Abstract
Prolactinomas are the most prevalent type of secreting pituitary tumors in humans and generally respond well to a medical therapy with dopamine agonists. However, for patients exhibiting resistance to dopaminergic drugs, alternative treatments are desired. Antiangiogenic strategies might represent a potential therapy for these tumors. Thrombospondin 1 (TSP-1) is a large multifunctional glycoprotein involved in multiple biological processes including angiogenesis, apoptosis, and activation of TGF-β1. Because tumors that overexpress TSP-1 grow more slowly, have fewer metastases, and have decreased angiogenesis, TSP-1 provides a novel target for cancer treatment. ABT-510 and ABT-898 are TSP-1 synthetic analogs that mimic its antiangiogenic action. In the present study, we explored the potential effect of ABT-510 and ABT-898 on experimental prolactinomas induced by chronic diethylstilbestrol (DES) treatment in female rats. We demonstrated that a 2-wk treatment with ABT-510 and ABT-898 counteracted the increase in pituitary size and serum prolactin levels as well as the pituitary proliferation rate induced by DES. These inhibitory effects on tumor growth could be mediated by the antiangiogenic properties of the drugs. We also demonstrated that ABT-510 and ABT-898, in addition to their described antiangiogenic effects, increased active TGF-β1 level in the tumors. We postulate that the recovery of the local cytokine activation participates in the inhibition of lactotrope function. These results place these synthetic TSP-1 analogs as potential alternative or complementary treatments in dopamine agonist-resistant prolactinomas.
In most human tumors, angiogenesis correlates with tumor behavior (1). Development of new blood vessels are needed for oxygen and the supply of basic energetic compounds (2). Therefore, the development of antiangiogenic agents that target tumor vasculature and interrupt tumor growth is of great interest (3).
Tissue angiogenesis depends on the balance of proangiogenic and antiangiogenic factors, and the interactions of these factors with extracellular matrix components allow new capillary development and endothelial cell migration.
Pituitary tumors are common benign adenomas that produce alterations of pituitary hormone secretion and compressive damage to adjacent tissues (4–6). Controversial results have been described referring to angiogenesis during pituitary adenoma generation because the normal pituitary is highly vascularized. On the other hand, differences in the angiogenic pattern have been described to be associated with hormonal phenotypes, size or invasion (7–9).
Among all secreting pituitary adenomas, prolactinomas are the most frequent tumors in adults accounting for 60% of all functioning pituitary tumors (10). They are usually benign and can be treated with dopaminergic agents. Nevertheless, 15% of these tumors may be resistant to classical pharmacological therapy, become invasive and aggressive, and require extirpation. The decrease or loss of response of dopamine D2 receptors has been described in these cases (11, 12), and alternative therapies would be desired for these tumors (13). The estrogen-treated rat is an interesting and well-known model of pituitary hyperplasia (14, 15). Increased pituitary weight, hyperprolactinemia, lactotrope hyperplasia, and reduced dopaminergic action at the pituitary level are found in chronically estrogenized female rats. Moreover, estrogen increases the local levels of potent proangiogenic factors like vascular endothelial growth factor (VEGF) and basic fibroblast growth factor (16), and the tumorigenic action of estradiol correlates with its ability to induce a direct arterial blood supply to the pituitary (17). Therefore, estrogen-induced prolactin-secreting pituitary tumors are highly angiogenic (17). Furthermore, tumor growth can be blocked by antiangiogenic agents (18, 19). In this regard we have demonstrated a significant decrease in vascularization and tumor growth using anti-VEGF strategies in dopamine-resistant prolactinomas (20).
Although anti-VEGF strategies have been used in many clinical trials, not all were successful (21, 22); therefore, the development of new antiangiogenic therapies have become of increasing interest. Among new drugs being studied are small peptides mimicking thrombospondin 1 (TSP-1), an antiangiogenic protein (23). TSP-1 is a large multifunctional matrix glycoprotein involved in multiple biological processes including angiogenesis, apoptosis, activation of TGF-β1, and platelet aggregation, among others (24). One of the most relevant functions is its role as an antiangiogenic factor, inhibiting the proliferation and migration of endothelial cells by interaction with its receptor CD36 expressed on the cell surface. Because tumors that overexpress TSP-1 grow slower, have fewer metastases, and exhibit less angiogenesis, TSP-1 provides a novel target for cancer treatment. Based on the CD36-binding peptide sequence from TSP-1, small molecules mimicking the antiangiogenic properties of TSP-1 were developed (23). These drugs act as antiangiogenic factors and slow tumor growth in preclinical models (25–27). ABT-510 and ABT-898 (Abbott Laboratories, Abbot Park, IL), two of such analogs, belong to the new generation of antiangiogenic drugs.
The role of TSP-1 in pituitary tumorogenesis is not well understood. Immunoreactive TSP-1 is distributed in the anterior pituitary, particularly in endothelial cells (28), and estradiol treatment for 2 and 4 wk decreased the total pituitary as well as the endothelial-cell specific immunoreactive TSP-1 levels in the gland (29).
On the other hand, TSP-1 is one of the main physiological activators of TGF-β1 in vitro and in vivo (30). TGF-β1 is a potent cytokine released from cells in a latent form coupled to latent TGF-β binding proteins (LTBP). The LTBP facilitate the secretion of latent TGF-β and mediate the targeting of the latent cytokine into extracellular matrix (ECM) structures, regulating localization, availability, and TGF-β1 activation (31). Activation of TGF-β1 involves the proteolytic release from its latent complex and due to the powerful actions of the cytokine, activation is a highly regulated process that precedes receptor binding. TGF-β1 signals through its transmembrane heteromeric Ser-Thr kinase receptor (type I: TβRI and type II: TβRII) that transduces intracellular signals via phosphorylation and nuclear translocation of Smad-2 and Smad3 proteins, which modulate the transcription of a large number of genes (32).
In the pituitary TGF-β1 is a known inhibitor of lactotrope function. Moreover, dopamine interacts with TGF-β1 to regulate lactotrope cell proliferation and prolactin (PRL) secretion. In female rats in vitro and in vivo, 17β-estradiol treatment decrease pituitary TGF-β1 and TβRII mRNA and protein, concomitantly with an increase in PRL levels (33–35). Therefore, the inhibition of TGF-β1 and TβRII might cooperate in the development of the prolactinoma induced by estradiol.
We hypothesized that because the decreased TSP-1, altered TGF-β1 pituitary expression, and increased angiogenesis are involved in prolactinoma development, compounds that mimic the TSP-1 antiangiogenic action would be effective in reducing tumor growth.
In the present study, we sought to investigate the in vivo effect of synthetic analogs of TSP-1 intramolecular small sequence (TSR-1) (ABT-510 and ABT-898), in estrogen-induced prolactinomas in female adult rats. We demonstrate that both ABT-510 and ABT-898 reduce tissue angiogenesis, pituitary weight, and serum prolactin levels in female rats with prolactinomas induced by chronic diethylstilbestrol (DES) treatment. In addition, we demonstrate that ABT-510 and ABT-898, independently of their described antiangiogenic effects, increase active TGF-β1 in the tumors and in an ex vivo assay using hemipituitaries in culture. These results place these synthetic TSP-1 analogs as potential alternative or complementary treatments in dopamine agonist-resistant prolactinomas.
Materials and Methods
Animals
Two-month-old female Sprague Dawley rats were housed in a temperature-controlled room with lights on at 0700 h and off at 1900 h with free access to laboratory chow and tap water. All experimental procedures were reviewed and approved by the Institutional Animal Care and Use Committee of the Instituto de Biología y Medicina Experimental, Buenos Aires (Division of Animal Welfare, Office for Protection of Research Risks, National Institutes of Health, A no. 5072-01).
Prolactinomas were induced by chronic exposure to estrogens using the following protocol: female rats were ovariectomized and sc implanted with a 20-mg DES pellet (DES group). This procedure was performed under anesthesia (ip 50 mg/kg ketamine, 10 mg/kg xylazine). After 4 wk prolactinomas were evaluated (see below).
Experimental protocol for ABT-510 and ABT-898 treatment in DES rats
Two weeks after ovariectomy and DES pellet implantation, animals were divided into three groups and treated with 100 mg/kg ABT-510 (ip) or 100 mg/kg ABT-898 or vehicle (5% dextrose, DES control). The drugs were injected three times per week during 2 wk. Forty-eight hours after the last injection, all animals were euthanized and trunk blood was collected. Pituitaries were carefully excised and weighed after removing the neurohypophysis. Hemianterior pituitaries were used for Western blot, immunohistochemistry, and quantitative real-time RT-PCR (Q-RT-PCR). Whole pituitaries were used for ELISA.
Short-term ABT-510 treatment in control and DES rats
Female cycling rats in diestrus were injected (ip) with 100 mg/kg ABT-510 or vehicle (5% dextrose) and euthanized after 30 min, 2 h, or 24 h to evaluate the short-term effect of the drug. DES control rats were injected with 100 mg/kg ABT-510 or vehicle and killed 2 h later. Trunk blood was collected for serum PRL determination, and the pituitary glands were excised and collected for ELISA.
Drugs
DES (Sigma, St Louis, MO), ABT-510, and ABT-898 peptides were generously supplied by Abbott Laboratories.
RIA
Serum PRL levels were measured by RIA using reagents provided by the National Institute of Diabetes and Digestive and Kidney Diseases, National Hormone and Pituitary Program (Dr. A. F. Parlow, Torrance, CA). Results are expressed in nanograms per milliliter in terms of rat PRL RP3. Intra- and interassay coefficients of variation were 6.9 and 11.6%, respectively.
Quantitative real-time RT-PCR
Hemipituitaries from different experimental groups were collected in RNA-later (Ambion, Austin, TX). Total RNA was extracted from the tissue using the RNeasy Protect minikit (QIAGEN, Valencia, CA). Reverse transcription was performed using 750 ng of total RNA and the resulting cDNA was used for Q-RT-PCR analysis. Q-RT-PCR were performed using specific primers and the QuantiFast SYBR green PCR kit (QIAGEN) on an iCycler thermal cycler (Bio-Rad, Hercules, CA). Different gene transcript expression was quantified by comparing the threshold cycle (CT) with that of β-actin by using the comparative CT method (ΔΔCT). Primers used were as follows: TGF-β1 forward, 5′-CAACAATTCCTGGCGTTACC-3′ and reverse, 5′-AGCCCTGTATTCCGTCTCCT-3′; LTBP1 forward, 5′-AGCACCGTCACCTCTGCTCT-3′ and reverse, 5′-ATCCTCGCAGTGGTCTCCAA-3′; LTBP3 forward, 5′-ATGGCCTCAGTTGCATAGAC-3′ and reverse, 5′-AAGGAGCCTGGTGTGTTCGT-3′; LTBP4 forward, 5′-CTGCGCGGAAGCGAATGTGC-3′ and reverse, 5′-TCCGCGCACGGGTGACAATC-3′; β-actin forward, 5′-CCACCAGTTCGCCATGGATGAC-3′ and reverse, 5′-GAGCATCGTCGCCCGCGAA-3′.
Detection of total and active TGF-β1
An ELISA was performed to quantify active or total TGF-β1 content in pituitary homogenates with the TGF-β1 DuoSet ELISA development system (DY1679, R&D Systems, Minneapolis, MN), following the manufacturer's instructions.
Entire anterior pituitary glands from different experimental groups were collected and processed as described for Western blotting. Equal amounts of protein were loaded per well, and TGF-β1 was expressed as picograms per milligram of protein.
To assay total TGF-β1, samples were acidified to pH 2.6 by adding 1 n HCl for 20 min at room temperature, followed by neutralization with 1 n NaOH to pH 7.6.
Immunohistochemistry
Hemianterior pituitaries from different experimental groups were fixed by immersion in 10% buffered formalin and subsequently embedded in paraffin. After deparaffinization, pituitary sections were microwaved in 10 mm sodium citrate buffer (pH 6) and left to cool for 20 min at room temperature. Endogenous peroxidase activity and nonspecific binding sites were blocked. Primary antibodies were incubated overnight at 4 C. After incubation with biotin-conjugated secondary antibodies for 1 h, the reactions were developed using a streptavidin-biotin peroxidase method and diaminobenzidine as a chromogen substrate. Samples were counterstained with hematoxylin and mounted with a permanent mounting medium. Negative controls replacing the primary antibody with PBS were included. The antibody used was the polyclonal antibody for cluster of differentiation 31 (CD31) detection (sc-1506, 1:100; Santa Cruz Biotechnologies Inc., Santa Cruz, CA).
Quantification of pituitary vascular area
Pituitary microvasculature was evaluated on CD31 immunostained sections. Images of randomly selected fields were recorded using an Axiostar Plus microscope (Carl Zeiss Inc., Thornwood, NY) and a PowerShot G6 digital camera (Canon, Lake Success, NY). Three parameters were evaluated: microvessel density was calculated by counting the number of CD31-positive vessels per square millimeter; the relative vascular area was calculated as the sum of the areas occupied by vessels expressed as percentage vessel area per total tissue area; and the size of the vessels was also evaluated by calculating the mean area of vessels. Three slides per pituitary were analyzed and at least four images were counted per slide using the image processing and analysis software (Image J, http://rsbweb.nih.gov/ij/).
A description of the Western blotting procedure, the in vitro assay procedure in the GH3 cell line, and the ex vivo experiment is detailed in Supplemental Data Materials and Methods, published on The Endocrine Society's Journals Online web site at http://endo.endojournals.org.
Statistical analysis
Results are expressed as means ± sem. The differences between means were evaluated by a Student's t test or a one-way ANOVA followed by a Tukey's honestly significant difference test. P < 0.05 was considered significant.
Results
ABT-510 and ABT-898 decreased prolactinoma growth induced by DES in female rats
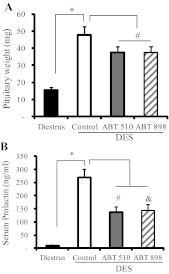
Experimental prolactinomas were induced in adult female rats by sc implantation of a 20-mg DES pellet (DES group). As previously described, pituitary weight and serum PRL levels were markedly increased after 4 wk of DES pellet implantation compared with rats in diestrus (Fig. 1, A and B). Cotreatment for 2 wk with ABT-510 or ABT-898 significantly reduced pituitary weight and serum PRL in DES-treated rats (Fig. 1, A and B). The effects of ABT-510 and ABT-898 were not different.
Fig. 1.
ABT-510 and ABT-898 reduced prolactinoma growth induced by DES. Pituitary tumors were induced in adult female rats by sc implantation of a 20-mg DES pellet. After 2 wk of DES pellet implantation, rats were injected three times a week during 2 wk with 100 mg/kg ABT-510, 100 mg/kg ABT-898, or vehicle (5% dextrose, DES control group). A, Pituitary weight increased 3-fold after DES treatment compared with untreated diestrous rats. *, P = 0.0002. Both ABT-510 and ABT-898 treatment significantly reduced pituitary weight; #, P = 0.03. B, Serum PRL levels were measured by RIA. DES increased serum PRL compared with diestrus levels. *, P = 0.0002. ABT-510 and ABT-898 decreased elevated serum PRL induced by DES; #, P = 0.036, &, P = 0.028, respectively (n = 11, 12, 14, and 15).
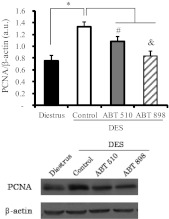
We next evaluated the effect of both analogs in the proliferating cell nuclear antigen (PCNA) expression by Western blot in pituitary homogenates, as an indirect parameter of cell proliferation. PCNA protein levels were higher in DES control pituitaries compared with those pituitaries from rats in diestrus as expected due to the mitogenic effect of estrogens on lactotrope and endothelial cells. Treatment with both TSP-1 analogs reduced PCNA expression in DES-treated pituitaries, and ABT-898 treatment was more successful (Fig. 2).
Fig. 2.
ABT-510 and ABT-898 treatment decreased PCNA expression induced by DES. Pituitaries of the different experimental groups were homogenized and protein expression was analyzed by Western blot. PCNA expression was normalized to β-actin. PCNA expression was higher in the DES control group compared with the diestrus group. *, P = 0.0002. Both ABT-510 and ABT-898 significantly reduced the PCNA protein levels. #, P = 0.03; &, P = 0.0002. Representative bands are shown (n = 6, 10, 11, and 10).
Antiangiogenic effect of ABT-510 and ABT-898 treatment
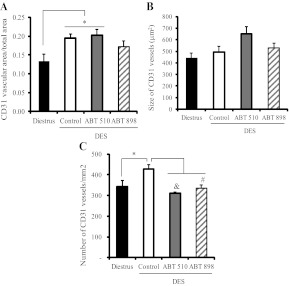
The reduction in pituitary weight, serum PRL, and PCNA expression observed after ABT treatment indicated a clear role of these peptides in inhibiting tumor growth in DES-induced prolactinomas. To elucidate whether this effect could be attributed to the antiangiogenic properties of the TSP-1 analogs, we analyzed the vascular area and microvessel density by immunohistochemical staining with the endothelial cell marker CD31. The vascular area, as well as the number of CD31-positive vessels per area, was increased in DES pituitaries, whereas the average size of the vessels was not modified (Fig. 3).
Fig. 3.
Antiangiogenic effect of ABT-510 and ABT-898 treatment in experimental prolactinomas. Pituitary vasculature was analyzed by CD31 immunostaining. A, Relative CD31-stained vascular area (CD31+ vascular area/total area) was higher in DES control and ABT-510-treated pituitaries compared with the pituitaries of rats in diestrus. *, P = 0.033; #, P = 0.037. B, Nonsignificant differences were found in the average size of CD31-stained vessels. C, Microvessel density (number of CD31 stained vessels per square millimeter) was increased in the DES control group compared with diestrus. *, P = 0.014. Treatment with ABT-510 and ABT-898 reduced microvessel density induced by DES; &, P = 0.01 and #, P = 0.027, respectively (n = 5, 7, 4, and 5).
ABT-510 and ABT-898 treatment significantly decreased microvessel density, without a significant effect on their average size. The increase in total vascular area induced by DES was not modified by ABT-510 but was prevented by ABT-898 (diestrus vs. ABT-898, P = 0.3, which was nonsignificant) (Fig. 3, A–C). Representative images can be found in Supplemental Fig. 1.
TSP-1 synthetic analogs normalize active TGF-β1 in DES-induced prolactinomas
We have previously reported that the activation of TGF-β1 was impaired in a mouse model of dopamine-resistant prolactinoma and that active TGF-β1 levels inversely correlated with serum PRL values (36). On the other hand, Sarkar et al. (29) showed that 4 wk of estradiol treatment in female rats decreased pituitary TGF-β1 synthesis, TGF-β1 receptor type II expression, and the total TSP-1 immunoreactive protein in the anterior pituitary.
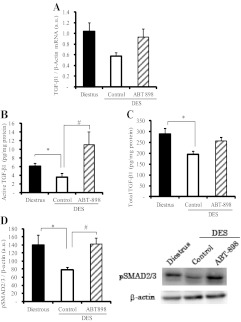
On this basis, we evaluated the effect of ABT-898 treatment on local active TGF-β1 and cytokine synthesis. No significant differences were found in pituitary TGF-β1 mRNA levels among groups despite a tendency to lower values in the DES control group (Fig. 4A).
Fig. 4.
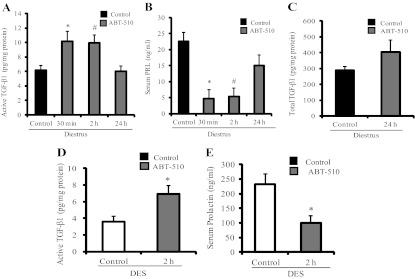
Treatment with TSP-1 analogs restored active TGF-β1 in DES-induced prolactinomas. A, Pituitary TGF-β1 mRNA expression was measured by Q-RT-PCR. Nonsignificant differences were found between groups (n = 4, 4, and 5). B, Active TGF-β1 content was determined by ELISA in the pituitary glands from the different experimental groups. ABT-898 treatment markedly restored active TGF-β1 decreased by DES. *, P = 0.036; #, P = 0.01. C, Total TGF-β1 was measured by ELISA after sample acidification. Total TGF-β1 content was lower in DES control pituitaries compared with pituitaries from rats in diestrus, whereas ABT-898 did not modify these levels. *, P = 0.04 (n = 8, 5, and 6). C, Phosphorylated SMAD2/3 (pSMAD2/3) protein expression, determined by Western blot in pituitary homogenates, was attenuated by DES and increased by ABT-898 treatment. *, P = 0.022; #, P = 0.002. Representative bands are shown. β-Actin was used as loading control (n = 9, 10, and 9).
Both active and total TGF-β1 content (measured by ELISA) was decreased in pituitaries from DES rats compared with female rats in diestrus. Less than 3% of total pituitary TGF-β1 was found in the active form, reflecting the high degree of regulation in the cytokine activation process. We found that ABT-898 treatment markedly enhanced active but not total TGF-β1 in DES-treated rats (Fig. 4, B and C).
When we analyzed phosphorylated (p) Smad2/3 expression, we found that the main signaling pathway triggered by TGF-β1 and therefore a parameter of cytokine activity was significantly decreased in DES control pituitaries. ABT-898 fully restored pSmad2/3 levels, indicating a recovery in TGF-β1 activity within the tumors (Fig. 4D). Similar results were observed with ABT-510 treatment (data not shown).
Given the alterations induced by DES in the pituitary TGF-β1 system and the fact that ABT-898 treatment restored local cytokine protein levels and activity, we next studied the impact of these synthetic TSR-1 analogs on other components of the TGF-β1 system, especially those involved in TGF-β1 secretion, storage, and availability in the ECM: LTBP1, LTBP3, and LTBP4 (31).
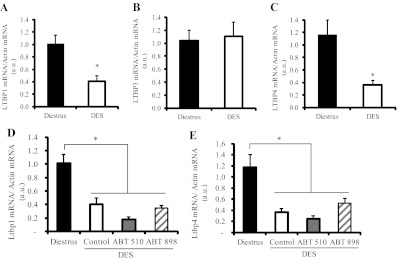
We demonstrated LTBP1, LTBP3, and LTBP4 mRNA expression in the pituitary glands of female rats. Interestingly, we found a differential effect of the steroid on the expression of the binding proteins: LTBP1 and LTBP4, but not LTBP3, expression was inhibited by DES treatment (Fig. 5A). Neither ABT-510 nor ABT-898 treatment restored LTBP1 or LTBP4 levels in DES-exposed pituitaries (Fig. 5, A–C).
Fig. 5.
Latent TGF-β binding protein mRNA are expressed in normal and tumoral pituitaries and differentially controlled by DES. LTBP1, LTBP3, and LTBP4 are expressed in normal pituitaries and in prolactinomas induced by DES. A, Pituitary LTBP1 mRNA was decreased after 4 wk DES pellet implantation in female rats. *, P = 0.009 (n = 4). B, LTBP3 mRNA expression was not altered by DES. C, LTBP4 mRNA was significantly reduced by DES. *, P = 0.01 (n = 4). D and E, Treatment with ABT-510 and ABT-898 did not restore either LTBP1 (D, *, P < 0.001, n = 4) or LTBP4 (E, *, P < 0.001, n = 4) expression in DES-induced prolactinomas.
Short-term effect of ABT-510 in pituitary TGF-β1 activation
Given that this is the first report of the effect of synthetic analogs of TSP-1 intramolecular small sequence molecules on TGF-β1 activation, we further characterized this finding by studying their impact on active TGF-β1 levels at short times. We evaluated pituitary active TGF-β1 by ELISA after 30 min, 2 h, and 24 h of ABT-510 injections (100 mg/kg) in control female rats in diestrus. Total cytokine was measured only at 24 h, the time at which synthesis could also be involved.
Although neither ABT-510 nor ABT-898 peptides contain any of the reported TGF-β1 activation sites, we found that ABT-510 increased local TGF-β1 activity as soon as 30 min after injection and that the effect was lost after 24 h (Fig. 6A). Interestingly, serum PRL values were significantly decreased after 30 min and 2 h of ABT-510 injections, concomitantly with the increase in TGF-β1 activity (Fig. 6B). Total pituitary TGF-β1 was not altered after 24 h of ABT 510 treatment (Fig. 6C).
Fig. 6.
Short-term effect of ABT-510 in pituitary TGF-β1 activation and serum PRL. A, Pituitary active TGF-β1 was evaluated by ELISA after 30 min, 2 h, or 24 h of a 100 mg/kg ABT-510 single injection in control female rats in diestrus. ABT-510 enhanced active TGF-β1 content in pituitary glands after 30 min and 2 h. *, P = 0.04; #, P = 0.02 (n = 10, 4, 8, and 6). B, Serum PRL levels measured by RIA were reduced after 30 min and 2 h of ABT-510 treatment. *, P = 0.006; #, P = 0.001 (n = 10, 4, 8, and 6). C, Total TGF-β1 content was not modified after 24 h of an ABT-510 injection in control rats in diestrus. D, Pituitary active TGF-β1 was also induced by ABT-510 after 2 h in a group of 4-wk DES-treated rats. *, P = 0.036 (n = 5). E, Serum PRL values were reduced by ABT-510 after 2 h in DES rats. *, P = 0.02 (n = 5).
We next evaluated whether the short-term effect of the TSP-1 analog on pituitary cytokine activation we observed in vivo in rats in diestrus was also found in DES-treated rats. As shown in Fig. 6, D and E, ABT-510 increased active TGF-β1 levels in DES pituitaries and lowered serum PRL values after 2 h of injection.
To determine whether the drugs could have a direct effect on lactotrope cells, we evaluated active TGF-β1 content and PRL secretion in the GH3 cell line after 5, 15, and 30 min of ABT-898 stimulation. We did not find differences between ABT-898-treated and control cells in any of the parameters evaluated (Supplemental Fig. 2, A and B). ABT-898 treatment had no effect in the proliferation rate of the GH3 cell line measured after 3 d either (Supplemental Fig. 2C).
However, when hemipituitaries from diestrous female rats were incubated in an ex vivo experimental model, in which the ECM structure is maintained intact, 1 μm ABT-898 caused a 21% increment on active TGF-β1 after 30 min in tissue homogenates (paired samples, Supplemental Fig. 3).
Discussion
In the present study, we explored the potential effect of the synthetic analogs of TSP-1, ABT-510 and ABT-898, in the treatment of experimental prolactinomas. We demonstrated that a 2-wk treatment with ABT-510 and ABT-898 counteracted the increase in pituitary size and serum prolactin levels as well as the pituitary proliferation rate induced by DES. The inhibitory effect of ABT-510 and ABT-898 on the tumor growth parameters evaluated was mediated, at least in part, by the antiangiogenic properties of these drugs because ABT-510 and ABT-898 decreased the number of CD31-positive vessels per area in DES-induced prolactinomas, indicating an antagonism of DES-induced angiogenesis. Interestingly, in addition to the antiangiogenic properties of these drugs, we found that they increased active TGF-β1 in the pituitary, possibly contributing to the reduction in serum prolactin release and in the inhibition of prolactinoma growth.
Previous work showed that TSP-1 is widely distributed in the anterior pituitary, and in particular it colocalizes with endothelial cell markers. Chronic estradiol treatment decreases the level of immunoreactive TSP-1 in the anterior pituitary, and the local decrease of this antiangiogenic factor (29) might be involved in the development of estradiol induced prolactinomas. Therefore, we postulated that mimicking the antiangiogenic activity of TSP-1 could reduce prolactinoma growth.
The two modified peptides we assayed in this study were developed based on the active sequence GVITRIR present in the second type I repeat of TSP-1, which was described to bind and activate CD36 (37). ABT-510, one of the first TSP-1 mimetic peptides developed, has potent proapoptotic activity in cultured cells and is clinically well tolerated with therapeutic benefits reported against several malignancies (23). This compound was evaluated in phase II clinical trials for the treatment of head and neck cancer, non-small-cell lung cancer, lymphoma, and renal cell carcinoma (23, 26, 38–41). The second-generation TSP-1 synthetic analog, ABT-898, was found to have greater potency than ABT-510 and is expected to have greater efficacy than the other available TSP-1 mimetic peptides (27, 42) due to its lower clearance rate. However, we found no differences between ABT-510 and ABT-898 potency in the reduction of pituitary weight or serum PRL levels, even though ABT-898 was more efficient in reducing PCNA expression
With regard to the antiangiogenic effect of these analogs in PRL-secreting tumors, we found a reduction in the microvascular density increased by DES, assessed as the number of CD31-stained vessels per square millimeter. Measurement of intratumoral microvasculature density has been used to investigate angiogenesis in different tumors. Microvascular density correlates with a poor prognosis in solid tumors and with the concentration and expression of proangiogenic growth factors, fibroblast growth factor-2 and VEGF, in several cancers (43). Our group and others have reported that VEGF is widely expressed in pituitary tumors (44, 45), with higher levels in macroprolactinomas (46–48), especially in dopamine agonist-resistant prolactinomas (12, 48, 49). Furthermore, a significant increase in microvessel density, assessed by CD31 staining, was observed in invasive compared with noninvasive prolactinomas (50).
Even though only ABT-898 treatment prevented the increase in the relative CD31 vascular area in DES tumors, both ABT-510 and ABT-898 diminished the number of vessels. This effect is likely due to the reported apoptotic action of these drugs on the microvasculature because the interaction of TSR-containing proteins with CD36 present in microvascular endothelium mediates their potent antiangiogenic and proapoptotic response (51). We cannot discard that apoptosis of secretory cells induced by hypoxia, as a result from the antiangiogenic therapies, could also collaborate with the reduction in pituitary size and serum prolactin levels observed in our in vivo experiments. However, there are conflicting findings regarding this topic because it was recently demonstrated that antiangiogenic agents can also normalize the abnormal structure and function of tumor vessels improving tumor oxygenation and drug delivery in cancer treatments (reviewed in Ref. 52). Supporting this, Campbell et al. (53) reported that a treatment with ABT-510 in advanced ovarian cancer not only decreased vascular density but also increased mature blood vessels and caused a reduction of tumor tissue hypoxia, suggesting a normalization of tumor vasculature. On this basis, other factors might be involved in the reduction of tumor size caused by TSP-1 analogs.
A very interesting and novel finding in this work is the fact that ABT-510 and ABT-898 reversed the inhibition of TGF-β1 activation in the pituitary mediated by DES. Because TGF-β1 is an inhibitor of lactotrope secretion and proliferation (33, 54), the enhanced cytokine activity elicited by ABT-510 and ABT-898 treatment might have a role in the prolactinoma regression. Of considerable importance is that less than 3% of total TGF-β1 was found in the active form. This is similar to what has been described in other tissues (55) and underlines the importance of evaluating biologically active TGF-β1 rather than cytokine expression, synthesis, or secretion of total TGF-β1. On the other hand, these data highlight the highly regulated activation process that enables the release of mature TGF-β1 and allows this potent cytokine to bind to its receptor.
It is important to note that the effects of a 2-wk treatment with ABT-510 and ABT-898 on TGF-β1 were observed only on active and not on total TGF-β1 levels. The increase in cytokine activation was also reflected in the recovery of pSmad2/3 expression. Moreover, we also demonstrated in vivo that TSP-1 analogs were able to increase pituitary active TGF-β1 at short term (30 min) with a concomitant reduction in serum prolactin levels in female control rats in diestrus as well as in the 4-wk DES pellet-implanted group. These results suggest that ABT-510 and ABT-898 might have a direct effect on TGF-β1 activation, namely releasing the mature cytokine from its latent complexes stored in the extracellular matrix, and disclose a novel property, which may underlie the therapeutic effects of the analogs.
We can hypothesize that the inhibition of PRL secretion and cell proliferation caused by TSP-1 analogs treatment may be mediated by the increment of active TGF-β1 levels or by a direct effect of the drug on lactotrope cells. We tested this second possibility by treating GH3 cells with ABT-898, and we did not find a direct effect of TSP-1 analog on active TGF-β1 levels, PRL secretion, or proliferation rate.
However, assays in pituitary cell lines do not always reflect what actually occurs in the pituitary tissue, in particular when changes in the extracellular matrix are involved, as in the case of TGF-β1 activation. We effectively demonstrated that ABT-898 was able to increase active TGF-β1 at short times in cultured ex vivo hemipituitaries, an experimental model in which ECM and tissue structures are maintained intact.
Even though we did not observe any effect of ABT-898 on GH3 cells, we cannot discard a direct effect of ABT-510 and ABT-898 on lactotropes because CD36 expression on nonendothelial cell types including macrophages, dendritic cells, hepatocytes, adipocytes, cardiac and skeletal myocytes, and specialized epithelia of the breast, kidney, and gut among others has been demonstrated (reviewed in Ref. 56).
The mechanism underlying TGF-β1 activation by these peptides in both in vivo and ex vivo assays remains to be elucidated. Interestingly, ABT-510 and ABT-898 sequences do not contain any of the reported TGF-β1 activation sites located in the TSP-1 type 1 repeats (57). However, because the GVITRIR sequence binds and activates CD36, and this receptor is present in endothelial cells, we speculate that these small peptides might participate in TGF-β1 activation through complexing with other ECM components once bound to its receptor. Supporting this, it has been described that the activation of the CD36 receptor enables its large extracellular domain to interact with other membrane receptors and integrins (51, 56).
To unravel the action of TSP-1 mimetic peptides on ECM components that might be involved in TGF-β activation, we measured, in the in vivo experiments, the impact of these drugs on the regulation of TGF-β latent binding proteins. LTBP facilitate the secretion of latent TGF-β and mediate the targeting of latent complexes into ECM structures, which is important for TGF-β activation and function (31). The different LTBP have only partially overlapping expression patterns. LTBP1 and LTBP3 associate with the propeptides of all three TGF-β isoforms, but LTBP4 binds only TGF-β1-LAP and more weakly than the other LTBP. Thus, it is possible that LTBP may also differentially contribute to TGF-β activation (31, 58, 59). Interestingly, we found that LTBP1, LTBP3, and LTBP4, the three LTBP that bind TGF-β1, are expressed in pituitary tissue, and they respond differentially to DES treatment. LTBP1 and LTBP4 levels, but not LTBP3, were reduced by DES treatment. This result could be related to the lower levels of active TGF-β1 found in this group. In accordance, reduced TGF-β activation in correlation with a decreased production and secretion of LTBP has been described by several transformed cells (60, 61). Furthermore, TGF-β1 enhances its own expression as well as LTBP1 levels in both normal and transformed human lung fibroblasts (62). We recently described reduced LTBP1 and LTBP4 but not LTBP3 levels in pituitaries from female mice lacking dopamine type 2 receptors (unpublished data). Therefore, we do not discard a local dopamine regulation on LTBP1 and LTBP4 mRNA levels. Supporting this last possibility, ABT-510 or ABT-898 administration did not normalize LTBP levels, even though TGF-β1 activity was restored. This is the first report of LTBP expression and regulation in normal and tumoral pituitary tissue.
As previously mentioned, prolactinomas are the most prevalent type of pituitary tumors in humans and generally respond well to a medical therapy with dopamine agonists. However, for patients exhibiting resistance to dopaminergic drugs, alternative therapies are desired. The definition, causes, behavior, and mechanisms of dopamine agonist-resistant prolactinomas were recently well reviewed (11, 12). Resistant prolactinomas tend to be large, invasive, and hyperangiogenic with high mitotic indexes. These conditions lead to the need of surgery and/or radiosurgery.
TGF-β1 has been postulated to mediate, in part, the inhibitory actions of dopamine on lactotrope cell proliferation (33, 54). We recently reported that pituitaries from mice with a null mutation in the dopamine type 2 receptor have lower active and total TGF-β1 compared with wild-type controls, highlighting the stimulatory role of dopamine on pituitary TGF-β1 (36). On the other hand Sarkar and colleagues (33–35) and our present results demonstrated decreased levels of pituitary TGF-β1 in female rats treated with estradiol, concomitantly with the increase in PRL levels. Moreover, the TSP-1 levels and its antiangiogenic effect are reduced in prolactinomas induced by estradiol. We suggest that recovering pituitary TSP-1 and TGF-β1 activities could reduce the progression of prolactinomas. Treatments that improve pituitary TGF-β1 activity would be an interesting alternative therapy in dopamine agonist-resistant prolactinomas.
Our results show that ABT-510 and ABT-898 decrease pituitary tumor angiogenesis, tumor size, proliferation rate, and serum prolactin values and restore pituitary TGF-β1 activity. We postulate that the increased cytokine activation participates in the inhibition of lactotrope function. The mechanisms involved in TGF-β1 activation induced by ABT-510 and ABT-898 are still unknown and have not been previously reported.
Our results suggest these antiangiogenic molecules as possible therapy in current treatments against prolactinomas, especially in those who are resistant to dopaminergic drugs.
Supplementary Material
Acknowledgments
We thank the National Institute of Diabetes and Digestive and Kidney Diseases's National Hormone and Pituitary Program and Dr. A. F. Parlow for prolactin RIA kits. We also thank Fulbright and Bunge and the Born Foundation for travel support. In addition, we thank Abbott Laboratories for the gift of ABT-510 and ABT-898.
This work was supported by the Consejo de Investigaciones Científicas y Técnicas Grant PIP 243, 2009 (to G.D.-T.) and PIP 640 (to D.B.-V.), Agencia Nacional de Promoción Científica y Técnica, Buenos Aires, Argentina (Grant PICT N206 and 459, 2006 and 2010 (to D.B.-V.), and the National Institutes of Health Grant R01 CA034282-25 (to D.B.R.).
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- CD31
- Cluster of differentiation 31
- CT
- threshold cycle
- DES
- diethylstilbestrol
- ECM
- extracellular matrix
- LTBP
- latent TGF-β binding proteins
- p
- phosphorylated
- PCNA
- proliferating cell nuclear antigen
- PRL
- prolactin
- Q-RT-PCR
- quantitative real-time RT-PCR
- TβRII
- TGF-β1 receptor type II
- TSP-1
- thrombospondin 1
- TSR-1
- TSP-1 intramolecular small sequence
- VEGF
- vascular endothelial growth factor.
References
- 1. Crawford Y, Ferrara N. 2009. VEGF inhibition: insights from preclinical and clinical studies. Cell Tissue Res 335:261–269 [DOI] [PubMed] [Google Scholar]
- 2. Folkman J, Shing Y. 1992. Angiogenesis. J Biol Chem 267:10931–10934 [PubMed] [Google Scholar]
- 3. Folkman J, Browder T, Palmblad J. 2001. Angiogenesis research: guidelines for translation to clinical application. Thromb Haemost 86:23–33 [PubMed] [Google Scholar]
- 4. Arafah BM, Nasrallah MP. 2001. Pituitary tumors: pathophysiology, clinical manifestations and management. Endocr Relat Cancer 8:287–305 [DOI] [PubMed] [Google Scholar]
- 5. Melmed S. 1999. Pathogenesis of pituitary tumors. Endocrinol Metab Clin North Am 28:1–12, v [DOI] [PubMed] [Google Scholar]
- 6. Saeger W, Lüdecke DK, Buchfelder M, Fahlbusch R, Quabbe HJ, Petersenn S. 2007. Pathohistological classification of pituitary tumors: 10 years of experience with the German Pituitary Tumor Registry. Eur J Endocrinol 156:203–216 [DOI] [PubMed] [Google Scholar]
- 7. Pizarro CB, Oliveira MC, Pereira-Lima JF, Leães CG, Kramer CK, Schuch T, Barbosa-Coutinho LM, Ferreira NP. 2009. Evaluation of angiogenesis in 77 pituitary adenomas using endoglin as a marker. Neuropathology 29:40–44 [DOI] [PubMed] [Google Scholar]
- 8. Schechter J. 1972. Ultrastructural changes in the capillary bed of human pituitary tumors. American Journal of Pathology 67:109–126 [PMC free article] [PubMed] [Google Scholar]
- 9. Turner HE, Nagy Z, Gatter KC, Esiri MM, Harris AL, Wass JA. 2000. Angiogenesis in pituitary adenomas and the normal pituitary gland. J Clin Endocrinol Metab 85:1159–1162 [DOI] [PubMed] [Google Scholar]
- 10. Ciccarelli A, Daly AF, Beckers A. 2005. The epidemiology of prolactinomas. Pituitary 8:3–6 [DOI] [PubMed] [Google Scholar]
- 11. Vasilev V, Daly AF, Vroonen L, Zacharieva S, Beckers A. 2011. Resistant prolactinomas. J Endocrinol Invest 34:312–316 [DOI] [PubMed] [Google Scholar]
- 12. Oh MC, Aghi MK. 2011. Dopamine agonist-resistant prolactinomas. J Neurosurg 114:1369–1379 [DOI] [PubMed] [Google Scholar]
- 13. Gürlek A, Karavitaki N, Ansorge O, Wass JA. 2007. What are the markers of aggressiveness in prolactinomas? Changes in cell biology, extracellular matrix components, angiogenesis and genetics. Eur J Endocrinol 156:143–153 [DOI] [PubMed] [Google Scholar]
- 14. Heaney AP, Fernando M, Melmed S. 2002. Functional role of estrogen in pituitary tumor pathogenesis. J Clin Invest 109:277–283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Heaney AP, Horwitz GA, Wang Z, Singson R, Melmed S. 1999. Early involvement of estrogen-induced pituitary tumor transforming gene and fibroblast growth factor expression in prolactinoma pathogenesis. Nat Med 5:1317–1321 [DOI] [PubMed] [Google Scholar]
- 16. Sarkar DK. 2006. Genesis of prolactinomas: studies using estrogen-treated animals. Front Horm Res 35:32–49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Elias KA, Weiner RI. 1984. Direct arterial vascularization of estrogen-induced prolactin-secreting anterior pituitary tumors. Proc Natl Acad Sci USA 81:4549–4553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Elias KA, Weiner RI. 1987. Inhibition of estrogen-induced anterior pituitary enlargement and arteriogenesis by bromocriptine in Fischer 344 rats. Endocrinology 120:617–621 [DOI] [PubMed] [Google Scholar]
- 19. Takechi A. 1994. Effect of angiogenesis inhibitor TNP-470 on vascular formation in pituitary tumors induced by estrogen in rats. Neurol Med Chir (Tokyo) 34:729–733 [DOI] [PubMed] [Google Scholar]
- 20. Luque GM, Perez-Millán MI, Ornstein AM, Cristina C, Becu-Villalobos D. 2011. Inhibitory effects of anti-VEGF strategies in experimental dopamine resistant prolactinomas. J Pharmacol Exp Ther 337:766–774 [DOI] [PubMed] [Google Scholar]
- 21. Burris H, 3rd, Rocha-Lima C. 2008. New therapeutic directions for advanced pancreatic cancer: targeting the epidermal growth factor and vascular endothelial growth factor pathways. Oncologist 13:289–298 [DOI] [PubMed] [Google Scholar]
- 22. Kerbel RS. 2008. Tumor angiogenesis. N Engl J Med 358:2039–2049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Haviv F, Bradley MF, Kalvin DM, Schneider AJ, Davidson DJ, Majest SM, McKay LM, Haskell CJ, Bell RL, Nguyen B, Marsh KC, Surber BW, Uchic JT, Ferrero J, Wang YC, Leal J, Record RD, Hodde J, Badylak SF, Lesniewski RR, Henkin J. 2005. Thrombospondin-1 mimetic peptide inhibitors of angiogenesis and tumor growth: design, synthesis, and optimization of pharmacokinetics and biological activities. J Med Chem 48:2838–2846 [DOI] [PubMed] [Google Scholar]
- 24. Lawler J. 2002. Thrombospondin-1 as an endogenous inhibitor of angiogenesis and tumor growth. J Cell Mol Med 6:1–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Anderson JC, Grammer JR, Wang W, Nabors LB, Henkin J, Stewart JE, Jr, Gladson CL. 2007. ABT-510, a modified type 1 repeat peptide of thrombospondin, inhibits malignant glioma growth in vivo by inhibiting angiogenesis. Cancer Biol Ther 6:454–462 [DOI] [PubMed] [Google Scholar]
- 26. Yang Q, Tian Y, Liu S, Zeine R, Chlenski A, Salwen HR, Henkin J, Cohn SL. 2007. Thrombospondin-1 peptide ABT-510 combined with valproic acid is an effective antiangiogenesis strategy in neuroblastoma. Cancer Res 67:1716–1724 [DOI] [PubMed] [Google Scholar]
- 27. Garside SA, Henkin J, Morris KD, Norvell SM, Thomas FH, Fraser HM. 2010. A thrombospondin-mimetic peptide, ABT-898, suppresses angiogenesis and promotes follicular atresia in pre- and early-antral follicles in vivo. Endocrinology 151:5905–5915 [DOI] [PubMed] [Google Scholar]
- 28. Burns G, Sarkar DK. 1993. Transforming growth factor-β1-like immunoreactivity in the pituitary gland of the rat: effect of estrogen. Endocrinology 133:1444–1449 [DOI] [PubMed] [Google Scholar]
- 29. Sarkar AJ, Chaturvedi K, Chen CP, Sarkar DK. 2007. Changes in thrombospondin-1 levels in the endothelial cells of the anterior pituitary during estrogen-induced prolactin-secreting pituitary tumors. J Endocrinol 192:395–403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Schultz-Cherry S, Ribeiro S, Gentry L, Murphy-Ullrich JE. 1994. Thrombospondin binds and activates the small and large forms of latent transforming growth factor-β in a chemically defined system. J Biol Chem 269:26775–26782 [PubMed] [Google Scholar]
- 31. Rifkin DB. 2005. Latent transforming growth factor-β (TGF-β) binding proteins: orchestrators of TGF-β availability. J Biol Chem 280:7409–7412 [DOI] [PubMed] [Google Scholar]
- 32. Mehra A, Wrana JL. 2002. TGF-β and the Smad signal transduction pathway. Biochem Cell Biol 80:605–622 [DOI] [PubMed] [Google Scholar]
- 33. Sarkar DK, Kim KH, Minami S. 1992. Transforming growth factor-β1 messenger RNA and protein expression in the pituitary gland: its action on prolactin secretion and lactotropic growth. Mol Endocrinol 6:1825–1833 [DOI] [PubMed] [Google Scholar]
- 34. De A, Morgan TE, Speth RC, Boyadjieva N, Sarkar DK. 1995. Pituitary lactotrope expresses transforming growth factor β (TGF-β) type II receptor mRNA and protein and contains I-TGF-β1 binding sites. J Endocrinol 149:19–27 [DOI] [PubMed] [Google Scholar]
- 35. Pastorcic M, De A, Boyadjieva N, Vale W, Sarkar DK. 1995. Reduction in the expression and action of transforming growth factor-β1 on lactotropes during estrogen-induced tumorigenesis. Cancer Res 55:4892–4898 [PubMed] [Google Scholar]
- 36. Recouvreux MV, Guida MC, Rifkin DB, Becu-Villalobos D, Díaz-Torga G. 2011. Active and total transforming growth factor-β1 are differentially regulated by dopamine and estradiol in the pituitary. Endocrinology 152:2722–2730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Guo NH, Krutzsch HC, Inman JK, Shannon CS, Roberts DD. 1997. Antiproliferative and antitumor activities of D-reverse peptides derived from the second type-1 repeat of thrombospondin-1. J Pept Res 50:210–221 [DOI] [PubMed] [Google Scholar]
- 38. Ebbinghaus S, Hussain M, Tannir N, Gordon M, Desai AA, Knight RA, Humerickhouse RA, Qian J, Gordon GB, Figlin R. 2007. Phase 2 study of ABT-510 in patients with previously untreated advanced renal cell carcinoma. Clin Cancer Res 13:6689–6695 [DOI] [PubMed] [Google Scholar]
- 39. Markovic SN, Suman VJ, Rao RA, Ingle JN, Kaur JS, Erickson LA, Pitot HC, Croghan GA, McWilliams RR, Merchan J, Kottschade LA, Nevala WK, Uhl CB, Allred J, Creagan ET. 2007. A phase II study of ABT-510 (thrombospondin-1 analog) for the treatment of metastatic melanoma. Am J Clin Oncol 30:303–309 [DOI] [PubMed] [Google Scholar]
- 40. Gordon MS, Mendelson D, Carr R, Knight RA, Humerickhouse RA, Iannone M, Stopeck AT. 2008. A phase 1 trial of 2 dose schedules of ABT-510, an antiangiogenic, thrombospondin-1-mimetic peptide, in patients with advanced cancer. Cancer 113:3420–3429 [DOI] [PubMed] [Google Scholar]
- 41. Nabors LB, Fiveash JB, Markert JM, Kekan MS, Gillespie GY, Huang Z, Johnson MJ, Meleth S, Kuo H, Gladson CL, Fathallah-Shaykh HM. 2010. A phase 1 trial of ABT-510 concurrent with standard chemoradiation for patients with newly diagnosed glioblastoma. Arch Neurol 67:313–319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Campbell N, Greenaway J, Henkin J, Petrik J. 2011. ABT-898 induces tumor regression and prolongs survival in a mouse model of epithelial ovarian cancer. Mol Cancer Ther 10:1876–1885 [DOI] [PubMed] [Google Scholar]
- 43. Hasan J, Byers R, Jayson GC. 2002. Intra-tumoural microvessel density in human solid tumours. Br J Cancer 86:1566–1577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Lloyd RV, Scheithauer BW, Kuroki T, Vidal S, Kovacs K, Stefaneanu L. 1999. Vascular endothelial growth factor (VEGF) expression in human pituitary adenomas and carcinomas. Endocr Pathol 10:229–235 [DOI] [PubMed] [Google Scholar]
- 45. McCabe CJ, Boelaert K, Tannahill LA, Heaney AP, Stratford AL, Khaira JS, Hussain S, Sheppard MC, Franklyn JA, Gittoes NJ. 2002. Vascular endothelial growth factor, its receptor KDR/Flk-1, and pituitary tumor transforming gene in pituitary tumors. J Clin Endocrinol Metab 87:4238–4244 [DOI] [PubMed] [Google Scholar]
- 46. Cristina C, Díaz-Torga G, Baldi A, Góngora A, Rubinstein M, Low MJ, Becú-Villalobos D. 2005. Increased pituitary vascular endothelial growth factor-A in dopaminergic D2 receptor knockout female mice. Endocrinology 146:2952–2962 [DOI] [PubMed] [Google Scholar]
- 47. Cristina C, Tornadú IG, Millán MIP, Díaz-Torga G, Becu-Villalobos D. 2006. Factores de crecimiento y antiangiogenesis en prolactinomas resistentes a dopamina. Anales de la Academia Nacional de Ciencias (Buenos Aires) 39:1–15 [Google Scholar]
- 48. Mallea-Gil MS, Cristina C, Perez-Millan MI, Villafañe AM, Ballarino C, Stalldecker G, Becu-Villalobos D. 2009. Invasive giant prolactinoma with loss of therapeutic response to cabergoline: expression of angiogenic markers. Endocr Pathol 20:35–50 [DOI] [PubMed] [Google Scholar]
- 49. Cristina C, Perez-Millan MI, Luque G, Dulce RA, Sevlever G, Berner SI, Becu-Villalobos D. 2010. VEGF and CD31 association in pituitary adenomas. Endocr Pathol 21:154–160 [DOI] [PubMed] [Google Scholar]
- 50. Turner HE, Nagy Z, Gatter KC, Esiri MM, Harris AL, Wass JA. 2000. Angiogenesis in pituitary adenomas—relationship to endocrine function, treatment and outcome. J Endocrinol 165:475–481 [DOI] [PubMed] [Google Scholar]
- 51. Febbraio M, Hajjar DP, Silverstein RL. 2001. CD36: a class B scavenger receptor involved in angiogenesis, atherosclerosis, inflammation, and lipid metabolism. J Clin Invest 108:785–791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Rice C, Huang LE. 2010. From antiangiogenesis to hypoxia: current research and future directions. Cancer Manag Res 3:9–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Campbell NE, Greenaway J, Henkin J, Moorehead RA, Petrik J. 2010. The thrombospondin-1 mimetic ABT-510 increases the uptake and effectiveness of cisplatin and paclitaxel in a mouse model of epithelial ovarian cancer. Neoplasia 12:275–283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Sarkar DK, Pastorcic M, De A, Engel M, Moses H, Ghasemzadeh MB. 1998. Role of transforming growth factor-β type I and TGF-β type II receptors in the TGF-β1 regulated gene expression in pituitary prolactin-secreting lactotropes. Endocrinology 139:3620–3628 [DOI] [PubMed] [Google Scholar]
- 55. Yoshinaga K, Obata H, Jurukovski V, Mazzieri R, Chen Y, Zilberberg L, Huso D, Melamed J, Prijatelj P, Todorovic V, Dabovic B, Rifkin DB. 2008. Perturbation of transforming growth factor (TGF)-β1 association with latent TGF-β binding protein yields inflammation and tumors. Proc Natl Acad Sci USA 105:18758–18763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Silverstein RL, Febbraio M. 2009. CD36, a scavenger receptor involved in immunity, metabolism, angiogenesis, and behavior. Sci Signal 2:re3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Young GD, Murphy-Ullrich JE. 2004. The tryptophan-rich motifs of the thrombospondin type 1 repeats bind VLAL motifs in the latent transforming growth factor-β complex. J Biol Chem 279:47633–47642 [DOI] [PubMed] [Google Scholar]
- 58. Miyazono K, Heldin CH. 1991. Latent forms of TGF-β: molecular structure and mechanisms of activation. Ciba Found Symp 157:81–89; discussion 89–92 [DOI] [PubMed] [Google Scholar]
- 59. Taipale J, Miyazono K, Heldin CH, Keski-Oja J. 1994. Latent transforming growth factor-β1 associates to fibroblast extracellular matrix via latent TGF-β binding protein. J Cell Biol 124:171–181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Taipale J, Saharinen J, Hedman K, Keski-Oja J. 1996. Latent transforming growth factor-β1 and its binding protein are components of extracellular matrix microfibrils. J Histochem Cytochem 44:875–889 [DOI] [PubMed] [Google Scholar]
- 61. Koski C, Saharinen J, Keski-Oja J. 1999. Independent promoters regulate the expression of two amino terminally distinct forms of latent transforming growth factor-β binding protein-1 (LTBP-1) in a cell type-specific manner. J Biol Chem 274:32619–32630 [DOI] [PubMed] [Google Scholar]
- 62. Weikkolainen K, Keski-Oja J, Koli K. 2003. Expression of latent TGF-β binding protein LTBP-1 is hormonally regulated in normal and transformed human lung fibroblasts. Growth Factors 21:51–60 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.