Abstract
Synthetic glucocorticoids are widely used for treatment of many inflammatory diseases. However, long-term glucocorticoid treatment can cause a variety of negative side effects. A genome-wide microarray analysis was performed in human lung A549 cells to identify genes regulated by both the antiinflammatory steroid dexamethasone (Dex) and the proinflammatory cytokine TNFα. Unexpectedly, we discovered that numerous genes were coregulated by treatment with both Dex and TNFα. We evaluated the mechanism of coregulation of one of these genes, serpinA3 (α-1 antichymotrypsin), a secreted, acute phase protein strongly associated with numerous inflammatory diseases. Up-regulation of serpinA3 requires the presence of both the glucocorticoid receptor and TNFα soluble receptor 1. Treatment with Dex or TNFα resulted in a 10- to 25-fold increase of serpinA3 mRNA, whereas coadministration of Dex and TNFα led to a synergistic increase in serpinA3 mRNA. The naturally occurring glucocorticoid, cortisol, also resulted in a synergistic increase in serpinA3 mRNA levels in A549 cells. Furthermore, in vivo treatment of C57BL/6 mice with Dex and TNFα resulted in coregulation of serpinA3 mRNA levels in both lung and liver tissues. Finally, chromatin immunoprecipitation analyses suggest that glucocorticoid receptor binding to the serpinA3 transcriptional start site can be enhanced by the combination of Dex plus TNFα treatment of A549 cells. These studies demonstrate that glucocorticoids and proinflammatory compounds can coregulate genes associated with human disease. This discovery may underlie the basis of some of the adverse effects associated with long-term glucocorticoid therapy.
Glucocorticoids are a class of steroid stress hormones that are required for life after birth. These hormones directly bind the glucocorticoid receptor (GR) to modulate its activity. GR is a member of the nuclear receptor superfamily of ligand-dependent transcription factors that can directly and indirectly regulate many genes involved in suppressing the immune response (1, 2). In the lung, inflammation defines the pathogenicity of many diseases, including chronic obstructive pulmonary disorder (COPD), cystic fibrosis, and asthma (3, 4). Synthetic glucocorticoids are commonly used for treatment of many airway inflammatory diseases. However, with chronic use, they can cause a variety of side effects, including weight gain, muscle breakdown, insulin resistance, osteoporosis (5), mood changes (6), and glaucoma (7), among others (8, 9).
Glucocorticoids are widely recognized and used as antagonistic regulators of inflammation induced by cytokines, and conventional wisdom and current dogma suggest that they would antagonize the majority of proinflammatory molecules throughout the genome. Interestingly, a small number of genes have previously been observed to be coregulated by glucocorticoids and cytokines (10, 11). However, the extent of this coregulation is largely unexplored. To identify on a genome-wide scale the genes coinduced by glucocorticoids and cytokines, we performed microarray analysis in A549 lung cells that were treated with the glucocorticoid dexamethasone (Dex) and the cytokine TNFα. Surprisingly, over 800 genes were significantly coregulated in A549 cells treated with Dex and TNFα as judged by genome-wide microarray analysis. We evaluated the regulation of serpinA3 [α1-antichymotrypsin (AACT)] because of its importance in lung inflammatory diseases, such as COPD (12, 13). This gene was up-regulated by coadministration of Dex and TNFα in A549 cells.
AACT (gene name, serpinA3) is a secreted, acute phase protein (14) that acts as a serine protease inhibitor. One major role of AACT is to protect tissues, including the lung, from damage by chymotrypsin released from phagocytes after pathogen invasion (15). The highest levels of AACT mRNA are found in hepatocytes in the liver, but it is also expressed in many other tissues, including the lung. The secreted AACT protein is primarily found in blood plasma, urine, and bronchoalveolar lavage fluid (14, 16). Mutation of AACT at Ala15 is associated with a higher incidence of COPD in heavy smokers (12). In addition, single nucleotide polymorphisms of AACT are also implicated in playing a role in the age of onset and severity of a number of other diseases, including Parkinson's disease (17, 18) and Alzheimer's disease (19–21), although the link to Parkinson's disease remains controversial (22–24). Previous research has indicated that cytokines such as IL-6, oncostatin M, and IL-1 increase the expression of AACT in hepatic cells (14). Furthermore, SerpinA3 production has been shown to increase after treatment with Dex and IL-6 in hepatocytes (25) and with Dex and TNFα in human astrocytomas (26). However, the underlying mechanism of regulation and the extent to which the synergistic regulation occurs are largely unknown.
In these studies we demonstrate that the up-regulation of SerpinA3 mRNA requires the presence of both the GR and TNFα soluble receptor 1 (TNFSR1). Furthermore, in vivo, treatment of mice with Dex and TNFα resulted in an additive increase in SerpinA3 mRNA levels in both lung and liver tissues, suggesting that this gene regulation may have physiological relevance to treatment after inflammation. Finally, chromatin immunoprecipitation (ChIP) analysis suggests that GR binding at the serpinA3 transcriptional start site is considerably more robust when cells are incubated with a combination of Dex and TNFα. Our studies demonstrate that glucocorticoids and cytokines can coregulate genes involved in inflammatory disease. We speculate that this observation may underlie a component of side effects seen under chronic glucocorticoid therapy.
Materials and Methods
Cell culture and treatments
A549 cells were purchased from American Type Culture Collection (Manassas, VA) and maintained as previously described (10) in DMEM/F-12 medium supplemented with 10% fetal calf serum, 100 IU of penicillin per milliliter, and 100 mg/ml of streptomycin. Dex (1,4-pregnadien-9-fluoro-16-methyl-11β,17,21-triol-3,20-dione) (Steraloids, Wilton, NH) was dissolved in water based on its known molecular extinction coefficient, and recombinant human TNFα (R&D Systems, Minneapolis, MN) was dissolved in PBS and both used at the indicated concentrations. Treatments also included mifepristone (RU486) (Steraloids) (1 μm), TNFα neutralizing/blocking monoclonal antibody (1 ng/ml; R&D Systems), actinomycin D (1 μg/ml; Sigma, St. Louis, MO), cortisol (100 nm; Steraloids), and cycloheximide (10 μg/ml; Sigma). All experiments were performed with a minimum of three biological replicates.
Mouse studies
Adrenalectomized C57BL/6 male mice were ip injected with vehicle (PBS), 1 mg/kg Dex (Steraloids), 1.5 μg of murine TNFα (R&D systems), or both Dex and TNFα for 6 h. Three animals were used per treatment for a total of 12 animals. Animals were killed by cervical dislocation and mRNA extracted using RNAlater (QIAGEN, Valencia, CA) and RNeasy kits (QIAGEN). mRNA levels were analyzed by quantitative real-time RT-PCR. All studies were approved by the National Institutes of Health animal care committee.
Microarray analysis
Gene expression analysis was conducted using Agilent Whole Human Genome 4 × 44 multiplex format oligo arrays (014850) (Agilent Technologies, Inc., Santa Clara, CA) following the Agilent 1-color microarray-based gene expression analysis protocol. Three biological replicates were analyzed for each condition for a total of 12 chips. Starting with 500 ng of total RNA, Cy3-labeled cRNA were fragmented and hybridized for 17 h in a rotating hybridization oven. Slides were washed and then scanned with an Agilent scanner. Data were obtained using the Agilent Feature Extraction software (version 9.5), using the 1-color defaults for all parameters. The Agilent Feature Extraction software performed error modeling, adjusting for additive and multiplicative noise. Principle component analysis was performed on all samples and all probes to characterize the variability present in the data. To identify differentially expressed probes, ANOVA was used to determine whether there was a statistical difference between the means of groups. In addition, we used a multiple test correction to reduce the number of false positives. Specifically, an error-weighted ANOVA and Benjamini-Hochberg multiple test correction with a P value of less than 0.01 was performed using Rosetta Resolver system (version 7.1). The resulting data were processed using Genespring GX (version 10.0.2; Agilent Technologies, Inc.) and ingenuity pathway analysis (IPA) (Ingenuity Systems, Redwood City, CA).
Quantitative (q) real-time RT-PCR
Predeveloped validated primer/probe sets were purchased for all genes analyzed (Applied Biosystems, Foster City, CA). Total RNA was extracted from cells and tissues with RNeasy kits (QIAGEN) according to the manufacturer's protocol. RT-PCR mixtures were composed of the following: TaqMan Universal PCR Master Mix, No AmpErase (Applied Biosystems), 1× concentrations of primer/probe set, 0.05–0.2 μg of RNA, 0.4 U/μl ribonuclease inhibitor (Roche Diagnostics, Indianapolis, IN), and 0.3 U/μl murine leukemia virus RT (Roche Diagnostics). Negative controls, consisting of all components except for RT enzyme, gave no signal in every case. Reactions were run in duplicate with a minimum of three biological replicates on an ABI Prism 7900HT with thermal cycling parameters specific for one-step RT-PCR. Target gene expression levels were normalized to an endogenous control (cyclophilin B), and data were plotted relative to control (hormone free) mRNA.
Short hairpin RNA (shRNA) and generation of stable cell lines
shRNA cloned into the lentivirus vector pLKO.1-puro were chosen from the human library (MISSION TRC-Hs 1.0) and purchased in glycerol stock form from Sigma. The control shRNA (nontarget shRNA vector, catalog no. SHC002; Sigma) contains a hairpin insert that will generate small interfering RNA but contains 4-bp mismatches to any known human or mouse gene. A set of three shRNA to GR (SHGLY-NM_000176; Sigma) and five shRNA to TNFα receptor 1 (TNFSR1A) (SHCLNG-NM_001065; Sigma) were tested for knockdown. One of the GR shRNA (TRCN0000019321; Sigma) and one of the TNFSR1A shRNA (TRCN0000058716; Sigma) were chosen for these experiments. The control shRNA, shRNA to GR, or shRNA to TNFSR1A lentivirus vectors were cotransfected with the packaging plasmids (Virapower System; Invitrogen, Carlsbad, CA) into 293 FT cells to generate lentivirus particles. These particles were then used to transduce A549 cells, and cells stably expressing the shRNA were selected with puromycin at a concentration of 2.5 μg/ml.
Western blot analysis
Cells were detached from plates using cell scrapers, lysed [in PBS containing 0.5% Nonidet P-40, 5% phosphatase inhibitors (phosphatase inhibitor cocktail II; EMD-Merck KGaA, Darmstadt, Germany), and protease inhibitors (complete mini tablet; Roche, Indianapolis, IN)], and sonicated with a cell disruptor (Branson Ultrasonic Co., Danbury, CT). Lysates were then centrifuged for 10 min (12,000 × g), and the resulting supernatants were used for Western blotting. Total protein was determined by colorimetric assay (Bio-Rad Protein Assay; Bio-Rad, Hercules, CA). Equivalent amounts of total protein were separated on SDS-PAGE and transferred to nitrocellulose membranes. Antibodies for immunoblot analysis include anti-GR 57 (previously described in Ref. 27), anti-TNFSR1A (Cell Signaling Technology, Beverly, MA), anti-AACT (ab20359; Abcam, Cambridge, MA), anti-β-actin (Millipore, Bedford, MA), antirabbit secondary (GE Biosciences, Princeton, NJ), and antimouse secondary (GE Biosciences). All Western blottings were representative of at least three different biological replicate samples.
Chromatin precipitation assays
ChIP assays were performed using the Magna ChIP A Chromatin Immunoprecipitation kit (Millipore) as previously described (28, 29). Briefly, cells were plated in 150-mm dishes and cultured for 24 h in DMEM/F12 supplemented with 10% charcoal dextran-treated (stripped) fetal bovine serum. Cells were then treated with 10 nm Dex, 10 μg/ml TNFα, or 10 nm of Dex + 10 μg/ml TNFα for 90 min. The cells were fixed, harvested, and nuclear contents were sonicated. Sheared chromatin was then immunoprecipitated overnight with 5 μl of 57-GR (previously described in Ref. 26) or 5 μl of p65 (catalog no. SC372X; Santa Cruz Biotechnology, Inc., Santa Cruz, CA), or 5 μl of active polymerase II antibodies (combination 1:1 of MMS-134R and MMS-126R; Covance, Richmond, CA). After elution of protein:DNA complexes and DNA purification, real-time PCR analysis was performed using the following primers: forward 5′-TCATTTCCAGTCCGAGAACAG-3′, probe 5-CACTTGGTTGTCCTGGCATTTCCC-3, and reverse 5′-GGATTTTCATGAATGCTGAGGC-3′ for serpinA3 transcriptional start site; forward 5′-TGC CCT GTG GTT TCA TCT GGT CAT-3′, probe 5′-TG AAT GGT GCT TGC AGA ACC GAA GGA-3′, and reverse 5′-ATT CCT GAC CTT CTC CCA GCC TCT TT-3′ for serpinA3 at 1700; forward 5′-AGGCTTGATGACCAGAGAGGTTTG-3′, probe 5′-ATTCAGCTGCAAGTCTGGCATGGGAA-3′, and reverse 5′-GCTCAGAGACTTTGTGGCTATTTGG-3′ for GILZ promoter; and forward 5′-AGTGTGATGACTCAGGTTTGCCCT-3′, probe 5′- TGGGCCATCAGTTGCAAATCGTGGAA-3′, and reverse 5′-TCCTAGAAGCTTGTGTGCTCTGCT-3′ for IL-8 promoter. All PCR ran for 40 cycles.
Statistical analysis
Statistical analyses were performed with one-way ANOVA. Where ANOVA indicated statistical significance (P < 0.05), Tukey HSD post hoc testing was applied.
Results
Identification and classification of Dex- and TNFα-regulated genes in A549 lung cells
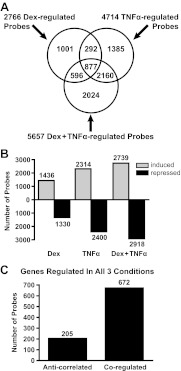
Glucocorticoids and cytokines are both known to regulate numerous genes involved in the inflammatory process where glucocorticoids have a major role in suppressing inflammation, whereas cytokines generally induce inflammation. It was not clear, however, whether glucocorticoids and cytokines regulate the same genes in an opposing matter or whether they regulated different genes to control inflammation. To distinguish between these possibilities, we treated A549 lung cells with the synthetic glucocorticoid Dex, the cytokine TNFα, or both Dex and TNFα, isolated the mRNA, and performed a microarray analysis. The genes significantly regulated by each condition compared and were visualized by Venn diagram (Fig. 1A). There were 2766 probes significantly regulated in cells treated with Dex, 4714 probes significantly regulated in cells treated with TNFα, and 5657 probes significantly regulated in cells treated with both Dex and TNFα. Interestingly, in cells treated with both Dex and TNFα, there were 2024 unique genes that were not regulated by either Dex or TNFα alone (Fig. 1A). A similar percentage of genes was up-regulated and down-regulated for each condition (Fig 1B), suggesting that there was no global induction or repression.
Fig. 1.
Identification and classification of Dex- and TNFα-regulated genes. A, Venn diagram of regulated transcripts indicating the overlap between transcripts regulated by the different treatments. B, Bar histogram of up- and down-regulated transcripts for each treatment. C, The 877 transcripts coregulated by Dex, TNFα, and Dex + TNFα were grouped based on their pattern of expression and classified as coregulated or anticorrelated.
To identify whether glucocorticoids and cytokines were regulating the same genes or different sets of genes, we examined overlap between genes regulated under each condition. As one would naturally predict based on current dogma, many of the genes that were regulated by Dex (1001/2766) were unaffected by TNFα and vice versa (1385/4714). However, 877 probes were regulated in all three conditions. These genes were further classified based on their regulation pattern (Fig. 1C). Interestingly, only 205 genes were antagonistically regulated by Dex and TNFα, whereas 672 probes were coregulated (Fig. 1C).
Pathway analysis of Dex- and TNF-regulated genes
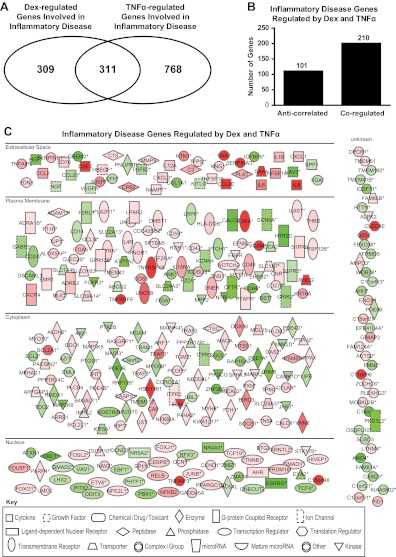
After identifying that such a large number of genes were coregulated by Dex and TNFα, we sought to determine the potential biological implications of the gene sets regulated by glucocorticoids and TNFα. To accomplish this goal, IPA was employed to identify signaling pathways that were significantly affected by Dex and TNFα. The IPA knowledge base identified molecules from the gene list associated with various biological processes; each molecule in the knowledge base is supported by at least one reference from the literature, from a textbook, or from canonical information. The IPA functional analysis identified the biological functions and/or diseases that were most significant to these data sets. Because we wanted to identify how many of the coregulated genes were involved in inflammation, the pathway associated with inflammatory disease was selected for further study. In this pathway, Dex treatment regulated 665 genes (Fig. 2A), and TNFα treatment regulated 1085 genes (Fig. 2A), with 312 genes overlapping between the two treatments (Fig. 2A). The entire list of significantly regulated genes is available in the Supplemental Data, published on The Endocrine Society's Journals Online web site at http://endo.endojournals.org. The overlapping genes were both anticorrelated and coregulated, with the majority of the genes (201/312) being coregulated by Dex and TNFα (Fig. 2B). The 312 common genes are displayed in their subcellular location in Fig. 2C. The intensity of the node color indicates the degree of up-regulation (red) or down-regulation (green). Nodes are displayed using various shapes that represent the functional class of the gene product. Based on these results, we conclude that Dex and TNFα coregulate rather than antagonistically regulate many genes involved in inflammatory disease. We speculate that some of these genes may be associated with side effects observed after long-term glucocorticoid treatment.
Fig. 2.
IPA of regulated genes. Genes regulated under the indicated conditions were subjected to IPA to predict potential effects on cellular functions. Genes regulated in inflammatory disorders were selected for further analysis. A, Venn diagram of Dex- or TNFα-regulated genes involved in inflammatory disorders indicating the overlap between the genes regulated by either of these compounds. B, Inflammatory disease genes regulated by Dex and TNFα were classified as coregulated or anticorrelated. C, Diagram listing of inflammatory genes regulated by Dex and TNFα. Green represents up-regulated genes, and red represents down-regulated genes; the brighter the color, the stronger the regulation. Each symbol represents a different class of gene products (see legend on figure). Asterisks indicate that multiple identifiers in the dataset file map to a single gene in the Global Molecular Network.
qRT-PCR validations of significantly regulated genes identified by microarray analysis
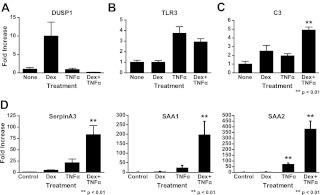
We identified several different categories of regulated genes in our microarray: Dex treatment regulated, TNFα treatment regulated, genes regulated only in the presence of both Dex and TNFα, and genes synergistically regulated by Dex and TNFα. To validate our microarray results, we selected representative genes from each category and performed qRT-PCR to determine mRNA levels on each corresponding gene. The representative genes from each of the categories were: Dusp1 (Fig. 3A), TLR3 (Fig. 3B), and C3 (Fig. 3C). We also validated three additional genes in the coregulated category because of the unexpected nature of this result (Fig. 3D): SAA1, SAA2, and serpinA3. Overall, the qRT-PCR results were consistent with the microarray results. Out of the genes synergistically regulated by Dex and TNFα, the acute phase protein serpinA3 (AACT) was selected for further mechanistic study because of its clinical relevance in inflammatory disease.
Fig. 3.
qRT-PCR validations of regulated genes. Gene expression patterns from the microarray were validated by one-step quantitative RT-PCR. Predeveloped and validated primer/probe sets were purchased for all genes analyzed (Applied Biosystems). A549 cells were treated with 10 nm of Dex, 10 μg/ml TNFα, or treated with both compounds, and RNA was isolated from these cells after 6 h of treatment. Target gene expression levels were normalized to an endogenous control (cyclophilin B) in all experiments, and data were plotted as fold change relative to control (hormone free) mRNA for statistical comparisons. A, Example of a Dex-up-regulated gene (Dusp1). B, Example of a TNFα-up-regulated gene (TLR3). C, Example of a gene regulated in cells treated with both Dex and TNFα (C3). D, Examples of genes coup-regulated by treatment with Dex and TNFα (SAA1, SAA2, and SerpinA3).
Characterization of serpinA3 regulation
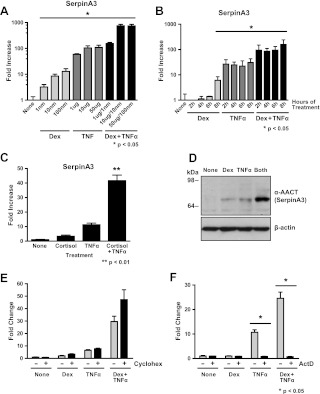
serpinA3 was one of the genes most highly up-regulated by Dex and TNFα in our microarray. serpinA3 encodes for a secreted, acute phase protein widely expressed in several different tissues, including lung, and because it was synergistically up-regulated by Dex and TNFα we performed a number of experiments to identify conditions where maximal expression of serpinA3 mRNA could be obtained. In these experiments, A549 lung cells were treated with increasing concentrations of Dex, TNFα, or both Dex and TNFα for 6 h. Significant induction of serpinA3 mRNA was seen with 1 nm of Dex and 1 μg/ml TNFα (Fig. 4A). Maximal levels of mRNA induction were seen with 10 nm of Dex and 10 μg/ml TNFα treatment, with no further increases at higher concentrations. Based on these results, the remaining experiments were conducted with 10 nm of Dex and 10ug/ml TNFα. Next, we analyzed the time course of serpinA3 induction. A549 cells were treated with Dex, TNFα, or both for up to 8 h (Fig. 4B). The levels of serpinA3 were significantly increased by 2 h of treatment and were maintained at a constant level for 8 h, suggesting that this increase may be a direct response to Dex and TNFα treatment. Thus, the remaining experiments were conducted at 6 h of treatment unless otherwise indicated.
Fig. 4.
Characterization of serpinA3 regulation. A549 cells were treated with Dex, TNFα, or both Dex and TNFα, and mRNA levels were analyzed by qRT-PCR. All samples were compared with control (none) for statistical analysis. A, Dose response. Cells were treated for 6 h with the indicated concentrations of Dex, TNFα, or both Dex and TNFα. Results are plotted in log scale. B, Time course of treatment. Cells were treated with Dex, TNFα, or both Dex and TNFα for the indicated lengths of time. Results are plotted in log scale. C, Cells were treated for 6 h with the indicated concentrations of cortisol instead of Dex. D, Western blot analysis of AACT (serpinA3) protein levels. β-Actin is used as a loading control. E, Cells were pretreated with either vehicle or cycloheximide, followed by treatment with Dex, TNFα, or both Dex and TNFα. serpinA3 mRNA levels were analyzed by qRT-PCR. F, Cells were pretreated with either vehicle or actinomycin D, followed by treatment with Dex, TNFα, or both Dex and TNFα. serpinA3 mRNA levels were analyzed by qRT-PCR.
Because Dex is a synthetic glucocorticoid, we wished to elucidate whether the synergistic regulation of serpinA3 was a physiologically relevant phenomenon rather than a specific response to the synthetic glucocorticoid. Thus, we analyzed the effect of the naturally occurring human glucocorticoid cortisol on serpinA3 gene regulation. Cells were treated with cortisol in the presence or absence of TNFα, and the levels of serpinA3 mRNA were determined by qRT-PCR. serpinA3 was significantly up-regulated by a moderate concentration of cortisol (100 nm) (Fig. 4C). Importantly, the synergistic regulation of serpinA3 was also observed by cotreatment with cortisol and TNFα. This concentration of cortisol chosen appeared to be saturating, because there was no further regulation at higher concentrations (data not shown).
Given that the levels of serpinA3 mRNA are increased after treatment with Dex and TNFα, we wished to understand whether there was also a corresponding increase in the levels of SerpinA3 (AACT) protein. Thus, we performed Western blot analysis to analyze AACT protein levels after treatment with Dex and TNFα. AACT was increased in the presence of Dex and TNFα (Fig. 4D). Furthermore, this increase in protein was greater than that seen in cells treated with either compound alone. Because both the protein level and mRNA expression increased after Dex and TNFα treatment, we conclude that the induction of SerpinA3 by both glucocorticoids and TNFα is physiologically relevant.
After establishing that the increase in serpinA3 occurred at both the mRNA and protein level after treatment with Dex and TNFα, we wanted to ascertain whether the up-regulation of serpinA3 required new protein synthesis or affected mRNA stability. To address the issue and determine whether the up-regulation was a direct or indirect event, we inhibited new protein synthesis by treating the cells with cycloheximide. A549 cells were pretreated with cycloheximide for 30 min before treatment with Dex, TNFα, or both for 2 h. Cell lysates were analyzed for serpinA3 mRNA levels by qRT-PCR (Fig. 4E). Our results clearly demonstrated that cycloheximide did not inhibit serpinA3 mRNA regulation, suggesting that regulation of serpinA3 is directly regulated by proteins already present within the cell rather than requiring new protein synthesis.
Finally, to examine whether there was a change in the stability of serpinA3 mRNA, we inhibited new transcription by treating the cells with actinomycin D. Cells were pretreated with actinomycin D, to inhibit new RNA synthesis, for 30 min before treatment with Dex, TNFα, or both Dex and TNFα. In the presence of actinomycin D, levels of serpinA3 mRNA were at or below baseline levels (Fig. 4F). Based on this result, we conclude that the increase in serpinA3 mRNA and protein is likely due to new transcription of serpinA3 gene and not due to stabilization of preexisting mRNA.
SerpinA3 gene regulation requires GR
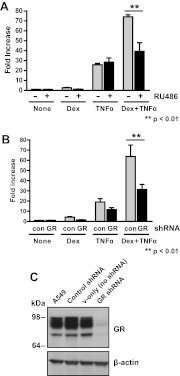
Dex is well known to directly bind GR to regulate gene expression, and because treatment with Dex induced serpinA3 expression, we hypothesized that GR was responsible for this regulation. To address this question, we determined whether the gene regulation was occurring through endogenous GR. For these experiments, A549 lung cells were pretreated with the GR antagonist RU486 along with Dex, TNFα, or both Dex and TNFα for 6 h, and the levels of serpinA3 mRNA were analyzed (Fig. 5A). RU486 significantly reduced the level serpinA3 mRNA in Dex-treated cells but not in TNFα-treated cells. serpinA3 mRNA levels were reduced by approximately 50% in the presence of Dex and RU486 or Dex + TNFα and RU486 (Fig. 5A). It is likely that the mRNA levels were not repressed completely, because RU486 is known to act as a partial agonist (30). Additionally, it may be possible that other factors that are unaffected by RU486, such as the TNFα receptor, are also required for serpinA3 regulation.
Fig. 5.
GR-dependent mechanism of action. A, A549 cells were treated with Dex, TNFα, or both Dex + TNFα for 6 h in conjunction with GR antagonist RU486, and mRNA was isolated. serpinA3 mRNA levels were analyzed by qRT-PCR. RU486 and control samples were compared in each treatment for the statistical analysis. B, A549 cells were treated with lentivirus containing shRNA for GR knockdown or control scrambled shRNA. A549 cells were treated with Dex, TNFα, or both Dex + TNFα for 6 h, and mRNA was isolated. serpinA3 mRNA levels were analyzed by qRT-PCR. Control and GR shRNA were compared in each treatment for the statistical analysis. C, Western blot analysis was performed on cells treated with lentivirus containing scrambled shRNA, vector only, or GR-specific shRNA to confirm reduction in GR protein levels. Con, Control.
To differentiate between RU486 agonist activities and alternative regulation by other factors, we reduced GR expression in these cells by employing shRNA. First, we created stable A549 cell lines transduced with lentiviruses containing shRNA to GR or a nonspecific scrambled shRNA. These cell lines were treated with Dex, TNFα, or both Dex and TNFα, RNA was isolated, and qRT-PCR was performed to determine the levels of serpinA3 mRNA (Fig. 5B). serpinA3 mRNA level was reduced by approximately 50% in GR knockdown cells when compared with the control shRNA cells. Thus, we conclude that GR is required for the induction of serpinA3, but other factors also appear to be required as well. GR protein levels (Fig. 5C) were reduced by at least 80% in GR shRNA-treated cells under our experimental conditions. Multiple bands seen for GR in this Western blot analysis reflect multiple GR isoforms (31) .
SerpinA3 gene regulation requires the TNFα receptor
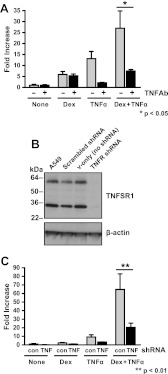
Our data suggest that GR was necessary for SerpinA3 up-regulation but that additional factors may also be involved in the regulation of this acute phase gene. In particular, we focused on TNFα, which regulated serpinA3 in our microarray data. To determine whether TNFα signaling was required for regulation of serpinA3, we treated cells with a blocking/neutralizing TNFα antibody, before treatment with Dex, TNFα, or both for 6 h (Fig. 6A). The TNFα antibody significantly reduced the level of serpinA3 regulation by TNFα but not Dex. Because the regulation of SerpinA3 appeared to be inhibited through blocking TNFα activity, we wanted to determine whether TNFα was signaling through the TNFα receptor (TNFSR1). To accomplish this goal, we next employed shRNA to reduce the level of TNFSR1 within cells. We established stable A549 cell lines transduced with lentiviruses containing shRNA to TNFSR1 or a nonspecific scrambled shRNA. TNFSR1 was reduced by approximately 90% in TNFSR1 shRNA-treated cells (Fig. 6B) under the conditions of our experiments. These cell lines were treated with Dex, TNFα, or both Dex and TNFα, RNA was isolated, and qRT-PCR was performed to determine the levels of serpinA3 mRNA (Fig. 6C). serpinA3 mRNA level was reduced by approximately 50% in cells lacking TNFSR1 compared with the control shRNA cells. Thus, we conclude that TNFSR1 is also required for the induction of serpinA3.
Fig. 6.
TNFR-dependent mechanism of action. A, A549 cells were treated with Dex, TNFα, or both Dex and TNFα in conjunction with TNFα antibody, and mRNA was isolated. serpinA3 mRNA levels were analyzed by qRT-PCR. TNFα antibody and control samples were compared in each treatment for the statistical analysis. B, Western blot analysis was performed on cells treated with lentivirus containing scrambled shRNA, vector only, or TNFR-specific shRNA to confirm reduction in TNFSR1A protein levels. C, A549 cells were treated with lentivirus containing shRNA for TNFR knockdown or control scrambled shRNA. A549 cells were treated with Dex, TNFα, or both Dex + TNFα for 6 h, and mRNA was isolated. serpinA3 mRNA levels were analyzed by qRT-PCR. Control and TNFR shRNA were compared in each treatment for the statistical analysis.
In vivo responses to Dex and TNFα treatment
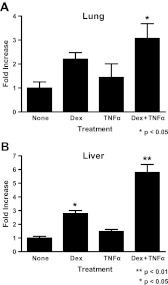
After observing an increase in serpinA3 mRNA and protein in the A549 cell culture model, we wished to determine whether the increase in serpinA3 would also occur in vivo in a physiologically relevant manner. To address this question, we treated adrenalectomized mice with Dex (1 mg/kg), TNFα (1.5 μg), or both Dex and TNFα for 6 h and analyzed SerpinA3 mRNA levels in different tissues by qRT-PCR. We first analyzed SerpinA3 levels in the lung (Fig. 7A) and then in the liver (Fig. 7B), because serpinA3 is highly produced in this tissue. In both tissues, we observed an additive increase in SerpinA3 mRNA levels when animals were treated with both Dex and TNFα. This increase in mRNA was not as large as expected given our results in the cell culture model, perhaps reflecting the metabolism of the steroid in this in vivo system.
Fig. 7.
In vivo responses to Dex and TNFα treatment. Mice were injected ip with 1 mg/kg Dex, 60 μg/kg TNFα, or both Dex and TNFα, and after 6 h, animals were killed, and mRNA was isolated from the indicated tissues. serpinA3 mRNA levels were analyzed by qRT-PCR. A, Lung. B, Liver.
GR ChIP analysis of serpinA3 transcriptional start site
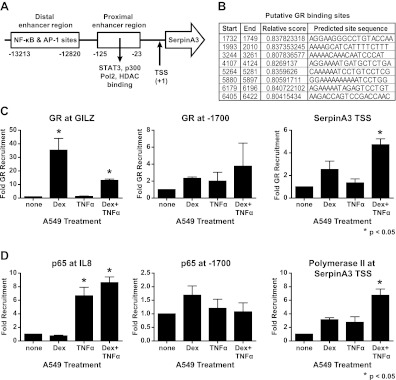
Because our data suggested that serpinA3 is directly regulated by GR, we next performed ChIP assays on the serpinA3 promoter to understand the mechanism of serpinA3 regulation. The SerpinA3 promoter was analyzed based on what was previously known of this region (Fig. 8A) (32, 33). Several putative GR binding sites upstream of the SerpinA3 transcriptional start site were identified by bioinformatics (Fig. 8B). We first verified that our GR pull-down was successful by confirming that GR could be recruited to a known region of the Gilz promoter (Fig. 8C) and then focused our attention on the SerpinA3 transcriptional start site, because ChIP analysis of nearby putative glucocorticoid response element were unsuccessful (Fig. 8C and data not shown). Primers covering the serpinA3 transcriptional start site were used to amplify serpinA3 DNA bound to GR. Importantly, we determined that GR was recruited to the SerpinA3 transcriptional start site (Fig. 8C) and probably reflects an interaction at the TATA box (33). The binding increased slightly in the presence of Dex or TNFα but was most prominently enhanced by the combination of Dex and TNFα. Based on these data, we conclude that GR is recruited to this region of the gene and is not constitutively present, because we were unable to detect binding after a shorter (30 min) treatment (data not shown). We confirmed that p65 was recruited to the IL-8 promoter in the presence of TNFα (Fig. 8D). However, p65 was not recruited to putative glucocorticoid response element (Fig. 8D and data not shown). Finally, we used RNA polymerase II binding as a positive control for GR recruitment to the SerpinA3 transcriptional start site (Fig. 8D). RNA polymerase II was recruited to a small extent to the SerpinA3 transcriptional start site in cells treated with either Dex or TNFα compared with untreated cells. This recruitment was significantly increased by the combination of Dex and TNFα.
Fig. 8.
ChIP of serpinA3 promoter. A549 cells were treated with 10 nm of Dex, 10 μg/ml TNFα, or both Dex and TNFα for 90 min. ChIP assays were performed on the indicated regions. A, Schematic of GR promoter region used for ChIP assay. B, Schematic of putative GR binding sites. C, ChIP assay using GR at the promoter region of Gilz, a site 1700 bp upstream of the SerpinA3 transcriptional start site, and at the transcriptional start site of serpinA3. D, ChIP assay using the p65 subunit of nuclear factor κB (NF-κB) at the promoter region of IL-8, at a site 1700 bp upsteam of the SerpinA3 transcriptional start site, and polymerase II at the transcriptional start site of SerpinA3. TSS, Transcriptional start site.
Discussion
Our studies define the set of genes regulated by the synthetic glucocorticoid Dex and the cytokine TNFα in A549 lung cells. Previous dogma suggested that Dex and TNFα primarily act in an opposing manner. However, we have now identified many genes coregulated by glucocorticoids and TNFα, indicating that glucocorticoids and cytokines can both regulate genes involved in inflammation (34–36). Microarray studies revealed the surprising result that the many of the genes regulated by both Dex and TNFα were coregulated rather than anticorrelated. The genes significantly up-regulated by treatment with Dex plus TNFα beyond that expected from treatment of either compound alone were particularly interesting. Not only were these genes being coregulated by two compounds previously thought to have opposing effects, but the synergistic up-regulation indicated that their signaling pathways were interacting in novel ways. It is possible that interaction between these signaling pathways may be responsible for some of the negative side effects of long-term glucocorticoid usage.
Inflammation resulting in an increase in TNFα and other cytokines is often treated in patients with a glucocorticoid such as Dex. Thus, genes regulated only in the presence of both Dex and TNFα may have important clinical implications. It has previously been shown that Dex treatment synergizes with TNFα to increase sodium absorption in the rat colon (37), suggesting that coregulation by glucocorticoids and cytokines occurs in vivo in other systems. Other studies have shown that SerpinA3 mRNA and protein is enhanced in cell culture after glucocorticoid and cytokine cotreatments in human astrocytoma cells and hepatoma cells (26, 38). However, this early work simply noted the synergistic regulation of SerpinA3 in the context of performing other experiments and did not characterize the mechanism of regulation. In this study, we characterized the increase in the synergistically regulated gene SerpinA3 through dose-response and time-course studies. Furthermore, we have shown that the SerpinA3 increase after Dex and TNFα treatment occurs in vivo, suggesting that this increase may have important physiological relevance. We also demonstrate that this regulation is direct, rapid, does not require new protein synthesis, and was not due to stabilization of mRNA. Finally, we have defined the mechanism of this regulation by determining that GR is significantly recruited to the SerpinA3 transcriptional start site by the combination of Dex plus TNFα.
We were surprised by our unexpected discovery that there was a large number of genes coregulated by Dex and TNFα. One potential explanation is that cross talk between glucocorticoids and proinflammatory cytokines may occur when the DNA near proinflammatory genes is more accessible. It is possible that when GR binds to the DNA, it changes local chromatin structure (39), and TNFα or other cytokines may promote local unwinding of DNA, allowing GR to bind and glucocorticoids to mediate their effects.
Overall, we demonstrate that there may be novel interactions and gene regulation by glucocorticoids and cytokines. This work has important physiological significance, because treatment with glucocorticoids is standard for many inflammatory diseases, and long-term glucocorticoid use has many negative side effects. Our discovery that coregulation of SerpinA3 by Dex and TNFα also occurred in vivo extends our results from cell culture to a variety of tissues and thus highlights the importance of our findings. Identification of genes regulated by both Dex and TNFα has also provided new information toward identification of signaling pathways that are involved after treatment with glucocorticoids. Systems biology analyses of these signaling pathways may help to discern why we see such a wide range of side effects from glucocorticoid treatment and why different people may respond so differently to a given course of treatment. Although we determined that many of the genes coregulated by Dex and TNFα are involved in inflammation, many of the genes regulated by both compounds are biologically diverse and perform functions currently unrelated to inflammation or their functions are unknown. Analysis of these genes and the mechanism by which they are regulated may provide clues to how to best mitigate the negative side effects from long-term glucocorticoid usage. Further study is needed to understand how and why glucocorticoids regulate proinflammatory genes.
Supplementary Material
Acknowledgments
We thank Robert Oakley and John Busillo for critical input to this manuscript.
Present address for E.A.L.: Prairie State College, 202 S. Halsted St., Chicago Heights, Illinois 60411.
Present address for A.J.G.-B.: Department of Anatomy and Physiology, KS State University, Manhattan, Kansas 66506.
This work was supported by National Institutes of Health intramural funding.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- AACT
- α1-Antichymotrypsin
- ChIP
- chromatin immunoprecipitation
- COPD
- chronic obstructive pulmonary disorder
- Dex
- dexamethasone
- GR
- glucocorticoid receptor
- IPA
- ingenuity pathway analysis
- q
- quantitative
- shRNA
- short hairpin RNA
- TNFSR1
- TNFα soluble receptor 1.
References
- 1. Revollo JR, Cidlowski JA. 2009. Mechanisms generating diversity in glucocorticoid receptor signaling. Ann NY Acad Sci 1179:167–178 [DOI] [PubMed] [Google Scholar]
- 2. Baschant U, Tuckermann J. 2010. The role of the glucocorticoid receptor in inflammation and immunity. J Steroid Biochem Mol Biol 120:69–75 [DOI] [PubMed] [Google Scholar]
- 3. Magnussen H, Watz H. 2009. Systemic inflammation in chronic obstructive pulmonary disease and asthma: relation with comorbidities. Proc Am Thorac Soc 6:648–651 [DOI] [PubMed] [Google Scholar]
- 4. Ross KR, Chmiel JF, Konstan MW. 2009. The role of inhaled corticosteroids in the management of cystic fibrosis. Paediatr Drugs 11:101–113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lane NE, Yao W. 2010. Glucocorticoid-induced bone fragility. Ann NY Acad Sci 1192:81–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Brown ES. 2009. Effects of glucocorticoids on mood, memory, and the hippocampus. Treatment and preventive therapy. Ann NY Acad Sci 1179:41–55 [DOI] [PubMed] [Google Scholar]
- 7. Gonzalez AV, Li G, Suissa S, Ernst P. 2010. Risk of glaucoma in elderly patients treated with inhaled corticosteroids for chronic airflow obstruction. Pulm Pharmacol Ther 23:65–70 [DOI] [PubMed] [Google Scholar]
- 8. Huscher D, Thiele K, Gromnica-Ihle E, Hein G, Demary W, Dreher R, Zink A, Buttgereit F. 2009. Dose-related patterns of glucocorticoid-induced side effects. Ann Rheum Dis 68:1119–1124 [DOI] [PubMed] [Google Scholar]
- 9. McDonough AK, Curtis JR, Saag KG. 2008. The epidemiology of glucocorticoid-associated adverse events. Curr Opin Rheumatol 20:131–137 [DOI] [PubMed] [Google Scholar]
- 10. Hermoso MA, Matsuguchi T, Smoak K, Cidlowski JA. 2004. Glucocorticoids and tumor necrosis factor α cooperatively regulate toll-like receptor 2 gene expression. Mol Cell Biol 24:4743–4756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Webster JC, Huber RM, Hanson RL, Collier PM, Haws TF, Mills JK, Burn TC, Allegretto EA. 2002. Dexamethasone and tumor necrosis factor-α act together to induce the cellular inhibitor of apoptosis-2 gene and prevent apoptosis in a variety of cell types. Endocrinology 143:3866–3874 [DOI] [PubMed] [Google Scholar]
- 12. Ishii T, Matsuse T, Teramoto S, Matsui H, Hosoi T, Fukuchi Y, Ouchi Y. 2000. Association between α-1-antichymotrypsin polymorphism and susceptibility to chronic obstructive pulmonary disease. Eur J Clin Invest 30:543–548 [DOI] [PubMed] [Google Scholar]
- 13. Sandford AJ, Chagani T, Weir TD, Paré PD. 1998. α1-antichymotrypsin mutations in patients with chronic obstructive pulmonary disease. Dis Markers 13:257–260 [DOI] [PubMed] [Google Scholar]
- 14. Baker C, Belbin O, Kalsheker N, Morgan K. 2007. SERPINA3 (aka α-1-antichymotrypsin). Front Biosci 12:2821–2835 [DOI] [PubMed] [Google Scholar]
- 15. Cichy J, Potempa J, Chawla RK, Travis J. 1995. Stimulatory effect of inflammatory cytokines on α1-antichymotrypsin expression in human lung-derived epithelial cells. J Clin Invest 95:2729–2733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Yanai I, Benjamin H, Shmoish M, Chalifa-Caspi V, Shklar M, Ophir R, Bar-Even A, Horn-Saban S, Safran M, Domany E, Lancet D, Shmueli O. 2005. Genome-wide midrange transcription profiles reveal expression level relationships in human tissue specification. Bioinformatics 21:650–659 [DOI] [PubMed] [Google Scholar]
- 17. Wang YC, Liu HC, Liu TY, Hong CJ, Tsai SJ. 2001. Genetic association analysis of α-1-antichymotrypsin polymorphism in Parkinson's disease. Eur Neurol 45:254–256 [DOI] [PubMed] [Google Scholar]
- 18. Lin JJ, Yueh KC, Chang CY, Chen CH, Lin SZ. 2004. The homozygote AA genotype of the α1-antichymotrypsin gene may confer protection against early-onset Parkinson's disease in women. Parkinsonism Relat Disord 10:469–473 [DOI] [PubMed] [Google Scholar]
- 19. Matsubara E, Amari M, Shoji M, Harigaya Y, Yamaguchi H, Okamoto K, Hirai S. 1989. Serum concentration of α1-antichymotrypsin is elevated in patients with senile dementia of the Alzheimer type. Prog Clin Biol Res 317:707–714 [PubMed] [Google Scholar]
- 20. Matsubara E, Hirai S, Amari M, Shoji M, Yamaguchi H, Okamoto K, Ishiguro K, Harigaya Y, Wakabayashi K. 1990. α1-Antichymotrypsin as a possible biochemical marker for Alzheimer-type dementia. Ann Neurol 28:561–567 [DOI] [PubMed] [Google Scholar]
- 21. Abraham CR. 2001. Reactive astrocytes and α1-antichymotrypsin in Alzheimer's disease. Neurobiol Aging 22:931–936 [DOI] [PubMed] [Google Scholar]
- 22. Muñoz E, Obach V, Oliva R, Martí MJ, Ezquerra M, Pastor P, Ballesta F, Tolosa E. 1999. α1-Antichymotrypsin gene polymorphism and susceptibility to Parkinson's disease. Neurology 52:297–301 [DOI] [PubMed] [Google Scholar]
- 23. Wang J, Zhao CY, Si YM, Liu ZL, Chen B, Yu L. 2002. ACT and UCH-L1 polymorphisms in Parkinson's disease and age of onset. Mov Disord 17:767–771 [DOI] [PubMed] [Google Scholar]
- 24. Tang G, Xie H, Xu L, Hao Y, Lin D, Ren D. 2002. Genetic study of apolipoprotein E gene, α-1 antichymotrypsin gene in sporadic Parkinson disease. Am J Med Genet 114:446–449 [DOI] [PubMed] [Google Scholar]
- 25. O'Riordain MG, Ross JA, Fearon KC, Maingay J, Farouk M, Garden OJ, Carter DC. 1995. Insulin and counterregulatory hormones influence acute-phase protein production in human hepatocytes. Am J Physiol 269:E323–E330 [DOI] [PubMed] [Google Scholar]
- 26. Machein U, Lieb K, Hüll M, Fiebich BL. 1995. IL-1β and TNFα, but not IL-6, induce α1-antichymotrypsin expression in the human astrocytoma cell line U373 MG. Neuroreport 6:2283–2286 [DOI] [PubMed] [Google Scholar]
- 27. Cidlowski JA, Bellingham DL, Powell-Oliver FE, Lubahn DB, Sar M. 1990. Novel antipeptide antibodies to the human glucocorticoid receptor: recognition of multiple receptor forms in vitro and distinct localization of cytoplasmic and nuclear receptors. Mol Endocrinol 4:1427–1437 [DOI] [PubMed] [Google Scholar]
- 28. Gross KL, Oakley RH, Scoltock AB, Jewell CM, Cidlowski JA. 2011. Glucocorticoid receptor α isoform-selective regulation of antiapoptotic genes in osteosarcoma cells: a new mechanism for glucocorticoid resistance. Mol Endocrinol 25:1087–1099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Galliher-Beckley AJ, Williams JG, Cidlowski JA. 2011. Ligand-independent phosphorylation of the glucocorticoid receptor integrates cellular stress pathways with nuclear receptor signaling. Mol Cell Biol 31:4663–4675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Fryer CJ, Kinyamu HK, Rogatsky I, Garabedian MJ, Archer TK. 2000. Selective activation of the glucocorticoid receptor by steroid antagonists in human breast cancer and osteosarcoma cells. J Biol Chem 275:17771–17777 [DOI] [PubMed] [Google Scholar]
- 31. Lu NZ, Cidlowski JA. 2005. Translational regulatory mechanisms generate N-terminal glucocorticoid receptor isoforms with unique transcriptional target genes. Mol Cell 18:331–342 [DOI] [PubMed] [Google Scholar]
- 32. Kalsheker N, Morley S, Morgan K. 2002. Gene regulation of the serine proteinase inhibitors α1-antitrypsin and α1-antichymotrypsin. Biochem Soc Trans 30:93–98 [DOI] [PubMed] [Google Scholar]
- 33. Kumar R, Volk DE, Li J, Lee JC, Gorenstein DG, Thompson EB. 2004. TATA box binding protein induces structure in the recombinant glucocorticoid receptor AF1 domain. Proc Natl Acad Sci USA 101:16425–16430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Langlais D, Couture C, Balsalobre A, Drouin J. 2008. Regulatory network analyses reveal genome-wide potentiation of LIF signaling by glucocorticoids and define an innate cell defense response. PLoS Genet 4:e1000224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Barnes PJ. 1998. Anti-inflammatory actions of glucocorticoids: molecular mechanisms. Clin Sci 94:557–572 [DOI] [PubMed] [Google Scholar]
- 36. Liberman AC, Druker J, Perone MJ, Arzt E. 2007. Glucocorticoids in the regulation of transcription factors that control cytokine synthesis. Cytokine Growth Factor Rev 18:45–56 [DOI] [PubMed] [Google Scholar]
- 37. Bergann T, Zeissig S, Fromm A, Richter JF, Fromm M, Schulzke JD. 2009. Glucocorticoids and tumor necrosis factor-α synergize to induce absorption by the epithelial sodium channel in the colon. Gastroenterology 136:933–942 [DOI] [PubMed] [Google Scholar]
- 38. Kasza A, Bugno M, Koj A. 1994. Long-term culture of HepG2 hepatoma cells as a model for liver acute phase response during chronic inflammation. Effects of interleukin-6, dexamethasone and retinoic acid. Biol Chem Hoppe Seyler 375:779–783 [DOI] [PubMed] [Google Scholar]
- 39. Adcock IM, Caramori G. 2001. Cross-talk between pro-inflammatory transcription factors and glucocorticoids. Immunol Cell Biol 79:376–384 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.