Abstract
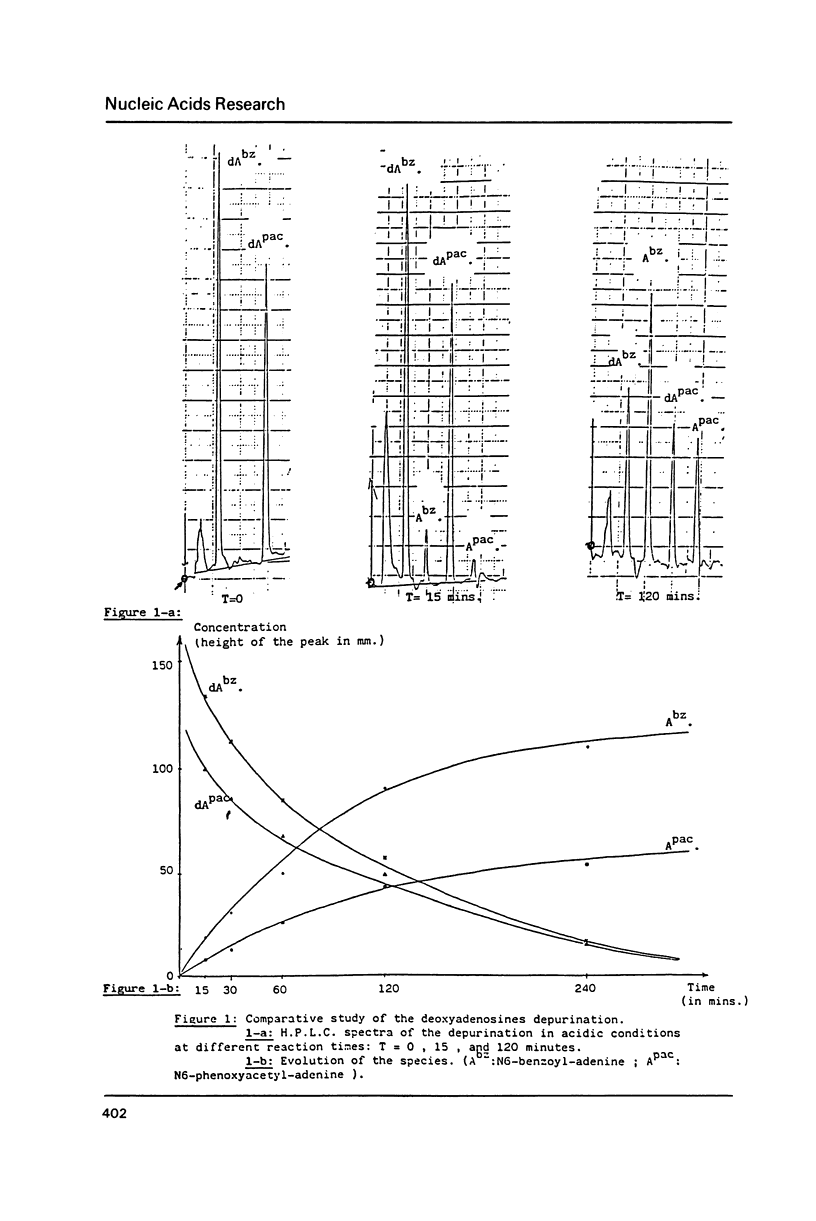
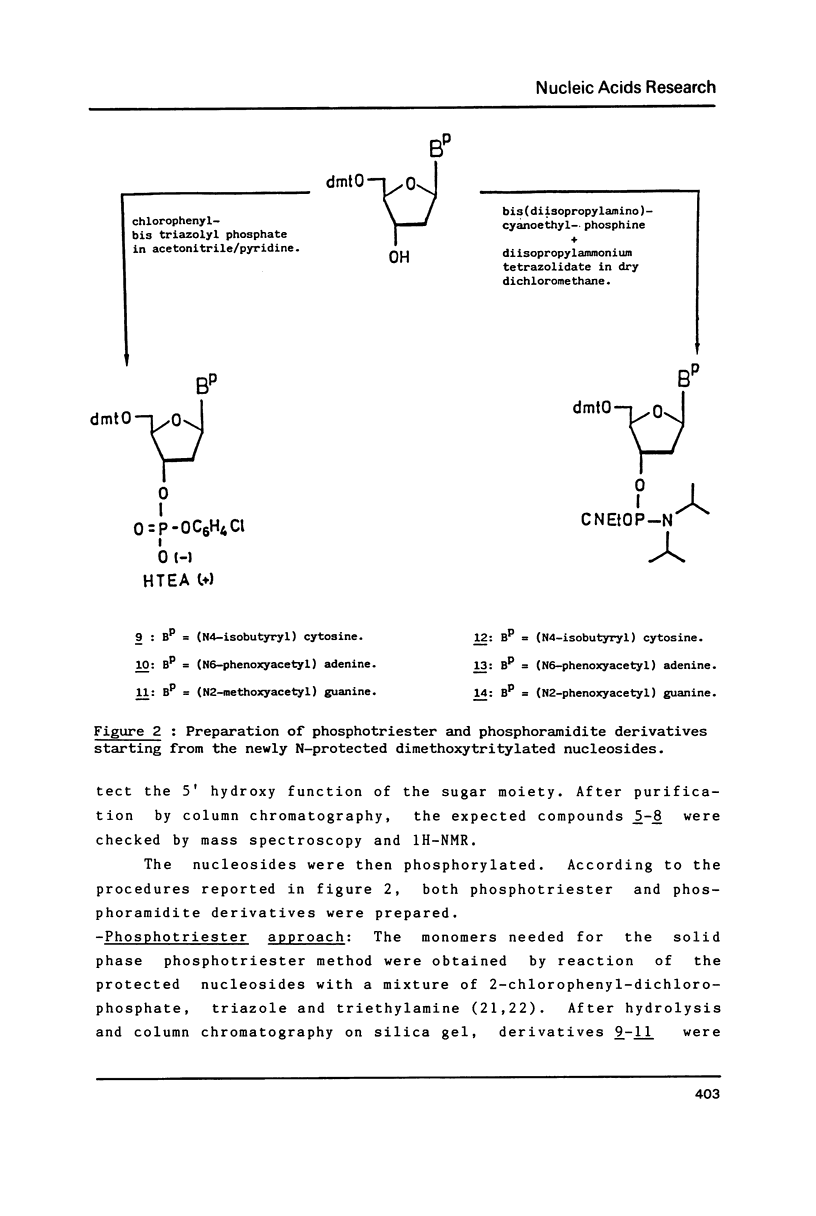
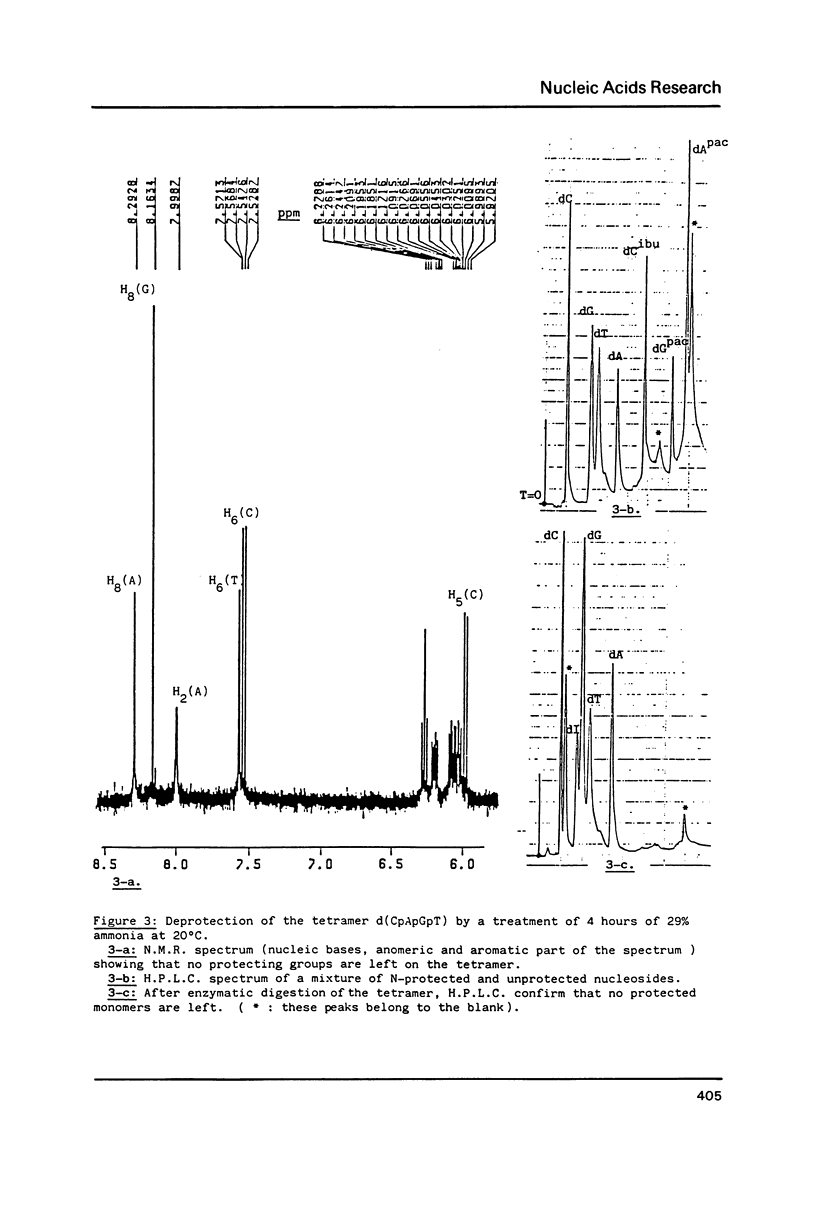
Phenoxyacetyl (pac) and methoxyacetyl (mac) for adenine and guanine, isobutyryl for cytosine, were successfully applied as amino protecting groups both in phosphotriester and phosphoramidite approaches. As shown by N.M.R. and H.P.L.C. analysis, they are completely deblocked in less than four hours in 29% ammonia at room temperature allowing the preparation of modified DNA containing alkali labile bases such as saturated pyrimidines. The stability of N6-phenoxyacetyl-deoxyadenosine versus depurination in acidic conditions used in the detritylation step was favorably compared with that of the classic N6-benzoyl protected adenine.
Full text
PDF















Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Banaszuk A. M., Deugau K. V., Sherwood J., Michalak M., Glick B. R. An efficient method for the sequence analysis of oligodeoxyribonucleotides. Anal Biochem. 1983 Feb 1;128(2):281–286. doi: 10.1016/0003-2697(83)90376-7. [DOI] [PubMed] [Google Scholar]
- Barone A. D., Tang J. Y., Caruthers M. H. In situ activation of bis-dialkylaminophosphines--a new method for synthesizing deoxyoligonucleotides on polymer supports. Nucleic Acids Res. 1984 May 25;12(10):4051–4061. doi: 10.1093/nar/12.10.4051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Büchi H., Khorana H. G. CV. Total synthesis of the structural gene for an alanine transfer ribonucleic acid from yeast. Chemical synthesis of an icosadeoxyribonucleotide corresponding to the nucleotide sequence 31 to 50. J Mol Biol. 1972 Dec 28;72(2):251–288. doi: 10.1016/0022-2836(72)90148-9. [DOI] [PubMed] [Google Scholar]
- Efimov V. A., Reverdatto S. V., Chakhmakhcheva O. G. New effective method for the synthesis of oligonucleotides via phosphotriester intermediates. Nucleic Acids Res. 1982 Nov 11;10(21):6675–6694. doi: 10.1093/nar/10.21.6675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Froehler B. C., Matteucci M. D. Dialkylformamidines: depurination resistant N6-protecting group for deoxyadenosine. Nucleic Acids Res. 1983 Nov 25;11(22):8031–8036. doi: 10.1093/nar/11.22.8031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heikkilä J., Balgobin N., Chattopadhyaya J. The 2-nitrophenylsulfenyl (Nps) group for the protection of amino functions of cytidine, adenosine, guanosine and their 2'-deoxysugar derivatives. Acta Chem Scand B. 1983;37(9):857–862. doi: 10.3891/acta.chem.scand.37b-0857. [DOI] [PubMed] [Google Scholar]
- Kume A., Iwase R., Sekine M., Hata T. Cyclic diacyl groups for protection of the N6-amino group of deoxyadenosine in oligodeoxynucleotide synthesis. Nucleic Acids Res. 1984 Nov 26;12(22):8525–8538. doi: 10.1093/nar/12.22.8525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Mishra R. K., Misra K. Improved synthesis of oligodeoxyribonucleotide using 3-methoxy-4-phenoxybenzoyl group for amino protection. Nucleic Acids Res. 1986 Aug 11;14(15):6197–6213. doi: 10.1093/nar/14.15.6197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyoshi K., Itakura K. Solid-phase synthesis of polynucleotides: V. Synthesis of oligodeoxyribonucleotides by the phosphomonotriazolide method. Nucleic Acids Symp Ser. 1980;(7):281–291. [PubMed] [Google Scholar]
- Sinha N. D., Biernat J., McManus J., Köster H. Polymer support oligonucleotide synthesis XVIII: use of beta-cyanoethyl-N,N-dialkylamino-/N-morpholino phosphoramidite of deoxynucleosides for the synthesis of DNA fragments simplifying deprotection and isolation of the final product. Nucleic Acids Res. 1984 Jun 11;12(11):4539–4557. doi: 10.1093/nar/12.11.4539. [DOI] [PMC free article] [PubMed] [Google Scholar]