Abstract
Cervical cancer is the second most common cancer in women worldwide. Persistent high-risk human papillomavirus (HPV) infection has been identified as the causative event for the development of this type of cancer. Recombinant adeno-associated viruses (rAAVs) are currently being developed and evaluated as vaccine vector. In previous work, we demonstrated that rAAVs administered intranasally in mice induced high titers and long-lasting neutralizing antibodies against HPV type 16 (HPV16). To extend this approach to a more human-related species, we immunized rhesus macaques (Macaca mulatta) with AAVs expressing an HPV16 L1 protein using rAAV5 and 9 vectors in an intranasal prophylactic setting. An rAAV5-L1 vector followed by a boost with rAAV9-L1 induced higher titers of L1-specific serum antibodies than a single rAAV5-L1 immunization. L1-specific antibodies elicited by AAV9 vector neutralized HPV16 pseudovirions and persisted for at least 7 months post immunization. Interestingly, nasal application of rAAV9 was immunogenic even in the presence of high AAV9 antibody titers, allowing reimmunization with the same serotype without prevention of the transgene expression. Two of six animals did not respond to AAV-mediated intranasal vaccination, although they were not tolerant, as both developed antibodies after intramuscular vaccination with HPV16 virus-like particles. These data clearly show the efficacy of an intranasal immunization using rAAV9-L1 vectors without the need of an adjuvant. We conclude from our results that rAAV9 vector is a promising candidate for a noninvasive nasal vaccination strategy.
In this study, Nieto and colleagues examine in rhesus macaques the impact of an adeno-associated viral (AAV) vector-based intranasal vaccine regimen on the induction of prophylactic immune responses against human papillomavirus (HPV). They demonstrate that intranasal immunization using AAV5 as the prime vector followed by AAV9 induces strong humoral responses against HPV16 and show that this approach can neutralize HPV16 pseudovirions.
Introduction
Despite the available knowledge and tools to reduce the incidence of cervical cancer, it remains the second most common cancer in women worldwide. Less-developed countries bear the greatest burden in terms of morbidity and mortality, largely due to the lack of organized screening programs (Arbyn et al., 2011). Persistent infection with high-risk human papillomavirus (HPV) is the main risk factor for the development of cervical cancer (Zur Hausen, 2006). At least 15 different (“high-risk”) HPV types are involved in the development of cancer of the cervix or other anogenital cancers (Muñoz et al., 2003; de Villiers et al., 2004). At present, there are two licensed prophylactic HPV vaccines with proven immunogenicity, safety, and efficacy (Villa, 2011). Both are based on the major viral coat protein L1, assembled into virus-like particles (VLPs). They induce neutralizing antibodies that have the capacity to prevent a large proportion of HPV infection and disease. The current costs of these vaccines and the requirement of refrigeration for storage and needles for injection are a major hurdle to their worldwide implementation. Future vaccines should therefore not only induce immunity against other high-risk HPV types, but also be more temperature-stable and allow delivery by a noninvasive route.
Recombinant adeno-associated virus (rAAV) vectors have become popular as potential gene-therapy vectors because of their ability to mediate stable, efficient, and long-term gene expression, combined with a favorable biological safety profile (for reviews, see Daya and Berns, 2008; Van Vliet et al., 2008; Zaiss and Muruve, 2008). In clinical trials, adeno-associated virus (AAV) has demonstrated great potential as a DNA-delivery vector to treat serious human diseases (for review, see Mingozzi and High, 2011). Remarkably, AAV can be delivered needle-free, which would overcome one of the challenges for vaccine delivery in the developing world (Xin et al., 2001, 2002; Kuck et al., 2006; Mouri et al., 2007; Nieto et al., 2009). In humans, a major problem is the presence of anti-AAV antibodies that effectively neutralize the vector; however, switching capsid serotypes might overcome this problem. To date, 12 different serotypes (AAV1–12) have been isolated from humans or nonhuman primates (Gao et al., 2004, 2005; Schmidt et al., 2008).
rAAVs have demonstrated serotype-specific vector biology and remarkable gene-transfer efficiency in preclinical studies, mainly in rodents (for review, see Van Vliet et al., 2008). In particular, rAAV5-based vectors perform very well in differentiated airway cultures from mice and lower primate species, as well as in in vivo mouse models (Auricchio et al., 2002; Sumner-Jones et al., 2006; Liu et al., 2007; Limberis et al., 2009; Buff et al., 2010; Flotte et al., 2010; Mattar et al., 2011). A number of gene-therapy approaches based on AAV have been investigated in nonhuman primates; however, intranasal (i.n.) delivery using serotype 9 has not been evaluated so far. Earlier, we demonstrated that a single dose of rAAV5-HPV L1 administered i.n. was sufficient to induce high titers of L1-specific serum antibodies, as well as mucosal antibodies in vaginal washes from mice (Kuck et al., 2006), and rAAV9-HPV L1 developed higher humoral responses than rAAV5-HPV L1 in mice (Nieto et al., 2009). We have extended our studies to rhesus macaques to address the impact of an AAV-based i.n. vaccine on the induction of prophylactic immune responses. Macaques immunized with HPV type 16 (HPV16) L1-expressing rAAV5 or rAAV9 showed that humoral responses evoked by i.n. application of rAAV5-L1 are highly increased after one or two boosts of rAAV9-L1. Preexisting anti-AAV9 antibodies do not prevent the generation of L1-specific neutralizing antibodies.
Materials and Methods
Animals
Healthy adult female rhesus macaques (Macaca mulatta) with a body weight between 5 and 6.5 kg were housed at the German Primate Center (Deutsches Primatenzentrum, Göttingen, Germany) under standard conditions. The animals were antibody-negative for simian T-lymphotropic virus type 1, simian D-type retrovirus, and simian immunodeficiency virus. The study was performed according to §8 of the German Animal Welfare Act, which complies with the European Union Directive 86/609/ECC and the European Union guidelines on the welfare of nonhuman primates used in research with the project license 33.11.42502-04-016/08 issued by the Lower Saxony State Office for Consumer Protection and Food Safety.
Immunization of macaques and sample collection
To collect blood samples, animals were anesthetized intramuscularly (i.m.) with 10 mg of ketamine per kilogram of body weight. For deeper anesthesia required for i.n. immunization, a mixture of 5 mg of ketamine hydrochloride, 1 mg of xylazine hydrochloride, and 0.01 mg of atropine sulfate per kilogram of body weight was injected i.m. A dose of 1×1013 genome-containing particles (gp) of viral vector was administered i.n. by spray application at total volumes between 0.28 and 1.0 ml. For this purpose, a spray device from Aptar Pharma (formerly Erich Pfeiffer GmbH, Radolfzell, Germany) was used; it consisted of a 3.5-ml glass flask with an inner conical shape onto which a baby nasal adapter with an integrated pump with a dose volume of 70 μl was screwed. To prove responsiveness against the HPV16 L1 antigen in two of the six macaques that failed to mount HPV-specific immune responses after nasal AAV application, these animals received subcutaneously 10 μg of baculo-derived HPV16 VLPs diluted in 1,300 μl of saline in equal volumes into both groins. The AAV5 recombinants were administered to all six macaques at week 0, three animals received a boost with rAAV9 at week 56, and the same vector was given to all monkeys at week 90, whereas VLPs were given at week 137 (two animals). To determine humoral immune responses, blood samples were taken at the time points indicated. All samples were stored at −20°C.
Cell lines and culture conditions
HeLa and 293T cells were maintained in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% heat-inactivated fetal calf serum, 100 U of penicillin/ml, and 100 μg of streptomycin/ml at 37°C in 5% CO2.
Vaccine vector production and purification
All AAV vectors used in this study were produced as described previously (Kuck et al., 2006). In brief, 293T cells were cotransfected with the packaging plasmid to produce pseudotyped AAV2/5 (pDP5) (Grimm et al., 2003) and AAV2/9 (Gao et al., 2002) and the vector plasmid UF3 L1h (Kuck et al., 2006). Self-complementary AAV9-EGFP (enhanced green fluorescent protein) was produced as described (Varadi et al., 2011) for neutralization assays. Cells were incubated for 48 hr at 37°C and 5% CO2, collected, dispersed in lysis buffer [50 mM Tris-HCl, 150 mM NaCl, 2 mM MgCl2 (pH 8.0)], and disrupted by three freeze–thaw cycles using liquid nitrogen. The tissue debris was removed by centrifugation, and the supernatant was digested with Benzonase (50 U/ml; Sigma, Taufkirchen, Germany) for 30 min at 37°C. AAV particles were purified from the cell lysates via an iodixanol step gradient (Zolotukhin et al., 1999). Samples were centrifuged in a Beckman Ti 50.2 rotor for 2 hr at 50,000 rpm at 4°C in Beckman Quickseal tubes. The fraction containing rAAV particles was further purified by a continuous iodixanol gradient, samples were diluted in phosphate-buffered saline (PBS) until refraction index 1.400, and centrifuged in a Beckman Ti 70.1 rotor for 8 hr at 70,000 rpm at 10°C in Beckman Quickseal tubes. Fractions containing full rAAV particles were pooled, and iodixanol solution was exchanged against PBS. Finally, virus stock was concentrated in a Vivaspin 20 (Vivascience, Göttingen, Germany) concentrator according to the manufacturer's protocol to a titer of 1×1013 gp/ml and stored at −80°C. Vector genome titers were determined by quantitative real-time PCR as described previously (Kuck et al., 2006).
Depletion of L1 particles in vector production
As described peviously (Nieto et al., 2009), expression of L1 gene was silenced using a stable 293T-sh5 cell line that represses expression of the L1 gene. HPV16 L1 particles formed due to incomplete L1 gene repression were further depleted as described previously (Nieto et al., 2009). In brief, dithiothreitol–trypsin was added to the crude cell lysate and incubated for 15 min at 37°C. Gradient fractions containing the rAAV were then treated overnight with carbonate buffer (200 mM Na2HCO3) for complete disruption of HPV L1 particles.
Measurement of antibody responses
The presence of L1-specific IgG antibodies in sera of immunized macaques was determined by VLP-ELISA. In brief, 96-well plastic plates were coated overnight at 4°C with VLP produced and purified according to a previously published method (Müller et al., 1997). After being washed with PBS/0.05% Tween, plates were blocked with MPBS-T (5% skim milk in PBS/0.05% Tween) for 1 hr at 37°C. Prediluted sera (in twofold dilutions starting from 1:50 to 1:51,200) were added, and plates were incubated for 1 hr at 37°C. After washing, plates were incubated for 1 hr at 37°C with 1:2,000 diluted horseradish peroxidase–coupled antihuman IgG-specific secondary antibody (Southern Biotechnology, Birmingham, MA) in MPBS-T; TMB (3,3′,5,5′-tetramethylbenzidine) substrate solution (Sigma, St. Louis, MO) was used as substrate. Optical density was measured in an ELISA reader at 450 nm after a 10-min incubation at room temperature. To detect anti-AAV5- or 9-specific IgG antibodies, sera were added into AAV5- or AAV9-precoated plates (1010 particles/well) following the same conditions as above. Nonspecific binding was determined by using the same dilutions on plates coated with PBS only.
Pseudovirion-based neutralization assay
Detection of neutralizing antibodies in sera of immunized animals was carried out as described previously (Kuck et al., 2006). In brief, pseudovirions were prepared by transfecting 293TT cells (cultivated in DMEM containing 50 μg of hygromycin/ml) with a plasmid encoding for the humanized HPV16 L1 and L2 genes, together with a plasmid containing the gene for secreted alkaline phosphatase (SEAP) under the control of the cytomegalovirus promoter. For pseudovirion extraction, cells were harvested 3–4 days later by trypsinization, washed once with PBS, and resuspended in 1 ml of PBS plus 1 mM CaCl2, 5.6 mM MgCl2 per 5×107 cells and lysed by 50 μl of Brij58 (Sigma) in the presence of Benzonase (250 U/ml) for 5 min on ice. The cellular lysate was centrifuged after the addition of NaCl to a final concentration of 710 mM, and the cleared supernatant containing the pseudovirions was used for infection of 293TT cells. For this purpose, pseudovirions were diluted 1:5,000 in DMEM and preincubated with the sera (1:50 to 1:100,000 dilution) for 15 min at room temperature. Pseudovirions were then added to the cells, followed by incubation at 37°C for 5 days. SEAP activity in cell-culture supernatant was measured by using a commercial assay (Roche, Mannheim, Germany) according to the manufacturer's recommendations.
AAV9 neutralization assay
Detection of AAV9-neutralizing antibodies in sera of immunized animals was determined as described previously (Varadi et al., 2011). In brief, a total of 2×104 gp/cell of rAAV9-GFP (green fluorescent protein) virus was preincubated with macaque sera (1:2 to 1:128 dilution) for 45 min at room temperature. A mixture of virus and sera was then added to 293T cells (1×104 cells/well) in a 96-well plate, followed by incubation at 37°C for 2 days. Transduction efficiency was analyzed by quantifying the cells expressing GFP. The percentage of GFP-positive cells was monitored by flow cytometry on a fluorescence-activated cell sorting Calibur device (Becton Dickinson, Heidelberg, Germany). Transduction efficiencies were evaluated with FlowJo software (v.7.6.1, Tree Star, Inc., Olten, Germany). Neutralization was assumed when transduction efficiency of samples treated with serum was reduced to 50% of that of mock-treated cells.
Results
Intranasal immunization using rAAV5-L1 as prime vector followed by AAV9-L1 induces strong humoral responses against HPV16 in rhesus macaques
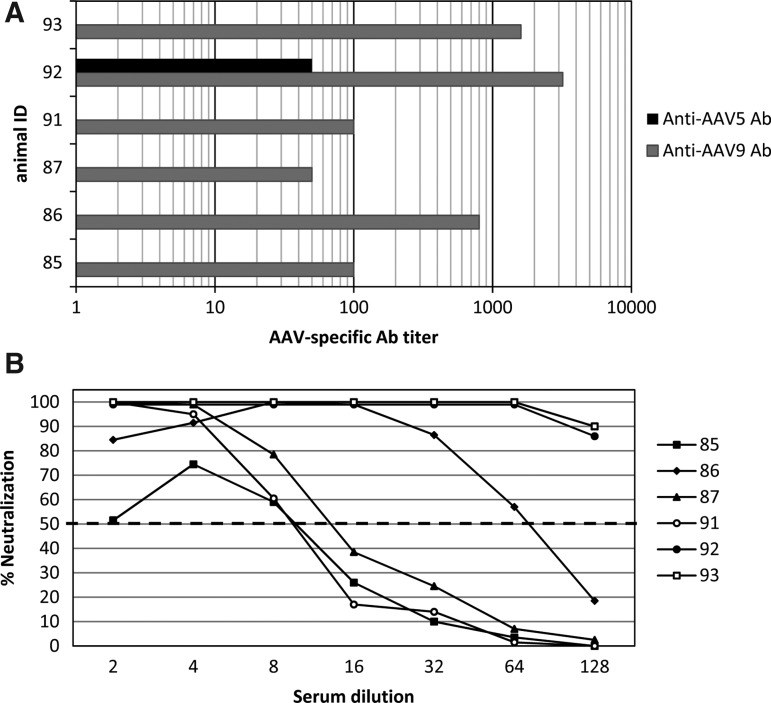
The aim of this study was to analyze the efficacy of genetic immunization by rAAV serotypes 5 and 9 in monkeys via the i.n. route. Those AAV serotypes were chosen following previous mouse studies demonstrating the best candidates for i.n. application (Nieto et al., 2009). As was done previously (Kuck et al., 2006; Nieto et al., 2009), we used the humanized gene of the major structural protein L1 of the HPV type 16. Six rhesus macaques were included in this study. As the presence of antibodies against a specific AAV serotype may prevent an efficient AAV-based effect, before vaccination animals were tested for the presence of serum antibodies reacting with AAV5 capsid and AAV9 capsid by an ELISA. As shown in Fig. 1A, all animals were AAV9-seropositive at baseline (titers from 50 to 3,200). We analyzed the sera also for neutralizing activity against rAAV9. As shown in Fig. 1B, there is a correlation between binding and neutralizing antibodies. Regarding AAV5-specific antibodies, only animal #92 had a measurable ELISA titer (1:50); the neutralizing activity was not determined. Because the prevalence of AAV5 antibodies was much lower, all animals were first immunized with rAAV5-L1.
FIG. 1.
Detection of natural AAV9- and AAV5-specific antibodies in rhesus macaques. (A) Sera of six preimmunized macaques were tested for detection of AAV9 capsid (gray bars) and AAV5 capsid (black bar) antibodies using an AAV-based ELISA. Data are expressed as reciprocal titers of the individual monkey. (B) Individual sera were also tested for neutralization of rAAV9-GFP vector-mediated transduction of cells in culture. Data are expressed as percent neutralization obtained after incubation with 1:2 to 1:128 dilutions of the sera. Fifty percent neutralization was set as the cutoff (dashed line).
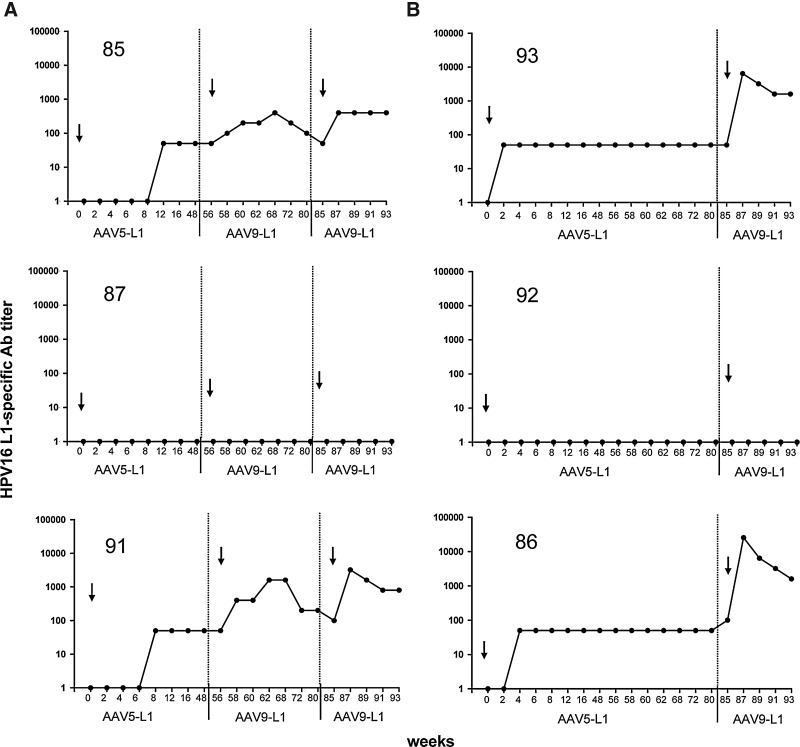
Each monkey received a single immunization with 1×1013 gp per dose of rAAV5-L1. Eight serum samples at 2–4-week intervals were taken with a total follow-up of 48 or 82 weeks. As shown in Fig. 2, animals #85, 93, 91, and 86 developed low but persistent titers of L1-specific antibodies. Animal #92, which had anti-AAV5 antibody titers prior to immunization (see Fig. 1), did not respond to the rAAV5-L1 vaccination. This suggests that possibly preexisting anti-AAV5 immunity prevented successful vaccination. The reason why animal #87 did not respond remained unclear.
FIG. 2.
Detection of HPV16 L1-specific antibody titers in rhesus macaques vaccinated with AAV-L1 vectors. Sera of six rhesus macaques were tested for HPV16 L1-specific antibodies using a VLP-based ELISA every 2 weeks after i.n. vaccination with rAAV5-L1 and either two (A) or one (B) immunizations with rAAV9-L1 (1×1013 gp/dose). Data are presented as the reciprocal dilutions of the individual sera. Arrows indicate the time of immunization, and numbers in the upper part of each graph indicate animal identification (ID).
To improve the humoral immune response, monkeys were reimmunized by switching the AAV serotype. According to our previous experience (Nieto et al., 2009), we choose rAAV9 as a promising candidate. Although AAV9 is of human origin, prevalent antibodies reacting with AAV9 capsids were detected in all animals (Fig. 1). However, titers differed substantially, ranging from 50 to >1,000. Assuming that a high titer of preexisting AAV9 antibodies would interfere with the efficacy of an rAAV9-L1 immunization, we first boosted the animals exhibiting low AAV9 antibody titers (#85, 87, and 91) with 1×1013 gp at week 56. As shown in Fig. 2A, the two animals (#85 and 91) that had developed measurable L1-specific antibodies after priming with rAAV5-L1 demonstrated an increase in titer up to 400 and 1,600, respectively. However, the titer decreased with time to the level obtained before the first immunization with rAAV9-L1. After decline in titers, monkeys #85 and 91 were boosted with 1×1013 gp of rAAV9-L1 at week 85. The HPV16 L1 antibody titers rose to about the same level, but at this time remained stable during the follow-up of another 7 weeks (Fig. 2A). At week 85, we decided to immunize also animals #93, 92, and 86, which had high titers of AAV9-specific antibodies with rAAV9-L1, in order to explore whether higher titers of preexisting antibodies might prevent vaccination with this same serotype.
Interestingly, two of the monkeys with high titers of preexisting AAV9-specific antibodies (#93 and 86) developed substantially higher levels of L1-specific antibodies (titer 6,400 and 25,600) as shown in Fig. 2B. The two animals, one with low (#87) and one with high (#92) AAV9 titers, that had not responded to the rAAV5-L1 vaccination did not develop an L1 antibody immune response despite the two or three immunizations, respectively. We conclude from these results that the humoral responses highly increased after boosting with rAAV9-L1, and that high titers of preexisting AAV9-specific antibodies do not preclude a successful i.n. immunization with AAV9-L1.
L1-specific antibodies elicited by rAAV9-L1 vectors neutralize HPV16 pseudovirions
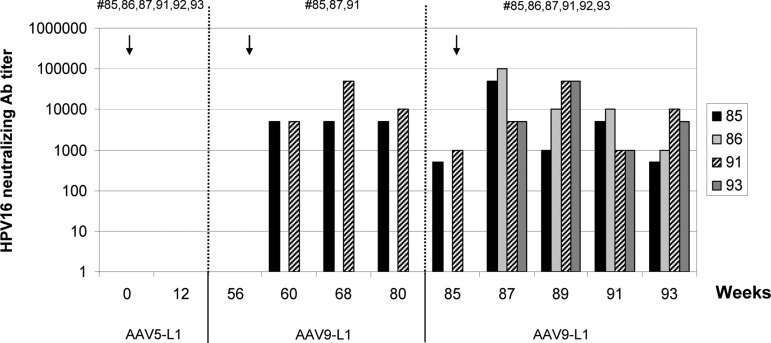
Macaque sera collected at selected time points (see Fig. 3) were analyzed for the presence of HPV16-specific neutralizing antibodies. Neutralizing activity was determined by the inhibition of gene transduction by HPV16 pseudovirions [pseudovirion-based neutralization assay (Pastrana et al., 2004)]. Gene transduction as a surrogate of infection was measured by the expression of SEAP. The sera were tested in serial dilutions from 1:50 to 1:100,000. None of the sera of animals that had developed L1 antibody titers after rAAV5-L1 vaccination exhibited neutralizing activities (Fig. 3). Using 50% reduction of SEAP activity as the cutoff, the sera of two animals, which developed L1-specific antibodies after one immunization with rAAV9-L1 (#85 and #91), were able to neutralize HPV16 pseudovirions 4 weeks after vaccination with AAV9-L1 (week 60) with a mean titer of 5,000, which was sustained for the follow-up of 24 weeks post immunization (Fig. 3). The neutralizing titer increased transiently to >10,000 after the third immunization. In addition, the two animals that received only one rAAV9-L1 dose (#86 and 93) developed neutralizing antibody titers (from 1,000 to 100,000).
FIG. 3.
Detection of neutralizing HPV16 L1 antibody titers in rhesus macaques vaccinated with AAV-L1 vectors. Neutralizing antibodies in individual sera of macaques vaccinated with AAV5-L1 and either one or two immunizations with AAV9-L1 were detected by inhibition of HPV16 pseudovirion infection. Data of responder animals are presented as the reciprocal of serum dilutions (1:50 to 1:100,000) exhibiting 50% neutralizating activity. Numbers in the upper part of the graph indicate animal ID.
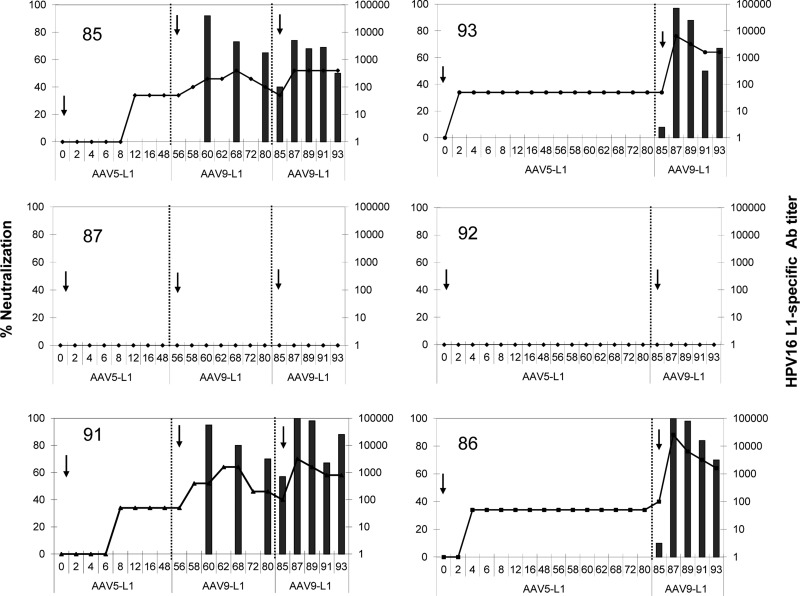
The antibody titers remained stable during the 8-week follow-up after the last immunization. Overall, four of the six macaques developed neutralizing titers of >1,000, which, however, only appeared after immunization with rAAV9-L1. Figure 4 shows a correlation between levels of L1-specific antibodies measured by ELISA and the neutralizing activity. This correlation indicates that the neutralizing antibody titers declined in each case after the first immunization with rAAV9-L1, whereas they were more stable after the second immunization (animals #85 and 91).
FIG. 4.
Comparative analysis of the L1-specific antibodies and neutralizing antibodies in rhesus macaques vaccinated with AAV-L1 vectors. Sera of macaques were tested for HPV16 L1-specific antibodies using a VLP-based ELISA every 2 weeks after i.n. vaccination with rAAV5-L1 and either one or two immunizations with rAAV9-L1 (1×1013 gp/dose). Data are expressed as the reciprocal dilutions of individual sera (1:10 to 1:100,000; right y-axis). Arrows show the time of immunization; the numbers in the upper part of each graph indicate animal ID. Detection of neutralizing antibodies in individual sera of macaques vaccinated with AAV-L1 was analyzed by HPV16 pseudovirion infection. Data are expressed as the percent neutralization obtained by incubation with a 1:1,000 dilution of the sera (left y-axis).
Natural and vector-directed AAV9 antibodies do not prevent the effect of additional vaccinations
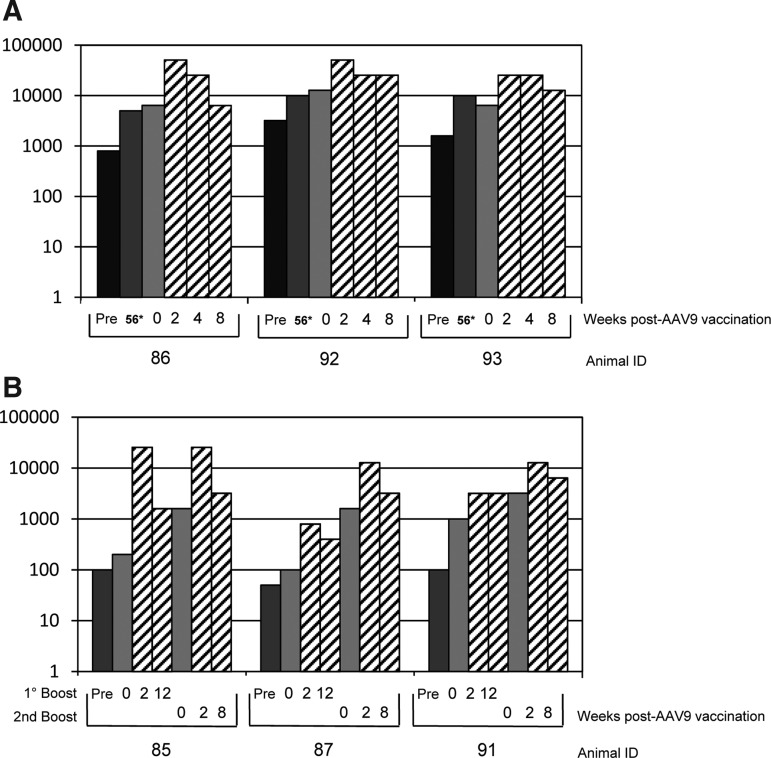
As reported previously, the presence of antibodies against a specific AAV serotype can influence the success of a repeated administration of vectors based on the same or another serotype. Although it has been reported that repeated immunization of mice with AAV9 vectors is possible (Limberis and Wilson, 2006; Nieto et al., 2009), this information is so far not available for nonhuman primates. Therefore, to assess the vector-specific immune response that had been induced by the AAV vector immunization, we measured serum antibodies against the AAV9 capsid in the animals by ELISA before and after application of rAAV9-L1. As shown above (Fig. 1), the macaques had naturally acquired AAV9, as indicated by the presence of low to high titers of AAV9-specific antibodies before immunization. As shown in Fig. 5A, immunization of monkeys with rAAV9-L1 having high AAV9 antibody titers only marginally (approximately twofold) (mean titer 43,000) increased AAV9-specific antibody levels further. The titers slightly declined to 15,000 by 8 weeks post immunization. The monkeys with low AAV9 antibody titers that were vaccinated twice with the rAAV9-L1 vector revealed an up to 10-fold increase of AAV9-specific antibodies after each immunization (Fig. 5B). Despite some variations, the titers remained elevated during the follow-up period of 8 weeks. Interestingly, also the two animals that did not develop L1 antibodies (#87 and 92) had similar anti-AAV9 antibody titers as the L1-positive animals. Therefore, we conclude that all immunizations were successful and that the failure of two animals to develop HPV16 L1-specific antibodies must have other reasons. Following up on this question, subcutaneous immunization of the two L1-negative animals #87 and 92 with HPV16 VLPs induced L1-specific antibodies, demonstrating that the two animals were not tolerant against the L1 antigen (data not shown).
FIG. 5.
AAV9-specific IgG antibodies in rhesus macaques vaccinated with AAV9-L1 vectors. Six rhesus macaques were immunized i.n. with rAAV5-L1 (1×1013 gp/dose) as a prime. Three monkeys received one (A) or two (B) doses of rAAV9-L1 (1×1013 gp/dose). Sera were tested for AAV9-specific antibodies using an AAV-based ELISA before and after immunization. Data are expressed as the reciprocal titer of the individual monkey. Dark bars show titers of naive animals (Pre, naive animals; 56*, 56 weeks after AAV5-L1 immunization); light gray bars show titers at the time of AAV9-L1 immunization; and hatched bars show titers at indicated weeks post vaccination.
Our data demonstrate that higher or lower preexisting antibody titers, either from natural exposure to AAV9 or from a first round of AAV9-dependent immunization with rAAV9 vectors, do not abrogate the induction of a humoral immune response against the antigen (HPV16 L1) encoded by the transgene within the AAV9 vector.
Discussion
Novel HPV vaccines could be improved in several aspects: Besides the broadening of preventive capacity against all or most high-risk HPV types and combining prophylactic and therapeutic properties, a next-generation vaccine candidate should be temperature-stable, thus avoiding maintenance of the cold chain, and preferentially be delivered by a noninvasive procedure. We have shown previously that rAAV is efficient as a vaccine-delivery system by nasal application that can provide prophylactic and therapeutic properties, and that it remains active after lyophilization and when kept under nonrefrigerated conditions (Kuck et al., 2006; Nieto et al., 2009). Here, for the first time, the rAAV vector system for nasal immunization of nonhuman primates was explored.
Rhesus macaques are one of the most frequently and thoroughly studied species of all nonhuman primates, because they are bred in captivity and readily available for biomedical research. Compared with rodents, macaques exhibit greater similarity to human physiology, neurobiology, and susceptibility to infectious and metabolic diseases (Gibbs et al., 2007). Most important, their response to infectious agents related to human pathogens, including simian immunodeficiency virus and influenza, has made macaques the preferred model for vaccine development (Gibbs et al., 2007). The i.n. administration of rAAV vectors that were used in this study proved to be safe and well tolerated by these animals.
Application of rAAV as a vaccine carrier has been widely studied in the past 10 years (Manning et al., 1997; Sarukhan et al., 2001; Lin et al., 2009; Quinn et al., 2011; Sipo et al., 2011). AAV vaccines provide long-term immunity and can meanwhile be produced in large quantities (Virag et al., 2009; Kotin, 2011). In general, immunization of humans against HPV was successful provided that a potent immunogen is used, such as VLPs of HPV16, which assemble spontaneously after expression of the L1 structural protein. However, a major limitation of the application of rAAV vectors is the frequent prevalence of preexisting AAV antibodies in the human population (Halbert et al., 2006; Calcedo et al., 2009, 2011; Boutin et al., 2010) and in laboratory animals (Rapti et al., 2011). Neutralizing antibodies against AAV may prevent or significantly dampen the development of a strong immune response against the transgene product (Lin et al., 2008). One way to circumvent this problem is the use of AAV serotypes for which a low prevalence of neutralizing antibodies is reported (Halbert et al., 2006; Calcedo et al., 2009; Boutin et al., 2010; Li et al., 2011). Besides AAV5, AAV12 and rh32.33, a hybrid AAV from two related rhesus macaque isolates, are promising candidates (Halbert et al., 2006; Calcedo et al., 2009; Lin et al., 2009; Boutin et al., 2010; Li et al., 2011; Quinn et al., 2011). Another option to solve the issue of preexisting AAV capsid antibodies is the use of rAAV9 vectors that can be repeatedly applied for gene transduction via the nasal route (Limberis and Wilson, 2006). Thus, for as yet unknown reasons, the immune response against AAV9 capsids does not seem to prevent successful gene delivery into the nasal epithelia. In addition, we previously reported that, in a mouse model, the immune response to AAV9 capsids was relatively low compared with the response to AAV5 capsids (Nieto et al., 2009). In monkeys, however, we detected higher antibody titers against the AAV9 capsids than against AAV5 capsids, and therefore we first immunized with rAAV5 vectors for which most animals were antibody-negative. Only when rAAV5 vaccination turned out to yield poor immune responses, we also immunized the animals with rAAV9 vectors. Surprisingly, a strong humoral immune response against HPV16 L1 was generated by nasal immunization with rAAV9 vectors, demonstrating that preexisting antibodies, derived either from natural exposure to AAV9 or from a first round of immunization with rAAV9 vectors, did not prevent the generation of a potent humoral immune response against the HPV16 L1 protein. These L1-specific antibodies elicited by AAV9 vectors proved to neutralize infection with HPV16 pseudovirions and were maintained for the complete time of follow-up (7 months post immunization; see Figs. 2 and 4).
In particular, in nasal immunization, the choice of the delivery system is of utmost importance (Slutter et al., 2008; Sharma et al., 2009; Jabbal-Gill, 2010). Among the currently used particulate carriers for nasal vaccines, viral vectors also are being explored as promising options. The primary function of such particles is to provide an antigen depot and promote uptake by antigen-presenting cells. Certain AAV vectors seem to exactly fulfill this function by releasing the antigen over a long period of time to the immune cells of the nasal tissue. A possible disadvantage of such a depot function is T-cell exhaustion, which has been observed for rAAV2 vectors (J. Lin et al., 2007; S.W. Lin et al., 2007). Our previous experience with rAAV9 vectors in mice, however, suggests no such effect with HPV16 L1 as a transgene (Nieto et al., 2009). In this study, the data we obtained from the T-cell analyses did not match with the humoral responses, and hence did not allow any conclusion (data not shown).
Recently, a new serotype—AAV12—showed a high transduction efficiency of airway epithelia of mice and, when applied i.n., also a good immune response to a protein encoded by the transgene (Schmidt et al., 2008; Quinn et al., 2011). So far, it is not known whether AAV12 transduces, in addition to the epithelial cells, the professional antigen-presenting cells resident in the nasal and lung compartments (CD11b+ and CD103+).
Altogether, our study demonstrates that the expression of HPV16 L1 gene using an rAAV9 vector elicits humoral immune responses after noninvasive application in rhesus macaques. As the biophysical properties of rAAV vectors also tolerate lyophilization and resuspension in water (Kuck et al., 2006), this delivery system meets at least some of the demands of future HPV vaccines, and thus a limitation of current vaccines—the requirement of cold storage—could be overcome. Especially, the possibility to readminister rAAV9 vectors with no loss in the generation of the B-cell response shows the advantage of AAV9 over other serotypes and opens a new field of further vaccine development based on this AAV serotype.
Acknowledgments
We would like to thank H. zur Hausen for his continuous support. J.A.K. and M.M. were supported by grant 107538 of the Deutsche Krebshilfe.
Author Disclosure Statement
No competing financial interests exist.
References
- Arbyn M. Castellsagué X. de Sanjosé S., et al. Worldwide burden of cervical cancer in 2008. Ann. Oncol. 2011;22:2675–2686. doi: 10.1093/annonc/mdr015. [DOI] [PubMed] [Google Scholar]
- Auricchio A. O'Connor E. Weiner D., et al. Noninvasive gene transfer to the lung for systemic delivery of therapeutic proteins. J. Clin. Invest. 2002;110:499–504. doi: 10.1172/JCI15780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boutin S. Monteilhet V. Veron P., et al. Prevalence of serum IgG and neutralizing factors against adeno-associated virus (AAV) types 1, 2, 5, 6, 8, and 9 in the healthy population: implications for gene therapy using AAV vectors. Hum. Gene Ther. 2010;21:704–712. doi: 10.1089/hum.2009.182. [DOI] [PubMed] [Google Scholar]
- Buff S.M. Yu H. McCall J.N., et al. IL-10 delivery by AAV5 vector attenuates inflammation in mice with Pseudomonas pneumonia. Gene Ther. 2010;17:567–576. doi: 10.1038/gt.2010.28. [DOI] [PubMed] [Google Scholar]
- Calcedo R. Vandenberghe L.H. Gao G., et al. Worldwide epidemiology of neutralizing antibodies to adeno-associated viruses. J. Infect. Dis. 2009;199:381–390. doi: 10.1086/595830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calcedo R. Morizono H. Wang L., et al. Adeno-associated virus antibody profiles in newborns, children, and adolescents. Clin. Vaccine Immunol. 2011;18:1586–1588. doi: 10.1128/CVI.05107-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daya S. Berns K.I. Gene therapy using adeno-associated virus vectors. Clin. Microbiol. Rev. 2008;21:583–593. doi: 10.1128/CMR.00008-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Villiers E.M. Fauquet C. Broker T.R., et al. Classification of papillomaviruses. Virology. 2004;324:17–27. doi: 10.1016/j.virol.2004.03.033. [DOI] [PubMed] [Google Scholar]
- Flotte T.R. Fischer A.C. Goetzmann J., et al. Dual reporter comparative indexing of rAAV pseudotyped vectors in chimpanzee airway. Mol. Ther. 2010;18:594–600. doi: 10.1038/mt.2009.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao G. Vandenberghe L.H. Alvira M.R., et al. Clades of adeno-associated viruses are widely disseminated in human tissues. J. Virol. 2004;78:6381–6388. doi: 10.1128/JVI.78.12.6381-6388.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao G. Vandenberghe L.H. Wilson J.M. New recombinant serotypes of AAV vectors. Curr. Gene Ther. 2005;5:285–297. doi: 10.2174/1566523054065057. [DOI] [PubMed] [Google Scholar]
- Gao G.P. Alvira M.R. Wang L., et al. Novel adeno-associated viruses from rhesus monkeys as vectors for human gene therapy. Proc. Natl. Acad. Sci. U.S.A. 2002;99:11854–11859. doi: 10.1073/pnas.182412299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbs R.A. Rogers J. Katze M.G., et al. Evolutionary and biomedical insights from the rhesus macaque genome. Science. 2007;316:222–234. doi: 10.1126/science.1139247. [DOI] [PubMed] [Google Scholar]
- Grimm D. Kay M.A. Kleinschmidt J.A. Helper virus-free, optically controllable, and two-plasmid-based production of adeno-associated virus vectors of serotypes 1 to 6. Mol. Ther. 2003;7:839–850. doi: 10.1016/s1525-0016(03)00095-9. [DOI] [PubMed] [Google Scholar]
- Halbert C.L. Miller A.D. McNamara S., et al. Prevalence of neutralizing antibodies against adeno-associated virus (AAV) types 2, 5, and 6 in cystic fibrosis and normal populations: implications for gene therapy using AAV vectors. Hum. Gene Ther. 2006;17:440–447. doi: 10.1089/hum.2006.17.440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jabbal-Gill I. Nasal vaccine innovation. J. Drug Target. 2010;18:771–786. doi: 10.3109/1061186X.2010.523790. [DOI] [PubMed] [Google Scholar]
- Kotin R.M. Large-scale recombinant adeno-associated virus production. Hum. Mol. Genet. 2011;20:R2–R6. doi: 10.1093/hmg/ddr141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuck D. Lau T. Leuchs B., et al. Intranasal vaccination with recombinant adeno-associated virus type 5 against human papillomavirus type 16 L1. J. Virol. 2006;80:2621–2630. doi: 10.1128/JVI.80.6.2621-2630.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C. Narkbunnam N. Samulski R.J., et al. Neutralizing antibodies against adeno-associated virus examined prospectively in pediatric patients with hemophilia. Gene Ther. 2011;19:288–294. doi: 10.1038/gt.2011.90. [DOI] [PubMed] [Google Scholar]
- Limberis M.P. Wilson J.M. Adeno-associated virus serotype 9 vectors transduce murine alveolar and nasal epithelia and can be readministered. Proc. Natl. Acad. Sci. U.S.A. 2006;103:12993–12998. doi: 10.1073/pnas.0601433103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Limberis M.P. Vandenberghe L.H. Zhang L., et al. Transduction efficiencies of novel AAV vectors in mouse airway epithelium in vivo and human ciliated airway epithelium in vitro. Mol. Ther. 2009;17:294–301. doi: 10.1038/mt.2008.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin J. Zhi Y. Mays L. Wilson J.M. Vaccines based on novel adeno-associated virus vectors elicit aberrant CD8+ T-cell responses in mice. J. Virol. 2007;81:11840–11849. doi: 10.1128/JVI.01253-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin J. Calcedo R. Vandenberghe L.H., et al. Impact of preexisting vector immunity on the efficacy of adeno-associated virus-based HIV-1 Gag vaccines. Hum. Gene Ther. 2008;19:663–669. doi: 10.1089/hum.2008.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin J. Calcedo R. Vandenberghe L.H., et al. A new genetic vaccine platform based on an adeno-associated virus isolated from a rhesus macaque. J. Virol. 2009;83:12738–12750. doi: 10.1128/JVI.01441-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin S.W. Hensley S.E. Tatsis N., et al. Recombinant adeno-associated virus vectors induce functionally impaired transgene product-specific CD8+ T cells in mice. J. Clin. Invest. 2007;117:3958–3970. doi: 10.1172/JCI33138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X. Luo M. Trygg C., et al. Biological differences in rAAV transduction of airway epithelia in humans and in Old World non-human primates. Mol. Ther. 2007;15:2114–2123. doi: 10.1038/sj.mt.6300277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manning W.C. Paliard X. Zhou S., et al. Genetic immunization with adeno-associated virus vectors expressing herpes simplex virus type 2 glycoproteins B and D. J. Virol. 1997;71:7960–7962. doi: 10.1128/jvi.71.10.7960-7962.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattar C.N. Nathwani A.C. Waddington S.N., et al. Stable human FIX expression after 0.9G intrauterine gene transfer of self-complementary adeno-associated viral vector 5 and 8 in macaques. Mol Ther. 2011;19:1950–1960. doi: 10.1038/mt.2011.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mingozzi F. High K.A. Therapeutic in vivo gene transfer for genetic disease using AAV: progress and challenges. Nat. Rev. Genet. 2011;12:341–355. doi: 10.1038/nrg2988. [DOI] [PubMed] [Google Scholar]
- Mouri A. Noda Y. Hara H., et al. Oral vaccination with a viral vector containing Aβ cDNA attenuates age-related Aβ accumulation and memory deficits without causing inflammation in a mouse Alzheimer model. FASEB J. 2007;21:2135–2148. doi: 10.1096/fj.06-7685com. [DOI] [PubMed] [Google Scholar]
- Müller M. Zhou J. Reed T.D, et al. Chimeric papillomavirus-like particles. Virology. 1997;234:93–111. doi: 10.1006/viro.1997.8591. [DOI] [PubMed] [Google Scholar]
- Muñoz N. Bosch F.X. de Sanjosé S., et al. Epidemiologic classification of human papillomavirus types associated with cervical cancer. N. Engl. J. Med. 2003;348:518–527. doi: 10.1056/NEJMoa021641. [DOI] [PubMed] [Google Scholar]
- Nieto K. Kern A. Leuchs B., et al. Combined prophylactic and therapeutic intranasal vaccination against human papillomavirus type-16 using different adeno-associated virus serotype vectors. Antivir. Ther. 2009;14:1125–1137. doi: 10.3851/IMP1469. [DOI] [PubMed] [Google Scholar]
- Pastrana D.V. Buck C.B. Pang Y.Y., et al. Reactivity of human sera in a sensitive, high-throughput pseudovirus-based papillomavirus neutralization assay for HPV16 and HPV18. Virology. 2004;321:205–216. doi: 10.1016/j.virol.2003.12.027. [DOI] [PubMed] [Google Scholar]
- Quinn K. Quirion M.R. Lo C.Y., et al. Intranasal administration of adeno-associated virus type 12 (AAV12) leads to transduction of the nasal epithelia and can initiate transgene-specific immune response. Mol. Ther. 2011;19:1990–1998. doi: 10.1038/mt.2011.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapti K. Louis-Jeune V. Kohlbrenner E., et al. Neutralizing antibodies against AAV serotypes 1, 2, 6, and 9 in sera of commonly used animal models. Mol Ther. 2011;20:73–83. doi: 10.1038/mt.2011.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarukhan A. Camugli S. Gjata B., et al. Successful interference with cellular immune responses to immunogenic proteins encoded by recombinant viral vectors. J. Virol. 2001;75:269–277. doi: 10.1128/JVI.75.1.269-277.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt M. Voutetakis A. Afione S., et al. Adeno-associated virus type 12 (AAV12): a novel AAV serotype with sialic acid- and heparan sulfate proteoglycan-independent transduction activity. J. Virol. 2008;82:1399–1406. doi: 10.1128/JVI.02012-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma S. Mukkur T.K. Benson H.A. Chen Y. Pharmaceutical aspects of intranasal delivery of vaccines using particulate systems. J. Pharm. Sci. 2009;98:812–843. doi: 10.1002/jps.21493. [DOI] [PubMed] [Google Scholar]
- Sipo I. Knauf M. Fechner H., et al. Vaccine protection against lethal homologous and heterologous challenge using recombinant AAV vectors expressing codon-optimized genes from pandemic swine origin influenza virus (SOIV) Vaccine. 2011;29:1690–1699. doi: 10.1016/j.vaccine.2010.12.037. [DOI] [PubMed] [Google Scholar]
- Slutter B. Hagenaars N. Jiskoot W. Rational design of nasal vaccines. J. Drug Target. 2008;16:1–17. doi: 10.1080/10611860701637966. [DOI] [PubMed] [Google Scholar]
- Sumner-Jones S.G. Davies L.A. Varathalingam A., et al. Long-term persistence of gene expression from adeno-associated virus serotype 5 in the mouse airways. Gene Ther. 2006;13:1703–1713. doi: 10.1038/sj.gt.3302815. [DOI] [PubMed] [Google Scholar]
- Van Vliet K.M. Blouin V. Brument N., et al. The role of the adeno-associated virus capsid in gene transfer. Methods Mol. Biol. 2008;437:51–91. doi: 10.1007/978-1-59745-210-6_2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varadi K. Michelfelder S. Korff T., et al. Novel random peptide libraries displayed on AAV serotype 9 for selection of endothelial cell-directed gene transfer vectors. Gene Ther. 2011 doi: 10.1038/gt.2011.143. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Villa L.L. HPV prophylactic vaccination: the first years and what to expect from now. Cancer Lett. 2011;305:106–112. doi: 10.1016/j.canlet.2010.12.002. [DOI] [PubMed] [Google Scholar]
- Virag T. Cecchini S. Kotin R.M. Producing recombinant adeno-associated virus in foster cells: overcoming production limitations using a baculovirus-insect cell expression strategy. Hum. Gene Ther. 2009;20:807–817. doi: 10.1089/hum.2009.092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xin K.Q. Urabe M. Yang J., et al. A novel recombinant adeno-associated virus vaccine induces a long-term humoral immune response to human immunodeficiency virus. Hum. Gene Ther. 2001;12:1047–1061. doi: 10.1089/104303401750214276. [DOI] [PubMed] [Google Scholar]
- Xin K.Q. Ooki T. Mizukami H., et al. Oral administration of recombinant adeno-associated virus elicits human immunodeficiency virus-specific immune responses. Hum. Gene Ther. 2002;13:1571–1581. doi: 10.1089/10430340260201662. [DOI] [PubMed] [Google Scholar]
- Zaiss A.K. Muruve D.A. Immunity to adeno-associated virus vectors in animals and humans: a continued challenge. Gene Ther. 2008;15:808–816. doi: 10.1038/gt.2008.54. [DOI] [PubMed] [Google Scholar]
- Zolotukhin S. Byrne B.J. Mason E., et al. Recombinant adeno-associated virus purification using novel methods improves infectious titer and yield. Gene Ther. 1999;6:973–985. doi: 10.1038/sj.gt.3300938. [DOI] [PubMed] [Google Scholar]
- Zur Hausen H. Infections Causing Human Cancer. Wiley-VCH Verlag GmbH; Weinheim, Germany: 2006. [Google Scholar]