Abstract
Vertical sleeve gastrectomy (VSG) has gained interest as a low morbidity bariatric surgery, which is effective in producing weight loss and causing type 2 diabetes resolution. However, the efficacy of VSG to prevent the onset of type 2 diabetes has not been previously investigated. VSG or sham surgery was performed on 2-month-old prediabetic male University of California Davis-type 2 diabetes mellitus rats. Sham-operated animals were either sham-operated ad libitum fed (S-AL) or were weight-matched to VSG-operated animals (S-WM). Diabetes onset was determined by weekly nonfasting blood glucose measurements. Animals underwent oral glucose tolerance tests at 1 and 4 months after surgery and indirect calorimetry at 1.5 months after surgery. VSG surgery significantly delayed diabetes onset compared with both S-AL and S-WM animals. VSG-operated animals ate 23% less and weighed 20% less than S-AL. Energy expenditure did not differ between VSG-operated animals and controls. Results from the oral glucose tolerance tests demonstrate improved glucose tolerance and islet function in VSG-operated animals compared with S-AL and S-WM. Nutrient-stimulated glucagon-like peptide (GLP)-1, GLP-2, and peptide YY excursions were greater in VSG-operated animals. VSG surgery resulted in decreased fasting plasma insulin, ghrelin and lipid concentrations, and markedly higher fasting plasma adiponectin and bile acid concentrations, independent of body weight. Increases of circulating bile acid concentrations were due to selective increases of taurine-conjugated bile acids. Thus, VSG delays type 2 diabetes onset in the University of California Davis-type 2 diabetes mellitus rat, independent of body weight. This is potentially mediated by increases of circulating bile acids, adiponectin, and nutrient-stimulated GLP-1 secretion and decreased circulating ghrelin concentrations.
Bariatric surgery is currently the most effective long-term treatment for obesity and often results in resolution of type 2 diabetes (1) and may also prevent or delay the onset of diabetes (2, 3). However, the mechanisms by which this occurs remain undefined. Identification of these mechanisms may provide new therapeutic targets for the treatment and prevention of type 2 diabetes. Vertical sleeve gastrectomy (VSG) has gained interest as a low morbidity bariatric surgery that is highly effective in producing weight loss and improving glucose homeostasis. VSG involves removal of the greater curvature of the stomach and thus has been considered to be primarily a restrictive surgery. However, retrospective studies have revealed that VSG reverses diabetes in 80–84% of patients with type 2 diabetes (4–6) with improvements of circulating insulin and glucose concentrations observed as early as 3 d postoperatively and before any significant weight loss (7). Interestingly, VSG has been shown to produce diabetes remission and improved glucose homeostasis to a similar degree as Roux en Y gastric bypass (RYGB) in both rodent and human clinical studies (4, 8). Furthermore, recent clinical studies in humans have shown that, similar to RYGB, metabolic improvements after VSG may be related to increases of nutrient-stimulated glucagon-like peptide (GLP)-1 and peptide YY (PYY) secretion (9). In addition, VSG results in marked decreases of circulating ghrelin concentrations, which have been postulated to contribute to greater early excess body weight loss (10).
In addition to the well-described effects of ghrelin to increase appetite, ghrelin also influences fat mass, growth hormone secretion, and glucose homeostasis. Ghrelin is primarily produced in the gastric mucosa and its receptor (GH secretagogue receptor-1a) is expressed on hypothalamic cells, pituitary, liver, adipocytes, and pancreas (11). Furthermore, GH secretagogue receptor-1a and ghrelin are both expressed in pancreatic α-, β-, and ϵ-cells (12–14). Recent studies suggest that excess ghrelin production could contribute to the development of type 2 diabetes, because ghrelin has been shown to impair glucose-stimulated insulin secretion and promote insulin resistance (15–17).
GLP-1 and PYY are secreted by L cells, located primarily in the distal small intestine, in response to direct stimulation by nutrients and by vagal stimulation (18, 19). PYY acts to decrease food intake and thus maintain weight loss (20). GLP-1 acts to decrease food intake, increase glucose-stimulated insulin secretion and improve insulin sensitivity, and may preserve islet integrity (18).
In the present study, we used the University of California Davis-type 2 diabetes mellitus (UCD-T2DM) rat model of type 2 diabetes to investigate the effects of VSG on the onset of diabetes. The UCD-T2DM rat model develops adult-onset polygenic obesity, insulin resistance, and hyperglycemia, without a defect in leptin signaling, making this model ideal for investigating the efficacy of VSG to delay type 2 diabetes onset (21). Previous rodent studies of VSG surgery have used models of obesity such as Zucker rats (22) and high-fat-fed Long-Evans rats (8). To our knowledge, this is the first study of the effects of VSG in an animal model of type 2 diabetes. Here, we report that VSG significantly delayed diabetes onset and was accompanied by significant improvements of insulin secretion and glucose and lipid metabolism. Furthermore, VSG-operated animals exhibited marked increases of nutrient-stimulated GLP-1, GLP-2, and PYY secretion, decreased fasting plasma ghrelin concentrations, and increased fasting plasma adiponectin and bile acid concentrations, which are likely contributors to the delay in diabetes onset.
Materials and Methods
Diets and animals
Male UCD-T2DM rats were individually housed in hanging wire cages in the animal facility in the Department of Nutrition at the University of California Davis and maintained on a 14-h light, 10-h dark cycle. At 2 months of age rats underwent sham or VSG surgery. Sham-operated animals were then divided into sham-operated ad libitum-fed (S-AL) or sham-operated weight-matched (S-WM) groups. Animals were followed until at least 8 months of age to determine the time to diabetes onset. Baseline body weights, measured on the day of surgery, were 385 ± 10, 385 ± 6, and 385 ± 7 g in S-AL (n = 14), S-WM (n = 16), and VSG-operated animals (n = 13), respectively. All animals received ground chow (no. 5012; Ralston Purina, Belmont, CA). Food intake and body weight were measured three times a week in S-AL and VSG-operated animals, and S-WM animals received daily rations of food to match their body weights with the VSG-operated animals. Nonfasting blood glucose was measured weekly with a glucometer (One-Touch Ultra; LifeScan, Milpitas, CA) at 1400–1600 h. Diabetes onset was defined as a nonfasted blood glucose value above 11.1 mmol/liter (200 mg/dl) for two consecutive weeks. Indirect calorimetry was performed at 1.5 months after surgery using an Integra ME system (AccuScan, Columbus, OH). The experimental protocols were approved by the University of California Davis Institutional Animal Care and Use Committee.
Bariatric procedures, including VSG surgery, can result in malabsorption of certain vitamins and minerals, including vitamin B12, often necessitating supplementation (23). In the rat, vitamin B12 deficiency can result from removal of the glandular portion of the stomach at 1–3 months after surgery, leading to the subsequent development of anemia. The glandular portion of the stomach is responsible for producing intrinsic factor, which is necessary for vitamin B12 absorption (24). To control for this, hematocrit was monitored, and when mild anemia was noted, vitamin B12 supplementation was initiated in VSG-operated animals. At 5 months after surgery, mild anemia was first noted in the VSG-operated animals (hematocrit, 34 ± 2%; rat reference range, 35–45%) (25). VSG-operated animals received 50 μg of cyanocobalamin sc once weekly thereafter (25). After one month of treatment, hematocrit values in VSG-operated animals were normalized to 40 ± 2% and remained stable thereafter.
VSG surgery
Rats were placed on a liquid diet (Boost, Novartis, Minneapolis, MN) 4 d before surgery and for 14 d after surgery and received enrofloxacin (20 mg/kg, sc once daily) and meloxicam (2 mg/kg sc once daily). Anesthesia was induced and maintained with isoflurane (2–5%). A midline abdominal incision was made, and connective tissue attachments to the liver and spleen were transected to allow isolation of the stomach outside the abdominal cavity. The stomach was packed-off with gauze sponges and 4–0 polydioxanone (PDS) suture (Ethicon, Cornelia, GA) was used to create a line of sutures encompassing both gastric walls just below the intended incision. Hemostats were placed just above the intended incision, and approximately 70% of the stomach (including the entire fundus) was removed by cutting along the greater curvature between the suture line and the hemostats to create a tubular remnant connecting the esophagus and pylorus. A second line of sutures was placed using 6–0 PDS (Ethicon) to reinforce apposition of the gastric mucosa. The gastric remnant was lavaged and placed back into the abdominal cavity. The abdominal cavity was closed in two layers using 4–0 PDS (Ethicon).
Sham-operated animals were treated in similar manner as the VSG group. Sham surgeries were performed by making a laparotomy incision and isolating the stomach outside of the abdominal cavity. A simple continuous pattern of suture was placed through one layer of the greater curvature of the stomach in the same location as the VSG-operated animals using 6–0 PDS (Ethicon) along both gastric walls. The stomach was lavaged and returned to the abdominal cavity.
Oral glucose tolerance tests (OGTT)
At 1 and 4 months after surgery, OGTT were performed. Animals were fasted overnight (12 h) and then received a 50% dextrose solution (1 g/kg body weight) by oral gavage. Blood was collected from the tail for measurement of glucose and insulin concentrations. A second aliquot of blood was placed in tubes containing EDTA, aprotinin, and a dipeptidyl peptidase-IV inhibitor and analyzed for GLP-1 and PYY. Serum glucose was measured using an enzymatic colorimetric assay for glucose (Thermo DMA, Louisville, CO). Serum insulin was measured by ELISA (Millipore, St. Charles, MO). Total GLP-1 was measured by sandwich electrochemiluminescence immunoassay (Meso Scale Discovery, Gaithersburg, MA). Plasma PYY was measured by RIA (Millipore).
Monthly fasted hormone and metabolite profiles
Baseline and monthly blood samples were collected after an overnight (12 h) fast from the tail. Plasma was assayed for glucose, insulin, triglycerides (TG), cholesterol, leptin, adiponectin, and ghrelin. Plasma glucose, cholesterol, and TG were measured using enzymatic colorimetric assays (Thermo DMA; L-type TG H kit, Wako Chemicals USA, Inc., Richmond, VA). Leptin and adiponectin were measured with rodent/rat-specific RIA (rat leptin, mouse adiponectin; Millipore). Insulin was measured by ELISA (Millipore). Ghrelin was measured by sandwich electrochemiluminescence immunoassay (Meso Scale Discovery).
Monthly fasting plasma bile acid profiles
Monthly bile acid profiles were analyzed as previously described (26, 27). An internal standard mixture of D4-cholate, D4-β-muricholate, D4-a-muricholate, D4-chenodeoxycholate, D4-deoxycholate, D4-hyodeoxycholate, D4-ursodeoxycholate, and D4-lithocholate, and their tauroconjugated and glycoconjugated counterparts, was prepared in acetonitrile. Eluted bile salts were detected by negative ion, electrospray mass spectrometry on a linear trap linear ion trap mass spectrometer (Thermo-Finnigan, San Jose, CA) using a data-dependent stage of detection triggering a qualitative 0 m/z neutral loss scan at 27% normalized collision energy. Peak areas from initial scans of individual bile salts at 514.3, 498.3, 464.3, 447.3, 411.3, 407.3, 395.3, 393.3, and 391.3 m/z were integrated, and response factors were defined by peak area ratios of analytes to that of internal, deuterated standards. The response factors were read against those obtained from standard curves in surrogate matrix, and molar levels of serum or plasma levels were interpolated from standard curves. Response factors for all samples were comprised of peak area ratios of nonlabeled salts normalized to the stable-labeled counterparts. Concentrations were interpolated by linear regression from curves of known standards.
Cell culture
3T3-L1 cells were maintained in high glucose (25 mm) DMEM supplemented with 10% newborn calf serum and penicillin-streptomycin. When confluent (designated d 0), cells were switched to high insulin (1.7 μm), high glucose DMEM supplemented 10% fetal bovine serum (FBS). Differentiation of 3T3-L1 preadipocytes was induced at d 2 by switching to high insulin, high glucose DMEM supplemented with 10% FBS, 1 μm dexamethasone, and 0.5 mm 3-isobutyl-l-methylxanthine. After 48 h, the medium was switched back to high insulin, high glucose DMEM supplemented 10% FBS. Differentiated adipocytes were exposed to one of three doses of acyl-ghrelin (AG) or desacyl-ghrelin (DAG) (Bachem; Torrance, CA) calculated to correspond with the concentrations seen in the VSG-operated animals (AG low, 22 pmol/liter; and DAG low, 89 pmol/liter) and the S-WM animals (AG medium, 65 pmol/liter; and DAG medium, 260 pmol/liter). A high dose (AG high, 130 pmol/liter; and DAG high, 520 pmol/liter) corresponding to a 2-fold higher dose than the medium dose was also included. The concentration of AG present in the circulation was estimated using the 1-month total ghrelin concentration data set and previously published data demonstrating a 1:5 ratio of acyl to total ghrelin in the circulation in rats (28). Cells were incubated with the appropriate dose of ghrelin for 24 h, and then media were collected for assessment of adiponectin secretion by RIA, and mRNA was extracted from cells using TRIzol reagent (Invitrogen, Carlsbad, CA) for assessment of adiponectin mRNA expression. cDNA was generated using high-capacity cDNA RT kit (Applied Biosystems, Foster City, CA). Adiponectin mRNA levels were determined by real-time PCR (iCycler; Bio-Rad, Hercules, CA) and normalized to TATA box-binding protein (TBP). Primers used were adiponectin forward, GTTGCAAGCTCTCCTGTTCC and adiponectin reverse, CTCCAGGAGTGCCATCTCT; TBP forward, GAAGCTGCGGTACAATTCCAG and TBP reverse, CCCCTTGTACCCTTCACCAAT.
Statistics and data analysis
Data are presented as mean ± sem. Statistical analyses were performed using GraphPad Prism 4.00 for Windows (GraphPad Software, San Diego, CA). The incidence of diabetes was analyzed by log-rank testing of Kaplan-Meier survival curves. All other data were analyzed by two-factor (time × treatment) repeated measures ANOVA followed by post hoc analysis with Bonferroni's multiple comparison test or Student's t test as indicated. Differences were considered significant at P < 0.05. Values are expressed as mean ± sem.
Results
VSG surgery delays the onset of diabetes
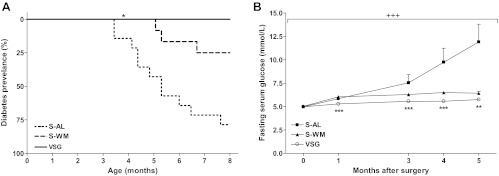
Compared with S-AL animals, the onset of diabetes was delayed in S-WM and VSG-operated animals by approximately 1 and 3.5 months, respectively. Furthermore, the age of diabetes onset was delayed in VSG-operated animals compared with S-WM animals by approximately 2.5 months. The mean ages of diabetes onset were 6.1 ± 0.5, 7.0 ± 0.2, and 9.6 ± 0.2 months in S-AL, S-WM, and VSG-operated animals, respectively (P < 0.05 VSG compared with S-WM and S-AL) (Table 1). By 8 months of age, none of the VSG-operated animals had become diabetic, whereas 3/16 S-WM and 11/14 S-AL animals were diabetic. Thus, within the first 8 months of age, VSG significantly delayed diabetes onset compared with both the S-AL and S-WM groups (P < 0.05) (Fig. 1A). At 1 yr of age, S-WM and VSG-operated animals both exhibited significantly delayed diabetes onset compared with S-AL animals (P < 0.001), but at this small sample size and longer time course, the time of onset was not significantly different between S-WM and VSG groups by Kaplan-Meier analysis. Similarly, diabetes-free days up to 1 yr of age were significantly greater in S-WM and VSG-operated animals compared with S-AL animals (P < 0.001) but did not differ between S-WM and VSG-operated animals (Table 1). The delay in the onset of diabetes corresponded with lower fasting serum glucose concentrations in S-WM and VSG-operated animals compared with S-AL animals (Fig. 1B).
Table 1.
Age of diabetes onset and incidence in animals followed to 1 yr of age
| S-AL | S-WM | VSG | |
|---|---|---|---|
| Age of onset (d) | 182 ± 16 | 210 ± 6 | 288 ± 23a |
| Incidence (%) | 100 | 19 | 23 |
| Diabetes-free days | 182 ± 16 | 336 ± 16b | 347 ± 9b |
| n | 14 | 16 | 13 |
Values are mean ± sem.
P < 0.05 compared with S-AL and S-WM.
P < 0.001 compared with S-AL by Student's t test.
Fig. 1.
Kaplan-Meier analysis of diabetes incidence in S-AL (n = 14), S-WM (n = 16), and VSG-operated animals up to 8 months of age (n = 13) (A). *, P < 0.05 compared with S-AL and S-WM by log-rank test. Fasting serum glucose concentrations (B). +++, P < 0.001 by two-factor repeated measures ANOVA. ***, P < 0.001; **, P < 0.01 by Bonferroni's post hoc test compared with S-AL.
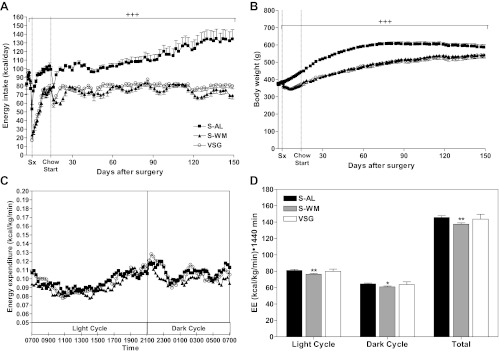
As expected, energy intake and body weight were higher in S-AL animals compared with S-WM and VSG-operated animals (P < 0.001) (Fig. 2, A and B). Energy intake was reduced by approximately 23% in VSG-operated animals compared with S-AL. Energy intake and body weight did not differ between S-WM and VSG-operated animals. Energy expenditure in VSG-operated animals did not differ compared with S-AL or S-WM groups (Fig. 2, C and D). However, energy expenditure was approximately 5% lower in S-WM animals compared with S-AL (P < 0.05). The observed decrease of energy expenditure with restriction of energy intake is similar to that reported in previous rodent and primate studies examining the effect of chronic energy intake restriction on energy expenditure (29, 30). It is interesting that surgically induced energy restriction did not produce a similar effect and suggests a mechanism by which bariatric surgery patients are better able to defend body weight loss after bariatric surgery compared with dieting alone (31).
Fig. 2.
Energy intake (A), body weight (B), energy expenditure (C), and AUC of energy expenditure (D) in S-AL (n = 14), S-WM (n = 16), and VSG-operated animals (n = 13). +++, P < 0.001 for S-WM and VSG compared with S-AL by two-factor repeated measures ANOVA with Bonferroni's post hoc test. **, P < 0.01; *, P < 0.05 compared with S-AL by Student's t test.
VSG surgery improves circulating lipids and hormones
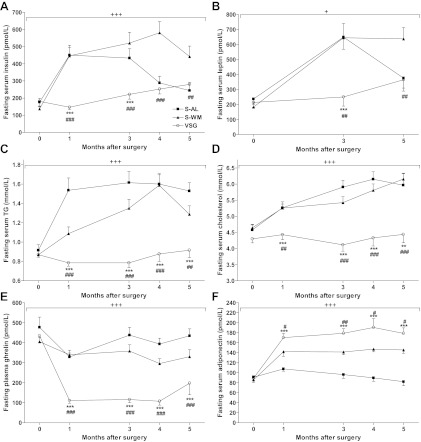
Monthly fasting plasma insulin remained significantly lower in VSG-operated animals compared with both S-AL and S-WM animals up to 3 months after surgery (P < 0.001), at which point, insulin values began to decline in S-AL animals due to the onset of diabetes and progressive β-cell decompensation (Fig. 3A). Circulating insulin concentrations continued to be lower in VSG-operated animals compared with S-WM up to 5 months after surgery, suggesting an improvement of insulin sensitivity, independent of body weight (P < 0.01). Fasting plasma leptin concentrations remained lower in VSG-operated animals compared with S-WM up to 5 months after surgery (P < 0.01) (Fig. 3B). We have previously reported that fasting plasma leptin concentrations increase in the UCD-T2DM rat before diabetes onset due to increasing adiposity and increasing circulating insulin concentrations. Conversely, fasting plasma leptin concentrations decrease after diabetes onset in the UCD-T2DM rat due to decreasing adipose mass and decreasing circulating insulin concentrations (21). Leptin concentrations in S-AL animals began to decline at 3 months after surgery, likely due to the increased prevalence of diabetes and corresponding decreases of circulating insulin concentrations and adipose mass.
Fig. 3.
Fasting serum insulin (A), fasting serum leptin (B), fasting serum TG (C), fasting serum cholesterol (D), fasting plasma ghrelin (E), and fasting serum adiponectin (F) in S-AL (n = 14), S-WM (n = 16), and VSG-operated animals (n = 13). +++, P < 0.001; +, P < 0.05 compared with S-AL and S-WM by two-factor repeated measures ANOVA. ***, P < 0.001; **, P < 0.01; *, P < 0.05 compared with S-AL; ###, P < 0.001 ##, P < 0.01; #, P < 0.05 compared with S-WM by Bonferroni's post hoc test.
Circulating lipids were markedly improved in VSG-operated animals compared with both S-AL and S-WM animals. Fasting plasma TG concentrations were 50% lower in VSG-operated animals compared with S-AL and S-WM up to 5 months after surgery (P < 0.001) (Fig. 3C). Furthermore, fasting plasma cholesterol concentrations were lower in VSG-operated animals compared with S-AL and S-WM up to 5 months after surgery (P < 0.001) (Fig. 3D).
Similar to previous studies in both rodents and humans, fasting plasma ghrelin concentrations were reduced by approximately 75% in VSG-operated animals compared with both S-AL and S-WM (P < 0.001) (Fig. 3E). Fasting plasma adiponectin concentrations were elevated in S-WM and VSG-operated animals compared with S-AL (P < 0.001) (Fig. 3F). However, fasting plasma adiponectin concentrations were further elevated in VSG-operated animals compared with S-WM, demonstrating a weight-independent increase of circulating adiponectin concentrations after VSG surgery (P < 0.05) (Fig. 3F).
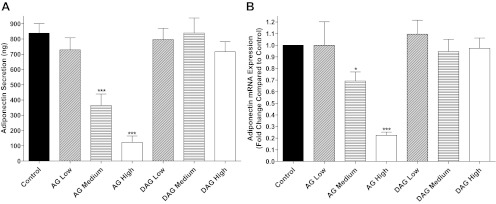
A previous study reported that incubation of brown adipocytes with ghrelin impairs adiponectin production (32). Thus, we hypothesized that decreases of circulating ghrelin in VSG-operated animals may have contributed to the body weight-independent augmentation of circulating adiponectin concentrations. To test this hypothesis, we exposed differentiated 3T3-L1 adipocytes to concentrations of acylated ghrelin calculated from the total ghrelin concentrations (28) measured in the VSG-operated animals (AG low) and from the ghrelin concentrations measured in the S-WM animals (AG medium) and a dose 2-fold higher than the medium dose of ghrelin (AG high). Adiponectin secretion and mRNA expression were not affected by exposure to AG concentrations similar to the concentrations found in VSG-operated animals. However, adiponectin secretion and mRNA expression were reduced in a step-wise manner with exposure to AG concentrations similar to the concentrations found in S-WM animals as well as by 2-fold higher AG concentrations. Incubation of differentiated 3T3-L1 adipocytes with similar concentrations of inactive DAG did not affect adiponectin secretion or adiponectin mRNA expression (Fig. 4).
Fig. 4.
Adiponectin secretion (A) and adiponectin mRNA expression (B) in fully differentiated 3T3-L1 cells exposed to acylated ghrelin (AG low, 22 pmol/liter; AG medium, 65 pmol/liter; and AG high, 130 pmol/liter) or desacylated ghrelin (DAG low, 89 pmol/liter; DAG medium, 260 pmol/liter; DAG high, 520 pmol/liter). Cells were incubated with ghrelin at the indicated concentrations for 24 h, and then media were collected and cells lysed for mRNA collection. *, P < 0.05; ***, P < 0.001 compared with Control by Student's t-test.
VSG surgery increases circulating bile acid concentrations
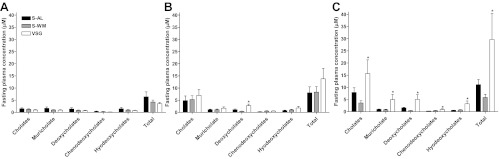
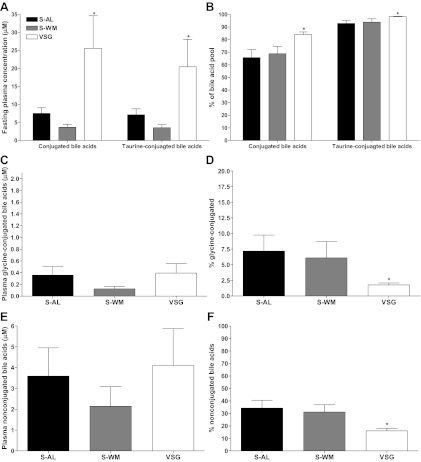
Circulating bile acid profiles did not differ between groups at baseline (Fig. 5A). At 3 months after surgery, there was a 2-fold increase of circulating deoxycholic acid concentrations (P < 0.05), but total circulating bile acid concentrations did not differ between groups (Fig. 5B). However, at 5 months after surgery, total bile acid concentrations and all bile acid subtype concentrations were elevated in VSG-operated animals compared with S-AL and S-WM (P < 0.05) (Fig. 5C). The elevation of circulating total bile acid concentrations in VSG-operated animals was primarily due to an increase of conjugated bile acids as both an absolute value and as a percentage of the total bile acid pool (P < 0.05) (Fig. 6, A and B). Specifically, taurine-conjugated bile acids were elevated approximately 3-fold in VSG-operated animals compared with S-AL and S-WM (P < 0.05) (Fig. 6, A and B). Glycine-conjugated bile acids did not differ between groups as an absolute value (Fig. 6C), but glycine-conjugated bile acids as a proportion of total conjugated bile acids were lower in VSG-operated animals compared with S-AL and S-WM (P < 0.05) (Fig. 6D). Fasting plasma-unconjugated bile acids did not differ between groups when expressed as an absolute value (Fig. 6E). However, unconjugated bile acids were proportionally lower in VSG-operated animals compared with S-AL and S-WM (P < 0.05) (Fig. 6F). Thus, VSG surgery results in a preferential increase of circulating taurine-conjugated bile acid concentrations.
Fig. 5.
Fasting plasma bile acid profile at baseline (A), 3 months after surgery (B) and 5 months after surgery (C) in S-AL (n = 14), S-WM (n = 16), and VSG-operated animals (n = 13). *, P < 0.05 compared with S-AL and S-WM by Student's t test.
Fig. 6.
Fasting plasma total-conjugated bile acid and taurine-conjugated bile acid concentrations (A). Conjugated bile acid concentrations expressed as a percentage of the total bile acid pool and taurine-conjugated bile acids expressed as a percentage of the total-conjugated bile acid pool (B). Fasting plasma glycine-conjugated bile acid concentrations (C). Glycine-conjugated bile acids expressed as a percentage of the total-conjugated bile acid pool (D). Fasting plasma-unconjugated bile acid concentrations (E). Unconjugated bile acids expressed as a percentage of the total bile acid pool (F). All values are shown at 5 months after surgery in S-AL (n = 14), S-WM (n = 16), and VSG-operated animals (n = 13). *, P < 0.05 by Student's t test.
VSG surgery improves insulin secretion and increases nutrient-stimulated GLP-1, GLP-2, and PYY secretion
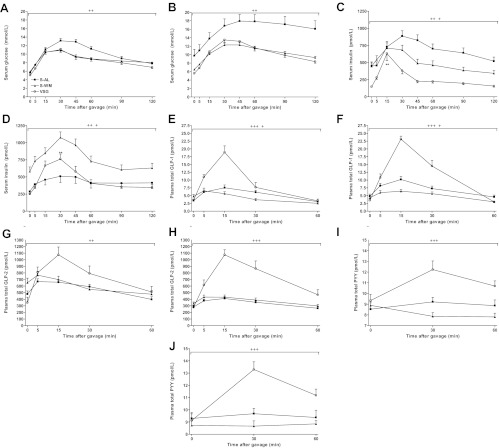
OGTT were performed at 1 and 4 months after surgery to assess changes of glucose tolerance and nutrient-stimulated gastrointestinal hormone secretion over time. Glucose excursions were significantly lower in S-WM and VSG-operated animals at both 1 and 4 months after surgery (P < 0.01) (Fig. 7, A and B). VSG resulted in significantly lower insulin excursions, as indexed by area under the curve (AUC), compared with S-WM at both 1 and 4 months after surgery, suggesting an improvement of insulin sensitivity (P < 0.05) (Fig. 7, C and D). At 4 months after surgery, insulin excursions were markedly blunted in S-AL animals due to the greater prevalence of diabetes in this group and the concomitant impairment of β-cell function (Fig. 7D). Importantly, the percent increase of plasma insulin concentrations from fasting to peak values was significantly higher in VSG-operated animals compared with both S-AL and S-WM during both the 1 and 4 month OGTT, demonstrating superior islet function in VSG-operated animals (P < 0.01) (Fig. 7, C and D).
Fig. 7.
Serum glucose at 1 (A) and 4 (B) months after surgery, serum insulin at 1 (C) and 4 (D) months after surgery, plasma total GLP-1 at 1 (E) and 4 (F) months after surgery, plasma total GLP-2 at 1 (G) and 4 (H) months after surgery, and plasma total PYY at 1 (I) and 4 (J) months after surgery, after an oral glucose gavage (1 g dextrose/kg body weight) in S-AL (n = 14), S-WM (n = 16), and VSG-operated animals (n = 13). +++, P < 0.001 for VSG compared with S-AL and S-WM; ++, P < 0.01 for VSG and S-WM compared with S-AL; +, P < 0.05 for S-AL compared with S-WM by Student's t test of the AUC. **, P < 0.01 for the percent change of serum insulin concentrations from baseline to peak values compared with S-AL and S-WM by Student's t-test.
Peak plasma GLP-1 concentrations were 3-fold higher in VSG-operated animals compared with S-AL and S-WM animals at both 1 and 4 months after surgery (P < 0.001) (Fig. 7, E and F). Furthermore, GLP-1 excursions were significantly higher at 4 months after surgery compared with at 1 month after surgery in VSG-operated animals, demonstrating preservation of postoperative increases of postprandial GLP-1 secretion (P < 0.05). The GLP-1 AUC was significantly lower in S-WM compared with S-AL animals during both the 1 and 4 month OGTT (P < 0.05). Similarly, nutrient-stimulated GLP-2 secretion was significantly elevated in VSG-operated animals compared with S-AL and S-WM (P < 0.05) (Fig. 7, G and H). This effect was preserved up to 4 months after surgery.
PYY excursions were markedly elevated in VSG-operated animals compared with S-AL and S-WM animals at both 1 and 4 months after surgery (P < 0.001) (Fig. 7, I and J). Although the PYY AUC in VSG-operated animals tended to be higher at 4 months after surgery compared with 1 month after surgery, this difference did not reach statistical significance. However, these data also demonstrate the durability of postoperative increases of postprandial PYY secretion in VSG-operated animals.
Discussion
In this study, we demonstrate that VSG performed in prediabetic male UCD-T2DM rats delays the onset of type 2 diabetes and results in long-lasting improvements of glucose and lipid metabolism. Postoperative decreases of food intake and body weight contributed to the delay in diabetes onset, as there was approximately a 5-month increase in the number of diabetes-free months in S-WM and VSG-operated animals compared with S-AL. However, metabolic and endocrine changes after VSG surgery likely contributed to the delay in diabetes onset, as VSG surgery resulted in a significantly higher age of diabetes onset and a significant delay in diabetes onset up to 8 months of age by Kaplan-Meier analysis compared with weight-matched controls. The weight-independent metabolic and endocrine changes that we observed after VSG surgery that likely contributed to the delay in diabetes onset include increased postprandial GLP-1 secretion, increased circulating bile acid and adiponectin concentrations, and decreased circulating ghrelin concentrations.
Glucose-stimulated GLP-1 and GLP-2 secretion were elevated at both 1 and 4 months after surgery in VSG-operated animals. Postprandial GLP-1 secretion in VSG-operated animals was further elevated at 4 months after surgery compared with 1 month after surgery, demonstrating that this response does not taper over time. The “hind-gut” hypothesis postulates that increases of postprandial GLP-1 secretion after bariatric surgery are due to increased delivery of incompletely absorbed nutrients to the distal small intestine, resulting in direct stimulation of L cells to release GLP-1 (33). Although VSG surgery increases gastric emptying (34), the ability of VSG surgery to increase the flux of incompletely absorbed nutrients in the distal small intestine is questionable, because it does not involve bypass of the proximal small intestine. Furthermore, human clinical studies have demonstrated similar postprandial GLP-1 secretion profiles after VSG compared with RYGB (9). This suggests that mechanisms other than that proposed in the hind-gut hypothesis may contribute to postoperative increases of postprandial GLP-1 secretion. One potential contributor is activation of a vagal reflex by nutrients in the proximal small intestine (19).
Increases of GLP-1 secretion likely contributed to the improvement of insulin sensitivity and islet function. Improvement of glucose-stimulated insulin secretion was observed during the OGTT at 1 and 4 months after surgery, in which VSG-operated animals exhibited approximately 2-fold greater glucose-stimulated insulin secretion compared with both S-AL and S-WM animals. As an incretin hormone, GLP-1 potentiates glucose-stimulated insulin secretion (18). Furthermore, GLP-1 has been shown to have beneficial effects on the islet by increasing insulin synthesis, stimulating β-cell proliferation, and preventing β-cell apoptosis (35). Increases of GLP-2 secretion may have contributed to the increases of nutrient-stimulated GLP-1 secretion, because GLP-2 is known to stimulate proliferation of intestinal cells and thus may contribute to an increase in L-cell number (36). Nutrient-stimulated GLP-2 secretion has been shown to be elevated after ileal interposition (IT) surgery in rats and early after RYGB and VSG in humans (37, 38). However, this study demonstrates that the increased GLP-2 response is maintained over time.
The decreases of fasting circulating insulin concentrations and decreased insulin AUC during both the 1 and 4 month OGTT compared with weight-matched animals suggest an improvement of insulin sensitivity that is independent of body weight. Increases of nutrient-stimulated GLP-1 secretion may have improved insulin sensitivity by reducing glucotoxicity and lipotoxicity. GLP-1 reduces glucotoxicity by improving islet function and reducing hepatic gluconeogenesis (18). GLP-1 may contribute to reductions in lipotoxicity by stimulating fat oxidation (39). VSG-operated animals exhibited improvements of lipid metabolism with markedly lower fasting plasma lipids.
Bile acids have been shown to be elevated after RYGB and IT surgery and may represent another mechanism involved in the metabolic improvements after bariatric surgery (40, 41). Here, we found marked increases of total circulating bile acid concentrations at 5 months after surgery. These increases were similar in magnitude to those reported in obese rats after IT surgery. However, bile acid profiles were different in VSG-operated animals compared with what has been previously reported after IT surgery (40). The increase of circulating bile acid concentrations after VSG surgery was primarily due to increased taurine-conjugated bile acids with proportional decreases of unconjugated bile acid concentrations. In contrast, Kohli et al. (40) report marked preferential increases of unconjugated bile acids along with increases of glycine and taurine-conjugated bile acid concentrations. These differences in circulating bile acid profiles between IT and VSG surgery suggest that the mechanisms responsible for postoperative increases of circulating bile acid concentrations likely differ based on the type of bariatric surgery performed.
The increases of circulating bile acid concentrations after VSG surgery may have contributed to the observed improvements of insulin sensitivity and circulating lipids. Bile acids have been shown to decrease hepatic gluconeogenesis and lipogenesis and increase insulin-mediated glucose disposal in adipocytes by signaling through farnesoid X receptor (42). Furthermore, administration of taurine-conjugated bile acids to rats has been shown to improve insulin signaling and decrease the expression of gluconeogenic genes, likely by signaling through farnesoid X receptor (43). Bile acids have also been shown to signal through TGR5 receptors located in the distal small intestine to increase GLP-1 secretion and through TGR5 receptors on skeletal muscle and brown adipose tissue to increase energy expenditure (42, 44). Energy expenditure did not differ between VSG-operated animals and controls. However, energy expenditure was measured at 1.5 months after surgery, before a significant increase of circulating bile acid concentrations was observed.
Similar to previous studies of RYGB and VSG, circulating adiponectin concentrations were significantly elevated after VSG surgery compared with ad libitum-fed controls (45). However, to our knowledge, this is the first study to demonstrate postoperative increases of circulating adiponectin concentrations compared with a weight-matched control, indicating that postoperative increases of circulating adiponectin are likely due to additional mechanisms beyond the reduction of body weight. We hypothesized that decreases of circulating ghrelin concentrations after VSG surgery contributed to the body weight-independent effects of VSG surgery to increase circulating adiponectin concentrations. This is supported by our in vitro results demonstrating significant inhibition of both adiponectin mRNA expression and secretion in 3T3-L1 adipocytes exposed to active acylated ghrelin concentrations similar to those of sham-operated animals. Increases of circulating adiponectin likely contributed to the improvement of insulin sensitivity and circulating lipids. Adiponectin is an adipokine that has been demonstrated to improve insulin sensitivity and lipid metabolism (46). Furthermore, circulating adiponectin concentrations are reduced in obesity and type 2 diabetes (47). Adiponectin has been proposed to improve insulin sensitivity by signaling in liver and skeletal muscle through activation of AMP kinase to promote decreased gluconeogenesis, increased insulin-mediated glucose uptake, and increased fatty acid oxidation resulting in decreased ectopic lipid deposition (46).
Similar to previous studies in rodents and humans, we demonstrated a marked decrease of circulating ghrelin concentrations after VSG surgery (10, 48). Decreased circulating ghrelin concentrations likely contributed to reduced food intake, improved glucose-stimulated insulin secretion, and improved insulin sensitivity. Ghrelin has been shown to promote food intake and increase adiposity (49). Reductions of circulating ghrelin likely contributed to improved islet function, because previous studies have demonstrated that both physiologic and pharmacologic doses of exogenous ghrelin impair glucose-stimulated insulin secretion in cell culture studies and in humans (15, 17). This has been suggested to be mediated by activation of voltage-dependent K+ channels resulting in decreased calcium-mediated insulin release (15). Ghrelin has also been shown to promote insulin resistance, because ablation of ghrelin in both the ob/ob mouse and high-fat-fed mice has been shown to improve insulin sensitivity (16). Furthermore, inhibition of ghrelin activation by ghrelin o-acyltransferase using a ghrelin o-acyltransferase antagonist results in improvement of glucose tolerance and body weight, making ghrelin an attractive pharmaceutical target for the treatment of type 2 diabetes (50).
In conclusion, we have shown for the first time that VSG surgery delays diabetes onset in the UCD-T2DM rat model of type 2 diabetes, an effect that is partially independent of reduced body weight. Furthermore, VSG surgery results in marked improvements of glucose and lipid metabolism with improved islet function and decreased circulating lipids. Increases of circulating adiponectin, bile acids, and GLP-1 concentrations and decreases of circulating ghrelin all likely contributed to the effect of VSG surgery to delay type 2 diabetes onset. Further studies on the effect of VSG surgery and other bariatric surgeries to delay type 2 diabetes onset in the UCD-T2DM rat will help to identify new pharmaceutical targets for the treatment and prevention of type 2 diabetes.
Acknowledgments
We thank Philip Sipes for technical assistance with bile acids analysis; Ruby Hsieh and Tak Hou Fong for assistance with study procedures; and Alison Huynh, Michelle Flores, Kelly Quilligan, Roel Vink, Wesley Wong, Sue Bennet, Cheryl Phillips, and the Meyer Hall Animal Facility for excellent animal care. We also thank Linda Jung and Meso Scale Discovery for the use of the Sector Imager 2400.
This work was supported by the University of California, Davis Veterinary Scientist Training Program, and by National Institutes of Health (NIH) Grants 1RC1DK087307-01, AT-002993, and AT-003545. P.J.H.'s laboratory also received funding during the project period from NIH Grants HL-075675, HL-091333, and R01-HL-107256, and a Multicampus Award from the University of California, Office of the President. F.G.H.'s laboratory was supported by JDRF Grant 1-2009-337 and NIH Grant RO1DK090492.
Disclosure Summary: B.P.C., A.B., J.L.G., K.L.S., F.G.H., and P.J.H. have nothing to disclose. M.K. and M.L.C. work for Eli Lilly, Inc.
Footnotes
- AG
- Acyl-ghrelin
- AUC
- area under the curve
- DAG
- desacyl-ghrelin
- FBS
- fetal bovine serum
- GLP
- glucagon-like peptide
- IT
- ileal interposition
- OGTT
- oral glucose tolerance test
- PYY
- peptide YY
- PDS
- polydioxanone
- RYGB
- Roux en Y gastric bypass
- S-AL
- sham-operated ad libitum fed
- S-WM
- sham-operated weight matched
- TBP
- TATA box-binding protein
- TG
- triglyceride
- UCD-T2DM
- University of California Davis-type 2 diabetes mellitus
- VSG
- vertical sleeve gastrectomy.
References
- 1. Buchwald H, Avidor Y, Braunwald E, Jensen MD, Pories W, Fahrbach K, Schoelles K. 2004. Bariatric surgery: a systematic review and meta-analysis. JAMA 292:1724–1737 [DOI] [PubMed] [Google Scholar]
- 2. Cummings BP, Strader AD, Stanhope KL, Graham JL, Lee J, Raybould HE, Baskin DG, Havel PJ. 2010. Ileal interposition surgery improves glucose and lipid metabolism and delays diabetes onset in the UCD-T2DM rat. Gastroenterology 138:2437–2446, 2446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Sjöström L, Lindroos AK, Peltonen M, Torgerson J, Bouchard C, Carlsson B, Dahlgren S, Larsson B, Narbro K, Sjöström CD, Sullivan M, Wedel H. 2004. Lifestyle, diabetes, and cardiovascular risk factors 10 years after bariatric surgery. N Engl J Med 351:2683–2693 [DOI] [PubMed] [Google Scholar]
- 4. Abbatini F, Rizzello M, Casella G, Alessandri G, Capoccia D, Leonetti F, Basso N. 2010. Long-term effects of laparoscopic sleeve gastrectomy, gastric bypass, and adjustable gastric banding on type 2 diabetes. Surg Endosc 24:1005–1010 [DOI] [PubMed] [Google Scholar]
- 5. Jacobs M, Bisland W, Gomez E, Plasencia G, Mederos R, Celaya C, Fogel R. 2010. Laparoscopic sleeve gastrectomy: a retrospective review of 1- and 2-year results. Surg Endosc 24:781–785 [DOI] [PubMed] [Google Scholar]
- 6. Vidal J, Ibarzabal A, Romero F, Delgado S, Momblán D, Flores L, Lacy A. 2008. Type 2 diabetes mellitus and the metabolic syndrome following sleeve gastrectomy in severely obese subjects. Obes Surg 18:1077–1082 [DOI] [PubMed] [Google Scholar]
- 7. Rizzello M, Abbatini F, Casella G, Alessandri G, Fantini A, Leonetti F, Basso N. 2010. Early postoperative insulin-resistance changes after sleeve gastrectomy. Obes Surg 20:50–55 [DOI] [PubMed] [Google Scholar]
- 8. Chambers AP, Jessen L, Ryan KK, Sisley S, Wilson-Pérez HE, Stefater MA, Gaitonde SG, Sorrell JE, Toure M, Berger J, D'Alessio DA, Woods SC, Seeley RJ, Sandoval DA. 2011. Weight-independent changes in blood glucose homeostasis after gastric bypass or vertical sleeve gastrectomy in rats. Gastroenterology 141:950–958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Peterli R, Wölnerhanssen B, Peters T, Devaux N, Kern B, Christoffel-Courtin C, Drewe J, von Flüe M, Beglinger C. 2009. Improvement in glucose metabolism after bariatric surgery: comparison of laparoscopic Roux-en-Y gastric bypass and laparoscopic sleeve gastrectomy: a prospective randomized trial. Ann Surg 250:234–241 [DOI] [PubMed] [Google Scholar]
- 10. Karamanakos SN, Vagenas K, Kalfarentzos F, Alexandrides TK. 2008. Weight loss, appetite suppression, and changes in fasting and postprandial ghrelin and peptide-YY levels after Roux-en-Y gastric bypass and sleeve gastrectomy: a prospective, double blind study. Ann Surg 247:401–407 [DOI] [PubMed] [Google Scholar]
- 11. Wren AM, Bloom SR. 2007. Gut hormones and appetite control. Gastroenterology 132:2116–2130 [DOI] [PubMed] [Google Scholar]
- 12. Date Y, Nakazato M, Hashiguchi S, Dezaki K, Mondal MS, Hosoda H, Kojima M, Kangawa K, Arima T, Matsuo H, Yada T, Matsukura S. 2002. Ghrelin is present in pancreatic α-cells of humans and rats and stimulates insulin secretion. Diabetes 51:124–129 [DOI] [PubMed] [Google Scholar]
- 13. Volante M, Allìa E, Gugliotta P, Funaro A, Broglio F, Deghenghi R, Muccioli G, Ghigo E, Papotti M. 2002. Expression of ghrelin and of the GH secretagogue receptor by pancreatic islet cells and related endocrine tumors. J Clin Endocr Metab 87:1300–1308 [DOI] [PubMed] [Google Scholar]
- 14. Wierup N, Svensson H, Mulder H, Sundler F. 2002. The ghrelin cell: a novel developmentally regulated islet cell in the human pancreas. Regul Peptides 107:63–69 [DOI] [PubMed] [Google Scholar]
- 15. Dezaki K, Kakei M, Yada T. 2007. Ghrelin uses Gαi2 and activates voltage-dependent K+ channels to attenuate glucose-induced Ca2+ signaling and insulin release in islet β-cells: novel signal transduction of ghrelin. Diabetes 56:2319–2327 [DOI] [PubMed] [Google Scholar]
- 16. Sun Y, Asnicar M, Saha PK, Chan L, Smith RG. 2006. Ablation of ghrelin improves the diabetic but not obese phenotype of ob/ob mice. Cell Metab 3:379–386 [DOI] [PubMed] [Google Scholar]
- 17. Tong J, Prigeon RL, Davis HW, Bidlingmaier M, Kahn SE, Cummings DE, Tschöp MH, D'Alessio D. 2010. Ghrelin suppresses glucose-stimulated insulin secretion and deteriorates glucose tolerance in healthy humans. Diabetes 59:2145–2151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Baggio LL, Drucker DJ. 2007. Biology of incretins: GLP-1 and GIP. Gastroenterology 132:2131–2157 [DOI] [PubMed] [Google Scholar]
- 19. Rocca AS, Brubaker PL. 1999. Role of the vagus nerve in mediating proximal nutrient-induced glucagon-like peptide-1 secretion. Endocrinology 140:1687–1694 [DOI] [PubMed] [Google Scholar]
- 20. Batterham RL, Cowley MA, Small CJ, Herzog H, Cohen MA, Dakin CL, Wren AM, Brynes AE, Low MJ, Ghatei MA, Cone RD, Bloom SR. 2002. Gut hormone PYY(3-36) physiologically inhibits food intake. Nature 418:650–654 [DOI] [PubMed] [Google Scholar]
- 21. Cummings BP, Digitale EK, Stanhope KL, Graham JL, Baskin DG, Reed BJ, Sweet IR, Griffen SC, Havel PJ. 2008. Development and characterization of a novel rat model of type 2 diabetes mellitus: the UC Davis type 2 diabetes mellitus UCD-T2DM rat. Am J Physiol Regul Integr Comp Physiol 295:R1782–R1793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lopez PP, Nicholson SE, Burkhardt GE, Johnson RA, Johnson FK. 2009. Development of a sleeve gastrectomy weight loss model in obese Zucker rats. J Surg Res 157:243–250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ziegler O, Sirveaux MA, Brunaud L, Reibel N, Quilliot D. 2009. Medical follow up after bariatric surgery: nutritional and drug issues. General recommendations for the prevention and treatment of nutritional deficiencies. Diabetes Metab 35:544–557 [DOI] [PubMed] [Google Scholar]
- 24. Nygaard K, Killander A, Myhre E, Helsingen N. 1966. Long-term observation of gastrectomized rats with regard to development of vitamin B12 deficiency. Scand J Haematol 3:213–221 [DOI] [PubMed] [Google Scholar]
- 25. Carpenter JW. ed. 2005 Exotic animal formulary. 3rd ed New York: Elsevier Saunders [Google Scholar]
- 26. Bootsma AH, Overmars H, van Rooij A, van Lint AE, Wanders RJ, van Gennip AH, Vreken P. 1999. Rapid analysis of conjugated bile acids in plasma using electrospray tandem mass spectrometry: application for selective screening of peroxisomal disorders. J Inherit Metab Dis 22:307–310 [DOI] [PubMed] [Google Scholar]
- 27. Torchia EC, Labonté ED, Agellon LB. 2001. Separation and quantitation of bile acids using an isocratic solvent system for high performance liquid chromatography coupled to an evaporative light scattering detector. Anal Biochem 298:293–298 [DOI] [PubMed] [Google Scholar]
- 28. Stengel A, Keire D, Goebel M, Evilevitch L, Wiggins B, Taché Y, Reeve JR., Jr 2009. The RAPID method for blood processing yields new insight in plasma concentrations and molecular forms of circulating gut peptides. Endocrinology 150:5113–5118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ballor DL. 1991. Effect of dietary restriction and/or exercise on 23-h metabolic rate and body composition in female rats. J Appl Physiol 71:801–806 [DOI] [PubMed] [Google Scholar]
- 30. Ramsey JJ, Roecker EB, Weindruch R, Kemnitz JW. 1997. Energy expenditure of adult male rhesus monkeys during the first 30 mo of dietary restriction. Am J Physiol 272:E901–E907 [DOI] [PubMed] [Google Scholar]
- 31. Mingrone G, Panunzi S, De Gaetano A, Guidone C, Iaconelli A, Leccesi L, Nanni G, Pomp A, Castagneto M, Ghirlanda G, Rubino F. 2012. Bariatric surgery versus conventional medical therapy for type 2 diabetes. N Engl J Med 366:1577–1585 [DOI] [PubMed] [Google Scholar]
- 32. Ott V, Fasshauer M, Dalski A, Meier B, Perwitz N, Klein HH, Tschöp M, Klein J. 2002. Direct peripheral effects of ghrelin include suppression of adiponectin expression. Horm Metab Res 34:640–645 [DOI] [PubMed] [Google Scholar]
- 33. Thaler JP, Cummings DE. 2009. Minireview: hormonal and metabolic mechanisms of diabetes remission after gastrointestinal surgery. Endocrinology 150:2518–2525 [DOI] [PubMed] [Google Scholar]
- 34. Horner KM, Byrne NM, Cleghorn GJ, Näslund E, King NA. 2011. The effects of weight loss strategies on gastric emptying and appetite control. Obes Rev 12:935–951 [DOI] [PubMed] [Google Scholar]
- 35. Brubaker PL, Drucker DJ. 2004. Minireview: glucagon-like peptides regulate cell proliferation and apoptosis in the pancreas, gut, and central nervous system. Endocrinology 145:2653–2659 [DOI] [PubMed] [Google Scholar]
- 36. Estall JL, Drucker DJ. 2006. Glucagon-like peptide-2. Annu Rev Nutr 26:391–411 [DOI] [PubMed] [Google Scholar]
- 37. Romero F, Nicolau J, Flores L, Casamitjana R, Ibarzabal A, Lacy A, Vidal J. 1 February 2012. Comparable early changes in gastrointestinal hormones after sleeve gastrectomy and Roux-En-Y gastric bypass surgery for morbidly obese type 2 diabetic subjects. Surg Endosc 10.1007/s00464-012-2166-y [DOI] [PubMed] [Google Scholar]
- 38. Thulesen J, Hartmann B, Kissow H, Jeppesen PB, Orskov C, Holst JJ, Poulsen SS. 2001. Intestinal growth adaptation and glucagon-like peptide 2 in rats with ileal-jejunal transposition or small bowel resection. Digest Dis Sci 46:379–388 [DOI] [PubMed] [Google Scholar]
- 39. Sancho V, Trigo MV, Martín-Duce A, Gonz Lez N, Acitores A, Arnés L, Valverde I, Malaisse WJ, Villanueva-Peñacarrillo ML. 2006. Effect of GLP-1 on D-glucose transport, lipolysis and lipogenesis in adipocytes of obese subjects. Int J Mol Med 17:1133–1137 [PubMed] [Google Scholar]
- 40. Kohli R, Kirby M, Setchell KD, Jha P, Klustaitis K, Woollett LA, Pfluger PT, Balistreri WF, Tso P, Jandacek RJ, Woods SC, Heubi JE, Tschoep MH, D'Alessio DA, Shroyer NF, Seeley RJ. 2010. Intestinal adaptation after ileal interposition surgery increases bile acid recycling and protects against obesity-related comorbidities. Am J Physiol Gastrointest Liver Physiol 299:G652–G660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Patti ME, HS, Bernier R, Bianco AC, Larsen PR, Holst JJ, Mun E, Auwerx J, Goldfine AB. 2007. Gastric bypass surgery increases bile acid levels: potential contribution to improved glucose tolerance. Diabetes S 6:A379 [Google Scholar]
- 42. Thomas C, Pellicciari R, Pruzanski M, Auwerx J, Schoonjans K. 2008. Targeting bile-acid signalling for metabolic diseases. Nat Rev Drug Discov 7:678–693 [DOI] [PubMed] [Google Scholar]
- 43. Cao R, Cronk ZX, Zha W, Sun L, Wang X, Fang Y, Studer E, Zhou H, Pandak WM, Dent P, Gil G, Hylemon PB. 2010. Bile acids regulate hepatic gluconeogenic genes and farnesoid X receptor via G(α)i-protein-coupled receptors and the AKT pathway. J Lipid Res 51:2234–2244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Watanabe M, Houten SM, Mataki C, Christoffolete MA, Kim BW, Sato H, Messaddeq N, Harney JW, Ezaki O, Kodama T, Schoonjans K, Bianco AC, Auwerx J. 2006. Bile acids induce energy expenditure by promoting intracellular thyroid hormone activation. Nature 439:484–489 [DOI] [PubMed] [Google Scholar]
- 45. Benedix F, Westphal S, Patschke R, Granowski D, Luley C, Lippert H, Wolff S. 2011. Weight loss and changes in salivary ghrelin and adiponectin: comparison between sleeve gastrectomy and Roux-en-Y gastric bypass and gastric banding. Obes Surg 21:616–624 [DOI] [PubMed] [Google Scholar]
- 46. Kadowaki T, Yamauchi T. 2005. Adiponectin and adiponectin receptors. Endocr Rev 26:439–451 [DOI] [PubMed] [Google Scholar]
- 47. Li S, Shin HJ, Ding EL, van Dam RM. 2009. Adiponectin levels and risk of type 2 diabetes: a systematic review and meta-analysis. JAMA 302:179–188 [DOI] [PubMed] [Google Scholar]
- 48. Shin AC, Zheng H, Townsend RL, Sigalet DL, Berthoud HR. 2010. Meal-induced hormone responses in a rat model of Roux-en-Y gastric bypass surgery. Endocrinology 151:1588–1597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Tschöp M, Smiley DL, Heiman ML. 2000. Ghrelin induces adiposity in rodents. Nature 407:908–913 [DOI] [PubMed] [Google Scholar]
- 50. Barnett BP, Hwang Y, Taylor MS, Kirchner H, Pfluger PT, Bernard V, Lin YY, Bowers EM, Mukherjee C, Song WJ, Longo PA, Leahy DJ, Hussain MA, Tschöp MH, Boeke JD, Cole PA. 2010. Glucose and weight control in mice with a designed ghrelin O-acyltransferase inhibitor. Science 330:1689–1692 [DOI] [PMC free article] [PubMed] [Google Scholar]