Abstract
Background
Patients with diabetes or cardiovascular disease are at risk of reduced renal function and frequently use drugs that interact with renal function. GPs monitor renal function in these patients. Computerised prescription systems produce alerts in patients labelled as having chronic kidney disease, but alerts are often ignored. If pharmacists use a pharmacy medication alert system (PMAS) based on renal function, they can provide the GP with therapeutic advice to optimise the medication. The extent of this advice and the feasibility in the clinical context are unknown.
Aim
To assess the therapeutic advice formulated by pharmacists with help of a PMAS based on the renal function of patients aged ≥70 years with diabetes or cardiovascular disease.
Design and setting
Observational study in primary health care in the Netherlands.
Method
GPs provided pharmacists with the renal function of older patients with diabetes or cardiovascular disease who were using target drugs, that is, drugs requiring therapeutic advice in patients with reduced renal function. With the help of a PMAS, pharmacists assessed the actual medication. The GP weighed the advice in relation to the clinical context of the individual patient.
Results
Six hundred and fifty patients were prescribed 1333 target drugs. Pharmacists formulated 143 therapeutic recommendations (11% of target drugs) concerning 89 patients (13.7% of study population). In 71 recommendations in 52 patients (8.0% of study population), the GP agreed immediately.
Conclusion
The use of a PMAS resulted in therapeutic advice in 11% of the target drugs. After weighing the clinical context, the GP agreed with half of the advice.
Keywords: aged, medication alert systems, medication errors, primary health care, renal insufficiency
INTRODUCTION
Chronic kidney disease (CKD) is a growing health problem, with a prevalence from 4.9% in general practice in the UK to up to 13% in the US population.1–3 The medical consequences of CKD are not only the risk of end-stage renal disease and cardiovascular morbidity, but also an increased risk of adverse drug events and medication-related hospital admissions.4,5
When renal function is reduced, the dosage of drugs that depend on renal excretion should be adjusted and nephrotoxic drugs should be avoided.6–8 Patients with diabetes and cardiovascular disease have an augmented risk of CKD and frequently use renally cleared drugs.1,9 Medication alerts systems warn prescribers of medication that can interact with impaired renal function, but these alerts are often ignored.10–13 A medication alert system that weighs the actual renal function of the patient could help to reduce medication errors.14–16
This observational study assessed the therapeutic advice formulated by the pharmacist with help of a medication alert system based on the renal function of patients aged ≥70 years with diabetes or cardiovascular disease.
METHOD
Setting and study population
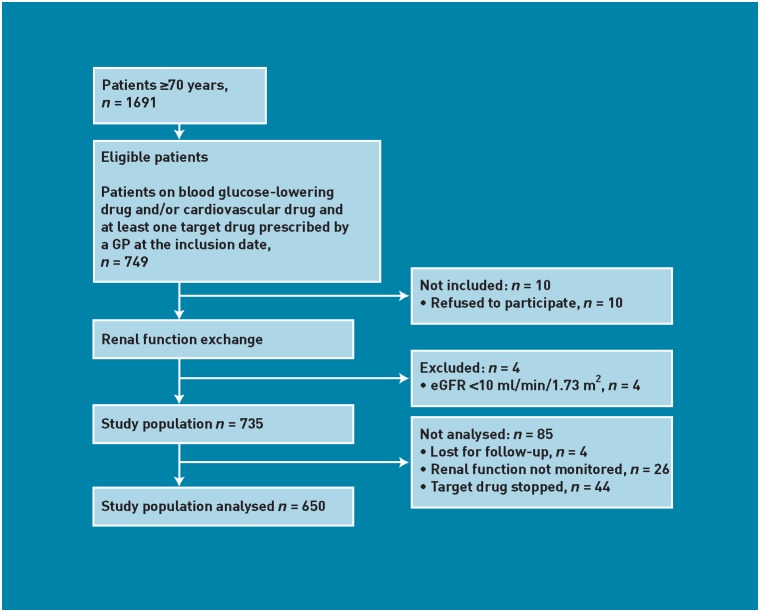
The study was conducted in Arnhem, a city in the East of the Netherlands with nearly 148 000 inhabitants. Seven GPs, belonging to the same pharmacotherapy audit meeting group, participated in the study. Five pharmacists who worked in close collaboration with this group selected the patients in their pharmacy computer system. Patients aged ≥70 years in the care of the participating GPs were eligible if they were on GP-prescribed maintenance therapy of blood-glucose-lowering or cardiovascular drugs (for example, digoxin, diuretics, or inhibitors of the renin–angiotensin system [RAS], including angiotensin-converting enzyme [ACE] inhibitors and angiotensin II receptor blockers [ARBs]). Patients also used at least one ‘target drug’ on the inclusion date of 4 January 2010. ‘Target drugs’ were defined as drugs requiring therapeutic advice in patients with decreased renal function considering the Dutch dosing guideline for impaired renal function.17 Patients with an estimated glomerular filtration rate (eGFR) <10 ml/min/1.73 m2 were excluded (Figure 1).
Figure 1.
Selection of the study population.
How this fits in
Patients who use drugs that interact with impaired renal function require monitoring of renal function to avoid medication errors. In daily practice, the adjustment of medication in relation to renal function does not always get the attention that it deserves. The use of a pharmacy medication alert system based on renal function may be of additional value. In this study, the use of such a system resulted in therapeutic advice concerning 11% of the drugs that interact with renal function, and the GP agreed with half of the therapeutic advice.
The GPs already used a computerised medication monitoring system. This system generated an alert when the GP prescribed a target drug in patients labelled as having CKD, but it could not consider the eGFR level.
The use of drug dispensing data and laboratory test results in this study complied with Dutch privacy regulations.
Renal function monitoring
Actual eGFR was defined as an eGFR value measured within the last 12 months. If an actual eGFR was unknown, the GP requested the patient to undergo a blood test for renal function. The laboratory provided serum creatinine and an eGFR (ml/min/1.73m2) calculated by the normalised four-variable Modification of Diet in Renal Disease (MDRD).18 Serum creatinine was measured enzymatically (Modular, Roche diagnostics) and was IDMS (isotope dilution mass spectrometry) calibrated. The actual values of eGFR data were provided to determine drug-specific risk.
Assessment of renal function alerts
In this study, the pharmacists used a pharmacy medication alert system (PMAS) built by one of the authors in a Microsoft® Access® database. The system, which was an addition to the current pharmacy computer system, assessed the medication in relation to the reported eGFR and provided an alert for target drugs according to the Dutch guidelines for drug administration in reduced renal function.17 These guidelines include drug-specific cut-off values for eGFR, accompanied by a therapy-adjustment advice.
After receiving a data file from the GP with the eGFR of the included patients, the pharmacists linked the eGFR in the PMAS. Simultaneously, the patient’s actual medication was electronically imported from the usual pharmacy computer system into the PMAS. An alert was generated to stipulate action if the eGFR was lower than the cut-off value of the target drug (Table 1). The software could not correct for invalid dose or dose interval, so the pharmacist assessed the alerts for these aspects based on the guideline recommendations presented in a text box. The pharmacist formulated therapeutic advice for either dosage adjustment, to stop the drug, or to substitute it by a non-contraindicated drug. Once a week, the pharmacist communicated the therapeutic advice to the GP by a list. The GP evaluated the therapeutic advice in relation to the clinical context of each individual patient, and responded with agreement or disagreement. Predefined reasons for disagreement could be checked on the list and the GP was asked to give supplementary comments in a free-text box. The list was returned to the pharmacist.
Table 1.
Predefined cut-off values top 10 target drugs with truncated guideline advice
| Therapeutic group | Drug name | Cut-off values, ml/min | Guideline advice |
|---|---|---|---|
| Blood-glucose-lowering drugs | Metformin | 30–50 | Initial dose 2 × 500 mg |
| <30 | Contraindicated | ||
| Glimepiride | 10–50 | Initial dose 50% | |
| Cardiac glycosides (digoxin) | Digoxin | 10–50 | Initial dose 50% |
| Low-ceiling diuretics, thiazides | Hydrochlorothiazide | 30–50 | Initial dose 12.5 mg |
| <30 | Contraindicated | ||
| High-ceiling diuretics | Furosemide | 10–30 | Dose higher |
| Potassium-sparing diuretics | Spironolactone | 10–50 | Monitor potassium |
| Amiloride | 30–50 | Monitor potassium | |
| <30 | Contraindicated | ||
| Diuretics combinations | Triamterene | 30–50 | Dose 50%, monitor potassium |
| <30 | Contraindicated | ||
| Epitizide | <30 | Contraindicated | |
| Beta-blockers | Sotalol | 30–50 | Max dose 160 mg/day |
| 10–30 | Max dose 80 mg/day | ||
| Angiotensin-converting enzyme inhibitors | Enalapril | 30–50 | Initial dose 5 mg |
| 10–30 | Initial dose 2.5 mg | ||
Outcome
The outcome of the study was the frequency of therapeutic advice formulated by the pharmacist (expressed as a proportion of the total number of target drugs). The management of the therapeutic advice by the GP was also studied.
Statistical analysis
All relevant patient data were entered into a Microsoft Access 2003 database and further analysed with SPSS Statistics (version 17.0) for descriptive statistics (mean, frequency, range).
RESULTS
On the inclusion date, 650 patients were included and analysed (Figure 1). These patients were prescribed 1333 target drugs (Table 2). An actual eGFR had been determined in 78.5% (n = 510) of the patients (range per GP = 66–89%). In the remaining patients, eGFR was determined after the inclusion date.
Table 2.
Characteristics of the analysed study population
| Characteristic | n | % |
|---|---|---|
| Patients | 650 | 100.0 |
| Female | 433 | 66.6 |
| Target drugs | 1333 | |
| Mean | SD (range) | |
| Age, years | 81 | 6.7 (70–101) |
| eGFR, ml/min/1.73 m2 | 63.3 | 17.0 (13–>95) |
| Number of drugs | 5.8 | 2.8 (1–17) |
| Number of target drugs | 2 | 1.1 (1–7) |
| Patients prescribed target drugs by therapeutic group | n | % |
| Blood glucose-lowering drugs | 156 | 24.0 |
| Cardiac glycosides digoxin | 73 | 11.2 |
| Low-ceiling diuretics thiazides | 259 | 39.8 |
| High-ceiling diuretics | 164 | 25.2 |
| Potassium sparing diuretics | 49 | 7.5 |
| Diuretics combinations | 46 | 7.1 |
| Beta-blocker sotalol | 33 | 5.1 |
| Beta-blockers atenolol/bisoprolol | 31 | 4.8 |
| RAS inhibitors | 224 | 5.1 |
eGFR = estimated glomerular filtration rate. RAS renin–angiotensin system. SD = standard deviation
Assessment of renal function alerts
The computer software generated 212 alerts (15.9%) in a total of 1333 target drugs, because the eGFR was lower than the predefined cut-off value of the target drug. After the pharmacist assessed the actual medication for correct dose and dose interval, 93 alerts (7.0%) appeared to be correct and seven alerts (0.5%) were missing. Therefore, action to adjust therapy was considered necessary in 112 prescriptions in 74 patients (8.4% of the target drugs, 11.4% of the patients). Additionally, pharmacists gave advice in 31 prescriptions of target drugs, even though the eGFR was just above the cut-off value. Eventually, 143 therapeutic recommendations (10.7% of the target drugs) concerning 89 patients (13.7% of analysed study population) were included for analysis of the GP responses. The drugs most frequently involved were diuretics (41.3% of therapeutic advice), blood-glucose-lowering drugs (14.0%), digoxin (11.2%), and RAS inhibitors (10.5%). Almost all prescriptions that received an alert were chronic prescriptions taken by the patient for a longer period of time.
GP response to pharmacist advice
The GP immediately agreed with 71 recommendations (49.7% of the therapeutic advice) concerning 52 patients (8.0% of the study population). The GP most frequently disagreed with the advice on diuretics, blood-glucose-lowering drugs, digoxin, and RAS-inhibitors. Within each of these therapeutic groups, the GP immediately disagreed with one-third of the advice. The responses of the GPs are shown in Table 3.
Table 3.
GP response
| Response | n (total N = 143) | % | Comments |
|---|---|---|---|
| Immediate agreement | 71 | 49.7 | 52 patients, 8% of study population |
| Postponed reaction | 20 | 14.0 | – |
| GP first wants to consult specialist | 12 | 8.4 | – |
| GP first wants to speak to patient | 6 | 4.2 | – |
| Further monitoring biomarker(s) | 2 | 1.4 | Potassium, creatinine |
| Disagreement | 38 | 26.6 | – |
| No standard reason indicated | 17 | 11.9 | No adverse reactions (n = 1), already low dose (n = 2) |
| Potassium normal | 5 | 3.5 | – |
| Disease is stable | 16 | 11.2 | Diabetes (n = 5), heart failure (n = 7), hypertension (n = 3), renal function (n = 1) |
| Specialist is treating patient | 14 | 9.8 | Specialist was responsible for the drug therapy (GP only prescribed the refill prescriptions) |
DISCUSSION
Summary
The use of a PMAS based on renal function resulted in therapeutic advice for a substantial number of drugs in older patients with diabetes or cardiovascular disease. The GP immediately agreed with half of the advice. Overall, in 5% of the prescriptions, the GP agreed to rectify the prescription.
The GPs used a medication monitoring system based on the Dutch G-standard,17 the national drug database, which is used by all professional parties in Dutch health care. Despite this monitoring system, pharmacists still formulated additive therapeutic advice in 11% of the target drugs. What could be the reasons for this? First, it is known that a high number of medication alerts may cause ‘alert fatigue’ in the prescriber.10 In the case of repeat prescriptions in particular, alerts were ignored. The extra effort to seek a renal function and to weigh the choice and dosage of the drug may cost too much time. Second, this observation could be explained because at the time of prescription, an actual eGFR was not available in more than 20% of the patients. Finally, it is important to consider the prescribing context. The alerts concerned chronic medication that the patient may have been using for a longer period of time, with an established clinical effect and with the patient accustomed to take them. Change of drug choice under these circumstances may disrupt the flow of treatment.
The use of a PMAS reduced the number of alerts compared to the current pharmacy computer system. A more sophisticated clinical decision support system could further reduce the number of irrelevant alerts by incorporating invalid dose or dose-interval algorithms that can weigh comorbidity and other patient-related risk factors that may affect the reliability of the eGFR,19 and by linking laboratory to pharmacy data. Currently, some of these principles are already incorporated in new versions of medication monitoring systems.
Strengths and limitations
This study revealed the benefit of therapeutic advice automatically generated by a PMAS based on renal function. The clinical relevance is substantial: prescribing of target drugs to older patients with diabetes or cardiovascular disease is a daily activity in primary care, and the risk of complications related to renal function is high.4 Primary care studies on compliance to dosing guidelines in patients with CKD are rare.6 Recently, Bhardwaja et al demonstrated in a large US study of 32 917 patients with an eGFR below 50 ml/min/1.73 m2, that an alert system in the pharmacy can result in a reduction of medication errors from 49% to 33%.14
The data may not be generalisable to other settings because of the small number of participating practices, but the underlying problem of medication safety in relation to renal function and the intervention of a PMAS is of general interest.
The advice given was based on a single eGFR value obtained not more 1 year previously. This was for pragmatic reasons: the renal function of patients who are in a diabetes or hypertension control system should be monitored yearly. However, variability in serum creatinine measurements necessitates at least two creatinine measurements,20,21 and even more frequent monitoring of renal function is needed in patients who are not stable.22
Comparison with existing literature
GPs immediately agreed with half of the therapeutic advice. This is in accordance with the acceptance rate in a study in which clinical pharmacists gave therapeutic recommendations to GPs based on the medical records of 200 patients with diabetes or hypertension.23 In a hospital setting, the acceptance rate was the same: 55% of the pharmacist advice was accepted by the clinician.15
Besides the predefined reasons for disagreement, the GPs were not very explicit with their comments in the free-text box. Disagreement could be explained by a difference between the dosage guidelines and clinical practice. An example is the advice to start with low doses of RAS inhibitors to prevent adverse drug reactions, whereas current clinical practice guidelines do advise to prescribe RAS inhibitors in high doses in order to protect kidney function (with monitoring of renal function and serum potassium).24–26 Meanwhile, the advice in the Dutch dosage guidelines has been adjusted to clinical practice.
Implications for research and practice
To optimise drug prescribing in patients with decreased renal function, many steps need to be taken: systematic renal function monitoring in patients on target drugs, linking the laboratory to the pharmacy, assessment of the alerts by both pharmacist and GP, and communication with the patient on the proposed prescription change. When implementing a PMAS, all above-mentioned steps deserve attention.
A PMAS based on renal function resulted in therapeutic advice in one of every nine target drugs in older patients on blood-glucose-lowering or cardiovascular drugs. After weighing the clinical context, the GP agreed with half of the advice. Collaboration between the GP and pharmacist, using their clinical and pharmacological expertise respectively, can contribute to patient safety.
Acknowledgments
The authors would like to thank Professor AC Egberts for his comments on the manuscript. We would very much like to thank all the pharmacists, FT Schroor, FJJM van der Leemputte, DC Sietses, F Dijkhuizen-Behr, B Klok, and the GPs MHGA van Wijk, JJ Hammink, WJ Marée-Wibbelink, RM Linders, MP Huizing-Mientjens, MMJ Hendriks, and M van Duivenboden, who participated in the study.
Funding
None.
Ethical approval
Ethics approval was not required, according to the accredited Medical Research Ethics Committee Arnhem/Nijmegen (number 2008/179).
Provenance
Freely submitted; externally peer reviewed.
Competing interests
The authors have declared no competing interests.
Discuss this article
Contribute and read comments about this article on the Discussion Forum: http://www.rcgp.org.uk/bjgp-discuss
REFERENCES
- 1.Coresh J, Selvin E, Stevens L, et al. Prevalence of chronic kidney disease in the United States. JAMA. 2007;298(17):2038–2047. doi: 10.1001/jama.298.17.2038. [DOI] [PubMed] [Google Scholar]
- 2.Levey AS, Coresh J, Balk E, et al. National Kidney Foundation practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Ann Intern Med. 2003;139(2):137–147. doi: 10.7326/0003-4819-139-2-200307150-00013. [DOI] [PubMed] [Google Scholar]
- 3.De Lusignan S, Chan T, Stevens P, et al. Identifying patients with chronic kidney disease from general practice computer records. Fam Pract. 2005;22(3):234–241. doi: 10.1093/fampra/cmi026. [DOI] [PubMed] [Google Scholar]
- 4.Leendertse AJ, Egberts ACG, Stoker LJ, et al. Frequency of and risk factors for preventable medication-related hospital admissions in the Netherlands. Arch Intern Med. 2008;168(17):1890–1896. doi: 10.1001/archinternmed.2008.3. [DOI] [PubMed] [Google Scholar]
- 5.Zhang M, Holman C, D’Arcy J, et al. Comorbidity and repeat admission to hospital for adverse drug reactions in older adults: retrospective cohort study. BMJ. 2009;338:a2752. doi: 10.1136/bmj.a2752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Long CL, Raebel MA, Price DW, et al. Compliance with dosing guidelines in patients with chronic kidney disease. Ann Pharmacother. 2004;38(5):853–858. doi: 10.1345/aph.1D399. [DOI] [PubMed] [Google Scholar]
- 7.Avery AJ, Dex GM, Mulvaney C, et al. Development of prescribing-safety indicators for GPs using the RAND Appropriateness Method. Br J Gen Pract. 2011 doi: 10.3399/bjgp11X588501. DOI: 10.3399/bjgp11X588501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vidal L, Shavit M, Fraser A, et al. Systematic comparison of four sources of drug information regarding adjustment of dose for renal function. BMJ. 2005;331(7511):263. doi: 10.1136/bmj.38476.471088.3A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.National Collaborating Centre for Chronic Conditions. Chronic kidney disease: national clinical guideline for early identification and management in adults in primary and secondary care. London: Royal College of Physicians; 2008. http://guidance.nice.org.uk/nicemedia/live/12069/42116/42116.pdf (accessed 3 Jul 2012). [PubMed] [Google Scholar]
- 10.Isaac T, Weissman JS, Davis RB, et al. Overrides of medication alerts in ambulatory care. Arch Intern Med. 2009;169(3):305–311. doi: 10.1001/archinternmed.2008.551. [DOI] [PubMed] [Google Scholar]
- 11.Raebel MA, Lyons EE, Chester EA, et al. Improving laboratory monitoring at initiation of drug therapy in ambulatory care: a randomized trial. Arch Intern Med. 2005;165(20):2395–2401. doi: 10.1001/archinte.165.20.2395. [DOI] [PubMed] [Google Scholar]
- 12.Van Dijk EA, Drabbe NRG, Kruijtbosch M, et al. Drug dosage adjustments according to renal function at hospital discharge. Ann Pharmacother. 2006;40(12):1254–1260. doi: 10.1345/aph.1G742. [DOI] [PubMed] [Google Scholar]
- 13.Weingart SN, Toth M, Sands DZ, et al. Physicians’ decisions to override computerized drug alerts in primary care. Arch Intern Med. 2003;163(21):2625–2631. doi: 10.1001/archinte.163.21.2625. [DOI] [PubMed] [Google Scholar]
- 14.Bhardwaja B, Carroll NM, Raebel MA, et al. Improving prescribing safety in patients with renal insufficiency in the ambulatory setting: the Drug Renal Alert Pharmacy (DRAP) program. Pharmacotherapy. 2011;31(4):346–356. doi: 10.1592/phco.31.4.346. [DOI] [PubMed] [Google Scholar]
- 15.Hassan Y, Al-Ramahi RJ, Aziz NA, et al. Impact of a renal drug dosing service on dose adjustment in hospitalized patients with chronic kidney disease. Ann Pharmacother. 2009;43(10):1598–1605. doi: 10.1345/aph.1M187. [DOI] [PubMed] [Google Scholar]
- 16.Schiff GD, Klass D, Peterson J, et al. Linking laboratory and pharmacy: opportunities for reducing errors and improving care. Arch Intern Med. 2003;163(8):893–900. doi: 10.1001/archinte.163.8.893. [DOI] [PubMed] [Google Scholar]
- 17.KNMP Royal Dutch Association for the Advancement of Pharmacy (ed.) Dutch dosing guideline for impaired renal function. The Hague: Thieme GrafiMedia; 2009. [Google Scholar]
- 18.Stevens LA, Nolin TD, Richardson MM, et al. Comparison of drug dosing recommendations based on measured GFR and kidney function estimating equations. Am J Kidney Dis. 2009;54(1):33–42. doi: 10.1053/j.ajkd.2009.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stevens LA, Coresh J, Greene T, et al. Assessing kidney function: measured and estimated glomerular filtration rate. N Engl J Med. 2006;354(23):2473–2483. doi: 10.1056/NEJMra054415. [DOI] [PubMed] [Google Scholar]
- 20.Myers GL, Miller WG, Coresh J, et al. Recommendations for improving serum creatinine measurement: a report from the laboratory working group of the national kidney disease education program. Clin Chem. 2006;52(1):5–18. doi: 10.1373/clinchem.2005.0525144. [DOI] [PubMed] [Google Scholar]
- 21.National Kidney Foundation. K/DOQI guidelines. New York, NY: NKF; http://www.kidney.org/professionals/kdoqi/guidelines.cfm (accessed 3 Jul 2012). [Google Scholar]
- 22.National Institute for Health and Clinical Excellence. Chronic kidney disease: early identification and management of chronic kidney disease in adults in primary and secondary care (GG23) London: NICE; 2008. http://www.nice.org.uk/nicemedia/pdf/CG073NICEGuideline.pdf (accessed 3 Jul 2012). [PubMed] [Google Scholar]
- 23.Patel HR, Pruchnicki MC, Hall LE. Assessment for chronic kidney disease service in high-risk patients at community health clinics. Ann Pharmacother. 2005;39(1):22–27. doi: 10.1345/aph.1E269. [DOI] [PubMed] [Google Scholar]
- 24.Ahmed A. Use of angiotensin-converting enzyme inhibitors in patients with heart failure and renal insufficiency: how concerned should we be by the rise in serum creatinine? J Am Geriatr Soc. 2002;50(7):1297–1300. doi: 10.1046/j.1532-5415.2002.50321.x. [DOI] [PubMed] [Google Scholar]
- 25.Bakris G, Weir M. Angiotensin-converting enzyme inhibitor-associated elevations in serum creatinine: is this a cause for concern? Arch Intern Med. 2000;160(5):685–693. doi: 10.1001/archinte.160.5.685. [DOI] [PubMed] [Google Scholar]
- 26.Brantsma AH, Bakker SJL, Hillege HL, et al. Cardiovascular and renal outcome in subjects with K/DOQI stage 1–3 chronic kidney disease: the importance of urinary albumin excretion. Nephrol Dial Transplant. 2008;23(12):3851–3858. doi: 10.1093/ndt/gfn356. [DOI] [PubMed] [Google Scholar]