Abstract
In LDLR−/− mice fed high-fat diabetogenic diets, osteogenic gene-regulatory programs are ectopically activated in vascular myofibroblasts and smooth muscle cells that promote arteriosclerotic calcium deposition. Msx2-Wnt signaling pathways previously identified as important for craniofacial skeletal development are induced in the vasculature by TNF, a prototypic cytokine mediator of the low-grade systemic inflammation of diabesity. To better understand this biology, we studied TNF actions on Msx2 in aortic myofibroblasts. TNF up-regulated Msx2 mRNA 4-fold within 3 h but did not regulate Msx1. Although IL-1β could also induce Msx2 expression, TNF-related apoptosis inducing ligand, receptor activator of nuclear factor-κB ligand, and IL-6 were inactive. Inhibition of nicotinamide adenine dinucleotide phosphate oxidase (Nox) activity and genetically induced Nox deficiency (p47phox−/−) reduced Msx2 induction, indicating contributions of reactive oxygen species (ROS) and redox signaling. Consistent with this, rotenone, an antagonist of mitochondrial complex I, inhibited TNF induction of Msx2 and Nox2, whereas pyruvate, an anapleurotic mitochondrial metabolic substrate, enhanced induction. Moreover, the glutathione peroxidase-mimetic ebselen abrogated this TNF response. Treatment of aortic myofibroblasts with hydrogen peroxide up-regulated Msx2 mRNA, promoter activity, and DNA-protein interactions. In vivo, SM22-TNF transgenic mice exhibit increased aortic Msx2 with no change in Msx1. Dosing SM22-TNF mice with either 20 ng/g Nox1 + 20 ng/g Nox2 antisense oligonucleotides or low-dose rotenone reduced arterial Msx2 expression. Aortic myofibroblasts from TNFR1−/− mice expressed levels of Msx2 that were 5% that of wild-type and were not inducible by TNF. Wnt7b and active β-catenin levels were also reduced. By contrast, TNF-inducible Msx2 expression was not reduced in TNFR2−/− cells. Finally, when cultured under mineralizing conditions, TNFR1−/− aortic myofibroblasts exhibited reduced calcification compared with wild-type and TNFR2−/− cells. Thus, ROS metabolism contributes to TNF induction of Msx2 and procalcific responses in myofibroblasts via TNFR1. Strategies that reduce vascular Nox- or mitochondrially activated ROS signals may prove useful in mitigating arteriosclerotic calcification.
Arteriosclerosis is the vascular stiffening that impairs conduit vessel function in diabetes, dyslipidemia, and uremia (1). Reductions in arterial Windkessel function with arteriosclerosis increase systolic blood pressure and myocardial workload and impair smooth distal tissue perfusion during the cardiac cycle (2). In type 2 diabetes (T2DM), arteriosclerotic stiffening is typically concentric, arising in part from the medial artery calcification and mural fibrosis that convey risk for lower extremity amputation, myocardial infarction, and stroke (3). Implementing a murine model of diet-induced T2DM, dyslipidemia, and vascular calcification, we identified that aortic activation of proosteogenic Msx2-Wnt signaling cascades contribute to arteriosclerotic disease (4, 5). The capacity of macrophage TNF, the prototypic inflammatory cytokine of so-called diabesity and innate immunity (6), to activate vascular mineralization directed by neighboring calcifying vascular cells was first identified in seminal studies by Tintut et al. (7). Our in vivo and in vitro studies subsequently demonstrated that TNF up-regulates aortic Msx2-Wnt signaling (8), a proosteogenic gene-regulatory program initially discovered as being important in craniofacial skeletal development (9). Similar processes have recently been shown to be active in calcifying human aortic valves (10, 11) and in the arterial calcification of patients suffering with chronic kidney disease (12). Very recent studies by Rajamannan (13) demonstrate that the Wnt receptor low density lipoprotein receptor-related protein-5 plays a critical role in mediating valve calcification in a murine disease model. Thus, rather than being a passive process, arteriosclerotic calcification has emerged as an actively regulated form of tissue biomineralization, entrained to vascular inflammatory cues (14).
We wanted to better understand the signaling mechanisms whereby TNF regulates Msx2 expression and elaboration of the vascular fibroosteogenic phenotype elaborated in arteriosclerosis. Therefore, we studied TNF actions in pharmacologically and genetically manipulated aortic myofibroblasts. We identify that reactive oxygen species (ROS) arising from nicotinamide adenine dinucleotide phosphate (NADPH) oxidase (Nox) activity and myofibroblast mitochondrial metabolism support TNF induction of procalcific Msx2 programs via TNFR1.
Materials and Methods
Reagents and Western blots
Routine biochemical, histochemical stains, custom oligodeoxynucleotides, and molecular biology reagents were obtained from Fisher (Fair Lawn, NJ), Sigma (St. Louis, MO), or Invitrogen (Carlsbad, CA). Recombinant rodent TNF, receptor activator of nuclear factor-κB ligand (RANKL), osteoprotegerin, IL-6, IL1β, noggin, endothelin, angiotensin II (AngII), Wnt3a, and TNF-related apoptosis inducing ligand (TRAIL) proteins were purchased from R & D Systems (Minneapolis, MN) or from Sigma-Aldrich (St. Louis, MO). Diphenyliodonium (DPI), rotenone, ebselen, 5,6-dichloro-1-β-d-ribofuranosylbenzimidazole, and actinomycin D were purchased from Sigma-Aldrich. Taqman gene expression assays for quantifying murine aortic mRNA by quantitative RT-PCR (RT-qPCR) were purchased from Applied Biosystems/Life Technologies Inc. (Foster City, CA). C3H10T1/2 cells were obtained from the American Type Culture Collection (no. CCL-226; Manassas, VA). Western blot analyses were carried out as recently detailed (15), using antibodies purchased from Santa Cruz Biotechnology, Inc. (Msx2, no. sc-15396; eIF2α, no. sc-133227; lamin B, no. sc-6217; Santa Cruz, CA) and from Cell Signaling Technology (non-phospho-β-catenin Ser33/Ser37/Thr41 antibody no. 4270; Beverly, MA). The alkaline phosphatase-conjugated secondary antibodies used were goat antimouse from Applied Biosystems (no. T2192) or goat antirabbit from Vector Laboratories (no. AP-1000; Burlingame, CA). Digitized gray scale images of Western blot autoradiograms were quantified using the gel analysis software tools of NIH ImageJ (16) (ImageJ64 for Mac OS X; National Institutes of Health, Bethesda, MD).
Genetically engineered mice
All protocols for handling and use of genetically engineered mice were of approved by the Washington University Animal Studies Committee. Breeding pairs of C57BL/6 (stock no. 000664), TNFR1−/− (stock no. 002818; B6.129-Tnfrsf1atm1Mak/J) and TNFR2−/− (stock no. 002620; B6.129S2-Tnfrsf1btm1Mwm/J) mice on the C57BL/6 background mice were obtained from the Jackson Laboratories (Bar Harbor, ME). The generation and characterization of our SM22-TNF transgenic mice on the C57BL/6 background have been previously detailed (8). Briefly, the 0.7-kb cDNA encoding the open reading frame of murine TNF (GenBank no. NM_013693) was placed downstream of the vascular smooth muscle cell-specific 0.5-kb murine SM22 promoter fragment −441 to +44 in plasmid DT58.11. After sequencing the insert, the plasmid was digested with XhoI and DrdI to release the 2.7-kb fragment encoding the SM22 promoter, mouse TNF cDNA, rabbit ß-globin 3′-untranslated region, and the polyA signal. Transgenic mice were made via male C57BL/6 pronuclear injection at the Washington University Mouse Genetics Core (Mia Wallace, director). PCR genotyping for the SM22-TNF transgene was directed toward uniquely juxtaposed murine TNF cDNA and rabbit ß-hemogolobin 3′-untranslated region sequences (genotyping primers 5′-ACA GAC TGC TCC AAC TTG GTG TCT TTC CCC-3′ and 5′-GGG ACC GAT CAC CCC GAA GTT CAG TAG ACA-3′), using the method of Stratman et al. (17).
Aortic adventitial myofibroblast cultures
Primary mouse aorta adventitial myofibroblasts were generated and maintained from male C57BL/6, TNFR1−/−, TNFR2−/−, and p47phox−/− (also known as NCF1−/−) mice as previously detailed (18). Briefly, six to 12 male mice (6–12 wk of age) were euthanized by exanguination under ketamine-xylazine general anesthesia following protocols approved by the Washington University Animal Studies Committee. Under sterile conditions, thoracic aortic segments from the level of the left subclavian artery to the diaphragm were resected en bloc, rinsed in normal saline supplemented with penicillin-streptomycin, and placed in sterile DMEM with 4 g/liter glucose in a 15-cm plastic tissue culture dish. Aortas were then transected to form multiple approximately 2-mm ring-like segments with a sterile no. 11 scalpel, transferred to two 10-cm plastic tissue culture dishes, and maintained in a minimal amount (∼3 ml/dish) of growth media (10% fetal calf serum in DMEM, high glucose, supplemented with 100 IU/ml penicillin and 100 μg/ml streptomycin). After 1–2 d of culture in a humidified chamber with 5% CO2, the now adherent tissue rings were supplemented with additional 5 ml of growth media and refed every 3 d. Two weeks after initial plating, a sterile Pasteur pipette was used to aspirate the residual aortic rings. The outgrowth of adherent myofibroblasts was harvested by trypsinization, and each dish was sequentially replated into three 10-cm tissue culture plates. Cells were amplified over the next 10–20 passages and then used for gene expression or mineralization assays as indicated. At least 90% of these cells myofibroblasts as based upon vascular smooth muscle cell (VSMC) α-actin staining (18) and express Msx2 (18, 19).
Cell culture, pharmacological treatments, biochemical and gene expression assays, transient transfections, and Alizarin Red histochemistry
For gene expression assays, aortic adventitial myofibroblasts were plated at 500,000 cells/well in six-well plastic tissue culture cluster dishes (9 cm2 per well). The following evening, the growth media were aspirated, and cells were maintained overnight in serum-free DMEM with high glucose (4 g/liter). The next morning, cultures were treated with either vehicle (1 mg/ml acetylated BSA in sterile PBS) or 10 ng/ml rat TNF in vehicle for the times indicated. At the end of the treatment period, media were aspirated, cultures rinsed once with ice-cold PBS, and RNA harvested into 600 μl of QIAGEN RNeasy minikit RLT buffer (QIAGEN catalog no. 74104; Valencia, CA), to which 10 μl/ml fresh β-mercaptoethanol was added just before use. After centrifugation through a QIAshredder (QIAGEN), total RNA is then isolated by spin column chromatography and eluted in 35 μl of nuclease-free sterile water. The RNA extract was treated with ribonuclease-free deoxyribonuclease to remove any small amount of contaminating genomic DNA (Ambion DNA free kit; catalog no. 1906; Austin, TX) and then repurified by QIAGEN RNeasy methodology per the manufacturer's instructions. Subsequently, cDNA was prepared using random hexamer priming (19). Quantitative fluorescence RT-qPCR was carried out as previously described (19), using commercially purchased TaqMan probe-based gene expression assays in conjunction with an Applied Biosystems ABI 7700 sequence detection system to quantify the indicated transcripts. The specific, inventoried TaqMan assays purchased are available upon request. The mRNA accumulation in duplicate aliquots was normalized to the signal provided by 18S ribosomal RNA in parallel duplicate aliquots and averaged, with a minimum of three independent culture wells per treatment averaged for statistical comparison. All experiments were repeated at least twice. Treatments with antisense oligodeoxynucleotides (ASO) (20) and lucigenin chemiluminescent assays for superoxide generation (15) were carried out as we have previously detailed (15, 20) using cells cultured in phenol red-free DMEM. For experiments evaluating the impact of pharmacological inhibitors, stock solutions of inhibitors were prepared in dimethyl sulfoxide (DMSO; vehicle) and then diluted greater than 1000-fold to achieve final concentrations indicated. All inhibitors (or DMSO vehicle control) were added 30 min before the addition of 10 ng/ml TNF or its vehicle control (1% BSA in PBS).
Preparation of Msx2 promoter-luciferase reporter constructs, transient transfection of C3H10T1/2 mesenchymal cells, luciferase assays, and EMSA to identify DNA binding activities were carried out as we have detailed (18, 19, 21–24). Briefly, mouse genomic DNA was used as template for PCR to generate the indicated Msx2 promoter fragments [numbering from start site of transcription (25, 26)] and introduce 5′-KpnI and 3′-MluI cognates as we have previously done for osteocalcin promoter-mapping studies (24, 27). After promoter fragment purification, digestions with KpnI and MluI, and vectorial ligation of into the KpnI/MluI sites in the polylinker of promoterless pGL2 Basic (Promega, Madison, WI) upstream of the luciferase reporter, Msx2 promoter fragments (−1586 to +26; −498 to +26; −69 to +26) were sequenced to verify insert fidelity. Preparation and characterization of our RSVLUC minimal promoter-luciferase reporter construct has been previously described (27).
Transcriptional regulation was carried out using murine C3H10T1/2 cells, a mesenchymal cell line that recapitulates key features of vascular myofibroblast (28, 29) and smooth muscle (30) regulatory programming; importantly, differentiating C3H10T1/2 cells can integrate into vessels in vivo as mural VSMC (30). Methods of cell culture and transiently transfection have been previously detailed (23). Dendrimer-based transfection was selected because dihyroethidium staining revealed less superoxide generation using this method compared with lipofection or electroporation (Lai, C. F. and D. A. Towler, unpublished observations). Briefly, C3H10T1/2 cells were passaged in 10% fetal bovine serum and basal medium Eagle and then plated at 70% of confluence in 24-well cluster plates (1.9 cm2 area/well). The following day, cells were transiently transfected with 500 ng of total plasmid DNA/well with 2.5 μl of SuperFect dendrimer reagent (QIAGEN) in 30 μl of OptiMEM (Invitrogen). After 4 h of transfection, cultures were treated with complete growth media and allowed to recover overnight. The following day, cultures were treated with vehicle, TNF, or H202 for 6 h as indicated and then harvested for luciferase reporter activity as previously detailed (18, 19). Methods for cellular protein extraction and gel shift analyses have been previously detailed in our studies of transcriptional regulation by fibroblast growth factor (21, 22) but with slight modification for specific TNF and H202 treatments. Briefly, C3H10T1/2 cells were plated into six-well cluster dishes (ca. 35 mm diameter) and allowed to recover. Cultures were serum depleted overnight in serum-free DMEM (high glucose) and then treated the next morning for 4 h with vehicles, 5 ng/ml TNF, or 200 μm H202 as indicated. Monolayers were rinsed twice with ice-cold PBS, cells scraped into 1 ml of ice-cold PBS and pelleted by centrifugation, and whole cell extracts prepared using 70 μl of Dignam nuclear extraction buffer C (31) supplemented with 0.5% Triton X-100, protease inhibitor cocktail (Sigma; P8340), and phosphatase inhibitors as we have detailed (21, 22). DNA binding was carried out precisely as detailed (21, 22) [viz., 10 μg of protein in 7 μl of modified buffer C, 1 μl of 1 μg/ml polydeoxyinosine-deoxycytosine, 1 μl of 10 mg/ml acetylated BSA, 1 pmol of 30,000 cpm 32P-radiolabeled annealed duplex oligonucleotide in 14 μl of Dignam buffer A (21, 22, 31)]. For cold competition studies, 125- to 250-molar excess of unlabeled (cold) annealed duplex oligonucleotide was included in the binding reaction as indicated. DNA binding activities were resolved by native gel electrophoresis on precast nondenaturing 4–20% acrylamide gradient gels (Novex/Life Technologies, San Diego, CA; preequilibrated in 0.375 × Tris-borate EDTA), gels dried, and complexes visualized by autoradiography as we have detailed (21, 22, 24).
For studies of culture mineralization (19), wild-type, TNFR1−/−, and TNFR2−/− primary aortic adventitial cells were maintained as above but plated into 12- or 24-well clusters at 100,000 cells/cm2. The day after plating, cultures were treated with mineralization media (DMEM supplemented with 1% fetal bovine serum, 50 μg/ml ascorbic acid, 5 mm β-glycerol phosphate, 100 U/ml penicillin, and 100 μg/ml streptomycin) supplemented with vehicles, 10 ng/ml TNF, 2.5 mm sodium phosphate, or both TNF and sodium phosphate as indicated with refeeding every 3 d for a total of 4 d. After the end of the culture period, monolayers were rinsed with Tris-buffered saline [50 mm Tris (pH 7.4)/0.15 m NaCl] and fixed in ice-cold 70% ethanol for 1 h. After two additional washes in water, 1 ml of filtered 0.5% Alizarin Red S calcium stain (19, 32) in distilled deionized H20 was added per well, and plates were incubated at room temperature for 30 min with gentle shaking. After aspiration, monolayers were then washed five times for 5 min in 3–4 ml water. Stained monolayers were then air dried and images of the stained cultures digitally captured. Image analysis was performed essentially as previously described (33, 34), quantifying the Alizarin Red calcium stain by measuring gray-scale intensity in the inverted green channel of the RGB-tagged image file format with NIH ImageJ64 (35) for Mac OS X.
In vivo pharmacological treatments, aortic RNA extraction, and gene expression assays
Two- to 3-month-old SM22-TNF transgenic mice were weighed and then divided into groups of equal average weight. Following a previously validated in vivo ASO knockdown protocol (36), mice were injected ip once daily with either 40 ng/g control ASO (20, 37) (scrambled p47phox sequence control; 5′-TGA GGC TCC GTC CGC TGG AGC G-3′) or with a combination of 20 ng/g mouse Nox1 ASO (5′-TTA ACC AGC CAG TTT CCC ATT G-3′) + 20 ng/g mouse Nox2 ASO (5′-TTC ACA GCC CAG TTC CCC ATG T-3′) for 5 d as indicated. All ASO targeted the sequence overlapping the initiator Met codon (38) and were made as the stable phosphorothioate derivatives by Integrated DNA Technologies Inc. (Coralville, IA). ASO stock solutions (5 μg/ml) were prepared in sterile phosphate buffer saline. Two hours after the fifth injection, animals were euthanized under general anesthesia as above and aortic tissues harvested for total RNA and RT-qPCR analysis precisely as previously detailed (15). Data are presented as the mean percent of value for the control ASO-treated cohorts, normalized to 18S (n = 6–8/group). For studies of in vivo rotenone responses, mice (n = 16/group, pooled from two separate injection sessions) were injected ip with either 1 μg/g rotenone (0.5 mg/ml stock) in DMSO or DMSO vehicle every other day for 5 d as previously described (39). Two hours after the third injection, aortas were harvested for total RNA and TaqMan probe-based gene expression assays as we have detailed (15).
Statistics
Statistical analyses were performed using GraphPad Instat Software (version 3.06; GraphPad Inc., San Diego, CA), implementing standard parametric Student's t test, and parametric ANOVA and nonparametric ANOVA methods as indicated. Post hoc analysis for significance between groups after one-way ANOVA was carried out using the Student-Neuman-Keuls multiple comparison test. All graphed gene expression and transcription data are presented as the mean ± sem values arising from at least three independent replicates. Experiments were repeated at least twice.
Results
TNF-regulated Msx2 expression in aortic myofibroblasts is dependent upon Nox and mitochondrial metabolism
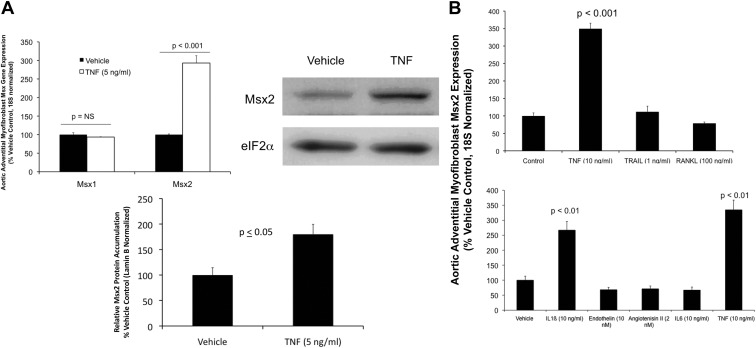
Msx2-Wnt signaling, a proosteogenic gene regulatory program initially discovered in craniofacial bone formation (9), is up-regulated in aortic tissues of both mice and humans with arteriosclerosis (4, 5). Signaling localizes to adventitial myofibroblasts, aortic valve interstitial myofibroblasts, and a subset of mural VSMC (4, 5). The expression of aortic Msx2 and Wnt is entrained in part to TNF activity in vivo (8), and TNF dose dependently increases Msx2 in aortic adventitial myofibroblasts in vitro (8). TNF selectively up-regulates Msx2 message (Fig. 1A) and protein (Fig. 1B; see also Supplemental Fig. 1, published on The Endocrine Society's Journals Online web site at http://endo.endojournals.org) levels in cultured adventitial myofibroblasts, without inducing Msx1 expression (Fig. 1A). Cytokines IL-6, RANKL, and TRAIL are inactive; however, like TNF, IL-1β also up-regulates Msx2 gene expression (Fig. 1, C and D). Endothelin and AngII are inactive in this assay (Fig. 1D).
Fig. 1.
TNF up-regulates Msx2 mRNA accumulation in primary aortic adventitial myofibroblasts. A, Upper left panel, Primary aortic myofibroblasts cultures were treated with TNF for 3 h and total cellular RNA harvested for gene expression analysis by RT-qPCR. Unlike Msx1, Msx2 is up-regulated by TNF (5 ng/ml; n = 3 per group; P < 0.05). Upper right and bottom panels, Msx2 protein levels as assessed by Western blot were also up-regulated by TNF after 4 h of treatment (1.8-fold, P < 0.05; n = 3 per group; see also Supplemental Fig. 1). B, Other inflammatory polypeptides and cytokines such as endothelin, AngII, IL-6, TRAIL, and RANKL do not increase Msx2 message, but IL-1β is active (n = 3 per treatment, P < 0.05).
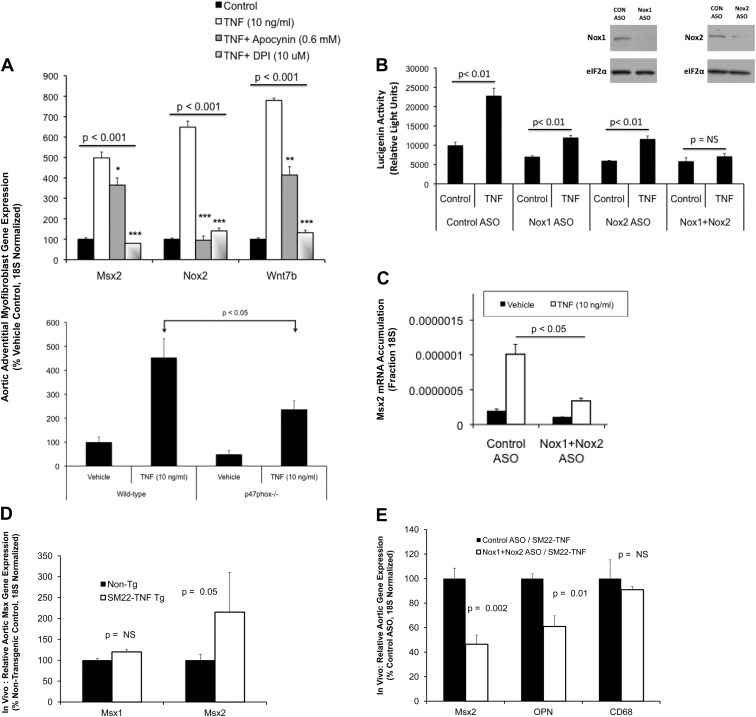
Because both TNF and IL1β are proinflammatory cytokines that signal via NADPH oxidase (Nox) activation (40–42), we examined the action of the two Nox inhibitors apocynin and DPI (11) on TNF-regulated Msx2-Wnt signaling. Both DPI and apocynin significantly inhibit Msx2 and Wnt7b induction, with DPI being most effective (Fig. 2A, upper panel). Apocynin reduces Nox activity via inhibition of p47phox subunit protein-protein interactions and ROS scavenging (43), and adventitial myofibroblasts deficient in Nox activation due to p47phox-deficiency (44) also exhibit reduced Msx2 induction in response to TNF (Fig. 2A, lower panel). ASO directed toward Nox1 and Nox2, the two major p47phox-dependent Nox enzymes in adventitial myofibroblasts (45, 46), reduce superoxide generation in response to TNF stimulation (Fig. 2B). Using the two ASO in combination was most effective in reducing TNF-induced superoxide levels (Fig. 2B), and Nox1+Nox2 ASO concomitantly inhibit Msx2 induction by TNF (Fig. 2C). ASO directed to Nox3 and Nox4 did not inhibit TNF induction (Supplemental Fig. 2). Mice for VSMC-specific TNF expression exhibit elevated levels of aortic Msx2 (Fig. 2D); as observed in vitro, administration of Nox1+Nox2 ASO to SM22-TNF transgenic mice down-regulate aortic Msx2 expression in vivo (Fig. 2E). Aortic OPN (osteopontin), another TNF target (20), is also decreased by Nox1+Nox2 ASO administration, whereas CD68, a marker of macrophage accumulation (47), is not affected (Fig. 2E). Thus, TNF activation of Msx2 expression depends in part on Nox activity.
Fig. 2.
TNF induction of Msx2 gene expression requires intact Nox and mitochondrial redox signaling. A, Treatment of primary aortic myofibroblasts with the Nox inhibitor apocynin partially reduced Msx2 while completely preventing TNF induction of Nox2. DPI, a dual inhibitor of Nox and mitochondrial respiration, abrogated TNF induction of both Msx2 and Nox2 (n = 3 per group; replicated > 10 times). Wnt7b responses parallel those of Msx2. Lower panel, Myofibroblasts from mice deficient for p47phox−/−, an obligatory coregulator of Nox1 and Nox2 activity and target of apocynin inhibition, exhibit significantly reduced Msx2 induction (n = 3 per treatment; P < 0.05). ASO directed toward Nox1 and Nox2 reduced TNF-induced oxidative stress (B) and Msx2 induction (C; n = 4 / group; replicated more than three times). Nox1 and Nox2 ASO concentrations were each at 0.25 μm and the control ASO (CON ASO) at 0.5 μm. B, inset, Western blot analyses confirm reductions in Nox1 and Nox2 protein levels with ASO treatment. Pharmacological inhibitors were introduced 30 min before treatment with TNF, and cultures were incubated overnight with the indicated ASO before TNF treatment. D, Mice transgenic for SM22-TNF express elevated aortic Msx2 but not Msx1 (n = 6–8 per genotype; P = 0.05). E, As in vitro, administration of Nox1+Nox2 ASO (n = 8) significantly reduces aortic Msx2 in vivo in SM22-TNF transgenic mice vs. control ASO (n = 6). OPN, another TNF target, is also reduced, whereas macrophage CD68 is not. *, P < 0.05 vs. TNF; **, P < 0.01 vs. TNF; ***, P < 0.001 vs.TNF.
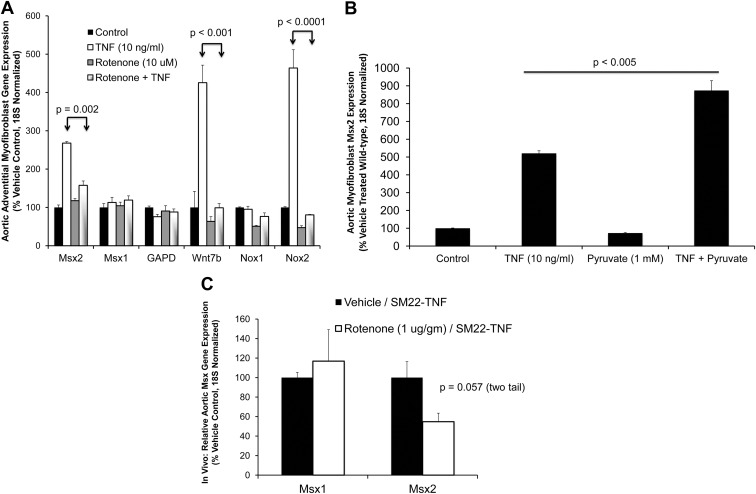
The greater efficacy of DPI vs. apocynin as an inhibitor of Msx2 induction by TNF prompted further consideration of DPI's mechanism of action. As an inhibitor of flavoenzyme-dependent oxidoreductases (48), DPI inhibits not only Nox activity but also complex I and complex II in the mitochondrial respiratory chain (49). Like Nox activity, complexes I and III of the mitochondrial respiratory chain generate ROS (50). Therefore, to address whether mitochondrial ROS signals might also contribute to TNF actions, we assessed the impact of rotenone, a specific inhibitor of mitochondrial respiratory activity at the level of complex I (49). Like DPI, rotenone inhibits TNF induction of Msx2 and Wnt7b, without altering expression of Msx1 or the housekeeping gene GAPDH (glyceraldehyde phosphate dehydrogenase; Fig. 3A). Basal and inducible levels of Nox2 are significantly reduced by rotenone, pointing to a feed-forward regulatory process dependent on mitochondrial ROS (51). Supplementation of culture media with pyruvate, an anapleurotic substrate for mitochondrial tricarboxylic acid cycle metabolism (50, 52), significantly enhances Msx2 induction with TNF treatment (Fig. 3B). Finally, in vivo low-dose rotenone administration selectively reduces Msx2 but not Msx1 expression in aortic tissues of SM22-TNF transgenic mice (Fig. 3C). Thus, TNF induction of Msx2 in aortic tissues requires intact NADPH/Nox activity and mitochondrial oxidative metabolism.
Fig. 3.
The mitochondrial respiratory chain inhibitor rotenone reduces aortic adventitial Msx2 expression in vitro and in vivo. A, Rotenone inhibits TNF induction of Msx2-Wnt signaling in primary aortic myofibroblast cultures (n = 6 per treatment). Msx1 and GAPDH (glyceraldehyde phosphate dehydrogenase) were not altered. Rotenone treatment was initiated 30 min before challenge with TNF. B, Conversely, pyruvic acid, an anapleurotic substrate for mitochondrial tricarboxylic cycle metabolism, significantly enhances TNF induction of Msx2 (n = 6 per treatment). C, In vivo rotenone treatment reduces aortic Msx2, but not Msx1, in SM22-TNF transgenic mice (n = 16 animals per treatment group).
Hydrogen peroxide signaling downstream of TNF-activated ROS generation mediates induction of Msx2 gene transcription
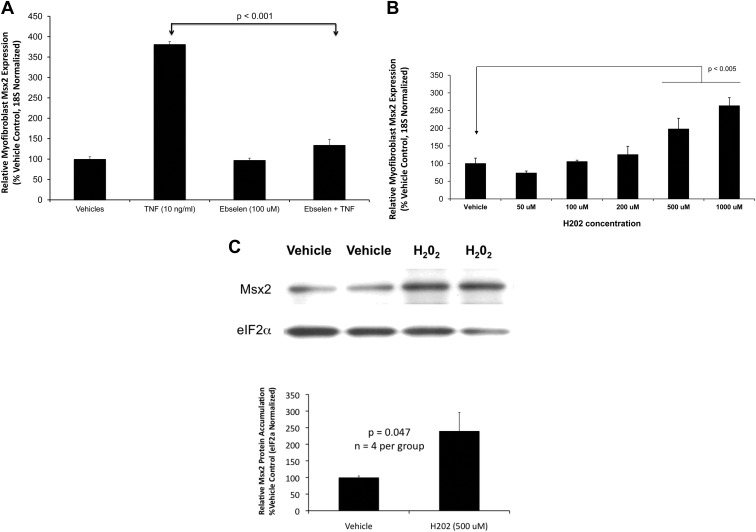
Superoxide is the initial, short-lived ROS generated by Nox and mitochondrial respiratory activity (45, 53). Dismutation reactions generate the longer-lived species hydrogen peroxide (H2O2), a bioactive second messenger metabolized by cellular enzymes including protein thiol peroxidases and glutathione peroxidases (53, 54). We wanted to determine whether H2O2 participates in TNF induction of Msx2 downstream of Nox and mitochondrial activity. Therefore, we assessed the impact of ebselen, an organoselenium-based glutathione peroxidase mimetic (55, 56). Ebselen treatment almost completely abrogates TNF induction of Msx2 (Fig. 4A). Furthermore, treatment of cultured aortic myofibroblasts with exogenous H2O2 dose dependently increases Msx2 mRNA accumulation (Fig. 4B) with concomitant increases in Msx2 protein levels (Fig. 4C; Supplemental Fig. 3). By contrast, Msx1 accumulation is not up-regulated by H2O2 treatment (Supplemental Fig. 4).
Fig. 4.
Hydrogen peroxide (H2O2) supports Msx2 induction in aortic adventitial myofibroblasts. A, The glutathione peroxidase-mimetic ebselen inhibits TNF induction of Msx2 (n = 4 per treatment group). Cultures were pretreated with ebselen for 30 min before treatment with TNF. B, Three hours of exogenous H2O2 treatment dose dependently increases Msx2 expression, phenocopying TNF (n = 3 per treatment group). C, Msx2 protein levels were also increased by H2O2 treatment (n = 4 per treatment group; P < 0.047; see also Supplemental Fig. 3).
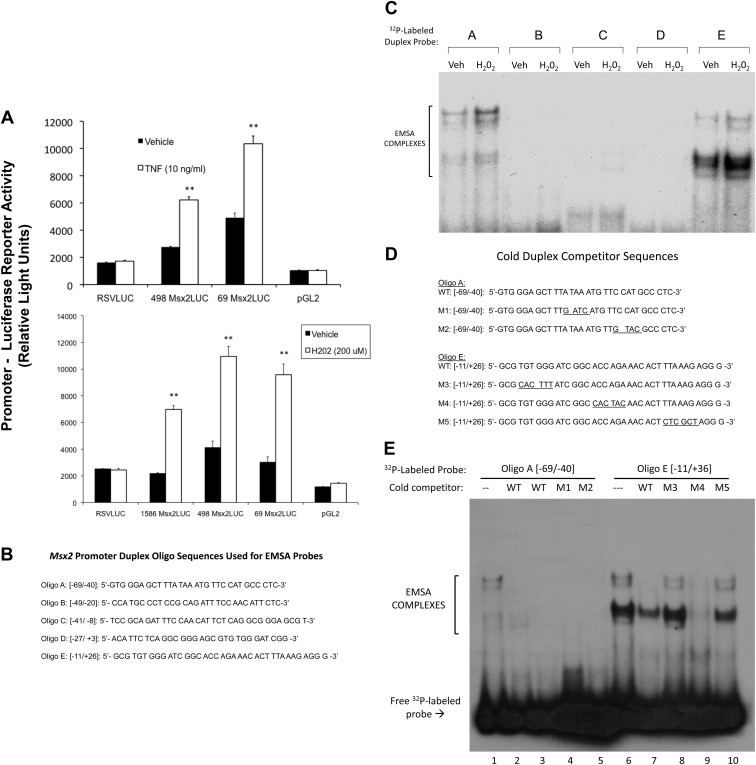
TNF induction of Msx2 is dependent on gene transcription inhibited by the RNA polymerase II inhibitors 5,6-dichloro-1-β-d-ribofuranosylbenzimidazole and actinomycin D (57). TNF up-regulates transcriptional activity driven by the proximal Msx2 promoter (luciferase reporter) in transiently transfected C3H10T1/2 cells (Fig. 5A), a useful cell culture model for studying growth factor and cytokine regulation of mural mesenchymal cell gene transcription including Msx2 (30, 58). Like TNF treatment, H2O2 also up-regulates transcription driven by the proximal Msx2 promoter fragment −1586 to +26 [Fig. 5A; numbering relative to the start site of Msx2 transcription (26)]. PCR-mediated 5′-promoter deletion analysis mapped activation to the proximal promoter fragment −69 to +26 (Fig. 5A), distinct from the upstream bone morphogenetic protein (BMP)-responsive enhancer (59). EMSA demonstrated that 4 h of H2O2 treatment increases cellular DNA binding activities recognizing Msx2 promoter regions −69 to −40 and −11 to +26 (Fig. 5, B and C). Inspection of the DNA sequence between −69 and −40 revealed TATA- and homeobox-like binding elements (Fig. 5D) that by competition analysis were not involved in assembly of these EMSA complexes (Fig. 5E, lanes 4 and 5; Supplemental Figs. 5 and 6, lanes 2–6). A putative Runx2 cognate (ACCAGA; nucleotides +5 to +10) in the Msx2 promoter region −11 to +26 was also identified (Fig. 5B); however, in competition assays, mutation of this cognate (Fig. 5D, M4) did not perturb assembly of protein-DNA complexes; M4 lacking this intact cognate completely inhibited binding to wild-type (WT) radiolabeled Msx2 probe −11 to +26 (Fig. 5E, lane 9). Moreover, antibodies to Runx binding factors did not disrupt or supershift any EMSA complexes binding Msx2 oligo E duplex probe −11 to +26 (Supplemental Fig. S7, lanes 6–8). However, intact TGTGGG (nucleotides −8 to −3) and CTTTAAAG (+15 to +22) elements embracing the ACCAGA Runx cognate were simultaneously required; mutant duplex oligos lacking either the former (M3; Fig. 5D) or the latter (M5, Fig. 5D) elements could not compete for complexes binding the radiolabeled native (WT) Msx2 promoter (Fig. 5E, lanes 8 and 10). Furthermore, unlabeled duplex oligo D, possessing the intact TGTGGG motif at nucleotides −8 to −3 (Fig. 5B), could compete for complex formation on radiolabeled oligo E (Supplemental Fig. 7, lane 4). Thus, H2O2 signaling cascades activated by TNF specifically up-regulate Msx2 gene transcription, proximal promoter activity, and Msx2 promoter DNA-protein interactions in mesenchymal cells.
Fig. 5.
Hydrogen peroxide up-regulates Msx2 promoter activity. A, upper panel, TNF treatment stimulates the Msx2 promoter-luciferase reporter activity in transiently transfected C3H10T1/2 mesenchymal cells via proximal promoter elements. A, lower panel, peroxide treatment also stimulates Msx2 promoter-luciferase reporter activity via proximal promoter elements. By contrast, the RSV (Rous sarcoma virus) proximal promoter was not stimulated by peroxide. Treatments were for 6 h, n = 3 per treatment group, and replicated more than three times. B, Upper-strand sequences of the Msx2 proximal promoter duplex oligos radiolabeled and used as EMSA probes. C, H2O2 treatment up-regulates DNA binding activity recognizing the proximal Msx2 promoter regions −69/−40 and −11/+26 detected by gel shift assay. Brackets indicate the EMSA complexes visualized by autoradiography. The unbound/unshifted radiolabeled duplex probes have been run off the bottom of the gel. Treatments were for 4 h. D, Upper-strand sequences of the native (WT) and mutant (M1–M5) Msx2 proximal promoter duplex oligos used for cold competition studies. E, Cold competition studies assessing binding specificity of complexes recognizing the Msx2 promoter region −69 to −40 (lanes 1–5) and −11 to +36 (lanes 6–10). The unbound, unshifted radiolabeled duplex probes are seen at the bottom of this gel (arrow). **, P < 0.01 vs. vehicle.
TNFR1 supports Msx2 expression and osteogenic mineralization directed by aortic adventitial myofibroblasts
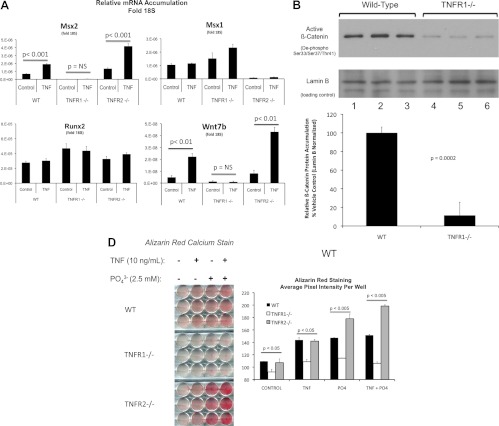
TNF initiates its biological actions via two cell surface receptors, TNFR1 and TNFR2 (60). To determine whether TNFR1, TNFR2, or both mediate TNF induction of Msx2, we analyzed primary aortic adventitial myofibroblasts prepared from male WT C57BL6, TNFR1−/−, and TNFR2−/− mice. Compared with WT aortic myofibroblasts, myofibroblasts from TNFR1−/− cells exhibit less than 5% the basal level of Msx2 and lack of TNF induction (Fig. 6A). Levels of Msx1 and Runx2 are not reduced in TNFR1−/− cultures, but basal expression and TNF induction of Wnt7b are concomitantly diminished along with Msx2. Furthermore, steady-state levels of dephosphorylated β-catenin, an index of activated canonical Wnt signaling, are reduced in myofibroblasts lacking TNFR1 (Fig. 6B). TNFR2−/− cells retain TNF induction of both Msx2 and Wnt7b (Fig. 6A). Unexpectedly, Msx1 is down-regulated in TNFR2−/− cultures with no alteration in Runx2.
Fig. 6.
Intact TNFR1 is required for Msx2 expression and robust mineralization in primary aortic myofibroblasts. Primary aortic myofibroblast cultures were established from WT, TNFR1−/−, and TNFR2−/− mice at high cell densities (6 × 105 per well), treated with either vehicle or 10 ng/ml TNF for 3 h, and RNA extracted for gene expression analysis. A, Loss of TNFR1 but not TNFR2 expression markedly reduced both basal and TNF induced Msx2 mRNA accumulation. Expression of the related homeodomain protein Msx1 was dependent on TNFR2, not TNFR1. The expression of osteogenic factor Wnt7b (84) parallels the expression of Msx2 (n = 3 per group; replicated more than two times). B, The dephosphorylated form of β-catenin, an index of activated canonical Wnt signaling (85), was reduced in TNFR1−/− aortic myofibroblasts as assessed by Western blot (n = 3 per group, P = 0.0002). C, Calcium deposition was assessed after treatment with vehicle, 10 ng/ml TNF, 2.5 mm supplemental NaPi, or TNF + NaPi in mineralization media (n = 3 per treatment, replicated more than three times). Alizarin Red calcium staining demonstrated exhibited significantly reduced calcification in TNFR1−/− cells compared with wild-type controls and TNFR2−/− cultures.
To assess the impact of specific TNFR subtypes on osteogenic calcification, we cultured WT, TNFR1−/−, and TNFR2−/− cells under mineralizing conditions with or without TNF treatment. Supplemental phosphate was provided as indicated to enhance and accelerate mineralization (61, 62). Compared with WT cells, TNFR1−/− cultures exhibit significantly reduced mineralization as revealed by Alizarin Red calcium staining (32) (Fig. 6C). When normalized to respective vehicle treated controls, the 31% increase in basal staining induced by TNF treatment of WT cells was reduced to 17% in TNFR1−/− cultures (P < 0.001). In contrast, like WT cells, TNFR2−/− cultures exhibit mineralization that is enhanced by TNF either in the presence or absence of phosphate supplementation (Fig. 6C). Thus, TNFR1 supports procalcific Msx2-Wnt expression and mineralization directed by primary aortic myofibroblasts.
Discussion
Diabetic arteriosclerosis is a significant contributor to the cardiovascular disease burden experienced by our aging, dysmetabolic populace (63). Arteriosclerotic vascular stiffening increases myocardial workload, conveys risk for end organ barotrauma, and reduces smooth distal tissue perfusion via impaired Windkessel physiology (64). To develop novel therapeutic strategies to prevent and treat diabetic arteriosclerosis, a better understanding of this disease biology is required. We previously identified that profibrotic, proosteogenic Msx2-Wnt signaling cascades are up-regulated by diabetogenic high-fat diet (HFD) in arterial myofibroblasts (4), dependent in part on TNF signals (8). Of note, other laboratories have now confirmed these results, extending observations to preadipocytes (65), VSMC (66, 67), and arteries of humans afflicted with the arteriosclerotic calcification in chronic kidney disease (12). In the setting of uremia, the expression patterns of vascular TNF and Msx2 are highly correlated in human arterial specimens, even before the onset of overt vascular mineral deposition (12).
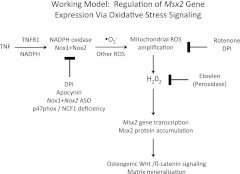
We characterize Msx2 as a direct target of TNF signaling and newly identify the critical role for Nox- and mitochondria-dependent ROS signaling on Msx2 promoter activation. In vitro and in vivo gene expression studies after genetic and pharmacological manipulation establish the role for inflammatory TNFR1-ROS signaling in vascular myofibroblast Msx2 gene expression. We demonstrate that H2O2 conveys these activating signals because of the following: 1) the glutathione peroxidase mimetic ebselen abrogates TNF induction of Msx2; 2) H2O2 directly stimulates Msx2 gene expression; and 3) H2O2 enhances Msx2 proximal promoter activity and up-regulates specific Msx2 promoter DNA-protein interactions. Because intracellular Nox-dependent ROS signaling is highly compartmentalized (68), unlike exogenous H2O2 administration, signal amplification and specificity can be achieved by receptor-mediated localization and initiation. Recently Kurabayashi and colleagues (82) identified that activation of the receptor for advanced glycosylation end-products (RAGE) can also up-regulate osteogenic Msx2 signaling in vascular mesenchymal progenitors. Intriguingly, ligand-induced protein-protein interactions between RAGE and Nox1 activate osteogenic differentiation of VSMC in vitro (57) and in vivo (58). Thus, our data begin to converge with that of others to highlight that vascular Msx2 expression is entrained to specific inflammatory pathways that activated oxidative stress signaling (Fig. 7). Moreover, these observations are in good agreement with the seminal studies of Chen et al. (69); they were the first to identify the important role for H2O2 in VSMC activation of Runx2, a Runt-domain factor necessary for orthotopic osteochondrogenic differentiation. Although the Msx2 proximal promoter region activated by H2O2 in myofibroblasts possesses a Runx2 ACCAGA cognate (70), this specific element appears not to be required for initial assembly of peroxide-regulated Msx2 promoter DNA-protein complexes. Future studies will identify the specific transcription factors supporting ostogenic Msx2 expression downstream of TNF and H2O2 signaling in aortic myofibroblasts.
Fig. 7.
A working model for regulation of vascular Msx2 gene expression via TNF and oxidative stress signaling. Inflammatory cytokine signaling initiated by TNF/TNFR1 engagement or IL-1β stimulation (not depicted) activates NAPDH oxidase (Nox) and mitochondrial ROS production. DPI-sensitive flavoenzymes in Nox and mitochondrial complexes generate ROS signals that activate Msx2 gene expression and downstream osteogenic Wnt/β-catenin cascades in vascular mesenchymal cells. Glutathione peroxidase mimetics such as ebselen (55) inhibit peroxide (H2O2)-dependent activation upstream of vascular osteogenic Wnt/β-catenin programs. Not depicted is the role for ROS-dependent vascular Runx2 activation, a transcription factor vital to the robust elaboration of osteogenic gene-regulatory programs during bone (86) and vascular (69) mineralization.
The significant impact of TNFR1 deficiency on myofibroblast mineralization responses to both TNF and phosphate supplementation is very intriguing; this suggests that TNFR1 deficiency conveys upon myofibroblasts a more generalized resistance to mineralizing stimuli. Conversely, TNFR2 deficiency increased mineralization responses to TNF and phosphate supplementation. Of note, TNF signaling has recently been shown to suppress expression of Ank (71), a plasma membrane transporter that reduces matrix calcification by increasing extracellular pyrophosphate, a mineralization inhibitor (72). Whether myofibroblast TNFR1 and TNFR2 differentially impact the elaboration of pyrophosphate, or other extracellular mineralization modulators, remains to be examined in detail.
During normal skeletal development, the two homeodomain transcription factors Msx1 and Msx2 play overlapping yet distinct roles in directing craniofacial mineralization and bone homeostasis (9). Although deficiency in Msx2 results in poor calvarial and tooth mineralization and global low-turnover bone disease, combined Msx1 and Msx2 deficiency is incompatible with life in great part due to the complete absence of craniofacial bone formation (9). Msx1 and Msx2 are coordinately regulated by BMP2 (73), the powerful bone morphogen necessary for maintenance of skeletal integrity and fracture repair (74). BMP2 signaling also plays an important role in diabetic vascular calcification (75). However, it is intriguing to note that Msx2 is selectively up-regulated by the ROS signals initiated by TNF and IL-1β, whereas Msx1 is not. This suggests that Msx2 expression has become entrained to inflammatory cues during evolution, potentially reflecting a unique role for dynamic Msx2 regulation during procalcific tissue responses to certain pathogens (76, 77). The role, if any, for Msx2 in innate immunity has yet to be assessed.
There are naturally limitations to our study. In this work, as an extension of our prior studies, we emphasize the TNF-dependent signals that control Msx2 expression (8). However, in vivo, other specific cytokines are certain to contribute (75). For example, we have now shown that IL-1β up-regulates Msx2 in myofibroblasts. Like TNF/TNFR1 signaling (42, 78), activation of IL-1RI by IL-1β promotes assembly of the redoxosome, a ligand-activated early endosome complex that promotes Nox-dependent ROS generation (40). Nox1 and Nox2 complexes are posttranscriptionally activated via protein-protein interactions with specific Rac1 family GTPases (41, 79); because TNF does not acutely regulate Nox1 mRNA expression, yet combined Nox1+Nox2 ASO reduces Msx2 induction, posttranscriptional activation of Nox1 and Nox2 enzyme activities are likely to be important to TNF and IL-1β signals. Of note the response is Nox1 and Nox2 specific because antisense directed to Nox3, Nox4, and Duox1 does not inhibit Msx2 induction. In diabetes, both TNF and IL-1β are up-regulated in the adventitia (80). Moreover, deletion of the receptor IL-1RI markedly reduces HFD-induced inflammation and diabetes (81); this suggests a vital role for IL-1β in addition to TNF in T2DM and its vascular complications. Matrix-associated RAGE agonists (82) also accrue with long-standing diabetes and advanced age (1). Thus, in vivo the multiplicity of low-grade inflammatory responses induced by HFD in diabetes- and dyslipidemia-prone LDLR−/− mice may obfuscate the singular contributions of TNF signaling in the initiation vs. progression of diabetic vascular disease. Finally, the specific nuclear transcription factor complexes mediating Msx2 activation in response to H2O2 remain to be characterized.
Of note, with age and long-standing diabetes, mitochondrial dysfunction can elaborate sufficient intracrine ROS signals capable of activating myofibroblast Msx2-Wnt programs in the absence of paracrine TNF and IL-1β cytokine cues (83). As such, points of convergence and shared signaling components in TNF, IL-1β, RAGE, and mitochondrial ROS pathways represent favored targets for therapeutic intervention. Furthermore, early data indicate that TNFR2 also exerts unique actions on β-catenin metabolism independent of Msx2 expression (Behrmann A., and D. A. Towler, unpublished observations). Although these regulatory pathways remain to be detailed, our data newly demonstrate that proosteogenic Msx2-Wnt signaling is a direct target of TNFR1 activation via ROS generation and H2O2 signaling in aortic myofibroblasts. Thus, strategies that inhibit vascular peroxide generation (e.g. Nox1/Nox2 inhibitors) or enhance peroxide degradation (e.g. glutathione peroxidase activators or mimetics) hold promise for treatment of diabetic arteriosclerosis (45, 55). Future pharmacological and biomechanical experimentation will directly test these notions.
Supplementary Material
Acknowledgments
This work was supported by National Institutes of Health Grants HL88651, HL81138, and HL69229 (to D.A.T.) and the Barnes-Jewish Hospital Foundation.
Disclosure Summary: D.A.T. has served on scientific advisory boards for Daichii-Sankyo, Eli Lilly & Co., and Merck & Co.
Footnotes
- AngII
- Angiotensin II
- ASO
- antisense oligodeoxynucleotide
- BMP
- bone morphogenetic protein
- DMSO
- dimethyl sulfoxide
- DPI
- diphenyliodonium
- HFD
- high-fat diet
- NADPH
- nicotinamide adenine dinucleotide phosphate
- Nox
- NADPH oxidase
- RAGE
- receptor for advanced glycosylation end-products
- RANKL
- receptor activator of nuclear factor-κB ligand
- ROS
- reactive oxygen species
- RT-qPCR
- quantitative RT-PCR
- T2DM
- type 2 diabetes
- TRAIL
- TNF-related apoptosis inducing ligand
- VSMC
- vascular smooth muscle cell
- WT
- wild type.
References
- 1. Greenwald SE. 2007. Ageing of the conduit arteries. J Pathol 211:157–17217200940 [Google Scholar]
- 2. Safar ME, Boudier HS. 2005. Vascular development, pulse pressure, and the mechanisms of hypertension. Hypertension 46:205–209 [DOI] [PubMed] [Google Scholar]
- 3. Shao JS, Cheng SL, Sadhu J, Towler DA. 2010. Inflammation and the osteogenic regulation of vascular calcification: a review and perspective. Hypertension 55:579–592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Shao JS, Cheng SL, Pingsterhaus JM, Charlton-Kachigian N, Loewy AP, Towler DA. 2005. Msx2 promotes cardiovascular calcification by activating paracrine Wnt signals. J Clin Invest 115:1210–1220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Towler DA, Bidder M, Latifi T, Coleman T, Semenkovich CF. 1998. Diet-induced diabetes activates an osteogenic gene regulatory program in the aortas of low density lipoprotein receptor-deficient mice. J Biol Chem 273:30427–30434 [DOI] [PubMed] [Google Scholar]
- 6. Devaraj S, Dasu MR, Jialal I. 2010. Diabetes is a proinflammatory state: a translational perspective. Expert Rev Endocrinol Metab 5:19–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Tintut Y, Patel J, Territo M, Saini T, Parhami F, Demer LL. 2002. Monocyte/macrophage regulation of vascular calcification in vitro. Circulation 105:650–655 [DOI] [PubMed] [Google Scholar]
- 8. Al-Aly Z, Shao JS, Lai CF, Huang E, Cai J, Behrmann A, Cheng SL, Towler DA. 2007. Aortic Msx2-Wnt calcification cascade is regulated by TNF-α-dependent signals in diabetic Ldlr−/− mice. Arterioscler Thromb Vasc Biol 27:2589–2596 [DOI] [PubMed] [Google Scholar]
- 9. Satokata I, Ma L, Ohshima H, Bei M, Woo I, Nishizawa K, Maeda T, Takano Y, Uchiyama M, Heaney S, Peters H, Tang Z, Maxson R, Maas R. 2000. Msx2 deficiency in mice causes pleiotropic defects in bone growth and ectodermal organ formation. Nat Genet 24:391–395 [DOI] [PubMed] [Google Scholar]
- 10. Caira FC, Stock SR, Gleason TG, McGee EC, Huang J, Bonow RO, Spelsberg TC, McCarthy PM, Rahimtoola SH, Rajamannan NM. 2006. Human degenerative valve disease is associated with up-regulation of low-density lipoprotein receptor-related protein 5 receptor-mediated bone formation. J Am Coll Cardiol 47:1707–1712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Miller JD, Chu Y, Brooks RM, Richenbacher WE, Peña-Silva R, Heistad DD. 2008. Dysregulation of antioxidant mechanisms contributes to increased oxidative stress in calcific aortic valvular stenosis in humans. J Am Coll Cardiol 52:843–850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Koleganova N, Piecha G, Ritz E, Schirmacher P, Müller A, Meyer HP, Gross ML. 2009. Arterial calcification in patients with chronic kidney disease. Nephrol Dial Transplant 24:2488–2496 [DOI] [PubMed] [Google Scholar]
- 13. Rajamannan NM. 2011. The role of Lrp5/6 in cardiac valve disease: experimental hypercholesterolemia in the ApoE−/− /Lrp5−/− mice. J Cell Biochem 112:2987–2991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Demer LL, Tintut Y. 2008. Vascular calcification: pathobiology of a multifaceted disease. Circulation 117:2938–2948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Cheng SL, Shao JS, Halstead LR, Distelhorst K, Sierra O, Towler DA. 2010. Activation of vascular smooth muscle parathyroid hormone receptor inhibits Wnt/β-catenin signaling and aortic fibrosis in diabetic arteriosclerosis. Circ Res 107:271–282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Rasband WS. 1997–2011. ImageJ. U.S. National Institutes of Health, Bethesda, MD, http://rsb.info.nih.gov/ij/ [Google Scholar]
- 17. Stratman JL, Barnes WM, Simon TC. 2003. Universal PCR genotyping assay that achieves single copy sensitivity with any primer pair. Transgenic Res 12:521–522 [DOI] [PubMed] [Google Scholar]
- 18. Bidder M, Shao JS, Charlton-Kachigian N, Loewy AP, Semenkovich CF, Towler DA. 2002. Osteopontin transcription in aortic vascular smooth muscle cells is controlled by glucose-regulated upstream stimulatory factor and activator protein-1 activities. J Biol Chem 277:44485–44496 [DOI] [PubMed] [Google Scholar]
- 19. Cheng SL, Shao JS, Charlton-Kachigian N, Loewy AP, Towler DA. 2003. MSX2 promotes osteogenesis and suppresses adipogenic differentiation of multipotent mesenchymal progenitors. J Biol Chem 278:45969–45977 [DOI] [PubMed] [Google Scholar]
- 20. Lai CF, Seshadri V, Huang K, Shao JS, Cai J, Vattikuti R, Schumacher A, Loewy AP, Denhardt DT, Rittling SR, Towler DA. 2006. An osteopontin-NADPH oxidase signaling cascade promotes pro-matrix metalloproteinase 9 activation in aortic mesenchymal cells. Circ Res 98:1479–1489 [DOI] [PubMed] [Google Scholar]
- 21. Newberry EP, Boudreaux JM, Towler DA. 1996. The rat osteocalcin fibroblast growth factor (FGF)-responsive element: an okadaic acid-sensitive, FGF-selective transcriptional response motif. Mol Endocrinol 10:1029–1040 [DOI] [PubMed] [Google Scholar]
- 22. Newberry EP, Willis D, Latifi T, Boudreaux JM, Towler DA. 1997. Fibroblast growth factor receptor signaling activates the human interstitial collagenase promoter via the bipartite Ets-AP1 element. Mol Endocrinol 11:1129–1144 [DOI] [PubMed] [Google Scholar]
- 23. Sierra OL, Towler DA. 2010. Runx2 trans-activation mediated by the MSX2-interacting nuclear target requires homeodomain interacting protein kinase-3. Mol Endocrinol 24:1478–1497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Towler DA, Bennett CD, Rodan GA. 1994. Activity of the rat osteocalcin basal promoter in osteoblastic cells is dependent upon homeodomain and CP1 binding motifs. Mol Endocrinol 8:614–624 [DOI] [PubMed] [Google Scholar]
- 25. Diamond E, Amen M, Hu Q, Espinoza HM, Amendt BA. 2006. Functional interactions between Dlx2 and lymphoid enhancer factor regulate Msx2. Nucleic Acids Res 34:5951–5965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Bell JR, Noveen A, Liu YH, Ma L, Dobias S, Kundu R, Luo W, Xia Y, Lusis AJ, Snead ML, et al. 1993. Genomic structure, chromosomal location, and evolution of the mouse Hox 8 gene. Genomics 16:123–131 [DOI] [PubMed] [Google Scholar]
- 27. Boudreaux JM, Towler DA. 1996. Synergistic induction of osteocalcin gene expression: identification of a bipartite element conferring fibroblast growth factor 2 and cyclic AMP responsiveness in the rat osteocalcin promoter. J Biol Chem 271:7508–7515 [DOI] [PubMed] [Google Scholar]
- 28. Small EM, Thatcher JE, Sutherland LB, Kinoshita H, Gerard RD, Richardson JA, Dimaio JM, Sadek H, Kuwahara K, Olson EN. 2010. Myocardin-related transcription factor-a controls myofibroblast activation and fibrosis in response to myocardial infarction. Circ Res 107:294–304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Qiu P, Feng XH, Li L. 2003. Interaction of Smad3 and SRF-associated complex mediates TGF-β1 signals to regulate SM22 transcription during myofibroblast differentiation. J Mol Cell Cardiol 35:1407–1420 [DOI] [PubMed] [Google Scholar]
- 30. Hirschi KK, Rohovsky SA, D'Amore PA. 1998. PDGF, TGF-β, and heterotypic cell-cell interactions mediate endothelial cell-induced recruitment of 10T1/2 cells and their differentiation to a smooth muscle fate. J Cell Biol 141:805–814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Dignam JD, Lebovitz RM, Roeder RG. 1983. Accurate transcription initiation by RNA polymerase II in a soluble extract from isolated mammalian nuclei. Nucleic Acids Res 11:1475–1489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Puchtler H, Meloan SN, Terry MS. 1969. On the history and mechanism of alizarin and alizarin red S stains for calcium. J Histochem Cytochem 17:110–124 [DOI] [PubMed] [Google Scholar]
- 33. Dallas SL, Veno PA, Rosser JL, Barragan-Adjemian C, Rowe DW, Kalajzic I, Bonewald LF. 2009. Time lapse imaging techniques for comparison of mineralization dynamics in primary murine osteoblasts and the late osteoblast/early osteocyte-like cell line MLO-A5. Cells Tissues Organs 189:6–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Winters G, Holzman R, Blekhman A, Beer S, Loya Y. 2009. Photographic assessment of coral chlorophyll contents: Implications for ecophysiological studies and coral monitoring. J Exp Mar Biol Ecol 380:25–35 [Google Scholar]
- 35. Collins TJ. 2007. ImageJ for microscopy. Biotechniques 43(Suppl 1):25–30 [DOI] [PubMed] [Google Scholar]
- 36. Wang FS, Ko JY, Lin CL, Wu HL, Ke HJ, Tai PJ. 2007. Knocking down dickkopf-1 alleviates estrogen deficiency induction of bone loss. A histomorphological study in ovariectomized rats. Bone 40:485–492 [DOI] [PubMed] [Google Scholar]
- 37. Xia L, Wang H, Goldberg HJ, Munk S, Fantus IG, Whiteside CI. 2006. Mesangial cell NADPH oxidase upregulation in high glucose is protein kinase C dependent and required for collagen IV expression. Am J Physiol Renal Physiol 290:F345–F356 [DOI] [PubMed] [Google Scholar]
- 38. Lebedeva I, Stein CA. 2001. Antisense oligonucleotides: promise and reality. Annu Rev Pharmacol Toxicol 41:403–419 [DOI] [PubMed] [Google Scholar]
- 39. Kwak HB, Lee BK, Oh J, Yeon JT, Choi SW, Cho HJ, Lee MS, Kim JJ, Bae JM, Kim SH, Kim HS. 2010. Inhibition of osteoclast differentiation and bone resorption by rotenone, through down-regulation of RANKL-induced c-Fos and NFATc1 expression. Bone 46:724–731 [DOI] [PubMed] [Google Scholar]
- 40. Oakley FD, Smith RL, Engelhardt JF. 2009. Lipid rafts and caveolin-1 coordinate interleukin-1β (IL-1β)-dependent activation of NFκB by controlling endocytosis of Nox2 and IL-1β receptor 1 from the plasma membrane. J Biol Chem 284:33255–33264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Li Q, Harraz MM, Zhou W, Zhang LN, Ding W, Zhang Y, Eggleston T, Yeaman C, Banfi B, Engelhardt JF. 2006. Nox2 and Rac1 regulate H2O2-dependent recruitment of TRAF6 to endosomal interleukin-1 receptor complexes. Mol Cell Biol 26:140–154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Li Q, Spencer NY, Oakley FD, Buettner GR, Engelhardt JF. 2009. Endosomal Nox2 facilitates redox-dependent induction of NF-κB by TNF-α. Antioxid Redox Signal 11:1249–1263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Mora-Pale M, Weïwer M, Yu J, Linhardt RJ, Dordick JS. 2009. Inhibition of human vascular NADPH oxidase by apocynin derived oligophenols. Bioorg Med Chem 17:5146–5152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Huang CK, Zhan L, Hannigan MO, Ai Y, Leto TL. 2000. P47(phox)-deficient NADPH oxidase defect in neutrophils of diabetic mouse strains, C57BL/6J-m db/db and db/+. J Leukoc Biol 67:210–215 [DOI] [PubMed] [Google Scholar]
- 45. Drummond GR, Selemidis S, Griendling KK, Sobey CG. 2011. Combating oxidative stress in vascular disease: NADPH oxidases as therapeutic targets. Nat Rev Drug Discov 10:453–471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Weaver M, Liu J, Pimentel D, Reddy DJ, Harding P, Peterson EL, Pagano PJ. 2006. Adventitial delivery of dominant-negative p67phox attenuates neointimal hyperplasia of the rat carotid artery. Am J Physiol Heart Circ Physiol 290:H1933–H1941 [DOI] [PubMed] [Google Scholar]
- 47. Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW., Jr 2003. Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest 112:1796–1808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. O'Donnell BV, Tew DG, Jones OT, England PJ. 1993. Studies on the inhibitory mechanism of iodonium compounds with special reference to neutrophil NADPH oxidase. Biochem J 290(Pt 1):41–49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Li Y, Trush MA. 1998. Diphenyleneiodonium, an NAD(P)H oxidase inhibitor, also potently inhibits mitochondrial reactive oxygen species production. Biochem Biophys Res Commun 253:295–299 [DOI] [PubMed] [Google Scholar]
- 50. Selivanov VA, Votyakova TV, Pivtoraiko VN, Zeak J, Sukhomlin T, Trucco M, Roca J, Cascante M. 2011. Reactive oxygen species production by forward and reverse electron fluxes in the mitochondrial respiratory chain. PLoS Comput Biol 7:e1001115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Dikalov S. 2011. Cross talk between mitochondria and NADPH oxidases. Free Radic Biol Med 51:1289–1301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Lambert AJ, Brand MD. 2004. Inhibitors of the quinone-binding site allow rapid superoxide production from mitochondrial NADH:ubiquinone oxidoreductase (complex I). J Biol Chem 279:39414–39420 [DOI] [PubMed] [Google Scholar]
- 53. Chen K, Craige SE, Keaney JF., Jr 2009. Downstream targets and intracellular compartmentalization in Nox signaling. Antioxid Redox Signal 11:2467–2480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Forman HJ, Maiorino M, Ursini F. 2010. Signaling functions of reactive oxygen species. Biochemistry 49:835–842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Bhabak KP, Mugesh G. 2010. Functional mimics of glutathione peroxidase: bioinspired synthetic antioxidants. Acc Chem Res 43:1408–1419 [DOI] [PubMed] [Google Scholar]
- 56. Sies H, Masumoto H. 1997. Ebselen as a glutathione peroxidase mimic and as a scavenger of peroxynitrite. Adv Pharmacol 38:229–246 [DOI] [PubMed] [Google Scholar]
- 57. Shao JS, Aly ZA, Lai CF, Cheng SL, Cai J, Huang E, Behrmann A, Towler DA. 2007. Vascular Bmp Msx2 Wnt signaling and oxidative stress in arterial calcification. Ann NY Acad Sci 1117:40–50 [DOI] [PubMed] [Google Scholar]
- 58. Brunelli S, Tagliafico E, De Angelis FG, Tonlorenzi R, Baesso S, Ferrari S, Niinobe M, Yoshikawa K, Schwartz RJ, Bozzoni I, Ferrari S, Cossu G. 2004. Msx2 and necdin combined activities are required for smooth muscle differentiation in mesoangioblast stem cells. Circ Res 94:1571–1578 [DOI] [PubMed] [Google Scholar]
- 59. Brugger SM, Merrill AE, Torres-Vazquez J, Wu N, Ting MC, Cho JY, Dobias SL, Yi SE, Lyons K, Bell JR, Arora K, Warrior R, Maxson R. 2004. A phylogenetically conserved cis-regulatory module in the Msx2 promoter is sufficient for BMP-dependent transcription in murine and Drosophila embryos. Development 131:5153–5165 [DOI] [PubMed] [Google Scholar]
- 60. Tracey D, Klareskog L, Sasso EH, Salfeld JG, Tak PP. 2008. Tumor necrosis factor antagonist mechanisms of action: a comprehensive review. Pharmacol Ther 117:244–279 [DOI] [PubMed] [Google Scholar]
- 61. Reynolds JL, Joannides AJ, Skepper JN, McNair R, Schurgers LJ, Proudfoot D, Jahnen-Dechent W, Weissberg PL, Shanahan CM. 2004. Human vascular smooth muscle cells undergo vesicle-mediated calcification in response to changes in extracellular calcium and phosphate concentrations: a potential mechanism for accelerated vascular calcification in ESRD. J Am Soc Nephrol 15:2857–2867 [DOI] [PubMed] [Google Scholar]
- 62. Jono S, McKee MD, Murry CE, Shioi A, Nishizawa Y, Mori K, Morii H, Giachelli CM. 2000. Phosphate regulation of vascular smooth muscle cell calcification. Circ Res 87:E10–E17 [DOI] [PubMed] [Google Scholar]
- 63. Weckbach S, Findeisen HM, Schoenberg SO, Kramer H, Stark R, Clevert DA, Reiser MF, Parhofer KG. 2009. Systemic cardiovascular complications in patients with long-standing diabetes mellitus: comprehensive assessment with whole-body magnetic resonance imaging/magnetic resonance angiography. Invest Radiol 44:242–250 [DOI] [PubMed] [Google Scholar]
- 64. Suzuki E, Yoshimura T, Omura Y, Sakaguchi M, Nishio Y, Maegawa H, Hisatomi A, Fujimoto K, Takeda J, Kashiwagi A. 2009. Higher arterial stiffness, greater peripheral vascular resistance and lower blood flow in lower-leg arteries are associated with long-term hyperglycaemia in type 2 diabetic patients with normal ankle-brachial index. Diabetes Metab Res Rev 25:363–369 [DOI] [PubMed] [Google Scholar]
- 65. Qadir AS, Lee HL, Baek KH, Park HJ, Woo KM, Ryoo HM, Baek JH. 2011. Msx2 is required for TNF-α-induced canonical Wnt signaling in 3T3-L1 preadipocytes. Biochem Biophys Res Commun 408:399–404 [DOI] [PubMed] [Google Scholar]
- 66. Villa-Bellosta R, Levi M, Sorribas V. 2009. Vascular smooth muscle cell calcification and SLC20 inorganic phosphate transporters: effects of PDGF, TNF-α, and Pi. Pflugers Arch 458:1151–1161 [DOI] [PubMed] [Google Scholar]
- 67. Lee HL, Woo KM, Ryoo HM, Baek JH. 2010. Tumor necrosis factor-α increases alkaline phosphatase expression in vascular smooth muscle cells via MSX2 induction. Biochem Biophys Res Commun 391:1087–1092 [DOI] [PubMed] [Google Scholar]
- 68. Ushio-Fukai M. 2009. Compartmentalization of redox signaling through NADPH oxidase-derived ROS. Antioxid Redox Signal 11:1289–1299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Byon CH, Javed A, Dai Q, Kappes JC, Clemens TL, Darley-Usmar VM, McDonald JM, Chen Y. 2008. Oxidative stress induces vascular calcification through modulation of the osteogenic transcription factor Runx2 by AKT signaling. J Biol Chem 283:15319–15327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Ducy P, Zhang R, Geoffroy V, Ridall AL, Karsenty G. 1997. Osf2/Cbfa1: a transcriptional activator of osteoblast differentiation. Cell 89:747–754 [DOI] [PubMed] [Google Scholar]
- 71. Zhao G, Xu MJ, Zhao MM, Dai XY, Kong W, Wilson GM, Guan Y, Wang CY, Wang X. 21 March 2012. Activation of nuclear factor-kappa B accelerates vascular calcification by inhibiting ankylosis protein homolog expression. Kidney Int 10.1038/ki.2012.40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Johnson K, Polewski M, van Etten D, Terkeltaub R. 2005. Chondrogenesis mediated by PPi depletion promotes spontaneous aortic calcification in NPP1−/− mice. Arterioscler Thromb Vasc Biol 25:686–691 [DOI] [PubMed] [Google Scholar]
- 73. Barlow AJ, Francis-West PH. 1997. Ectopic application of recombinant BMP-2 and BMP-4 can change patterning of developing chick facial primordia. Development 124:391–398 [DOI] [PubMed] [Google Scholar]
- 74. Tsuji K, Bandyopadhyay A, Harfe BD, Cox K, Kakar S, Gerstenfeld L, Einhorn T, Tabin CJ, Rosen V. 2006. BMP2 activity, although dispensable for bone formation, is required for the initiation of fracture healing. Nat Genet 38:1424–1429 [DOI] [PubMed] [Google Scholar]
- 75. Boström KI, Jumabay M, Matveyenko A, Nicholas SB, Yao Y. 2011. Activation of vascular bone morphogenetic protein signaling in diabetes mellitus. Circ Res 108:446–457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Chan ED, Morales DV, Welsh CH, McDermott MT, Schwarz MI. 2002. Calcium deposition with or without bone formation in the lung. Am J Respir Crit Care Med 165:1654–1669 [DOI] [PubMed] [Google Scholar]
- 77. Abedin M, Tintut Y, Demer LL. 2004. Vascular calcification: mechanisms and clinical ramifications. Arterioscler Thromb Vasc Biol 24:1161–1170 [DOI] [PubMed] [Google Scholar]
- 78. Yazdanpanah B, Wiegmann K, Tchikov V, Krut O, Pongratz C, Schramm M, Kleinridders A, Wunderlich T, Kashkar H, Utermöhlen O, Brüning JC, Schütze S, Krönke M. 2009. Riboflavin kinase couples TNF receptor 1 to NADPH oxidase. Nature 460:1159–1163 [DOI] [PubMed] [Google Scholar]
- 79. Ushio-Fukai M. 2006. Localizing NADPH oxidase-derived ROS. Sci STKE 2006:re8. [DOI] [PubMed] [Google Scholar]
- 80. Thandavarayan RA, Giridharan VV, Sari FR, Arumugam S, Veeraveedu PT, Pandian GN, Palaniyandi SS, Ma M, Suzuki K, Gurusamy N, Watanabe K. 2011. Depletion of 14-3-3 protein exacerbates cardiac oxidative stress, inflammation and remodeling process via modulation of MAPK/NF-kB signaling pathways after streptozotocin-induced diabetes mellitus. Cell Physiol Biochem 28:911–922 [DOI] [PubMed] [Google Scholar]
- 81. McGillicuddy FC, Harford KA, Reynolds CM, Oliver E, Claessens M, Mills KH, Roche HM. 2011. Lack of interleukin-1 receptor I (IL-1RI) protects mice from high-fat diet-induced adipose tissue inflammation coincident with improved glucose homeostasis. Diabetes 60:1688–1698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Suga T, Iso T, Shimizu T, Tanaka T, Yamagishi S, Takeuchi M, Imaizumi T, Kurabayashi M. 2011. Activation of receptor for advanced glycation end products induces osteogenic differentiation of vascular smooth muscle cells. J Atheroscler Thromb 18:670–683 [DOI] [PubMed] [Google Scholar]
- 83. Piconi L, Quagliaro L, Ceriello A. 2003. Oxidative stress in diabetes. Clin Chem Lab Med 41:1144–1149 [DOI] [PubMed] [Google Scholar]
- 84. Hu H, Hilton MJ, Tu X, Yu K, Ornitz DM, Long F. 2005. Sequential roles of Hedgehog and Wnt signaling in osteoblast development. Development 132:49–60 [DOI] [PubMed] [Google Scholar]
- 85. Wu G, He X. 2006. Threonine 41 in β-catenin serves as a key phosphorylation relay residue in β-catenin degradation. Biochemistry 45:5319–5323 [DOI] [PubMed] [Google Scholar]
- 86. Karsenty G. 2001. Minireview: transcriptional control of osteoblast differentiation. Endocrinology 142:2731–2733 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.