Abstract
Cumulus cell-oocyte complex (COC) expansion is obligatory for LH-induced ovulation and is initiated by LH induction of the epidermal growth factor (EGF)-like factors that mediate the synthesis of the hyaluronan-rich matrix and hyaluronan-stabilizing factors. COC expansion also involves the movement of cumulus cells within the matrix by mechanisms that have not been characterized. We document herein that two proteases, calpain 2 and to a lesser extent calpain 1, are expressed in cumulus cells and that the proteolytic activity of these enzymes is rapidly and significantly increased in COC isolated from human chorionic gonadotropin-induced ovulatory follicles in vivo. Stimulation of calpain activity was associated with proteolytic degradation of paxillin and talin (two components of focal adhesion complexes), cell detachment, and the formation of cell surface bleb-like protrusions. Injection of a calpain inhibitor in vivo reduced 1) human chorionic gonadotropin-stimulated calpain enzyme activity, 2) cell detachment, 3) membrane protrusion formation, and 4) COC expansion by mechanisms that did not alter Has2 expression. During EGF-like factor induction of COC expansion in culture, calpain activity was increased by ERK1/2 and intracellular Ca2+ signaling pathways. Inhibition of calpain activity in cultured COC blocked cumulus cell detachment, protrusion formation, and the vigorous movement of cumulus cells. As a consequence, COC expansion was impaired. Collectively, these results show that two highly coordinated processes control COC expansion. One process involves the synthesis of the hyaluronan matrix, and the other mediates cumulus cell detachment and movement. The latter are controlled by calpain activation downstream of the EGF receptor activation of the Ca2+ pathway and ERK1/2 pathways.
The mammalian preovulatory follicle contains a mature oocyte that is enclosed by the somatic cumulus cells forming the cumulus cell-oocyte complex (COC). In this special niche, the cumulus cells are tightly connected to each other and to the oocyte via cell adhesion complexes and gap junctions (1, 2). The cumulus cells and the oocyte produce specific factors that act by paracrine mechanisms and gap junctions to control oocyte meiotic arrest and cumulus cell functions (3, 4).
The LH surge causes dramatic functional and structural changes in the COC that lead to the production of a hyaluronan-rich matrix, a process called mucification (5). During this process, LH induces the rapid expression of the the epidermal growth (EGF)-like factors amphiregulin (Areg), epiregulin (Ereg), betacellulin (Btc), and neuregulin-1 (Nrg1) in granulosa cells that act on their cognate receptors, EGF receptor (EGFR) and v-erb-b2 erythroblastic leukemia viral oncogene, homolog 2/3, expressed on granulosa cells and cumulus cells (6–8). The LH surge also stimulates the rapid expression of prostaglandin (PG) synthase 2 (Ptgs2), leading to increased production of PGE2, which via its receptors maintains EGF-like factor expression in granulosa cells and cumulus cells (7, 9). The EGF-like factors and PGE2 synergistically stimulate cumulus cells to produce hyaluronan (via Has2) and hyaluronan-stabilizing factors such as Tnfaip6 and Ptx3 (10). As the cumulus cells secrete and make the matrix, they detach from one another and move away from the oocyte by a process called expansion (11). Additionally, during COC expansion, cumulus cells exhibit morphological changes, including membrane protrusions that are observed at 2–3 h before rupture of the follicle wall (12).
Although the molecular mechanisms and factors that control COC expansion have been studied extensively, much less is known about what factors control cumulus cell movement. In migratory fibroblasts and cancer cells, the EGF-like factors initiate events that destabilize components of focal adhesion complexes within the cell surface membrane and alter the cytoskeleton (13). The focal adhesion complex is comprised of specific proteins including paxillin, talin, and focal adhesion kinase (14–16). These components bind to integrins and actin filaments to provide a stable cell structure (16). Degradation of focal adhesion components can be induced by two proteinases, μ-calpain (calpain 1; CAPN1) and/or m-Calpain (calpain 2; CAPN2) (17). Calpain 1 is activated by changes in intracellular calcium, whereas calcium-induced calpain 2 activity is accelerated by ERK1/2 (18). Calpain 1 and calpain 2 are 100-kDa proteins that are cleaved by autolysis to an 80-kDa catalytic subunit. The modified catalytic subunit is further cleaved by autolysis to smaller products that exhibit increased enzyme activity (17). In migratory cells, EGF activates both calpain 1 by Ca2+-dependent mechanisms and calpain 2 by ERK1/2 and/or Ca2+-dependent mechanisms (19, 20). Because EGF can activate ERK1/2 and increase Ca2+ uptake in cumulus cells of cultured COC (21, 22), we sought to determine whether the EGF-like factors could activate calpains in cumulus cells and whether the activated calpains were critical for cumulus cell detachment and movement during COC expansion.
Therefore, in this study, we analyzed which calpains are expressed and activated in cumulus cells during expansion and furthermore 1) whether calpain activation was increased by EGF-like factors or PGE2, 2) whether changes in the focal adhesion components occurred in cumulus cells, and 3) whether calpain inhibitors could block the movement of cumulus cells in vitro and in vivo and thereby impair COC expansion.
Materials and Methods
Materials
Equine chorionic gonadotropin (eCG) was purchased from Calbiochem (La Jolla, CA) or from Asuka Seiyaku (Tokyo, Japan). Human chorionic gonadotropin (hCG) was from Organon Special Chemicals (West Orange, NJ) or Asuka Seiyaku. AG1478 and U0126, calpain inhibitor I (CI-1) and calpain inhibitor III (CI-3) were purchased from Calbiochem. 1,2-bis(2-aminophenoxy)ethane N,N,N′,N′-tetraacetic and acetoxymethyl ester (BAPTA-AM) was purchased from Sigma Chemical Co. (St. Louis, MO). AREG was obtained from R&D Systems, Inc. (Minneapolis, MN). PGE2 was purchased from Cayman Chemical Co. (Ann Arbor, MI) or Sigma. DMEM/F12 medium with penicillin/streptomycin was obtained from Invitrogen (Carlsbad, CA), fetal bovine serum (FBS) from Life Technologies Inc. (Grand Island, NY), oligonucleotide poly-deoxythymidine from Invitrogen, and avian myeloblastosis virus reverse transcriptase from Promega (Madison, WI). Anti-calpain 1 and anti-calpain 2 antibodies and anti-α-actin antibody were purchased from Abcam (Cambridge MA). Antitalin antibody was obtained Sigma. Antipaxillin antibody was purchased from BD Transduction Laboratories (Franklin Lakes, NJ). Routine chemicals and reagents were obtained from Fisher Scientific (Pittsburgh, PA), Nakarai Chemical Co. (Osaka, Japan), or Sigma.
Animals
Immature female C57BL/6 mice were obtained from Harlan, Inc. (Indianapolis, IN), or Clea Japan (Tokyo, Japan). At 23 d of age, female mice were injected ip with 4 IU eCG to stimulate follicular growth, followed 48 h later with 5 IU hCG to stimulate cumulus expansion and ovulation. Animals were housed under a 14-h light, 10-h dark cycle schedule in the Experimental Animal Center at Hiroshima University or the Center for Comparative Medicine at Baylor College of Medicine and provided with food and water ad libitum. Animals were treated in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals, as approved by the Animal Care and Use Committee at Baylor College of Medicine or at Hiroshima University.
COC isolation and culture
The procedures used for in vivo COC isolation and in vitro COC culture were as described previously (23). Briefly, COC and granulosa cells from preovulatory follicles of mice primed with eCG for 48 h were released into the culture medium by needle puncture of the ovary. The COC were collected separately from the granulosa cells by pipette, pooled, and treated as described (24). For the calpain activity assay and Western blot analyses, 100 COC were cultured in 300 μl DMEM/F12 medium with 5% FBS. The COC were then treated with specific reagents for the time intervals indicated in the text. For the time-lapse assay, 50 COC were cultured in 3 ml COC medium in a 35-mm dish. The COC were cultured for the indicated times before the onset of video recording. Image-capture and time-lapse recording of the cultured COC were performed using a Keyence BZ-9000 microscope (Keyence Co., Osaka, Japan) equipped with a CO2 incubation chamber unit maintained at 37 C. Three cells on the outer side of each COC that were clearly focused were analyzed. Three COC were used for the analysis in each culture. The study was repeated three times in each treatment group. Motion Analyzer software (Keyence) was used to track the x–y coordinates of the cells in time-lapse images and to calculate the displacement of the cells from their respective start points.
Calpain activity assay
Calpain activity was measured using the SensoLyte520 fluorometric calpain activity assay kit (AnaSpec, Freemont, CA) according to the manufacturer's instructions. The assay employs a novel internally quenched 5-FAM/OXLTM 520 FRET substrate (AnaSpec) to increase the sensitivity of the measurements. Activated calpain cleaved the FRET substrate, yielding the release of fluorescent 5-FAM, which was monitored at 520-nm emission and 490-nm excitation using a 96-well fluorescence microplate reader (BD Biosciences, Franklin Lakes, NJ).
Detection of intracellular Ca2+ ([Ca2+]i) in cumulus cells of COC
A total of 50 COC were incubated with fluo-2/AM (final concentration, 5 μm; Calbiochem) and 1 μl (0.02%) Pluronic F-127 (Invitrogen). After 30-min in culture under dark conditions, the COC were washed two times to remove excess fluo-2/AM in the medium. The COC were moved to 100 μl DMEM/F12 medium with 5% FBS in 96-well plates. The intensity of fluorescence was detected at 488 nm by a fluorescence microplate reader.
RNA extraction and quantitative PCR analyses
Total RNA was obtained from mouse ovaries using the RNeasy Mini Kit (QIAGEN Sciences, Germantown, MD) according to the manufacturer's instructions. Total RNA was reverse transcribed using 500 ng poly-deoxythymidine and 0.25 U avian myeloblastosis virus reverse transcriptase at 42 C for 75 min and 95 C for 5 min. Quantitative real-time PCR analyses were performed as previously (25). Briefly, cDNA and primers [Has2 (forward, 5′-AGACATTCAATGGGGGTTGG-3′; reverse, 5′-CCACACAAAGCATGGCAAGT-3′) and L19 (forward, 5′-CTGAAGGTCAAAGGGAATGTG-3′, reverse, 5′-GGACACAGTCTTGATGATCTC-3)] were added to 15 μl total reaction volume of the Power SYBR Green PCR Master Mix (Applied Biosystems, Foster City, CA). PCR were then performed using the StepOne real-time PCR system (Applied Biosystems). Conditions were set to the following parameters: 10 min at 95 C followed by 45 cycles each of 15 sec at 95 C and 1 min at 64 C.
Western blot analyses
COC were lysed with RIPA buffer [20 mm Tris (pH 7.5), 150 mm NaCl, 1% Nonidet P-40, 0.5% sodium deoxycholate, 1 mm EDTA, and 0.1% sodium dodecyl sulfate] containing complete protease inhibitors (Roche, Indianapolis, IN). Western blot analyses were performed according to our previous study (9). Briefly, extracts (10 μg protein) were resolved by SDS-PAGE (7.5 or 10%) and transferred to polyvinylidene difluoride membranes (GE Bioscience). Membranes were blocked in Tris-buffered saline and Tween 20 [10 mm Tris (pH7.5), 150 mm NaCl, and 0.05% Tween 20] containing 5% nonfat Carnation instant milk (Nestle Co., Solon, OH). Blots were incubated with primary antibodies (ll the primary antibodies were used at 1:1000 dilutions) overnight at 4 C. After washing in Tris-buffered saline and Tween 20, enhanced chemiluminescence detection was performed by using the enhanced chemiluminescence system according the manufacturer's specifications (GE Bioscience) and appropriate exposure of the blots to Fuji x-ray film (Fujifilm, Tokyo, Japan).
Immunofluorescence
Ovaries were collected and fixed in 4% (wt/vol) paraformaldehyde/PBS, embedded in paraffin, and processed by routine procedures. Sections were probed with primary antibodies (1:350 dilution of anti-calpain 2 antibody, 1:350 of antipaxillin antibody, 1:100 of anti-α-actin antibody) and visualized with Alexa Fluor 488- and 594-conjugated goat antirabbit/mouse IgG (1:250 dilution; Molecular Probes, Eugene, OR). Digital images were captured using a Zeiss Axiphot microscope with ×40–68 objectives. For all the experiments, the exposure times were kept the same.
Pharmacological assay of calpain inhibitor III
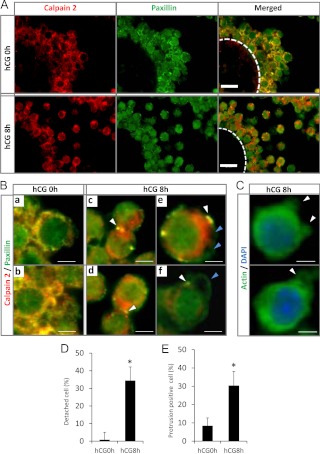
CI-3 (Calbiochem) was dissolved in dimethylsulfoxide (DMSO) at a concentration of 12.5 or 25.0 mg/ml. The stock solution was diluted to 10-fold in saline solution (0.9% NaCl) (26, 27). Doses of 12.5 or 25.0 mg/kg were injected ip at 4 or 8 h after the injection of hCG. For the calpain activity assay and Western blot analyses, 100 COC were collected from ovary at 6.5 or 10 h after hCG injection. Morphological changes in the whole ovary were determined by routine histological procedures, paraffin embedding, and preparing sections (4 μm) stained with hematoxylin and eosin (24) or immunostained for α-actin. To determine the area of each COC, an area-measurement system associated with the microscope (Keyence) was used. To determine the number of detached cells in each COC, the number of detached cells and total number of cells in each COC (n = 5) per ovary (n = 5) were counted (total number of COC was 25) in hematoxylin- and eosin-stained sections. To determine the percentage of protrusion-positive cells, the number of cells that formed protrusions and the total number of cells in each COC (n = 5) per ovary (n = 5) were counted. The protrusion formation was judged by the actin staining. Actin was accumulated within protrusions (see Fig. 2C).
Fig. 2.
The localization of calpain 2 and paxillin in cumulus cells of COC during cumulus expansion in vivo. A, Cross-sections of mouse COC were stained with antibodies to visualize either calpain 2 (red) or paxillin (green) at 0 and 8 h after hCG (scale bars, 20 μm). Circle with white line shows the localization of the oocyte. B, High-magnification views of cumulus cells immunostained for calpain 2 (red) and paxillin (green) corresponding to the ones shown in A; a and b, double immunostaining of calpain 2 and paxillin in cumulus cells from eCG-injected mice (hCG 0 h) (scale bars, 5 μm); c and d, calpain 2 and paxillin colocalize to cell-cell adhesion sites (white arrowheads) in cumulus cells at 8 h after hCG injection (scale bars, 5 μm); e and f, calpain 2 and paxillin colocalize to specific sites at the base of cell surface protrusions (white arrowheads) but not on the surface of protrusion (blue arrowheads) in cumulus cells at 8 h after hCG injection (scale bars, 3 μm). C, The localization of α-actin in cumulus cells of COC recovered from mice at 8 h after hCG injection. Immunofluorescent images localize α-actin (green) and nuclei [4′,6-diamidino-2-phenylindole (DAPI); blue]. α-actin is accumulated within protrusions (white arrowheads). D, The percentage of detached cumulus cells in COC, five COC in each section (section number is five, total 25 COC) were used for this analysis. *, The number of detached cells was significantly higher in COC recovered from mice 8 h after hCG injection as compared with that in control COC at 0 h hCG (P < 0.01). Values are mean ± sem of three replicates. E, The number of cumulus cells with protrusions in COC, five COC in each section (section number is five, total 25 COC) were used for this analysis. *, The number of cells with protrusions was significantly higher in COC recovered from mice 8 h after hCG injection as compared with that in COC of hCG 0-h mice (P < 0.01). Values are mean ± sem of three replicates.
Statistics
Statistical analyses of all data from three or four replicates for comparison were carried out by one-way ANOVA followed by Duncan's multiple-range test (Statview; Abacus Concepts, Inc., Berkeley, CA). All percentage data were subjected to arcsine transformation before ANOVA.
Results
The expression of calpains in cumulus cells and their enzyme activity after hCG in vivo
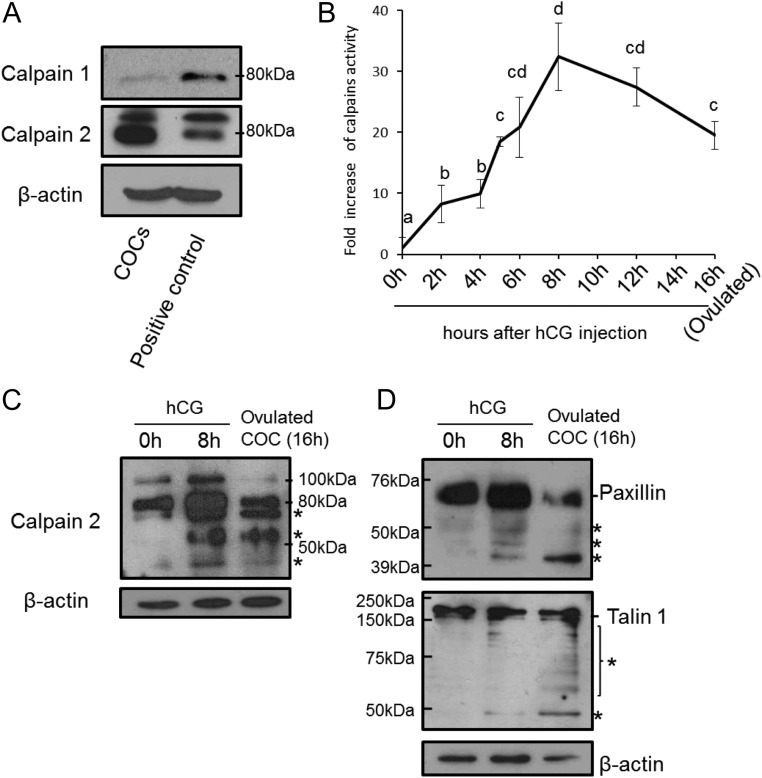
To determine whether either calpain 1 or calpain 2 was expressed in cumulus cells, COC were collected from ovaries of eCG-primed immature mice, and cell extracts were prepared for Western blots. An immunopositive signal for calpain 1 was detected in COC, but the level was lower than that observed in brain (used as a positive control) (Fig. 1A). By contrast, the immunopositive signal for calpain 2 in COC was similar to that observed in brain (Fig. 1A). Moreover, calpain 2 was detected only in cumulus cells; it was not in the oocyte (Supplemental Fig. 1, published on The Endocrine Society's Journals Online web site at http://endo.endojournals.org). mRNA transcripts expressing both calpain family members were also detected in cumulus cells (Supplemental Fig. 2).
Fig. 1.
Expression and activation of calpain isoforms in ovulating COC recovered from eCG- and hCG-treated mice. A, Levels of calpain 1 and calpain 2 protein in the COC or brain (positive control) from mice 48 h after eCG priming were analyzed by immunoblot. β-Actin was used as a loading control. B, Calpain activity in COC as assessed by fluorescent determination of the cleavage of a specific calpain substrate (5-FAM/OXLTM). a–d, No common letters are significant (P < 0.05). Values represent the mean ± sem of three replicates. *, For reference, the value of the 0-h sample was set as 1, and the data are presented as fold increase. C, Immunoblot analysis of calpain 2 cleavage forms in COC recovered from eCG-primed mice before (hCG 0 h) or 8 or 16 h after hCG. The asterisks indicate the cleaved forms of calpain 2 and the low-molecular-mass forms of calpain are known to contain the activated catalytic region (28). β-Actin was used as a loading control. D, Immunoblot analysis of calpain substrates paxillin and talin in COC recovered from eCG-primed mice before (0 h) or at 8 or 16 h after hCG. The asterisks indicate cleavage products. β-Actin was used as a loading control.
Calpain enzyme activity in nonexpanded COC collected from preovulatory follicles of eCG-primed mice was low (0 h). However, calpain activity increased significantly at 2 and 5 h after hCG and reached a maximum level (30-fold induction) at 8 h after hCG (Fig. 1B). Calpain enzyme activation is associated with cleavage of pro-calpain (17, 28). In agreement with this, two high-molecular-mass (100 and 80 kDa) immunoreactive calpain 2 bands were detected in COC before hCG stimulation (0 h) (Fig. 1C). At 8 h, the amount of pro-calpain increased. In addition, lower-molecular-mass (less than 75 kDa to about 30 kDa, denoted by the asterisks) cleaved forms of calpain 2 appeared (Fig. 1B). Because these cleaved forms of calpain 2 are known to be activated (28), the appearance of cleaved forms in the COC samples is consistent with their being the active forms. The low-molecular-mass activated forms of calpain 2 were also observed in ovulated COC (Fig. 1C).
Two focal adhesion proteins, paxillin and talin, are known targets of calpain 2. Both proteins were detected in COC recovered from ovary before hCG stimulation. After hCG injection, lower-molecular-mass immunopositive bands for both paxillin and talin were detected (Fig. 1D), suggesting that both proteins were proteolytically cleaved after hCG stimulation. Cleavage was most extensive in the ovulated COC at 16 h after hCG (Fig. 1D). These results show that calpain 2 is mainly expressed in cumulus cells of COC, that its enzyme activity is dramatically increased after hCG stimulation, and that its activation is associated temporally with the degradation of paxillin and talin.
The intracellular localization of calpain 2 and paxillin change in cumulus cells during COC expansion
To localize calpain 2 and paxillin during COC expansion in vivo, ovaries were collected from eCG-primed mice before (0 h) and 8 h after hCG stimulation. In preovulatory follicles (0 h), immunoreactive calpain 2 (red) and paxillin (green) were predominantly colocalized to the cumulus cell surface membrane (Fig. 2, A and Ba and -b). Paxillin was also detected in the cytoplasm of cumulus cells as has been observed in hepatocellular carcinoma cells (29). Neither protein was detected in oocytes (Fig. 2A). After hCG stimulation (8 h), paxillin and calpain 2 were still localized in cytoplasm of cumulus cells; however, intense signals (yellow) were detected in specific punctate regions of cell surface membrane, indicating specific colocalization of paxillin and calpain 2 at cell-cell adhesion sites (Fig. 2B, c and d). In cumulus cells that had detached from each other, paxillin and calpain 2 colocalized (yellow) at the base of specific protrusions (Fig. 2B, e and f). Actin accumulated within the protrusions (Fig. 2C). The marked changes in the immunolocalization of paxillin and calpain 2 at 8 h after hCG were associated with an increased number of either detached cells or cells exhibiting protrusion formation compared with COC before hCG injection (Fig. 2, D and E).
Inhibition of calpain activity prevents cumulus expansion in vivo
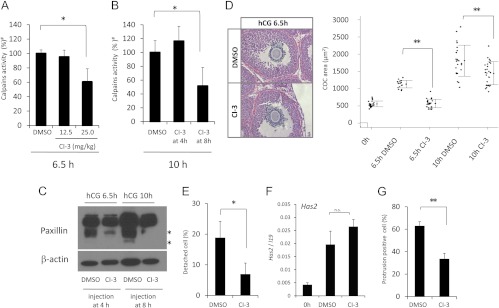
To determine the role of calpain activity in COC during ovulation in vivo, we tested the pharmacological effects of a calpain inhibitor (CI-3) on calpain activity and COC expansion in vivo. The inhibitory activity of Cl-3 in vivo is completely depleted within 4 h after ip injections (26, 27). Therefore, we injected 12.5 and 25 mg/kg CI-3 into mice at 4 h after hCG and measured calpain enzyme activity in COC collected 2.5 and 6 h later (i.e. at 6.5 and 10 h after hCG). To determine the effects of CI-3 on protrusion formation in cumulus cells, the mice were injected with the inhibitor at 8 h after hCG when cumulus cells were detached from one another and protrusions were observed (Fig. 2C).
When 25 mg/kg CI-3 was injected 4 h after hCG stimulation, calpain activity was significantly decreased at 6.5 h compared with that in the control group (DMSO injection) (Fig. 3A). Furthermore, the calpain inhibitor 1) suppressed the degradation of paxillin (Fig. 3C), 2) reduced COC expansion (area of COC) (Fig. 3D), and 3) decreased the number of detached cells compared with that in COC in the control group (Fig. 3E). However, the calpain inhibitor did not significantly suppress the expression of Has2 in cumulus cells at 6.5 h after hCG injection (Fig. 3F).
Fig. 3.
Pharmacological inhibition of calpain activity using CI-3 in vivo. A, Calpain activity in COC recovered from mice 6.5 h after hCG injection, Calpain activity in COC was assessed by fluorescent determination of the cleavage of a specific calpain substrate (5-FAM/OXLTM). Mice (n > 3 for each group) were treated at 4 h after hCG with DMSO or with either 12.5 or 25.0 mg/kg CI-3. At 2.5 h later, the mice were scarified for use in the calpain activity assay. *, The injection of 25 mg/kg CI-3 significantly decreased calpain activity as compared with that in control mice injected with DMSO (P < 0.05). #, The value of the control sample (DMSO) was set as 100, and the data are presented as a ratio. Values are mean ± sem of three replicates. B, Calpain activity in COC collected 10 h after hCG injection. Mice were injected with DMSO or 25 mg/kg of CI-3 at 4 or 8 h after hCG. Treatment of COC with CI-3 at 4 h did not significantly suppress calpain activity at 10 h after hCG. However, the same treatment at 8 h significantly decreased enzyme activity at 10 h after hCG injection (CI-3 at 8 h) as compared with that in control (DMSO injection) (*, P < 0.05). Values are mean ± sem of three replicates. C, The effects of a calpain inhibitor on the cleavage of paxillin. Mice were injected with DMSO or 25 mg/kg CI-3 at 4 or 8 h after hCG, and then COC were recovered at 6.5 or 10 h, respectively. Total lysates of COC obtained from mice treated with DMSO and CI-3 (25 mg/kg) were separated by SDS-PAGE, and paxillin was detected by immunoblotting. The asterisks indicate cleavage products. β-Actin was used as a loading control. D, The left panel shows an image of COC expansion at 6.5 h after hCG injection when DMSO or 25 mg/kg of CI-3 was injected at 4 h after hCG. The right scatter graph shows the individual areas of COC in sections containing an oocyte with a nucleus. Sections were obtained from mice treated with DMSO or CI-3 (25 mg/kg) (**, P < 0.01; n = 3 animals per condition). E, The number of detached cumulus cells in each COC, five COC in each ovary (section number is five, total 25 of COC in each treatment group) were used for this analysis (*, P < 0.05). The ovaries were collected from mice injected at 4 h after hCG treatment with DMSO or 25 mg/kg CI-3 and killed at 6.5 h after hCG. Values are mean ± sem of five replicates. F, The expression levels of Has2 in cumulus cells of COC recovered from mice 6.5 h after hCG injection when DMSO or 25 mg/kg of CI-3 was injected at 4 h after hCG. (0 h) COC were recovered from eCG-primed mice. Values are mean ± sem of three replicates. G, The number of cumulus cells with protrusions in each COC, five COC in each ovary (section number is five, total 25 of COC in each treatment group) were used for this analysis (**, P < 0.01). The ovaries were collected from mice at 10 h after hCG injection when DMSO or 25 mg/kg CI-3 was injected at 8 h after hCG. Values are mean ± sem of five replicates.
Likewise, when the CI-3 (25 mg/kg) was injected once at 8 h, calpain activity and the degradation of paxillin were suppressed at 10 h after hCG injection (Fig. 3, B and C). The number of cells with protrusions was significantly decreased by CI-3 as compared with that in the control group (DMSO) (Fig. 3G). The expansion of COC was also significantly reduced by CI-3 at 10 h after hCG (Fig. 3D).
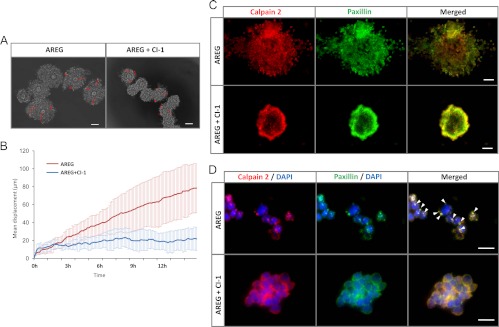
The movement of cumulus cells of COC during expansion process in vitro
To determine the role of calpain activity in the movement of cumulus cells during COC expansion in more detail, COC were collected from ovaries of eCG-primed mice and cultured with AREG with or without 5 μm calpain inhibitor 1 (CI-1), the calpain inhibitor used more commonly for in vitro studies (20). When COC were cultured with AREG for 12 h, a maximal degree of expansion was observed (Fig. 4A). When we analyzed the movement of individual cumulus cells at 6-min intervals during expansion by time-lapse microscopy (Supplement Movie 1), cumulus cell motility was higher in the AREG-treated COC than in control COC until 8 h. By contrast, the addition of CI-1 to the AREG-containing medium blocked COC expansion and significantly reduced cumulus cell motility (P < 0.001, Fig. 4, A and B, and Supplemental Movie 2). Additionally, the CI-1 inhibitor significantly blocked calpain activation induced by AREG (Fig. 5B). Furthermore, when COC were cultured with AREG, calpain and paxillin were colocalized on the cell membrane in discrete, punctate structures as was observed in COC expanded in vivo (Figs. 2B and 4, C and D). The colocalization of calpain and paxillin was blocked by the calpain inhibitor; the immunopositive signals for calpain 2 and paxillin were detected evenly at the cell membrane (Fig. 4D).
Fig. 4.
CI-1 impedes cumulus cell movement and expansion. A, Movement tracks of cumulus cells (nine cells for each treatment) from 4–16 h are displayed as a red line. Time-lapse images, taken at 6-min intervals, were captured for 12 h from 4–16 h (Supplemental Movies 1 and 2). Scale bars, 100 μm. B, Displacement of cumulus cells plotted against time (hour). Red line, mean motility of nine cumulus cells in the AREG treatment group from time-lapse imaging; blue line, mean motility of nine cumulus cells in the AREG + CI-1 treatment from time-lapse imaging. C, COC were pretreated with or without CI-1 and then stimulated with AREG for 8 h. After culture, COC were fixed and stained with anti-calpain 2 antibody (red) and paxillin (green) antibody. Scale bars, 50 μm. D, To observe the localization of calpain 2 and paxillin in more detail, COC were fixed by 4% (wt/vol) paraformaldehyde/PBS, and then some clusters of cumulus cells were gently separated from COC using a pipette. The clusters were mounted on glass slides to obtain the magnified images. Scale bars, 10 μm. Protrusions on the cumulus cell surface are indicated with white arrowheads. Nuclei were stained by 4′,6-diamidino-2-phenylindole (DAPI) (blue).
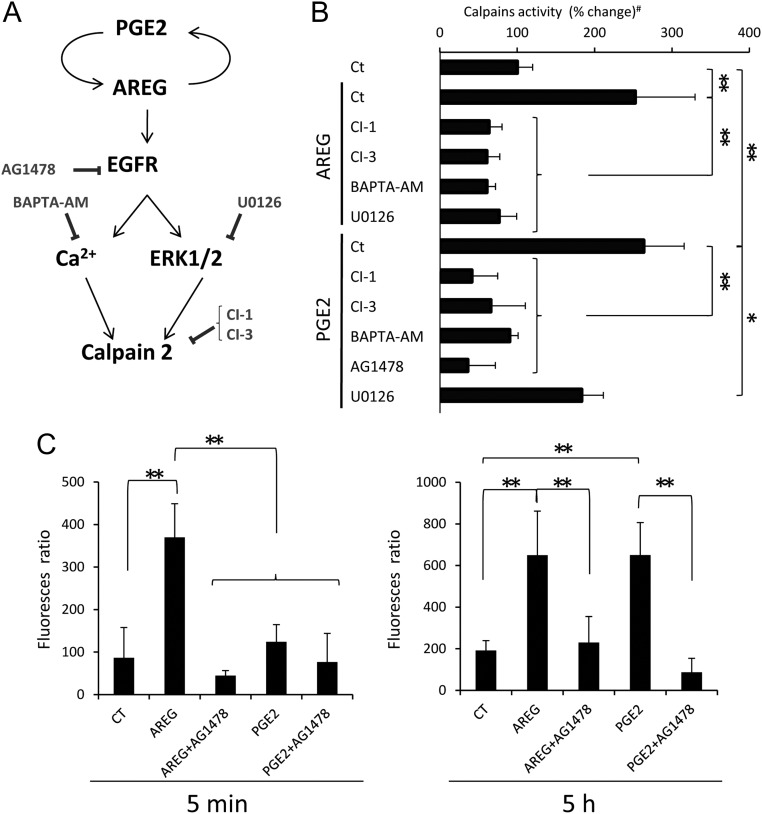
Fig. 5.
Cumulus expansion is regulated by EGFR-Ca2+-induced calpain activity. A, Schematic diagram shows the signaling pathways in COC and inhibitors that might activate or block calpain activity and COC expansion (7). B, Total calpain activity was measured in COC that were precultured with the EGFR tyrosine kinase inhibitor (AG1478), calcium chelator (BAPTA-AM), MEK1/2 inhibitor (U0126), or calpain inhibitors (CI-1 or CI-3) for 30 min and then cultured with either AREG or PGE2 for 5 h. AREG significantly increased the activity of calpain (**, P < 0.01). This induction was significantly suppressed by CI-1, CI-3, BAPTA-AM, or U0126, respectively (**, P < 0.01). PGE2 also significantly increased the level of calpain activity (**, P < 0.01). The induction was significantly and dramatically suppressed by CI-1, CI-3, BAPTA-AM, or AG1478 (**, P < 0.01). U0126 also slightly but significantly decreased the calpain activity (*P < 0.05). Values are mean ± sem of three replicates. #, The value of control sample (COC were cultured without any agonists and inhibitors) was set as 100, and the data are presented as percentage. C, The levels of Ca2+ in cumulus cells of COC. COC were preincubated with Fura2-acetoxymethylester and then cultured with AREG or PGE2 for 5 min (left) or 5 h (right). After culture, the level of Ca2+ uptake was measured as the level of 334-nm/365-nm fura-2 fluorescence ratios. Values are mean ± sem of three replicates. At 5 min, the level of Ca2+ in COC was significantly increased by AREG treatment as compared with that in COC without any agonist (control) (**, P < 0.01). The AREG-induced increase of Ca2+ was significantly suppressed by AG1478 (**, P < 0.01). PGE2 did not significantly increase the level of Ca2+ at 5 min. After 5 h culture, not only AREG but also PGE2 significantly increased the level of Ca2+ (**, P < 0.01). The inductions were significantly decreased by AG1478 (**, P < 0.01).
The regulation of calpain activity by EGFR-induced Erk1/2 pathway and the increase of Ca2+
Calpain 2 activity can be increased by EGFR pathway activation of ERK1/2 and/or Ca2+ in fibroblast cells (19, 20). To examine which pathway(s) was (were) required to increase calpain enzyme activity in cumulus cells, COC were treated with a Ca2+ chelator (BAPTA-AM), a MAPK kinase-1/2 (MEK-1/2) inhibitor (U0126) or an EGFR tyrosine kinase inhibitor (AG1478) in the presence of either AREG or PGE2 (Fig. 5A). As shown, both agonists increased calpain activity in cultured COC (Fig. 5B). BAPTA-AM significantly suppressed calpain activity in COC cultured with either AREG or PGE2 (Fig. 5B), suggesting that Ca2+ is essential for the induction of calpain activity. In fact, the level of intracellular Ca2+ was significantly increased within 5 min by AREG but not by PGE2 (Fig. 5C). However, after 5 h of culture with either AREG or PGE2, both agonists significantly increased the intracellular Ca2+ levels in cumulus cells (Fig. 5C). Moreover, AG1478 significantly decreased both the level of intracellular Ca2+ and calpain activity (Fig. 5, B and C). U0126 slightly but significantly suppressed calpain activity induced by PGE2 (P < 0.05, Fig. 5B) but dramatically decreased the enzyme activity induced by AREG in cumulus cells (P < 0.01, Fig. 5B).
Discussion
It is now well established that LH induces expression of the EGF-like factors in granulosa cells and that these factors, in turn, mediate the EGFR signaling pathway in cumulus cells to induce COC expansion and oocyte maturation (6, 30, 31). One downstream target of the EGFR pathway in cumulus cells is ERK1/2 that regulates the expression of genes involved in hyaluronan synthesis and accumulation (32–34). However, not all of the effects of the EGF-like factors require transcriptional activation of selected genes to mediate events in COC. Sela-Abramovich et al. (35) reported recently that activation of ERK1/2 in cumulus cells regulated oocyte maturation by mechanisms independent of gene transcription. Specifically, ERK1/2 mediated the phosphorylation of connexin-43, a component of gap junctions. As a consequence, communication between cumulus cells and the oocyte was disrupted, and the repression of meiosis was relieved. In this study, we document for the first time that activation of the calpain proteinases mediates rapid, nontranscriptional responses in cumulus cells that lead to the detachment and movement of cumulus cell during the expansion of COC.
The detachment of cells from each other and the formation of the extracellular matrix are obligatory first steps for migration of fibroblasts and epithelial-derived cancer cells (36). Fibroblasts and epithelial cells are normally attached to each other via transmembrane proteins, such as integrins and cadherins. These membrane proteins bind to focal adhesion proteins that, in turn, bind actin filaments (18). Thus, the degradation and disruption of the focal adhesion complex by calpain family members can initiate cell detachment (13). In the present study, we show that cumulus cells express predominantly calpain 2 and to a lesser extent calpain 1, that calpain activity is increased during LH/hCG-induced ovulation in vivo, and that activated calpain is associated with proteolytic cleavage of two key focal adhesion proteins, paxillin and talin, in cumulus cells. Calpain activity increased significantly within 4 h after hCG injection and reached maximum levels at 8 h when most of the cumulus cells were detached from one another and developed protrusions in which actin accumulated.
The accumulation of actin within membrane protrusions is known to be characteristic of bleb-like structures (37), and bleb formation is essential for cell movement in three-dimensional space (38). When white blood cells were cultured in three-dimensional matrices, the cells moved using bleb-like protrusions, suggesting that the movement of cells using bleb-like protrusions is different from the migration of cells using lamellipodia in a two-dimensional space (39). Importantly, lamellipodia-like structures were not detected in cumulus cells, even after hCG injection. Moreover, when the calpain inhibitor (CI-3) was injected at 4 or 8 h after hCG in vivo, the detachment of cumulus cells and the formation of protrusions (blebs) on the cumulus cell surface were suppressed, and expansion of the COC was also decreased. However, the calpain inhibitor did not significantly decrease the expression level of Has2, a gene that encodes the enzyme producing hyaluronan. These results indicate that COC expansion is mediated not only by hyaluronan synthesis and matrix formation but also by calpain-dependent cumulus cell movement. Additionally, the characteristics of cumulus cell movement during the process of COC expansion are similar to that of cancer cells in which cell detachment and cell-surface bleb formation precede cell motility in a three-dimensional culture system (40).
The role of calpain in regulating cumulus cell detachment in vivo was also documented in vitro. When COC were cultured with either AREG or PGE2, the level of calpain activity increased and was associated with the increased levels of intracellular Ca2+ in cumulus cells. Calpain activity was suppressed not only by a well-known MEK1/2-ERK1/2 pathway inhibitor (U0126) but also by a Ca2+ chelator (BAPTA-AM). It has been reported that calpain 1 is activated primarily by changes in intracellular calcium, whereas calcium-induced calpain 2 activity is accelerated by ERK1/2 (18). Because both Ca2+ and MEK1/2-ERK1/2 were required for maximal calpain activity in cumulus cells and because the levels of calpain 2 are greater than those of calpain 1, it is likely that calpain 2 is the major enzyme controlling these specific proteolytic events in cumulus cells during the ovulation process.
Fan et al. (41) reported that ERK1/2 were activated by an EGF-like factor-EGFR-rat sarcoma viral oncogene homolog-dependent mechanism in cumulus cells and granulosa cells in periovulatory follicles. In the present study, we show that the EGF-like factor, AREG, and PGE2 increase intracellular Ca2+ levels. Whereas AREG directly binds and activates the EGFR, PGE2 likely acts indirectly by its ability to induce expression of AREG in cumulus cells (7, 42), thereby leading to increased levels of Ca2+ via an autocrine mechanism. In cumulus cells, Ca2+ is released into the cytoplasm after inositol tri-phosphate (IP3)-stimulated receptors present in endoplasmic reticulum, and the sensitivity of IP3-mediated Ca2+ release increases during the in vitro maturation of COC (43, 44). EGF also increases the intracellular Ca2+ levels in cumulus cells of bovine COC via an EGFR-dependent manner (45). IP3 is produced from phosphatidylinositol-diphosphate by the phospholipase C (PLC) family. PLCγ has Src homology 2 domains that selectively bind to phosphorylated tyrosine on the EGFR (46). Although the expression and function of PLCγ in cumulus cells of COC remains to be determined, human granulosa cells express the γ-isoform of PLC (47). Therefore, in ovulating follicles, EGF-like factors induced by LH in granulosa cells appear to act rapidly on cumulus cells to stimulate both the EGFR-PLCγ-Ca2+-releasing pathway and EGFR-rat sarcoma viral oncogene homolog-ERK1/2 pathway. Both of these pathways are required for activation of calpain 2 in the cells.
The biochemical mechanisms by which cumulus cells form bleb-like protrusions of the cell surface appear to involve intracellular Ca2+ activation of calpain 2 that degrades paxillin and talin and thereby disrupts focal adhesion complexes. This mechanism differs from that in melanoma cells where GTP-loaded Rho activates its effector kinase, Rho-associated kinase (ROCK). Activated ROCK directly phosphorylates myosin light chain and causes bleb formation (48, 49). However, in cumulus cells, the Rho-ROCK pathway is down-regulated by PGE2 during ovulation (50). In Pger2-mutant mice, the Rho-ROCK pathway is activated in cumulus cells, and fibronectin accumulates within the cumulus matrix, resulting in deficient cumulus expansion (50). Thus, in cumulus cells during ovulation, calpain 2 may be an essential factor for bleb-like protrusion formation. This hypothesis is supported by studies of Drosophila embryos where the phenotype of the talin-knockout flies exhibited spontaneous cell protrusions that were similar to that of integrin-knockout flies (51). Moreover, in rat hepatocytes where tissue injury leads to oxidase stress, increased levels of Ca2+ uptake activate calpain and bleb formation (52).
In summary, the results of this study provide new and important evidence that activation of calpain, mainly calpain 2, is a key factor mediating the movement of cumulus cells during expansion of the COC in vivo and in vitro. Ca2+- and ERK1/2-activated calpain 2 leads to the degradation of the focal adhesion proteins paxillin and talin, destabilizes the adhesion complex, and causes cumulus cells to detach. Thus, cumulus cells not only make a hyaluronan-rich matrix but also appear to use the matrix for their movement away from the oocyte. Based on these observations, we conclude that COC expansion is mediated by two highly coordinated processes. The formation of hyaluronan-rich matrix in cumulus cells is induced primarily by the EGFR-ERK1/2 pathway, whereas the movement of cumulus cells is controlled by both the EGFR-ERK1/2 pathway and EGFR-Ca2+-calpain 2 pathway.
Supplementary Material
Acknowledgments
We are grateful to Y. Tomoda and T. Kawai, Hiroshima University, for giving technical assistance to analysis of time-lapse images.
This work was supported by NIH-HD-16229 and HD-07495 (Project III, Specialized Cooperative Program in Reproductive Research) (to J.S.R), Grant-in-Aid for Scientific Research (21688019, 21028014, and 21248032) (to M.S.), and Young Research Fellowship (09J04118) (to I.K.) from the Japan Society for the Promotion of Science.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- AREG
- Amphiregulin
- BAPTA-AM
- 1,2-bis(2-aminophenoxy)ethane N,N,N′,N′-tetraacetic acid acetoxymethyl ester
- CI-1
- calpain inhibitor I
- COC
- cumulus cell-oocyte complex
- DMSO
- dimethylsulfoxide
- eCG
- equine chorionic gonadotropin
- EGF
- epidermal growth factor
- EGFR
- EGF receptor
- FBS
- fetal bovine serum
- hCG
- human chorionic gonadotropin
- IP3
- inositol tri-phosphate
- MEK1/2
- MAPK kinase-1/2
- PG
- prostaglandin
- PLC
- phospholipase C
- ROCK
- Rho-associated kinase.
References
- 1. Eppig JJ. 1991. Inter communication between mammalian oocytes and companion somatic cells. Bioessays 13:569–574 [DOI] [PubMed] [Google Scholar]
- 2. Kidder GM, Mhawi AA. 2002. Gap junctions and ovarian folliculogenesis. Reproduction 123:613–620 [DOI] [PubMed] [Google Scholar]
- 3. Larsen WJ, Wert SE, Brunner GD. 1987. Differential modulation of rat follicle cell gap junction populations at ovulation. Dev Biol 122:61–71 [DOI] [PubMed] [Google Scholar]
- 4. Zhang M, Su YQ, Sugiura K, Xia G, Eppig JJ. 2010. Granulosa cell ligand NPPC and its receptor NPR2 maintain meiotic arrest in mouse oocytes. Science 330:366–369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Dekel N, Kraicer PF. 1978. Induction in vitro of mucification of rat cumulus oophorus by gonadotrophins and adenosine 3′,5′-monophosphate. Endocrinology 102:1797–1802 [DOI] [PubMed] [Google Scholar]
- 6. Park JY, Su YQ, Ariga M, Law E, Jin SL, Conti M. 2004. EGF-like growth factors as mediators of LH action in the ovulatory follicle. Science 303:682–684 [DOI] [PubMed] [Google Scholar]
- 7. Shimada M, Hernandez-Gonzalez I, Gonzalez-Robayna I, Richards JS. 2006. Paracrine and autocrine regulation of epidermal growth factor-like factors in cumulus oocyte complexes and granulosa cells: key roles for prostaglandin synthase 2 and progesterone receptor. Mol Endocrinol 20:1352–1365 [DOI] [PubMed] [Google Scholar]
- 8. Noma N, Kawashima I, Fan HY, Fujita Y, Kawai T, Tomoda Y, Mihara T, Richards JS, Shimada M. 2011. LH-induced neuregulin 1 (NRG1) type III transcripts control granulosa cell differentiation and oocyte maturation. Mol Endocrinol 25:104–116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sirois J, Richards JS. 1992. Purification and characterization of a novel, distinct isoform of prostaglandin endoperoxide synthase induced by human chorionic gonadotropin in granulosa cells of rat preovulatory follicles. J Biol Chem 267:6382–6388 [PubMed] [Google Scholar]
- 10. Richards JS. 2005. Ovulation: new factors that prepare the oocyte for fertilization. Mol Cell Endocrinol 234:75–79 [DOI] [PubMed] [Google Scholar]
- 11. Dekel N, Hillensjö T, Kraicer PF. 1979. Maturational effects of gonadotropins on the cumulus-oocyte complex of the rat. Biol Reprod 20:191–197 [DOI] [PubMed] [Google Scholar]
- 12. Dekel N, Phillips DM. 1979. Maturation of the rat cumulus oophorus. A scanning electron microscopic study. Biol Reprod 21:9–18 [DOI] [PubMed] [Google Scholar]
- 13. Glading A, Lauffenburger DA, Wells A. 2002. Cutting to the chase: calpain proteases in cell motility. Trends Cell Biol 12:46–54 [DOI] [PubMed] [Google Scholar]
- 14. Carragher NO, Levkau B, Ross R, Raines EW. 1999. Degraded collagen fragments promote rapid disassembly of smooth muscle focal adhesions that correlates with cleavage of pp125(FAK), paxillin, and talin. J Cell Biol 147:619–630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Plopper G, Ingber DE. 1993. Rapid induction and isolation of focal adhesion complexes. Biochem Biophys Res Commun 193:571–578 [DOI] [PubMed] [Google Scholar]
- 16. Geiger B, Bershadsky A, Pankov R, Yamada KM. 2001. Transmembrane crosstalk between the extracellular matrix–cytoskeleton crosstalk. Nat Rev Mol Cell Biol 2:793–805 [DOI] [PubMed] [Google Scholar]
- 17. Goll DE, Thompson VF, Li H, Wei W, Cong J. 2003. The calpain system. Physiol Rev 83:731–801 [DOI] [PubMed] [Google Scholar]
- 18. Franco SJ, Huttenlocher A. 2005. Regulating cell migration: calpains make the cut. J Cell Sci 118:3829–3838 [DOI] [PubMed] [Google Scholar]
- 19. Glading A, Chang P, Lauffenburger DA, Wells A. 2000. Epidermal growth factor receptor activation of calpain is required for fibroblast motility and occurs via an ERK/MAP kinase signaling pathway. J Biol Chem 275:2390–2398 [DOI] [PubMed] [Google Scholar]
- 20. Huttenlocher A, Palecek SP, Lu Q, Zhang W, Mellgren RL, Lauffenburger DA, Ginsberg MH, Horwitz AF. 1997. Regulation of cell migration by the calcium-dependent protease calpain. J Biol Chem 272:32719–32722 [DOI] [PubMed] [Google Scholar]
- 21. O'Donnell JB, Jr, Hill JL, Gross DJ. 2004. Epidermal growth factor activates cytosolic [Ca2+] elevations and subsequent membrane permeabilization in mouse cumulus-oocyte complexes. Reproduction 127:207–220 [DOI] [PubMed] [Google Scholar]
- 22. Fan HY, Liu Z, Shimada M, Sterneck E, Johnson PF, Hedrick SM, Richards JS. 2009. MAPK3/1 (ERK1/2) in ovarian granulosa cells are essential for female fertility. Science 324:938–941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ochsner SA, Day AJ, Rugg MS, Breyer RM, Gomer RH, Richards JS. 2003. Disrupted function of tumor necrosis factor-α-stimulated gene 6 blocks cumulus cell-oocyte complex expansion. Endocrinology 144:4376–4384 [DOI] [PubMed] [Google Scholar]
- 24. Liu Z, Fan HY, Wang Y, Richards JS. 2010. Targeted disruption of Mapk14 (p38MAPKα) in granulosa cells and cumulus cells causes cell-specific changes in gene expression profiles that rescue COC expansion and maintain fertility. Mol Endocrinol 24:1794–1804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Shimada M, Yanai Y, Okazaki T, Yamashita Y, Sriraman V, Wilson MC, Richards JS. 2007. Synaptosomal-associated protein 25 gene expression is hormonally regulated during ovulation and is involved in cytokine/chemokine exocytosis from granulosa cells. Mol Endocrinol 21:2487–2502 [DOI] [PubMed] [Google Scholar]
- 26. Ma W, Han W, Greer PA, Tuder RM, Toque HA, Wang KK, Caldwell RW, Su Y. 2011. Calpain mediates pulmonary vascular remodeling in rodent models of pulmonary hypertension, and its inhibition attenuates pathologic features of disease. J Clin Invest 121:4548–4566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Mingorance-Le Meur A, O'Connor TP. 2009. Neurite consolidation is an active process requiring constant repression of protrusive activity. EMBO J 28:248–260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Moldoveanu T, Gehring K, Green DR. 2008. Concerted multi-pronged attack by calpastatin to occlude the catalytic cleft of heterodimeric calpains. Nature 20:404–408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Wong CC, Wong CM, Ko FC, Chan LK, Ching YP, Yam JW, Ng IO. 2008. Deleted in liver cancer 1 (DLC1) negatively regulates Rho/ROCK/MLC pathway in hepatocellular carcinoma. PLoS One 23:e2779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Downs SM, Chen J. 2008. EGF-like peptides mediate FSH-induced maturation of cumulus cell-enclosed mouse oocytes. Mol Reprod Dev 75:105–114 [DOI] [PubMed] [Google Scholar]
- 31. Reizel Y, Elbaz J, Dekel N. 2010. Sustained activity of the EGF receptor is an absolute requisite for LH-induced oocyte maturation and cumulus expansion. Mol Endocrinol 24:402–411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Hsieh M, Lee D, Panigone S, Horner K, Chen R, Theologis A, Lee DC, Threadgill DW, Conti M. 2007. Luteinizing hormone-dependent activation of the epidermal growth factor network is essential for ovulation. Mol Cell Biol 27:1914–1924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Hsieh M, Thao K, Conti M. 2011. Genetic dissection of epidermal growth factor receptor signaling during luteinizing hormone-induced oocyte maturation. PLoS One 6:e21574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Panigone S, Hsieh M, Fu M, Persani L, Conti M. 2008. Luteinizing hormone signaling in preovulatory follicles involves early activation of the epidermal growth factor receptor pathway. Mol Endocrinol 22:924–936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Sela-Abramovich S, Chorev E, Galiani D, Dekel N. 2005. Mitogen-activated protein kinase mediates luteinizing hormone-induced breakdown of communication and oocyte maturation in rat ovarian follicles. Endocrinology 146:1236–1244 [DOI] [PubMed] [Google Scholar]
- 36. Storr SJ, Carragher NO, Frame MC, Parr T, Martin SG. 2011. The calpain system and cancer. Nat Rev Cancer 11:364–374 [DOI] [PubMed] [Google Scholar]
- 37. Takesono A, Heasman SJ, Wojciak-Stothard B, Garg R, Ridley AJ. 2010. Microtubules regulate migratory polarity through Rho/ROCK signaling in T cells. PLoS One 5:e8774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Charras G, Paluch E. 2008. Blebs lead the way: how to migrate without lamellipodia. Nat Rev Mol Cell Biol 9:730–736 [DOI] [PubMed] [Google Scholar]
- 39. Keller H, Rentsch P, Hagmann J. 2002. Differences in cortical actin structure and dynamics document that different types of blebs are formed by distinct mechanisms. Exp Cell Res 15:161–172 [DOI] [PubMed] [Google Scholar]
- 40. Fackler OT, Grosse R. 2008. Cell motility through plasma membrane blebbing. J Cell Biol 181:879–884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Fan HY, Shimada M, Liu Z, Cahill N, Noma N, Wu Y, Gossen J, Richards JS. 2008. Selective expression of KrasG12D in granulosa cells of the mouse ovary causes defects in follicle development and ovulation. Development 135:2127–2137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Fan HY, O'Connor A, Shitanaka M, Shimada M, Liu Z, Richards JS. 2010. β-Catenin (CTNNB1) promotes preovulatory follicular development but represses LH-mediated ovulation and luteinization. Mol Endocrinol 24:1529–1542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Boni R, Cocchia N, Silvestre F, Tortora G, Lorizio R, Tosti E. 2008. Juvenile and adult immature and in vitro matured ovine oocytes evaluated in relation to membrane electrical properties, calcium stores, IP3 sensitivity and apoptosis occurrence in cumulus cells. Mol Reprod Dev 75:1752–1760 [DOI] [PubMed] [Google Scholar]
- 44. Silvestre F, Boni R, Fissore RA, Tosti E. 2011. Ca2+ signaling during maturation of cumulus-oocyte complex in mammals. Mol Reprod Dev 78:744–756 [DOI] [PubMed] [Google Scholar]
- 45. Zhao Z, Garbett D, Hill JL, Gross DJ. 2005. Epidermal growth factor receptor downregulation in cultured bovine cumulus cells: reconstitution of calcium signaling and stimulated membrane permeabilization. Reproduction 130:517–528 [DOI] [PubMed] [Google Scholar]
- 46. Wang Z, Glück S, Zhang L, Moran MF. 1998. Requirement for phospholipase C-γ1 enzymatic activity in growth factor-induced mitogenesis. Mol Cell Biol 18:590–597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Carrasco MP, Asbóth G, Phaneuf S, López Bernal A. 1997. Activation of the prostaglandin FP receptor in human granulosa cells. J Reprod Fertil 111:309–317 [DOI] [PubMed] [Google Scholar]
- 48. Hagmann J, Burger MM, Dagan D. 1999. Regulation of plasma membrane blebbing by the cytoskeleton. J Cell Biochem 73:488–499 [PubMed] [Google Scholar]
- 49. Pinner S, Sahai E. 2008. PDK1 regulates cancer cell motility by antagonising inhibition of ROCK1 by RhoE. Nat Cell Biol 10:127–137 [DOI] [PubMed] [Google Scholar]
- 50. Yodoi R, Tamba S, Morimoto K, Segi-Nishida E, Nishihara M, Ichikawa A, Narumiya S, Sugimoto Y. 2009. RhoA/Rho kinase signaling in the cumulus mediates extracellular matrix assembly. Endocrinology 150:3345–3352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Wang Y, Litvinov RI, Chen X, Bach TL, Lian L, Petrich BG, Monkley SJ, Kanaho Y, Critchley DR, Sasaki T, Birnbaum MJ, Weisel JW, Hartwig J, Abrams CS. 2008. Loss of PIP5KIγ, unlike other PIP5KI isoforms, impairs the integrity of the membrane cytoskeleton in murine megakaryocytes. J Clin Invest 118:812–819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Miyoshi H, Umeshita K, Sakon M, Imajoh-Ohmi S, Fujitani K, Gotoh M, Oiki E, Kambayashi J, Monden M. 1996. Calpain activation in plasma membrane bleb formation during tert-butyl hydroperoxide-induced rat hepatocyte injury. Gastroenterology 110:1897–1904 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.