the neurotrophin BDNF inhibits IGF-I-stimulated proliferation in primary chondrocytes and enhances differentiation in ATDC5 cells, as does CNP (Fig. 2 and Ref. 9). Because p38 inhibition also enhances proliferation (Fig. 1) and suppresses differentiation (Fig. 2B) in chondrocytes, we questioned whether the effects of BDNF on chondrocytes are in fact mediated by the p38 pathway. To see whether BDNF activated p38, we exposed isolated primary bovine chondrocytes to combinations of IGF-I and BDNF and then assayed p38 activation by Western blotting using an antiserum to the dually phosphorylated TGY motif required for p38 kinase activity. IGF-I and BDNF in combination increased p38 phosphorylation 10-fold (10.4 ± 2.1), but neither protein alone had any effect. A representative blot is shown in Fig. 5A. We also assessed the ability of CNP, a known regulator of growth plate chondrocyte differentiation, to increase p38 activity; CNP alone had little effect, whereas p38 activation by IGF-I plus CNP was 8.1 ± 1.3-fold greater than seen with IGF-I alone.
Fig. 5.
Activity of p38 in primary chondrocytes in response to IGF-I, BDNF, and CNP. Serum-starved isolated bovine reserve/proliferative cells (A) were exposed to BDNF, CNP, or vehicle for 30 min, then to IGF-I (or vehicle) for 30 min. Serum-starved prehypertrophic bovine cells (B) were given IGF-I for the times shown. C, Lysates were made from serum-starved bovine chondrocytes from each fraction (Fx 4, Reserve zone; Fx 1, hypertrophic zone). Activation of p38 in whole-cell lysates was assessed by Western blotting for the dually phosphorylated form of the kinase; membranes were then stripped, and loading efficiency was assessed by blotting for total p38 protein content in each sample.
Because (as seen in Fig. 4B) increased ERK activity resulting from overexpression of the active MEK mutant reduced p38 activity, we asked whether IGF-I alone could similarly affect p38. The activity of p38 is very low in reserve zone chondrocytes and increases with further development, with the activity in hypertrophic zone cells 43 ± 6.4 times that of reserve zone cells (Fig. 5C). Isolated primary bovine chondrocytes corresponding to the prehypertrophic zone (fraction 2) were serum starved for 2 h and then exposed to 100 ng/ml IGF-I for 1 h. This treatment indeed reduced p38 phosphorylation to 15.3 ± 2.1% of baseline by 60 min (Fig. 5B). After 90 and 120 min of IGF-I addition, p38 phosphorylation was undetectable (data not shown). All Western blottings shown are representative of several independent experiments.
BDNF and CNP are not equally dependent on p38 for inhibition of ERK1/2
IGF-I-stimulated chondrocyte proliferation requires ERK1/2 activation, which is reduced by both BDNF and CNP (9). To determine whether this ERK inhibition involves p38, we exposed reserve/proliferative zone bovine chondrocytes to the p38 inhibitor SB203580, followed by either BDNF or CNP, and finally to IGF-I. ERK1/2 and MEK activation were assessed by Western blotting using antisera to the respective specifically phosphorylated proteins. As seen previously (9), although IGF-I alone increased ERK activity by a factor of 21.4 ± 2.7, both BDNF (Fig. 6A) and CNP (Fig. 6B) reduced ERK1/2 activity to 30–50% of baseline levels in primary chondrocytes. In cells pretreated with the p38 inhibitor, ERK activation by IGF-I was increased to 43.7 ± 10.1 over baseline, whereas BDNF plus IGF-I resulted in ERK activation 38.7 ± 7.8 over baseline in the presence of the p38 inhibitor. The pattern of MEK activation was similar: IGF-I alone increased MEK activity by 8.9 ± 3.1-fold, IGF-I plus BDNF reduced MEK activity to 50% of baseline, and pretreatment with the p38 inhibitor changed the fold activation of MEK to 16.5 ± 8.6 and 16.1 ± 6.7 over baseline, respectively.
Fig. 6.
Role of p38 in the attenuation of ERK activity by BDNF and CNP. Isolated bovine primary reserve/proliferative chondrocytes were serum starved and then exposed to the p38 inhibitor SB203580 or vehicle for 30 min, followed by either BDNF (A) or CNP (B) for 30 min, then IGF-I or vehicle for 15 min. Activation of ERK1/2 and MEK in whole-cell lysates was assessed by Western blotting for the phosphorylated forms of the kinases; the membranes were then stripped and blotted for total ERK1/2 or MEK protein. Similar results were seen in ATDC5 cells (data not shown).
In contrast, p38 inhibition only partly relieved the inhibition of ERK by CNP (Fig. 6B), because ERK activity in cells pretreated with BS203580 then exposed to IGF-I plus CNP was only 12.5 ± 2.9-fold over baseline compared with 27.6 ± 7.1 over baseline without CNP. Moreover, p38 inhibition did not reverse the ability of CNP to block MEK activation by IGF-I.
BDNF inhibits the ERK pathway via p38-dependent inhibition of raf-1 kinase
We hypothesized that the reduction of ERK1/2 activity by BDNF occurs via inhibition of upstream activators or by activation of various phosphatases that inactivate ERK. To explore the former hypothesis, we assessed the activity of the kinase directly upstream of MEK, raf-1. Primary bovine chondrocytes were treated as above, first with the p38 inhibitor, followed by either BDNF or CNP, and lastly with IGF-I. Endogenous raf-1 was immunoprecipitated from whole-cell lysates and raf-1 kinase activity assayed using kinase-defective MEK as substrate. Figure 7 shows that both BDNF and CNP inhibit IGF-I-stimulated raf-1 activity. However, although p38 inhibition almost completely relieves the inhibition of raf-1 by BDNF, it does not affect inhibition of raf-1 by CNP. Similar results were seen when ATDC5 cell lysates were used.
Fig. 7.
Involvement of p38 in the regulation of raf-1 activity by BDNF and CNP. Isolated bovine reserve/proliferative chondrocytes were treated as in Fig. 5; whole-cell lysates normalized for total protein content were immunoprecipitated with antisera to raf-1. The resulting immunoprecipitates were used in in vitro kinase assays; raf-1 kinase activity was measured as incorporated 32P into catalytically inactive MEK1 protein. *, P < 0.01; **, P < 0.03 as compared with no insulin control. Data points represent the mean ± sd of six samples, with kinase assays run in duplicate.
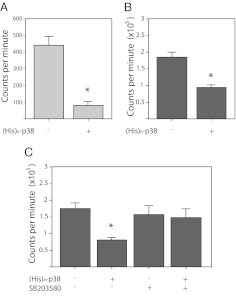
To explore the possibility that p38 directly inhibits raf-1 kinase activity, we incubated immunoprecipitates of endogenous raf-1 (from the lysates of ATDC5 cells stimulated with IGF-I) with (His)6-p38 protein (purified from bacteria) in the presence of 10 μm ATP for 30 min, followed by the addition of kinase-defective GST-MEK1 protein and γ32P-ATP. Note that we did not preactivate the p38 protein by incubation with MEK3/6. The p38 protein reduced the ability of immunoprecipitated, endogenous raf-1 to phosphorylate MEK1 by 80% (Fig. 8A). Similarly, when p38 protein was coincubated with purified, recombinant, constitutively active raf-1 EE, the activity of raf-1 toward MEK1 is reduced by almost 50% (Fig. 8B).
Fig. 8.
Inhibition of raf-1 kinase activity by p38. ATDC5 cells were stimulated with IGF-I (100 ng/ml) for 15 min; cell lysates immunoprecipitated for endogenous raf-1 protein were incubated with or without recombinant (His)6-p38 protein for 30 min, then kinase inactive MEK1 and γ32P-ATP were added for another 30 min (A). Alternatively, recombinant, constitutively active raf-1 EE was treated with or without p38 (B). C, (His)6-p38 was preincubated with or without SB203580, then immunoprecipitated and washed before the addition of raf-1 EE protein. In each panel, raf-1 kinase activity was measured as incorporated 32P into catalytically inactive MEK1 protein. *, P < 0.01, as compared with raf-1 alone. Data points represent the mean ± sd of eight samples, with kinase assays run in duplicate.
To demonstrate that the inhibition of raf-1 is dependent on p38 kinase activity, we sought to use the p38 inhibitor SB203580, which is highly selective for p38α/β due to the presence of a threonine residue at position 106. However, due to the presence of a threonine at the analogous position in the raf proteins, they are also subject to inhibition by SB203580, with an in vitro IC50 of 2 μm (17). To inhibit p38 without affecting raf-1 activity, p38 protein was preincubated with 10 μm SB203580, then bound to anti-p38 antisera and protein-A agarose. After washing, the treated p38 was incubated with raf-1 EE, and its activity toward MEK1 assessed as above. Figure 8C shows that although p38 pretreated without the inhibitor reduced raf-1 activity by almost 50%, the p38 pretreated with SB203580 was unable to significantly affect raf-1 activity toward MEK1.
Discussion
In the present study, we describe how the ERK1/2 and p38 MAPK signaling pathways interact to regulate growth plate chondrocyte proliferation (in primary bovine reserve/proliferative zone chondrocytes and murine ATDC5 cells), differentiation (in ATDC5 cells), and the transition between these two very different developmental programs.
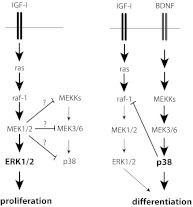
We found that ERK and p38 are both crucial to chondrogenesis, but the two pathways play distinct roles and appear to be mutually antagonistic. ERK activity is absolutely required for all stages of chondrocyte development, but the level of ERK activation varies greatly. ERK activity must be high during proliferation but must be low (but not nil) for differentiation to proceed. CNP and BDNF both attenuate ERK activity while stimulating ATDC5 cell differentiation. Too much ERK activity (as is seen in the case of overexpression of wild-type ERK2 and MEK R4F), or interference with ERK's ability to activate downstream targets (as is seen in the case of overexpression of a kinase dead mutant), will ultimately impair the pace of differentiation, seen in reduced Col2a1 and Agc1 mRNA levels. (Agc1 mRNA changes parallel that of Col2a1 in these experiments.) We believe that the reason there were not significant changes in either ColX or Lox mRNA levels in these experiments was due to the nature of the timing; ATDC5 cells were only allowed to incubate with insulin for 3 d, and during this time, although Col2a1 and Agc1 mRNA levels rise significantly, late-phase differentiation markers ColX and Lox are not expected to change (9). In contrast, p38 activity is absolutely required for hypertrophic differentiation in ATDC5 cells but is inhibitory to proliferation in primary chondrocytes and ATDC5 cells. Moreover, IGF-I suppresses p38 activity in primary chondrocytes. Thus, our data are consistent with a model wherein unopposed IGF-I produces high ERK/p38 activity ratios favoring proliferation, whereas the addition of either BDNF or CNP reduces the ERK/p38 activity ratio, favoring differentiation in chondrocytes (see Fig. 9).
Fig. 9.
Proposed model of interaction between ERK and p38 MAPK pathways in growth plate chondrocyte development. Unopposed IGF-I favors proliferation via a high ERK/p38 activity ratio; the inhibition of the pathway upstream of p38 by MEK1/2 is speculative (as designated by a question mark). IGF-I and BDNF in combination activates p38, which inhibits raf-1 activity, leading to a low ERK/p38 activity ratio that favors differentiation.
We also found that the interaction between the two pathways is at least partly responsible for the transition from the high ERK/p38 ratios seen in the early proliferative stages to later low ERK/p38 activity ratios favoring differentiation. Overexpression of constitutively active MEK1 prevents p38 activation, and IGF-I suppresses p38 activity by activating MEK. The mechanism whereby the ERK pathway suppresses p38 activation is unknown. The observation that overexpression of the kinase-inactive ERK2 did not affect p38 activity, whereas overexpression of constitutively active MEK1 does reduce p38 activity, implicates MEK1/2 in the suppression of p38 activation, but this is yet to be shown. The ability of the ERK pathway to negatively regulate p38 fits in our model, wherein unopposed IGF-I favors proliferation over differentiation by keeping p38 activity in check (Fig. 9).
In primary bovine chondrocytes we showed that BDNF and CNP attenuate ERK activity by blocking activation of the upstream kinase raf-1. This finding is consistent with previous reports that CNP blocks ERK activation at the level of raf-1 in growth plate chondrocytes (18, 19). We also demonstrated that suppression of raf-1 activation by BDNF is p38 dependent, raising the possibility that raf-1 might be a direct target of p38. Indeed, we found that p38 directly inhibits raf-1 kinase activity in vitro and that this inhibition is most likely via direct phosphorylation of raf-1 by p38, because p38 pretreated with SB203580, which blocks ATP binding at the active site of the kinase, abrogates the ability of p38 to affect raf-1 kinase activity. Although we have yet to define the specific phosphorylation site on raf-1, it is unlikely to be in the ras-binding domain, because the constitutively active form of raf-1 used in our in vitro experiments lacks that region. Previous reports have noted that SB203580 activates raf-1 in whole cells, despite the fact that this p38 inhibitor acts as a raf-1 inhibitor in vitro (17, 20), suggesting that p38 activity does play a role in the modulation of raf-1 activity. However, to our knowledge, it has not been demonstrated previously that raf-1 is a direct target of p38.
The requirement of p38 for chondrogenesis has been reported in rat mesenchymal cells (13), chick embryo limb buds (21), and ATDC5 cells (22), wherein pharmacological inhibition of p38 activity delays hypertrophic differentiation. At least some of the effects of CNP at the growth plate are reported to be p38 dependent (18). The observation that BDNF reverses the effect of IGF-I on p38 activity suggests a complex interaction between pathways downstream of the IGF-I receptor and the receptor for BDNF, TrkB. TrkB is a transmembrane receptor that includes in its intracellular region a Shc-binding site near the cell membrane and tyrosine kinase and phospholipase C-γ-binding domains distally. The TrkB inhibitors used in the present studies target its tyrosine kinase activity, but other pathways mediating BDNF/TrkB effects cannot be ruled out as yet.
The precise role of the ERK MAPK pathway in chondrocyte development has been unclear. In embryonic chick limb buds, pharmacological inhibition of MEK resulted in increased expression of transcripts for collagen II and aggrecan (23). A transgenic mouse expressing constitutively active MEK1 at the growth plate displayed incomplete hypertrophic differentiation and dwarfism (24). In these reports, the ERK MAPK pathway was interpreted as being inhibitory to chondrocyte development. However, in mice lacking both ERK1 and ERK2 in osteo-chondroprogenitor cells, the growth plates had extremely small proliferative zones, widened hypertrophic zones, and very little active proliferation (25). The nearly absent proliferative zone and widened hypertrophic zone of these mice suggest that ERKs are required for proliferation but inhibitory to terminal differentiation. These results are consistent with our model, wherein ERK1/2 activation is absolutely required for IGF-I-stimulated proliferation in primary chondrocytes, whereas prodifferentiation factors decrease ERK1/2 activity and increase p38 activity to favor differentiation over proliferation. However, the failure of ATDC5 cells to differentiate in the face of ERK and MEK inhibition suggests that some amount of ERK1/2 activity is required for the complete chondrocyte developmental program.
Precise patterns of activation of the ERK and p38 pathways and interactions between them are crucial to a wide variety of developmental programs, but how the two pathways interact in different tissues varies. In 3T3-L1 cells, inhibition of either p38 or ERK blocks adipogenesis (26, 27), and both pathways are similarly required for neurite outgrowth in PC12 cells (28). Tubulogenesis in renal epithelial MDCK cells requires the sustained activation of ERK as well as p38 activation (29, 30). Coordination of the timing of activation of the ERK and p38 pathways is critical to proper development. For example, during smooth muscle cell development, growth factors such as platelet-derived growth factor stimulate high levels of ERK activity that promote proliferation while suppressing the expression of muscle-specific genes by displacing transcription factors such as myocardin from muscle-specific promoter regions (31–33). Because ERK activity is required for the early proliferative stage, but antagonistic to later stages of differentiation in both developing myocytes and growth plate chondrocytes, the timing of the activation of the MAPK pathways must be tightly regulated in these cells. In chondrocytes, this timing is at least partly regulated by growth factors such as CNP and BDNF, which increase p38 activity to attenuate ERK activity to promote the transition from a proliferative program to a differentiation program. Interestingly, not all factors that influence chondrocyte differentiation do so by altering p38 activity. We found that PTH-related peptide, which inhibits the pace of differentiation in prehypertrophic chondrocytes, did not affect p38 activity in prehypertrophic stage primary chondrocytes or ATDC5 cells (data not shown).
In summary, our observations indicate that BDNF and CNP act in chondrocytes to promote the transition from proliferation to differentiation by coordinating the attenuation of ERK1/2 activity while increasing that of p38, but some amount of ERK activity is required for further chondrocyte differentiation. The JNK MAPK pathway and PI3K are not involved in this process. The transition from a high ERK activity/proliferative program to a low ERK activity/differentiation program requires the activation of p38, which directly inhibits raf-1 kinase activity. Understanding the precise mechanisms by which the ERK and p38 MAPK pathways interact during chondrocyte development should enhance our understanding of development in other tissues.
Acknowledgments
I thank Michelle Castro for her technical help with RT-PCR analysis and Perrin C. White for his critical review of the manuscript.
This work was supported by the National Institutes of Health Grant DK073447.
Disclosure Summary: The author has nothing to disclose.
Footnotes
- Agc
- Aggrecan
- BDNF
- brain-derived neurotrophic factor
- CNP
- C-type natriuretic peptide
- Col2a1
- collagen II, alpha 1
- COLX
- type X collagen
- GSK
- glutathione S-transferase
- ITS
- insulin/transferrin/selenium
- JNK
- c-Jun N-terminal kinase
- Lox
- lysyl oxidase
- MEK
- MAPK kinase
- PI3K
- phosphatidylinositol 3 kinase
- SDS
- sodium dodecyl sulfate
- TrkB
- neurotrophin kinase receptor B.
References
- 1. Hunziker EB. 1994. Mechanism of longitudinal bone growth and its regulation by growth plate chondrocytes. Microsc Res Tech 28:505–519 [DOI] [PubMed] [Google Scholar]
- 2. Kronenberg HM. 2003. Developmental regulation of the growth plate. Nature 423:332–336 [DOI] [PubMed] [Google Scholar]
- 3. Chen Z, Gibson TB, Robinson F, Silvestro L, Pearson G, Xu B, Wright A, Vanderbilt C, Cobb MH. 2001. MAP kinases. Chem Rev 101:2449–2476 [DOI] [PubMed] [Google Scholar]
- 5. Cobb MH. 1999. MAP kinase pathways. Prog Biophys Mol Biol 71:479–500 [DOI] [PubMed] [Google Scholar]
- 5. Johnson GL, Lapadat R. 2002. Mitogen-activated protein kinase pathways mediated by ERK, JNK, and p38 protein kinases. Science 298:1911–1912 [DOI] [PubMed] [Google Scholar]
- 6. Kyriakis JM, Avruch J. 1996. Protein kinase cascades activated by stress and inflammatory cytokines. Bioessays 18:567–577 [DOI] [PubMed] [Google Scholar]
- 7. Robinson MJ, Cobb MH. 1997. Mitogen-activated protein kinase pathways. Curr Opin Cell Biol 9:180–186 [DOI] [PubMed] [Google Scholar]
- 8. Pearson G, Robinson F, Beers Gibson T, Xu BE, Karandikar M, Berman K, Cobb MH. 2001. Mitogen-activated protein (MAP) kinase pathways: regulation and physiological functions. Endocr Rev 22:153–183 [DOI] [PubMed] [Google Scholar]
- 9. Hutchison MR, Bassett MH, White PC. 2010. SCF, BDNF, and Gas6 are regulators of growth plate chondrocyte proliferation and differentiation. Mol Endocrinol 24:193–203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hutchison MR, Bassett MH, White PC. 2007. Insulin-like growth factor-I and fibroblast growth factor, but not growth hormone, affect growth plate chondrocyte proliferation. Endocrinology 148:3122–3130 [DOI] [PubMed] [Google Scholar]
- 11. Cuenda A, Rouse J, Doza YN, Meier R, Cohen P, Gallagher TF, Young PR, Lee JC. 1995. SB 203580 is a specific inhibitor of a MAP kinase homologue which is stimulated by cellular stresses and interleukin-1. FEBS Lett 364:229–233 [DOI] [PubMed] [Google Scholar]
- 12. Goedert M, Cuenda A, Craxton M, Jakes R, Cohen P. 1997. Activation of the novel stress-activated protein kinase SAPK4 by cytokines and cellular stresses is mediated by SKK3 (MKK6); comparison of its substrate specificity with that of other SAP kinases. EMBO J 16:3563–3571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Li J, Zhao Z, Liu J, Huang N, Long D, Wang J, Li X, Liu Y. 2010. MEK/ERK and p38 MAPK regulate chondrogenesis of rat bone marrow mesenchymal stem cells through delicate interaction with TGF-β1/Smads pathway. Cell Prolif 43:333–343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bashey RI, Iannotti JP, Rao VH, Reginato AM, Jimenez SA. 1991. Comparison of morphological and biochemical characteristics of cultured chondrocytes isolated from proliferative and hypertrophic zones of bovine growth plate cartilage. Differentiation 46:199–207 [DOI] [PubMed] [Google Scholar]
- 15. Olney RC, Smith RL, Kee Y, Wilson DM. 1993. Production and hormonal regulation of insulin-like growth factor binding proteins in bovine chondrocytes. Endocrinology 133:563–570 [DOI] [PubMed] [Google Scholar]
- 16. Shukunami C, Shigeno C, Atsumi T, Ishizeki K, Suzuki F, Hiraki Y. 1996. Chondrogenic differentiation of clonal mouse embryonic cell line ATDC5 in vitro: differentiation-dependent gene expression of parathyroid hormone (PTH)/PTH-related peptide receptor. J Cell Biol 133:457–468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hall-Jackson CA, Goedert M, Hedge P, Cohen P. 1999. Effect of SB 203580 on the activity of c-Raf in vitro and in vivo. Oncogene 18:2047–2054 [DOI] [PubMed] [Google Scholar]
- 18. Agoston H, Khan S, James CG, Gillespie JR, Serra R, Stanton LA, Beier F. 2007. C-type natriuretic peptide regulates endochondral bone growth through p38 MAP kinase-dependent and -independent pathways. BMC Dev Biol 7:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Krejci P, Masri B, Fontaine V, Mekikian PB, Weis M, Prats H, Wilcox WR. 2005. Interaction of fibroblast growth factor and C-natriuretic peptide signaling in regulation of chondrocyte proliferation and extracellular matrix homeostasis. J Cell Sci 118:5089–5100 [DOI] [PubMed] [Google Scholar]
- 20. Kalmes A, Deou J, Clowes AW, Daum G. 1999. Raf-1 is activated by the p38 mitogen-activated protein kinase inhibitor, SB203580. FEBS Lett 444:71–74 [DOI] [PubMed] [Google Scholar]
- 21. Stanton LA, Sabari S, Sampaio AV, Underhill TM, Beier F. 2004. p38 MAP kinase signalling is required for hypertrophic chondrocyte differentiation. Biochem J 378:53–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Nakamura K, Shirai T, Morishita S, Uchida S, Saeki-Miura K, Makishima F. 1999. p38 mitogen-activated protein kinase functionally contributes to chondrogenesis induced by growth/differentiation factor-5 in ATDC5 cells. Exp Cell Res 250:351–363 [DOI] [PubMed] [Google Scholar]
- 23. Bobick BE, Kulyk WM. 2004. The MEK-ERK signaling pathway is a negative regulator of cartilage-specific gene expression in embryonic limb mesenchyme. J Biol Chem 279:4588–4595 [DOI] [PubMed] [Google Scholar]
- 24. Murakami S, Balmes G, McKinney S, Zhang Z, Givol D, de Crombrugghe B. 2004. Constitutive activation of MEK1 in chondrocytes causes Stat1-independent achondroplasia-like dwarfism and rescues the Fgfr3-deficient mouse phenotype. Genes Dev 18:290–305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Matsushita T, Chan YY, Kawanami A, Balmes G, Landreth GE, Murakami S. 2009. Extracellular signal-regulated kinase 1 (ERK1) and ERK2 play essential roles in osteoblast differentiation and in supporting osteoclastogenesis. Mol Cell Biol 29:5843–5857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Engelman JA, Lisanti MP, Scherer PE. 1998. Specific inhibitors of p38 mitogen-activated protein kinase block 3T3-L1 adipogenesis. J Biol Chem 273:32111–32120 [DOI] [PubMed] [Google Scholar]
- 27. Sale EM, Atkinson PG, Sale GJ. 1995. Requirement of MAP kinase for differentiation of fibroblasts to adipocytes, for insulin activation of p90 S6 kinase and for insulin or serum stimulation of DNA synthesis. EMBO J 14:674–684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Morooka T, Nishida E. 1998. Requirement of p38 mitogen-activated protein kinase for neuronal differentiation in PC12 cells. J Biol Chem 273:24285–24288 [DOI] [PubMed] [Google Scholar]
- 29. O'Brien LE, Tang K, Kats ES, Schutz-Geschwender A, Lipschutz JH, Mostov KE. 2004. ERK and MMPs sequentially regulate distinct stages of epithelial tubule development. Dev Cell 7:21–32 [DOI] [PubMed] [Google Scholar]
- 30. Albig AR, Schiemann WP. 2005. Identification and characterization of regulator of G protein signaling 4 (RGS4) as a novel inhibitor of tubulogenesis: RGS4 inhibits mitogen-activated protein kinases and vascular endothelial growth factor signaling. Mol Biol Cell 16:609–625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Wang Z, Wang DZ, Hockemeyer D, McAnally J, Nordheim A, Olson EN. 2004. Myocardin and ternary complex factors compete for SRF to control smooth muscle gene expression. Nature 428:185–189 [DOI] [PubMed] [Google Scholar]
- 32. Gille H, Kortenjann M, Thomae O, Moomaw C, Slaughter C, Cobb MH, Shaw PE. 1995. ERK phosphorylation potentiates Elk-1-mediated ternary complex formation and transactivation. EMBO J 14:951–962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Bennett AM, Tonks NK. 1997. Regulation of distinct stages of skeletal muscle differentiation by mitogen-activated protein kinases. Science 278:1288–1291 [DOI] [PubMed] [Google Scholar]