Abstract
The hypothalamus plays a critical role in the regulation of energy balance. Neuroanatomical and mouse genetic data have defined a core circuitry in the hypothalamus that mediates many of the effects of leptin on feeding and energy balance regulation. The present study used 5-bromo-2′-deoxyuridine (a marker of dividing cells) and a neuronal marker to systematically examine neurogenesis in the mouse embryonic hypothalamus, particularly the birth of neurons that relay leptin signaling. The vast majority of neurons in hypothalamic nuclei known to control energy balance is generated between embryonic days (E) 12 and E16, with a sharp peak of neurogenesis occurring on E12. Neurons in the dorsomedial and paraventricular nuclei and the lateral hypothalamic area are born between E12 and E14. The arcuate and ventromedial nuclei exhibit a relatively longer neurogenic period. Many neurons in these nuclei are born on E12, but some neurons are generated as late as E16. We also examined the birth of leptin-activated cells by coupling the 5-bromo-2′-deoxyuridine staining with cFos immunohistochemistry. Remarkably, the majority of leptin-activated cells in the adult hypothalamus were also born during a discrete developmental window on E12. These results provide new insight into the development of hypothalamic neurons that control feeding and identify important developmental periods when alterations in the intrauterine environment may affect hypothalamic neurogenesis and produce long-term consequences on hypothalamic cell numbers.
The hypothalamus has been the traditional focus of feeding regulation because this brain region contains sets of neurons that are devoted to metabolic regulation and respond directly to peripheral hormonal and nutritional signals. Empirical experiments using physical lesions of specific hypothalamic structures, and, more recently, studies using sophisticated transgenic approaches have specifically revealed the importance of neurons within the arcuate nucleus (ARH), ventromedial nucleus (VMH), dorsomedial nucleus (DMH), paraventricular nucleus (PVH), and lateral hypothalamic area (LHA) in the regulation of body weight and glucose levels (1–7) (and see Refs. 8 and 9 for reviews). The ARH is the predominant site for the integration of peripheral blood-borne signals, including endocrine and metabolic factors. ARH neurons that coproduce neuropeptide Y (NPY) and agouti-related peptide (AgRP) or that contain proopiomelanocortin (POMC)-derived peptides directly respond to peripheral hormonal signals, such as the adipocyte-derived hormone leptin. Both NPY/AgRP- and POMC-containing neurons project extensively to other key hypothalamic nuclei, including the PVH, DMH, and LHA. These target nuclei contain sets of neurons that play a crucial role in energy balance regulation and include neurons that produce anorexigenic peptides, such as TRH, CRH, and oxytocin in the PVH, and express orexigenic neuropeptides, such as orexins and melanin-concentrating hormone (MCH) in the LHA.
Impairments of hypothalamic development during perinatal life may result in lifelong metabolic dysregulations due to the importance of the hypothalamus in the control of eating and energy balance (10–12 and see Refs. 13–16 for reviews). However, a good understanding of the timing of normal hypothalamic development in species that are used for the study of metabolic programming, such as the mouse, is particularly critical to adequately assess the influence of perinatal environmental factors on hypothalamic development. The formation of the hypothalamus is characterized by various developmental processes that fall into three major categories: 1) the birth of new neurons (i.e. neurogenesis); 2) the migration of these cells to their final destination; and 3) the formation of functional circuits, which includes axon growth and synaptogenesis (see Refs. 17 and 18 for reviews). Axonal tract tracing experiments in mice have revealed that ARH projections develop postnatally and reach their target nuclei within distinct temporal domains. Innervation of the DMH occurs on postnatal day (P) 6 followed by innervation of the PVH on P8–10. Projections to the LHA are established on P12, and the pattern of ARH projections does not resemble that of an adult mouse until P18 (19).
Surprisingly little is known regarding the embryonic development of hypothalamic neurons despite a tremendous increase in the interest in the influence of prenatal factors on lifelong metabolic regulation and hypothalamic development. Even less information on the precise birthdate of the hypothalamic neurons that mediate leptin's action is available. Much of the current knowledge on the generation of hypothalamic nuclei has been inferred from [3H]thymidine studies, which cannot be used to determine the chemical phenotype of the generated cells (20–22). The present study used the analog of thymidine 5-bromo-2′-deoxyuridine (BrdU; a marker of dividing cells), with various cellular markers to systematically examine the birthdates of hypothalamic neurons in key hypothalamic nuclei and other areas that are implicated in energy balance regulation, with particular attention on the birthdate of cells that relay leptin signaling.
Materials and Methods
Animals
Time-pregnant C57BL6/J wild-type mice (Jackson Laboratories, Sacamento, CA) were produced in our breeding colony. The mice were mated around midnight and checked for a vaginal plug the next day. The day of conception (sperm positive vaginal smear) was designated as embryonic day (E) zero (E0). Animals were provided food and water ad libitum and housed in a 12-h light, 12-h dark cycle (lights on at 0700 h), temperature-controlled (21–22 C) environment. Animal use was in compliance with and approved by the Institutional Animal Care and Use Committee of the Saban Research Institute of Childrens Hospital of Los Angeles. The day of birth was designated as P0. Litter size was culled to six to eight pups (average litter size after culling was seven pups per litter) at P1 to assure adequate and standardized nutrition until P22 (weaning). Animals were provided ad libitum access to standard laboratory chow [Research Diet (New Brunswick, NJ)] after weaning. Only male offspring were studied, and each experimental group in all experiments included offspring from four litters.
5-Bromo-2-deoxyuridine injections
Pregnant mice received a single ip injection of BrdU (Sigma, St. Louis, MO; 160 mg/kg body weight, dissolved in 0.007 n NaOH) (23) on E10, E12, E14, E16, or E18. For the experiments combining BrdU and cFos stainings, pregnant mice received ip injections of BrdU (50 m/kg) on E12, E14, or E16 three times a day (at 0800, 1000, and 1200 h).
Leptin injections
Adult (P60) offspring of BrdU-injected dams received ip injections of either recombinant murine leptin (3 mg/kg body weight; Prepro Tech, Rocky Hill, NJ) or pathogen-free 5 mm Na citrate buffer (pH 4.0; n = 4 per group). These offspring were perfused 2 h later as described below.
Tissue preparation
Male offspring of the BrdU-injected dams were examined on P10 in most experiments. The measurement of BrdU and cFos double labeling was examined on P60. Mice (n = 4–6 per group) were deeply anesthetized with tribromoethanol and perfused transcardially with 0.9% saline, followed by an ice-cold 4% paraformaldehyde solution made in 0.1 m phosphate buffer (pH 7.4). The brains were quickly removed from each perfused animals, postfixed in the same fixative containing 20% sucrose for 2 h at 4 C, and immersed in 20% sucrose in a 0.02 m potassium PBS (KPBS) solution at 4 C overnight. Frozen coronal sections (25 μm thick, typically at a frequency of 1 in 3) from each brain were then cut using a cryostat, and every section was mounted onto gelatin-subbed, poly-l-lysine-coated microscope slides and stored in antifreeze at −20 C until use.
Immunohistochemistry
The sections were processed for double immunofluorescence after washing in KPBS as described previously (19, 24). A special procedure was used to visualize BrdU. Antigen retrieval was performed using a microwave. Briefly, the slides were slow boiled for 10 min, incubated in 0.01 m citrate buffer (pH 6.0) for 30 min, and rinsed in 0.02 m KPBS for 15 min. The sections were placed in 2% normal serum + 0.3% Triton X-100 overnight before incubation with either rat anti-BrdU (1:100; Abcam, Inc., Cambridge, MA) and mouse anti-human neuronal protein HuC/HuD (HuC/D) antibodies (neuronal marker; 1:250; Invitrogen, Carlsbad, CA) or rat anti-BrdU (1:100; Abcam) and rabbit anti-cFos antibodies (1:1000; Calbiochem, La Jolla, CA) for 48 h at 4 C in 2% normal serum + 0.3% Triton X-100. A goat antirat IgG conjugated to Alexa Fluor 488 (1:200; Invitrogen, Eugene, OR) was used to visualize the anti-BrdU. A goat antimouse or goat antirabbit IgG conjugated to Alexa Fluor 568 antibody (1:200; Invitrogen) was used to visualize the anti-HuC/D and anti-cFos antibodies, respectively. The sections were counterstained with bis-benzamide (1:3000; Invitrogen) to visualize cell nuclei and the morphological limits of each nucleus. The slides were coverslipped with buffered glycerol (pH 8.5) and observed under a Zeiss Imager Z1 microscope with a ×20 objective (Carl Zeiss, New York, NY).
Data analysis and quantification
Two independent observers analyzed the sections using a Zeiss Axio Imager Z1 microscope. Each region of interest was identified with the aid of a standard brain atlas, an in-house library of age-matched Nissl-stained sections, and a bis-benzamide counterstain (Figs. 1–5A). The number of immunoreactive cells for each animal was quantified manually using Image J (National Institutes of Health, Bethesda, MD) in three sections through the ARH, VMH, DMH, PVH, and LHA. Only brightly or heavily labeled neurons were considered to ensure the accurate determination of neuronal birthdate and proliferation. Only BrdU-positive cells with a corresponding bis-benzamide-stained nucleus were included in our quantification. Two categories of labeled cells were quantified: 1) relative numbers of BrdU-labeled cells and 2) relative numbers of double-labeled BrdU/HuC/D neurons. The relative numbers of cFos and cFos+BrdU were assessed in the ARH, VMH, DMH, LHA, and PVH of each group. The results are expressed as the percentage of BrdU/cFos nuclei among the cFos-immunopositive cells. Our measurements were not intended to provide absolute cell counts but rather to assess relative changes in cell numbers between experimental groups. Percentages of cFos-IR cells containing BrdU were compared between groups by two-way ANOVA with embryonic day and treatment group as factors. Fisher's least significant difference test was used for post hoc comparisons. A P ≥ 0.05 was defined as significant.
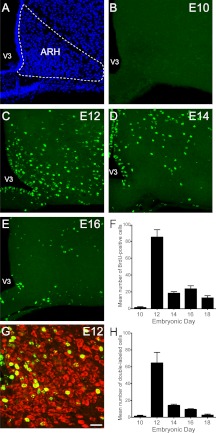
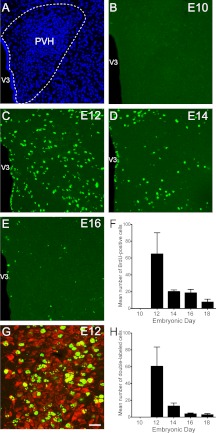
Fig. 1.
Embryonic birthdate of neurons in the ARH. A, Confocal image illustrating nuclear (bis-benzamide) staining used to recognize the morphological limits of the ARH. B–E, Confocal images illustrating the presence of BrdU (green)-positive cells in the ARH of P10 mice that were injected with BrdU at E10 (B), E12 (C), E14 (D), or E16 (E). F, Quantification of the numbers of BrdU-positive cells in the ARH of P10 mice that were injected with BrdU at E10, E12, E14, E16, or E18 (n = 4–6 per group; three sections per animal). G, Confocal image showing the presence of HuC/D immunoreactivity (a neuronal marker, red) in ARH BrdU (green)-positive cells of a P10 mouse that was injected with BrdU at E12. H, Quantification of the numbers of BrdU+HuC/D double-labeled cells in the ARH of P10 mice that were injected with BrdU at E10, E12, E14, E16, or E18 (n = 4–6 per group; three sections per animal). V3, Third ventricle. Values are the means ± sem. Scale bar, 50 μm (A–E) and 30 μm (G).
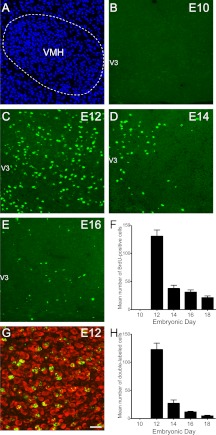
Fig. 2.
Embryonic birthdate of neurons in the VMH. A, Confocal image illustrating nuclear (bis-benzamide) staining used to recognize the morphological limits of the VMH. B–E, Confocal images showing the presence of BrdU (green)-positive cells in the VMH of P10 mice that were injected with BrdU at E10 (B), E12 (C), E14 (D), or E16 (E). F, Quantification of the numbers of BrdU-positive cells in the VMH of P10 mice that were injected with BrdU at E10, E12, E14, E16, or E18 (n = 4–6 per group; three sections per animal). G, Confocal image showing the presence of HuC/D immunoreactivity (a neuronal marker, red) in VMH BrdU (green)-positive cells of a P10 mouse that was injected with BrdU at E12. H, Quantification of the numbers of BrdU+HuC/D double-labeled cells in the VMH of P10 mice that were injected with BrdU at E10, E12, E14, E16, or E18 (n = 4–6 per group; three sections per animal). V3, Third ventricle. Values are the means ± sem. Scale bar, 50 μm (A–E) and 30 μm (G).
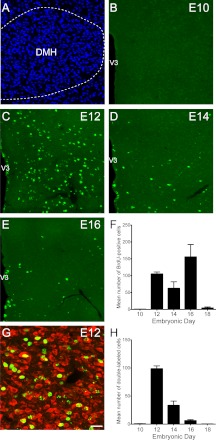
Fig. 3.
Embryonic birthdate of neurons in the DMH. A, Confocal image illustrating nuclear (bis-benzamide) staining used to recognize the morphological limits of the DMH. B–E, Confocal images showing the presence of BrdU (green)-positive cells in the DMH of P10 mice that were injected with BrdU at E10 (B), E12 (C), E14 (D), or E16 (E). F, Quantification of the numbers of BrdU-positive cells in the DMH of P10 mice that were injected with BrdU at E10, E12, E14, E16, or E18 (n = 4–6 per group; three sections per animal). G, Confocal image showing the presence of HuC/D immunoreactivity (a neuronal marker, red) in DMH BrdU green)-positive cells (of a P10 mouse that was injected with BrdU at E12. H, Quantification of the numbers of BrdU+HuC/D double-labeled cells in the DMH of P10 mice that were injected with BrdU at E10, E12, E14, E16, or E18 (n = 4–6 per group; three sections per animal). V3, Third ventricle. Values are the means ± sem. Scale bar, 50 μm (A–E) and 30 μm (G).
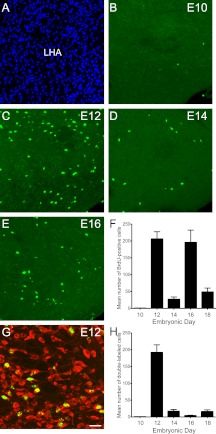
Fig. 4.
Embryonic birthdate of neurons in the lateral hypothalamic LHA. A, Confocal image illustrating nuclear (bis-benzamide) staining used to recognize the morphological limits of the LHA. B–E, Confocal images showing the presence of BrdU- (green)-positive cells in the LHA of P10 mice that were injected with BrdU at E10 (B), E12 (C), E14 (D), or E16 (E). F, Quantification of the number of BrdU-positive cells in the LHA of P10 mice that were injected with BrdU at E10, E12, E14, E16, or E18 (n = 4–6 per group; three sections per animal). G, Confocal image showing the presence of HuC/d-immunoreactivity (a neuronal marker, red) in LHA BrdU (green)-positive cells of a P10 mouse that was injected with BrdU at E12. H, Quantification of the number of BrdU+HuC/D double-labeled cells in the LHA of P10 mice that were injected with BrdU at E10, E12, E14, E16, or E18 (n = 4–6 per group; three sections per animal). V3, Third ventricle. Values are the means ± sem. Scale bar, 50 μm (A–E) and 30 μm (G).
Fig. 5.
Embryonic birthdate of neurons in the PVH. A, Confocal image illustrating nuclear (bis-benzamide) staining used to recognize the morphological limits of the PVH. B–E, Confocal images showing the presence of BrdU (green)-positive cells in the PVH of P10 mice that were injected with BrdU at E10 (B), E12 (C), E14 (D), or E16 (E). F, Quantification of the numbers of BrdU-positive cells in the PVH of P10 mice that were injected with BrdU at E10, E12, E14, E16, or E18 (n = 4–6 per group; three sections per animal). G, Confocal image showing the presence of HuC/D immunoreactivity (a neuronal marker, red) in PVH BrdU (green)-positive cells of a P10 mouse that was injected with BrdU at E12. H, Quantifications of the numbers of BrdU+HuC/D double-labeled cells in the PVH of P10 mice that were injected with BrdU at E10, E12, E14, E16, or E18 (n = 4–6 per group; three sections per animal). V3, Third ventricle. Values are the means ± sem. Scale bar, 50 μm (A–E) and 30 μm (G).
Results
Pregnant dams were injected with BrdU at 10, 12, 14, 16, or 18 d after conception to assess the birthdate of cells in key hypothalamic nuclei that are involved in energy balance regulation. The offspring were killed on either P10 (for BrdU and HuC/D labelings) or P60 (for leptin induced cFos), which is when nearly all cells have migrated to their final destinations and differentiated, as well as when the markers that we studied are expressed at adult levels. BrdU produced a stereotypical labeling of cell nuclei throughout the hypothalamus at all of the ages studied. However, the degree and timing of neurogenesis varied among the various hypothalamic nuclei.
Birthdate of neurons in the arcuate nucleus of the hypothalamus
Very few BrdU-labeled cells were identified in the ARH of mice that were injected with BrdU at E10 (Fig. 1, B and F). However, BrdU injection in E12-E18 animals produced distinctly labeled cells throughout the rostrocaudal and dorsoventral aspects of the nucleus. The animals that received BrdU on E12 exhibited the highest numbers of BrdU-labeled cells (average number of labeled cells at E12: 85 ± 9) (Fig. 1, C and F). The number of BrdU-positive cells in the ARH decreased by approximately 4- to 5-fold between E12 and E14 mice (average number of labeled cells at E14: 18 ± 2), and cell numbers remained relatively low in animals that received BrdU at older ages (average number of cells at E16: 23 ± 4; E18: 13 ± 3) (Fig. 1, D–F). We next performed double-labeling experiments using antibodies against HuC/D, a marker for differentiated neurons, to investigate the chemical phenotype of arcuate BrdU-positive cells. Approximately 60–70% of E12 and E14-labeled BrdU cells also contained HuC/D-immunoreactivity (Fig. 1, G and H). However, only 40 and 20% of E16- and E18-labeled BrdU cells were HuC/D-immunopositive, respectively (Fig. 1H). These results indicate that neurons in the ARH were generated between E12 and E18, with a peak of neurogenesis occurring at E12.
Birthdate of neurons in the ventromedial nucleus of the hypothalamus
No BrdU-labeled cells were observed in the VMH of mice that received BrdU on E10 (Fig. 2, B and F). In contrast, animals that received BrdU on E12-E18 exhibited labeled cells throughout the VMH. The animals that received BrdU on E12 exhibited the highest numbers of BrdU-labeled cells (average number of labeled cells at E12: 130 ± 11) (Fig. 2, C and F), which is similar to the ARH results. The numbers of BrdU-labeled cells on E14-E18 were moderate and 3–6 times lower than those in the E12 animals (average number of cells at E14: 37 ± 6; E16: 30 ± 4; and E18: 21 ± 3) (Fig. 2, D–F). Double-labeling experiments using the neuronal marker HuC/D revealed that 90 and 70% of BrdU cells that were labeled on E12 and E14, respectively, also contained HuC/D immunoreactivity (Fig. 2, G and H). However, only 40 and 20% of BrdU cells labeled on E16 and E18, respectively, were HuC/D immunopositive (Fig. 2H). These data show that VMH neurons were generated between E12 and E18 with a peak on E12. Notably, each subdivision of the VMH followed the same temporal pattern of neurogenesis with a peak of neuronal formation occurring on E12 (Table 1).
Table 1.
Mean number of BrdU+HuC/D double-labeled cells in distinct anatomical components of the VMH and PVH
| E10 | E12 | E14 | E16 | E18 | |
|---|---|---|---|---|---|
| VMH | |||||
| Dorsomedial part | 0 ± 0 | 59.8 ± 11.5 | 22.5 ± 5.7 | 4.0 ± 1.8 | 2.3 ± 1.3 |
| Central part | 0 ± 0 | 37.5 ± 5.0 | 3.0 ± 0.7 | 3.8 ± 1.5 | 1.5 ± 0.9 |
| Ventrolateral part | 0 ± 0 | 25.2 ± 3.0 | 1.2 ± 0.9 | 3.7 ± 0.9 | 0.75 ± 0.75 |
| PVN | |||||
| Posterior magnocellular part, lateral zone | 0 ± 0 | 14.7 ± 1.9 | 0.2 ± 0.2 | 0.5 ± 0.5 | 0.7 ± 0.3 |
| Medial parvicellular part, ventral zone | 0 ± 0 | 14.0 ± 3.8 | 0.8 ± 0.2 | 1.2 ± 0.5 | 1.0 ± 0.0 |
| Medial parvicellular part, dorsal zone | 0 ± 0 | 35.0 ± 6.1 | 5.2 ± 2.7 | 1.5 ± 0.6 | 2.0 ± 1.5 |
| Dorsal parvicellular part | 0 ± 0 | 16.7 ± 5.7 | 6.8 ± 1.9 | 0.5 ± 0.5 | 0 ± 0 |
Birthdate of neurons in the dorsomedial nucleus of the hypothalamus
Virtually no BrdU-labeled cells were identified in the DMH of mice that received BrdU on E10 (Fig. 3, B and F). The injection of BrdU on E12-E16 produced a high number of labeled cells in the DMH (average number of cells at E12: 105 ± 6; E14: 62 ± 20; and E16: 155 ± 37) (Fig. 3, C–F). However, few BrdU-labeled cells were observed in the DMH of animals that received BrdU on E18 (average number of cells at E18: 4 ± 3) (Fig. 3F). Double-labeling experiments show that 90 and 60% of cells that were labeled with BrdU on E12 and E14 displayed HuC/ D-IR, respectively (Fig. 3, G and H). However, only a fraction (<5%) of the DMH cells that were generated on E16 acquired a neuronal phenotype. Similarly, less than 2% of DMH BrdU cells that were labeled with BrdU on E18 displayed HuC/D immunoreactivity (Fig. 3H). These data show that the majority of neurons in the DMH were born between E12 and E14.
Birthdate of neurons located in the LHA
No BrdU-labeled cells were observed in the LHA of mice that received BrdU on E10 (Fig. 4, B and F). However, clear BrdU incorporation was identified in the LHA of animals that received BrdU on E12-E18. The highest numbers of BrdU-labeled cells were identified in animals that received BrdU on E12 (average number of labeled cells at E12: 206 ± 21) (Fig. 4, C and F). The number of BrdU-positive cells in the LHA decreased by approximately 8-fold between E12 and E14 mice (average number of labeled cells at E14: 26 ± 7). Animals that received BrdU on E16 displayed high numbers of positive cells in the LHA (average number of cells at E16: 195 ± 36) (Fig. 4, D–F), and the number of BrdU-immunoreactive cells was dramatically reduced on E18 (average number of cells at E18: 48 ± 11) (Fig. 4F). Double-labeling experiments using the HuC/D and BrdU antibodies demonstrated that approximately 90 and 60% of the cells that were generated on E12 and E14 acquired a neuronal phenotype, respectively (Fig. 4, G and H). However, less than 3% of the BrdU-positive cells that were generated on E16 were HuC/D immunopositive (Fig. 4H). Approximately 30% of the cells in the LHA that were generated on E18 displayed a neuronal phenotype (Fig. 4H). These results indicated that cells in the LHA were generated primarily during two sharp peaks on E12 and E16 but that neurogenesis in the LHA was largely restricted on E12.
Birthdate of neurons in the paraventricular nucleus of the hypothalamus
No BrdU-labeled cells were observed in the PVH of mice that received BrdU on E10 (Fig. 5, B and F). In contrast, numerous BrdU-labeled cells were identified in the VMH of animals that received BrdU on E12-E18. Animals that received BrdU on E12 exhibited the highest numbers of BrdU-labeled cells in the PVH (average number of labeled cells at E12: 64 ± 25) (Fig. 5, C and F). The numbers of BrdU-labeled cells in animals that received BrdU on E14-E16 were moderate and approximately three times lower than those in E12 animals (average number of cells at E14: 20 ± 2; E16: 18 ± 4) (Fig. 5, D–F). The numbers of labeled cells in the PVH further decreased in the animals that received BrdU on E18 (average number of cells at E18: 7 ± 3) (Fig. 5F). Double-labeling experiments using the neuronal marker HuC/D demonstrated that 70 and 60% of BrdU-positive cells that were labeled on E12 and E14 also contained HuC/D immunoreactivity, respectively (Fig. 5, G and H). However, only 20 and 30% of BrdU-positive cells that were labeled on E16 and E18 were HuC/D immunopositive, respectively (Fig. 5H). These data show that PVH neurons were generated between E12 and E18, with a sharp peak on E12. Notably, each subdivision of the PVH followed the same temporal pattern of neurogenesis with a peak of neuronal formation occurring on E12 (Table 1).
Birthdate of leptin-activated neurons in the hypothalamus
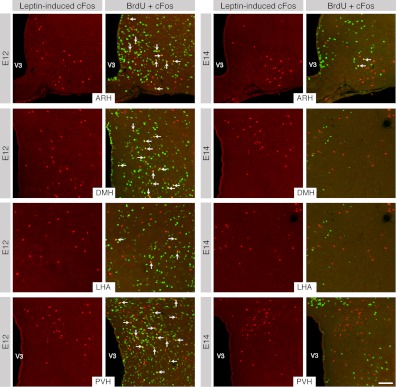
Leptin primarily acts within the hypothalamus to mediate its actions on feeding and body weight regulation (2, 25, 26). We repeated the BrdU injections described above and also injected the adult animals with either leptin (3 mg/kg, ip) or vehicle alone to determine when hypothalamic neurons that convey leptin signaling during adult life are born. The brains were stained for cFos because changes in cFos staining are generally taken to represent an increase in neuronal activity that can be conveyed either directly by leptin or through transynaptic activation (27). Leptin treatment produced a marked induction of cFos-immunoreactivity in the ARH, DMH, LHA, and PVH of adult mice (Supplemental Figs. 1 and 2, published on The Endocrine Society's Journals Online web site at http://endo.endojournals.org) as reported previously (19, 28). Double-staining experiments with BrdU indicated that 30–50% of leptin-sensitive neurons in the adult hypothalamus, including in the ARH, DMH, LHA, and PVH, were born during a short developmental period that is restricted on E12 (Figs. 6 and 7). Only a small proportion of hypothalamic leptin-activated cells (1–10%) were born during later embryonic stages (Figs. 6 and 7).
Fig. 6.
Embryonic birthdate of leptin-activated neurons. Confocal images showing leptin-induced cFos immunoreactivity (red), BrdU immunoreactivity (green), and cFos+BrdU double staining (yellow) in the ARH, DMH, LHA, and PVH of P60 mice that were injected with BrdU at E12 or E14. V3, Third ventricle. Scale bar, 50 μm.
Fig. 7.
Birthdate of hypothalamic leptin-activated neurons. Quantification of the percentage of cFos-immunoreactive cells containing BrdU in the ARH (A), DMH (B), LHA (C), and PVH (D) of adult mice that were injected with BrdU at E12, E14, or E16 and with leptin (3 mg/kg) or vehicle at P60 (n = 4 per group; three sections per animal). Values are the means ± sem. *, P < 0.05 vs. vehicle; #, P < 0.05 vs. E12.
Discussion
The developmental process of a functional hypothalamic nucleus begins with the birth or terminal mitosis of the neurons within that nucleus. In their landmark analyses of the fetal rodent hypothalamus, Altman and Bayer (20) and Shimada and Nakamura (21) reported using [3H]thymidine, which indicated that cells in the hypothalamic nuclei are primarily derived from precursors that originate in the proliferative zone surrounding the lower portion of the third ventricle. This proliferative zone is also known as the neuroepithelium of the third ventricle. These authors further demonstrated that numerous cells in the hypothalamus were born embryonically in rats (20) and mice (21). These studies have provided valuable information on the numbers and timing of hypothalamic cell production, but they have not investigated the chemical identity of the cells that are born at a given time. The present study used BrdU to examine the birthdate of hypothalamic neurons in nuclei that play a key role in energy balance. Moreover, we performed a detailed analysis of embryonic hypothalamic neurogenesis in the mouse, which is a widely used model in molecular genetic studies of feeding physiology. The advantages of using BrdU, instead of [3H]thymidine, include a lower background, intranuclear labeling, and the visualization of the uridine analog with immunofluorescence, which provides a convenient, simultaneous visualization of multiple markers. It is worth noting that high doses of BrdU can lead to teratogenic malformations and behavioral changes. However, we did not observe any major physiological or behavioral alterations nor any gross morphological changes in our BrdU-injected animals. These observations suggest that it was unlikely that the regime of BrdU administration used in these studies caused significant neurotoxicity.
Our present findings show that the vast majority of neurons in hypothalamic nuclei that control energy homeostasis in mice are born between E12 and E14 with peak birthdates on E12. These findings suggest that the proliferation rate in the neuroepithelium of the third ventricle is high at E12 and decreases after E14. They also contrast with those reported in rats, which exhibit a relatively long neurogenic period that is initiated on E11 and continues until E17, with peak birthdates on E13-E15 (17, 20). Reports on human fetal chemoarchitecture and cytoarchitecture have also suggested that early hypothalamic neurogenesis is limited to the ninth and 10th weeks of gestation (29–33).
The chemical phenotype of the nonneuronal HuC/D- BrdU+ cells remains to be determined. Only a minority of glial fibrillary acidic protein-positive astrocytes was born embryonically (data not shown). However, based on the observation that the hypothalamus is one of the most vascularized brain region, it is likely that some of these nonneuronal cells born embryonically become vascular endothelial cells. Remarkably, the majority of leptin-activated cells in the adult hypothalamus (as revealed by cFos-IR after leptin administration) were born during a developmental period that was largely restricted to E12. We chose to use cFos staining to detect leptin-activated cells, instead of more direct leptin receptor signaling markers such as phosphorylated signal transducer and activator of transcription-3 or phosphorylated ERK1/2 because this protooncogene labels neurons that are both directly and transynaptically activated by leptin and identifies both first- and second-order leptin-activated neurons. Brischoux et al. (34) previously reported that MCH neurons in the rat LHA were born between E12 and E13 and that MCH mRNA was detected in the LHA as early as E13. Moreover, recent birthdating studies indicate that POMC neurons in the ARH are born primarily on E12, and in situ hybridization studies have demonstrated that neurons express POMC mRNA on E12 (35, 36). These anatomical observations are consistent with an early determination of cell fate. In addition, specific sets of hypothalamic neurons may share a common ontological lineage. For example, Padilla et al. (36) showed that a subpopulation of embryonic Pomc-expressing precursors subsequently adopts a NPY phenotype in adult mice. These studies also identified an important developmental period when alterations in the intrauterine environment may affect hypothalamic neurogenesis and produce enduring structural consequences in the hypothalamus.
A second important hypothalamic developmental period in rodents occurs during the first few weeks of postnatal life when neurons send their axonal projections to their target sites. Projections from the ARH in mice and rats are immature at birth and primarily develop postnatally. The development of axonal projections from the ARH to each of their target nuclei is not initiated until P6, and these projections are not fully mature, i.e. they do not resemble an adult, until P18 (19). Similar results have been observed for the development of AgRP-containing fibers in mice and rats (37, 38), which originate exclusively from NPY-containing neurons in the ARH (39, 40). Various parts of the hypothalamus extend axonal projections at different times. For example, efferent projections from the DMH and VMH develop before projections from the ARH, and these connections are established by P6 (19). These previous studies and the present data indicate that the developing hypothalamus is exposed to two different and successive environments: an intrauterine environment in which hypothalamic cell numbers are determined, and an extrauterine environment in which neuronal connections are being established. These developmental windows also represent periods of vulnerability for hypothalamic development, and alterations in the neonatal environment may perturb normal hypothalamic development and subsequent function.
One unique property of hypothalamic development compared with the development of other brain structures, such as the cortex and hippocampus, is that this region is largely activity independent; it is controlled by physiological signals that reflect environmental conditions. Alterations in the hormonal or nutritional environment during critical periods of development may exert lasting effects on the architecture of the hypothalamic circuits that regulate feeding (see Refs. 14, 15, 41, and 42 for reviews). For example, maternal high-fat feeding increases hypothalamic cellular proliferation in rats, which yields higher numbers of neurons that contain orexigenic neuropeptides, such as galanin, enkephalin, dynorphin, MCH, and orexin, in the PVH and LHA (43). In contrast maternal malnutrition is associated with a reduced number of orexigenic neurons in the hypothalamus of the offspring, including a reduction of NPY-immunoreactive neurons in the ARH (44). Whether this change in neuronal cell numbers is the result of a reduction of neurogenesis and cellular migration or an enhancement of programmed cell death must be investigated.
The capacity of hypothalamic cells to generate neurons is not restricted to embryonic life, as recent evidence has suggested that neurogenesis occurs in the adult hypothalamus (45). However, the degree of hypothalamic neurogenesis differs markedly between adults and embryos. Low rates of neurogenesis are observed in the mature hypothalamus under basal conditions compared with neurogenesis in other neurogenic structures, such as the hippocampus and the subventricular zone (45, 46). However, constitutive hypothalamic neurogenesis may be enhanced by external cues. Injections of ciliary neurotrophic factor (CNTF) into adult obese mice induce marked neurogenesis in the hypothalamus (47). This CNTF-induced neurogenesis has consequences on energy balance regulation, and it participates in the weight loss effects of CNTF in obese mice (47). Hypothalamic neurogenesis can also be induced in neurodegenerative conditions, such as in mutant mice in which AgRP neurons undergo progressive neurodegeneration due to the deletion of mitochondrial transcription factor A (48). These data illustrate the functional relevance of adult neurogenesis and indicate that it may also serve as a compensatory mechanism that contributes to the plastic control of energy balance in response to environmental insults.
In conclusion, the results of these studies provide detailed and novel information regarding the spatiotemporal pattern of neuronal birth in metabolically relevant hypothalamic nuclei in mice. These morphological data identified an important developmental period that was largely restricted to E12, which is when alterations of the intrauterine environment may affect hypothalamic neurogenesis and produce long-term consequences on the number of hypothalamic neurons that are involved in feeding and metabolism.
Supplementary Material
Acknowledgments
We thank Mrs. Li Liu for her expert technical assistance.
This work was supported by National Institute of Health Grant DK84142 (to S.G.B.), the Fondation pour la Recherche Médicale (to S.G.B.), the European Union FP7 integrated project (Grant Agreement 266408, “Full4Health,” to S.G.B.), and the Agence Nationale de la Recherche Grant ANR-08-JCJC-0055-01 (to S.G.B.).
Disclosure Summary: The authors (Y.I. and S.G.B.) have nothing to disclose.
Footnotes
- AgRP
- Agouti-related peptide
- ARH
- arcuate nucleus
- BrdU
- 5-bromo-2′-deoxyuridine
- CNTF
- ciliary neurotrophic factor
- DMH
- dorsomedial nucleus
- E
- embryonic day
- HuC/D
- human neuronal protein HuC/HuD
- KPBS
- potassium PBS
- LHA
- lateral hypothalamic area
- MCH
- melanin-concentrating hormone
- NPY
- neuropeptide Y
- P
- postnatal day
- POMC
- proopiomelanocortin
- PVH
- paraventricular nucleus
- VMH
- ventromedial nucleus.
References
- 1. Hetherington AW, Ranson SW. 1940. Hypothalamic lesions and adiposity in the rat. Anat Rec 78 149–172 [Google Scholar]
- 2. Elmquist JK, Coppari R, Balthasar N, Ichinose M, Lowell BB. 2005. Identifying hypothalamic pathways controlling food intake, body weight, and glucose homeostasis. J Comp Neurol 493:63–71 [DOI] [PubMed] [Google Scholar]
- 3. Dhillon H, Zigman JM, Ye C, Lee CE, McGovern RA, Tang V, Kenny CD, Christiansen LM, White RD, Edelstein EA, Coppari R, Balthasar N, Cowley MA, Chua S, Jr, Elmquist JK, Lowell BB. 2006. Leptin directly activates SF1 neurons in the VMH, and this action by leptin is required for normal body-weight homeostasis. Neuron 49:191–203 [DOI] [PubMed] [Google Scholar]
- 4. Ring LE, Zeltser LM. 2010. Disruption of hypothalamic leptin signaling in mice leads to early-onset obesity, but physiological adaptations in mature animals stabilize adiposity levels. J Clin Invest 120:2931–2941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hill JW, Elias CF, Fukuda M, Williams KW, Berglund ED, Holland WL, Cho YR, Chuang JC, Xu Y, Choi M, Lauzon D, Lee CE, Coppari R, Richardson JA, Zigman JM, Chua S, Scherer PE, Lowell BB, Brüning JC, Elmquist JK. 2010. Direct insulin and leptin action on pro-opiomelanocortin neurons is required for normal glucose homeostasis and fertility. Cell Metabolism 11:286–297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Balthasar N, Dalgaard LT, Lee CE, Yu J, Funahashi H, Williams T, Ferreira M, Tang V, McGovern RA, Kenny CD, Christiansen LM, Edelstein E, Choi B, Boss O, Aschkenasi C, Zhang CY, Mountjoy K, Kishi T, Elmquist JK, Lowell BB. 2005. Divergence of melanocortin pathways in the control of food intake and energy expenditure. Cell 123:493–505 [DOI] [PubMed] [Google Scholar]
- 7. Segal-Lieberman G, Bradley RL, Kokkotou E, Carlson M, Trombly DJ, Wang X, Bates S, Myers MG, Jr, Flier JS, Maratos-Flier E. 2003. Melanin-concentrating hormone is a critical mediator of the leptin-deficient phenotype. Proc Natl Acad Sci USA 100:10085–10090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sawchenko PE. 1998. Toward a new neurobiology of energy balance, appetite, and obesity: the anatomists weigh in. J Comp Neurol 402:435–441 [PubMed] [Google Scholar]
- 9. Berthoud HR. 2006. Homeostatic and non-homeostatic pathways involved in the control of food intake and energy balance. Obesity 14:197S–200S [DOI] [PubMed] [Google Scholar]
- 10. Bouret SG, Gorski JN, Patterson CM, Chen S, Levin BE, Simerly RB. 2008. Hypothalamic neural projections are permanently disrupted in diet-induced obese rats. Cell Metab 7:179–185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Coupé B, Amarger V, Grit I, Benani A, Parnet P. 2010. Nutritional programming affects hypothalamic organization and early response to leptin. Endocrinology 151:702–713 [DOI] [PubMed] [Google Scholar]
- 12. Kirk SL, Samuelsson AM, Argenton M, Dhonye H, Kalamatianos T, Poston L, Taylor PD, Coen CW. 2009. Maternal obesity induced by diet in rats permanently influences central processes regulating food intake in offspring. PLoS ONE 4:e5870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Horvath TL, Bruning JC. 2006. Developmental programming of the hypothalamus: a matter of fat. Nat Med 12:52–53 [DOI] [PubMed] [Google Scholar]
- 14. Levin B. 2006. Metabolic imprinting: critical impact of the perinatal environment on the regulation of energy homeostasis. Philos Trans R Soc Lond B Biol Sci 361:1107–1121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Plagemann A. 2006. Perinatal nutrition and hormone-dependent programming of food intake. Horm Res 65:83–89 [DOI] [PubMed] [Google Scholar]
- 16. Martin-Gronert MS, Ozanne SE. 2005. Programming of appetite and type 2 diabetes. Early Hum Dev 81:981–988 [DOI] [PubMed] [Google Scholar]
- 17. Markakis EA. 2002. Development of the neuroendocrine hypothalamus. Front Neuroendocrinol 23:257–291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bouret SG. 2010. Development of hypothalamic neural networks controlling appetite. Forum Nutr 63:84–93 [DOI] [PubMed] [Google Scholar]
- 19. Bouret SG, Draper SJ, Simerly RB. 2004. Formation of projection pathways from the arcuate nucleus of the hypothalamus to hypothalamic regions implicated in the neural control of feeding behavior in Mice. J Neurosci 24:2797–2805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Altman J, Bayer SA. 1986. The development of the rat hypothalamus. Adv Anat Embryol Cell Biol 100:1–178 [PubMed] [Google Scholar]
- 21. Shimada M, Nakamura T. 1973. Time of neuron origin in mouse hypothalamic nuclei. Exp Neurol 41:163–173 [DOI] [PubMed] [Google Scholar]
- 22. Ifft JD. 1972. An autoradiographic study of the time of final division of neurons in rat hypothalamic nuclei. J Comp Neurol 144:193–204 [DOI] [PubMed] [Google Scholar]
- 23. Markakis EA, Swanson LW. 1997. Spatiotemporal patterns of secretomotor neuron generation in the parvicellular neuroendocrine system. Brain Res Rev 24:255–291 [DOI] [PubMed] [Google Scholar]
- 24. Caron E, Sachot C, Prevot V, Bouret SG. 2010. Distribution of leptin-sensitive cells in the postnatal and adult mouse brain. J Comp Neurol 518:459–476 [DOI] [PubMed] [Google Scholar]
- 25. Myers MG, Jr, Münzberg H, Leinninger GM, Leshan RL. 2009. The geometry of leptin action in the brain: more complicated than a simple ARC. Cell Metab 9:117–123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ahima RS, Saper CB, Flier JS, Elmquist JK. 2000. Leptin regulation of neuroendocrine systems. Front Neuroendocrinol 21:263–307 [DOI] [PubMed] [Google Scholar]
- 27. Armstrong RC, Montminy MR. 1993. Transsynaptic control of gene expression. Annu Rev Neurosci 16:17–29 [DOI] [PubMed] [Google Scholar]
- 28. Elmquist JK, Ahima RS, Maratos-Flier E, Flier JS, Saper CB. 1997. Leptin activates neurons in ventrobasal hypothalamus and brainstem. Endocrinology 138:839–842 [DOI] [PubMed] [Google Scholar]
- 29. Bugnon C, Fellmann D, Bresson JL, Clavequin MC. 1982. Immunocytochemical study of the ontogenesis of the CRH-containing neuroglandular system in the human hypothalamus. C R Seances Acad Sci III 294:599–604 [PubMed] [Google Scholar]
- 30. Burford GD, Robinson IC. 1982. Oxytocin, vasopressin and neurophysins in the hypothalamo-neurohypophysial system of the human fetus. J Endocrinol 95:403–408 [DOI] [PubMed] [Google Scholar]
- 31. Ackland J, Ratter S, Bourne GL, Rees LH. 1983. Characterization of immunoreactive somatostatin in human fetal hypothalamic tissue. Regul Pept 5:95–101 [DOI] [PubMed] [Google Scholar]
- 32. Mai JK, Lensing-Höhn S, Ende AA, Safroniew MV. 1997. Developmental organization of neurophysin neurons in the human brain. J Comp Neurol 385:477–489 [PubMed] [Google Scholar]
- 33. Koutcherov Y, Mai JK, Ashwell KW, Paxinos G. 2002. Organization of human hypothalamus in fetal development. J Comp Neurol 446:301–324 [DOI] [PubMed] [Google Scholar]
- 34. Brischoux F, Fellman D, Risold PY. 2001. Ontogenetic development of the diencephalic MCH neurons: a hypothalamic 'MCH area' hypothesis. Eur J Neurosci 13:1733–1744 [DOI] [PubMed] [Google Scholar]
- 35. Khachaturian H, Alessi NE, Lewis ME, Munfakh N, Fitzsimmons MD, Watson SJ. 1985. Development of hypothalamic opioid neurons: a combined immunocytochemical and [3H]thymidine autoradiographic study. Neuropeptides 5:477–480 [DOI] [PubMed] [Google Scholar]
- 36. Padilla SL, Carmody JS, Zeltser LM. 2010. Pomc-expressing progenitors give rise to antagonistic neuronal populations in hypothalamic feeding circuits. Nat Med 16:403–405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Grove KL, Allen S, Grayson BE, Smith MS. 2003. Postnatal development of the hypothalamic neuropeptide Y system. Neuroscience 116:393–406 [DOI] [PubMed] [Google Scholar]
- 38. Nilsson I, Johansen JE, Schalling M, Hökfelt T, Fetissov SO. 2005. Maturation of the hypothalamic arcuate agouti-related protein system during postnatal development in the mouse. Dev Brain Res 155:147–154 [DOI] [PubMed] [Google Scholar]
- 39. Broberger C, Johansen J, Johansson C, Schalling M, Hökfelt T. 1998. The neuropeptide Y/agouti gene-related protein (AGRP) brain circuitry in normal, anorectic, and monosodium glutamate-treated mice. Proc Natl Acad Sci USA 95:15043–15048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Bagnol D, Lu XY, Kaelin CB, Day HE, Ollmann M, Gantz I, Akil H, Barsh GS, Watson SJ. 1999. Anatomy of an endogenous antagonist: relationship between Agouti-related protein and proopiomelanocortin in brain. J Neurosci 19:RC26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Bouret SG. 2010. Role of early hormonal and nutritional experiences in shaping feeding behavior and hypothalamic development. J Nutr 140:653–657 [DOI] [PubMed] [Google Scholar]
- 42. Sullivan EL, Grove KL. 2010. Metabolic imprinting in obesity. Forum Nutr 63:186–194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Chang GQ, Gaysinskaya V, Karatayev O, Leibowitz SF. 2008. Maternal high-fat diet and fetal programming: increased proliferation of hypothalamic peptide-producing neurons that increase risk for overeating and obesity. J Neurosci 28:12107–12119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. García AP, Palou M, Priego T, Sánchez J, Palou A, Picó C. 2010. Moderate caloric restriction during gestation results in lower arcuate nucleus NPY- and alpha MSH-neurons and impairs hypothalamic response to fed/fasting conditions in weaned rats. Diabetes Obes Metab 12:403–413 [DOI] [PubMed] [Google Scholar]
- 45. Kokoeva MV, Yin H, Flier JS. 2007. Evidence for constitutive neural cell proliferation in the adult murine hypothalamus. J Comp Neurol 505:209–220 [DOI] [PubMed] [Google Scholar]
- 46. Zhao C, Deng W, Gage FH. 2008. Mechanisms and functional implications of adult neurogenesis. Cell 132:645–660 [DOI] [PubMed] [Google Scholar]
- 47. Kokoeva MV, Yin H, Flier JS. 2005. Neurogenesis in the hypothalamus of adult mice: potential role in energy balance. Science 310:679–683 [DOI] [PubMed] [Google Scholar]
- 48. Pierce AA, Xu AW. 2010. De novo neurogenesis in adult hypothalamus as a compensatory mechanism to regulate energy balance. J Neurosci 30:723–730 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.