Abstract
L-Glutamate (Glu) is the main excitatory neurotransmitter in the mammalian central nervous system, and it is involved in most aspects of normal brain function, including cognition, memory and learning, plasticity, and motor movement. Although micro-dialysis techniques have been used to study Glu, the slow temporal resolution of the technique may be inadequate to properly examine tonic and phasic Glu. Thus, our laboratory has developed an enzyme-based microelectrode array (MEA) with fast response time and low detection limits for Glu. We have modified the MEA design to allow for reliable measures in the brain of awake, freely moving mice. In this study, we chronically implanted the MEA in prefrontal cortex (PFC) or striatum (Str) of awake, freely moving C57BL/6 mice. We successfully measured Glu levels 7 days postimplantation without loss of MEA sensitivity. In addition, we determined resting (tonic) Glu levels to be 3.3 μM in the PFC and 5.0 μM in the Str. Resting Glu levels were subjected to pharmacological manipulation with tetrodotoxin (TTX) and DL-threo-β-hydroxyaspartate (THA). TTX significantly (p < 0.05) decreased resting Glu by 20%, whereas THA significantly (p < 0.05) increased resting Glu by 60%. Taken together, our data show that chronic recordings of tonic and phasic clearance of exogenously applied Glu can be carried out in awake mice for at least 7 days in vivo, allowing for longer term studies of Glu regulation.
L-Glutamate (Glu) is the major excitatory neurotransmitter in the mammalian central nervous system. Glu is involved in most aspects of normal brain function, including cognition, memory and learning, plasticity, and motor movement. As a result, altered pathophysiology of Glu is implicated in several neurodegenerative disorders, including Huntington’s disease, Alzheimer’s disease, Parkinson’s disease, and amyotrophic lateral sclerosis (Danbolt, 2001).
At this time, in vivo microdialysis is the most commonly used technique to study extracellular Glu in the intact brain. Microdialysis uses semipermeable membranes to restrict the diffusion of extracellular molecules or neurotransmitters along their concentration gradients (Ungerstedt, 1984; Di Chiara et al., 1996). There are numerous microdialysis studies conducted in awake mouse models (Holmer et al., 2005a,b; Shakil et al., 2005; Win-Shwe et al., 2007). With microdialysis, several analytes can be measured simultaneously, allowing for studies of multiple neurotransmitter systems. However, the temporal and spatial resolution of this technique may be inadequate to properly measure the fast dynamics of Glu neurotransmission. Strides have been made to increase the temporal resolution into intervals of several seconds by coupling microdialysis with capillary electrophoresis (Tucci et al., 1997; Kennedy et al., 2002; Rossell et al., 2003). Even with these recent improvements, it is unlikely that microdialysis can achieve the second-by-second response time necessary for studying in vivo Glu dynamics due to diffusion processes required for molecules to cross the dialysis membrane. Although it has been demonstrated that collection and analysis of microdialysis samples corresponding to 1-s intervals is possible (Rossell et al., 2003), work by Kennedy’s group has demonstrated that band broadening of the sample within the tubing prevents this technique from achieving response times faster than 10 s (Lada et al., 1997). Because Glu neurotransmission occurs on a subsecond-to-second time scale (Kinney et al., 1997), faster recording techniques are necessary to understand its release and uptake dynamics. Furthermore, several microdialysis studies have reported that the Glu overflow measured with microdialysis is not tetrodotoxin (TTX)-dependent (Timmerman and Westerink, 1997; Baker et al., 2002; Melendez et al., 2005). This supports that Glu signals measured from microdialysis were not neuronally derived, which does not fulfill the classical criteria for exocytotic release and questions the source of resting (tonic) levels of Glu.
Within the past decade, several groups have developed Glu-selective microelectrodes to address fast glutamatergic physiology (Hu et al., 1994; Rahman et al., 2005), and some studies using these electrodes have shown that Glu is TTX-dependent (Kulagina et al., 1999; Day et al., 2006; Oldenziel et al., 2006). Our laboratory has developed an enzyme-based microelectrode array (MEA) that is capable of detecting low levels of Glu on a subsecond time scale (500–800 ms) that is free from central nervous system interferents such as 3,4-dihydroxyphenylacetic acid (DOPAC) and ascorbic acid (AA) (Burmeister and Gerhardt, 2001; Burmeister et al., 2002). These MEAs have been successfully used by our laboratory to measure Glu dynamics in anesthetized and awake Fischer 344 rats (Nickell et al., 2005; Day et al., 2006; Rutherford et al., 2007). The present study concerns the adaptation and validation of these MEAs for selective Glu recordings in awake, freely moving mice. With the development of several transgenic mouse models for Glu metabolism, our laboratory is interested in studying Glu dynamics in inbred mouse strains. Because there is a greater than 99% genomic conservation between mice and humans (Waterston et al., 2002), the rapidly expanding library of transgenic mouse models provides researchers with a animal system to study pertinent human diseases, including diabetes, cancer, immunological disorders, and neurological disorders. Detailed understanding of Glu neurotransmission in the mouse model is needed for researchers to understand their transgenic mouse systems.
For these experiments, we have adapted our methodology for chronic recordings of resting (tonic) and phasic (rapid clearance of exogenously applied Glu) changes in Glu in awake mice. Second, we investigated the reproducibility of such methods for measures in the mouse prefrontal cortex (PFC) and striatum (Str) The data presented show differences in resting Glu levels between the brain regions of the mouse and when compared with similar brain regions in the Long Evans rat (Rutherford et al., 2007). Finally, we studied the pharmacological effects of 100 μM DL-threo-β-hydroxyaspartate (THA), a transportable, competitive inhibitor for all excitatory amino acid transporters (EAATs), and 1 μM TTX, a potent sodium channel blocker, on resting Glu levels in the mouse Str. These are the first studies using the MEA technology to examine Glu dynamics in the PFC and Str of awake, freely moving mice.
Materials and Methods
Animals
Male C57BL/6 mice (Harlan, Indianapolis, IN) 20 to 24 weeks of age and weighing 28 to 32 g at the time of implantation, were used for all experiments. After chronic implantation, mice were individually housed in a 12-h light/dark cycle with food and water ad libitum according to the Association for Assessment and Accreditation of Laboratory Animal Care International-approved animal resource center at the University of Kentucky. Upon arrival to the University of Kentucky animal resource center, and before implantation, mice were allowed to acclimate to their environment for at least 1 week. All appropriate animal care (e.g., food, water, bedding, and cage cleaning) was performed by the Animal Resource Center staff. There were no procedures involving undue discomfort to the animals. After surgery, mice were individually housed under the same conditions. Animal care was approved by the University of Kentucky Institutional Animal Care and Use Committee, and it was in accordance with the Guide for the Care and Use of Laboratory Animals.
Enzyme-Based Microelectrode Array Design
Ceramic-based MEAs were assembled and prepared for in vivo recordings as described previously (Burmeister et al., 2000, 2002; Nickell et al., 2005; Hascup et al., 2006; Rutherford et al., 2007). The four platinum recording sites were dip-coated with Nafion (Sigma-Aldrich, St. Louis, MO) to repel anions such as DOPAC and AA (Burmeister and Gerhardt, 2001). Next, the bottom pair of recording sites (sites 1 and 2) were coated with a Glu oxidase (GluOx) (Seikagaku America, East Falmouth, MA) containing a final concentration of 1% bovine serum albumin (BSA) (Sigma-Aldrich), 0.125% glutaraldehyde (Sigma-Aldrich), and 1% GluOx. The BSA and glutaraldehyde help induce cross-linking of the GluOx onto the platinum recording sites. GluOx is required for our recording technique to measure Glu because it metabolizes Glu to α-ketoglutarate and the reporter molecule H2O2. When a potential of +0.7 V versus a silver/silver chloride reference electrode was applied to the MEA, the H2O2 was oxidized and transferred two electrons to our recording surface. The resulting change in current was then amplified and digitized by a Fast Analytical Sensing Technology (FAST)16 mkII recording system (Quanteon, LLC, Nicholasville, KY). The top pair of recording sites (sites 3 and 4) was coated with only BSA and glutaraldehyde, which is referred to as the sentinel sites (Burmeister and Gerhardt, 2001, 2003). This technique treats the MEA as a dual recording microelectrode that allows us to remove possible interferents from the Glu signal. The current on sites 3 and 4 was subtracted from the current on sites 1 and 2, resulting in Glu measures free from noise artifacts and possible interferents such as dopamine, which was not repelled by Nafion. Self-referencing methods were used throughout this study to ensure that resting and peak responses were predominantly due to changes in extracellular Glu levels.
MEA Preparation for Chronic Recordings
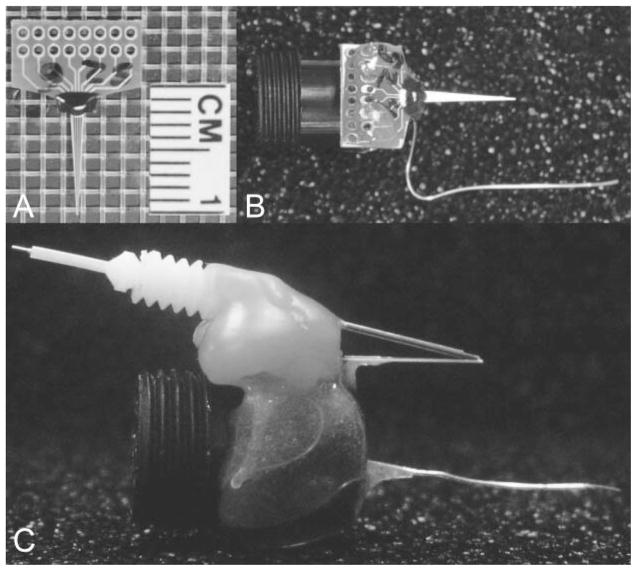
Preparation of the mouse pedestal system (including modifications of the MEA, attachment to the miniature connector containing a silver/silver chloride reference electrode, assembly of the guide cannula, and recording apparatus) were similar to previously published methods by our laboratory for use in awake, freely moving rats (Rutherford et al., 2007). Photographs of the MEA modification process are shown in Fig. 1.
Fig. 1.
MEA modification: photographs of the MEA modification process for chronic implantation in the freely moving mouse. A, stub MEA with a 1-cm ruler for size comparison. B, copper wires are soldered onto the paddle, one for each corresponding platinum recording site. Female, gold sockets are soldered to the other ends of the copper wire, which are inserted into a miniature black connector along with a miniature silver/silver chloride wire. Five-minute epoxy is applied to hold the apparatus together and keep the ceramic tip of the MEA parallel to the miniature black connector for stereotaxic placement. This entire apparatus is now referred to as the pedestal MEA. C, side view of B with an attached stainless steel guide cannula. Sticky Wax (Kerr Brand, Romulus, MI) was used to hold a guide cannula in place that positions an internal cannula among the four platinum recording sites resting approximately 100 μm above the recording surface.
Electrode Calibration
Microelectrodes were calibrated at a constant applied potential of +0.7 V versus a silver/silver chloride reference electrode to determine their sensitivity to Glu and selectivity against AA as described previously (Nickell et al., 2005; Hascup et al., 2006). Constant potential amperometry (final gain of pA/mV) was performed using a FAST16 mkII system designed for four-channel simultaneous recordings. The tip of the MEA attached to the pedestal was placed in a continuously stirred solution of 0.05 M phosphate-buffered saline, pH 7.4. A recirculating water bath (Gaymar Industries, Inc., Orchard Park, NY) was used to maintain a constant buffer temperature of 37°C to simulate in vivo temperature for proper enzyme activity. Aliquots of 20 mM ascorbic acid (500 μl) and 20 mM Glu (40 μl) were used to obtain final buffer concentrations of 250 μM ascorbic acid and 20, 40, and 60 μM Glu for all calibrations. Selectivity ratios for Glu over AA were calculated in addition to the slope (sensitivity for Glu), limit of detection, and linearity (R2) for all Glu MEAs. Reported as mean ± S.E.M., the MEAs had average slopes of −8.6 ± 0.7 pA/μM, limit of detections of 0.9 ± 0.2 μM, and selectivity ratios of 146 ± 20 to 1 (n = 31 MEAs with 61 platinum recording sites). The MEAs were also tested to compare the recording capability among the four platinum recording sites using dopamine (2 μM, final buffer concentration) and H2O2 (8.8 μM, final buffer concentration) as test substances. For self-referencing data analysis, MEAs were only used if all four platinum recordings sites had in vitro responses to H2O2 that were within 10% of each other.
Electrode Implantation
All surgeries were performed in a Vertical Laminar Flow Workstation (Microzone Corporation, Ottawa, ON, Canada), which filtered lab air through a high efficiency particulate air filter. Mice were anesthetized with 2% isoflurane using a SurgiVet anesthetic vaporizer (Smiths Medical PM, Inc., Waukesha, WI) and placed in a stereotaxic apparatus fitted with a mouse adapter (David Kopf Instruments, Tujunga, CA). Animal body temperature was maintained at 37°C with a heating pad (Braintree Scientific, Braintree, MA). The eyes of a mouse were coated with artificial tears (The Butler Company, Columbus, OH) to help maintain moisture and to prevent infection. Before incision, the skin on top of the head of a mouse was wiped with Betadine solution to keep the incision area clean and to help prevent infection. The skin on top of the skull of a mouse was reflected, making as small an incision as possible. The mice underwent a 2- × 2-mm craniotomy, and they were implanted with a Glu-selective MEA pedestal assembly into either the right Str (AP, +0.9 mm; ML, 1.5 mm; DV, −3.85 mm versus bregma) or the right PFC (AP, +1.54 mm; ML, 0.3 mm; DV, −2.45 mm versus bregma) based on the coordinates from Paxinos and Franklin (2004). Three small holes were also drilled in the skull. Two holes for a small, stainless steel screw (Small Parts, Inc., Miami Lakes, FL), which served as anchors. The final hole was used for placement of the miniature silver/silver chloride reference electrode. The assembly was secured with approximately four layers of dental acrylic (Lang Dental MFG, Wheeling, IL).
After surgery, mice were placed on a heating pad to help maintain body temperature until recovering from anesthesia (approximately 30 min). One injection of 0.005 mg/g body weight of carpofen was given to each mouse immediately after surgery to help with inflammation and pain. Mice were allowed to recover for a minimum of 2 days before initial recordings.
Recording Protocol
Drug naive animals were used for each series of experiments. Before connecting the mouse pedestal with the FAST16 mkII recording system, mice were allowed to freely roam around the recording chamber for at least 30 min to acclimate to their environment. Once connected to the recording system, the mice underwent a minimum 60-min acclimation period to establish a stable baseline recording. Once a stable baseline was established, the dummy cannula was removed and the internal cannula (connected to a 10-μl Hamilton Syringe with a repeating dispenser; Hamilton Co., Reno, NV) was inserted into the guide cannula on recording sessions when chemicals were locally applied. After the internal cannula was inserted, another acclimation period of 30 min was allowed to reestablish baseline. After this period, resting Glu and the effects of locally applied Glu (1 mM; 0.5 μl) were tested on days 3, 5, and 7 postimplantation. For experiments using TTX or THA, Glu was locally applied on day 3 postimplantation to ensure functional MEAs. Either TTX and citrate control or THA and saline control were locally applied on day 4 postimplantation, and volumes were kept constant at 1.0 μl. Solutions were locally applied over 2- to 3-s intervals using the repeating dispenser. Local application of solutions in this manner is similar to convection-enhanced delivery. Numerous studies have shown that convection-enhanced delivery of molecules into the brain of rats, cats, primates, and humans did not result in any clinical deterioration, raised intracranial pressure, or histological evidence of disrupted cytoarchitecture other than at the site of cannula insertion (Occhiogrosso et al., 2003). What was often observed was pressure or dilution artifact due to local application of control solutions (saline or citrate) from cannula placed in proximity (50–100 μm) of the working electrode. This has been observed by our laboratory (Burmeister and Gerhardt, 2001) and other laboratories using microelectrodes (Oldenziel et al., 2006). These changes were not from the solutions evoking Glu release, but rather they were from introduction of exogenous fluid into the extracellular space surrounding the MEA.
After completion of all recording sessions (after multiple days of recording), mice were anesthetized with isoflurane and transcardially perfused with 0.9% saline followed by 4% paraformaldehyde. The brain was removed and stored in 4% paraformaldehyde for 3 days followed by storage in 0.1 M phosphate buffer (10% sucrose) for sectioning and staining to confirm MEA placement.
Data Analysis
The FAST16 mkII recording system saved amperometric data, time, and local application data marks for all four recording channels. Unless otherwise noted, calibration data, in conjunction with a modified Microsoft Excel spreadsheet program (Jason J. Burmeister, Center for Microelectrode Technology, Lexington, KY) was used to determine parameters for the signal differences recorded between data marks. Measures derived from the data file included 1) maximum amplitude, the peak concentration of the signal; and 2) t80, the time in seconds from maximum amplitude to 80% decay of the signal. An analysis of variance with a Tukey’s post hoc test was used to analyze the significance of signals over days. A two-tailed t test was used to compare changes in Glu concentration between control and either TTX or THA. All statistical analysis was performed on GraphPad Prism 4 (GraphPad Software Inc., San Diego, CA), and significance was defined as p < 0.05. All data are represented as S.E.M.
Results
Validation of Glu Signals in Vivo
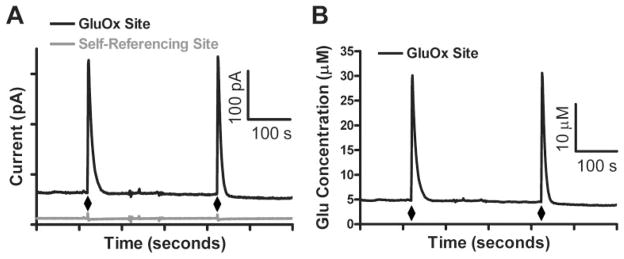
The FAST16 mkII recording system along with our modified MEA (Fig. 1) allowed for reliable second-by-second recordings of Glu in the Str and PFC of awake, freely behaving mice for as long as 2 weeks postimplantation. Local application of an isotonic solution of Glu (1 mM; 0.5 μl), pH 7.4, through an internal cannula was used to verify Glu recordings in vivo. In Fig. 2A, the top, black tracing shows a GluOx-coated site (Glu recording), whereas the bottom, gray tracing shows the self-referencing, or sentinel site. When we use our offline self-referencing technique (Burmeister and Gerhardt, 2001), the concentration of Glu is obtained that is free of interferents and noise artifacts (Fig. 2B). Glu measures were repeatable within brain regions, and over multiple days (Fig. 3). This self-referencing subtracting technique (Burmeister and Gerhardt, 2001) was also used to measure resting Glu levels as described below.
Fig. 2.
Representative traces from local application of Glu in the PFC. A, representative current tracing of locally applied Glu as shown on a GluOx-coated site (black) and a sentinel recording site (gray) in the PFC. Glu (0.5 μl; 1 mM) was locally applied at the time points indicated by diamonds. A rapid spike in current was recorded on the GluOx-coated site, with very little to no change in current observed on the self-referencing site. B, self-referenced Glu signals obtained from A. Differences in maximum amplitude suggests actual in vivo Glu signal from local application of Glu (diamonds), whereas differences in baseline measures suggests resting Glu levels.
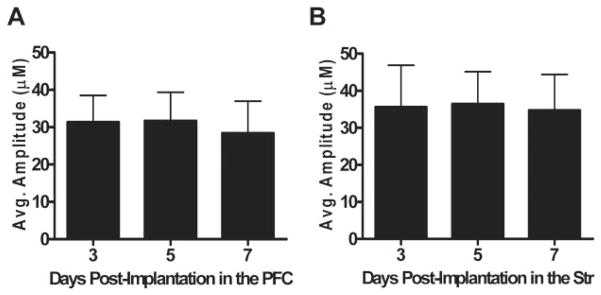
Fig. 3.
Local application of Glu in the PFC and Str: locally applied Glu data for day 3, 5, and 7 postimplantation in the PFC (A; n = 6) and Str (B; n = 8). Substantial and stable Glu recordings were observed over all days measured, indicating reliable, in vivo Glu measurements for at least 1 week postimplantation. Average maximum amplitude over days between the PFC (30.5 ± 7.8) and the Str (35.6 ± 9.9) was not significantly different.
Longevity of the Chronically Implanted MEAs in Awake Mice
An isotonic solution of Glu (1 mM; 0.5 μl), pH 7.4, was locally applied in the PFC and Str of male C57BL/6 mice on days 3, 5, and 7 postimplantation. As shown in Fig. 3, Glu signals were reproducible over multiple days within the PFC (Fig. 3A) and Str (Fig. 3B) and similar between the two brain regions. When the control for Glu (0.9% saline), pH 7.4, was locally applied, we did not observe any release of Glu (data not shown), which was consistent with previously published data (Pomerleau et al., 2003).
Resting Glu Measures
Resting Glu measures in awake mice were measured in the PFC and Str of male, C57BL/6 mice on days 3 through 7 postimplantation using the self-referencing technique described above. In brief, the self-referencing electrode current was subtracted from the Glu recording site current (and divided by the Glu recording site slope obtained from in vitro calibration) during unstimulated recordings. We observed no significant difference in resting Glu over days in the PFC or the Str as shown in Table 1. However, the average resting Glu levels showed a decrease (p = 0.30; two-tailed t test) in the PFC compared with the Str. To determine whether the MEA was still selective for the analyte of interest, the current on the GluOx- and sentinel-coated sites was compared between day 3 and day 7 postimplantation. Current values did not significantly change as a function of time on the GluOx and sentinel sites in both the Str and PFC (data not shown).
TABLE 1.
Resting Glu measures on days 3 to 7 postimplantation
Resting Glu levels on days 3 to 7 postimplantation were measured in the PFC and Str of male C57BL/6 mice.
| Brain Region | Days Postimplantation
|
Average | |||||
|---|---|---|---|---|---|---|---|
| 3 | 4 | 5 | 6 | 7 | |||
| Resting Glu (μM) | PFC (n = 8) | 3.3 ± 1.3 | 3.2 ± 1.1 | 4.0 ± 0.9 | 3.0 ± 0.9 | 3.0 ± 1.0 | 3.3 ± 1.0 |
| Str (n = 10) | 5.5 ± 1.3 | 5.0 ± 1.0 | 5.1 ± 1.1 | 4.4 ± 1.1 | 4.9 ± 1.2 | 5.0 ± 1.2 | |
Increase in Resting Glu Levels after Local Application of THA
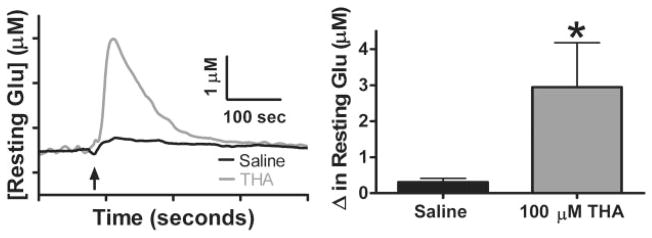
We wanted to determine how blocking the high-affinity transporters (Danbolt, 2001) affects resting Glu levels in the Str. To test this, we locally applied 100 μM THA, a transportable, competitive inhibitor for all EAATs (Anderson et al., 2001; Chatton et al., 2001; Gaillet et al., 2001), in the Str of C57BL/6 mice. Figure 4A shows Glu tracings after local application of 100 μM THA (1.0 μl) in 0.9% saline, pH 7.4, and 0.9% saline control (1.0 μl), pH 7.4. Local application of 100 μM THA in saline caused a significant increase (*, p < 0.05) in resting Glu levels (2.9 ± 1.2 μM; n = 7) compared with saline control (0.3 ± 0.1 μM; n = 7) (Fig. 4B). This was approximately a 60% increase in resting Glu levels in the Str compared with our study in Table 1.
Fig. 4.
Local application of THA: effects of local application of THA. A, 10-s boxcar averaged tracings of local application of THA (1.0 μl; 100 μM) and 0.9% saline control (1.0 μl) in the Str of C57BL/6 mice. B, change in resting Glu levels were significantly increased with local application of THA (2.9 ± 1.2 μM; n = 7) compared with saline control (0.3 ± 0.1 μM; n = 7) (*, p < 0.05).
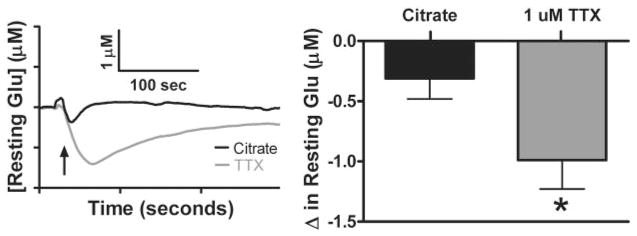
Decrease in Resting Glu Levels after Local Application of TTX
We further investigated the source of resting Glu by estimating the extent of exocytotic release from neurons. To test this, we locally applied TTX, a sodium channel blocker that prevents depolarization and subsequently inhibits the action potential-dependent vesicular release of Glu. Figure 5A shows Glu tracings after local application of 1 μM TTX (1.0 μl), pH 7.4, in an isotonic solution of citrate, in the Str of C57BL/6. TTX (1 μM) in the Str of C57BL/6 mice caused a significant decrease (*, p < 0.05) in resting Glu levels (−1.0 ± 0.2 μM; n = 6) compared with an isotonic solution of citrate control (1.0 μl), pH 7.4 (−0.3 ± 0.1 μM; n = 6) as shown in Fig. 5B. This was approximately a 20% decrease in resting Glu in the Str compared with our study in Table 1.
Fig. 5.
Local application of TTX: effects of local application of TTX. A, 10-s boxcar averaged tracings of local application of TTX (1.0 μl; 1 μM) and citrate control (1.0 μl) in the Str of C57BL/6 mice. B, change in resting Glu levels were significantly decreased with local application of TTX (−1.0 ± 0.2 μM; n = 6) compared with citrate control (−0.3 ± 0.1 μM; n = 6) (*, p < 0.05).
Discussion
We adapted our current method for enzyme-based second-by-second Glu detection for use in awake, freely moving mice. The small size and lightweight design of the pedestal allowed for chronic recordings with limited effect on the behavior of a mouse. Our enzyme-based MEAs reliably measured Glu for 7 days postimplantation with 80% of implanted MEAs functioning to this time point. The small size of our MEAs and our self-referencing capability (Burmeister and Gerhardt, 2001) allowed us to measure resting Glu levels to determine that a portion was TTX-dependent, whereas modulation of the high-affinity Glu transporters by THA increased extracellular Glu levels.
When using amperometry for in vivo studies, selectively identifying the molecule of interest is questioned; however, our laboratory has overcome this issue. The enzyme-based MEAs differentially recorded Glu, because of the high substrate selectivity of GluOx (Kusakabe et al., 1983). Varying the applied potential to the platinum recording surface is another way we identify the molecule of interest (Day et al., 2006; Rutherford et al., 2007). Finally, the ability to subtract off interferents from the Glu signal further enhances the selectivity of our MEA.
Local application of an isotonic solution of Glu (0.5 μl; 1 mM) in the PFC and Str of the C57BL/6 mouse on days 3, 5, and 7 postimplantation was used to determine the longevity of the enzyme-based MEA in vivo. There was no significant change in MEA sensitivity for Glu in either brain region on days 3 to 7 postimplantation. The Str (35.6 ± 9.9 μM) had slightly elevated responses to local application of 1 mM Glu compared with the PFC (30.5 ± 7.8 μM). Rutherford et al. (2007) observed similar in vivo MEA longevity to Glu in the PFC and Str of the Long Evans rat strain. Because there was no decline in MEA response for Glu, we concluded that GluOx, when adhered to our MEA, is viable for 7 days in vivo. However, we do not know whether the MEA loses sensitivity immediately after implantation, nor has a complete analysis of the Glu response past day 7 postimplantation been completed. Complete loss of response to Glu is not due to failure of the MEA itself, because the MEAs respond to H2O2 for 90 days postimplantation (Rutherford et al., 2007); therefore, the most likely cause is failure of the enzyme layer. Additional studies are being conducted to address this issue. Nevertheless, the ability to measure Glu over multiple days in vivo has important implications as we progress this technology to study behavioral-related changes in extracellular Glu in the awake, freely moving mouse.
Our MEA self-referencing recording techniques can also be used to measure resting Glu levels (Day et al., 2006; Rutherford et al., 2007). Data in Table 1 indicate neither a significant change in resting Glu levels over days within brain regions nor when averages were compared between the PFC (3.3 ± 1.0 μM) and Str (5.0 ± 1.2 μM) (p = 0.31). If MEA selectivity was deteriorating in vivo, we should observe an increase in resting current levels on the sentinel and GluOx recording channels as a function of time. However, we do not see changes in these levels, indicating that the MEA is still effective at repelling interferents such as DOPAC and AA. If the MEA was becoming less selective, it would occur on both the GluOx and sentinel sites. Even if this would occur, the advantage of the self-referencing subtraction method allows us to subtract off any chemical interferents that may contribute to a portion of the Glu signal.
The limited number of publications examining resting Glu levels using microdialysis in freely moving C57BL/6 mice makes it difficult to compare techniques. We are unaware of studies using microdialysis in the PFC of freely moving C57BL/6 mice, but Meshul’s group has examined resting Glu in the Str (Holmer et al., 2005a,b; Shakil et al., 2005). Resting Glu levels obtained with our MEA technology in the Str of freely moving C57BL/6 mice were double compared with what Meshul’s group observed using microdialysis (Table 2). These discrepancies may be explained by the difference in sampling time and method and also by the origin of the Glu pool being sampled. The small size and smooth edges of our MEA tips (333 × 200 × 125 μm) cause less damage compared with a microdialysis probe (Clapp-Lilly et al., 1999; Borland et al., 2005; Rutherford et al., 2007). Furthermore, a faster sampling rate allows us to measure more synaptic Glu and subsequently the neuronal pool of Glu (Drew et al., 2004). The Glu that diffuses from the synapse to our MEA was able to be detected due to our enhanced temporal resolution.
TABLE 2.
Comparison of resting Glu levels in the striatum of C57BL/6 mice
Resting Glu levels in the Str of C57BL/6 mice when measured using MEAs compared with studies using microdialysis probes.
| Reference | Technique | Sampling Interval | Resting Glu |
|---|---|---|---|
| μM | |||
| Hascup et al. (2006) | MEA | 1 s | 5.0 ± 1.2 |
| Holmer et al. (2005b) | Microdialysis | 15 min | 3.5 ± 0.1 to 4.2 ± 0.1 |
| Holmer et al. (2005a) | Microdialysis | 15 min | ~2.75 |
| Shakil et al. (2005) | Microdialysis | 15 min | 1.2 ± 0.1 to 1.8 ± 0.1 |
It is interesting to note that resting Glu values obtained in the C57BL/6 PFC are 1 order of magnitude lower than those obtained in the Long Evans PFC, but striatal resting Glu levels between the two species are similar (Rutherford et al., 2007). We have also observed that resting Glu levels in the PFC and Str of Fisher 344 rats are double compared with the respective brain regions in C57BL/6 mice (Rutherford et al., 2007). Because of these observations, we think that resting Glu levels can vary within brain regions and across strains and species of animals. This is an important aspect of Glu dynamics we are pursuing.
Our laboratory determined how resting Glu levels in the Str were affected by blocking all EAATs using the transportable, competitive inhibitor THA (Fig. 4). Local application of an isotonic solution of 100 μM THA (1.0 μl), pH 7.4, caused a significant (p < 0.05) increase (2.9 ± 1.2 μM) in resting Glu levels compared with 0.9% saline control (0.3 ± 0.1 μM). This is an approximate 60% increase in resting Glu levels compared with average Str values from Table 1. Furthermore, local application of THA resulted in sustained Glu elevation compared with our data from local application of exogenous Glu. This is expected because resting Glu is now competing for uptake with THA, whereas exogenously applied Glu induces an immediate effect without competition for uptake. Heteroexchange of Glu from astrocytes is possible with transportable inhibitors; however, even nontransportable inhibitors such as DL-threo-β-benzyloxyaspartate induce heteroex-change at similar concentrations (Anderson et al., 2001).
To determine the neuronal contribution to resting Glu levels in the Str, the sodium channel blocker TTX was locally applied. Local application of an isotonic solution of 1 μM TTX (1.0 μl), pH 7.4 (−1.0 ± 0.2 μM), caused a significant decrease (p < 0.05) in resting Glu levels compared with citrate control (1.0 μl), pH 7.4 (−0.3 ± 0.1 μM). This was an approximate 20% decrease in resting Glu levels in the Str compared with averaged values from Table 1. This observation contrasts several microdialysis studies that do not show TTX dependence in rats (Timmerman and Westerink, 1997) and further supports findings conducted in rats with microelectrodes (Kulagina et al., 1999; Day et al., 2006; Oldenziel et al., 2006). Unlike other TTX studies using Glu-selective microelectrodes in anesthetized rats, our freely moving mouse studies did not completely attenuate resting Glu levels for two possible reasons. First, anesthetics exert their effects by inhibiting excitatory neurotransmission (Hara and Harris, 2002), and our laboratory demonstrated that urethane significantly decreased resting Glu levels in rats by 58% (Rutherford et al., 2007). This suggests that resting Glu levels are altered in anesthetized rodents, which may explain why TTX did not, completely attenuate resting Glu levels in the freely moving mouse. Second, our concentration of TTX used was 100-fold lower compared with those used in the anesthetized rat studies (Kulagina et al., 1999; Day et al., 2006; Oldenziel et al., 2006), and it may not have resulted in complete inhibition of action potentials to all glutamatergic synapses surrounding the MEA.
Our laboratory has observed similar decreases (~20%) in resting Glu after local application of TTX in freely moving rats (Rutherford et al., 2006). This indicates that other sources are contributing to resting Glu levels, including the metabotropic Glu receptors and the cystine-Glu exchanger (Baker et al., 2002). Our laboratory has data indicating that local application of inhibitors of presynaptic metabotropic Glu receptors and the cystine-Glu exchanger can decrease resting Glu levels in the freely moving rat (Rutherford et al., 2006). Additional studies are being conducted to determine their exact contribution to resting Glu levels and other potential sources.
In summary, the present study indicated MEAs configured for second-by-second Glu measurements were viable for 7 days postimplantation in the awake, freely moving mouse. We observed a nonsignificant difference in resting Glu levels between the PFC and Str of awake C57BL/6 mice. In addition, local application of THA significantly increased resting Glu levels, whereas TTX significantly decreased resting Glu levels. These findings provide evidence that the high spatial and fast temporal resolution provided by our MEA technology is necessary to better understand resting Glu levels. Furthermore, the ability to monitor Glu for 7 days postimplantation allows us to design behavioral paradigms to better understand physiological release and uptake of Glu in the central nervous system.
Acknowledgments
This work was supported by DARPA Grant N66001-02-C-8085, National Science Foundation Grant DBI-0352848, U.S. Public Health Service Grant DA017186, and National Institute of Health training Grant 1 T32 DA022738-01.
ABBREVIATIONS
- Glu
L-glutamate
- TTX
tetrodotoxin
- MEA
microelectrode array
- DOPAC
3,4-dihydroxyphenylacetic acid
- AA
ascorbic acid
- PFC
prefrontal cortex
- Str
striatum
- THA
DL-threo-β-hydroxyaspartate
- EAAT
excitatory amino acid transporter
- GluOx
L-glutamate oxidase
- BSA
bovine serum albumin
- FAST
fast analytical sensing technology
References
- Anderson CM, Bridges RT, Chamberlin AR, Shimamoto K, Yasuda-Kamatani Y, Swanson RA. Differing effects of substrate and non-substrate transport inhibitors on glutamate uptake reversal. J Neurochem. 2001;79:1207–1216. doi: 10.1046/j.1471-4159.2001.00668.x. [DOI] [PubMed] [Google Scholar]
- Baker DA, Xi ZX, Shen H, Swanson CJ, Kalivas PW. The origin and neuronal function of in vivo nonsynaptic glutamate. J Neurosci. 2002;22:9134–9141. doi: 10.1523/JNEUROSCI.22-20-09134.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borland LM, Shi G, Yang H, Michael AC. Voltammetric study of extra-cellular dopamine near microdialysis probes acutely implanted in the striatum of the anesthetized rat. J Neurosci Methods. 2005;146:149–158. doi: 10.1016/j.jneumeth.2005.02.002. [DOI] [PubMed] [Google Scholar]
- Burmeister JJ, Moxon K, Gerhardt GA. Ceramic-based multisite microelectrodes for electrochemical recordings. Anal Chem. 2000;72:187–192. doi: 10.1021/ac9907991. [DOI] [PubMed] [Google Scholar]
- Burmeister JJ, Gerhardt GA. Self-referencing ceramic-based multisite microelectrodes for the detection and elimination of interferences from the measurement of L-glutamate and other analytes. Anal Chem. 2001;73:1037–1042. doi: 10.1021/ac0010429. [DOI] [PubMed] [Google Scholar]
- Burmeister JJ, Gerhardt GA. Ceramic based-multisite microelectrode arrays for in vivo electrochemical recordings of glutamate and other neurochemicals. Trends Anal Chem. 2003;22:498–502. [Google Scholar]
- Burmeister JJ, Pomerleau F, Palmer M, Day BK, Huettl P, Gerhardt GA. Improved ceramic-based multisite microelectrode for rapid measurements of L-glutamate in the CNS. J Neurosci Methods. 2002;119:163–171. doi: 10.1016/s0165-0270(02)00172-3. [DOI] [PubMed] [Google Scholar]
- Clapp-Lilly KL, Roberts RC, Duffy LK, Irons KP, Hu Y, Drew KL. An ultrastructural analysis of tissue surrounding a microdialysis probe. J Neurosci Methods. 1999;90:129–142. doi: 10.1016/s0165-0270(99)00064-3. [DOI] [PubMed] [Google Scholar]
- Chatton J, Shimamoto K, Magistretti PJ. Effects of glial glutamate transporter inhibitors on intracellular Na+ in mouse astrocytes. Brain Res. 2001;893:46–52. doi: 10.1016/s0006-8993(00)03286-8. [DOI] [PubMed] [Google Scholar]
- Danbolt NC. Glutamate uptake. Prog Neurobiol. 2001;65:1–105. doi: 10.1016/s0301-0082(00)00067-8. [DOI] [PubMed] [Google Scholar]
- Day BK, Pomerleau F, Burmeister JJ, Huettl P, Gerhardt GA. Microelectrode array studies of basal and potassium-evoked release of L-glutamate in the anesthetized rat brain. J Neurochem. 2006;96:1626–1635. doi: 10.1111/j.1471-4159.2006.03673.x. [DOI] [PubMed] [Google Scholar]
- Di Chiara G, Tanda G, Carboni E. Estimation of in-vivo neurotransmitter release by brain microdialysis: the issue of validity. Behav Pharmacol. 1996;7:640–657. [PubMed] [Google Scholar]
- Drew KL, Pehek EA, Rasley BT, Ma YL, Green TK. Sampling glutamate and GABA with microdialysis: suggestion on how to get the dialysis membrane closer to the synapse. J Neurosci Methods. 2004;140:127–131. doi: 10.1016/j.jneumeth.2004.04.039. [DOI] [PubMed] [Google Scholar]
- Gaillet S, Plachez C, Malaval F, Bézine MF, Récasens M. Transient increase in the high affinity [3H]-L-glutamate uptake activity during in vitro development of hippocampal neurons in culture. Neurochem Int. 2001;38:293–301. doi: 10.1016/s0197-0186(00)00098-x. [DOI] [PubMed] [Google Scholar]
- Hara K, Harris RA. The anesthetic mechanism of urethane: the effects on neurotransmitter-gated ion channels. Anesth Analg. 2002;94:313–318. doi: 10.1097/00000539-200202000-00015. [DOI] [PubMed] [Google Scholar]
- Hascup KN, Rutherford EC, Quintero JE, Day BK, Nickell JR, Pomerleau F, Huettl P, Burmeister JJ, Gerhardt GA. Second-by-Second Measures of L-glutamate and other neurotransmitters using enzyme-based microelectrode arrays. In: Michael AC, Borland LM, editors. Electrochemical Methods for Neuroscience. CRC Press; Boca Raton, FL: 2006. pp. 407–450. [PubMed] [Google Scholar]
- Holmer HK, Keyghobadi M, Moore C, Menashe RA, Meshul CK. Dietary restriction affects striatal glutamate in the MPTP-induced mouse model of nigrostriatal degeneration. Synapse. 2005a;57:100–112. doi: 10.1002/syn.20163. [DOI] [PubMed] [Google Scholar]
- Holmer HK, Keyghobadi M, Moore C, Meshul CK. L-dopa-induced reversal in striatal glutamate following partial depletion of nigrostriatal dopamine with 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine. Neuroscience. 2005b;136:333–341. doi: 10.1016/j.neuroscience.2005.08.003. [DOI] [PubMed] [Google Scholar]
- Hu Y, Mitchell KM, Albahadily FN, Michaelis EK, Wilson GS. Direct measurement of glutamate release in the brain using a dual enzyme-based electrochemical sensor. Brain Res. 1994;659:117–125. doi: 10.1016/0006-8993(94)90870-2. [DOI] [PubMed] [Google Scholar]
- Kennedy RT, Watson CJ, Haskins WE, Powell DH, Strecker RE. In vivo neurochemical monitoring by microdialysis and capillary separations. Curr Opin Chem Biol. 2002;6:659–665. doi: 10.1016/s1367-5931(02)00373-3. [DOI] [PubMed] [Google Scholar]
- Kinney GA, Overstreet LS, Slater NT. Prolonged physiological entrapment of glutamate in the synaptic cleft of cerebellar unipolar brush cells. J Neurophysiol. 1997;78:1320–1333. doi: 10.1152/jn.1997.78.3.1320. [DOI] [PubMed] [Google Scholar]
- Kulagina NV, Shanker L, Michael AC. Monitoring glutamate and ascorbate in the extracellular space of brain tissue with electrochemical microsensors. Anal Chem. 1999;71:5093–5100. doi: 10.1021/ac990636c. [DOI] [PubMed] [Google Scholar]
- Kusakabe H, Midorikawa Y, Fujishim T, Kuninaka A, Yoshino H. Purification and properties of a new enzyme, L-glutamate oxidase, from Streptomyces sp. X-119-6 grown on wheat bread. Agric Biol Chem. 1983;47:1323–1328. [Google Scholar]
- Lada MW, Vickroy TW, Kennedy RT. High temporal resolution monitoring of glutamate and aspartate in vivo using microdialysis on-line with capillary electrophoresis with laser-induced fluorescence detection. Anal Chem. 1997;69:4560–4565. doi: 10.1021/ac970518u. [DOI] [PubMed] [Google Scholar]
- Melendez RI, Vuthiganon J, Kalivas PW. Regulation of extracellular glutamate in the prefrontal cortex: focus on the cysteine glutamate exchanger and group I metabotropic glutamate receptors. J Pharmacol Exp Ther. 2005;314:139–147. doi: 10.1124/jpet.104.081521. [DOI] [PubMed] [Google Scholar]
- Nickell J, Pomerleau F, Allen J, Gerhardt GA. Age-related changes in the dynamics of potassium-evoked L-glutamate release in the striatum of Fischer 344 rats. J Neural Transm. 2005;112:87–96. doi: 10.1007/s00702-004-0151-x. [DOI] [PubMed] [Google Scholar]
- Occhiogrosso G, Edgar MA, Sandberg DI, Souweidane MM. Prolonged convection-enhanced delivery into the rat brainstem. Neurosurgery. 2003;52:388–394. doi: 10.1227/01.neu.0000043696.83722.8d. [DOI] [PubMed] [Google Scholar]
- Oldenziel WH, Dijkstra G, Cremers TIFH, Westerink BHC. In vivo monitoring of extracellular glutamate in the brain with a microsensor. Brain Res. 2006;1118:34–42. doi: 10.1016/j.brainres.2006.08.015. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Franklin KBJ. The Mouse Brain in Sterotaxic Coordinates. Academic Press; San Diego, CA: 2004. [Google Scholar]
- Pomerleau F, Day BK, Huettl P, Burmeister JJ, Gerhardt GA. Real time in vivo measures of L-glutamate in the rat central nervous system using ceramic-based multisite microelectrode arrays. Ann N Y Acad Sci. 2003;1003:454–457. doi: 10.1196/annals.1300.051. [DOI] [PubMed] [Google Scholar]
- Rahman A, Kwon NH, Won MS, Choe ES, Shim YB. Functionalized conducting polymer as an enzyme-immobilizing substrate: an amperometric glutamate microbiosensor for in vivo measurements. Anal Chem. 2005;77:4854–4860. doi: 10.1021/ac050558v. [DOI] [PubMed] [Google Scholar]
- Rutherford EC, Pomerleau F, Huettl P, Strömberg I, Gerhardt GA. Chronic second-by-second measures of L-glutamate in the CNS of freely moving rats. J Neurochem. 2007;102:712–722. doi: 10.1111/j.1471-4159.2007.04596.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutherford EC, Pomerleau F, Huettl P, Strömberg I, Johnson KW, Gerhardt GA. Second-by-second enzyme-based microelectrode recordings of basal L-glutamate in the prefrontal cortex of awake rats. In: Di Chiara G, Carboni E, Valentini V, Acquas E, Bassareo V, Cadoni C, editors. Monitoring Molecules in Neuroscience; 2006 11th International Conference; 2006 May; Sardinia, Italy. Cagliari, Italy: University of Cagliari; 2006. pp. 334–336. [Google Scholar]
- Rossell S, Gonzalez LE, Hernández L. One-second time resolution brain microdialysis in fully awake rats: protocol for the collection, separation and sorting of nanoliter dialysate volumes. J Chromatogr B. 2003;784:385–393. doi: 10.1016/s1570-0232(02)00826-7. [DOI] [PubMed] [Google Scholar]
- Shakil SS, Holmer HK, Moore C, Abernathy AT, Jakowec MW, Petzinger GM, Meshul CK. High and low responders to novelty show differential effects in striatal glutamate. Synapse. 2005;58:200–207. doi: 10.1002/syn.20198. [DOI] [PubMed] [Google Scholar]
- Timmerman W, Westerink BHC. Brain microdialysis of GABA and glutamate: what does it signify? Synapse. 1997;27:242–261. doi: 10.1002/(SICI)1098-2396(199711)27:3<242::AID-SYN9>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- Tucci S, Rada P, Sepúlveda MJ, Hernandez L. Glutamate measured by 6-s resolution brain microdialysis: capillary electrophoretic and laser-induced fluorescence detection application. J Chromatogr B. 1997;694:343–349. doi: 10.1016/s0378-4347(96)00488-4. [DOI] [PubMed] [Google Scholar]
- Ungerstedt U. Measurement of neurotransmitter release by intracranial dialysis. In: Marsden CA, editor. Measurement of Neurotransmitter Release in Vivo. John Wiley & Sons; New York: 1984. pp. 81–105. [Google Scholar]
- Waterston RH, Lindblad-Toh K, Birney E, Rogers J, Abril JF, Agarwal P, Agarwala R, Ainscough R, Alexandersson M, An P, et al. Initial sequencing and comparative analysis of the mouse genome. Nature. 2002;420:520–562. doi: 10.1038/nature01262. [DOI] [PubMed] [Google Scholar]
- Win-Shwe TT, Misushima D, Nakajima D, Ahmed S, Yamamoto S, Tsukahara S, Kakeyama M, Goto S, Fujimaki H. Toluene induces rapid and reversible rise of hippocampal glutamate and taurine neurotransmitter levels in mice. Toxicol Lett. 2007;198:75–82. doi: 10.1016/j.toxlet.2006.10.017. [DOI] [PubMed] [Google Scholar]