Abstract
Although the effectiveness of nuclear hormone-receptor complexes is known to depend on coregulator partner proteins, relatively little is known about the roles of coregulators in uterine development and early stages of pregnancy and implantation. Because conventional genetic deletion of the coregulator, repressor of estrogen receptor activity (REA), was embryonic lethal, we here study REA conditional knockout mice generated by cre-loxP recombination, in which REA function was abrogated only in progesterone receptor-expressing tissues, to define the roles of REA in postembryonic stages and in a tissue-specific manner. We find that REA has gene dose-dependent activity impacting uterine development and fertility. Conditional homozygous mutant (REAd/d) mice developed to adulthood and showed normal ovarian function, but females were infertile with severely compromised uterine development and function characterized by cell cycle arrest, apoptosis, and altered adenogenesis (endometrial gland morphogenesis), resulting in failure of implantation and decidualization. By contrast, mice heterozygous for REA (REAf/d) had a very different phenotype, with estradiol treatment resulting in hyperstimulated, large uteri showing increased proliferation of luminal epithelial cells, and enhanced fluid imbibition associated with altered regulation of aquaporins. These REAf/d female mice showed a subfertility phenotype with reduced numbers and sizes of litters. These findings highlight that uterine development and regulation of estrogen receptor activities show a bimodal dependence on the gene dosage of REA. Optimal uterine development and functional activities require the normal gene dosage of REA, with partial or complete deletion resulting in hyperresponsiveness or underresponsiveness to hormone and subfertility or infertility, respectively.
Nuclear receptors act as ligand-activated transcription factors that mediate the actions of many hormones and some nonhormonal ligands. The state of receptor activation or suppression is modulated not only by the nature of the ligand but also by a delicate balance between coactivators and corepressors in a cell (1–4). Repressor of estrogen receptor (ER) activity (REA) was initially identified as a coregulator of the ER that repressed the activity of estradiol (E2) (5–7). Our previous conventional genetic disruption of both alleles of REA resulted in embryonic lethality (8), suggesting that REA had fundamentally important cellular functions. Therefore, to address the role of REA in uterine development and fertility, we have generated and characterized mice with conditional tissue-selective deletion of REA.
REA, also known as prohibitin (PHB)2, is a highly conserved protein (9). In addition to binding to the ER, REA has been shown to interact with cellular proteins associated with chromatin remodeling and transcriptional repression, such as enhancer of zeste homolog 2, a chromatin modifying polycomb group histone methyltransferase (10–12), and histone deacetylase 1. It also competes with coactivators, such as steroid receptor coactivator-1, for binding to chromatin of estrogen-regulated genes (6). In addition to its role as a brake on estrogen-ER regulated gene expression in the nucleus, REA has also been demonstrated to exist in a complex with PHB1 in the mitochondrial inner membrane (9), and these mitochondrial protein complexes have been implicated in mitochondrial biogenesis and cell senescence (9, 13, 14). These findings imply that REA may have pivotal roles in several key cellular processes and may act in different cellular compartments, as reported recently for the coregulator small heterodimer partner (SHP) (15). However, despite accumulating data, the physiological functions that REA controls during development and in reproductive health remain elusive.
In the present study, to circumvent the embryonic lethality encountered in conventional REA knockout mice and to enable the study of REA function in postembryonic tissues, we have used conditional REA knockout mice (REAd/d) generated by cre-loxP recombination, in which REA function was abrogated only in progesterone (P4) receptor (PR)-expressing cell types. Our findings in animals with conditional loss of both alleles of REA or only one allele of REA highlight the importance of the correct gene dosage of REA for proper uterine development, response to E2, and reproductive function.
Materials and Methods
Generation of conditional REA knockout mice
Animals were maintained in accordance with the National Institutes of Health Guide for Care and Use of Laboratory Animals, and all procedures described here were approved by the University of Illinois and Baylor College of Medicine Institutional Animal Care and Use Committees.
Conditional REA knockout mice were generated and characterized as described by us recently (16). In brief, we employed a cre-loxP-mediated recombination strategy, in which the targeting vector eliminated REA exons 2 through 6, which are required for interaction with ER and repressive activity on ER target genes (5). Positive ES cells harboring the targeted allele were confirmed by Southern blot screening and injected into blastocysts donated from a C57BL/6 strain and implanted into pseudopregnant female mice. The resulting chimeric animals were bred to C57BL/6 females, and F1 REAflox-neo/+ mice were mated with Flpe mice (17) for deletion of the neomycin resistance gene cassette and generation of REAf/+ mice.
Because of our interest in delineating REA actions on fertility, we used mice expressing Cre recombinase under control of the PR promoter (PRcre/+) to delete REA in postembryonic reproductive tissues (18). PR-Cre knockin (PRcre/+) mice have been used successfully to eliminate targeted genes in uterine cells (11, 19–21). The REAf/f PRCre/+ mice were finally generated by crossing of the PRcre/+ with REAf/f mice. Ablation of REA alleles in uteri and mammary glands, PR-expressing tissues, was confirmed by genotying PCR (16).
The female REAflox/+ mice were mated with the male PRcre/cre knockin mice (18) to generate the REAflox/+ PRcre/+ bigenic mouse model. To create the REAflox/flox PRcre/+ (denoted REAd/d) mice for deletion of the REA gene in both alleles, several pairs of REAflox/+ PRcre/+ mice were mated.
Superovulation, implantation, and decidualization analyses
The details of these methods have been previously described (19, 20, 22). Briefly, superovulation was induced in 24-d-old mice by administering 5 IU of pregnant mares' serum gonadotropin ip (EMD Biosciences, Inc., San Diego, CA), followed by 5 IU of human chorionic gonadotropin (Sigma-Aldrich Co., St. Louis, MO) given ip 48 h later. Oocytes were flushed from the oviducts 24 h after human chorionic gonadotropin injection and counted.
Implantation sites at 5.5 d postcoitum (dpc) were visualized by an iv injection of 1% Chicago Blue 6B (Sigma-Aldrich Co.) dye solution, and the number of implantation sites was counted.
To induce decidual reactions, ovariectomized mice were primed with once daily sc injections of 100 ng of E2 for 3 d. After 2 d rest, mice were treated with daily sc injection of 1 μg of P4 and 6.7 ng of E2 in 0.1 ml of sesame oil. One uterine horn was mechanically stimulated by an intraluminal injection of 50 μl of sesame oil 6 h after the third injection. The right horn was not stimulated. Daily injections of P4 and E2 were administered until d 5. Mice were killed at 0 and 2 d after stimulation.
Isolation of uterine stromal cells and induction of decidualization in vitro
Uterine stromal cells were isolated as previously described (23, 24). Briefly, uterine horns of d-4 pregnant female mice were dissected longitudinally and cut into 5- to 6-mm pieces. The tissues then were washed with Hanks' balanced salt solution (HBSS) and digested with dispase (6 g/liter) and pancreatin (25 g/liter) for 1 h at room temperature, followed by 10 min at 37 C. The tissue-digestion mixture was gently mixed, and the luminal epithelial containing supernatant was discarded. The partially digested tissues were then washed once in HBSS and redigested with collagenase (0.5 g/liter) for 45 min at 37 C. Digested tissues were mixed until the supernatant became turbid with dispersed stromal cells. The cell suspension was filtered through a 70-μm pore-size mesh (BD Biosciences, San Diego, CA) and centrifuged at 2000 rpm for 5 min. Cells were then resuspended in DMEM-F12 with 2% fetal bovine serum and seeded in six-well culture plates. Isolated uterine stromal cells were grown in this medium containing 1 μm P4 and 10 nm E2 for 96 h to induce in vitro decidual reactions.
Recombinant adenovirus preparation
The Cre-expressing recombinant adenovirus was constructed using an AdEasy XL adenoviral vector system kit (Stratagene, La Jolla, CA) according to the manufacturer's protocols. Cre coding region containing nuclear localization signal was amplified from PGK-Cre-bpA plasmids (25) obtained from Addgene (plasmid 115430), and the REA coding region was retrieved from pCMV-REA (6) and cloned into the pShuttle-CMV (cytomegalovirus) vector. The pShuttle-CMV-Cre plasmids and pShuttle-CMV-REA-Flag were linearlized with PmeI restriction enzyme and then transformed into BJ5183-AD-1 cells. AdEasy-Cre plasmids were selected with PacI enzyme after homologous recombination in BJ5183-AD-1 cells. AdEasy-Cre plasmids then were amplified, linearized, and transfected into human AD-293 cells. After transfection, cells were cultured in DMEM with 10% fetal bovine serum until 80–90% of cells were detached from flasks. Adenoviruses were collected and purified by CsCl gradient protocol as described (26).
Uterine stromal cells were isolated from d-4 pregnant mice and grown as described above. 5 × 105 cells were seeded in six-well plates and cultivated in 5% CO2 for 8 h at 37 C. After the cells were allowed to attach for 8 h, the unattached cells were removed by washing with HBSS and then incubated with either control adenovirus (AdCMV) or Cre adenovirus (AdCre) at different multiplicities of infection.
Uterine bioassays
Ovariectomized (8 wk of age) or immature (21 d old) female mice were injected sc daily with E2 (0.5 μg/10 g of body weight/d) for 4 d. E2 was dissolved in dimethylsulfoxide and then diluted 1:10 in sesame oil. At 24 h after the last injection, uteri were removed and weighed after removal of associated fat and expression of any luminal fluid. One uterine horn from each uterus was stored for RNA isolation, and the other horn was fixed for histology.
Histology and immunostaining
Hematoxylin and eosin staining or immunohistochemistry was performed as previously described (22). In brief, the tissues were fixed in 10% buffered formalin phosphate for 24 h at room temperature, transferred to 70% ethanol, and then embedded in paraffin. Sections (4 μm) were incubated with antibodies to REA (07-234; Millipore, Billerica, MA), PR (A0098; Dako Co., Glostrup, Denmark), p21 (556430; BD Biosciences), or caspase-3 (AF835; R&D Systems, Minneapolis, MN). For assessment of 5-bromo-2-deoxyuridine (BrdU) incorporation, mice were injected ip with 30 μg/g of body weight BrdU (BD Biosciences) at 2 h before killing. Uteri were fixed, embedded in paraffin, and tissue sections were stained with BrdU antibody (3D4; BD Biosciences). Terminal deoxynucleotidyl transferase 2′-deoxyuridine, 5′-triphosphate nick end labeling (TUNEL) staining for apoptosis used the In Situ Cell Death Detection kit (Roche, Indianapolis, IN).
Western blot analysis
Immunoblotting was performed as previously described (8). Uterine stromal cell extracts were prepared using ice-cold lysis buffer [25 mm Tris-HCl (pH 7.4), 150 mm NaCl, 1% Nonidet P-40, 1% sodium deoxycholate, and 1% sodium dodecyl sulfate] supplemented with protease inhibitors (complete EDTA free; Roche) and phosphatase inhibitors (Phopho-stop; Roche). Protein concentrations were determined by the bicinchoninic acid protein assay system (Pierce, Rockford, IL). Proteins (20–50 μg) were separated on SDS-PAGE gels, transferred onto nitrocellulose membranes, and subjected to immunoblotting with anti-REA (07-234; Millipore), anti-Cre (PRB-106C-200; Covance, Inc., Madison, WI), and anti-β-actin (A2228; Sigma-Aldrich Co.).
RNA isolation and real-time PCR
Total RNA was isolated from whole uterine tissue or uterine stromal cells using TRIzol reagent (Invitrogen, Carlsbad, CA). One microgram of total RNA was reverse transcribed and analyzed by real-time PCR as described previously (8). Primers for the genes studied are listed in Supplemental Table 1, published on The Endocrine Society's Journals Online web site at http://endo.endojournals.org.
Results
Conditional REA heterozygous (REAf/d) and REA homozygous (REAd/d) animals exhibit different uterine phenotypes and responsiveness to E2
To examine the impact of REA on uterine development and function, we generated REA conditional knockout animals, in which the REA gene was selectively deleted by Cre-mediated excision in PR-expressing cell lineages. Generation of REA conditional knockout mice was described in detail recently (16), and genotyping documented that REA was specifically deleted in the uterus, mammary gland, and ovary (Supplemental Fig. 1).
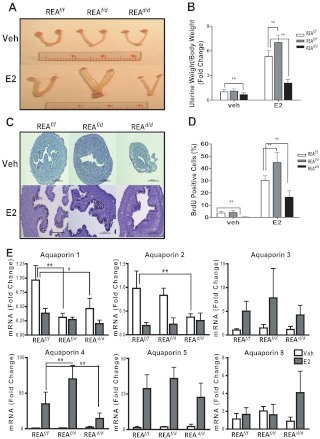
To determine whether REA function might be gene dose dependent, we examined conditional homozygous (REAd/d) animals and also conditional heterozygous REA animals (REAf/d), the latter generated by crossing REAd/d and REAf/f animals. We first monitored uterine morphology and uterine growth in response to E2 in immature (21 d old) REAf/f, REAf/d, and REAd/d animals treated for 4 d with E2 or control vehicle. Strikingly, uteri with conditional loss of one allele of REA (REAf/d) exhibited a phenotype and response to E2 very different from that of conditional homozygous mutant (REAd/d) mice. Although REAd/d uteri were smaller than the REAf/f uteri, the vehicle-treated conditional REA heterozygous (REAf/d) uteri were similar in size to that of the wild-type (REAf/f) mice (Fig. 1, A and B). Treatment with E2 resulted in markedly larger and fluid-filled uteri in REAf/d mice (Fig. 1, A and 1B). Of note, the luminal area of uteri in REAf/d mice was considerably greater than that of REAf/f or REAd/d animals (Fig. 1C), but we found no significant difference in luminal epithelial cell height. Also, the E2-induced uterine weight increase was significantly greater in REAf/d animals compared with wild-type REAf/f animals, and uterine weight was low in REAd/d animals after E2 treatment (Fig. 1B).
Fig. 1.
Uterine growth and cell proliferation in response to E2 are enhanced in REAf/d (heterozygous) mice and reduced in REAd/d (homozygous) mice and are accompanied by changes in the expression of AQP genes. A, Uteri from vehicle or E2-treated REAf/f, REAf/d, and REAd/d mice. Mice, 21 d of age, were injected once daily with control vehicle or E2 for 4 d, and uteri were harvested 24 h after the last injection. B, Uterine weight gain in response to E2 was monitored and normalized to each animal's body weight. C, BrdU immunohistochemistry in uteri from REAf/f, REAf/d, and REAd/d mice treated with vehicle (Veh) or E2 for 4 d and injected ip with BrdU at 2 h before harvesting of uteri (magnification, ×20). Scale bar, 200 μm. D, Percentage of BrdU-positive epithelial cells/total cells monitored in at least six ×40 fields. E, mRNA levels of AQP known to be expressed in the mouse uterus were monitored by qRT-PCR in mice at age 21 d treated with control vehicle or E2 for 4 d. Uteri were harvested and RNA isolated at 24 h after the last injection of E2 or vehicle. The data are mean ± sd (n = 10 per group), and mRNA levels are illustrated as relative expression normalized to 36B4 by vehicle-treated wild type. *, P < 0.05; **, P < 0.01.
To investigate whether this increased E2-stimulated uterine weight gain in REAf/d mice was a result of increased cell proliferation, mice were treated with E2 or vehicle for 4 d and then injected with BrdU at 2 h before killing. As shown in Fig. 1C, the majority of BrdU positive cells was seen in luminal epithelial cells in response to E2 treatment, and the percent of BrdU positive cells was significantly greater in REAf/d vs. REAf/f or homozygous (REAd/d) uteri (Fig. 1D).
Because the uteri of conditional heterozygous REA mutant animals treated with E2 were filled with a large amount of fluid, whereas REAd/d uteri were small and reduced in their fluid imbibition, we examined whether the regulation of genes responsible for uterine water imbibition might be altered in REAf/d and REAd/d uteri. It has been shown that E2 stimulates water imbibition in the uterine endometrium, in part, through water channel proteins termed aquaporins (AQP) (27). To date, 13 AQP have been identified in mammals, and specific AQP isotypes have been shown to be expressed in male and female reproductive tissues (28). To examine whether REA might alter the expression of AQP, we examined the expression of AQP reported to be expressed in the mouse uterus (28). As shown in Fig. 1E, among the six tested, two AQP (AQP1 and AQP2) were down-regulated by E2, and three AQP (AQP3–AQP5) were up-regulated by E2 in wild-type (REAf/f) uteri. Interestingly, the expression of uterine AQP4 in response to E2, which was one of the two most up-regulated of the AQP, was significantly greater in REAf/d vs. REAf/f animals and was greatly reduced in REAd/d uteri. Expression of AQP2 and AQP1 was reduced in REAd/d uteri such that no further decrease by E2 treatment was observed, and the same was seen for AQP1 expression in REAf/d uteri. These findings suggest that the altered fluid accumulation in the uteri of REAf/d and REAd/d animals might be associated with changes in AQP expression and their responsiveness to E2.
Conditional heterozygous (REAf/d) female mice show reduced fertility, whereas conditional homozygous (REAd/d) female mice are infertile—Analysis of ovulation and implantation
To investigate whether the uterine hyperresponsiveness to E2 observed in conditional heterozygous REA females impacts reproductive capability, REAf/d females were mated with wild-type males, and their fertility was monitored in continuous breeding studies over 6 months. As presented in Table 1, REAf/d females showed a significant decrease in litter size compared with control REAf/f mice (6.1 ± 2.5 vs. 9.0 ± 2.1 pups per litter, P < 0.01) and had fewer numbers of pregnancies (4.3 ± 1.8 vs. 6.4 ± 0.5, P < 0.01) over the 6-month period compared with control mice.
Table 1.
REAf/d females are subfertile
| Genotype | Sample size | Deliveries/dam·6 monthsa | Pups | Litters | Pups per littera |
|---|---|---|---|---|---|
| REAf/f | 7 | 6.4 ± 0.5 | 403 | 45 | 9.0 ± 2.1 |
| REAf/d | 7 | 4.3 ± 1.8b | 184 | 30 | 6.1 ± 2.5b |
Mean ± sd.
P < 0.01.
In contrast to the reduced fertility of the REAf/d females, the conditional REA homozygous (REAd/d) female mice were found to be completely infertile. No pups were born from REAd/d females mated with wild-type males over the 6-month period (Table 2). Because aggressive mating behavior has been reported in ERα knockout mice (29, 30), we examined this, but no abnormal sexual behaviors were observed in the mutant females, and copulatory plugs were detected at the normal frequency, indicating normal mating behavior of REAd/d animals.
Table 2.
REAd/d females are infertile
| Genotype | Sample size | Deliveries/dam·6 monthsa | Pups | Litters | Pups per littera |
|---|---|---|---|---|---|
| REAf/f | 9 | 6.7 ± 0.5 | 549 | 60 | 9.1 ± 1.0 |
| REAd/d | 10 | 0 | 0 | 0 |
Mean ± sd.
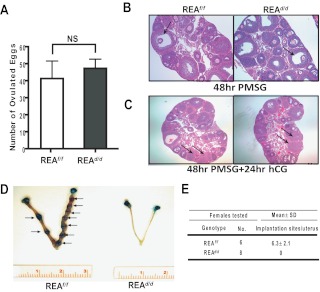
To further explore the cause of infertility of the REAd/d females, we examined the estrous cycle of REAd/d mice as a measure of the integrity of the hypothalamic-pituitary-ovarian reproductive axis, and as reported (16), vaginal epithelial cytology demonstrated that these females cycled normally. Genotyping of tissues from REA conditional knockout mice demonstrated knockout of REA in ovary, uterus, and mammary gland (Supplemental Fig. 1). We therefore investigated whether the ovaries of REAd/d mice were responsive to exogenous gonadotropin treatment and found that the number of ovulated eggs was similar to that observed in control mice (Fig. 2A). Histological studies on ovaries isolated from the superovulation treatment revealed normal follicle maturation and corpora lutea formation (Fig. 2, B and C), further supporting that REAd/d females had no obvious ovarian defects. We also performed superovulation studies in mature mice and found that the number of ovulated eggs was similar in REAf/f and REAd/d mice (Supplemental Fig. 2A). Ovarian histology was also similar in mature mice (Supplemental Fig. 2B).
Fig. 2.
Early pregnancy events in REAf/f and REAd/d mice: superovulation and implantation activities. A, Female mice for each genotype at 24 d of age were subjected to a superovulatory dose of the gonadotropins PMSG and hCG. Oocytes were then collected from their oviducts and counted. B, Follicular development of the ovary was assessed by histologic examination after treatment with PMSG only for 48 h. C, Formation of corpora lutea (CL) was assessed by histologic examination after complete superovulation treatment. Note the presence of numerous mature follicles and CL (indicated as arrow) in wild-type and mutant ovaries. D, Representative uteri from REAf/f and REAd/d mice at 5.5 dpc. Arrows indicate sites of implantation. E, Implantation sites were visually counted by the localized retention of Chicago Blue dye. PMSG, Pregnant mare's serum goandotropin; hCG, human chorionic goandotropin.
We also examined whether blastocysts could properly attach to the uterine luminal epithelium to initiate the implantation process. On the morning of pregnancy d 5–6 (4.5–5.5 dpc), females were injected by Chicago Blue B dye solution, and implantation sites were counted. As shown in Fig. 2, D and E, no implantation sites were detected in the uteri of REAd/d females, whereas the normal number of implantation sites was observed in the REAf/f controls. We also tried to examine early embryo development during pregnancy, but we could not detect any blastocysts or embryos in uterine flushings in REAd/d uteri.
Uterine decidual response is impaired in REAd/d females
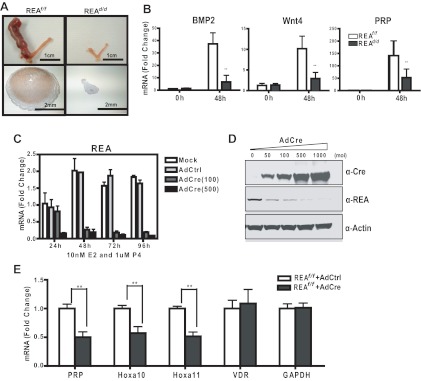
Successful implantation is followed by proliferation and differentiation of endometrial stromal cells into decidual cells (23, 24). We therefore examined decidual response in the uteri of REAd/d animals, which can be induced in the absence of embryo implantation by providing an artificial stimulation to E2- and P4-primed uteri (31). REAf/f and REAd/d animals were ovariectomized and treated with a well-defined 13-d regimen of exposure to exogenous E2 and P4 (see Materials and Methods), and oil was infused into the lumen of the left uterine horn to initiate a decidual response, with the right horn not manipulated to serve as the control. As shown in Fig. 3A, the uteri of REAf/f animals exhibited a full decidual response accompanied by a dramatic increase in uterine size and the characteristic enlargement of decidual cell layers. By contrast, the stimulated uterine horn of REAd/d mice failed to undergo a decidual response, and expression of the decidualization marker genes bone morphogenetic protein 2 (BMP2), wingless-related MMTV integration site 4, and prolactin-related protein was markedly lower in these uteri (Fig. 3B).
Fig. 3.
REAd/d mice show impaired uterine decidual response in vivo and in vitro. A, Representative gross anatomy of uteri from REAf/f and REAd/d mice after decidual stimulation. The left horn was stimulated, and the right horn was not stimulated (see Materials and Methods). Note the dramatic increase in size of the left horn of REAf/f uteri after stimulation. Lower panels show REA in the stimulated REAf/f left horn by immunohistochemistry (IHC). Note high expression of REA in the decidual zone. B, Expression of molecular markers for decidualization measured by qRT-PCR at 0 h and 48 h after stimulation. C, In vitro decidual response of uterine stromal cells. Mouse stromal cells from d-4 pregnant uteri were infected by mock or control adenovirus or different multiplicity of infection (MOI) of AdCre, and then exposed to 10 nm E2 and 1 μm P4 for times up to 96 h. REA mRNA (C) and protein levels (D) were then analyzed by qRT-PCR (24–96 h) and immunoblotting (96 h), respectively. E, Molecular markers specific to stromal cells were monitored at 96 h by qRT-PCR. Data are mean ± sd. *, P < 0.05; **, P < 0.01. Wnt4, Wingless-related MMTV integration site 4; PRP, prolactin-related protein; VDR, vitamin D receptor; GAPDH, glyceraldehyde-3-phosphate dehydrogenase.
To investigate the specific requirement of REA in the uterine stroma for functional and morphological decidual reactions, we examined decidualization of uterine stromal cells in vitro after exposure to E2 and P4. This well-characterized protocol allowed us to examine the role of REA in the stromal compartment only, as well as enabling strict control of Cre-mediated loss of REA by adenoviral infection of cells (23, 24). Primary stromal cells isolated from uteri of d-4 REAf/f pregnant mice were infected with either control adenovirus or AdCre to excise REA alleles. As shown in Fig. 3, C and D, AdCre effectively depleted REA mRNA and protein, and this Cre-mediated REA ablation in stromal cells led to a significant reduction in expression of the stromal differentiation markers, prolactin-related protein, homeobox A (Hoxa)10, and Hoxa11, whereas the expression of other genes not associated with decidualization, such as vitamin D receptor and glyceraldehyde-3-phosphate dehydrogenase, remained unaltered (Fig. 3E). These results demonstrate that REA is a critical factor for successful decidualization both in the uterus in vivo and in uterine stromal cells in vitro.
REAd/d females exhibit uterine developmental defects and altered expression of developmental genes
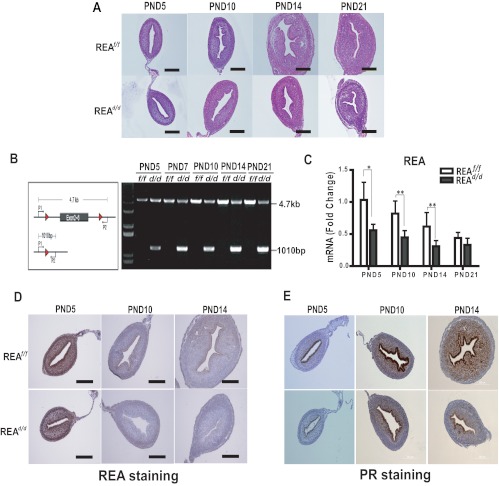
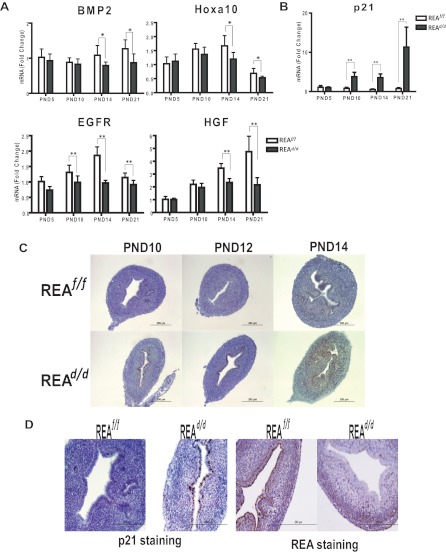
To examine the basis of the altered uterine growth in REAd/d mice, we analyzed uteri throughout different stages of postnatal development for gene expression and histologic phenotypes. The mature uterus is comprised of the endometrium and myometrium, and development of the endometrial glandular epithelium (GE) as well as differentiation of endometrial stroma and myometrium from the mesenchyme occurs during the neonatal stages after birth and is established by postnatal day (PND)15 (32–34). Our evaluations revealed that uteri from REAd/d mutants had a morphology similar to that of wild-type animals until PND10, but that an altered uterine phenotype became apparent by PND14 (Fig. 4A). To determine whether this abnormal uterine development was the result of loss of REA, we examined the REA expression level during postnatal development. Genotyping revealed that targeted REA exons were excised by PR-driven Cre activity as early as PND5 (Fig. 4B). Depletion of REA mRNA and protein was also confirmed by quantitative (q)RT-PCR (Fig. 4C) and immunohistochemistry (Fig. 4D). Of note, reduction of REA expression was first observed in the luminal epithelial cells beginning at PND5, and REA loss was extended to the stromal compartment after PND5 and was observed until PND14 (Fig. 4D). As illustrated in Fig. 4A, the uteri of REAd/d mice contained few endometrial glands, whereas the uteri of wild-type (REAf/f) mice contained many glands in the endometrium. Additionally, wild-type uteri showed a continuous increase in growth of the uterine stroma until PND14, but the uteri of REAd/d mice showed little if any further stroma growth after PND10. These findings indicate that ablation of REA in the developing uterus after birth impedes endometrial gland morphogenesis and stromal proliferation.
Fig. 4.
REAd/d mice show altered uterine growth and maturation. A, Uterine morphology was examined in hematoxylin- and eosin-stained sections from uteri of REAf/f and REAd/d mice at PND5, PND10, PND14, and PND21. B, REA gene is excised in the uteri of REAd/d mice from as early as age 5 d (PND5). REA deletion was confirmed by genotyping. C, REA mRNA level was monitored by qRT-PCR in uteri from PND5, PND10, PND14, and PND21. Values are mean ± sd (n = 10 per group), and mRNA levels are illustrated as relative expression normalized to 36B4 by wild-type PND5. *, P < 0.05; **, P < 0.01. Immunohistochemical detection of REA (D) and PR from PND5, PND10, and PND14 uteri of REAf/f and REAd/d mice (E). Magnification is the same for D and E. Scale bar, 200 μm.
Consistent with this age- and tissue-specific loss of REA, PR expression was only detected in the epithelial cells at PND5, with stromal expression occurring between PND5 and PND10 (Fig. 4E). Therefore, PR-dependent, Cre-mediated loss of REA was well correlated with the endogenous PR expression. These results indicate that loss of REA expression leads to altered uterine development and adversely affects implantation and pregnancy maintenance.
We also examined the expression of several genes implicated in the regulation of postnatal uterine development (34, 35), including BMP2, Hoxa10, epidermal growth factor receptor (EGFR), and hepatocyte growth factor (HGF). As shown in Fig. 5A, expression of all of these genes was significantly reduced in the uteri of REAd/d mice at PND14 and PND21, compared with REAf/f mice.
Fig. 5.
Uterine developmental gene expression is altered in REAd/d mutant mice. A, mRNA levels of molecular markers (BMP2, Hoxa10, EGFR, and HGF) implicated in uterine development as monitored by qRT-PCR. B, The cell cycle inhibitor p21 mRNA expression levels are elevated in the uterus of REAd/d mice, as measured by qRT-PCR. C, p21 protein levels are elevated in the REAd/d uterus as observed by immunohistochemistry (IHC). D, Reduction of REA expression is correlated with increased p21 expression as measured by IHC (PND10 uterus). Scale bar, 200 μm. Values are mean ± sd (n = 10 per group), and mRNA levels are illustrated as relative expression normalized to 36B4 by wild-type PND5. *, P < 0.05; **, P < 0.01.
Neonatal uterine cell proliferation is decreased and apoptosis is increased in uteri of conditional homogygous REA mutant mice
Our findings of uterine hypoplasia in conditional homozygous REA mutant mice led us to investigate cell proliferation and apoptosis. The RNA and protein expression level of p21, a regulator of cell cycle progression at G1, was found to be significantly increased in the uteri of REAd/d mice compared with that of wild-type mice (Fig. 5, B and C), and this elevated p21 expression closely corresponded with the tissues in which the expression of REA was lost (Fig. 5D). It is noteworthy that increased expression of p21 was detected only in the luminal epithelial cells at PND10, but its expression extended to the stroma by PND14. The pattern of increased expression of p21 inversely correlating with the spatial and temporal expression pattern of REA (Fig. 5D) implies that increased p21 expression in cells lacking REA may result in cell cycle arrest and impede uterine growth.
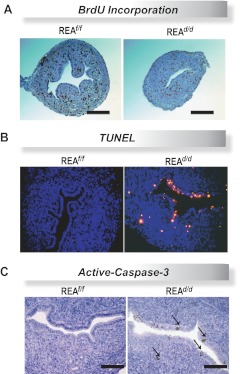
To examine cell proliferation, specifically S-phase activity of the cell cycle, REAf/f and REAd/d mice at 5, 10, and 14 d of age were injected with BrdU 2 h before killing, and uteri were collected. As shown in Fig. 6A, REA mutant uteri exhibited decreased BrdU incorporation, indicating that DNA replication was impaired in uteri of REAd/d mice. We also assessed DNA fragmentation, one of the hallmarks of apoptosis, by TUNEL analysis on wild-type and conditional REAd/d mutant uteri at 14 d of age. We observed markedly increased apoptotic cell death in the REAd/d uterine epithelium and stroma, as evidenced by increased fluorescence (Fig. 6B). We also examined activation of the apoptosis-promoting executioner caspase-3 in REAf/f and REAd/d uterine cells by immunohistochemistry. Because caspase-3 is known to be cleaved from the 32-kDa procaspase-3 to the 18 kDa of caspase-3 to be active, we examined the level of the cleaved form of caspase-3 (36). As shown in Fig. 6C, cells positive for active caspase-3 were present only in the REAd/d uteri and were not observed in REAf/f uterine cells. Collectively, these data suggest that the hypoplasia seen in the REAd/d uterus is likely the result of a combination of increased G1-S-phase cell cycle arrest and also increased apoptosis. Consonant with these findings, in the mature uterus, we observed greatly reduced stimulation of the E2-regulated genes complement C3 and lactoferrin in REAd/d vs. REAf/f uteri (Supplemental Fig. 3).
Fig. 6.
REAd/d mice have decreased neonatal uterine cell proliferation and increased apoptosis. A, Representative histologic sections of BrdU immunohistochemistry in uteri of REAf/f and REAd/d d-14 mice. Note decreased BrdU incorporation in the REAd/d uterus. Scale bar, 200 μm. B, Representative fluorescence images of TUNEL staining in uterine sections from REAf/f and REAd/d d-14 mice. C, Immunohistochemical detection of active-caspase-3 in uteri of REAf/f and REAd/d d-14 mice. Scale bar, 100 μm.
Discussion
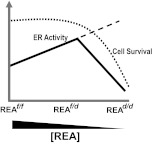
Our findings reveal a key role for REA in cell proliferation, cell survival, and adenogenesis to enable proper uterine development and functional activities. Although conditional loss of both REA alleles resulted in infertility due to severely compromised uterine development, conditional loss of only one REA allele resulted in increased uterine cell proliferation and hyperresponsiveness to E2 that also impacted fertility and resulted in a subfertility phenotype. This in vivo study thus highlights, as schematized in the model shown in Fig. 7, that the correct gene dosage and level of REA are crucial for optimal uterine function.
Fig. 7.
Model for the relationship between REA gene dosage and ER activity and optimal uterine function and fertility. Estrogen activity in the uterus was enhanced with reduction of REA level in REAf/d mice, thereby allowing reduced repression of E2-driven ER activity, resulting in an increased uterine weight gain and enhanced cell proliferation and gene expression with E2 treatment. However, further depletion of REA (REAd/d mice) does not elicit a further enhancement of E2 stimulation (long dashes), because this is associated with loss of an essential cell survival function of REA that results in cell cycle arrest and apoptosis, leading to the impairment of REA-dependent activities essential for ER action, and uterine development and function. Thus, the normal gene dosage of REA is required for optimal fertility, with hyperresponsiveness to E2 in REAf/d mice and underresponsiveness to hormone in REAd/d mice resulting in subfertility or infertility, respectively.
Uterine developmental defects of homozygous REA knockout animals: Enhanced cell cycle arrest and altered uterine adenogenesis
Normal uterine function is required for fertility, with reproductive health dependent on proper embryonic and perinatal development, as evidenced by exposure of neonates to endocrine disruptors resulting in adult infertility (37). The uterus begins to develop from the Müllerian duct during embryonic developmental stages, but development is not completed until after birth. Postnatal uterine development involves massive cell proliferation and differentiation to establish the mature endometrium and myometrium of the uterus (32–35). We observed that the uteri of conditional homozygous REA mutants displayed an immature, hypoplastic morphology. This uterine hypoplasia was associated with cell cycle arrest, as evidenced by elevated cell cycle inhibitor p21 expression and reduced BrdU uptake, and increased apoptosis of uterine epithelial and stromal cells. Loss of REA also resulted in altered expression of a number of genes implicated in uterine development. However, we do not currently know whether the defects in adenogenesis and in other aspects of uterine development indicate a direct role of REA or are consequent to REA depletion.
Our examination of postnatal wild-type uteri showed a continuous increase in growth and size of the uterus until PND14, and this growth was positively correlated with increased levels of growth factors and growth factor receptors. The uterine defects seen in REAd/d animals may be, at least in part, associated with a decreased level of EGFR and growth factors such as HGF. Moreover, these uteri contained notably fewer endometrial glands. The endometrial glands secrete and transport diverse cellular factors, such as leukemia inhibitory factor and calcitonin, required for the establishment of uterine receptivity and nourishment of the developing conceptus. Endometrial gland development from the luminal epithelium, termed adenogenesis, which is predominantly a postnatal process, involves a series of morphogenic events: budding of GE from the luminal epithelium, penetration of stroma by tubes of GE, and coiling and branching of GE. In the rodent uterus, adenogenesis proceeds from PND9 through PND15 (34). Interestingly, the window of time at which the altered uterine phenotype becomes apparent in REAd/d animals (between d 10 and 14) corresponds with the period of endometrial gland development. Our observation that loss of REA expression started from the luminal epithelium and was associated with cell death suggests that the early apoptosis of luminal epithelial cells in these REA knockout animals likely contributes to the failure of successful uterine adenogenesis.
REA function as a nuclear receptor coregulator
The current studies on the impact of conditional tissue-specific REA homozygous (REAd/d) and heterozygous (REAf/d) deletion demonstrate that REA is a gene dosage-dependent coregulator able to profoundly impact steroid receptor function. Female REA heterozygous animals showed a greater uterine weight gain in response to E2, which was due, in part, to increased E2-induced cell proliferation, as evidenced by BrdU incorporation, providing strong support for REA being a coregulator that can repress the activities of E2. Strikingly, treatment of REAf/d animals with E2 resulted not only in larger but also very fluid-filled uteri. Our findings suggest that the exaggerated fluid accumulation seen in the uteri of REAf/d animals might be through altered regulation of AQP expression, especially of interest because changes in the expression and regulation of AQP have been strongly associated with some reproductive disorders (28). The marked enhancement of fluid uptake in response to E2 in d 21 age REAf/d mice was, however, much less obvious in mature ovariectomized REAf/d uteri, perhaps because the myometrium is usually more developed and thicker, leading to less fluid uptake due to the resistance offered by the thicker uterine wall. The hyperresponsiveness to E2 that we observed in REAf/d mice also influenced female reproductive function and was associated with a subfertility phenotype. Litter size and also the frequency of pregnancies were significantly decreased in REAf/d animals, implying that REA plays an important role in establishing the tightly regulated estrogen responsiveness essential for optimal fertility.
REA is a protein with multiple functions in the uterus
It is increasingly appreciated that many cellular proteins have more than one intracellular localization and can carry out several functions. For example, the ERα is localized primarily in the nucleus but is also found at extranuclear locations, suggested to be cytoplasmic, mitochondrial, or membrane associated, and to have effects on gene expression and also on activation of protein kinases and the regulation of energy metabolism (38–40). Numerous connections have also now been demonstrated between mitochondrial and nuclear activities, with evidence of mitochondrial-nuclear communication, the latter involving transcription factors and coactivators that regulate both nuclear and mitochondrial gene expression (41). Indeed, mitochondrial biogenesis requires a coordination of expression of nuclear and mitochondrial genome-encoded proteins and therefore cross talk between these cellular organelles (41). In this regard, it is of interest that recent studies have documented the nuclear and mitochondrial localization of the coregulator, SHP, and roles for SHP at both of these sites in regulating the actions of SHP and the bile acid receptor FXR in hepatic carcinogenesis (15).
REA is an evolutionarily conserved protein and has increasingly been found to be involved in diverse cellular processes, including transcription, mitochondrial biogenesis, and replicative senescence (6, 9, 12, 42). Our lab previously identified REA in a yeast two-hybrid screen using ERα as bait and characterized REA as a coregulator that repressed the transcriptional activity of this nuclear hormone receptor (5–8). In addition to its role as a transcriptional coregulator, REA has been shown to exist in a protein complex with PHB1, and the REA/PHB1 complex appears to be important for maintenance of mitochondrial integrity (9, 14, 42–45).
Our findings provide evolving understanding of REA as a nuclear transcriptional coregulator and also support a mitochondrial function for REA, because complete deletion of REA induced apoptosis and increased caspase-3 activation in the uterus. Because REA can form oligomers with PHB1, it is of interest that PHB1 has been shown to act like REA to repress ERα activity and that its complete deletion severely impacted uterine function as also observed in PHB1d/d mice (46, 47).
The studies presented here provide new insight into REA functions, and assist in understanding the key roles of REA in uterine development and fertility, supporting implantation and uterine decidualization early in pregnancy. We have demonstrated, as shown schematically in Fig. 7, that REA is a negative regulator of E2-stimulated activities, ensuring optimal physiological actions of this hormone by serving as a brake to keep E2-stimulated activities in check. This corepressor activity of REA is observed in uteri of REAf/d mice, where the partial loss of REA diminishes the restraining effect of REA on E2-driven activity. Thus, these heterozygous conditional REAf/d mice showed hyperresponsiveness to E2 that was associated with a subfertility phenotype. With further loss of REA, in homozygous conditional REAd/d mice, it is not possible to examine coregulator functions of REA, because this loss of REA leads to loss of a critical cell survival function, as demonstrated by the impaired uterine cell proliferation and increased apoptosis in these animals, and their complete infertility resulting from uterine developmental defects. These findings provide new insight into gene dose-dependent physiological roles of REA in uterine development and reproductive capability. They highlight that the normal gene dosage of REA is required for optimal uterine development and function and that hyperresponsiveness to E2 or underresponsiveness to hormone results in alteration and impairment of fertility.
Supplementary Material
Acknowledgments
This work was supported by National Institutes of Health (NIH) Grants U54 HD055787 as part of the Eunice Kennedy Shriver National Institute of Child Health and Human Development/NIH Centers Program in Reproduction and Infertility Research (to B.S.K., F.J.D., and M.K.B.) and P50 AT006268 from the National Center for Complementary and Alternative Medicine, the Office of Dietary Supplements and the National Cancer Institute (B.S.K.), and U54 HD07495 (to B.W.O.), R01 DK058242 (to J.X.), and R01 CA077530 (to J.P.L.).
Disclosure Summary: The authors have nothing to disclose.
For editorial see page 3555
- AQP
- Aquaporin
- BMP2
- bone morphogenetic protein 2
- BrdU
- 5-bromo-2-deoxyuridine
- CMV
- cytomegalovirus
- dpc
- dpostcoitum
- E2
- estradiol
- EGFR
- epidermal growth factor receptor
- ER
- estrogen receptor
- GE
- glandular epithelium
- HBSS
- Hanks' balanced salt solution
- HGF
- hepatocyte growth factor
- Hoxa
- homeobox A
- P4
- progesterone
- PHB
- prohibitin
- PND
- postnatal day
- PR
- P4 receptor
- q
- quantitative
- REA
- repressor of ER activity
- SHP
- small heterodimer partner
- TUNEL
- terminal deoxynucleotidyl transferase 2′-deoxyuridine, 5′-triphosphate nick end labeling.
References
- 1. Hewitt SC, Korach KS. 2003. Oestrogen receptor knockout mice: roles for oestrogen receptors α and β in reproductive tissues. Reproduction 125:143–149 [DOI] [PubMed] [Google Scholar]
- 2. Dey SK, Lim H, Das SK, Reese J, Paria BC, Daikoku T, Wang H. 2004. Molecular cues to implantation. Endocr Rev 25:341–373 [DOI] [PubMed] [Google Scholar]
- 3. McKenna NJ, O'Malley BW. 2002. Combinatorial control of gene expression by nuclear receptors and coregulators. Cell 108:465–474 [DOI] [PubMed] [Google Scholar]
- 4. Katzenellenbogen BS, Montano MM, Ediger TR, Sun J, Ekena K, Lazennec G, Martini PG, McInerney EM, Delage-Mourroux R, Weis K, Katzenellenbogen JA. 2000. Estrogen receptors: selective ligands, partners, and distinctive pharmacology. Recent Prog Horm Res 55:163–193; discussion 194–165 [PubMed] [Google Scholar]
- 5. Delage-Mourroux R, Martini PG, Choi I, Kraichely DM, Hoeksema J, Katzenellenbogen BS. 2000. Analysis of estrogen receptor interaction with a repressor of estrogen receptor activity (REA) and the regulation of estrogen receptor transcriptional activity by REA. J Biol Chem 275:35848–35856 [DOI] [PubMed] [Google Scholar]
- 6. Montano MM, Ekena K, Delage-Mourroux R, Chang W, Martini P, Katzenellenbogen BS. 1999. An estrogen receptor-selective coregulator that potentiates the effectiveness of antiestrogens and represses the activity of estrogens. Proc Natl Acad Sci USA 96:6947–6952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Mussi P, Liao L, Park SE, Ciana P, Maggi A, Katzenellenbogen BS, Xu J, O'Malley BW. 2006. Haploinsufficiency of the corepressor of estrogen receptor activity (REA) enhances estrogen receptor function in the mammary gland. Proc Natl Acad Sci USA 103:16716–16721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Park SE, Xu J, Frolova A, Liao L, O'Malley BW, Katzenellenbogen BS. 2005. Genetic deletion of the repressor of estrogen receptor activity (REA) enhances the response to estrogen in target tissues in vivo. Mol Cell Biol 25:1989–1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Mishra S, Murphy LC, Murphy LJ. 2006. The Prohibitins: emerging roles in diverse functions. J Cell Mol Med 10:353–363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hwang C, Giri VN, Wilkinson JC, Wright CW, Wilkinson AS, Cooney KA, Duckett CS. 2008. EZH2 regulates the transcription of estrogen-responsive genes through association with REA, an estrogen receptor corepressor. Breast Cancer Res Treat 107:235–242 [DOI] [PubMed] [Google Scholar]
- 11. Kurihara I, Lee DK, Petit FG, Jeong J, Lee K, Lydon JP, DeMayo FJ, Tsai MJ, Tsai SY. 2007. COUP-TFII mediates progesterone regulation of uterine implantation by controlling ER activity. PLoS Genet 3:e102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kurtev V, Margueron R, Kroboth K, Ogris E, Cavailles V, Seiser C. 2004. Transcriptional regulation by the repressor of estrogen receptor activity via recruitment of histone deacetylases. J Biol Chem 279:24834–24843 [DOI] [PubMed] [Google Scholar]
- 13. Artal-Sanz M, Tavernarakis N. 2009. Prohibitin and mitochondrial biology. Trends Endocrinol Metab 20:394–401 [DOI] [PubMed] [Google Scholar]
- 14. Merkwirth C, Dargazanli S, Tatsuta T, Geimer S, Löwer B, Wunderlich FT, von Kleist-Retzow JC, Waisman A, Westermann B, Langer T. 2008. Prohibitins control cell proliferation and apoptosis by regulating OPA1-dependent cristae morphogenesis in mitochondria. Genes Dev 22:476–488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Zhang Y, Soto J, Park K, Viswanath G, Kuwada S, Abel ED, Wang L. 2010. Nuclear receptor SHP, a death receptor that targets mitochondria, induces apoptosis and inhibits tumor growth. Mol Cell Biol 30:1341–1356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Park S, Zhao Y, Yoon S, Xu J, Liao L, Lydon J, DeMayo F, O'Malley BW, Katzenellenbogen BS. 2011. Repressor of estrogen receptor activity (REA) is essential for mammary gland morphogenesis and functional activities: studies in conditional knockout mice. Endocrinology 152:4336–4349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Farley FW, Soriano P, Steffen LS, Dymecki SM. 2000. Widespread recombinase expression using FLPeR (flipper) mice. Genesis 28:106–110 [PubMed] [Google Scholar]
- 18. Soyal SM, Mukherjee A, Lee KY, Li J, Li H, DeMayo FJ, Lydon JP. 2005. Cre-mediated recombination in cell lineages that express the progesterone receptor. Genesis 41:58–66 [DOI] [PubMed] [Google Scholar]
- 19. Mukherjee A, Soyal SM, Fernandez-Valdivia R, Gehin M, Chambon P, Demayo FJ, Lydon JP, O'Malley BW. 2006. Steroid receptor coactivator 2 is critical for progesterone-dependent uterine function and mammary morphogenesis in the mouse. Mol Cell Biol 26:6571–6583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lee KY, Jeong JW, Wang J, Ma L, Martin JF, Tsai SY, Lydon JP, DeMayo FJ. 2007. Bmp2 is critical for the murine uterine decidual response. Mol Cell Biol 27:5468–5478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Daikoku T, Hirota Y, Tranguch S, Joshi AR, DeMayo FJ, Lydon JP, Ellenson LH, Dey SK. 2008. Conditional loss of uterine Pten unfailingly and rapidly induces endometrial cancer in mice. Cancer Res 68:5619–5627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kim J, Sato M, Li Q, Lydon JP, Demayo FJ, Bagchi IC, Bagchi MK. 2008. Peroxisome proliferator-activated receptor γ is a target of progesterone regulation in the preovulatory follicles and controls ovulation in mice. Mol Cell Biol 28:1770–1782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Li Q, Kannan A, Wang W, Demayo FJ, Taylor RN, Bagchi MK, Bagchi IC. 2007. Bone morphogenetic protein 2 functions via a conserved signaling pathway involving Wnt4 to regulate uterine decidualization in the mouse and the human. J Biol Chem 282:31725–31732 [DOI] [PubMed] [Google Scholar]
- 24. Shimizu A, Maruyama T, Tamaki K, Uchida H, Asada H, Yoshimura Y. 2005. Impairment of decidualization in SRC-deficient mice. Biol Reprod 73:1219–1227 [DOI] [PubMed] [Google Scholar]
- 25. Soriano P, Montgomery C, Geske R, Bradley A. 1991. Targeted disruption of the c-src proto-oncogene leads to osteopetrosis in mice. Cell 64:693–702 [DOI] [PubMed] [Google Scholar]
- 26. Luo J, Deng ZL, Luo X, Tang N, Song WX, Chen J, Sharff KA, Luu HH, Haydon RC, Kinzler KW, Vogelstein B, He TC. 2007. A protocol for rapid generation of recombinant adenoviruses using the AdEasy system. Nat Protoc 2:1236–1247 [DOI] [PubMed] [Google Scholar]
- 27. Jablonski EM, McConnell NA, Hughes FM, Jr, Huet-Hudson YM. 2003. Estrogen regulation of aquaporins in the mouse uterus: potential roles in uterine water movement. Biol Reprod 69:1481–1487 [DOI] [PubMed] [Google Scholar]
- 28. Huang HF, He RH, Sun CC, Zhang Y, Meng QX, Ma YY. 2006. Function of aquaporins in female and male reproductive systems. Hum Reprod Update 12:785–795 [DOI] [PubMed] [Google Scholar]
- 29. Nelson RJ, Demas GE, Huang PL, Fishman MC, Dawson VL, Dawson TM, Snyder SH. 1995. Behavioural abnormalities in male mice lacking neuronal nitric oxide synthase. Nature 378:383–386 [DOI] [PubMed] [Google Scholar]
- 30. Couse JF, Korach KS. 1999. Estrogen receptor null mice: what have we learned and where will they lead us? Endocr Rev 20:358–417 [DOI] [PubMed] [Google Scholar]
- 31. Lydon JP, DeMayo FJ, Funk CR, Mani SK, Hughes AR, Montgomery CA, Jr, Shyamala G, Conneely OM, O'Malley BW. 1995. Mice lacking progesterone receptor exhibit pleiotropic reproductive abnormalities. Genes Dev 9:2266–2278 [DOI] [PubMed] [Google Scholar]
- 32. Yin Y, Ma L. 2005. Development of the mammalian female reproductive tract. J Biochem 137:677–683 [DOI] [PubMed] [Google Scholar]
- 33. Brody JR, Cunha GR. 1989. Histologic, morphometric, and immunocytochemical analysis of myometrial development in rats and mice: II. Effects of DES on development. Am J Anat 186:21–42 [DOI] [PubMed] [Google Scholar]
- 34. Gray CA, Bartol FF, Tarleton BJ, Wiley AA, Johnson GA, Bazer FW, Spencer TE. 2001. Developmental biology of uterine glands. Biol Reprod 65:1311–1323 [DOI] [PubMed] [Google Scholar]
- 35. Hu J, Gray CA, Spencer TE. 2004. Gene expression profiling of neonatal mouse uterine development. Biol Reprod 70:1870–1876 [DOI] [PubMed] [Google Scholar]
- 36. Riedl SJ, Salvesen GS. 2007. The apoptosome: signalling platform of cell death. Nat Rev Mol Cell Biol 8:405–413 [DOI] [PubMed] [Google Scholar]
- 37. Spencer TE, Hayashi K, Hu J, Carpenter KD. 2005. Comparative developmental biology of the mammalian uterus. Curr Topics Dev Biol 68:85–122 [DOI] [PubMed] [Google Scholar]
- 38. Hammes SR, Levin ER. 2007. Extranuclear steroid receptors: nature and actions. Endocr Rev 28:726–741 [DOI] [PubMed] [Google Scholar]
- 39. Madak-Erdogan Z, Kieser KJ, Kim SH, Komm B, Katzenellenbogen JA, Katzenellenbogen BS. 2008. Nuclear and extranuclear pathway inputs in the regulation of global gene expression by estrogen receptors. Mol Endocrinol 22:2116–2127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Madak-Erdogan Z, Lupien M, Stossi F, Brown M, Katzenellenbogen BS. 2011. Genomic collaboration of estrogen receptor α and extracellular signal-regulated kinase 2 in regulating gene and proliferation programs. Mol Cell Biol 31:226–236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Ryan MT, Hoogenraad NJ. 2007. Mitochondrial-nuclear communications. Annu Rev Biochem 76:701–722 [DOI] [PubMed] [Google Scholar]
- 42. Osman C, Merkwirth C, Langer T. 2009. Prohibitins and the functional compartmentalization of mitochondrial membranes. J Cell Sci 122:3823–3830 [DOI] [PubMed] [Google Scholar]
- 43. Merkwirth C, Langer T. 2009. Prohibitin function within mitochondria: essential roles for cell proliferation and cristae morphogenesis. Biochim Biophys Acta 1793:27–32 [DOI] [PubMed] [Google Scholar]
- 44. Nijtmans LG, de Jong L, Artal Sanz M, Coates PJ, Berden JA, Back JW, Muijsers AO, van der Spek H, Grivell LA. 2000. Prohibitins act as a membrane-bound chaperone for the stabilization of mitochondrial proteins. EMBO J 19:2444–2451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Tatsuta T, Model K, Langer T. 2005. Formation of membrane-bound ring complexes by prohibitins in mitochondria. Mol Biol Cell 16:248–259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. He B, Feng Q, Mukherjee A, Lonard DM, DeMayo FJ, Katzenellenbogen BS, Lydon JP, O'Malley BW. 2008. A repressive role for prohibitin in estrogen signaling. Mol Endocrinol 22:344–360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. He B, Kim TH, Kommagani R, Feng Q, Lanz RB, Jeong JW, DeMayo FJ, Katzenellenbogen BS, Lydon JP, O'Malley BW. 2011. Estrogen-regulated prohibitin is required for mouse uterine development and adult function. Endocrinology 152:1047–1056 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.