Abstract
An appropriate concentration of intracellular T3 is a critical determinant of placenta development and function and is mainly controlled by the activity of type II deiodinase (D2). The levels of this enzyme are finely regulated in different tissues by coordinated transcriptional mechanisms, which rely on dedicated promoter sequences (e.g. cAMP response element and TATA elements) that impart inducibility and tissue specificity to Dio2 mRNA expression. Here we show that CCAAT enhancer-binding proteins α and β (C/EBPα and C/EBPβ) promote Dio2 expression in the trophoblastic cell line JEG3 through a conserved CCAAT element, which is a novel key component of the Dio2 promoter code that confers tissue-specific expression of D2 in these cells. Increased C/EBPs levels potently induce Dio2 transcription, whereas their ablation results in loss of Dio2 mRNA. By measuring the activity of several deletion and point mutant promoter constructs, we have identified the functional CCAAT element responsible for this effect, which is located in close proximity to the most 5′ TATA box. Notably, this newly identified sequence is highly conserved throughout the species and binds in vivo and in vitro C/EBP, indicating the relevance of this regulatory mechanism. Together, our results unveil a novel mechanism of regulation of D2 expression in a trophoblastic cell line, which may play a relevant role during placenta development.
During gestation, the maintenance of appropriate intracellular T3 concentrations is a critical determinant for proper development of some tissues, including fetal brain and maternal placenta (1, 2). In these areas, the intracellular T3 levels are promptly adjusted to the cellular requirements by several mechanisms aimed at preventing the severe consequences of gestational hypothyroidism (e.g. cretinism, premature delivery, and abortion). The intracellular conversion of T4 to T3, catalyzed by type II deiodinase (D2), represents a critical regulated mechanism of this process. D2 catalyzes the removal of a single iodine from the outer ring of T4 in the 5′ position and is expressed in several tissues, including brain, muscle, fat, thyroid, bone, and placenta (3). The regulation of D2 levels and activity is a critical step to control local T3 concentrations and has been characterized in different tissues (3). D2 can be regulated at the posttranslational level by ubiquitination and de-ubiquitination mechanisms (4). Additionally, a tight regulation of D2 levels in specific tissues and in response to different stimuli is achieved by a fine-tuned transcriptional control of the Dio2 gene. The promoter region of the Dio2 gene has been carefully characterized in different contexts. Several cis-acting elements have been identified as responsible for the response to different stimuli or as determinants of tissue specificity (5). In humans, one of the tissues where the Dio2 gene is specifically expressed and regulated is the placenta. Indeed, D2 levels are elevated in the cytotrophoblast during the first trimester of gestation (6), possibly as a consequence of an increased need for T3 in this tissue at this stage to support the endocrine function of placenta (7). For this reason, D2 levels in early pregnancy appear to be carefully regulated by multiple mechanisms. In our previous work, we have characterized the promoter architecture of the human Dio2 gene and its regulation in the choriocarcinoma cell line JEG3, which presents many of the biological and biochemical features associated with early trophoblast. These cells express the α- and β-subunits of the chorionic gonadotropin (CG) gene and harbor a significant 5′-deiodination activity (8, 9). In analogy with the α-subunit of CG (αCG), a paradigmatic placenta-specific gene expressed mostly during the first trimester of gestation, Dio2 promoter is synergistically regulated by epidermal growth factor (EGF) and cAMP, acting through a conserved promoter cAMP response element (CRE) and a TATA element (9, 10). Because EGF is secreted by placenta during early gestation and cytotrophoblast expresses EGF receptor (11), we have hypothesized that this autocrine mechanism may contribute to ensure high levels of placental D2, αCG, and other critical genes during the early stage of pregnancy.
In addition to the CRE-TATA unit, there are other important determinants of placental expression in the αCG promoter, such as CCAAT boxes, GATA elements, and upstream regulatory elements (12).
In particular, CCAAT boxes appear to play a major role in determining the tissue specificity to αCG gene. CCAAT elements bind a bZIP family of transcription factors, called CCAAT enhancer-binding proteins (C/EBPs) (13). There are six members of C/EBPs (C/EBPα, C/EBPβ, C/EBPδ, C/EBPϵ, C/EBPγ, and C/EBPζ) that play different roles in different tissues (14). It has been demonstrated that C/EBPα and -β are pivotal regulators of development and function of placenta (15) and that their combined ablation results in failure to develop the trophoblast (16). Because we noticed several putative CCAAT elements in the promoter region of the human Dio2 gene, the aim of this study was to determine whether C/EBPs directly regulate Dio2 mRNA levels and participate in the orchestrated regulation of Dio2 expression in the JEG3 placenta cell line.
Materials and Methods
Plasmids
Human reporter plasmids 1299 ATG Dio2 Luc and 805 ATG Dio2 Luc have been previously described (8). A series of 5′-deletion promoter constructs, all lacking the region downstream of the transcriptional start site (TSS), were constructed by PCR using the 1.3-kb Dio2 Luc plasmid as template. The following constructs were generated and named based on their distance to the TSS: 590 TSS, 120 TSS, 101 TSS, 81 TSS, 61 TSS, and 41 TSS Dio2 Luc.
The following primers were used: 590 TSS forward, 5′-CTGGGATGGTAC-3′; 120 TSS forward, 5′-CTGGCCAAAGTAAAGCCCT-3′; 101 TSS forward, 5′-CCCAAGCTTCTTTCTCAATGACGTCAAGA-3′; 81 TSS forward, 5′-CCCAAGCTTTCTTTACCAAGATTAGGCTT-3′; 61 TSS forward, 5′-CCCAAGCTTTCACTTCTCTATTGCAGCAA-3′; 41 TSS forward, 5′-CCCAAGCTTTTAGCCAGGGAATGTATAAAAG-3′; and TSS reverse, 5′-GCTATCTGTCTGTGGTGCA-3′.
Promoter sequences were amplified by PCR with GoTaq Green Master Mix (Promega, Madison, WI), together with forward and reverse primers that contained a 5′ HindIII adaptor sequence. PCR products were cloned into the HindIII site of plasmid pSVOALΔ5′, a luciferase expression vector (8).
The expression vectors pcDNA3-HA-C/EBPα and pcDNA3-Flag-C/EBPβ were obtained by PCR amplification of human placental cDNA and cloning into pcDNA3-HA and pcDNA3-Flag vectors. The constructs have been sequenced to verify the absence of PCR-dependent errors.
Cell culture, transient and stable transfections, and luciferase assays
JEG3 cells were cultured in MEM supplemented with 10% fetal bovine serum and 1% l-glutamine. The day before transfection, 200,000 cells per well were seeded in six-well plates and grown overnight. For transfection, cells were incubated overnight in Opti-MEM (Invitrogen, Carlsbad, CA) with 0.9 μg luciferase reporter plasmid, 1 μg of the indicated expression vectors, and 5 μl Lipofectin per well (Invitrogen). Plasmid RSV β-gal (0.1 μg/well), which expresses β-galactosidase under the control of the RSV promoter, was used in all transfections to normalize the luciferase activity. The empty expression vector pcDNA3 (Invitrogen) was used, when necessary, to maintain a total of 2 μg f plasmid DNA. After transfection, cells were lysed in 0.5% Triton X-100, 0.25 m Tris (pH 8), and luciferase activity was measured.
To generate stably overexpressing C/EBPα (JEG3α) and -β (JEG3β) cell lines, JEG3 cells were transfected with pcDNA3-HA-C/EBPα and pcDNA3-Flag-C/EBPβ plasmids (see above). Stably transfected cells were selected by incubation with 0.8 mg/ml Geneticin (Life Technologies, Grand Island, NY), and single clonal cells were isolated and grown. The level of expression of C/EBPα or -β proteins in the clonal cells was verified by Western blot assay.
RNA isolation and semiquantitative and quantitative RT-PCR
Total RNA was isolated with RNeasy mini Kit (QIAGEN, Valencia, CA) according to the manufacturer's instructions. Reverse transcription was performed with 1 μg total RNA using Superscript II and random hexamers (Invitrogen). Semiquantitative PCR was performed as previously described (9), using primers specific to Dio2 and β-actin cDNA.
The primers used to amplify the human Dio2 cDNA were forward 5′-GCATGCTGACCTCAGAGGGACTGCGCTGCGTCTGG-3′ and reverse 5′-AACCAGCTAATCTAGTTTTCTTTCATCTCTTGCTG-3′. For human β-actin, the following primers were used: forward 5′-CTACAATGAGCTGCGTGTGG-3′ and reverse 5′-CGGTGAGGATCTTCATGAGG-3′.
Quantitative real-time PCR was performed as described previously (17) with the following primers: human Dio2, forward 5′-AGCTTCCTCCTCGATGCCTAC-3′ and reverse 5′-CCACTGTTGTCACCTCCTTCTGT-3′, and human GAPDH, forward 5′-GTATTCCCCCAGGTTTACAT-3′ and reverse 5′-TTCTGTCTTCCCTCACTCC-3′.
mRNA stability
To determine the stability of Dio2 mRNA, cells were incubated with 5 μg/ml actinomycin D (Sigma Chemical Co., St. Louis, MO) for the indicated times. RNA was extracted, and semiquantitative RT-PCR was performed as described above. Densitometric analysis was performed using NIH ImageJ software.
RNA interference
RNA interference experiments were performed using the following small interfering RNA (siRNA) smart pools (Dharmacon, Lafayette, CO): 100 nm human C/EBPα (M006422030005), 100 nm human C/EBPβ (M006423030005), and 100 nm nontargeting SMART pool (D-001206-13). siRNA transfections were performed by incubating JEG3 cells with Lipofectamine 2000 (Invitrogen) in Opti-MEM for 6 h. After transfection, cells were grown for another 48 h. The efficacy of the knockdown was monitored by C/EBP Western blot analysis and was always greater than 95%.
PCR-based mutagenesis
Plasmid 1299 ATG TATA MUT Dio2 Luc has been previously described (10). To generate point mutations of the CCAAT box sequence in the 1299 ATG Dio2 Luc plasmid, the QuikChange Site-Directed Mutagenesis Kit (Stratagene, La Jolla, CA) was used. The primer sequences containing the mutations were human Dio2 CCAAT MUT, forward 5′-TTTCACTTCTCTATGGCAGCACTTAGCCAGGG-3′ and reverse 5′-CCCTGGCTAAGTGCTGCCATAGAGAAGTGAAA-3′.
Western Blot
To perform Western blot experiments on C/EBPα and C/EBPβ JEG3 stable lines, cells were lysed in sodium dodecyl sulfate/urea buffer (17) and sonicated. Forty micrograms of cell lysates were loaded, and Western blot was performed following standard techniques. Antibodies against C/EBPα (SC-61) and C/EBPβ (SC-150) were from Santa Cruz Biotechnology (Santa Cruz, CA).
Chromatin immunoprecipitation
Chromatin immunoprecipitation was performed as previously described (9). Briefly, cells were incubated with 1% formaldehyde for 20 min at room temperature to cross-link proteins to DNA. After cross-linking, cells were lysed [3% sarkosyl, 5 mm EDTA, 50 mm Tris-HCl (pH 8.1), plus protease inhibitors] and sonicated to reduce the genomic DNA into small fragments (400–600 bp). The lysates were precleared with protein A-agarose and nonspecific antibodies for 2 h at 4 C. After preclearing, the lysates were incubated with control IgG, C/EBPα, and C/EBPβ antisera (Santa Cruz Biotechnology) overnight at 4 C. At the end of the incubation, the immunocomplexes were washed extensively and eluted, the cross-linking was reversed, and the DNA purified, precipitated, and resuspended. Eluted DNA was analyzed with quantitative real-time PCR using primers encompassing Dio2 or the unrelated GAPDH gene. Quantitative ChIP was performed as already described (9). The following primers were used: human Dio2 CCAAT WT (primers A), forward 5′- GCCAAAGTAAAGCCCTCTTTCTC-3′ and reverse 5′-GCCTTTTATACATTCCCTGGCTAA-3′; human Dio2 intron (primers B), forward 5′-TGATTTTCGCCCTGGTCTTT-3′ and reverse 5′-CATTCTTCTCAAGCCTGGTTGA-3′; and GAPDH, forward 5′-AGAACATCATCCCTGCCTCT-3′ and reverse 5′-CACCTGGTGCTCAGTGTAG-3′.
Recombinant proteins
C/EBPα and C/EBPβ recombinant proteins have been generated with TNT T7 Coupled Reticulocyte Lysate System Kit (Promega), according to the manufacturer's instructions, using pcDNA3-HA-C/EBPα and pcDNA3-Flag-C/EBPβ plasmids.
Electrophoretic mobility shift assay
Binding reactions mixture contained 0.6 ng 32P-labeled oligonucleotide probe, 15 μg recombinant proteins, 2 μg poly (deoxyinosine-deoxycytosine), 200 mm KCl, 75 mm HEPES (pH 7.9), 5 mm EDTA, 2.5 mm dithiothreitol, and 25% glycerol in a total volume of 25 ml. To perform supershift experiments, 2 μg of the anti-C/EBPβ antibody (Santa Cruz Biotechnology) were added to the binding reaction mixture. In competition experiments, 100-fold molar excess of cold oligonucleotides was added to the binding reaction mixture. The following oligonucleotide probes were used: human Dio2 CAAT WT, forward 5′-TTTCACTTCTCTATTGCAGCAATTAGCCAGGG-3′ and reverse 5′-CCCTGGCTAATTGCTGCAATAGAGAAGTGAAA-3′; and human Dio2 CAAT MUT, forward 5′-TTTCACTTCTCTATGGCAGCACTTAGCCAGGG-3′ and reverse 5′-CCCTGGCTAAGTGCTGCCATAGAGAAGTGAAA-3′. Reaction mixtures were incubated for 30 min at room temperature, resolved on nondenaturing 4% polyacrylamide gel, dried, and exposed to autoradiography.
Statistical analysis
The results presented in figures are representative of at least three independent experiments with comparable results.
Statistical differences were tested using the Mann-Whitney U test when treatment and control samples were analyzed or with nonparametric ANOVA (Kruskal-Wallis test) when more than two treatments were analyzed. Where significant differences were observed (P < 0.05) using ANOVA, pairwise comparisons were carried out using Dunn's multiple-comparisons test. All statistical tests are indicated in the figure legends. Differences were considered significant at P < 0.05. Statistical analyses were performed using Instat GraphPad version 3.06 statistical software (GraphPad Inc., San Diego, CA).
Results
Dio2 gene is regulated by C/EBPs
The promoter region of the human Dio2 gene contains potential binding sites for C/EBP proteins (Table 1), and the activity of these transcription factors have been linked to the regulation of key genes during early trophoblast development (15, 16). To determine whether C/EBPs could also regulate transcription of Dio2 gene in a placenta cell line, we first performed a luciferase assay on the 1.3-kb region of the Dio2 promoter in JEG3 cells. Previous studies have shown that C/EBPα and -β are predominantly expressed in placenta cells (15). Therefore, we focused our studies on these two isoforms.
Table 1.
Potential C/EBP sites in Dio2 promoter
| C/EBP site | |
|---|---|
| 1 | TCAAT (−1002/−997 ATG) (−291 TSS −286 TSS) |
| 2 | GCAAT (−833/−828 ATG) (−122 TSS −117 TSS) |
| 3 | TCAAT (−803/−798 ATG) (−92 TSS −87 TSS) |
| 4 | ATTGCAGCAAT (−760/−749 ATG) (−49 TSS −44 TSS) (−43 TSS −37 TSS) |
| 5 | GCAAT (−503/−498 ATG) |
| 6 | ACCAT (−461/−456 ATG) |
Numbers in parentheses indicate the positions of the different sites from the translational start site (ATG) and from the most 5' transcriptional start site (TSS).
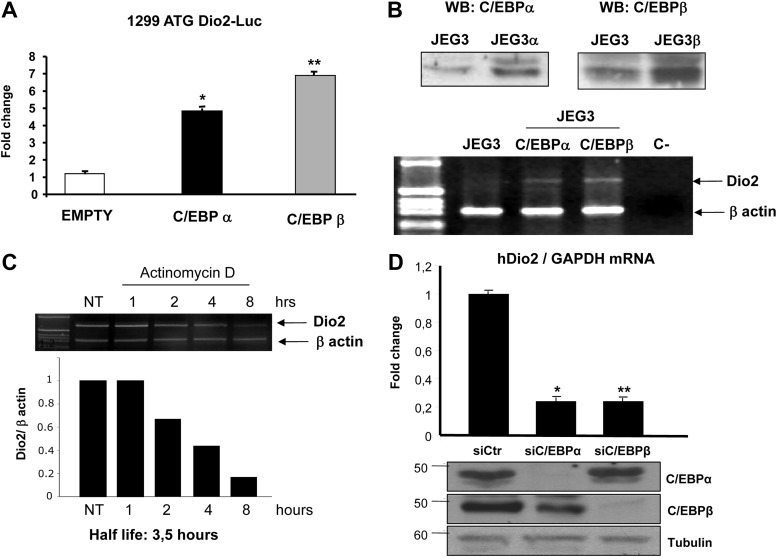
As shown in Fig. 1A, overexpression of both C/EBPα and C/EBPβ strongly induced Dio2 promoter. To verify that C/EBPs increase the levels of endogenous Dio2 mRNA, we performed RT-PCR studies in JEG3 cells stably expressing C/EBPα and C/EBPβ (JEG3α and JEG3β; Fig. 1B). As shown in Fig. 1B, Dio2 mRNA levels were higher in JEG3α and JEG3β cells overexpressing the transcription factors compared with control JEG3 cells transfected with empty vector.
Fig. 1.
A, Dio2 gene is induced by C/EBPs: luciferase assay of 1299 ATG Dio2 luc promoter construct in JEG3 cells. Cells were seeded in six-well plates and transfected with 1299 ATG Dio2 Luc, RSV β-gal, C/EBPα, and C/EBPβ plasmids. At the end of the incubation, luciferase and β-galactosidase assays were performed. ANOVA test: *, C/EBPα vs. control, P < 0.05; **, C/EBPβ vs. control, P < 0.001. Results represent the average value ± sd of three different experiments, each performed in triplicate. B, Endogenous expression of Dio2 mRNA in JEG3-C/EBPα and JEG3-C/EBPβ stable cell lines; top, Western blot (WB) with C/EBPα and C/EBPβ antisera; bottom, RT-PCR analysis, using primers specific for Dio2 and β-actin cDNA. C, Stability of C/EBP-induced transcript. C/EBPβ stable cell line (JEG3β) cells were incubated with 5 μg/ml actinomycin D for the indicated times. At the end of the incubation, total RNA was extracted and reverse transcribed, and the cDNA was amplified with primers complementary to Dio2 and β-actin genes (top); bottom, densitometric analysis of the gel. D, Effect of siRNA-mediated knockdown of C/EBPs on Dio2 mRNA expression; top, quantitative RT-PCR of RNA extracted from JEG3 cells transfected with control (siCtr) and C/EBPα and C/EBPβ siRNA, with Dio2 mRNA levels normalized to GAPDH mRNA levels; bottom, Western blot with C/EBPα, C/EBPβ, or tubulin antisera to show the efficacy of siRNA-mediated knockdown. ANOVA test: *, C/EBPα vs. control, P < 0.001; **, C/EBPβ vs. control, P < 0.001. Results represent the average value ± sd of three different experiments, each performed in triplicate.
Previous studies have shown that the stability of Dio2 mRNA is differentially affected by different stimuli and transcription factors (9). To measure the stability of the C/EBP-induced transcript, we incubated JEG3β cells with actinomycin D for different times. The calculated half-life for this C/EBP-induced transcript was 3.5 h (Fig. 1C). Similar data were obtained with JEG3α cells (not shown).
To understand the physiological relevance of the C/EBPs regulation on Dio2 gene expression, we next examined the consequences of siRNA-mediated knockdown of both transcription factors in JEG3 cells. After 48 h from the siRNA transfection, we observed an almost complete loss of both C/EBPα and C/EBPβ proteins (Fig. 1D, bottom). Remarkably, Dio2 mRNA levels were strongly reduced in both C/EBPα- and C/EBPβ-deficient cells (Fig. 1D, top), as assessed by real-time quantitative PCR. These data demonstrate that both transcription factors are essential to basal Dio2 mRNA expression in these cells.
Mapping of the C/EBP-responsive sequence
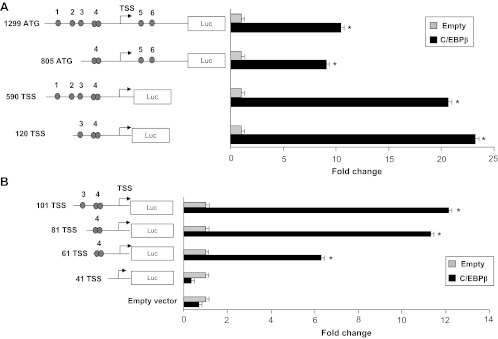
Because the promoter region of Dio2 gene has multiple potential CCAAT boxes (Table 1 and Fig. 2), we sought to identify the sequence involved in the response to C/EBPs. We first tested the 5′ and 3′ deletion mutant constructs depicted in Fig. 2A. All the constructs tested showed a significant response to C/EBPβ, including the smaller plasmid, starting at nucleotide −120 relative to the TSS.
Fig. 2.
A, Luciferase assay of Dio2-Luc promoter constructs in JEG3 cells. Cells were seeded in six-well plates and transfected with 1299 ATG, 805 ATG, 590 TSS, 120 TSS Dio2 Luc (schematic representation on the left), RSV β-gal, and C/EBPβ plasmids. At the end of the incubation, luciferase and β-galactosidase assays were performed. *, C/EBPβ vs. control, P < 0.05, Mann-Whitney test U test. Results represent the average value ± sd of three different experiments, each performed in triplicate. The numbered circles represent the potential Dio2 CCAAT elements. B, Luciferase assay of Dio2-Luc promoter constructs in JEG3 cells. Cells were seeded in six-well plates and transfected with 101 TSS, 81 TSS, 61 TSS, 41 TSS Dio2 Luc, RSV β gal and C/EBPβ plasmids. At the end of the incubation, luciferase and β-galactosidase assays were performed. *, C/EBPβ vs. control, P < 0.05, Mann-Whitney test U test. Results represent the average value ± sd of three different experiments, each performed in triplicate.
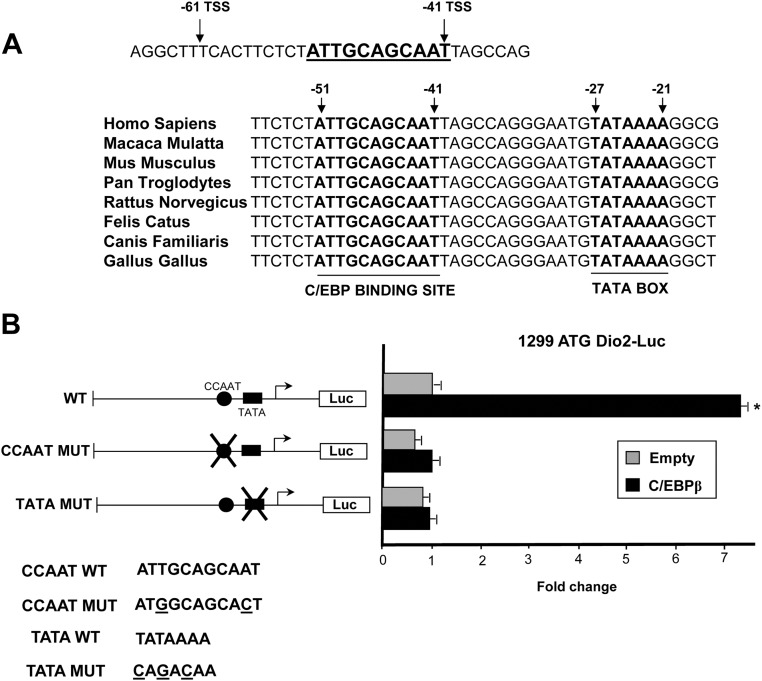
Therefore, we generated and tested a series of micro-5′-deletion mutants, starting from the 120 TSS constructs. With this approach, we isolated a 20-bp region, between nucleotides −61 and −41, that was essential to confer the response to C/EBPβ (Fig. 2B). Sequence analysis of this 20-nucleotide fragment revealed the presence of a C/EBP-binding site (ATTGCAGCAAT; Fig. 3A), which differs from a canonical consensus site (ATTGCGCAAT) only for a single nucleotide insertion and is separated from the most 5′ Dio2 TATA box by 13 nucleotides. Sequence alignment showed that the putative C/EBP site, the downstream region, and the TATA box are highly conserved throughout the species (Fig. 3A).
Fig. 3.
A, Top, sequence of the 20-nucleotide region (−61 to −41) containing the CCAAT element (underlined bold) in human Dio2 promoter; bottom, sequence alignment of this region in various species; bold indicates C/EBP binding sites and TATA boxes. The numbers indicate the position of the two elements, relative to the most 5′ TSS in human Dio2. B, Luciferase assay of 1299 ATG Dio2 Luc promoter constructs in JEG3 cells. Cells were seeded in six-well plates and transfected with 1299 ATG WT Dio2 Luc, 1299 ATG CCAAT MUT Dio2 Luc, 1299 ATG TATA MUT Dio2 Luc, RSV β-gal, and C/EBPβ plasmids. At the end of the incubation, luciferase and β-galactosidase assays were performed. *, C/EBPβ vs. control, P < 0.001, Mann-Whitney test U test. Results represent the average value ± sd of three different experiments, each performed in triplicate. Bottom, Wild-type and mutant sequences (the underlined letters indicate the point mutations).
To determine whether the putative site is functional, we introduced two point mutations in the CCAAT site (see Fig. 3B) by site-directed mutagenesis followed by luciferase assay. As shown in Fig. 3B, mutation of the CCAAT site in the context of the 1299 ATG Dio2 Luc construct resulted in the complete loss of C/EBPβ response, demonstrating that C/EBPβ induces Dio2 promoter exclusively through the identified CCAAT element.
The 5′-flanking region human Dio2 gene has a complex organization and contains multiple TATA boxes and TSS (8, 18). Therefore, we wondered whether C/EBPβ works in concert with the nearby TATA box (the most 5′ TATA box) or other TATA or potential non-TATA elements. To this end, we tested the response of the 1299 Dio2 region mutated in the most 5′ TATA box (TATA MUT). As shown in Fig. 3B, C/EBPβ was unable to induce transcription of the mutant construct, thus demonstrating that the CCAAT unit is functionally dependent on the integrity of the most 5′ TATA box.
Analysis of the binding of C/EBPs to Dio2 promoter
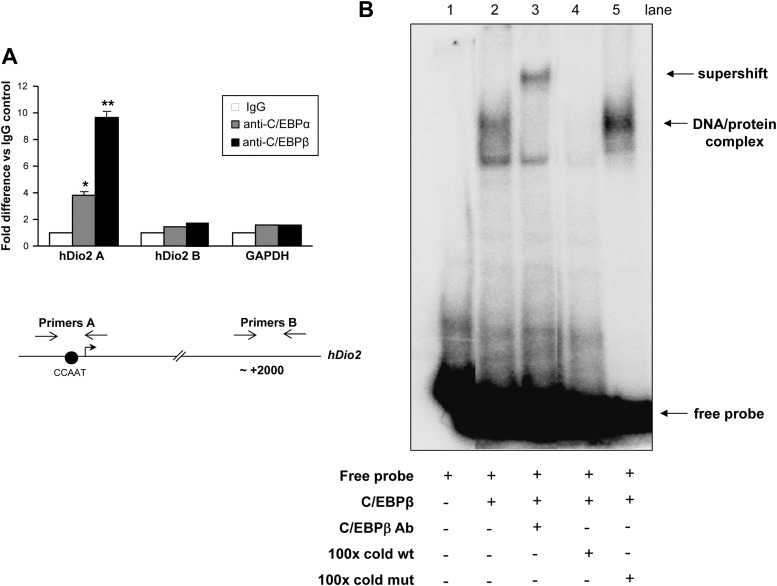
To determine whether C/EBPs are recruited to Dio2 promoter in vivo, we performed quantitative chromatin immunoprecipitation experiments. As shown in Fig. 4A, when the PCR was performed with primers encompassing the Dio2 CCAAT promoter region (primers A, Fig. 4A, bottom), a strong enrichment was detected in C/EBPα and C/EBPβ immunoprecipitation samples, compared with IgG control. In contrast, no enrichment was detected when using primers amplifying an intronic region located 2000 nt downstream from the TSS (primers B, Fig. 4A, bottom) or with primers amplifying the coding region of the GAPDH gene. Thus, these data demonstrate that C/EBPα and -β efficiently bind Dio2 promoter in vivo.
Fig. 4.
A, Quantitative chromatin immunoprecipitation. JEG3 cells were cross-linked with 1% formaldehyde and lysed. The lysates were sonicated and immunoprecipitated with control IgG, C/EBPα, and C/EBPβ antisera. At the end of the incubation, the immunocomplexes were washed extensively and eluted, the cross-linking was reversed, and the DNA was purified, precipitated, and resuspended in Tris-EDTA buffer. Quantitative PCR was performed using primers amplifying Dio2 CCAAT sequence (primers A), an intronic region of Dio2 gene (primers B), and the unrelated GAPDH gene. ANOVA test: *, C/EBPα vs. control, P < 0.05; **, C/EBPβ vs. control, P < 0.001. Results represent the average value ± sd of three different experiments, each performed in triplicate. hDio2, Human Dio2. B, EMSA. 32P-Labeled Dio2 CCAAT oligonucleotide probe, recombinant flag-tagged C/EBPβ, anti-C/EBPβ antibody, 100-fold molar excess of cold wild-type (wt) and cold mutated (mut) oligonucleotides were added to the reaction, as indicated. The free probe is indicated. The indicated retarded bands represent the complex between recombinant C/EBPβ and the Dio2 CCAAT probe (DNA-protein complex) or the supershift upon addition of antisera (supershift).
To study whether C/EBPs bind directly to the identified responsive sequence, we performed EMSA (Fig. 4B). The addition of recombinant flag-tagged C/EBPβ to a radiolabeled oligonucleotide containing the Dio2 C/EBP site caused the formation of a DNA-protein complex (see lane 2). The band was specifically supershifted upon addition of anti-C/EBPβ antibodies (lane 3). The addition of 100× excess cold wild-type oligonucleotide completely displaced the complex (lane 4), whereas addition of 100× excess cold mutated oligo (same mutation of Fig. 3B) failed to compete with the labeled oligonucleotide (lane 5).
Taken together, these data demonstrated that C/EBPs bind in vivo and in vitro Dio2 promoter.
Discussion
In the present work, we have demonstrated the relevance of C/EBP transcription factors in the regulation of Dio2 gene expression in a human placenta cell line.
The time-restricted expression of several genes in specific tissues is governed by the concerted action of multiple transcription factors, working in an orchestrated fashion on specific promoter modules. One of these genes appears to be Dio2, which is expressed in selected tissues as a result of dedicated mechanisms acting at the promoter level.
A well-known case of such regulation occurs in human thyroid, where it has been demonstrated that human Dio2 gene is highly expressed thanks to the presence of a thyroid transcription factor-1 response element located in the enhancer region of human but not mouse gene (19). Another mechanism of tissue-specific expression of Dio2 gene has been elucidated in cardiac muscle. In cardiomyocytes, Dio2 expression is regulated by the combined action of two transcription factors, NKX 2.5 and GATA4, that impart tissue specificity to the gene through the binding to specific promoter sequences (20).
In our previous studies, we have sought to understand how Dio2 gene is regulated and selectively expressed in JEG3, a cell line recapitulating several features of the early trophoblast. We have compared Dio2 gene with a paradigmatic example of a placenta-specific gene: the α-subunit of CG (αCG). Interestingly, analogies between the two genes have emerged from our studies. Both genes are regulated by the cAMP-protein kinase A signaling through activation of the transcription factor CRE-binding protein, which specifically binds to conserved CRE sites located in their promoter regions (8, 10, 18, 21). In both cases, the CRE is located in proximity to a functional TATA box, which is a critical feature needed to confer a full response to cAMP agonists (22). In addition, both genes are similarly regulated by EGF, which acts synergistically with cAMP agonists through activation of a composite transcription factor module, consisting of CRE-binding protein, cJun, and cfos, which are activated and recruited to the CRE in a single complex (9, 12).
With this work, we have added further complexity to this model. We have demonstrated that the specific expression of Dio2 gene in trophoblastic cells is governed by an additional common promoter sequence, represented by the CCAAT enhancer binding element, which is remarkably conserved throughout the species. In analogy with the αCG promoter, this functional CCAAT element is located nearby the TATA box and needs its integrity to properly function (23).
We have demonstrated that C/EBPα and C/EBPβ, which are expressed in the early trophoblast, bind in vivo and in vitro to this CCAAT box and potently induce Dio2 mRNA expression in JEG3 cells. In addition, a strong reduction of Dio2 transcription is observed in JEG3 cells upon ablation of both transcription factors or mutation of the CCAAT box, indicating a crucial role of this mechanism for the physiological maintenance of basal Dio2 mRNA levels in this placenta cell line.
The maintenance of proper levels of T3 in human placenta has been suggested to be critical during the early stage of gestation to support the proliferation and the endocrine functions of the expanding trophoblast (7). Because C/EBPs are mediators of the response to a number of extracellular cues (e.g. cytokines, insulin, and thyroid hormones) (14), it may be speculated that the Dio2 CCAAT box could also be involved in the response to proliferating and differentiating signals acting at placenta level during the early stage of trophoblast development. One such signal could be the leukemia inhibitory factor cytokine, which activates C/EBP in some cells, such as the adipocytes (24), and has been found to be crucial for trophoblast development and embryo implantation (25, 26).
Another relevant issue that stems from these data is whether the effect of C/EBP on Dio2 gene expression is important in other areas. In this regard, previous studies have shown that mice lacking C/EBPα display impaired thermogenesis and decreased T3 content in brown adipose tissue, due to low D2 activity (27). Therefore, this observation can now be explained with a direct effect of C/EBP on Dio2 expression in brown adipose tissue, a tissue where C/EBPα and D2 act as master regulators of tissue development and heat production. In addition, it has been recently shown (28) that overexpressed C/EBPα and -β induce rat Dio2 gene expression in glial cells. Although the responsive sequence has not been finely mapped in that report, it was shown that the C/EBP-mediated regulation occurs in a promoter fragment mapping between nucleotides −83 and −37 (relative to the TSS), which overlaps with the region that we have identified in this study.
In conclusion, we have described a novel mechanism of regulation of Dio2 gene in a placenta cell line, whereby CCAAT elements are critical components of a composite promoter code, which imparts tissue specificity to this gene. The Dio2 promoter shares several features with αCG promoter, a critical placenta-specific gene, thus indicating that these two genes are part of a common transcriptional program and suggesting that this mechanism could also play a relevant role in vivo during the early stage of trophoblast development.
Acknowledgments
This work was supported by grants from “Sapienza” University of Rome (University Grants prot. 0006345).
Disclosure Summary: The authors have no conflicts of interest to declare.
Footnotes
- C/EBP
- CCAAT enhancer-binding protein
- CG
- chorionic gonadotropin
- CRE
- cAMP response element
- D2
- type II deiodinase
- EGF
- epidermal growth factor
- siRNA
- small interfering RNA
- TSS
- transcriptional start site.
References
- 1. Porterfield SP, Hendrich CE. 1993. The role of thyroid hormones in prenatal and neonatal neurological development–current perspectives. Endocr Rev 14:94–106 [DOI] [PubMed] [Google Scholar]
- 2. Burrow GN, Fisher DA, Larsen PR. 1994. Maternal and fetal thyroid function. N Engl J Med 331:1072–1078 [DOI] [PubMed] [Google Scholar]
- 3. Bianco AC, Salvatore D, Gereben B, Berry MJ, Larsen PR. 2002. Biochemistry, cellular and molecular biology, and physiological roles of the iodothyronine selenodeiodinases. Endocr Rev 23:38–89 [DOI] [PubMed] [Google Scholar]
- 4. Gereben B, Zavacki AM, Ribich S, Kim BW, Huang SA, Simonides WS, Zeold A, Bianco AC. 2008. Cellular and molecular basis of deiodinase-regulated thyroid hormone signalling. Endoc Rev 29:898–938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Stoytcheva ZR, Berry MJ. 2009. Transcriptional regulation of mammalian selenoprotein expression. Biochim Biophys Acta 1790:1429–1440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Chan S, Kachilele S, Hobbs E, Bulmer JN, Boelaert K, McCabe CJ, Driver PM, Bradwell AR, Kester M, Visser TJ, Franklyn JA, Kilby MD. 2003. Placental iodothyronine deiodinase expression in normal and growth-restricted human pregnancies. J Clin Endocrinol Metab 88:4488–4495 [DOI] [PubMed] [Google Scholar]
- 7. Maruo T, Matsuo H, Mochizuki M. 1991. Thyroid hormone as a biological amplifier of differentiated trophoblast function in early pregnancy. Acta Endocrinol (Copenh) 125:58–66 [DOI] [PubMed] [Google Scholar]
- 8. Canettieri G, Celi FS, Baccheschi G, Salvatori L, Andreoli M, Centanni M. 2000. Isolation of human type 2 deiodinase gene promoter and characterization of a functional cyclic adenosine monophosphate response element. Endocrinology 141:1804–1813 [DOI] [PubMed] [Google Scholar]
- 9. Canettieri G, Franchi A, Della Guardia M, Morantte I, Santaguida MG, Harney JW, Larsen PR, Centanni M. 2008. Activation of thyroid hormone is transcriptionally regulated by epidermal growth factor in human placenta-derived JEG-3 cells. Endocrinology 149:695–702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Canettieri G, Franchi A, Sibilla R, Guzmán E, Centanni M. 2004. Functional characterisation of the CRE/TATA box unit of type 2 deiodinase gene promoter in a human choriocarcinoma cell line. J Mol Endocrinol 33:51–58 [DOI] [PubMed] [Google Scholar]
- 11. Amemiya K, Kurachi H, Adachi H, Morishige KI, Adachi K, Imai T, Miyake A. 1994. Involvement of epidermal growth factor (EGF)/EGF receptor autocrine and paracrine mechanism in human trophoblast cells: functional differentiation in vitro. J Endocrinol 143:291–301 [DOI] [PubMed] [Google Scholar]
- 12. Roberson MS, Ban M, Zhang T, Mulvaney JM. 2000. Role of the cyclic AMP response element binding complex and activation of mitogen-activated protein kinases in synergistic activation of the glycoprotein hormone alpha subunit gene by epidermal growth factor and forskolin. Mol Cell Biol 20:3331–3334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Osada S, Yamamoto H, Nishihara T, Imagawa M. 1996. DNA binding specificity of the CCAAT/enhancer-binding protein transcription factor family. J Biol Chem 271:3891–3896 [DOI] [PubMed] [Google Scholar]
- 14. Ramji DP, Foka P. 2002. CCAAT/enhancer-binding proteins: structure, function and regulation. Biochem J 365:561–575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bamberger AM, Makrigiannakis A, Schroder M, Bamberger CM, Relakis C, Gellersen B, Milde-Langosch K, Loning T. 2004. Expression pattern of the CCAAT/enhancer-binding proteins C/EBP-alpha, C/EBP-beta and C/EBP-delta in the human placenta. Virchows Arch 444:149–152 [DOI] [PubMed] [Google Scholar]
- 16. Begay V, Smink J, Leuz A. 2004. Essential requirement of CCAAT/Enhancer binding proteins in embryogenesis. Mol Cell Biol 24:9744–9751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Canettieri G, Coni S, Della Guardia M, Nocerino V, Antonucci L, Di Magno L, Screaton R, Screpanti I, Giannini G, Gulino A. 2009. The coactivator CRTC1 promotes cell proliferation and transformation via AP-1. Proc Natl Acad Sci U S A 106:1445–1450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bartha T, Kim SW, Salvatore D, Gereben B, Tu HM, Harney JW, Rudas P, Larsen PR. 2000. Characterization of the 5′-flanking and 5′-untranslated regions of the cyclic adenosine 3′,5′-monophosphate-responsive human type 2 iodothyronine deiodinase gene. Endocrinology 141:229–237 [DOI] [PubMed] [Google Scholar]
- 19. Gereben B, Salvatore D, Harney JW, Tu HM, Larsen PR. 2001. The human, but not rat, dio2 gene is stimulated by thyroid transcription factor-1 (TTF-1). Mol Endocrinol 15:112–124 [DOI] [PubMed] [Google Scholar]
- 20. Dentice M, Morisco C, Vitale M, Rossi G, Fenzi G, Salvatore D. 2003. The different cardiac expression of the type 2 iodothyronine deiodinase gene between human and rat is related to the differential response of the Dio2 genes to Nkx-2.5 and GATA-4 transcription factors. Mol Endocrinol 17:1508–1521 [DOI] [PubMed] [Google Scholar]
- 21. Deutsch PJ, Jameson JL, Habener JF. 1987. Cyclic AMP responsiveness of human gonadotropin-alpha gene transcription is directed by a repeated 18-base pair enhancer. Alpha-promoter receptivity to the enhancer confers cell-preferential expression. J Biol Chem 262:12169–12174 [PubMed] [Google Scholar]
- 22. Conkright MD, Guzmán E, Flechner L, Su AI, Hogenesch JB, Montminy M. 2003. Genome-wide analysis of CREB target genes reveals a core promoter requirement for cAMP responsiveness. Mol Cell 11(4):1101–1108 Erratum in: Mol Cell 11:1417, 2003 May [DOI] [PubMed] [Google Scholar]
- 23. Andersen B, Kennedy GC, Nilson JH. 1990. A cis-acting element located between the cAMP response elements and CCAAT box augments cell-specific expression of the glycoprotein hormone alpha subunit gene. J Biol Chem 265:21874–21880 [PubMed] [Google Scholar]
- 24. Belmonte N, Phillips BW, Massiera F, Villageois P, Wdziekonski B, Saint-Marc P, Nichols J, Aubert J, Saeki K, Yuo A, Narumiya S, Ailhaud G, Dani C. 2001. Activation of extracellular signal-regulated kinases and CREB/ATF-1 mediate the expression of CCAAT/enhancer binding proteins beta and -delta in preadipocytes. Mol Endocrinol 15:2037–2049 [DOI] [PubMed] [Google Scholar]
- 25. Stewart CL, Kaspar P, Brunet LJ, Bhatt H, Gadi I, Köntgen F, Abbondanzo SJ. 1992. Blastocyst implantation depends on maternal expression of leukaemia inhibitory factor. Nature 359:76–79 [DOI] [PubMed] [Google Scholar]
- 26. Nachtigall MJ, Kliman HJ, Feinberg RF, Olive DL, Engin O, Arici A. 1996. The effect of leukemia inhibitory factor (LIF) on trophoblast differentiation: a potential role in human implantation. J Clin Endocrinol Metab 81:801–806 [DOI] [PubMed] [Google Scholar]
- 27. Carmona MC, Iglesias R, Obregon MJ, Darlington GJ, Villarroya F, Giralt M. 2002. Mitochondrial biogenesis and thyroid status maturation in brown fat require CCAAT/Enhancer-binding protein α. J Biol Chem 277:21489–21498 [DOI] [PubMed] [Google Scholar]
- 28. Lamirand A, Ramaugé M, Pierre M, Courtin F. 2011. Bacterial lipopolysaccharide induces type 2 deiodinase in cultured rat astrocytes. J Endocrinol 208:183–192 [DOI] [PubMed] [Google Scholar]