Abstract
Although prolactinomas can be effectively treated with dopamine agonists, about 20% of patients develop dopamine resistance or tumor recurrence after surgery, indicating a need for better understanding of underlying disease mechanisms. Although estrogen-induced rat prolactinomas have been widely used to investigate the development of this tumor, the extent that the model recapitulates features of human prolactinomas is unclear. To prioritize candidate genes and gene sets regulating human and rat prolactinomas, microarray results derived from human prolactinomas and pituitaries of estrogen-treated ACI rats were integrated and analyzed. A total of 4545 differentially expressed pituitary genes were identified in estrogen-treated ACI rats [false discovery rate (FDR) < 0.01]. By comparing pituitary microarray results derived from estrogen-treated Brown Norway rats (a strain not sensitive to estrogen), 4073 genes were shown specific to estrogen-treated ACI rats. Human prolactinomas exhibited 1177 differentially expressed genes (FDR < 0.05). Combining microarray data derived from human prolactinoma and pituitaries of estrogen-treated ACI rat, 145 concordantly expressed genes, including E2F1, Myc, Igf1, and CEBPD, were identified. Gene set enrichment analysis revealed that 278 curated pathways and 59 gene sets of transcription factors were enriched (FDR < 25%) in estrogen-treated ACI rats, suggesting a critical role for Myc, E2F1, CEBPD, and Sp1 in this rat prolactinoma. Similarly increased Myc, E2F1, and Sp1 expression was validated using real-time PCR and Western blot in estrogen-treated Fischer rat pituitary glands. In summary, characterization of individual genes and gene sets in human and in estrogen-induced rat prolactinomas validates the model and provides insights into genomic changes associated with this commonly encountered pituitary tumor.
Prolactinoma is the most common adult pituitary tumor accounting for 60% of functional pituitary adenomas (1, 2). Prolactinoma causes hyperprolactinemia, resulting in impaired reproduction, decreased libido, amenorrhea, and galactorrhea. Adenoma growth leads to compressive mass effects resulting in headache, visual disturbances, cranial nerve palsies, and hypopituitarism. Prolactinomas express abundant levels of dopamine D2 receptor (D2R) and can be effectively treated with dopaminergic drugs, reducing both prolactin levels and tumor volume (1). However, about 20% of prolactinomas may exhibit either dopamine agonist resistance or high recurrence rates after surgery (3), indicating a need for better understanding the disease and developing improved therapies.
Evaluation of new drugs and understanding prolactinoma development have been assessed using high-mobility group protein A (HMGA)-1 and HMGA2 transgenic mice, D2R knockout mice and estrogen-treated rats (3–7). HMGA1 and HMGA2 transgenic mice develop pituitary tumors that secrete both GH and prolactin (PRL) at approximately 12–16 months of age (4, 5). D2R-deficient mice form pituitary lactotroph adenomas at approximately 17–20 months (6). In contrast to these transgenic models, some rat strains rapidly develop prolactinoma when exposed to estrogen (7). The Fischer 344 (F344)-inbred rat is the most sensitive strain to form prolactinomas after receiving estrogen (7). Continuous estrogen treatment of F344 rats induces rapid pituitary growth within a few days, and the pituitary enlarges up to 10-fold after 8–12 wk of treatment. PRL overproduction by these estrogen-induced pituitary tumors results in circulating hyperprolactinemia increasing up to approximately 220-fold (7).
Rapid prolactinoma development in rats makes them useful for drug evaluation and for studying tumorigenesis. Drug studies have included those evaluating cysteamine (8), SMS 201–995 (9), fumagillin and its analog TNP-470, terguride, flutamide, and tamoxifen (10), estrogen receptor (ER) antagonist ICI-182780 (11), thalidomide, octreotide (12), and most recently epidermal growth factor receptor (EGFR) tyrosine kinase inhibitors. Bromocriptine and cabergoline potently suppress prolactinoma growth, validating the usefulness of this model in evaluating drug efficacy (12, 13). Aberrant pathways discovered using this model include expression of estrogen-induced pituitary tumor transforming gene (PTTG1) and fibroblast growth factor as early events in prolactinoma pathogenesis (14), bone morphogenetic protein-4 (BMP4) promotion of PRL secretion and lactotroph proliferation (15), reduced retinaldehyde dehydrogenase 1 synthesis (16), and melatonin induction of apoptosis of prolactinoma cells (17).
Although estrogen-induced rat prolactinomas have been widely studied, the underlying pathogenesis of human prolactinoma formation remains elusive. Importantly, the fidelity by which rat models recapitulate human prolactinoma is not clear. Accordingly, we used global profiling with microarray technology to investigate these questions in rat and human prolactinomas and found that both species share expression of several genes. We also identified novel genes and gene sets, which may be important for prolactinoma development.
Materials and Methods
Microarray data
Experiments to yield rat pituitary microarray data were previously reported (7), and microarray data was downloaded from Gene Expression Omnibus (access no. GSE4028). Briefly, male ACI or Brown Norway (BN) rats were previously treated with diethylstilbestrol (DES; a synthetic nonsteroidal estrogen, 5 mg for 12 wk), and pituitaries were analyzed using Affymetrix Rat Genome 230 version 2.0 arrays (Affymetrix, Santa Clara, CA). For each strain, four pituitaries of the DES-treated and three pituitaries of the vehicle-treated were analyzed.
The microarray data for human prolactinomas were derived from the Oyesiku laboratory (18). Briefly, human prolactinomas (n = 4, three males and one female) were obtained during transsphenoidal surgery as part of an ongoing accession of human pituitary tumors (18). The study was approved by the Institutional Review Board of Emory University, and informed consent was obtained for all subjects. Tumors were microdissected and removed using the surgical microscope, rinsed in sterile saline, snap frozen in liquid nitrogen, and stored (−80 C) until analysis. Each tumor fragment was confirmed independently by a neuropathologist by histology and immunohistochemistry before molecular analysis. Three cadaveric pituitary glands (two males and one female) were obtained from the National Resource Center (National Disease Research Interchange, www.ndriresource.org) and confirmed to be normal by histology. Each human tissue sample was analyzed using Affymetrix Human Genome U95Av2 arrays and data uploaded to Gene Expression Omnibus (access no. GSE36314).
Microarray data analysis
Microarray data were imported into Genespring GX11 (Agilent Technologies, Palo Alto, CA) according to the manual. All genes were normalized to their median, and data quality was assessed using a principle component analysis and sample clustering. Differentially expressed genes were identified by parametric testing (not assuming equal variance). Results were subjected to multiple testing correction using the Benjamini and Hochberg method. Genes with a false discovery rate (FDR) less than 0.01 were considered as differentially expressed. The gene symbols of differentially expressed genes were used to query the PubMed database together with key words such as prolactinoma, invasion, drug resistance, and recurrence using LocoySpider 2010 software (Locoy, Hefei, China) according to the manual. Microarray results were validated by real-time PCR.
Gene set enrichment analysis
Analyses were performed using gene set enrichment analysis (GSEA) desktop software from the Broad Institute (Massachusetts Institute of Technology, Cambridge, MA) (19). Briefly, the data matrix was exported from Genespring GX11 and further analyzed according to the manual (Agilent Technologies). Gene sets for curated pathways (c2.all.v2.5.symbols.gmt) and gene sets derived from motifs of transcription factors (c3.tft.v2.5.symbols.gmt) were used for analysis. Results were ranked according to the FDR. The FDR of 25% was used as a cutoff as suggested in the user guide of the GSEA software (Broad Institute).
Rat prolactinoma model
Animal protocols were approved by the Institutional Animal Care and Use Committee at Cedars-Sinai Medical Center (Los Angeles, CA) (20). After isoflurane inhalational anesthesia, 17β-estradiol-filled SILASTIC brand capsules (Dow Corning Corp., Midland, MI; medical grade tubing special; length 3 cm; outer diameter 0.125 in.; inner diameter 0.062 in.) were implanted sc into 4- to 5-wk ovariectomized female F344 rats (Harlan Sprague Dawley, Inc., Indianapolis, IN). Five hundred microliters of blood were collected by retroorbital bleeding every 2 wk for hormone assessment. Rats were euthanized 2 months after estrogen implantation and cardiac blood and pituitary glands collected and analyzed. Fragments of each pituitary were fixed in formalin, embedded in paraffin, and either preserved in RNA Later solution (Ambion, Grand Island, NY) or frozen in liquid nitrogen.
Western blot
Protein extracts were resolved by Nupage 4–12% Bis-Tris Gel (Invitrogen, Grand Island, NY), samples were electroblotted onto polyvinyl difluoride membrane (Invitrogen), and membranes blocked and incubated with primary antibody. Antibody targeting C-Myc (5605, 1:1000) was purchased from Cell Signaling (Danvers, MA). E2F1 (sc-193X, 1:500) and Sp1 (sc-59, 1:200) antibodies were from Santa Cruz Biotechnology (Santa Cruz, CA). Donkey antirabbit (1:2000) or antimouse (1:2000) (GE Healthcare, Waukesha, WI) antibodies were conjugated to horseradish peroxide to reveal immunocomplexes by enhanced chemiluminescence (Pierce, Rockford, IL). Detected bands were quantified using Image J version 1.43 (National Institutes of Health, Bethesda, MD) as instructed in the software manual.
Real-time quantitative PCR
Total RNA was isolated by an RNAeasy kit (QIAGEN, Hilden, Germany) according to the manual. Reverse transcription was carried out by a Superscript III first-strand cDNA synthesis kit (Invitrogen) and PCR amplifications with SYBR Green PCR master mix. Real-time quantitative PCR was performed according to the manufacturer's protocol (Bio-Rad Laboratories, Hercules, CA) in a Bio-Rad IQ5 multicolor real-time PCR detection system (Bio-Rad). A standard curve was used to quantify expression levels and the amount of each gene normalized to glyceraldehyde-3-phosphate dehydrogenase (GAPDH) mRNA. Primer sequences are available on request.
Results
Rat prolactinoma
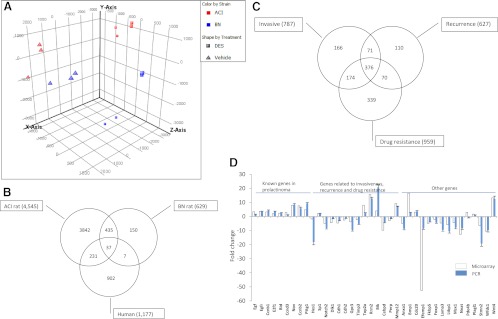
Estrogen-treated ACI and BN rats exhibited distinct gene expression patterns as indicated by principle component analysis (Fig. 1A). A total of 4545 genes (FDR < 0.01) were differentially expressed in pituitaries of DES-treated ACI rats compared with vehicle treatment (Fig. 1B and Supplemental Fig. 1, published on The Endocrine Society's Journals Online web site at http://endo.endojournals.org). In contrast, only 629 pituitary genes (FDR < 0.01) were differentially expressed in DES-treated BN rats (Fig. 1B and Supplemental Fig. 2). A total of 472 genes were found to be differentially expressed in both DES-treated ACI and BN rats (Fig. 1B). Because DES-treated BN rats did not develop prolactinomas, these overlapping genes may not be critical for estrogen-induced prolactinoma development. Of the 4545 genes, 2711 were up-regulated and 1834 down-regulated in DES-treated ACI rats (Supplemental Fig. 1). When searching the literature, 58 of 4545 genes are known to be aberrantly expressed in prolactinomas (Table 1), including Pttg1, Gal, Egf, Egfr, Ccnb1/b2, Igfbp3, Nrg1, and Myc.
Fig. 1.
Microarray analysis of prolactinomas. A, Microarray data derived from pituitaries of either vehicle- or DES-treated ACI or BN rats were imported into Genespring GX11. Principle component analysis was performed according to the software manual. B, Differentially expressed genes were identified using Genespring GX11 and a Venn diagram generated to unmask overlapping genes. C, Genes related to invasion, recurrence, and drug resistance were identified and applied to the Venn diagram. D, A total of 38 genes was selected from 4545 differentially expressed genes in DES-treated ACI rats (empty bar). Expression of these genes was validated using real-time PCR (solid bar) in prolactinomas from estrogen-treated Fischer rats. The y-axis represents fold change in arithmetic scale.
Table 1.
Differentially expressed pituitary genes in DES-treated ACI rats that are also aberrantly expressed in human prolactinomas
| Gene symbol | Gene title | ACI |
|
|---|---|---|---|
| Fold | P value | ||
| Gal | Galanin prepropeptide | 214.0 | 1.16E-04 |
| Vip | Vasoactive intestinal peptide | 28.7 | 2.36E-03 |
| Cfp | Complement factor properdin | 12.8 | 6.13E-03 |
| Lpl | Lipoprotein lipase | 12.8 | 1.63E-03 |
| Nupr1 | Nuclear protein 1 | 10.7 | 2.70E-03 |
| Nov | Nephroblastoma overexpressed gene | 8.0 | 1.17E-03 |
| Pgr | Progesterone receptor | 7.9 | 2.41E-04 |
| Sstr3 | Somatostatin receptor 3 | 7.5 | 1.97E-04 |
| Mki67 | Antigen identified by monoclonal antibody Ki-67 | 7.0 | 2.25E-03 |
| Cdc2 | Cell division cycle 2, G1 to S and G2 to M | 6.5 | 2.52E-03 |
| Tnf | TNF (TNF superfamily, member 2) | 6.4 | 4.74E-03 |
| Bag1 | BCL2-associated athanogene | 5.8 | 1.74E-04 |
| Lgals3 | Lectin, galactoside-binding, soluble, 3 | 5.4 | 3.73E-04 |
| Myc | Myelocytomatosis oncogene | 4.9 | 1.82E-03 |
| Pttg1 | Pituitary tumor-transforming 1 | 4.8 | 2.31E-03 |
| Ntrk2 | Neurotrophic tyrosine kinase, receptor, type 2 | 4.7 | 1.66E-04 |
| Mpg | N-methylpurine-DNA glycosylase | 4.3 | 3.31E-04 |
| Egfr | Epidermal growth factor receptor | 3.7 | 8.57E-03 |
| Egf | Epidermal growth factor | 3.4 | 4.20E-04 |
| Ccnb1 | Cyclin B1 | 3.3 | 6.32E-04 |
| Igfbp3 | IGF binding protein 3 | 3.2 | 5.05E-03 |
| Sla | Src-like adaptor | 3.0 | 1.96E-04 |
| Ccnd3 | Cyclin D3 | 2.9 | 2.58E-03 |
| Igf1 | IGF-I | 2.9 | 7.64E-03 |
| Cope | Coatomer protein complex, subunit-ϵ | 2.7 | 3.09E-03 |
| Aga | Aspartylglucosaminidase | 2.5 | 9.19E-04 |
| Tgfb3 | TGFβ3 | 2.4 | 7.01E-04 |
| Bid | BH3 interacting domain death agonist | 2.3 | 8.34E-04 |
| Cs | Citrate synthase | 2.2 | 4.66E-03 |
| Pts | 6-Pyruvoyl-tetrahydropterin synthase | 2.1 | 9.92E-03 |
| E2f1 | E2F transcription factor 1 | 2.0 | 8.57E-03 |
| Guk1 | Guanylate kinase 1 | 1.9 | 1.97E-03 |
| Poll | Polymerase (DNA directed), λ | 1.9 | 3.63E-03 |
| Grb2 | Growth factor receptor bound protein 2 | 1.6 | 2.97E-03 |
| Chgb | Chromogranin B | 1.5 | 8.98E-03 |
| Prkar1a | Protein kinase, cAMP dependent regulatory, type I, α | 1.5 | 1.94E-03 |
| Preb | Prolactin regulatory element binding | 1.4 | 8.11E-03 |
| Cdkn1b | Cyclin-dependent kinase inhibitor 1B | 1.4 | 1.18E-03 |
| D2R | Dopamine D2 receptor | 1.4 | 4.53E-03 |
| Ncl | Nucleolin | 1.4 | 6.79E-03 |
| Gpi | Glucose phosphate isomerase | 1.4 | 6.39E-03 |
| Ctnna1 | Catenin (cadherin associated protein), α1 | −1.5 | 3.54E-03 |
| Pou1f1 | POU class 1 homeobox 1 | −1.5 | 4.80E-03 |
| Slc20a1 | Solute carrier family 20 (phosphate transporter), member 1 | −1.7 | 1.40E-03 |
| Pitx1 | Paired-like homeodomain 1 | −1.8 | 9.03E-03 |
| Dst | Dystonin | −1.9 | 3.56E-03 |
| Jup | Junction plakoglobin | −2.1 | 6.92E-03 |
| Cp | Ceruloplasmin | −2.3 | 4.02E-03 |
| Ghr | GH receptor | −2.7 | 3.19E-03 |
| Gstm1 | Glutathione S-transferase μ 1 | −2.7 | 5.06E-03 |
| Chga | Chromogranin A | −2.9 | 1.36E-03 |
| Sms | Spermine synthase | −3.1 | 1.94E-03 |
| Prlr | Prolactin receptor | −3.7 | 8.18E-03 |
| Nrg1 | Neuregulin 1 | −5.0 | 7.01E-04 |
| Rab3b | RAB3B, member RAS oncogene family | −18.2 | 6.35E-04 |
| Ret | Ret protooncogene | −21.3 | 3.75E-03 |
| Spp1 | Secreted phosphoprotein 1 | −44.5 | 1.56E-03 |
Analysis of human prolactinomas identified 1177 differentially expressed genes (FDR < 0.01) (Fig. 1B and Supplemental Fig. 3), of which 32 were previously reported in prolactinomas. When combining the results derived from pituitaries of DES-treated ACI rats and human prolactinomas, 268 genes were identified (Fig. 1B), whereas 44 genes were identified when combining results derived from pituitaries of DES-treated BN rats and human prolactinomas (Fig. 1B), suggesting that pituitary glands derived from DES-treated ACI rats are more similar to human prolactinomas. Of the 268 genes identified, 235 are well annotated. A total of 145 of 235 genes were concordantly expressed between pituitaries of DES-treated ACI rats and human prolactinomas, and the remaining genes were inversely expressed. Concordantly expressed genes included Myc, Ccng1, Igf1, Faim3, Atf6, E2f1, Akt2, ATF1, Ccne1, Bcr, Hes1, Mdm1, Sox2, Cdh1 and Cdh2, Igfbp5, Gpc3, Dlk1, Cebpd, and Nnat. A representative list is presented in Table 2 (see Supplemental Fig. 4 for a full list). The inversely expressed genes included Gal, Igfbp3, Pik3ca, Gpc4, stat2, Nrg2, and Acin1. Other genes that are likely important for prolactinoma development but not differentially expressed in the ACI rat pituitary include Hmga1 and Hmga2.
Table 2.
Representative pituitary genes concordantly expressed in DES-induced ACI rat and human prolactinomas
| Gene symbol | Gene title | ACI rat |
Human |
||
|---|---|---|---|---|---|
| Fold | P value | Fold | P value | ||
| Cgref1 | Cell growth regulator with EF hand domain 1 | 211.3 | 1.9E-05 | 1.3 | 3.2E-02 |
| Plaur | Plasminogen activator, urokinase receptor | 49.0 | 1.2E-04 | 1.3 | 8.4E-03 |
| Ptprc | Protein tyrosine phosphatase, receptor type, C | 13.3 | 8.3E-03 | 1.2 | 1.4E-02 |
| Spc25 | SPC25, NDC80 kinetochore complex component, homolog (Saccharomyces cerevisiae) | 5.6 | 1.1E-03 | 1.1 | 6.1E-03 |
| Myc | Myelocytomatosis oncogene | 4.9 | 1.8E-03 | 1.4 | 1.8E-02 |
| Ccng1 | Cyclin G1 | 4.3 | 3.0E-04 | 1.4 | 2.5E-02 |
| Scamp5 | Secretory carrier membrane protein 5 | 3.5 | 7.9E-03 | 1.4 | 3.5E-02 |
| Ptprn | Protein tyrosine phosphatase, receptor type, N | 3.2 | 4.4E-04 | 1.8 | 1.4E-03 |
| Faim3 | Fas apoptotic inhibitory molecule 3 | 3.0 | 3.9E-03 | 1.3 | 8.1E-03 |
| Igf1 | IGF-I | 2.9 | 7.6E-03 | 1.2 | 1.2E-02 |
| Ern1 | Endoplasmic reticulum to nucleus signaling 1 | 2.8 | 1.8E-04 | 1.1 | 2.2E-02 |
| Eif4ebp1 | Eukaryotic translation initiation factor 4E binding protein 1 | 2.5 | 1.8E-03 | 1.2 | 3.7E-02 |
| Spink5 | Serine peptidase inhibitor, Kazal type 5 | 2.4 | 6.0E-03 | 1.1 | 1.7E-02 |
| Atf6 | Activating transcription factor 6 | 2.3 | 2.3E-03 | 1.2 | 3.3E-02 |
| Atp2a3 | ATPase, Ca2+ transporting, ubiquitous | 2.2 | 9.9E-03 | 1.1 | 2.2E-02 |
| Polr2e | Polymerase (RNA) II (DNA directed) polypeptide E, 25 kDa | 2.1 | 3.0E-04 | 1.3 | 3.5E-02 |
| Rala | V-ral simian leukemia viral oncogene homolog A (ras related) | 2.1 | 4.2E-04 | 1.4 | 8.5E-03 |
| E2f1 | E2F transcription factor 1 | 2.0 | 8.6E-03 | 1.2 | 1.3E-02 |
| Akt2 | V-akt murine thymoma viral oncogene homolog 2 | 2.0 | 7.9E-03 | 1.2 | 3.4E-02 |
| Atf1 | Activating transcription factor 1 | 2.0 | 4.8E-03 | 1.3 | 5.3E-03 |
| Mcm6 | Minichromosome maintenance complex component 6 | 1.9 | 1.9E-03 | 1.4 | 4.9E-02 |
| Npr2 | Natriuretic peptide receptor B/guanylate cyclase B (atrionatriuretic peptide receptor B) | 1.8 | 4.3E-03 | 1.2 | 2.3E-02 |
| Dnajb5 | DnaJ (Hsp40) homolog, subfamily B, member 5 | 1.7 | 6.0E-03 | 1.8 | 1.3E-02 |
| Fntb | Farnesyltransferase, CAAX box, β | 1.7 | 3.3E-03 | 1.2 | 3.8E-02 |
| Bcr | Breakpoint cluster region | −1.4 | 5.0E-03 | −1.3 | 2.4E-02 |
| Add1 | Dducing 1 (α) | −1.6 | 1.8E-03 | −1.1 | 2.4E-03 |
| Hes1 | Hairy and enhancer of split 1 (Drosophila) | −1.8 | 3.7E-03 | −1.6 | 4.3E-03 |
| Ddit4 | DNA damage-inducible transcript 4 | −1.9 | 3.0E-03 | −3.4 | 6.6E-03 |
| Map3k4 | MAPK kinase kinase 4 | −2.0 | 6.4E-03 | −1.4 | 9.6E-03 |
| Plch2 | Phospholipase C, η2 | −2.0 | 8.4E-03 | −1.5 | 4.1E-03 |
| Hspa2 | Heat shock protein 2 | −2.0 | 5.8E-03 | −2.8 | 4.4E-06 |
| Mdm1 | Mdm1 nuclear protein homolog (mouse) | −2.2 | 3.2E-03 | −1.8 | 1.1E-02 |
| Sox2 | SRY (sex determining region Y)-box 2 | −2.3 | 9.0E-03 | −1.3 | 1.1E-02 |
| Ghrhr | GHRH receptor | −2.3 | 7.0E-03 | −1.8 | 2.0E-02 |
| Prkci | Protein kinase C, iota | −2.4 | 6.7E-03 | −1.7 | 4.8E-02 |
| Cdh2 | Cadherin 2 | −2.5 | 3.2E-03 | −1.9 | 7.1E-03 |
| Rorb | RAR-related orphan receptor B | −3.0 | 8.5E-03 | −1.2 | 1.1E-02 |
| Igfbp5 | IGF binding protein 5 | −3.3 | 2.6E-03 | −10.8 | 1.4E-03 |
| Gpc3 | Glypican 3 | −3.8 | 2.6E-03 | −12.0 | 3.0E-05 |
| Lpin1 | Lipin 1 | −4.3 | 1.7E-04 | −1.9 | 2.0E-02 |
| Dlk1 | δ-Like 1 homolog (Drosophila) | −4.4 | 1.3E-03 | −18.9 | 1.1E-04 |
| Cdh1 | Cadherin 1 | −4.7 | 5.5E-03 | −2.6 | 7.3E-04 |
| Dhcr24 | 24-Dehydrocholesterol reductase | −5.1 | 5.8E-04 | −1.3 | 1.3E-02 |
| Wfdc2 | WAP four-disulfide core domain 2 | −5.3 | 5.1E-04 | −2.4 | 1.6E-06 |
| Itpr1 | Inositol 1,4,5-triphosphate receptor, type 1 | −6.4 | 2.6E-04 | −2.2 | 3.6E-03 |
| Stmn2 | Stathmin-like 2 | −6.6 | 1.7E-04 | −2.2 | 4.4E-06 |
| Fam107a | Family with sequence similarity 107, member A | −8.2 | 1.4E-03 | −1.7 | 2.4E-03 |
| Tmem30b | Transmembrane protein 30B | −8.5 | 1.9E-03 | −1.7 | 5.7E-03 |
| Cebpd | CCAAT/enhancer binding protein (C/EBP), δ | −9.8 | 1.4E-03 | −4.3 | 4.9E-05 |
| Nnat | Neuronatin | −12.8 | 6.8E-04 | −9.9 | 2.8E-04 |
| Efemp1 | EGF-containing fibulin-like extracellular matrix protein 1 | −52.5 | 8.9E-03 | −1.4 | 1.3E-02 |
Genes related to prolactinoma invasion, drug resistance, and recurrence
The major challenges of prolactinoma treatment include drug resistance and rapid recurrence after surgery. We searched the literature to determine candidate genes that may relate to tumor invasiveness, drug resistance, and rapid recurrence. Among 4545 genes, 787 appear related to invasiveness (Fig. 1C), 959 to drug resistance (Fig. 1C), and 627 to tumor recurrence. The presence of tumor stem cells may contribute to drug resistance and tumor recurrence, and of 4545 genes, 1225 were known stem cell-associated markers. A total of 376 genes were identified from combining genes related to drug resistance and recurrence, of which 360 were also present in the list of 1225 stem-cell related genes (Fig. 1C). Notable gene expressions associated with stem cell regulation and tumor invasion included Notch2, Cd44, Cd14, Cd55, Pten, Tgfb3, Mdm2, Bcr, Abr, stat1, sox2, and Sp1 (Table 3 and Supplemental Fig. 5).
Table 3.
Representative pituitary genes involved in stem cell regulations, regulation of invasion, tumor recurrence, and drug resistance
| Gene symbol | Gene title | Fold | P value |
|---|---|---|---|
| Gale | UDP-galactose-4-epimerase | 12.3 | 1.1E-04 |
| G6pd | Glucose-6-phosphate dehydrogenase | 2.9 | 1.6E-04 |
| Efs | Embryonal Fyn-associated substrate | −4.0 | 2.3E-04 |
| Ccr5 | Chemokine (C-C motif) receptor 5 | 22.7 | 4.1E-04 |
| Dcn | Decorin | −20.3 | 4.6E-04 |
| Cd68 | Cd68 molecule | 5.1 | 5.8E-04 |
| Il4ra | IL-4 receptor, α | 28.6 | 6.8E-04 |
| Cd14 | CD14 molecule | 4.3 | 8.2E-04 |
| Cd44 | Cd44 molecule | 2.9 | 8.5E-04 |
| Cad | Carbamoyl-phosphate synthetase 2, aspartate transcarbamylase, and dihydroorotase | 3.0 | 1.0E-03 |
| Abr | Active BCR-related gene | −2.1 | 1.1E-03 |
| Cd55 | Cd55 molecule | 18.5 | 1.3E-03 |
| Crop | Cisplatin resistance-associated overexpressed protein | −2.0 | 1.3E-03 |
| Dlk1 | δ-Like 1 homolog (Drosophila) | −4.4 | 1.3E-03 |
| Spn | Sialophorin | −2.6 | 1.4E-03 |
| Mdm2 | Mdm2 p53 binding protein homolog (mouse) | 1.5 | 1.8E-03 |
| Notch2 | Notch homolog 2 (Drosophila) | −4.9 | 1.9E-03 |
| Numb | Numb homolog (Drosophila) | −1.6 | 2.1E-03 |
| Cd9 | CD9 molecule | −2.0 | 2.2E-03 |
| Lox | Lysyl oxidase | 6.1 | 2.3E-03 |
| Evi1 | Ecotropic viral integration site 1 | −3.0 | 2.3E-03 |
| Ace | Angiotensin I converting enzyme (peptidyl-dipeptidase A) 1 | −5.4 | 2.5E-03 |
| App | Amyloid β (A4) precursor protein | 2.1 | 2.6E-03 |
| Anxa5 | Annexin A5 | 1.6 | 2.6E-03 |
| Tnfrsf11a | TNF receptor superfamily, member 11a | 10.4 | 2.7E-03 |
| Ptpn11 | Protein tyrosine phosphatase, nonreceptor type 11 | −1.7 | 2.8E-03 |
| Ky | Kyphoscoliosis peptidase | −4.2 | 3.2E-03 |
| Hmox1 | Heme oxygenase (decycling) 1 | 9.5 | 3.6E-03 |
| Hes1 | Hairy and enhancer of split 1 (Drosophila) | −1.8 | 3.7E-03 |
| Dcx | Doublecortin | −4.3 | 3.9E-03 |
| Cdk4 | Cyclin-dependent kinase 4 | 1.6 | 4.1E-03 |
| Ptpn6 | Protein tyrosine phosphatase, nonreceptor type 6 | 1.4 | 4.1E-03 |
| Sp1 | Sp1 transcription factor | 2.1 | 4.5E-03 |
| Rars | Arginyl-tRNA synthetase | 1.7 | 4.7E-03 |
| Itgb2 | Integrin-β2 | 1.9 | 4.8E-03 |
| Bcr | Breakpoint cluster region | −1.4 | 5.0E-03 |
| Dhfr | Dihydrofolate reductase | 1.8 | 6.0E-03 |
| Lef1 | Lymphoid enhancer binding factor 1 | −1.9 | 6.6E-03 |
| Ckap4 | Cytoskeleton-associated protein 4 | 3.9 | 6.9E-03 |
| Stat1 | Signal transducer and activator of transcription 1 | −1.7 | 7.5E-03 |
| Ptprc | Protein tyrosine phosphatase, receptor type, C | 13.3 | 8.3E-03 |
| Pten | Phosphatase and tensin homolog | 2.3 | 8.8E-03 |
| Sox2 | SRY (sex determining region Y)-box 2 | −2.3 | 9.0E-03 |
| Bdnf | Brain derived neurotrophic factor | 11.4 | 9.6E-03 |
| Rad51 | RAD51 homolog (RecA homolog, E. coli) (S. cerevisiae) | 2.1 | 9.6E-03 |
| Wars | Tryptophanyl-tRNA synthetase | 1.7 | 9.6E-03 |
| Cdc42 | Cell division cycle 42 (GTP binding protein) | 1.2 | 9.6E-03 |
| Ril | Reversion induced LIM gene | −2.2 | 9.7E-03 |
| Rac1 | Ras-related C3 botulinum toxin substrate 1 | 2.0 | 9.8E-03 |
To validate the microarray results, the expression of 38 genes was measured by real-time PCR. As depicted in Fig. 1D, this technique validated microarray expression data for 36 of 38 investigated genes observed in rat prolactinomas, suggesting approximately 95% validation using real-time PCR. Due to low availability of human prolactinoma tissues, the microarray results were not validated with quantitative RT-PCR. To avoid overinterpretation of the human tissue results, we performed a conservative data analysis by analyzing genes with a fold change larger or smaller than 2. This analysis indicated 24 genes (Igsf1, Gja1, Ddit4, Blcap, Cd9, Hspa2, Mdk, Dsp, Igfbp5, Aldh2, Cnn3, Gpc3, Tshb, Dlk1, Cdh1, Wfdc2, Itpr1, Stmn2, Ppl, Tbx19, Cebpd, Nnat, Fshb, and Lhb), which exhibit convergent expression between human prolactinomas and rat models.
Gene set enrichment analysis
Cellular processes are affected by sets of genes acting in concert (19). Using 3272 gene sets derived from curated pathways (pathway gene sets), 669 were found up-regulated in DES-treated ACI rats, of which 278 gene sets had a FDR less than 25% and 19 had a FDR less than 10% (Table 4 and Supplemental Fig. 6A). A total of 526 gene sets were up-regulated in vehicle-treated ACI rats, with 211 gene sets having a FDR less than 25% and 67 having a FDR less than 10% (Supplemental Fig. 6B). In the BN rat pituitary gland, 622 gene sets were up-regulated after vehicle treatment with 47 gene sets at FDR less than 25%, and two at FDR less than 10% (Table 4 and Supplemental Fig. 6C). A total of 573 gene sets were up-regulated in DES-treated rats, with 15 gene sets at FDR less than 25% but none at FDR of 10% (Supplemental Fig. 6D). In human prolactinomas 615 gene sets were up-regulated but none at FDR less than 25% (Table 4 and Supplemental Fig. 6E). A total of 753 gene sets were up-regulated in the normal rat pituitary, with 102 sets significantly enriched at FDR less than 25% with five at FDR less than10% (Table 4 and Supplemental Fig. 6F).
Table 4.
Enriched pathway gene sets for each group
| Source | Type | Pathway gene sets |
Transcription factor gene sets |
||||
|---|---|---|---|---|---|---|---|
| Total enriched | FDR <25% | FDR <10% | Total enriched | FDR <25% | FDR <10% | ||
| ACI rat | DES | 669 | 278 | 19 | 186 | 59 | 7 |
| Vehicle | 526 | 211 | 67 | 404 | 260 | 111 | |
| BN rat | DES | 622 | 47 | 2 | 310 | 57 | 0 |
| Vehicle | 573 | 15 | 0 | 280 | 41 | 0 | |
| Human | Prolactinoma | 615 | 0 | 0 | 279 | 0 | 0 |
| Normal pituitary | 753 | 102 | 5 | 309 | 14 | 1 | |
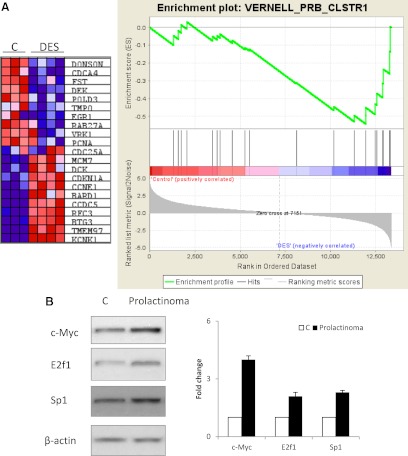
Of the top 20 pathway gene sets enriched in DES-treated ACI rats, 19 were also found to be enriched in pituitary gland of DES-treated BN rats (Supplemental Fig. 6G), suggesting that the two samples are similar. In contrast, only nine were also enriched in human prolactinomas. None of the 19 gene sets enriched in DES-treated BN rats passed the FDR 25% cutoff, suggesting the degree of alteration of these gene sets may determine development of rat prolactinoma. Altered gene sets were grossly categorized into four categories: 1) cell death/transformation/invasion (VERNELL_pRB_CLSTR1, BCL2_FAMILY_AND_REG_NETWORK, OLDAGE_DN, CMV_24HRS_UP, CROONQUIST_RAS_STROMA_UP, and EMT_UP); 2) cell differentiation (HSC_INTERMEDIATEPROGENITORS_FETAL, LIAN_MYELOID_DIFF_RECEP-TORS, HSC_INTERMEDIATE-PROGENITORS_SHARED, and HSC_INTERMEDIATE-PROGENITORS_ADULT); 3) epigenetic disruption (HDACI_COLON_BUT30MIN_DN, TSA_PANC50_UP, TSA_CD4_UP, and HDACI_COLON_SUL16HRS_DN); and 4) metabolism (NADLER_OBESITY_HYPERGLYCEMIA and HSA01030_GLYCAN_STRUCTURES_BIOSYNTHESIS_1). Enrichment of the VERNELL_pRB_CLSTR1 gene set was of special interest because Rb is known to play an important role in pituitary tumorigenesis (21). A representative figure for the VERNELL_pRB_CLSTR1 is shown in Fig. 2A.
Fig. 2.
Microarray data for either rat or human prolactinomas were exported from Genespring GX11 and imported to GSEA software according to the manual. GSEA was performed using pathway gene sets. A, VERNELL_pRB_CLSTR1 gene set enrichment. B, Western blot for c-Myc, E2F1, and Sp1 proteins in pituitary glands from vehicle- or estrogen-treated Fischer rats.
Because Rb regulates E2F activity, enrichment of the Rb pathway gene set is also supported by observed enrichment of the E2F1 gene set (REN_E2F1_TARGETS, rank 56; Supplemental Fig. 6A). Enrichment of pituitary gene sets was attributed to altered expression of corresponding genes. For example, enrichment of the epidermal growth factor (EGF) gene sets (EGFPATHWAY, rank 33; CARDIACEGFPATHWAY, rank 51; and EGF_HDMEC_UP, rank 264) supports the overexpression of EGF (3.4-fold, P = 4.20E-04) and EGFR (3.7-fold, P = 8.57E-03). Enrichment of the WNT gene set (LIN_WNT_UP, rank 106; Supplemental Fig. 6A) supports the up-regulation of WNT4 (13.3-fold, P = 6.07E-4). Enrichment of Myc gene sets (SCHUMACHER_MYC_UP, rank 61; ZELLER_MYC_UP, rank 119; MYC_TARGETS, rank 141; LEE_MYC_TGFA_UP, rank 227; MYC_ONCOGENIC_SIGNATURE, rank 255; YU_CMYC_UP, rank 260; and FERNANDEZ_MYC_TARGETS, rank 262; Supplemental Fig. 6A) supports the up-regulation of Myc (4.9-fold, P = 1.8E-3) in prolactinomas. Changes in the ATF/CREB pathway were supported by enrichment of the CREB gene set (CREBPATHWAY, rank 117; Supplemental Fig. 6A). Down-regulation of CEBPD (9.8-fold, P = 1.4E-3, Fig. 2B) was reflected in enrichment of the CEBP gene set (HALMOS_CEBP_DN, rank 123; Supplemental Fig. 6A). Up-regulation of TNF (6.4 fold, P = 4.7E-3) was supported by enrichment of the gene set (SANA_TNFA_ENDOTHELIAL_UP, rank 208; NEMETH_TNF_UP, rank 236). Moreover, enrichment of the stem cell gene sets (STEMCELL_COMMON_DN, rank 118; and STEMCELL_COMMON_UP, rank 154; Supplemental Fig. 6A), suggesting involvement of tumor stem cell in rat prolactinoma development. Enrichment of the EMT gene sets (EMT_UP, rank 20; and JECHLINGER_EMT_UP, rank 98; Supplemental Fig. 6A) support involvement of invasion pathways.
We also analyzed the data using 615 gene sets derived from those with a transcription factor (TF) motif (TF gene sets). One hundred eighty-six gene sets were up-regulated in the DES-treated ACI rats, with 59 gene sets at FDR less than 25% and seven at FDR less than 10% (Table 4 and Supplemental Fig. 7A). Four hundred four gene sets were up-regulated in the vehicle-treated ACI rats with 260 at FDR less than 25% and 111 at FDR less than 10% (Table 4 and Supplemental Fig. 7B). Analyzing the BN rats indicated that 310 gene sets were up-regulated in vehicle-treated rats with 57 gene sets at FDR less than 25% (Table 4 and Supplemental Fig. 7C). Two hundred eighty gene sets were up-regulated in the DES-treated rat with 41 gene sets at FDR less than 25% (Table 4 and Supplemental Fig. 7D). None of the gene sets was enriched in the vehicle- or DES-treated BN rats at FDR less than 10% (Table 4). In the human data set, 279 gene sets were up-regulated in prolactinomas but none at FDR less than 25% (Table 4 and Supplemental Fig. 7E). Three hundred nine gene sets were up-regulated in the normal rat pituitary, with 14 gene sets at FDR less than 25% and one gene set at FDR less than 10% (Table 4 and Supplemental Fig. 7F).
Enrichment of the TF gene sets suggested that activities of the corresponding transcription factor may be altered. The top 20 enriched gene sets (Supplemental Fig. 7G) correspond to 13 known transcription factors. Three of 13 (ETS1, ATF1, and CREB) known to regulate prolactin expression (20, 22, 23) suggest that multiple pathways are involved in regulating prolactin expression in estrogen-induced rat prolactinoma. Analysis of transcription factor gene sets were supported by gene expression microarray results, which indicated that gene expression levels of eight of 13 transcription factors (c-Myc, Sp1, ATF1, AP4s1, CREB3, USF1, ETS1, and CEBPD) were significantly altered (Supplemental Fig. 7H). Altered expression of the eight transcription factors were further validated by real-time PCR (Supplemental Fig. 7H). Our results indicated an increased E2F1 mRNA and enrichment of the E2F1 gene set, and expression of Sp1, c-Myc, and E2F1 was validated at the protein level by Western blotting (Fig. 2B). These results were also supported by previous analysis of pathway gene sets. For example, enrichment of the Myc TF gene set (V$MYC_Q2) was supported by several gene sets for the Myc pathways (rank 61, FDR = 0.14; rank 119, FDR = 0.17; rank 141, FDR = 0.18; Supplemental Fig. 7A). The E2F TF gene set (V$E2F_Q6_01) was ranked 56 in the enriched TF gene sets (FDR = 0.23) and is supported by pathway gene sets VERNELL_pRB_CLSTR1 (rank 3) and REN_E2F1_TARGETS (rank 56; Supplemental Fig. 7A). The enrichment of the CEBPD (V$CEBPDELTA_Q6) TF gene set was supported by the pathway gene set (HALMOS_CEBP_DN, rank 123; Supplemental Fig. 7A). Enrichment of the ATF1 (V$ATF_01) and CREB (V$CREB_01) TF gene sets were supported by the CREB pathway gene set (CREBPATHWAY, rank 117; Supplemental Fig. 7A).
Discussion
This study integrally analyzed microarray results derived from rat and human prolactinomas at the single gene as well as gene set levels and provides insights in the genomic profile of rat and human prolactinomas.
When using single gene-based analysis, results obtained from pituitaries of estrogen-treated rats exhibited commonly regulated genes also observed in human prolactinomas, suggesting that the microarray captured some common prolactinoma features, which may play roles in regulating prolactinomas. Microarray data derived from DES-treated ACI rats confirmed that several genes such as Pttg1, Egf, Egfr, E2f1, Myc, Ccnb1, and Ccnb2 are aberrantly expressed in prolactinoma. PTTG1 overexpression is found in most pituitary adenomas and particularly in invasive hormone-secreting tumors (24). EGF increases prolactinoma cell proliferation and prolactin expression (25). Inhibition of EGFR or its family members using small molecular inhibitors blocks cell proliferation as well as prolactin expression. Earlier studies found that Ccnb2 was overexpressed in pituitary tumors (26, 27), and this gene was induced by HMGA1 and HMGA2 (27). The E2F1 pathway is frequently enhanced in pituitary tumors (28) and Rb knockout induces these tumors in transgenic mice (28). Up-regulation of these genes was further supported by subsequent GSEA analysis, suggesting that these genes affect tumor growth in a gene set-specific manner.
By searching the literature, we also identified 376 genes that might play important roles in regulating drug resistance and prolactinoma recurrence. Most genes (360 of 376) also regulate stem cells, suggesting that prolactinoma tumor stem cells may play a role in prolactinoma treatment resistance. These stem cell related genes include Notch2, Cd44, Cd14, Pten, Tgfb3, Mdm2, and Abr. Involvement of stem cells in prolactinoma development was also supported by the pathway gene sets analysis, which exhibited enrichment of both stem cell gene sets and cell differentiation gene sets.
HMGA1 and HMGA2 regulate pituitary tumor development (4, 5, 27). HMGA1 and HMGA2 transgenic mice develop PRL- and GH-secreting pituitary tumors associated with a high expression of Ccnb2 (4, 5, 27). We detected up-regulation of Ccnb1 and Ccnb2 but no change in HMGA1 and HMGA2, suggesting that other pathways may be involved in regulating Ccnb1/2. It is also interesting that some genes are discordantly expressed in rat and human prolactinomas. For example, galanin (Gal) knockout mice exhibit reduced prolactin, and transgenic mice overexpressing Gal develop pituitary adenomas and increased secretion of PRL and GH (29). Gal was up-regulated 214-fold in DES-treated ACI rats but was down-regulated 55-fold in human prolactinomas. Similarly, expression of Igfbp3, Pik3ca, Gpc4, stat2, Nrg2, and Acin1 was discordant in rat and human prolactinomas. These results might reflect either differences in tumor types or aberrant species-specific mechanisms involved in prolactinoma development.
When using gene set-based analysis, we identified several gene sets significantly enriched (FDR < 10%) in DES-treated ACI rats but not enriched (FDR < 10%) in human prolactinomas. No overlap of gene sets between prolactinomas derived from ACI rats and human prolactinomas suggested that underlying pathways for prolactinoma development may differ between the two species. However, this could also reflect differences in experimental systems. Rat prolactinomas develop in homogenously inbred rat strains, allowing rigorous results with high statistical power using only a few samples. In contrast, human prolactinomas are highly heterogeneous, leading to low statistical power and subsequently constraining identification of pathways that might be important for disease pathogenesis. Therefore, analyses of human samples require greater sample size to gain genomic insights into the disease.
Despite the lack of gene set overlap between rat and human prolactinomas, gene sets enriched in estrogen-treated rats confirmed known pathways in pituitary tumor development. For example, the Rb/E2F pathway is known to play an important role in pituitary tumorigenesis (30). Rb heterozygous mice develop spontaneous pituitary tumors (21, 30). We identified Rb (VERNELL_pRB_CLSTR1) and E2F (REN_E2F1_TARGETS) pathway gene sets. Moreover, involvement of the Rb/E2F pathway was further supported by enrichment of the TF gene set derived from the E2F binding motif (V$E2F_Q6_01). Finally, increased E2F1 expression was validated by Western blot. Similarly, several Myc pathway gene sets were enriched in DES-treated ACI rats. Activation of Myc was also supported by the GSEA analysis using TF gene sets. Increased Myc expression was validated by real-time PCR and Western blot. The role of the EGF pathways was similarly supported by pathway gene set analysis. Altered in ATF1, CREB3, Sp1, and Ets1 were supported by pathway and TF gene set analysis and validated by real-time PCR. The CEBPD pathway involvement was supported by TF gene set analysis, and CEBPD down-regulation was validated by real-time PCR.
This study focused on gene sets enriched in prolactinomas. Because the pituitary gland comprises more than six cell types, gene sets enriched in control groups could be due to artificial effects introduced by unrelated, nontarget cells (31). Care should be taken to interpret gene set results in the normal pituitary, especially because microarrays may not be sufficiently sensitive and poor microarray probes may introduce errors. For example, in all microarray studies for prolactinoma, prolactin gene expression levels were not significantly changed, which was not consistent with real-time PCR results. Using next-generation sequencing may help address this challenge. Estrogen-induced rat prolactinomas may exhibit limited invasiveness and are responsive to treatment with dopamine agonists (12, 13), indicating that these identified changes in gene expression are not wholly sufficient to cause prolactinoma invasion or drug resistance. This analysis is based on previous knowledge of these genes, and genes with little previous study could therefore be missed. Importantly, because human pituitary microarray data were not validated with quantitative RT-PCR, all fold change of less than 2 should be considered with caution. When discarding genes with fold change less than 2, only 24 exhibited convergent expression between human prolactinomas and rat models. We validated the microarray data derived from DES-treated ACI rats with estrogen-induced prolactinomas in F344 rats, indicating that these results can be generalized to other rat strains. Indeed, rat strains exhibit different sensitivities to estrogen, and Quantitative trait locus (QTL) has been used to map loci responsible for observed differential estrogen sensitivity in ACI and BN strains (32). By using pituitary mass as a quantitative trait, two loci (Ept 5 and 7) on rat chromosome 4 were found to exert significant effects on 17β-estradiol (E2)-induced pituitary growth. D4Rat103, Ghrhr, Tgfa, Trh, Ghr1, Ret, Ccnd2, Cdkn1b, and D4Rat204 reside in the Ept5 locus and D7Rat44, Cdk4, Trhr, Myc, and D7Rat15 in the Ept 7 locus. Among these genes, five (Ghrhr, Ret, Cdkn1b, Cdk4, and Myc) exhibited altered gene expression in pituitaries of ACI rat models and two (Ghrhr and Myc) exhibited convergent expression between human prolactinomas and rat models (Table 2).
This study generated thousands of differentially expressed genes associated with prolactinoma development. Here we prioritized these candidate genes for further investigation. At the single-gene level, we identified 149 genes concordantly expressed in both rat and human prolactinomas. GSEA identified hundreds of enriched gene sets and dozens were directly supported by gene expression changes at the single gene level. These candidate genes or gene sets may be worthy of further investigation.
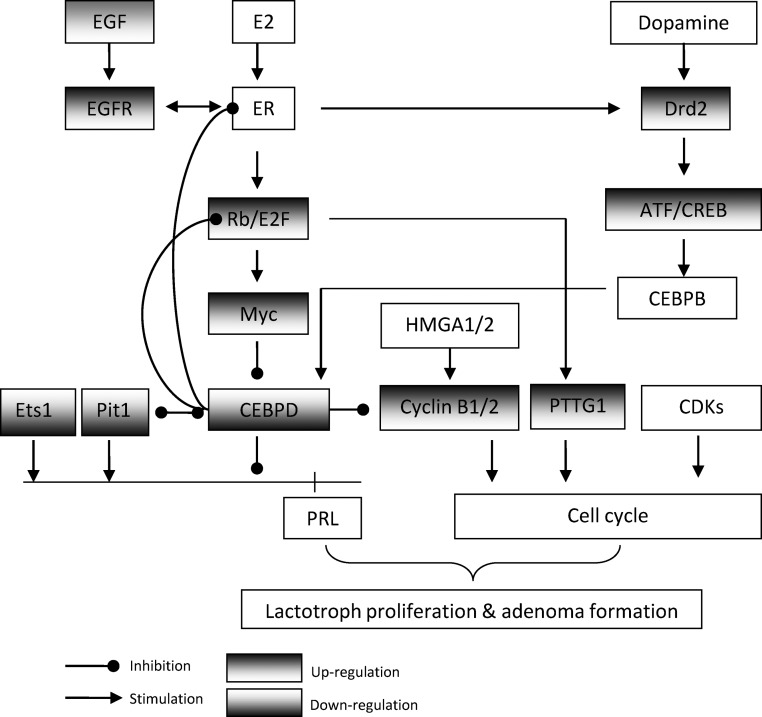
Several identified genes and gene sets have been shown to regulate pituitary tumorigenesis and/or prolactin expression. These include EGF/EGFR cross talk with the E2/ER pathway to up-regulate prolactin expression and lactotroph cell proliferation (33, 34), Rb/E2F1 regulation of pituitary tumorigenesis (35), CEBPD coordination of prolactin expression and lactotroph proliferation (20), Pit1 and Ets1 regulation of prolactin transcription (22), dopamine and D2R suppression of prolactin (36), and CREB induction of prolactin (37). We further searched the literature for evidence of interaction between identified genes and gene sets. Indeed, these genes and gene sets appear to regulate each other and form a complex signaling network (Fig. 3). E2/ER activates Rb/E2F1 (38), which may subsequently activate Myc (39). Myc also suppresses CEBPD expression (40), which may subsequently up-regulate prolactin and lactotroph proliferation (20). CEBPD in turn suppresses E2/ER and Rb/E2F1 activity by direct interaction (41). Interestingly, D2R expression is up-regulated in estrogen-induced rat prolactinomas, which may in turn constrain lactotroph tumorigenesis. How dopamine/D2R interacts with E2/ER-Rb/E2F1-Myc-CEBPD is unclear. CREB may activate CEBPB (42), which may subsequently activate CEBPD.
Fig. 3.
Proposed scheme for interactions between identified genes and gene sets in estrogen-induced rat prolactinomas.
In summary, we analyzed rat and human prolactinomas at the levels of single genes and gene sets. We confirmed known genes and pathways in the pituitaries of DES-treated ACI rats and also unraveled new candidate genes and gene sets that may play important roles in regulating prolactinomas, forming a complicated signaling network. This knowledge may result in enhanced use of rat models to investigate genomic profiles of human prolactinomas.
Supplementary Material
Acknowledgments
Y.T. designed and carried out the experiments and wrote the manuscript; J.Z. assisted in the bioinformatic analysis; Y.Z. did the real-time PCR; N.M.O. provided the microarray data derived from human prolactinoma; H.P.K. was involved in the data interpretation and discussion of results; and S.M. was pivotal in the experimental design, data interpretation, discussion of the results, and writing the manuscript.
This work was supported by National Institutes of Health Grant K99CA138914 (to Y.T.), Grant CA 75979 (to S.M.), and The Doris Factor Molecular Endocrinology Laboratory (to S.M.); Grant CA026038-32 (to H.P.K.), and an A*STAR Investigator Grant (to H.P.K.).
Disclosure Summary: The authors declare no commercial conflicts.
Footnotes
- BMP4
- Bone morphogenetic protein-4
- BN
- brown Norway
- DES
- diethylstilbestrol
- D2R
- dopamine D2 receptor
- E2
- 17β-estradiol
- EGF
- epidermal growth factor
- EGFR
- EGF receptor
- ER
- estrogen receptor
- F344
- Fischer 344
- FDR
- false discovery rate
- Gal
- galanin
- GAPDH
- glyceraldehyde-3-phosphate dehydrogenase
- GEO
- Gene Expression Omnibus
- GSEA
- gene set enrichment analysis
- HMGA
- high-mobility group protein A
- PRL
- prolactin
- PTTG1
- pituitary tumor transforming gene
- QTL
- Quantitative trait locus
- TF
- transcription factor.
References
- 1. Melmed S, Casanueva FF, Hoffman AR, Kleinberg DL, Montori VM, Schlechte JA, Wass JA. 2011. Diagnosis and treatment of hyperprolactinemia: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab 96:273–288 [DOI] [PubMed] [Google Scholar]
- 2. Gillam MP, Molitch ME, Lombardi G, Colao A. 2006. Advances in the treatment of prolactinomas. Endocr Rev 27:485–534 [DOI] [PubMed] [Google Scholar]
- 3. Oh MC, Aghi MK. 2011. Dopamine agonist-resistant prolactinomas. J Neurosurg 114:1369–1379 [DOI] [PubMed] [Google Scholar]
- 4. Fedele M, Pentimalli F, Baldassarre G, Battista S, Klein-Szanto AJ, Kenyon L, Visone R, De Martino I, Ciarmiello A, Arra C, Viglietto G, Croce CM, Fusco A. 2005. Transgenic mice overexpressing the wild-type form of the HMGA1 gene develop mixed growth hormone/prolactin cell pituitary adenomas and natural killer cell lymphomas. Oncogene 24:3427–3435 [DOI] [PubMed] [Google Scholar]
- 5. Fedele M, Battista S, Kenyon L, Baldassarre G, Fidanza V, Klein-Szanto AJ, Parlow AF, Visone R, Pierantoni GM, Outwater E, Santoro M, Croce CM, Fusco A. 2002. Overexpression of the HMGA2 gene in transgenic mice leads to the onset of pituitary adenomas. Oncogene 21:3190–3198 [DOI] [PubMed] [Google Scholar]
- 6. Kelly MA, Rubinstein M, Asa SL, Zhang G, Saez C, Bunzow JR, Allen RG, Hnasko R, Ben-Jonathan N, Grandy DK, Low MJ. 1997. Pituitary lactotroph hyperplasia and chronic hyperprolactinemia in dopamine D2 receptor-deficient mice. Neuron 19:103–113 [DOI] [PubMed] [Google Scholar]
- 7. Spady TJ, Harvell DM, Lemus-Wilson A, Strecker TE, Pennington KL, Vander Woude EA, Birt DF, McComb RD, Shull JD. 1999. Modulation of estrogen action in the rat pituitary and mammary glands by dietary energy consumption. J Nutr 129:587S–590S [DOI] [PubMed] [Google Scholar]
- 8. Jeitner TM, Oliver JR. 1990. Possible oncostatic action of cysteamine on the pituitary glands of oestrogen-primed hyperprolactinaemic rats. J Endocrinol 127:119–127 [DOI] [PubMed] [Google Scholar]
- 9. Hyde JF, Howard G. 1992. Regulation of galanin gene expression in the rat anterior pituitary gland by the somatostatin analog SMS 201–995. Endocrinology 131:2097–2102 [DOI] [PubMed] [Google Scholar]
- 10. Leung G, Tsao SW, Wong YC. 2002. The effect of flutamide and tamoxifen on sex hormone-induced mammary carcinogenesis and pituitary adenoma. Breast Cancer Res Treat 72:153–162 [DOI] [PubMed] [Google Scholar]
- 11. Heaney AP, Fernando M, Melmed S. 2002. Functional role of estrogen in pituitary tumor pathogenesis. J Clin Invest 109:277–283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gruszka A, Kunert-Radek J, Pawlikowski M. 2004. The effect of octreotide and bromocriptine on expression of a pro-apoptotic Bax protein in rat prolactinoma. Folia Histochem Cytobiol 42:35–39 [PubMed] [Google Scholar]
- 13. Palmeri CM, Petiti JP, Sosa Ldel V, Gutiérrez S, De Paul AL, Mukdsi JH, Torres AI. 2009. Bromocriptine induces parapoptosis as the main type of cell death responsible for experimental pituitary tumor shrinkage. Toxicol Appl Pharmacol 240:55–65 [DOI] [PubMed] [Google Scholar]
- 14. Heaney AP, Horwitz GA, Wang Z, Singson R, Melmed S. 1999. Early involvement of estrogen-induced pituitary tumor transforming gene and fibroblast growth factor expression in prolactinoma pathogenesis. Nat Med 5:1317–1321 [DOI] [PubMed] [Google Scholar]
- 15. Labeur M, Páez-Pereda M, Haedo M, Arzt E, Stalla GK. 2010. Pituitary tumors: cell type-specific roles for BMP-4. Mol Cell Endocrinol 326:85–88 [DOI] [PubMed] [Google Scholar]
- 16. Fujiwara K, Davaadash B, Yatabe M, Kikuchi M, Horiguchi K, Kusumoto K, Kouki T, Yashiro T. 2008. Reduction of retinaldehyde dehydrogenase 1 expression and production in estrogen-induced prolactinoma of rat. Med Mol Morphol 41:126–131 [DOI] [PubMed] [Google Scholar]
- 17. Yang QH, Xu JN, Xu RK, Pang SF. 2006. Inhibitory effects of melatonin on the growth of pituitary prolactin-secreting tumor in rats. J Pineal Res 40:230–235 [DOI] [PubMed] [Google Scholar]
- 18. Evans CO, Moreno CS, Zhan X, McCabe MT, Vertino PM, Desiderio DM, Oyesiku NM. 2008. Molecular pathogenesis of human prolactinomas identified by gene expression profiling, RT-qPCR, and proteomic analyses. Pituitary 11:231–245 [DOI] [PubMed] [Google Scholar]
- 19. Subramanian A, Tamayo P, Mootha VK, Mukherjee S, Ebert BL, Gillette MA, Paulovich A, Pomeroy SL, Golub TR, Lander ES, Mesirov JP. 2005. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc Natl Acad Sci USA 102:15545–15550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Tong Y, Zhou J, Mizutani J, Fukuoka H, Ren SG, Gutierrez-Hartmann A, Koeffler HP, Melmed S. 2011. CEBPD suppresses prolactin expression and prolactinoma cell proliferation. Mol Endocrinol 25:1880–1891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hu N, Gutsmann A, Herbert DC, Bradley A, Lee WH, Lee EY. 1994. Heterozygous Rb-1 Δ20/+mice are predisposed to tumors of the pituitary gland with a nearly complete penetrance. Oncogene 9:1021–1027 [PubMed] [Google Scholar]
- 22. Schweppe RE, Gutierrez-Hartmann A. 2001. Pituitary Ets-1 and GABP bind to the growth factor regulatory sites of the rat prolactin promoter. Nucleic Acids Res 29:1251–1260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Liang J, Kim KE, Schoderbek WE, Maurer RA. 1992. Characterization of a non-tissue-specific, 3′,5′-cyclic adenosine monophosphate-responsive element in the proximal region of the rat prolactin gene. Mol Endocrinol 6:885–892 [DOI] [PubMed] [Google Scholar]
- 24. Vlotides G, Eigler T, Melmed S. 2007. Pituitary tumor-transforming gene: physiology and implications for tumorigenesis. Endocr Rev 28:165–186 [DOI] [PubMed] [Google Scholar]
- 25. Vlotides G, Siegel E, Donangelo I, Gutman S, Ren SG, Melmed S. 2008. Rat prolactinoma cell growth regulation by epidermal growth factor receptor ligands. Cancer Res 68:6377–6386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Raverot G, Wierinckx A, Dantony E, Auger C, Chapas G, Villeneuve L, Brue T, Figarella-Branger D, Roy P, Jouanneau E, Jan M, Lachuer J, Trouillas J. 2010. Prognostic factors in prolactin pituitary tumors: clinical, histological, and molecular data from a series of 94 patients with a long postoperative follow-up. J Clin Endocrinol Metab 95:1708–1716 [DOI] [PubMed] [Google Scholar]
- 27. De Martino I, Visone R, Wierinckx A, Palmieri D, Ferraro A, Cappabianca P, Chiappetta G, Forzati F, Lombardi G, Colao A, Trouillas J, Fedele M, Fusco A. 2009. HMGA proteins up-regulate CCNB2 gene in mouse and human pituitary adenomas. Cancer Res 69:1844–1850 [DOI] [PubMed] [Google Scholar]
- 28. Chesnokova V, Zonis S, Kovacs K, Ben-Shlomo A, Wawrowsky K, Bannykh S, Melmed S. 2008. p21(Cip1) restrains pituitary tumor growth. Proc Natl Acad Sci USA 105:17498–17503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Perumal P, Vrontakis ME. 2003. Transgenic mice over-expressing galanin exhibit pituitary adenomas and increased secretion of galanin, prolactin and growth hormone. J Endocrinol 179:145–154 [DOI] [PubMed] [Google Scholar]
- 30. Jacks T, Fazeli A, Schmitt EM, Bronson RT, Goodell MA, Weinberg RA. 1992. Effects of an Rb mutation in the mouse. Nature 359:295–300 [DOI] [PubMed] [Google Scholar]
- 31. Melmed S. 2003. Mechanisms for pituitary tumorigenesis: the plastic pituitary. J Clin Invest 112:1603–1618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Shull JD, Lachel CM, Murrin CR, Pennington KL, Schaffer BS, Strecker TE, Gould KA. 2007. Genetic control of estrogen action in the rat: mapping of QTLs that impact pituitary lactotroph hyperplasia in a BN x ACI intercross. Mamm Genome 18:657–669 [DOI] [PubMed] [Google Scholar]
- 33. Fukuoka H, Cooper O, Mizutani J, Tong Y, Ren SG, Bannykh S, Melmed S. 2011. HER2/ErbB2 receptor signaling in rat and human prolactinoma cells: strategy for targeted prolactinoma therapy. Mol Endocrinol 25:92–103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Ben-Jonathan N, Chen S, Dunckley JA, LaPensee C, Kansra S. 2009. Estrogen receptor-α mediates the epidermal growth factor-stimulated prolactin expression and release in lactotrophs. Endocrinology 150:795–802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Parisi T, Bronson RT, Lees JA. 2009. Inhibition of pituitary tumors in Rb mutant chimeras through E2f4 loss reveals a key suppressive role for the pRB/E2F pathway in urothelium and ganglionic carcinogenesis. Oncogene 28:500–508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Filopanti M, Lania AG, Spada A. 2010. Pharmacogenetics of D2 dopamine receptor gene in prolactin-secreting pituitary adenomas. Expert Opin Drug Metab Toxicol 6:43–53 [DOI] [PubMed] [Google Scholar]
- 37. Ishida M, Mitsui T, Yamakawa K, Sugiyama N, Takahashi W, Shimura H, Endo T, Kobayashi T, Arita J. 2007. Involvement of cAMP response element-binding protein in the regulation of cell proliferation and the prolactin promoter of lactotrophs in primary culture. Am J Physiol Endocrinol Metab 293:E1529–E1537 [DOI] [PubMed] [Google Scholar]
- 38. Stender JD, Frasor J, Komm B, Chang KC, Kraus WL, Katzenellenbogen BS. 2007. Estrogen-regulated gene networks in human breast cancer cells: involvement of E2F1 in the regulation of cell proliferation. Mol Endocrinol 21:2112–2123 [DOI] [PubMed] [Google Scholar]
- 39. Oswald F, Lovec H, Möröy T, Lipp M. 1994. E2F-dependent regulation of human MYC: trans-activation by cyclins D1 and A overrides tumour suppressor protein functions. Oncogene 9:2029–2036 [PubMed] [Google Scholar]
- 40. Mink S, Mutschler B, Weiskirchen R, Bister K, Klempnauer KH. 1996. A novel function for Myc: inhibition of C/EBP-dependent gene activation. Proc Natl Acad Sci USA 93:6635–6640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Chang W, Parra M, Centrella M, McCarthy TL. 2005. Interactions between CCAAT enhancer binding protein Δ and estrogen receptor α control insulin-like growth factor I (igf1) and estrogen receptor-dependent gene expression in osteoblasts. Gene 345:225–235 [DOI] [PubMed] [Google Scholar]
- 42. Ruffell D, Mourkioti F, Gambardella A, Kirstetter P, Lopez RG, Rosenthal N, Nerlov C. 2009. A CREB-C/EBPβ cascade induces M2 macrophage-specific gene expression and promotes muscle injury repair. Proc Natl Acad Sci USA 106:17475–17480 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.