Abstract
Chicken ovalbumin upstream promoter transcription factor (COUP-TF)II has been shown to play a major role in endothelial cell growth and regulation of the Notch signaling pathway to confer vein identity. However, the underlying mechanisms for COUP-TFII regulation in these pathways remain to be defined. Here we employed a genomic approach by using microarray analysis to identify downstream targets in human umbilical vein endothelial cells (HUVEC) cells and found the expression of many genes in the cell cycle pathway and Notch signaling pathway are significantly altered in the COUP-TFII-depleted cells. The expression of E2F transcription factor 1 (E2F1), a key transcription factor that regulates the expression of cell cycle regulators, is reduced in the absence of COUP-TFII. Using chromatin immunoprecipitation experiments, we showed that COUP-TFII directly regulates the expression of E2F1 through tethering to the Sp1 binding sites in the promoter of E2F1 to modulate cell proliferation. In addition, we also demonstrate that Foxc1 and Np-1, two upstream genes of Notch signaling and Hey2, a downstream effector of Notch signaling, are direct targets of COUP-TFII. Furthermore, COUP-TFII suppresses the expression of EphrinB2, an arterial marker, while enhancing the expression of ephrin receptor B4, a venous marker, supporting our in vivo findings that COUP-TFII regulates vein identity by suppressing the Notch signal pathway.
Chicken ovalbumin upstream promoter transcription factor (COUP-TF)II, a member of the nuclear receptor superfamily, plays a critical role in angiogenesis during both developmental and pathological conditions (1–3). Targeted deletion of the COUP-TFII gene results in embryonic lethality with defects in angiogenesis and heart development. COUP-TFII mutants are also defective in remodeling the primitive capillary plexus into large and small microcapillaries (1). Conditional knockout of COUP-TFII in adult mice severely compromises tumor neoangiogenesis and limits tumor growth in various mouse tumor models, including xenograft, spontaneous mammary gland, and pancreatic islet tumor models (2, 3).
Various signal pathways and molecular regulators have been identified to participate in the angiogenic process (4–8). We have shown previously that COUP-TFII plays a cell-autonomous role in the endothelial cells to stimulate endothelial cell sprouting by regulating endothelial cell proliferation and migration and the eventual angiogenesis (3). One underlying mechanism was attributed to COUP-TFII's ability to stimulate vascular endothelial growth factor (VEGF)/VEGF receptor (VEGFR)2 signaling by inhibiting the expression of VEGFR1, a decoy receptor of VEGF signaling (9–11). However, knockdown of VEGFR1 in COUP-TFII-depleted human umbilical vein endothelial cells (HUVEC) cells can only partially rescue the sprouting defect (3), suggesting that other alternative pathways may also contribute to COUP-TFII's function in regulating endothelial cell sprouting and angiogenesis. Because endothelial cell sprouting requires cell proliferation, the cell cycle arrest upon depletion of COUP-TFII in HUVEC cells suggests that COUP-TFII also regulates cell proliferation to control angiogenesis.
Employing microarray analysis as an unbiased approach to identify downstream targets that may mediate COUP-TFII function, we found that the expression of many cell cycle-regulated genes is significantly changed in COUP-TFII-depleted cells. Interestingly, the expression of E2F transcription factor 1 (E2F1), a major G1/S transition phase regulator (12, 13), is significantly reduced in the absence of COUP-TFII. Using chromatin immunoprecipitation (ChIP), we further illustrated that COUP-TFII is recruited to the promoter region of E2F1 to stimulate the expression of E2F1, which, in turn, regulates cell proliferation.
In addition to endothelial cell proliferation, Notch signaling is also shown to be involved in angiogenesis and the establishment of the vein/artery identity in the zebra fish and mouse (14–18). Mutant mice with target disruption in components of the Notch pathway, including Notch1, Notch1/Notch4, Jagged1, and Dll1, are embryonically lethal due to vascular defects and hemorrhage (15, 19, 20). In addition, disruption of Notch downstream components hairy-and-enhancer-of-split related (Hey1)/Hey2 in the mouse also leads to embryonic lethality with vascular defects and the loss of expression of arterial marker EphrinB2 (21). Foxc1 and Foxc2, two closely related Fox transcription factors, have also been shown to be required for artery specification in mice. The compound Foxc1/Foxc2 mutant exhibits arteriovenous malformations and a lack of induction of arterial markers (21, 22). Importantly, Foxc1/Foxc2 acts upstream of the Notch pathway by directly regulating Dll4 expression (23).
We showed previously that COUP-TFII is essential for the specification of vein identity. COUP-TFII is expressed in venous endothelial cells but not arterial endothelial cells. Endothelial-specific knockout of COUP-TFII showed that the mutant vein acquires arterial characteristics by expressing many arterial markers, including the ectopic expression of the arterial markers neuropilin 1 (Np-1), Jagged1, Notch1, Hey1, and ephrinB2 (24). Moreover, overexpression of COUP-TFII in the endothelium resulted in embryonic lethality with defective angiogenesis. The arterial markers Np-1 and Jagged 1 was greatly reduced in the large fused, disorganized vein-like vessel plexus in the transgenic embryos. All these results indicate that COUP-TFII regulates vein identity by repressing Notch signaling (24).
Although we have shown that the Notch signaling component is repressed by COUP-TFII in vein endothelial cells, the mechanism by which COUP-TFII regulates this pathway has yet to be elucidated. Microarray analysis showed that the expression of many genes within the Notch signaling pathway is altered in the COUP-TFII-depleted cells. Among the numerous targets, we identified Foxc1, Np-1, and Hey2 as the direct COUP-TFII downstream target genes at the transcriptional level. Collectively, our results demonstrate that COUP-TFII antagonizes Notch signaling through direct regulation of players at multiple steps of the Notch cascade to maintain vein identity.
Materials and Methods
Cell cultures and reagents
Human Embryonic Kidney 293T (293T) cells were maintained in DMEM. 293T were transfected with pCNX plasmid containing the COUP-TFII open reading frame by using lipofectin (Invitrogen, Grand Island, NY) according to the manufacturer's instruction. Cells were collected 24 h later for ChIP assay. PC3 cells were maintained in RPMI-1640. HUVEC cells were obtained from Lonza and maintained in EGM-2 (Lonza, Basel, Switzerland). SMART pool small interfering RNA (siRNA) duplexes targeting COUP-TFII and Sp1 were purchased from Dharmacon (Lafayette, CO). HUVEC cells were transfected with siRNA duplexes (50 nm) by using Oligofectamine (Invitrogen) according to manufacturer's instruction. PC3 cells were transfected with siRNA duplexes (50 nm) by using Dharmacon transfection III (Dharmacon) according to manufacturer's instruction. N-[N-(3,5-difluorophenylacetyl)-l-alanyl]-S-phenylglycine t-butyl ester (DAPT) (8 μmol/ml; Sigma, St. Louis, MO) was used to treat the HUVEC cells for 8 h, and RNA was then isolated.
Microarray
HUVEC cells were transfected with COUP-TFII or control siRNA., RNA was extracted 48 h after transfection using the RNeasy Plus mini kit (Qiagen, Valencia, CA). mRNA expression profiling in HUVEC cells was carried out on Affymetrix GeneChip Human Genome U133 Plus 2.0 Array (Affymetrix, Santa Clara, CA) per the manufacturer's protocol. Microarray data were analyzed with DNA-Chip Analyzer dChip software. We selected differentially expressed genes using three sample comparisons according to the following criteria: lower bound of 90% confidence interval of fold change greater than 1.2, and absolute value of difference between group means greater than 50. Differentially expressed genes were classified according to Gene Ontology function using Affymetrix annotation and Ingenuity Pathways Analysis (Ingenuity Systems, Redwood, CA). Raw data can be found in the GEO database as accession no. GSE33301.
Quantitative real-time RT-PCR (qRT-PCR)
Total RNA was extracted by Trizol (Invitrogen) according to the manufacturer's protocol and reverse transcribed using Reverse Transcription Reagents (Invitrogen). Real-time PCR was performed using TaqMan Universal PCR Master Mix or SYBR Green PCR Master Mix (Applied Biosystems, Carlsbad, CA) according to the manufacturer's instructions. Gene expression assay was performed on the ABI PRISM 7500 Sequence Detector System (Applied Biosystems). The primer sequences are shown in Supplemental Table 1 published on The Endocrine Society's Journals Online web site at http://mend.endojournals.org. All mRNA expression levels were normalized against 18S RNA.
ChIP Assays
ChIP assays were performed using an assay kit (Millipore Corp., Billerica, MA) following the manufacturer's recommendation. Antibodies used for immunoprecipitating protein-DNA complexes were monoclonal anti-COUP-TFII (Perseus Proteomics, Tokyo, Japan) and rabbit-anti-Sp1 antibody (Millipore). The primer sequences used in ChIP assay are listed in Supplemental Table 2.
Luciferase reporter assay
E2F1 promoter constructs (−728/+77) and (−122/+77) were provided by Joseph R. Nevins (Durham, NC) (33). Hey2, Foxc1, and different deletion constructs were cloned into PGL3 basic plasmids. 293 cells seeded at 70% confluence in 12 wells were transfected with 100 ng of reporter and expression plasmid using Fugene HD (Roche, Nutley, NJ). Luciferase-reporter activities were measured using a reporter assay kit (Promega Corp., Madison, WI).
Statistics
Results are presented as mean ± sd. Statistical analysis was carried out by Student's t test. P values < 0.05 were considered significant.
Results
COUP-TFII regulates cell cycle pathway gene expression
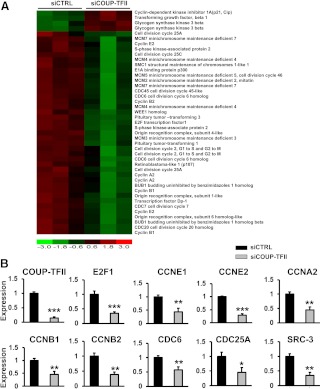
We have reported previously that depletion of COUP-TFII enhanced the expression of VEGFR1, a decoy receptor by sequestering VEGF ligands and thereby inhibiting VEGF/VEGFR2 signaling (3). The disruption of VEGF/VEGFR2 signaling in COUP-TFII-depleted cells compromises VEGF signaling and hinders cell migration and cell growth. Because inhibition of VEGFR1 fails to completely restore the sprouting and growth defects of COUP-TFII-depleted cells, other signals must also contribute to the COUP-TFII-dependent growth defects. To identify COUP-TFII-regulated genes that influence cell growth, we employed a genomic approach by using microarray analysis in primary HUVEC cells. We first treated HUVEC cells with scrambled control siRNA or COUP-TFII-specific siRNA to deplete COUP-TFII expression and analyze the affected genes by microarray analysis. The knockdown efficiency of COUP-TFII is 80–90% using either the pool siRNA and single siRNA, which have similar effects (3). We performed microarray analyses in triplicates of each sample using Affymetrix GeneChip Human Genome U133 Plus 2.0. Because COUP-TFII is a transcription factor of the nuclear receptor superfamily, it is not surprising that the expression of many genes in different signal pathways is altered in the COUP-TFII-depleted cells. One pathway that stands out among all, is the cell cycle signaling pathway, including the cyclin genes, minichromosome maintenance genes, cell division cycle genes, SRC-3, and transcription factor E2F1, all of which are down-regulated in the COUP-TFII-depleted HUVEC cells (Fig. 1A). For validation of the microarray analysis, we examined the expression of many genes that have been shown to be important for the cell cycle regulation by qRT-PCR. We showed that the expression of E2F1, cyclin (CCN)A2, CCNB1, CCNB2, CCNE1, CCNE2, CDC25A, CDC6, and SRC-3 are all significantly reduced in the COUP-TFII-depleted cells, consistent with the notion that COUP-TFII plays an important role in cell cycle progression (Fig. 1B).
Fig. 1.
Up-regulation of members of the cell cycle signaling pathway in the COUP-TFII knockdown HUVEC cells. A, Heat map of microarray analysis of members of the cell cycle signaling pathway in HUVEC cells treated with COUP-TFII-specific siRNA or scrambled control siRNA. B, qRT-PCR analysis of CyclinA2, Cyclin B1, Cyclin B2, Cyclin E1, Cyclin E2, CDC6, CDC25A, SRC-3, E2F1, and COUP-TFII expression in control and COUP-TFII-depleted HUVEC cells. Error bars indicate sem. *, P < 0.05; **, P < 0.005; ***, P < 0.001.
Direct transcriptional regulation of E2F1 by COUP-TFII in endothelial cells
We have shown previously that endothelial cells are arrested in G1/S1 transition upon COUP-TFII depletion, which compromises cell proliferation (3). E2F1 has been shown to play an important role in the G1/S transition through its direct regulation of the expression of Cyclin B, Cyclin E, CDC6, and CDC25A (12, 13, 25–28). The fact that E2F1 expression is greatly reduced in the COUP-TFII knockdown cells leads us to hypothesize that COUP-TFII might directly regulate E2F1 expression to modulate cell growth.
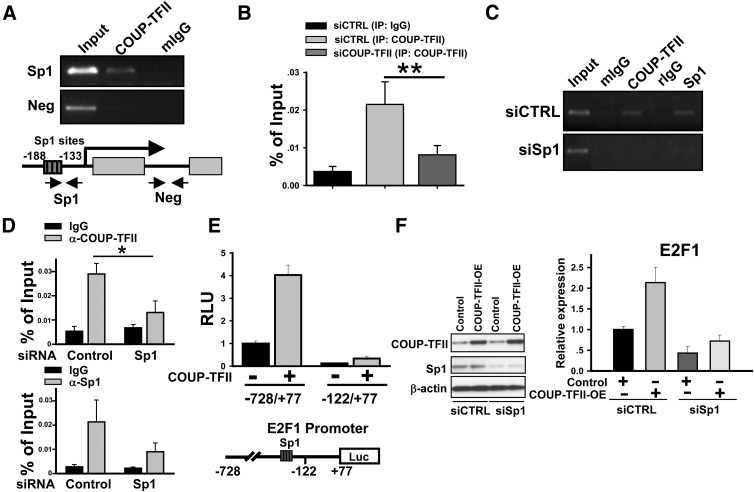
We and others had previously demonstrated that COUP-TFII activates gene expression by tethering to Sp1 and recruiting coactivators to enhance the expression of target genes (2, 29–32). Analysis of the E2F1 gene revealed multiple Sp1 binding sites present in the promoter region of E2F1 (33). To assess whether COUP-TFII regulates E2F1 promoter activity, we used ChIP analysis and observed that COUP-TFII was recruited to the E2F1 promoter region containing the clustered Sp1 binding sites in HUVEC cells (Fig. 2A) In contrast, there was no significant recruitment to an intragenic region lacking the Sp1 binding site. In addition, we also performed quantitative PCR (qPCR) analysis to quantify the above observation and the results were shown in Fig. 2B. As indicated in this figure, depletion of COUP-TFII in HUVEC abolished the recruitment of COUP-TFII to the E2F1 promoter, further confirming the specificity of our ChIP results (Fig. 2B). We next showed that Sp1 was also recruited to the same region in a manner similar to COUP-TFII recruitment. To further clarify whether recruitment of COUP-TFII to the E2F1 promoter is Sp1 dependent, siRNA against Sp1 was employed to deplete endogenous Sp1 expression. As expected, we observed that the recruitment of COUP-TFII was also significantly attenuated concomitant with the reduction of Sp1 recruitment to the E2F1 promoter in the Sp1 knockdown cells (Fig. 2C). Similarly, we also performed ChIP assay in PC3 cells, which also highly express COUP-TFII. As shown in Fig. 2D, COUP-TFII was recruited to the E2F1 promoter in PC3 cells in a manner similar to that of HUVEC cells, whereas depletion of Sp1 in PC3 cells diminishes COUP-TFII recruitment to the E2F1 promoter. Together, all these results indicate that COUP-TFII binds to E2F1 in a Sp1-dependent manner.
Fig. 2.
COUP-TFII regulates E2F1 expression in HUVEC cells. A, ChIP analysis shows that COUP-TFII binds to the region containing Sp1 binding sites in the E2F1 promoter. The region in the downstream without a Sp1 binding site served as a negative control. B, ChIP and qPCR analysis of COUP-TFII binding to E2F1 promoter in control and COUP-TFII -depleted HUVEC cells. C, HUVEC cells were transfected with Sp1-specific siRNA to knock down Sp1 expression. ChIP assay was performed using anti-COUP-TFII or Sp1 antibody to test whether COUP-TFII was recruited to the E2F1 promoter in a Sp1-depedent manner. D, ChIP and qRT-PCR analysis shows that both COUP-TFII and Sp1 bind to the region containing a Sp1 binding site in the E2F1 promoter in PC3 cells. E, Relative luciferase activity of the E2F1 promoter-driven reporter with or without cotransfection of COUP-TFII. F, Western blotting analysis of E2F1 expression in control and COUP-TFII overexpression cells with or without Sp1 depletion. Neg, Negative. *, less than 0.05; **, less than 0.01.
Consistent with these observations, cotransfection of COUP-TFII enhanced the activity of the E2F1 promoter-driven luciferase-reporter, whereas the truncated promoter without Sp1 binding sites abolished the regulation of the E2F1 promoter by COUP-TFII (Fig. 2E). To further validate that COUP-TFII regulates E2F1 expression in a Sp1-dependent manner, we performed rescue experiments to examine E2F1 expression in COUP-TFII overexpressing cells with or without Sp1 depletion. As shown in Fig. 2F, knockdown of Sp1 expression eliminated the induction of E2F1 expression elicited by COUP-TFII overexpression. Together, these results indicate that COUP-TFII tethers to Sp1 on the Sp1 binding sites in the E2F1 promoter to enhance E2F1 expression.
Transcriptional regulation of genes in the Notch signaling pathway by COUP-TFII
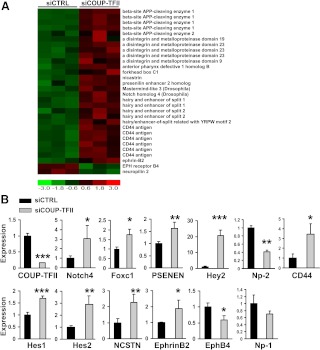
We showed previously that COUP-TFII determines vein identity by suppressing the Notch signaling pathway in vivo (24). However, the direct downstream targets and the mechanism of how COUP-TFII represses the Notch signaling pathways remain largely undefined. Consistent with our in vivo findings, gene expression array analysis showed that expression of numerous components within the Notch signaling pathway are elevated in the COUP-TFII-depleted cells. This includes the Notch downstream genes Hey2, hairy-and-enhancer-of-split (Hes)1, and Hes2, the γ-secretase complex components presenilin enhancer 2 homolog (PSENEN) and nicastrin (NCSTN), the Notch receptor, Notch 4, the Notch upstream regulator, Foxc1, and others (Fig. 3A). In accordance with the enhanced activity of the Notch signaling, we also found that depletion of COUP-TFII increased the expression of arterial markers, Ephrin B2 and CD44, while reducing the expression of vein marker ephrin receptor B4 (EphB4) and Np-2 (Fig. 3A). Together, the activation of the Notch signaling pathway in the COUP-TFII-depleted HUVEC cells resembles our previous observation in mouse models that COUP-TFII suppresses notch signaling in vein endothelial cells in vivo (24).
Fig. 3.
Up-regulation of Notch signaling pathway genes in the COUP-TFII knockdown of HUVEC cells. A, Heat map of microarray analysis of members of the Notch pathway in HUVEC cells treated with COUP-TFII-specific siRNA or scrambled control siRNA. B, qRT-PCR analysis of Notch4, Foxc1, Hey2, Hes1, Hes2, PSENEN, NCSTN, EphrinB2, EphB4, CD44, Np-1, Np-2, and COUP-TFII expression in control and COUP-TFII-depleted HUVEC cells. Error bars indicate sd. *, P < 0.05; **, P <0.005; ***, P < 0.001.
Next we performed qRT-PCR to validate our microarray data. As expected, the expression of Hes1 (1.7-fold), Hes2 (2.9-fold), Hey2 (20-fold), PSENEN (1.6-fold), NCSTN (2.3-fold), Notch4 (3.0-fold), CD44 (3.2-fold), and Foxc1 (1.7-fold) were all elevated in the COUP-TFII-depleted endothelial cells. As a consequence of enhanced expression of Notch signaling components, a significant induction of EphrinB2 expression and a decrease in EphB4 expression were observed upon COUP-TFII depletion (Fig. 3B). Furthermore, we observed that Np-2, a direct transcription target of COUP-TFII (32), was decreased accordingly, whereas Np-1 expression did not differ significantly between control and COUP-TFII-depleted cells (Fig. 3B).
Inhibition of Notch signaling with γ-secretase inhibitor reveals that Hey2 expression may be regulated by other molecules in addition to Notch signaling
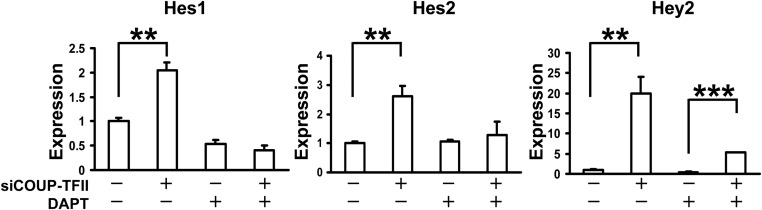
Notch signaling is initiated with interactions of the Notch receptors with their transmembrane ligands. Upon ligand binding, the receptors undergo proteolytic cleavage by presenlins, release the Notch intracellular domains, and translocate to the nucleus to activate the Notch downstream genes, such as Hes and Hey (34–36). Treatment with the γ-secretase inhibitor blocks the cleavage of the Notch receptor and inhibits Notch signaling. To address whether the up-regulation of Hes1, Hes2, and Hey2 expression in the COUP-TFII knockdown cells was due to the up-regulation of Notch signaling, γ-secretase inhibitor DAPT was added to the COUP-TFII-depleted and control HUVEC cells. The up-regulation of Hes1 and Hes2 expression, quantified by qRT-PCR, in COUP-TFII-depleted cells was completely blocked by DAPT treatment (Fig. 4), consistent with the notion that the expression of Hes proteins is under the control of canonical Notch signaling. To our surprise, we still observed an increase in the expression of Hey2 with DAPT treatment, although at a reduced level, in the COUP-TFII-depleted cells (Fig. 4), suggesting that other COUP-TFII-dependent factors or COUP-TFII itself may regulate Hey2 expression in addition to the Notch signaling.
Fig. 4.
qRT-PCR analysis of Hes1, Hes2, and Hey2 expression in control and COUP-TFII-depleted HUVEC cells after DAPT and DMSO treatment. Error bars indicate sd. **, P < 0.005; ***, P < 0.001.
Identification of COUP-TFII direct target genes within Notch signaling
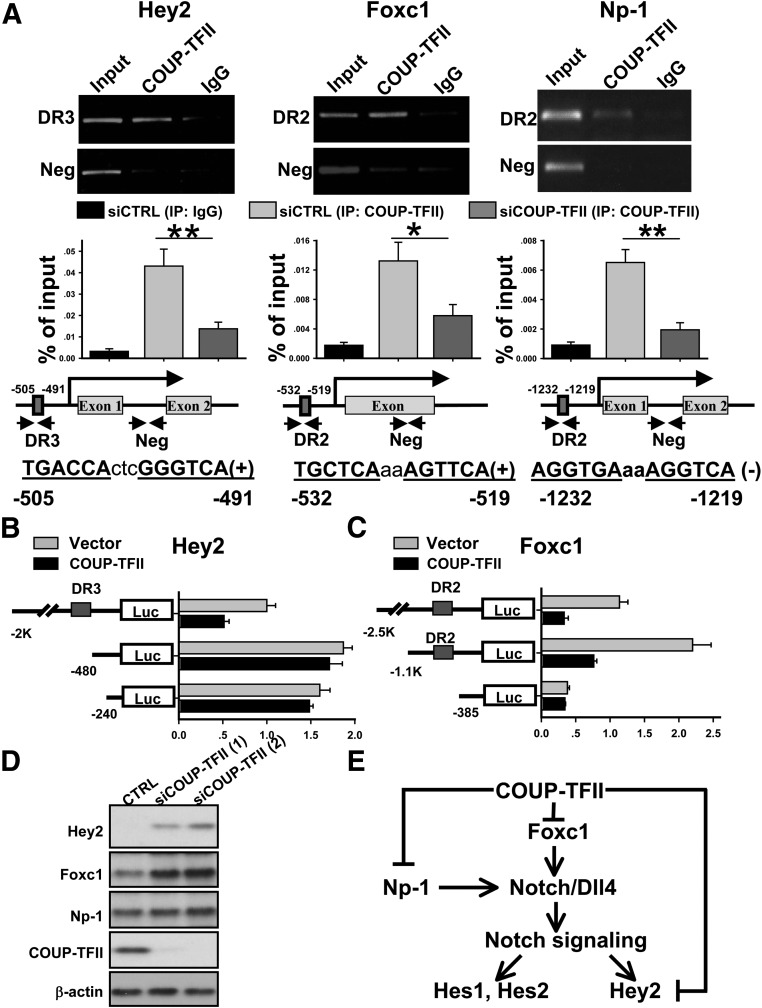
In an effort to identify COUP-TFII direct target genes in the Notch signaling pathway, we set out to analyze COUP-TFII response elements in potential direct COUP-TFII target genes. As a transcription repressor, COUP-TFII has been shown to directly bind to the AGGTCAnAGGTCA direct repeat (DR) sequences located in the genomic locus of its downstream target genes (37). The increase in Notch signaling in the COUP-TFII knockdown cells suggests that COUP-TFII might directly bind to the DR sequences in the genomic locus of the Notch signal components to silence their expression. We first used bioinformatic analysis to search for the evolutionally conserved COUP-TFII DR-binding elements in the Notch signaling molecules. Next, we designed the primer to encompass these sites (Fig. 5A) and performed ChIP analysis to examine the possible recruitments of COUP-TFII to the target promoters. To facilitate the detection of COUP-TFII recruitment to a target gene of interest, we first overexpressed COUP-TFII in 293T cells and used a COUP-TFII-specific antibody to pull down COUP-TFII-associated regulatory elements. We observed that COUP-TFII was preferentially recruited to DR sites of the promoters of Hey2, Np-1, and Foxc1 in 293T cells (Supplemental Fig. 1). We also scanned 5 kb upstream of the promoter of notch4 with 10 pairs of primers, and no significant recruitment of COUP-TFII to that region was detected (Supplemental Fig. 1).
Fig. 5.
COUP-TFII represses the transcription of Hey2, Foxc1, and Np-1 in HUVEC cells. A, Endogenous COUP-TFII in HUVEC cells binds to the region containing conserved DR3 binding site in the Hey2 promoter and DR2 binding sites in the Foxc1 and Np-1 promoters. Promoter region in the downstream without COUP-TFII binding site served as negative control. qPCR results of ChIP assay were shown in the bottom of the figure. B, Relative luciferase activity of Hey2 promoter-driven reporter (−2 kb, −480 bp, and –240 bp) with or without cotransfection of COUP-TFII. C, Relative luciferase activity of Foxc1 promoter-driven reporter (−2.5 kb, −1.1 kb, and –385 bp) with or without cotransfection of COUP-TFII. D, Western blotting analysis of Hey2, Foxc1, Np-1, and COUP-TFII expression in control and COUP-TFII-depleted HUVEC cells. E, Model depicts how COUP-TFII regulates genes in the Notch signaling pathway. COUP-TFII directly and negatively regulates Foxc1 and Np-1 expression. In the absence of COUP-TFII, an increase in Foxc1 and Np-1 expression will stimulate Dll4 expression and subsequently activate Notch signaling. In addition, COUP-TFII directly binds to the Hey2 promoter to repress Hey2 expression to ensure that Notch signaling is silent. Neg, Negative; *P < 0.05; **, P < 0.01.
To confirm this regulation in endothelial cells, we carried out ChIP analysis in HUVEC cells. As shown in Fig. 5A, endogenous COUP-TFII was preferentially recruited to the conserved DR3 binding element in the Hey2 promoter, DR2 binding element in the Foxc1 promoter and Np-1 promoter in comparison with nonspecific IgG or the intragenic region lacking COUP-TFII binding sites. In addition, we also performed qPCR analysis to quantify COUP-TFII recruitments to those genes, respectively, and employed the additional COUP-TFII knockdown cells as a negative control (Fig. 5A). The results confirmed that COUP-TFII was preferentially recruited to Hey2, Foxc1, and Np-1 promoters (Fig. 5A). Consistent with this observation, cotransfection of COUP-TFII repressed Hey2 and Foxc1-promoter driven Luciferase-reporter activity, whereas deletion of the DR sites at the Hey2 and Foxc1 promoter abolished the regulation elicited by COUP-TFII (Fig. 5, B and C). Finally, in agreement with the increased transcripts of Hey2 and Foxc1 in COUP-TFII-depleted cells, we observed that loss of COUP-TFII resulted in the significant increase of Hey2 and Foxc1 expression on protein levels, whereas Np-1 expression displays a marginal increase similar to the minimal changes of RNA transcripts observed (Fig. 3B). Taken together, our results indicated that COUP-TFII represses the transcription of Hey2, Foxc1, and Np-1 at different steps of the Notch signaling cascade to ensure that the Notch signaling pathway is silent to confer vein identity (Fig. 5E).
Discussion
We previously demonstrated that COUP-TFII plays a cell-autonomous function to regulate endothelial sprouting by affecting endothelial proliferation and migration (3). Our microarray data confirm this observation that many cell cycle signaling genes were down-regulated in the COUP-TFII-depleted HUVEC cells. Among all of these, E2F1 has been shown to be a key transcription factor that regulates the cyclin and CDC to promote G1/S transition and cell proliferation (12, 13, 25, 27, 28). Here we showed that COUP-TFII directly binds to the promoter region of E2F1 in a Sp1-dependent manner to stimulate endothelial cell proliferation. Given the fact that COUP-TFII directly participated in endothelial proliferation through regulation of E2F1 expression, it could explain in part why knockdown of VEGFR-1 to restore VEGFR-2 signaling only partially rescues endothelial-sprouting defects.
The establishment of arteriovenous identity is a tightly regulated process. Works from zebrafish and the mouse have demonstrated that Notch signaling is essential for arterial fate decision. Mice lacking Notch1/4, Dll4, Rbpj, Mib, or Hey1/2 exhibit defects not only in vascular development, but also in arterial specification (15, 21, 38–42). Vascular endothelial growth factor (VEGF) functions upstream from Notch signaling to induce artery identity. VEGF interacts with the VEGF receptor 2 (VEGFR2)-Np-1 complex to activate downstream phospholipase Cγ-1 (Plc-γ)-Erk, and Notch signaling pathways, thereby inducing arterial marker expression, such as ephrin B2 (42–44). Our previous works showed that COUP-TFII functions as a key player that confers vein identity by repressing Notch signaling (24). COUP-TFII is expressed in endothelial cells of the vein, not in the artery. Endothelial-specific knockout of COUP-TFII in mice illustrated the mutant veins acquire many arterial characteristics, including the ectopic expression of Notch signaling components, such as Np-1, Jagged1 and Notch1, which further induce ectopic expression of the downstream arterial markers, ephrin-B2 and Hey1. Additionally, ectopic expression of COUP-TFII in the endothelial cells results in the fusion of arteries and veins, similar to phenotypes observed in Np-1−/− or Notch1−/− mice (45, 46), suggesting that COUP-TFII might regulate venous identity by repressing the Notch signaling pathway through regulation of Np-1 and Notch1 expression. Consistent with this notion, here we showed that COUP-TFII is recruited to the promoter of Np-1 even though we did not detect significant changes of Np-1 expression in the COUP-TFII-depleted cells. It is likely that Np-1 is regulated by other factors in addition to COUP-TFII. Indeed, Np-1 is shown to be positively regulated by E2F1 (47), and E2F1 is a direct downstream target of COUP-TFII. Therefore, the down-regulation of E2F1 in the absence of COUP-TFII might counterbalance the up-regulation of Np-1 upon COUP-TFII deletion and nullify the Np-1 up-regulation as a consequence.
Forkhead transcription factors, Foxc1 and Foxc2, have been recently shown to control arterial specification by activating Notch signaling. Mice with compound mutant for Foxc1 and Foxc2 exhibit arterial-venous malformations and decreased expression of arterial markers (Dll4, Jag1, Notch1, Notch4, Hey2, and Efnb2), whereas expression of the venous markers COUP-TFII and Ephb4 are not altered (22). Furthermore, Foxc1 and Foxc2 function upstream of the Notch pathway to regulate artery specification (23). Our data demonstrates that COUP-TFII represses Foxc1 expression in HUVEC cells. The repression of Foxc1 expression by COUP-TFII contributes to how COUP-TFII plays an autonomous role in endothelial cells to control Notch signaling. Although Foxc2 has a similar function as Foxc1 in the mouse, neither the expression change nor the recruitment of COUP-TFII to the promoter of Foxc2 was detected in our experiments (data not shown).
The HES and Hey transcriptional repressors are downstream of the canonical Notch signaling (48). Here we show that the expression of Hes1, Hes2, and Hey2 increase in the COUP-TFII knockdown cells, supporting the notion that COUP-TFII represses Notch signaling. Canonical Notch signaling usually can be blocked by γ-secretase inhibitors, such as DAPT. This is the case for the expression of Hes1 and Hes2, in which DAPT treatment completely blocks their enhanced expression in COUP-TFII-depleted cells. The fact that DAPT can only partially block the enhanced expression of Hey2 in the COUP-TFII-depleted cells suggested that COUP-TFII might directly regulate Hey2 expression. Indeed, we show that COUP-TFII is recruited to the DR element in the Hey2 promoter, suggesting that its expression is also directly regulated by COUP-TFII in addition to through the COUP-TFII-regulated Notch signaling pathway. The dual regulation of Hey2 by both canonical Notch signaling and COUP-TFII implicates its central role in the regulation of artery-vein identity. Indeed, in the zebrafish dorsal aorta, loss of gridlock (grl), a homolog of mammalian Hey2, leads to failure to express the arterial cell marker ephrinB2 and an increase in expression of the venous marker EphB4 (14, 49). In the mouse, targeted disruption of Hey1/Hey2 leads to embryonic lethality with vascular defects in the placenta, yolk sac, and embryo itself, suggesting that Hey genes are the essential effectors within the Notch signaling cascade for arterial identity (21).
In conclusion, our results demonstrate that COUP-TFII directly regulates the proliferation of endothelial cells by stimulating the expression of E2F1. COUP-TFII also directly regulates multiple genes, Np-1, Foxc1, and Hey2, at different steps of the Notch signaling cascade to antagonize Notch signaling. This stringent control of the Notch pathway by COUP-TFII ensures that the vein identity is maintained during development. Together with our previous results (2, 3), which demonstrated that COUP-TFII also regulates VEGF signaling and paracrine signaling, angiopoietin-1, in the smooth muscle cells, we have clearly demonstrated that COUP-TFII is one of the key regulators of angiogenesis. Because COUP-TFII is a member of the nuclear receptor superfamily, it is likely that screening of chemical libraries for this receptor may yield an antagonist to inhibit angiogenesis for disease intervention.
Supplementary Material
Acknowledgments
We thank Dr. Joseph R. Nevins (Duke, Durham, NC) for E2F1 promoter constructs (−728/+77) and (−122/+77). We thank Ms. Jodie R. Hebert for reading this manuscript.
This work was supported by grants from the National Institutes of Health, HL076448 (to S.Y.T.), DK45641 (to M.J.T.), HD07165 (to M.J.T.), and DK059820 (to S.Y.T. and M.J.T.). The microarray experiment was carried out by the Baylor Chip Core partially supported by Diabetes and Endocrinology Research Center (P30 DK079638) and headed by Ms. Lisa White.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- CCN
- Cyclin
- COUP-TFII
- chicken ovalbumin upstream promoter transcription factor
- DAPT
- N-[N-(3,5-difluorophenylacetyl)-l-alanyl]-S-phenylglycine t-butyl ester
- DR
- direct repeat
- E2F1
- E2F transcription factor 1
- EphB4
- ephrin receptor B4
- HES
- hairy-and-enhancer-of-split
- Hey
- hairy-and-enhancer-of-split related
- HUVEC
- human umbilical vein endothelial cell
- NCSTN
- nicastrin
- Np-1 and -2
- neuropilin-1 and -2
- PSENEN
- presenilin enhancer 2 homolog
- qPCR
- quantitative PCR
- qRT-PCR
- quantitative real-time RT-PCR
- siRNA
- small interfering RNA
- VEGF
- vascular endothelial growth factor
- VEGFR
- VEGF receptor.
References
- 1. Pereira FA, Qiu Y, Zhou G, Tsai MJ, Tsai SY. 1999. The orphan nuclear receptor COUP-TFII is required for angiogenesis and heart development. Genes Dev 13:1037–1049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Qin J, Chen X, Xie X, Tsai MJ, Tsai SY. 2010. COUP-TFII regulates tumor growth and metastasis by modulating tumor angiogenesis. Proc Natl Acad Sci USA 107:3687–3692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Qin J, Chen X, Yu-Lee LY, Tsai MJ, Tsai SY. 2010. Nuclear receptor COUP-TFII controls pancreatic islet tumor angiogenesis by regulating vascular endothelial growth factor/vascular endothelial growth factor receptor-2 signaling. Cancer Res 70:8812–8821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ferrara N, Gerber HP, LeCouter J. 2003. The biology of VEGF and its receptors. Nat Med 9:669–676 [DOI] [PubMed] [Google Scholar]
- 5. Dickson MC, Martin JS, Cousins FM, Kulkarni AB, Karlsson S, Akhurst RJ. 1995. Defective haematopoiesis and vasculogenesis in transforming growth factor-β 1 knock out mice. Development 121:1845–1854 [DOI] [PubMed] [Google Scholar]
- 6. Adams RH, Alitalo K. 2007. Molecular regulation of angiogenesis and lymphangiogenesis. Nat Rev Mol Cell Biol 8:464–478 [DOI] [PubMed] [Google Scholar]
- 7. Hamada K, Sasaki T, Koni PA, Natsui M, Kishimoto H, Sasaki J, Yajima N, Horie Y, Hasegawa G, Naito M, Miyazaki J, Suda T, Itoh H, Nakao K, Mak TW, Nakano T, Suzuki A. 2005. The PTEN/PI3K pathway governs normal vascular development and tumor angiogenesis. Genes Dev 19:2054–2065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Suri C, Jones PF, Patan S, Bartunkova S, Maisonpierre PC, Davis S, Sato TN, Yancopoulos GD. 1996. Requisite role of angiopoietin-1, a ligand for the TIE2 receptor, during embryonic angiogenesis. Cell 87:1171–1180 [DOI] [PubMed] [Google Scholar]
- 9. Fischer C, Mazzone M, Jonckx B, Carmeliet P. 2008. FLT1 and its ligands VEGFB and PlGF: drug targets for anti-angiogenic therapy? Nat Rev Cancer 8:942–956 [DOI] [PubMed] [Google Scholar]
- 10. Cao Y. 2009. Positive and negative modulation of angiogenesis by VEGFR1 ligands. Sci Signal 2:re1. [DOI] [PubMed] [Google Scholar]
- 11. Jinnin M, Medici D, Park L, Limaye N, Liu Y, Boscolo E, Bischoff J, Vikkula M, Boye E, Olsen BR. 2008. Suppressed NFAT-dependent VEGFR1 expression and constitutive VEGFR2 signaling in infantile hemangioma. Nat Med 14:1236–1246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Johnson DG, Schwarz JK, Cress WD, Nevins JR. 1993. Expression of transcription factor E2F1 induces quiescent cells to enter S phase. Nature 365:349–352 [DOI] [PubMed] [Google Scholar]
- 13. Wu L, Timmers C, Maiti B, Saavedra HI, Sang L, Chong GT, Nuckolls F, Giangrande P, Wright FA, Field SJ, Greenberg ME, Orkin S, Nevins JR, Robinson ML, Leone G. 2001. The E2F1–3 transcription factors are essential for cellular proliferation. Nature 414:457–462 [DOI] [PubMed] [Google Scholar]
- 14. Zhong TP, Childs S, Leu JP, Fishman MC. 2001. Gridlock signalling pathway fashions the first embryonic artery. Nature 414:216–220 [DOI] [PubMed] [Google Scholar]
- 15. Krebs LT, Xue Y, Norton CR, Shutter JR, Maguire M, Sundberg JP, Gallahan D, Closson V, Kitajewski J, Callahan R, Smith GH, Stark KL, Gridley T. 2000. Notch signaling is essential for vascular morphogenesis in mice. Genes Dev 14:1343–1352 [PMC free article] [PubMed] [Google Scholar]
- 16. Lawson ND, Scheer N, Pham VN, Kim CH, Chitnis AB, Campos-Ortega JA, Weinstein BM. 2001. Notch signaling is required for arterial-venous differentiation during embryonic vascular development. Development 128:3675–3683 [DOI] [PubMed] [Google Scholar]
- 17. Lawson ND, Vogel AM, Weinstein BM. 2002. sonic hedgehog and vascular endothelial growth factor act upstream of the Notch pathway during arterial endothelial differentiation. Dev Cell 3:127–136 [DOI] [PubMed] [Google Scholar]
- 18. Weinstein BM, Lawson ND. 2002. Arteries, veins, Notch, and VEGF. Cold Spring Harb Symp Quant Biol 67:155–162 [DOI] [PubMed] [Google Scholar]
- 19. Xue Y, Gao X, Lindsell CE, Norton CR, Chang B, Hicks C, Gendron-Maguire M, Rand EB, Weinmaster G, Gridley T. 1999. Embryonic lethality and vascular defects in mice lacking the Notch ligand Jagged1. Hum Mol Genet 8:723–730 [DOI] [PubMed] [Google Scholar]
- 20. Hrabĕ de Angelis M, McIntyre J, II, Gossler A. 1997. Maintenance of somite borders in mice requires the Delta homologue DII1. Nature 386:717–721 [DOI] [PubMed] [Google Scholar]
- 21. Fischer A, Schumacher N, Maier M, Sendtner M, Gessler M. 2004. The Notch target genes Hey1 and Hey2 are required for embryonic vascular development. Genes Dev 18:901–911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Seo S, Fujita H, Nakano A, Kang M, Duarte A, Kume T. 2006. The forkhead transcription factors, Foxc1 and Foxc2, are required for arterial specification and lymphatic sprouting during vascular development. Dev Biol 294:458–470 [DOI] [PubMed] [Google Scholar]
- 23. Hayashi H, Kume T. 2008. Foxc transcription factors directly regulate Dll4 and Hey2 expression by interacting with the VEGF-Notch signaling pathways in endothelial cells. PLoS One 3:e2401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. You LR, Lin FJ, Lee CT, DeMayo FJ, Tsai MJ, Tsai SY. 2005. Suppression of Notch signalling by the COUP-TFII transcription factor regulates vein identity. Nature 435:98–104 [DOI] [PubMed] [Google Scholar]
- 25. DeGregori J, Kowalik T, Nevins JR. 1995. Cellular targets for activation by the E2F1 transcription factor include DNA synthesis- and G1/S-regulatory genes. Mol Cell Biol 15:4215–4224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ohtani K, DeGregori J, Nevins JR. 1995. Regulation of the cyclin E gene by transcription factor E2F1. Proc Natl Acad Sci USA 92:12146–12150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ohtani K, Tsujimoto A, Ikeda M, Nakamura M. 1998. Regulation of cell growth-dependent expression of mammalian CDC6 gene by the cell cycle transcription factor E2F. Oncogene 17:1777–1785 [DOI] [PubMed] [Google Scholar]
- 28. Vigo E, Müller H, Prosperini E, Hateboer G, Cartwright P, Moroni MC, Helin K. 1999. CDC25A phosphatase is a target of E2F and is required for efficient E2F-induced S phase. Mol Cell Biol 19:6379–6395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kim BJ, Takamoto N, Yan J, Tsai SY, Tsai MJ. 2009. Chicken ovalbumin upstream promoter-transcription factor II (COUP-TFII) regulates growth and patterning of the postnatal mouse cerebellum. Dev Biol 326:378–391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Pipaón C, Tsai SY, Tsai MJ. 1999. COUP-TF upregulates NGFI-A gene expression through an Sp1 binding site. Mol Cell Biol 19:2734–2745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Rohr O, Aunis D, Schaeffer E. 1997. COUP-TF and Sp1 interact and cooperate in the transcriptional activation of the human immunodeficiency virus type 1 long terminal repeat in human microglial cells. J Biol Chem 272:31149–31155 [DOI] [PubMed] [Google Scholar]
- 32. Lin FJ, Chen X, Qin J, Hong YK, Tsai MJ, Tsai SY. 2010. Direct transcriptional regulation of neuropilin-2 by COUP-TFII modulates multiple steps in murine lymphatic vessel development. J Clin Invest 120:1694–1707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Johnson DG, Ohtani K, Nevins JR. 1994. Autoregulatory control of E2F1 expression in response to positive and negative regulators of cell cycle progression. Genes Dev 8:1514–1525 [DOI] [PubMed] [Google Scholar]
- 34. Fortini ME. 2002. γ-Secretase-mediated proteolysis in cell-surface-receptor signalling. Nat Rev Mol Cell Biol 3:673–684 [DOI] [PubMed] [Google Scholar]
- 35. Mumm JS, Kopan R. 2000. Notch signaling: from the outside in. Dev Biol 228:151–165 [DOI] [PubMed] [Google Scholar]
- 36. Bray SJ. 2006. Notch signalling: a simple pathway becomes complex. Nat Rev Mol Cell Biol 7:678–689 [DOI] [PubMed] [Google Scholar]
- 37. Tsai SY, Tsai MJ. 1997. Chick ovalbumin upstream promoter-transcription factors (COUP-TFs): coming of age. Endocr Rev 18:229–240 [DOI] [PubMed] [Google Scholar]
- 38. Kokubo H, Miyagawa-Tomita S, Johnson RL. 2005. Hesr, a mediator of the Notch signaling, functions in heart and vessel development. Trends Cardiovasc Med 15:190–194 [DOI] [PubMed] [Google Scholar]
- 39. Krebs LT, Shutter JR, Tanigaki K, Honjo T, Stark KL, Gridley T. 2004. Haploinsufficient lethality and formation of arteriovenous malformations in Notch pathway mutants. Genes Dev 18:2469–2473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Duarte A, Hirashima M, Benedito R, Trindade A, Diniz P, Bekman E, Costa L, Henrique D, Rossant J. 2004. Dosage-sensitive requirement for mouse Dll4 in artery development. Genes Dev 18:2474–2478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Koo BK, Lim HS, Song R, Yoon MJ, Yoon KJ, Moon JS, Kim YW, Kwon MC, Yoo KW, Kong MP, Lee J, Chitnis AB, Kim CH, Kong YY. 2005. Mind bomb 1 is essential for generating functional Notch ligands to activate Notch. Development 132:3459–3470 [DOI] [PubMed] [Google Scholar]
- 42. Lin FJ, Tsai MJ, Tsai SY. 2007. Artery and vein formation: a tug of war between different forces. EMBO Rep 8:920–924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Hong CC, Peterson QP, Hong JY, Peterson RT. 2006. Artery/vein specification is governed by opposing phosphatidylinositol-3 kinase and MAP kinase/ERK signaling. Curr Biol 16:1366–1372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Ng YS, Krilleke D, Shima DT. 2006. VEGF function in vascular pathogenesis. Exp Cell Res 312:527–537 [DOI] [PubMed] [Google Scholar]
- 45. Kawasaki T, Kitsukawa T, Bekku Y, Matsuda Y, Sanbo M, Yagi T, Fujisawa H. 1999. A requirement for neuropilin-1 in embryonic vessel formation. Development 126:4895–4902 [DOI] [PubMed] [Google Scholar]
- 46. Huppert SS, Le A, Schroeter EH, Mumm JS, Saxena MT, Milner LA, Kopan R. 2000. Embryonic lethality in mice homozygous for a processing-deficient allele of Notch1. Nature 405:966–970 [DOI] [PubMed] [Google Scholar]
- 47. Jiang SX, Sheldrick M, Desbois A, Slinn J, Hou ST. 2007. Neuropilin-1 is a direct target of the transcription factor E2F1 during cerebral ischemia-induced neuronal death in vivo. Mol Cell Biol 27:1696–1705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Iso T, Kedes L, Hamamori Y. 2003. HES and HERP families: multiple effectors of the Notch signaling pathway. J Cell Physiol 194:237–255 [DOI] [PubMed] [Google Scholar]
- 49. Zhong TP, Rosenberg M, Mohideen MA, Weinstein B, Fishman MC. 2000. gridlock, an HLH gene required for assembly of the aorta in zebrafish. Science 287:1820–1824 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.