Abstract
Congenitally athymic (nude) female mice show severe ovarian dysgenesis after puberty, which seems to be consequential to a number of neuroendocrine derangements described in these mutants. Thus, considerable evidence suggests that thymulin, a thymic peptide, may be involved in thymus-pituitary communication. In order to clarify the relevance of thymulin for the maturation of the female reproductive system, we assessed at hypothalamic, pituitary, ovarian, and uterine level the preventive action of neonatal thymulin gene therapy (NTGT) on the changes that typically occur after puberty in congenitally athymic female mice. We injected (im) an adenoviral vector harboring a synthetic DNA sequence encoding a biologically active analog of thymulin, methionine-serum thymic factor, in newborn nude mice (which are thymulin deficient) and killed the animals at 70–71 d of age. NTGT in the athymic mice restored the serum thymulin levels. Morphometric analysis revealed that athymic nudes have reduced numbers of brain GnRH neurons and pituitary gonadotropic cells as compared with heterozygous controls. NTGT prevented these changes and also rescued the premature ovarian failure phenotype typically observed in athymic nude mice (marked reduction in the number of antral follicles and corpora lutea, increase in atretic follicles). Serum estrogen, but not progesterone, levels were low in athymic nudes, a reduction that was partially prevented by NTGT. Little to no morphological changes were observed in the endometrium of female nudes. The delay in the age of vaginal opening that occurs in athymic nudes was significantly prevented by NTGT. Our results suggest that thymulin plays a relevant physiologic role in the thymus-hypothalamo-pituitary-gonadal axis.
During perinatal life, the integrity of the thymus is necessary for a proper maturation of the pituitary-gonadal axis as revealed by the endocrine alterations caused by neonatal thymectomy or congenital absence of the thymus in mice. In effect, congenitally athymic (nude) female mice show significantly reduced levels of circulating gonadotropins, a fact that seems to be causally related to a number of reproductive derangements described in these mutants (1). In homozygous (nu/nu) females, the times of vaginal opening and first ovulation are delayed (2), fertility is reduced (3), and follicular atresia is increased, such that premature ovarian failure results (3, 4). Similar abnormalities result from neonatal thymectomy in normal female mice (5, 6). Neonatal thymus grafting prevents the consequences of the lack of thymus in the above models (2).
Thymulin is a well-characterized thymic factor discovered, purified, and sequenced during the 1970s (7). It consists of a biologically inactive nonapeptide component, FTS (facteur thymique serique, an acronym for serum thymus factor in French), coupled in an equimolecular ratio to the ion zinc (8), which confers biological activity to the molecule (9).
Thymulin has been shown to stimulate gonadotropin release from dispersed rat pituitary cells in a dose-related manner, an effect that declined with the age of the pituitary cell donors (10). The gonadotropin-releasing activity of thymulin has been also shown in rat pituitary fragments and in primary rat pituitary cell cultures (11, 12). There is evidence suggesting that thymulin plays a role in the regulation of female puberty (13) and that it may exert a modulatory action on gonadotropin-induced steroidogenesis in the ovary (14) and testis (15).
We have previously shown that serum thymulin immunoneutralization from birth to postnatal day (P)8–P9 in normal mice induces a significant fall in serum gonadotropin levels at puberty. Conversely, in the same study, it was demonstrated that neonatal thymulin gene therapy (NTGT) in nude female mice (nude mice have undetectable circulating levels of thymulin) elicited long-term restoration of serum thymulin in these mutants. This treatment, which used a synthetic gene encoding the FTS analog methionine-FTS (metFTS) (5′-ATGCAGGCCAAGTCGCAGGGGGGGTCG-AACTAGTAG-3′), was able to prevent the deficits in serum LH and FSH that typically appear in adult female nudes (16). In the present study, we assessed, at hypothalamic, pituitary, ovarian, and uterine level, the preventive action of NTGT on the changes that typically occur after puberty in congenitally athymic female mice.
Materials and Methods
Animals and experimental procedures
The offspring of NIH homozygous (nu/nu) nude male and heterozygous (nu/+) female mice were used. The parent mice were purchased from the Animal Core Facility of the Ezeiza Atomic Center (Ezeiza, Argentina). All mice were maintained on a γ-irradiate chow diet and sterilized water. Animals had free access to food and water and were kept at 22 C with a 12-h light, 12-h dark cycle. All animal experiments were done following the Animal Welfare Guidelines of the National Institutes of Health (Instituto de Investigaciones Bioquímicas de La Plata's Animal Welfare Assurance No. A5647-01).
On P1, each experimental pup (both nu/nu and nu/+) received a single bilateral im (hindlegs) injection of 108 plaque forming units recombinant adenoviral (RAd)-FTS or RAd-green fluorescent protein (GFP) (a control vector; see below) in 10 μl of vehicle (5 μl per side). On P13, three mice from each group were bled for thymulin determination. Beginning on P 22, animals were daily checked for vaginal opening.
On P70–P71, mice were bled and immediately killed by cervical dislocation or perfusion with fixative as appropriate. The brain, pituitary gland, ovaries, and uterus were removed and stored in fixative solution for morphologic assessment.
Adenoviral vectors
Recombinant adenoviral-FTS
A DNA sequence (5′-ATGCAGGCCAAGTCGCAGGGGGGGTCGAACTAGT AG-3′) coding for the biologically active thymulin analog metFTS, here referred to as synthetic gene for thymulin, was constructed as previously reported (17). A RAd vector harboring the synthetic gene for thymulin was constructed by a variant of the two-plasmid method (18) employing the AdMax plasmid kit (Microbix, Toronto, Canada). This kit uses a shuttle plasmid (pDC515) containing a FRT recognition site for the yeast FLP recombinase. This cassette is flanked by sequences of the adenovirus type 5 (Ad5) E1 region. The second plasmid of the kit, the genomic plasmid pBHGfrt(del)E1,3 FLP, consists of the entire genome of Ad5, containing deletions in the regions E1 and E3. Upstream the E1 deletion, the genomic plasmid contains an expression cassette for the gene of yeast FLP recombinase, and immediately downstream the E1 deletion, a FRT recognition site has been inserted. Once the thymulin synthetic gene was inserted into the shuttle, both plasmids were cotransfected into HEK293 cells. In cotransfected HEK293 cells, FLP recombinase is readily expressed and efficiently catalyzes the site-directed recombination of the expression cassette of pDC515-metFTS into the left end of pBHGfrt(del)E1,3 FLP, thus generating the genome of the desired RAd vector, RAd-FTS (Fig. 1A). The newly generated RAd was rescued from HEK293 cell lysates and plaque purified. It was further purified by ultracentrifugation in CsCl gradient and titrated by a serial dilution plaque assay.
Fig. 1.
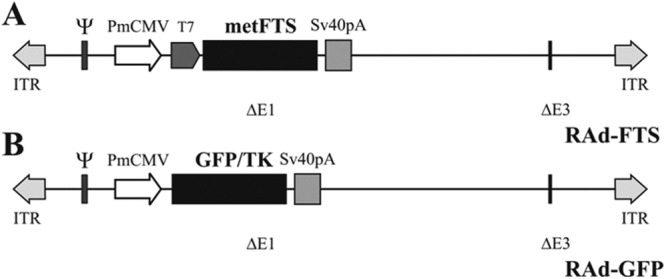
Recombinant adenovectors expressing metFTS (thymulin) and GFP. A, A DNA sequence coding for the thymulin analog metFTS was cloned in a first generation adenovector backbone, thus generating RAd-FTS. B, A DNA sequence encoding a chimeric variant of enhanced GFP (GFP/TK) was cloned in an adenovector thus generating RAd-GFP, a control vector. PmCMV, Mouse cytomegalovirus promoter; T7, phage T7 promoter primer binding site; ITR, inverted terminal repeats; ΔE1 and ΔE3, deletions in the Ad5 genome; Sv40pA, simian virus 40 polyadenylation signal; ψ, packaging signal.
Recombinant adenoviral-GFP
An adenoviral vector termed RAd-GFP was also constructed following the general procedures outlined above and was used as a control vector (Fig. 1B). The vector harbors a DNA sequence encoding a chimeric variant of the Aequorea victoria-enhanced GFP fused to HSV-1 thymidine kinase (GFP/TK), a kind gift from Jacques Galipeau (McGill University, Montreal, Canada).
Hormone assays
Thymulin bioassay
Biologically active thymulin was measured in serum by a rosette bioassay described in detail elsewhere (19). This method is based on the ability of thymulin to restore the inhibitory effect of azathioprine on rosette formation in spleen cells from thymectomized mice. The inhibitory activity of samples was compared with that of a standard curve using synthetic thymulin. Serum values were expressed as femtograms per milliliter bioactive thymulin. This bioassay has been previously validated against an ELISA for thymulin (17).
Steroid hormone assays
Serum β-estradiol (E2) and progesterone (P4) levels were measured by RIA using a commercial kit (Coat-A-Count; Diagnostic Products Corp., Los Angeles, CA).
Histology and immunohistochemistry
Brain GnRH immunofluorescence and morphometry
Animals were placed under deep anesthesia and intracardially perfused with cold phosphate-buffered paraformaldehyde 4% (pH 7.4) fixative. Each brain was removed and serially cut into 30-μm-thick coronal sections on a freezing microtome. For each brain, one out of every four serial sections was selected to obtain four sets of noncontiguous serial sections spanning the whole brain. For counting purposes, each set was considered as representative of the whole brain taken between coordinates 2.22 up to −2.46 from the bregma (20). For each animal, one set of brain sections was processed for immunofluorescence using the free floating technique (21). Briefly, sections were incubated for 45 min with a 1:3000 dilution of anti-GnRH monoclonal antibody LHR 13 (Institute for Molecular and Cellular Regulation, Maebashi, Japan), washed twice with PBS, and incubated for 45 min with a 1:300 dilution of an Alexa Fluor 488 antimouse Fc (Invitrogen BA, Buenos Aires, Argentina). Images were captured using an Olympus DP70 digital camera attached to an Olympus BX51 microscope (Olympus, Tokyo, Japan) and were analyzed using the ImagePro Plus version 5.1 image analysis software (Media Cybernetics, Silver Spring, MA). GnRH immunoreactive perikarya were counted. The distribution and density of GnRH fibers were also recorded.
Anterior pituitary immunohistochemistry
Immunohistochemical analysis was used to evaluate the effects of treatments on the population of luteotrope and folliculotrope cells in pituitary tissue.
Histological and histomorphometric assessment of ovaries and uterus
Ovaries and uteri were removed, fixed in 4% formaldehyde, and embedded in paraffin. Serial ovarian and transversal uterine horn 4-μm-thick sections were stained with hematoxylin and eosin. Micrographs of ovarian and uterine sections were taken with a digital camera, and corresponding images were analyzed using the ImagePro Plus software. The number of antral follicles, corpora lutea, and atretic follicles per square millimeter was determined using 20 images per animal taken with a ×10 objective. The height of the uterine epithelium was assessed counting 100 cells in a total of 10 images per uterus taken with a ×40 objective.
Statistical analysis
Data are expressed as mean ± sem, unless otherwise indicated. Statistical comparisons among experimental groups were performed by ANOVA followed by the Dunnett test when the ANOVA was significant. For serum E2 data, the Tukey test was used. Differences with a P > 0.05 were considered nonsignificant.
Results
Restorative effect of RAd-FTS administration on serum thymulin in nude mice
A single neonatal im injection of RAd-FTS, but not RAd-GFP (a control vector), increased the circulating levels of biologically active thymulin in both heterozygous and homozygous nude mice tested at 70–71 d of age (Table 1). At P13, the RAd-FTS-treated nu/nu mice achieved serum thymulin levels only slightly lower than those of control nu/+ animals, whereas RAd-FTS-treated heterozygous mice showed thymulin levels comparable with those of control counterparts.
Table 1.
Serum thymulin (fg/ml) in experimental and control nude mice
| Experimental group | nu/+ | nu/nu | ||
|---|---|---|---|---|
| Age (days) | RAd-GFP | RAd-FTS | RAd-GFP | RAd-FTS |
| 13 | 150 ± 30 (3)a | 171 ± 43 (3)a | 8 ± 1.2 (3) | 107 ± 21(3)a |
| 70–71 | 117 ± 11 (8)a | 427 ± 127 (8)a | 8.7 ± 1.6 (8) | 256 ± 81 (8)a |
Data are expressed as mean ± sem. Numbers in parentheses represent the N value per group.
Significant differences vs. RAd-GFP nu/nu; P < 0.001.
Preventive action of NTGT on GnRH-producing neurons and pituitary gonadotrophic cells in nude mice
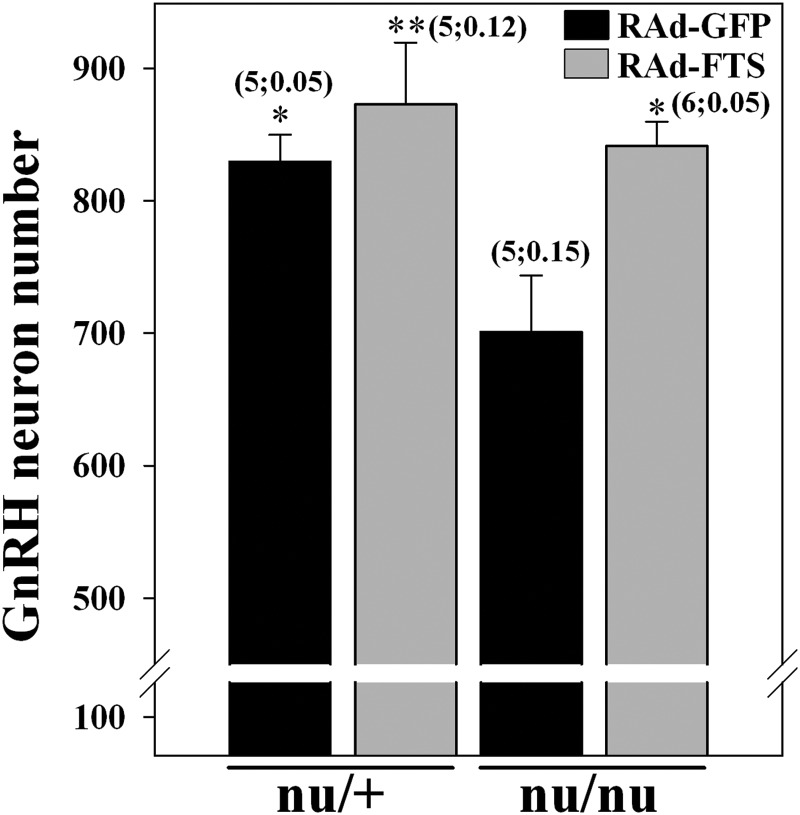
Congenital athymia induced a significant (P < 0.05) reduction in the number of brain GnRH-producing neurons, a phenotype rescued by NTGT (Fig. 2). This preventive action was clear for GnRH neuron bodies located mainly in the preoptic and anterior hypothalamic area. The fiber density of GnRH-producing neurons in the median eminence and its vicinity was similar in thymulin gene-treated athymic animals compared with heterozygous controls (Supplemental Fig. 1, published on The Endocrine Society's Journals Online web site at http://endo.endojournals.org). NTGT prevented the occurrence of low numbers of anterior pituitary luteotropic and folliculotropic cells typically observed in adult athymic female nudes (Supplemental Fig. 2).
Fig. 2.
GnRH-producing neuron numbers in the brain of control and experimental hetero and homozygous nude females. GnRH perikarya were counted in one every four coronal sections of the whole brain, and the sum of all counted sections per mouse was multiplied by 4 (thus, values represent an estimate of total brain GnRH-producing neurons). Numbers above columns are in the format (N;CV) and represent the N value and the coefficient of variation per group, respectively. Asterisks refer to differences vs. corresponding control nu/nu; **, P < 0.01; *, P < 0.05.
NTGT prevents ovarian dysgenesis in nude mice
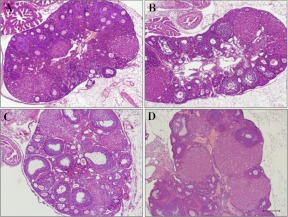
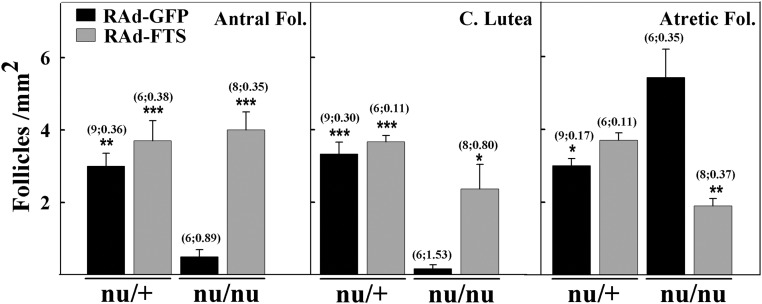
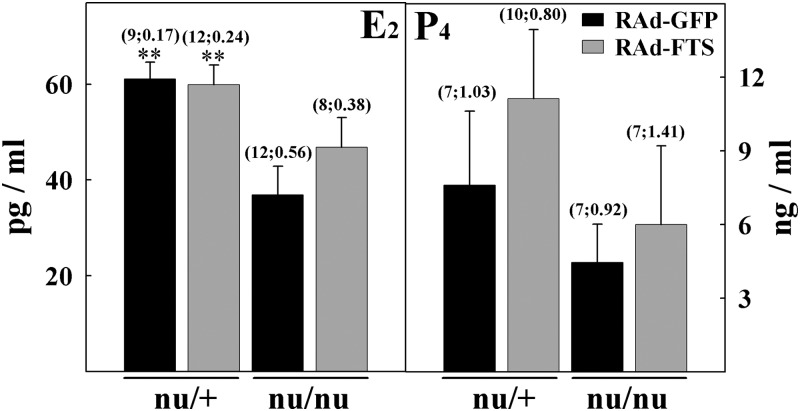
Heterozygous nudes had normal ovaries showing follicles in all developmental stages as well as normal corpora lutea. As expected, control homozygous nude females displayed multiple ovarian anomalies, characterized by reduced numbers of antral (secondary and tertiary) follicles (P < 0.01) and corpora lutea (P < 0.001) as well as increased numbers of atretic follicles (P < 0.05), compared with normal age-matched counterparts (Figs. 3 and 4). Preovulatory follicles were not observed in athymic controls, but there were numerous follicles displaying development of the granulosa cell layer containing a substantial number of dead cells. Most of these follicles lacked oocytes and were classified as atretic. Lymphocyte infiltration was observed neither in the ovaries of homozygous nor heterozygous nudes whether control or RAd-FTS treated (Fig. 3). NTGT prevented the incidence of ovarian alterations in homozygous nude females (Figs. 3D and 4). The ovaries of treated nudes showed a number of developing follicles, corpora lutea, and atretic follicles comparable with that of control heterozygous nudes. The number of primary follicles was unaffected by athymia or thymulin gene therapy (data not shown). Low serum levels of estrogen (P < 0.01) were observed in control nu/nu females but not in nudes submitted to NTGT (Fig. 5). P4 levels were not significantly different in athymic nudes and heterozygous mice and were not affected by NTGT (Fig. 5).
Fig. 3.
Effect of thymulin gene therapy on ovarian morphology in nude mice. H&E-stained sections of ovaries from nu/+ (A) and nu/nu (C) mice treated with the control vector (RAd-GFP) and nu/+ (B) and nu/nu (D) mice submitted to NTGT. Scale bar, 200 μm.
Fig. 4.
Histomorphometric assessment of ovaries from control and experimental nude mice. The number of antral (secondary and tertiary) and atretic follicles (Fol.) as well as corpora lutea was assessed in the ovaries from nu/+ and nu/nu mice treated with the control vector (RAd-GFP) and nu/+ and nu/nu mice submitted to NTGT. Asterisks refer to differences vs. corresponding control nu/nu; ***, P < 0.001; **, P < 0.01; *, P < 0.05. Other details are as in Fig. 2.
Fig. 5.
Serum E2 and P4 levels in control and experimental nude mice. Steroids were measured in the serum of RAd-GFP- and RAd-FTS-treated heterozygous and homozygous female nude mice. Asterisks refer to differences vs. corresponding control nu/+; **, P < 0.01. Serum E2 levels in metFTS gene-treated nu/nu and control nu/+ mice did not differ significantly (Tukey test). Other details are as in Fig. 2.
Effects of thymulin gene therapy on uterine morphology in nude mice
No inflammatory changes in the uteri of control nude mice were detected (Supplemental Fig. 3). A trend toward decreased (P = 0.09) epithelial cell height was observed in the endometrium of homozygous female nudes, although it was not statistically significant (Supplemental Figs. 3 and 4). The epithelial height of RAd-FTS-treated athymic nudes was comparable with that of heterozygous animals.
NTGT and puberty in nude female mice
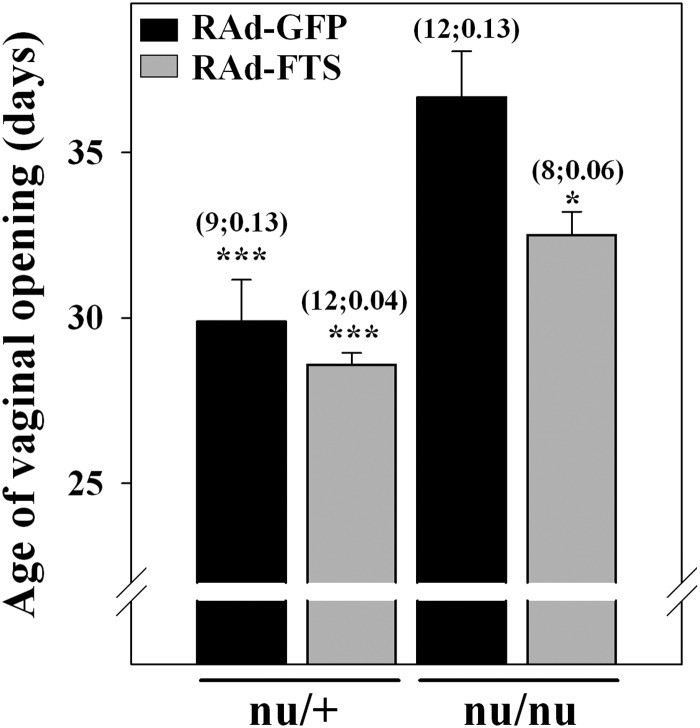
As expected, homozygous female nudes exhibited a significant (P < 0.001) delay in the age of vaginal opening. NTGT significantly (P < 0.05) prevented this delay (Fig. 6). Nevertheless, the age of vaginal opening in RAd-FTS-treated athymic nudes still showed a slight delay compared with control heterozygous mice.
Fig. 6.
Effect of thymulin gene therapy on the age of vaginal opening in nude mice. The age of vaginal opening was assessed in RAd-GFP- and RAd-FTS-treated heterozygous and homozygous nude mice. Asterisks refer to differences from corresponding control nu/nu; ***, P < 0.001; *, P < 0.05. Other details are as in Fig. 2.
Discussion
Thymulin is probably the best characterized of all putative thymic hormones and seems to play a physiologic role in thymus-pituitary communication, particularly during perinatal life (22). Interest in the therapeutic use of thymulin flourished during the 1970s and 1980s, when efforts where almost exclusively focused on using thymulin (and other thymic peptides) for the treatment of autoimmune pathologies as well as cancer (23, 24). Subsequent studies, most of them carried out during the last 20 yr, established that thymulin is active on the hypophysis and the brain. This awareness and the availability of a synthetic gene for thymulin (17) provide new avenues for functional studies of this metallopeptide in the endocrine system. The nude female mouse provides a suitable model of reproductive dysgenesis associated with the absence of the thymus and was therefore chosen in this study for the assessment of the physiological relevance of thymulin for the maturation of the hypothalamo-pituitary-ovarian axis.
To our knowledge, there is no previous documentation of the impact of congenital athymia on the GnRH neuron population in the mouse brain. Our data demonstrate a small (15%) but significant reduction in the number of GnRH neurons in athymic females. This reduction, which was prevented by NTGT, is likely to be associated with a reduced synthesis and secretion of GnRH into the anterior pituitary portal system, which in turn could contribute to the reduced size of the gonadotropic cell population in the pituitary of nude females. This is in line with studies reporting that injection of thymulin in the medial, but not in the anterior hypothalamus, of normal prepubertal mice (20 d old) reversed the blocking effect of ether anesthesia on ovulation (25). Although hypothalamic GnRH content in congenitally athymic mice has been reported to be similar to that of their heterozygous counterparts (26, 27), athymic nudes (21 d old) failed to increase their serum LH levels in response to ovariectomy but did respond to GnRH injection (26), suggesting an impaired GnRH neuron function in homozygous nude mice. In previous studies, we demonstrated that in 51- to 52-d-old homozygous nude females, the gonadotropic pituitary cell population and serum gonadotropin levels are reduced compared with their heterozygous counterparts and that these changes were prevented by NTGT (16, 28). Because thymulin possesses gonadotropin-releasing activity in vitro (10–12), it is likely that in vivo, the peptide exerts its stimulatory effect on gonadotropin secretion by a direct action on the pituitary gland and also by facilitating the release of GnRH. Another thymic peptide, thymosin β4, has also been shown to possess LH-releasing activity (29), which suggests that the thymus gland probably secretes a number of hypophysiotropic peptides, especially during perinatal life.
Our results confirm that nude female mice develop severe ovarian dysgenesis and demonstrate that NTGT can significantly prevent this alteration. Although it is well established that neonatal thymus grafting can fully prevent the ovarian dysgenesis of nude mice (2), this is, to our knowledge, the first report showing that such a preventive effect can be achieved with a single thymic peptide, although in this case, ovarian dysgenesis was largely but not fully prevented.
In adult homozygous nude female mice, circulating E2 and P4 levels have been reported to be lower than in the heterozygous counterparts (30), whereas in normal female mice thymectomized at 10 d of age, serum E2, but not P4, levels were lower than in intact counterparts (31). In our control homozygous females, we found a significant reduction in serum E2 but no change in P4 levels. NTGT was only partially able to prevent the low serum estrogen levels of homozygous nudes (i.e. serum estrogen levels in the experimental nu/nu mice were not significantly different from either the control nu/+ or the control nu/nu groups), whereas the treatment had no significant effect on serum P4 levels. Our E2 data are consistent with a study showing that in congenitally athymic female mice, neonatal grafting of a thymus prevents serum estrogen deficiency (30). The partial preventive effect of thymulin gene therapy on serum E2 supports the former notion and suggests an endocrine function of the thymus in regards to maturation of the reproductive system. It is also important to note that more than one thymic factor may be necessary to completely prevent ovarian dysgenesis and deficiencies in steroid production. Although we did not observe significant changes in the uterine epithelium height of homozygous nudes, there seems to be a trend toward a reduced height in control nu/nu females, which would be consistent with the low estrogen levels of these mutants. Although an early study reported that about half of homozygous nude mice show inflammatory changes in their uteri (31), none of our mice showed signs of uterine inflammation. This discrepancy may be due to differences in housing or other breeding conditions in the two studies.
In mice, it has been reported that congenital athymia and neonatal or infantile thymectomy are associated with a significant delay in the age of vaginal opening (2, 32), a well-established sign of the attainment of puberty. Taking into account that neonatal thymus grafting in nude or neonatally thymectomized mice fully prevents the delay in the age of vaginal opening (2), our results point to thymulin as a thymus effector on the maturation of the reproductive system in mice.
Taken together, our results strongly suggest that thymulin is a relevant physiologic mediator of the influence of the thymus on the reproductive system. Immunoneutralization of circulating thymulin levels from P1 to P8 in normal mice has been shown to be sufficient to cause severely reduced serum gonadotropin levels at 45 d of age (16), which is consistent with the well-established fact that in mice, the presence of the thymus is critical for neuroendocrine maturation only during the first few days of postnatal life (30, 6).
Thymulin is a highly conserved peptide, whose amino acid sequence is the same in pigs, mice, and humans, the only three species in which the sequence has been determined. This observation and the fact that thymulin affects the release of all anterior pituitary hormones suggest that this ancestral peptide may act as a general modulator of pituitary (and other) hormonal responses to specific releasing or inhibiting factors. This idea is in line with evidence that thymulin modulates GnRH-stimulated gonadotropin release (10, 11), TRH-stimulated TSH and prolactin release (33), and GHRH-stimulated release of GH (34) in rat pituitary cells. Such a multiple modulatory effect on hormone secretion has been reported for another thymic factor (35–38).
The present study complements our previous findings that NTGT prevents the well-documented deficit of circulating gonadotropins in adult nudes (16) and supports the hypothesis that thymulin is an important player in the thymus-reproductive axis. Additionally, our data suggest that thymulin gene therapy may be an effective strategy to approach reproductive deficits associated with thymus dysfunction.
Supplementary Material
Acknowledgments
P.C.R., C.G.B., and R.G.G. are Consejo Nacional de Investigaciones Científicas y Técnicas career researchers. G.M.C. is career researcher of CIC-PBA.
This work was supported in part by the National Institutes of Health Grant R01AG029798-3, the Agencia Nacional de Promoción cientifica y tecnológica Grant PICT08-369, and the Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET) Grant PIP2378 (to R.G.G.) and by the CONICET and the Institut National de la Santé et de la Recherche Médicale, France (M.D. and R.G.G.).
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- Ad5
- Adenovirus type 5
- E2
- β-estradiol
- FTS
- facteur thymique serique
- GFP
- green fluorescent protein
- metFTS
- methionine-FTS
- NTGT
- neonatal thymulin gene therapy
- P
- postnatal day
- P4
- progesterone
- RAd
- recombinant adenoviral.
References
- 1. Rebar RW, Morandini IC, Erickson GF, Petze JE. 1981. The hormonal basis of reproductive defects in athymic mice. Endocrinology 108:120–126 [DOI] [PubMed] [Google Scholar]
- 2. Besedovsky HO, Sorkin E. 1974. Thymus involvement in female sexual maturation. Nature 249:356–358 [DOI] [PubMed] [Google Scholar]
- 3. Flanagan SP. 1966. Nude, a new hairless gene with pleiotropic effects in the mouse. Genet Res 8:295–309 [DOI] [PubMed] [Google Scholar]
- 4. Lintern-Moore S, Pantelouris EM. 1975. Ovarian development in athymic nude mice. The size and composition of the follicle population. Mech Ageing Dev 4:385–390 [DOI] [PubMed] [Google Scholar]
- 5. Michael SD, Taguchi O, Nishizuka Y. 1980. Effects of neonatal thymectomy on ovarian development and plasma LH, FSH, GH and PRL in the mouse. Biol Reprod 22:343–350 [DOI] [PubMed] [Google Scholar]
- 6. Nishizuka Y, Sakakura T. 1971. Ovarian dysgenesis induced by neonatal thymectomy in the mouse. Endocrinology 89:889–893 [DOI] [PubMed] [Google Scholar]
- 7. Bach JF. 1983. Thymulin (FTS-Zn). Clin Immunol Allergy 3:133–156 [Google Scholar]
- 8. Gastinel LN, Dardenne M, Pleau JM, Bach JF. 1984. Studies on the zinc-binding site to the serum thymic factor. Biochim Biophys Acta 797:147–155 [DOI] [PubMed] [Google Scholar]
- 9. Dardenne M, Pléau JM, Nabarra B, Lefrancier P, Derrien M, Choay J, Bach JF. 1982. Contribution of zinc and other metals to the biological activity of serum thymic factor (FTS). Proc natl Acad Sci USA 79:5370–5373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Brown OA, Sosa YE, Dardenne M, Pléau JM, Goya RG. 2000. Studies on the gonadotropin-releasing activity of thymulin: changes with age. J Gerontol A Biol Sci Med Sci 55:B170–B176 [DOI] [PubMed] [Google Scholar]
- 11. Hinojosa L, García L, Domínguez R, Romano MC, Damián-Matsumura PG, Castillo L, Rosas P. 2004. Effects of thymulin and GnRH on The release of gonadotropins by pituitary cells obtained from rat in each day of estrous cycle. Life Sci 76:795–804 [DOI] [PubMed] [Google Scholar]
- 12. Zaidi SA, Kendall MD, Gillham B, Jones MT. 1988. The release of LH from pituitaries perifused with thymic extracts. Thymus 12:253–264 [PubMed] [Google Scholar]
- 13. Hinojosa L, Chavira R, Domínguez R, Rosas P. 1999. Effects of thymulin on spontaneous puberty and gonadotrophin-induced ovulation in prepubertal normal and hypothymic mice. J Endocrinol 163:255–260 [DOI] [PubMed] [Google Scholar]
- 14. Ledwitz-Rigby F, Scheid PG. 1991. Thymulin modulates porcine granulosa cell responsiveness to gonadotrophins in vitro. In: Gibori G, ed. Signaling messages and gene expression in the ovary. New York: Springer Verlag; 473–478 [Google Scholar]
- 15. Wise T. 1998. In vitro and in vivo effects of thymulin on rat testicular steroid synthesis. J Steroid Biochem Mol Biol 66:129–135 [DOI] [PubMed] [Google Scholar]
- 16. Goya RG, Reggiani PC, Vesenbeckh SM, Pléau JM, Sosa YE, Cónsole GM, Schade R, Henklein P, Dardenne M. 2007. Thymulin gene therapy prevents the reduction in circulating gonadotropins induced by thymulin deficiency in mice. Am J Physiol Endocrinol Metab 293:E182–E187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Reggiani PC, Hereñú CB, Rimoldi OJ, Brown OA, Pléau JM, Dardenne M, Goya RG. 2006. Gene therapy for long-term restoration of circulating thymulin in thymectomized mice and rats. Gene Ther 13:1214–1221 [DOI] [PubMed] [Google Scholar]
- 18. Hitt M, Bett A, Prevec L, Graham FL. 1998. Construction and propagation of human adenovirus vectors. In: Celis J, ed. Cell biology: a laboratory handbook. New York: Academic Press; 1500–1512 [Google Scholar]
- 19. Dardenne M, Bach JF. 1975. The sheep cell rosette assay for the evaluation of thymic hormones. In: Van Bekkum DW, Kruisbeek AM, eds. Biological activity of thymic hormones. International Workshop and Symposium Rotterdam, The Netherlands: Kooyker Scientific Publications, Halsted Press Division, Wiley; 235–243 [Google Scholar]
- 20. Paxinos G, Franklin KBJ. 2001. The mouse brain in stereotaxic coordinates. 2nd ed San Diego: Academic Press [Google Scholar]
- 21. Imboden H, Felix D. 1995. Immunohistochemistry in brain tissue. Method Neurosci 24:236–260 [Google Scholar]
- 22. Reggiani PC, Poch B, Cónsole GM, Rimoldi OJ, Schwerdt JI, Tüngler V, Garcia-Bravo MM, Dardenne M, Goya RG. 2011. Thymulin-based gene therapy and pituitary function in animal models of aging. Neuroimmunomodulation 18:350–356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Bach JF, Dardenne M, Goldstein AL. 1984. Clinical aspects of thymulin (FTS). In: Goldstein AL, ed. Thymic hormones and lymphokines. Basic chemistry and clinical applications. New York: Plenum Press; 593–600 [Google Scholar]
- 24. Sztein MB, Goldstein AL. 1986. Thymic hormones-a clinical update. Springer Semin Immunopathol 9:1–18 [DOI] [PubMed] [Google Scholar]
- 25. García L, Hinojosa L, Domínguez R, Chavira R, Rosas P. 2005. Effects of injecting thymulin into the anterior or medial hypothalamus or the pituitary on induced ovulation in prepubertal mice. Neuroimmunomodulation 12:314–320 [DOI] [PubMed] [Google Scholar]
- 26. Weinstein Y. 1978. Impairment of the hypothalamo-pituitary-ovarian axis of the athymic “nude” mouse. Mech Ageing Dev 8:63–68 [DOI] [PubMed] [Google Scholar]
- 27. Strich G, Petze JE, Silva de Sa MF, Rebar RW. 1985. The effects of thymus-derived peptides on hypothalamic LRF and pituitary gonadotropin content in prepubertal congenitally athymic nude mice and their normal heterozygous littermates. J Reprod Immunol 7:351–359 [DOI] [PubMed] [Google Scholar]
- 28. Reggiani P, Martines E, Ferese C, Goya R, Cónsole G. 2009. Morphological restoration of gonadotrope population by thymulin gene therapy in nude mice. Histol Histopathol 24:729–735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Rebar RW, Miyake A, Low TL, Goldstein AL. 1981. Thymosin stimulates secretion of luteinizing hormone. Science 214:669–671 [DOI] [PubMed] [Google Scholar]
- 30. Pierpaoli W, Besedovsky HO. 1975. Role of the thymus in programming of neuroendocrine functions. Clin Exp Immunol 20:323–338 [PMC free article] [PubMed] [Google Scholar]
- 31. Alten HE, Groscurth P. 1975. The postnatal development of the ovary in the “nude” mouse. Anat Embryol 148:35–46 [DOI] [PubMed] [Google Scholar]
- 32. García L, Hinojosa L, Domínguez R, Chavira R, Rosas P. 2000. Effects of infantile thymectomy on ovarian functions and gonadotrophin-induced ovulation in prepubertal mice: role of thymulin. J Endocrinol 166:381–387 [DOI] [PubMed] [Google Scholar]
- 33. Brown OA, Sosa YE, Dardenne M, Pléau J, Goya RG. 1999. Growth hormone-releasing activity of thymulin: effects of age. Neuroendocrinology 69:20–27 [DOI] [PubMed] [Google Scholar]
- 34. Brown OA, Sosa YE, Bolognani F, Goya RG. 1998. Thymulin stimulates prolactin and thyrotropin release in an age-related manner. Mech Age Devel 104:249–262 [DOI] [PubMed] [Google Scholar]
- 35. Aguilera G, Romano MC. 1989. Influence of the thymus on steroidogenesis by rat ovarian cells in vitro. J Endocr 123:367–373 [DOI] [PubMed] [Google Scholar]
- 36. Mendoza ME, Romano MC. 1989. Prepubertal rat thymus secretes a factor that modulates gonadotropin secretion in cultured rat pituitary cells. Thymus 14:233–242 [PubMed] [Google Scholar]
- 37. Hiriart M, Romano MC. 1986. Human chorionic gonadotropin binding to rat testis receptors is inhibited by a thymus factor. Life Sci 38:789–795 [DOI] [PubMed] [Google Scholar]
- 38. Reyes-Esparza JA, Romano MC. 1989. An age-dependent thymic secretion modulates testicular function. J Steroid Biochem 34:541–545 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.