Abstract
Gastric bypass leads to the remission of type 2 diabetes independently of weight loss. Our hypothesis is that changes in bile flow due to the altered anatomy may partly explain the metabolic outcomes of the operation. We prospectively studied 12 patients undergoing gastric bypass and six patients undergoing gastric banding over a 6-wk period. Plasma fibroblast growth factor (FGF)19, stimulated by bile acid absorption in the terminal ileum, and plasma bile acids were measured. In canine and rodent models, we investigated changes in the gut hormone response after altered bile flow. FGF19 and total plasma bile acids levels increased after gastric bypass compared with no change after gastric banding. In the canine model, both food and bile, on their own, stimulated satiety gut hormone responses. However, when combined, the response was doubled. In rats, drainage of endogenous bile into the terminal ileum was associated with an enhanced satiety gut hormone response, reduced food intake, and lower body weight. In conclusion, after gastric bypass, bile flow is altered, leading to increased plasma bile acids, FGF19, incretin. and satiety gut hormone concentrations. Elucidating the mechanism of action of gastric bypass surgery may lead to novel treatments for type 2 diabetes.
A link between bile acids and glycemic control has been suggested by studies showing improved blood glucose in patients with type 2 diabetes when receiving the bile acid sequestrants cholestyramine and colesevelam (1–5). Several possible mechanisms have been proposed, including the disruption of enterohepatic circulation of bile acids and a number of different effects through the farnesoid X receptor (FXR) pathway, which is the intracellular signaling pathway for bile acids (4, 6). Bile acids also activate the cell-membrane G protein-coupled receptor, TGR5, which has been shown to stimulate the incretin, glucagon-like peptide-1 (GLP-1) in vitro in an FXR-independent manner (7). GLP-1 in turn stimulates β-cells in the pancreas to release insulin (8).
Gastric bypass surgery is being used as a treatment for type 2 diabetes, although the mechanism of action remains largely unclear (9–11). The improved glycemic control in the immediate postoperative period is weight loss independent, because simultaneous improved insulin secretion and reduced insulin resistance have been observed between d 2 and 7 after gastric bypass (9). The early increase in insulin secretion was associated with an enhanced GLP-1 response (9), which may partly be explained by L-cell stimulation from bile acids (12–14). Moreover, the decrease in insulin resistance may also be the result of increased fasting plasma bile acid levels after gastric bypass surgery (15, 16), because firstly, bile acids inhibit gluconeogenesis in an FXR dependent and independent manner (17–20) and bind to TGR5, leading to cAMP generation and activation of the intracellular type 2 thyroid hormone deiodinase (21). Secondly, bile acids also act via the phosphatidylinositol 3 kinase/serine-threonine kinase pathway directly promoting insulin signaling and glycogen synthase activation, thus aiding insulin-dependent control of glucose metabolism in the liver (22). Thirdly, possible effects of bile acids on fibroblast growth factor (FGF)19 could lead to enhanced mitochondrial activity, which improves insulin resistance (23). Recently FGF19 has been shown to regulate glycogen metabolism in an insulin-independent manner (24). Furthermore, FGF19 has been shown to correlate with nutritional status (25). Finally, tauroursodeoxycholic acid has also been shown to protect against the onset of insulin resistance in obese and diabetic mice by alleviating stress in the endoplasmic reticulum (26).
The prolonged improvements in glycemic control after gastric bypass are further aided by the substantial and maintained weight loss. The attenuated appetite may be partly explained by enhanced satiety gut hormones from the endocrine L cell, such as peptide YY (PYY), GLP-1, and oxyntomodulin (27, 28). Bile acids are also implicated in the release of these L-cell hormones.
We hypothesized that the altered anatomy after gastric bypass affects bile delivery to the terminal ileum and lead to elevated plasma bile acids. We postulated that changes in bile flow result in increased satiety gut hormone responses, reduced food intake, and weight loss. Our aim was to test this hypothesis in humans after gastric bypass surgery and to explore further the potential mechanisms involved in two animal models of altered bile flow.
Materials and Methods
The human studies were performed according to the principles of the Declaration of Helsinki. The Somerset Research and Ethics committee approved the study (LREC protocol no. 05/Q2202/96). The canine studies were approved by the ethics committee of Onderstepoort Vetinary School (University of Pretoria). The rat studies were approved by the Home Office United Kingdom (PL 70-6669).
Human studies
Written informed consent was obtained from all participants. Exclusion criteria included pregnancy, substance abuse, and more than two alcoholic drinks per day. Twelve gastric bypass patients (seven females and five males) with mean age of 45.2 ± 2.7 yr and body mass index 49.8 ± 1.5 as well as six gastric banding patients (four females and two males), with mean age 45.4 ± 2.6 yr and body mass index 44 ± 2.0 kg/m2 were recruited. All patients were prescribed a 2-wk preoperative diet of 1000 kcal before surgery. Operations were performed laparoscopically by one surgeon. The technique for the gastric bypass has been described previously (29). For gastric banding, the Swedish Adjustable Gastric band (Ethicon Endo-Surgery, London, UK) and the LAP-BAND (Allergan, Marlow, UK) bands were used with the pars flaccida dissection technique and gastro-gastric tunnelating sutures (30). Using an enhanced recovery protocol postoperatively, all patients were allowed free fluids on return to the ward, and diet was recommenced when tolerated. The recommended postoperative diet for the first week was the same for the patients with banding and bypass. After a 12-h fast, blood was obtained in tubes containing EDTA and aprotinin. Samples were immediately centrifuged and stored in a −80 C freezer until analysis.
Canine studies
Four male and four female Beagles were fasted overnight and then given a standard 400 g of test meal of dog chow (Husky, Purina, South Africa). The composition was 7.5% protein, 2% fat, 1% fiber, 7.5% crude ash, and 82% moisture. Five milliliters of blood were collected (in tubes containing EDTA and aprotinin) every 30 min from 30 min before the meal up to 150 min postprandially.
The next day, the dogs were prepared for theater by placing an overnight fentanyl patch and withholding food overnight. The common bile duct was transected. An 8 French Foley catheter was placed into the gall bladder. An 8 French feeding tube was advanced through the pylorus to the duodenum with the most distal point being 5–8 cm distal to the pylorus, close to the level of the ampulla of Vater (Fig. 1A). For the first 12 h after the surgery, the dogs were given ad libitum access to water but no food.
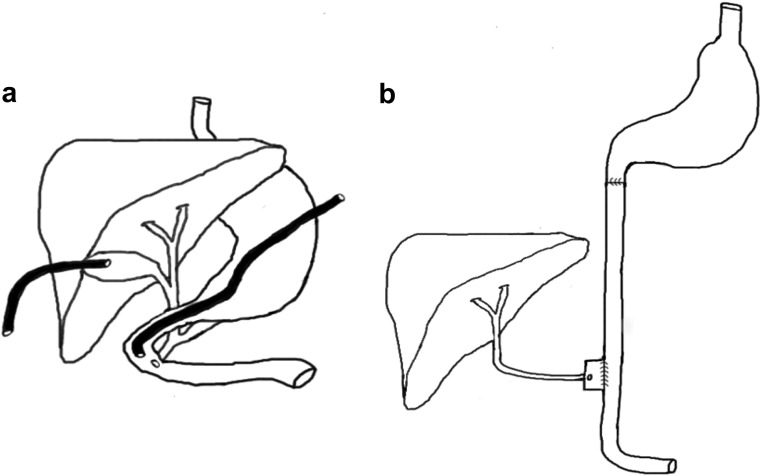
Fig. 1.
A, Schematic illustration of the anatomy and canulation of the canine model. A gastrostomy tube was placed into the duodenum close to the ampulla of Vater. The common bile duct was ligated and the gallbladder canulated to allow drainage of bile. B, Schematic illustration of the functional anatomy of the bile in ileum group. Transections 1 cm proximal and distal to the drainage point of the common bile duct were performed. The proximal and distal ends of the transected duodenum were anastomosed end to end and continuity restored. The segment of the duodenum containing the common bile duct was anastomosed side to side to the distal jejunum, 10 cm proximally to the terminal ileum.
Only one dog was terminated after showing signs of jaundice and infection. The dogs received a standard meal of 400 g of normal chow at the start of the light phase. At this time, as much bile as possible was aspirated from the Foley catheter and injected through the gastrostomy tube, followed by a 5-ml flush of saline. This prevented the dogs from becoming jaundiced and allowed normal digestion.
On d 4–6, the dogs were randomized to a 180-min crossover designed protocol of venous blood collection every 30 min after 1) a standard meal of 400 g of dog food only without bile; 2) bile only, without food; or 3) 400 g of dog food and bile in combination.
Rodent studies
Sixteen male Wistar obese rats were randomized to a sham operation, which maintained the normal bile delivery to the duodenum or to an operation that would deliver bile to the ileum. The bile-in-duodenum group underwent transections 1 cm proximal and distal to the drainage point of the common bile duct and reanastomosis to maintain the normal anatomy but allow for a similar surgical insult. The bile-in-ileum group had the same transection of the duodenum, but the proximal and distal ends of the transected duodenum were anastomosed end to end and continuity restored (Fig. 1B). The segment of the duodenum containing the common bile duct was anastomosed side to side to the distal jejunum, 10 cm proximally to the terminal ileum. This allowed bile and pancreatic juices to bypass the duodenum and most of the jejunum.
Body weight and food consumption was measured daily at the beginning of the light phase for 28 d. Feces were collected over a 24-h period on d 25. The rats were then fasted for 12 h before they were terminated, and blood samples were collected.
FGF19 assay
Plasma FGF19 concentration was measured using a quantitative sandwich ELISA technique (FGF19 Quantikine ELISA kit, catalog no. DF1900; R&D Systems, Minneapolis, MN).
Bile acids assay
The measurement of fractionated plasma bile acids was performed with liquid chromatography tandem mass spectrometry (31). The method allowed 12 different bile acids [cholic acid (CA), chenodeoxycholic acid (CDC), deoxycholic acid (DC), glycocholic acid (GCA), glycodeoxycholic acid (GCD), glycochenodeoxycholic acid (GCDC), glycolithocholic acid (GLC), glycoursodeoxycholic acid (GUDC), lithocholic acid (LC), taurocholic acid (TCA), taurochenodeoxycholic acid (TCDC), and taurodeoxycholic acid (TDC)] to be measured within the range of 0.1–10 μm.
GLP-1 and PYY assay
All samples were assayed in duplicate. Analysis was performed with an established GLP-1 RIA (7). PYY-like immunoreactivity was measured with a specific and sensitive RIA, which measures both the full length (PYY1-36) and the fragment (PYY3-36) (32).
Bomb calorimetry
To evaluate nutrient absorption, feces were collected over 24 h on postoperative d 25 from all rats. Feces were dried in an oven and weighed; calorie content was measured using an established ballistic bomb calorimeter technique (33).
Rodent C-reactive protein (CRP) assay
To assess inflammation serum CRP levels were measured (Rat Serum CRP ELISA kit catalog no. 1010; Alpha Diagnostics International, San Antonio, TX).
Statistical analysis
Results were analyzed using GraphPad Prism version 5.00 for Windows (GraphPad Software, San Diego, CA). Data are expressed as means ± sem when the data follow a Gaussian distribution. When the data did not follow a Gaussian distribution, the median (range) is used. Values for the area under the curve (AUC) were calculated with the use of the trapezoidal rule. End points were compared with the use of two-tailed, paired Student's t tests for parametric data and Mann-Whitney U test for nonparametric data. Results were considered significant if P < 0.05.
Results
Human study FGF19
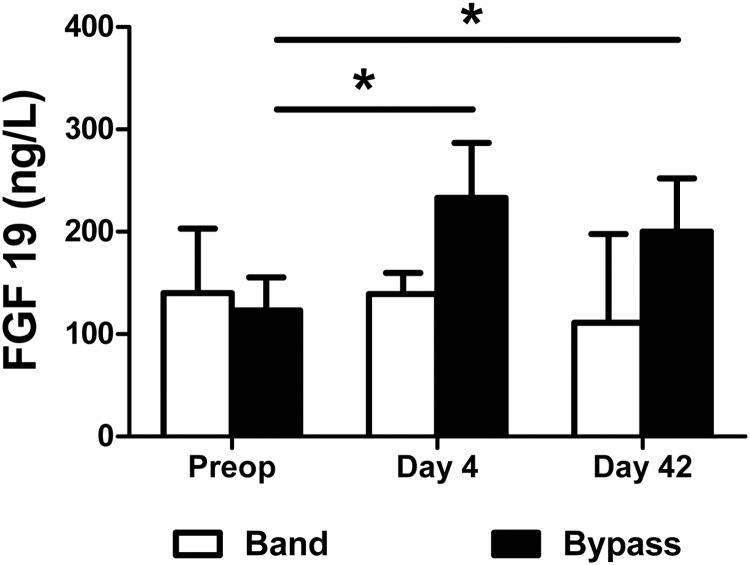
Preoperative fasted plasma FGF19 levels did not differ between gastric banding and gastric bypass patients [median 140 ng/liter range (26–466) vs. 123 (41–406), respectively; P = 0.37]. However, these values were lower than those found in nonobese controls (34). In the gastric banding group, there was no significant change from preoperative values for fasting FGF19 at d 4 or 42 after surgery. In the gastric bypass group, fasting levels of plasma FGF19 were significantly increased as early as 4 d after gastric bypass compared with preoperative values (P < 0.01) (Fig. 2). The enhanced FGF19 levels were sustained at 42 d postoperatively (P < 0.05).
Fig. 2.
Fasting plasma FGF19 concentrations (median and interquartile ranges) at d 0, 4, and 42 in six gastric banding patients (white bars) and 12 gastric bypass patients (black bars). *, P < 0.05 Mann-Whitney U test. Preop, Preoperatively.
Human study plasma bile acids
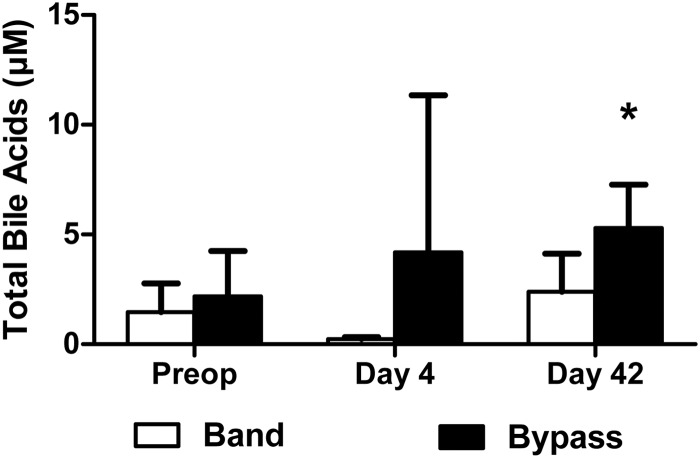
Fasting concentrations of total plasma bile acids measured in banding and bypass patients preoperatively were not different (Fig. 3). On d 4, fasting bile acids were increased after gastric bypass, and there was a significant difference compared with the banding group. By d 42 after gastric bypass fasting, total bile acid concentrations were higher compared with preoperative levels. There was no difference in the banding group.
Fig. 3.
Fasting total plasma bile acid concentrations at d 0, 4, and 42 in six gastric banding patients (white bars) and 12 gastric bypass patients (black bars). *, P < 0.05 Mann-Whitney U test. Preop, Preoperatively.
A similar pattern of results (increase in the gastric bypass group but not in the banding group) was previously shown for GLP-1 and PYY in these same patients (9).
Canine studies
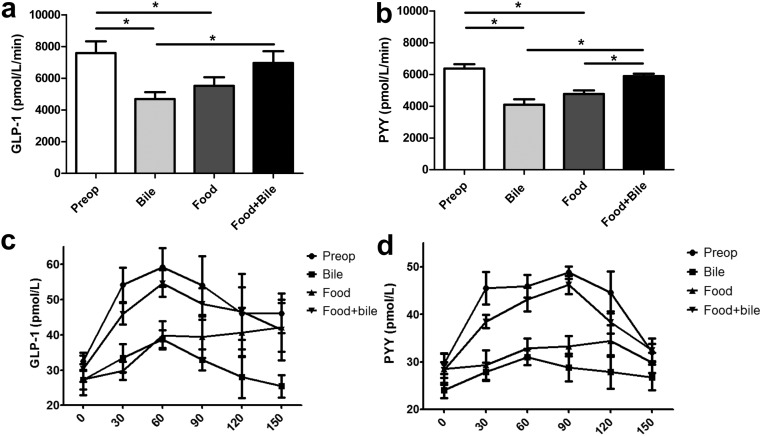
The responses of GLP-1 and PYY were studied in this model after stimulation with food or bile alone or a combination of both. Unfortunately, at the time of these experiments, there was no available assay for canine FGF19. Baseline GLP-1 and PYY levels were the same before and after the operation (6936 vs. 7290 for GLP-1 and 6386 vs. 5856 for PYY). Figure 4 shows the AUC and the time course over 150 min for the postprandial GLP-1 and PYY response after the standard meal of 400 g of dog food. In the operated dogs, both food alone and bile alone lead to a significant GLP-1 and PYY response from baseline, although the responses were attenuated. The response to bile and or food alone was inferior to the combination of food and bile either pre- or postoperatively.
Fig. 4.
A, AUC for the postprandial GLP-1. B, AUC for postprandial PYY response after 400 g of food in dogs pre- or postoperatively either receiving food alone without bile (food), bile alone without food (bile), or food and bile in combination (food + bile).*, P < 0.05. The time course of the postprandial response for GLP-1 (C) and PYY (D).
Rodent studies
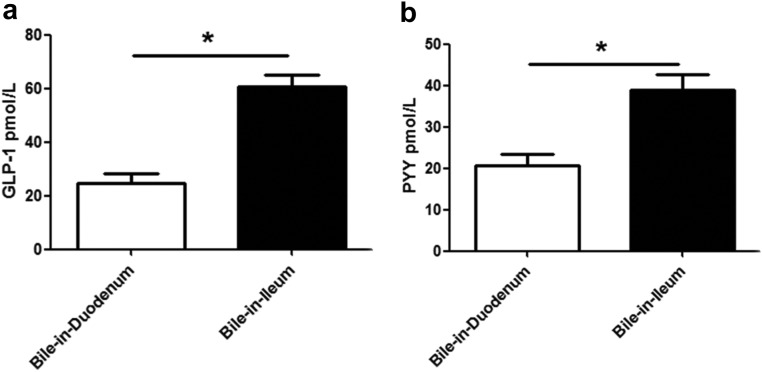
In this model, rats had bile draining into their ileum or duodenum. Both the fasting plasma GLP-1 levels (Fig. 5A) and the plasma PYY levels (Fig. 5B) were higher in the bile-in-ileum group than in the bile-in-duodenum group (P < 0.05).
Fig. 5.
Plasma GLP-1 (A) and PYY (B) levels in rats that had bile draining into their duodenum or bile draining into their ileum.
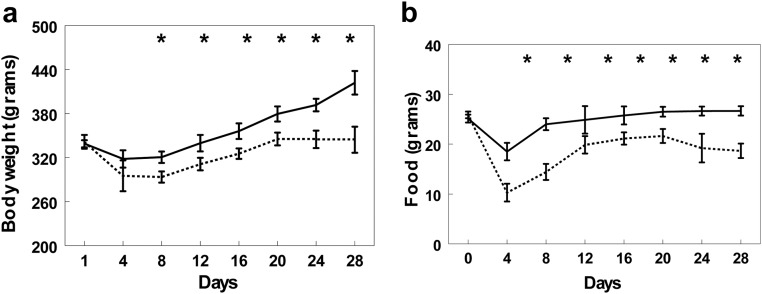
Figure 6A demonstrates that both the bile-in-ileum and bile-in-duodenum groups lost a similar initial amount of weight during the first 4 d while recovering from surgery. The bile-in-duodenum group, however, reached their preoperative weight within 8 d. The bile-in-ileum group weighed significantly less than the bile-in-duodenum group on d 6 (P < 0.05) and continued to have a lower bodyweight for the duration of the study (P < 0.05). Figure 6B shows that the rats in the bile-in-ileum group ate significantly less than the bile-in-duodenum group (P < 0.05).
Fig. 6.
A, Weight of rats in bile-in-duodenum (solid line) and bile-in-ileum (broken line) groups before and up to 28 d after surgery. B, Food intake of bile-in-duodenum (solid line) and bile-in-ileum (broken line) rats before and up to 28 d after surgery. *, P < 0.05.
Fecal parameters at 25 d after the operation did not reveal differences between the bile-in-ileum and the bile-in-duodenum groups at the end of the experiment in dry weight (4.12 ± 0.20 vs. 4.28 ± 0.18 g, P = 0.55) or in calorific content (3.58 ± 0.4 vs. 3.58 ± 0.4 fecal kcal/24 h, P = 0.91). There was no evidence of increased inflammation in the bile-in-ileum compared with the bile-in-duodenum group either by white cell count (11.5 ± 0.32 × 1000/μl vs. 11.64 ± 0.42 × 1000/μl, P = 0.79) or CRP (370.88 ± 26.03 vs. 378.5 ± 21.71 μg, P = 0.83).
Discussion
We showed in obese patients that FGF19 and plasma total bile acids were increased after gastric bypass but not after gastric banding. We have shown previously that GLP-1 and PYY are affected in the same way as FGF19 in these patients (9). How could the alterations in gastro-intestinal physiology produce these changes?
Both food and bile on their own can release GLP-1 and PYY as shown in the canine model, but the combination of food and bile (before surgery or after surgery) resulted in greater PYY responses compared with food alone, whereas GLP-1 also showed a trend to be higher after the combination of food and bile. Endogenous bile acids and pancreatic juices delivered in the rat model 10 cm proximally to the terminal ileum were also associated with elevated plasma GLP-1 and PYY, reduced food intake, and lower body weight. Taken together, these data suggest that one of the mechanisms by which a gastric bypass leads to beneficial elevations of GLP-1 and PYY may be through the undiluted flow of bile through the biliopancreatric limb and the altered delivery of bile to the terminal ileum.
Well-matched patients undergoing gastric banding were used as a control group, because they have a similar laparoscopic surgical insult and identical preoperative and immediate postoperative diets compared with bypass, but in the gastric banding operation, there is no change in the anatomy of the gut that would affect bile flow. Exogenous bile salts have been shown to be the most potent stimulus of gut hormones from the endocrine L cells, such as PYY in rabbit colon explants (12), in vivo in conscious dogs (13), and in humans (14). GLP-1, PYY, and bile are released postprandially and in proportion to the amount of calories consumed. Although early arrival of nutrients at the terminal ileum still remains a possibility, the reduced gut motility and early peak GLP-1 and PYY responses postprandially suggest other mechanisms, which may involve bile in the release of L-cell hormones.
The gallbladder is not usually removed during Roux-en-Y gastric bypass, and there is no indication that gallbladder contraction is adversely affected after gastric bypass. Previous studies suggested an increase in cholecystokinin after jejunoileal bypass, which may result in enhanced bile flow from the gallbladder or liver (34). After gastric bypass, the length of small bowel from the ampulla of Vater to the terminal ileum is reduced by 100–150 cm, and bile may thus reach the terminal ileum before the ingested food, which triggers the release of bile. We postulate that due to the anatomical changes after gastric bypass, bile progresses down the biliopancreatic limb to the distal L cells in an undiluted state. This could lead to increased availability of bile acids in the distal intestine with the potential to engage TGR5 on L cells. Bile acid activation of TGR5 has been found to stimulate GLP-1 production in vitro and may explain the early and exaggerated release of incretin gut hormones, such as GLP-1 and subsequently insulin (8). Bile acids would normally be more bound up in micelles due to the presence of nutrients and therefore less likely to stimulate L cells for peptide secretion.
Although the canine studies should be interpreted with caution, especially because the anatomical changes are dissimilar to gastric bypass, we would suggest that both bile and food contribute to the postprandial gut hormone response. The role of bile can be attributed to the fact that bile conjugated with food facilitates better digestion of complex ingested fats by intestinal lipases into smaller lipid subunits, therefore leading to a more effective stimulation of L cells (35). Inhibition of intestinal lipases leads to attenuated postprandial GLP-1 and PYY associated with increased appetite (35). However, the results of the rodent studies do not support this hypothesis, because the distal intestinal delivery of bile should interfere with the intestinal digestion of fats and hence lead to reduced L-cell responses. As the opposite was demonstrated, the effect of bile on digestion of ingested fats cannot explain the enhanced gut hormone response, suggesting that bile acids may play a role as signaling molecules.
Bile acids may also influence glucose metabolism by altering body weight. Weight loss may result from enhanced satiety, which may be facilitated via L cell-derived gut hormones (9, 25). In addition, bile acids increase energy expenditure in brown adipose tissue, thus preventing obesity and insulin resistance via induction of the cAMP-dependent thyroid hormone-activating enzyme type 2 iodothyronine deiodinase (21). This is achieved via the TGR5 and is consistent with recent findings that in rat models, gastric bypass prevented the decrease in energy expenditure after weight loss (36, 37).
Activation of the FXRα may also mediate the effects of bile acids on energy homeostasis via FGF19 released from ileal enterocytes, leading to improved metabolic rate and decreased adiposity (38, 39). FGF19 has recently been shown to inhibit hepatic gluconeogenesis (40). FGF19 was only assayed in humans, because unfortunately there are no reliable assays for use in dogs or rats. FGF19 and the FGF15 ortholog in rodents are thought to provide feedback inhibition of bile acid synthesis in the liver. Why the increased levels of FGF19 after gastric bypass are associated with an increase in total bile acids is not immediately apparent, unless the changes in ileal bile acid absorption and FXR-mediated FGF19 production are much greater than the effects on the liver.
Some of the beneficial metabolic effect of Roux-en-Y gastric bypass on glycemic control may be attributed to changes in bile acids (15). Our prospective study confirms the previous cross sectional observation that total plasma bile acids are elevated after gastric bypass. We went on to show that this happens as early as 42 d after surgery. We propose an additional mechanism for the observed improvements in glycemic control involving altered bile flow after gastric bypass. The changes facilitated by bile may increase satiety and improved glycemic control via gut hormones as well as a direct effect on insulin resistance. Therefore, bile may be one of the key products of the proximal gut, which transfers a message to the distal gut and to other metabolically active tissues.
Limitations of our study include the lack of randomization in the human studies. However, the groups were well matched for preoperative patient characteristics. Portal vein levels of FGF19 and bile acids were not measured, and the relationship between portal veins and systemic levels after gastric bypass is not currently known. Furthermore only fasting levels of FGF19 and bile acids were measured. Postprandial changes may also occur and are the subject of future studies. In the canine experiment, a dislodgement of either the feeding tube or the Foley catheter could have lead to chemical peritonitis affecting the results. However, the postmortem examination confirming the position of the tubes makes this unlikely. In the rat experiment, the difference in the severity of the operative procedures may have caused different inflammatory response, but the lack of difference in the biochemical and histological markers of inflammation mitigates against this.
In conclusion, Roux-en-Y gastric bypass causes changes in bile flow resulting in increased total plasma bile acids concentrations, FGF19, GLP-1, and PYY. Altering the flow of endogenous bile leads to an increase in gut hormone responses, which can be further enhanced by the synergistic delivery of food. Bile may partly explain the pleotrophic metabolic effects seen after gastric bypass surgery and could be a therapeutic target for novel surgical devices or pharmaceuticals.
Acknowledgments
This work was supported by a National Institute of Health Research Clinician scientist award (C.W.l.R.) and by NIHR Biomedical Research Centre funding scheme to Imperial College London.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- AUC
- Area under the curve
- CRP
- C-reactive protein
- FGF
- fibroblast growth factor
- FXR
- farnesoid X receptor
- GLP-1
- glucagon-like peptide-1
- PYY
- peptide YY.
References
- 1. Claudel T, Staels B, Kuipers F. 2005. The farnesoid X receptor: a molecular link between bile acid and lipid and glucose metabolism. Arterioscler Thromb Vasc Biol 25:2020–2030 [DOI] [PubMed] [Google Scholar]
- 2. Garg A, Grundy SM. 1994. Cholestyramine therapy for dyslipidemia in non-insulin-dependent diabetes mellitus. A short-term, double-blind, crossover trial. Ann Intern Med 121:416–422 [DOI] [PubMed] [Google Scholar]
- 3. Fonseca VA, Rosenstock J, Wang AC, Truitt KE, Jones MR. 2008. Colesevelam HCl improves glycemic control and reduces LDL cholesterol in patients with inadequately controlled type 2 diabetes on sulfonylurea-based therapy. Diabetes Care 31:1479–1484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Goldberg RB, Fonseca VA, Truitt KE, Jones MR. 2008. Efficacy and safety of colesevelam in patients with type 2 diabetes mellitus and inadequate glycemic control receiving insulin-based therapy. Arch Intern Med 168:1531–1540 [DOI] [PubMed] [Google Scholar]
- 5. Bays HE, Goldberg RB, Truitt KE, Jones MR. 2008. Colesevelam hydrochloride therapy in patients with type 2 diabetes mellitus treated with metformin: glucose and lipid effects. Arch Intern Med 168:1975–1983 [DOI] [PubMed] [Google Scholar]
- 6. Guzelian P, Boyer JL. 1974. Glucose reabsorption from bile: evidence for a biliohepatic circulation. J Clin Invest 53:526–535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kreymann B, Williams G, Ghatei MA, Bloom SR. 1987. Glucagon-like peptide-1 7-36: a physiological incretin in man. Lancet 2:1300–1304 [DOI] [PubMed] [Google Scholar]
- 8. Katsuma S, Hirasawa A, Tsujimoto G. 2005. Bile acids promote glucagon-like peptide-1 secretion through TGR5 in a murine enteroendocrine cell line STC-1. Biochem Biophys Res Commun 329:386–390 [DOI] [PubMed] [Google Scholar]
- 9. Pournaras DJ, Osborne A, Hawkins SC, Vincent RP, Mahon D, Ewings P, Ghatei MA, Bloom SR, Welbourn R, le Roux CW. 2010. Remission of type 2 diabetes after gastric bypass and banding: mechanisms and two year outcomes. Ann Surg 252:966–971 [DOI] [PubMed] [Google Scholar]
- 10. Polyzogopoulou EV, Kalfarentzos F, Vagenakis AG, Alexandrides TK. 2003. Restoration of euglycemia and normal acute insulin response to glucose in obese subjects with type 2 diabetes following bariatric surgery. Diabetes 52:1098–1103 [DOI] [PubMed] [Google Scholar]
- 11. Pournaras DJ, le Roux CW. 2009. Obesity, gut hormones, and bariatric surgery. World J Surg 33:1983–1988 [DOI] [PubMed] [Google Scholar]
- 12. Ballantyne GH, Longo WE, Savoca PE, Adrian TE, Vukasin AP, Bilchik AJ, Sussman J, Modlin IM. 1989. Deoxycholate-stimulated release of peptide YY from the isolated perfused rabbit left colon. Am J Physiol 257:G715–G724 [DOI] [PubMed] [Google Scholar]
- 13. Izukura M, Hashimoto T, Gomez G, Uchida T, Greeley GH, Jr, Thompson JC. 1991. Intracolonic infusion of bile salt stimulates release of peptide YY and inhibits cholecystokinin-stimulated pancreatic exocrine secretion in conscious dogs. Pancreas 6:427–432 [DOI] [PubMed] [Google Scholar]
- 14. Adrian TE, Ballantyne GH, Longo WE, Bilchik AJ, Graham S, Basson MD, Tierney RP, Modlin IM. 1993. Deoxycholate is an important releaser of peptide YY and enteroglucagon from the human colon. Gut 34:1219–1224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Patti ME, Houten SM, Bianco AC, Bernier R, Larsen PR, Holst JJ, Badman MK, Maratos-Flier E, Mun EC, Pihlajamaki J, Auwerx J, Goldfine AB. 2009. Serum bile acids are higher in humans with prior gastric bypass: potential contribution to improved glucose and lipid metabolism. Obesity 17:1671–1677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Jansen PL, van Werven J, Aarts E, Berends F, Janssen I, Stoker J, Schaap FG. 2011. Alterations of hormonally active fibroblast growth factors after Roux-en-Y gastric bypass surgery. Dig Dis 29:48–51 [DOI] [PubMed] [Google Scholar]
- 17. Thomas C, Pellicciari R, Pruzanski M, Auwerx J, Schoonjans K. 2008. Targeting bile-acid signalling for metabolic diseases. Nat Rev Drug Discov 7:678–693 [DOI] [PubMed] [Google Scholar]
- 18. De Fabiani E, Mitro N, Gilardi F, Caruso D, Galli G, Crestani M. 2003. Coordinated control of cholesterol catabolism to bile acids and of gluconeogenesis via a novel mechanism of transcription regulation linked to the fasted-to-fed cycle. J Biol Chem 278:39124–39132 [DOI] [PubMed] [Google Scholar]
- 19. Yamagata K, Daitoku H, Shimamoto Y, Matsuzaki H, Hirota K, Ishida J, Fukamizu A. 2004. Bile acids regulate gluconeogenic gene expression via small heterodimer partner-mediated repression of hepatocyte nuclear factor 4 and Foxo1. J Biol Chem 279:23158–23165 [DOI] [PubMed] [Google Scholar]
- 20. Ma K, Saha PK, Chan L, Moore DD. 2006. Farnesoid X receptor is essential for normal glucose homeostasis. J Clin Invest 116:1102–1109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Watanabe M, Houten SM, Mataki C, Christoffolete MA, Kim BW, Sato H, Messaddeq N, Harney JW, Ezaki O, Kodama T, Schoonjans K, Bianco AC, Auwerx J. 2006. Bile acids induce energy expenditure by promoting intracellular thyroid hormone activation. Nature 439:484–489 [DOI] [PubMed] [Google Scholar]
- 22. Han SI, Studer E, Gupta S, Fang Y, Qiao L, Li W, Grant S, Hylemon PB, Dent P. 2004. Bile acids enhance the activity of the insulin receptor and glycogen synthase in primary rodent hepatocytes. Hepatology 39:456–463 [DOI] [PubMed] [Google Scholar]
- 23. Tomlinson E, Fu L, John L, Hultgren B, Huang X, Renz M, Stephan JP, Tsai SP, Powell-Braxton L, French D, Stewart TA. 2002. Transgenic mice expressing human fibroblast growth factor-19 display increased metabolic rate and decreased adiposity. Endocrinology 143:1741–1747 [DOI] [PubMed] [Google Scholar]
- 24. Kir S, Beddow SA, Samuel VT, Miller P, Previs SF, Suino-Powell K, Xu HE, Shulman GI, Kliewer SA, Mangelsdorf DJ. 2011. FGF19 as a postprandial, insulin-independent activator of hepatic protein and glycogen synthesis. Science 331:1621–1624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Mráz M, Lacinová Z, Kaválková P, Haluzíková D, Trachta P, Drápalová J, Hanušová V, Haluzík M. 2011. Serum concentrations of fibroblast growth factor 19 in patients with obesity and type 2 diabetes mellitus: the influence of acute hyperinsulinemia, very-low calorie diet and PPAR-α agonist treatment. Physiol Res 60:627–636 [DOI] [PubMed] [Google Scholar]
- 26. Ozcan U, Yilmaz E, Ozcan L, Furuhashi M, Vaillancourt E, Smith RO, Görgün CZ, Hotamisligil GS. 2006. Chemical chaperones reduce ER stress and restore glucose homeostasis in a mouse model of type 2 diabetes. Science 313:1137–1140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. le Roux CW, Welbourn R, Werling M, Osborne A, Kokkinos A, Laurenius A, Lönroth H, Fändriks L, Ghatei MA, Bloom SR, Olbers T. 2007. Gut hormones as mediators of appetite and weight loss after Roux-en-Y gastric bypass. Ann Surg 246:780–785 [DOI] [PubMed] [Google Scholar]
- 28. Laferrère B, Swerdlow N, Bawa B, Arias S, Bose M, Oliván B, Teixeira J, McGinty J, Rother KI. 2010. Rise of oxyntomodulin in response to oral glucose after gastric bypass surgery in patients with type 2 diabetes. J Clin Endocrinol Metab 95:4072–4076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Pournaras DJ, Jafferbhoy S, Titcomb DR, Humadi S, Edmond JR, Mahon D, Welbourn R. 2010. Three hundred laparoscopic Roux-en-Y gastric bypasses: managing the learning curve in higher risk patients. Obes Surg 20:290–294 [DOI] [PubMed] [Google Scholar]
- 30. O'Brien PE, Dixon JB, Laurie C, Anderson M. 2005. A prospective randomized trial of placement of the laparoscopic adjustable gastric band: comparison of the perigastric and pars flaccida pathways. Obes Surg 15:820–826 [DOI] [PubMed] [Google Scholar]
- 31. Tagliacozzi D, Mozzi AF, Casetta B, Bertucci P, Bernardini S, Di Ilio C, Urbani A, Federici G. 2003. Quantitative analysis of bile acids in human plasma by liquid chromatography-electrospray tandem mass spectrometry: a simple and rapid one-step method. Clin Chem Lab Med 41:1633–1641 [DOI] [PubMed] [Google Scholar]
- 32. Batterham RL, Cohen MA, Ellis SM, Le Roux CW, Withers DJ, Frost GS, Ghatei MA, Bloom SR. 2003. Inhibition of food intake in obese subjects by peptide YY3-36. N Engl J Med 349:941–948 [DOI] [PubMed] [Google Scholar]
- 33. Jackson RJ, Davis WB, Macdonald I. 1977. The energy values of carbohydrates: should bomb calorimeter data be modified? Proc Nutr Soc 36:90A. [PubMed] [Google Scholar]
- 34. Näslund E, Grybäck P, Hellström PM, Jacobsson H, Holst JJ, Theodorsson E, Backman L. 1997. Gastrointestinal hormones and gastric emptying 20 years after jejunoileal bypass for massive obesity. Int J Obes Relat Metab Disord 21:387–392 [DOI] [PubMed] [Google Scholar]
- 35. Ellrichmann M, Kapelle M, Ritter PR, Holst JJ, Herzig KH, Schmidt WE, Schmitz F, Meier JJ. 2008. Orlistat inhibition of intestinal lipase acutely increases appetite and attenuates postprandial glucagon-like peptide-1-(7-36)-amide-1, cholecystokinin, and peptide YY concentrations. J Clin Endocrinol Metab 93:3995–3998 [DOI] [PubMed] [Google Scholar]
- 36. Bueter M, Löwenstein C, Olbers T, Wang M, Cluny NL, Bloom SR, Sharkey KA, Lutz TA, le Roux CW. 2010. Gastric bypass increases energy expenditure in rats. Gastroenterology 138:1845–1853 [DOI] [PubMed] [Google Scholar]
- 37. Stylopoulos N, Hoppin AG, Kaplan LM. 2009. Roux-en-Y gastric bypass enhances energy expenditure and extends lifespan in diet-induced obese rats. Obesity 17:1839–1847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Inagaki T, Choi M, Moschetta A, Peng L, Cummins CL, McDonald JG, Luo G, Jones SA, Goodwin B, Richardson JA, Gerard RD, Repa JJ, Mangelsdorf DJ, Kliewer SA. 2005. Fibroblast growth factor 15 functions as an enterohepatic signal to regulate bile acid homeostasis. Cell Metab 2:217–225 [DOI] [PubMed] [Google Scholar]
- 39. Holt JA, Luo G, Billin AN, Bisi J, McNeill YY, Kozarsky KF, Donahee M, Wang DY, Mansfield TA, Kliewer SA, Goodwin B, Jones SA. 2003. Definition of a novel growth factor-dependent signal cascade for the suppression of bile acid biosynthesis. Genes Dev 17:1581–1591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Potthoff MJ, Boney-Montoya J, Choi M, He T, Sunny NE, Satapati S, Suino-Powell K, Xu HE, Gerard RD, Finck BN, Burgess SC, Mangelsdorf DJ, Kliewer SA. 2011. FGF15/19 regulates hepatic glucose metabolism by inhibiting the CREB-PGC-1α pathway. Cell Metab 13:729–738 [DOI] [PMC free article] [PubMed] [Google Scholar]