Abstract
In the past two decades, human gene transfer research has been translated from a laboratory technology to clinical evaluation. The success of adoptive transfer of tumor-reactive lymphocytes to treat the patients with metastatic melanoma has led to new strategies to redirect normal T cells to recognize tumor antigens by genetic engineering with tumor antigen-specific T cell receptor (TCR) genes. This new strategy can generate large numbers of defined antigen-specific cells for therapeutic application. Much progress has been made to TCR gene transfer systems by optimizing gene expression and gene transfer protocols. Vector and protein modifications have enabled excellent expression of introduced TCR chains in human lymphocytes with reduced mis-pairing between the introduced and endogenous TCR chains. Initial clinical studies have demonstrated that TCR gene-engineered T cells could mediate tumor regression in vivo. In this review, we discuss the progress and prospects of TCR gene-engineered T cells as a therapeutic strategy for treating patients with melanoma and other cancers.
Keywords: T cell receptor, Gene therapy, Cancer immunotherapy
1. Introduction
Adoptive cellular immunotherapy (ACT), the administration of autologous or allogenic tumor-reactive T cells into patients, is a promising cancer therapy. It has been successful in achieving tumor regression in transplant-related malignancies, leukemia, and melanoma. In melanoma, this process involves the identification of lymphocytes with high tumor recognition that can be expanded in vitro, and administered back to patients [1]. In pioneering clinical studies at the Surgery Branch, US National Cancer Institute, it was shown that using selected tumor-reactive infiltrating lymphocytes (TIL) mediated a 50–70% response rate in patients with metastatic melanoma when combined with lymphodepleting chemotherapy and IL-2 administration [2,3]. However, the generation of such patient-specific tumor-reactive lymphocytes is time-consuming and technically demanding, limiting the number of cancer patients that receive treatment.
The success of ACT for the treatment of metastatic melanoma patients laid the foundation for the current interest in genetic engineering with T cell receptors to redirect effector T cell reactivity. Genes encoding TCRs can be isolated from tumor-reactive T cell clones and introduce into circulating lymphocytes for therapeutic application. This approach has opened the possibility to treat patients with a variety of cancer types other than melanoma. In addition to the generation of anti-tumor effector cells, genetic engineering of T cell also offers a strategy to introduce molecules that can augment T cell function or overcome tumor evasion mechanisms, such as adding genes encoding homeostatic or pro-inflammatory cytokines, chemokine receptors and costimulatory factors, as well as elements to silence inhibitory molecules.
During the past decade, progresses have been made to improve the efficiency of TCR gene transfer and expression with subsequent enhanced function of the TCR gene-engineered cells. The aim of this review is to discuss the development of TCR gene transfer and update the clinical application of this cell-based drug delivery.
2. TCR as a therapeutic tool
The T cell antigen receptor is the protein expressed on the surface of T lymphocytes and mediates recognition of antigenic peptides presented by major histocompatability complex (MHC) proteins. Antigen specificity is determined by the TCR heterodimer, which is composed of two-chains, either αβ or γδ. Genes encoding the TCR α and β chains are molecularly cloned from highly reactive T cells that recognize and lyse target tumor cells and can be inserted into gene transfer vectors such as, retroviral or lentiviral vectors using recombinant DNA techniques. The genetic transfer of TCR α and β chains directed against specific tumor antigens can create antigen-specific T cells from any normal T cell.
The identification of large numbers of tumor antigens has been essential to the development of TCR based immunotherapy [4]. The most widely studied antigens recognized by tumor infiltrating lymphocytes in human melanoma include; melanocyte differentiation antigens, tumor-specific antigens, and normal proteins highly over-expressed in tumors [5]. Recently, many investigators have focused on cancer testis (CT) antigens as targets for therapeutic cancer vaccines and TCR based adoptive immunotherapy. More than 110 CT antigens have been identified and these are normally expressed only in the human germ line, but are also expressed in various tumor types [6,7]. Targeting T cells to tumor-associated CT antigens might selectively eliminate tumor cells and avoid or reduce toxicity to normal tissue. Identification of suitable target antigens for TCR gene therapy will be a high priority for the coming years.
The first TCR gene transfer to human peripheral blood lymphocytes conferring anti-tumor reactivity was reported in 1999 using a melanoma-antigen specific TCR [8]. Since then several reports demonstrated that transfer of a tumor antigen-specific TCR into T cells results in an antigen-specific T cell population [9–11]. This approach bypasses the need to isolate tumor-specific T effector cells from individual cancer patients as the TCR-transduced T cells display tumor-specific recognition. It has demonstrated in vitro that TCR gene-engineered T cells secrete immunostimulatory cytokines (IFN-γ, IL-2, and TNFα) upon encounter with antigen positive tumor cells, exhibit antigen-specific cytotoxicity, and are able to expand in response to the antigen stimulation [11,12]. In early clinical studies, circulating lymphocytes from cancer patients were engineering with TCRs against melanocyte differentiation antigens MART-1 and gp100 and the adoptive transfer of these cells into the lymphodepleted patients was shown to mediate cancer regression [13,14]. The clinical success of adoptive cell transfer therapy of TCR engineered T cells offers the possibility to treat patients who do not have natural tumor-reactive T cells.
2.1. Improving transgenic T cell receptor gene expression
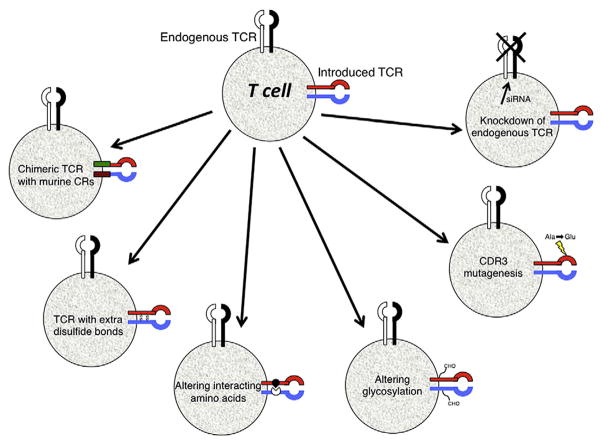
It is known that TCR function depends on the expression level of the α and β TCR chains. Initial TCR gene transfer studies generated small numbers of effector T cells with poor function most likely caused by low-level expression of the introduced TCR chains. Much progress has been made to improve the TCR gene transfer efficacy by optimizing the transduction protocol and by modifying TCR genes to improve specific chain pairing and expression (Fig. 1).
Fig. 1.
Strategies to improve TCR activity. Shown are the varieties of different strategies that can be used to potentially improve TCR activity (see text for details). These methods include creating hybrid molecules containing the constant region (CR) from murine TCR chains, as well as adding extra disulfide bonds, and altering amino acids or glycosylation sites within the TCR chains. Mutagenesis of amino acids in the complementarity determining region 3 (CDR3) and knocking down endogenous TCR gene expression using short interfering RNAs (siRNA) can also increase the overall reactivity of the introduced TCR.
One of the potential problems in ectopic expression of tumor-specific TCR is the formation of mixed dimmers between endogenous and introduced TCR chains. Theoretically, co-expression of two α and two β chains could form four different TCRs: (i) the endogenous αβ heterodimer; (ii) the introduced αβ heterodimer; (iii) the introduced α chain paired with endogenous β chain; and (iv) the endogenous α chain paired with the introduced β chain. The mixed pairing of TCR chains will not target tumor antigens and, in theory, could result in T cells with novel reactivity that may be deleterious to the host. Though there is no evidence of toxicity or autoimmunity in reported clinical trials, several strategies have been developed to prevent mixed dimer formation while increasing TCR expression.
The first strategy to lessen mixed dimmer formation was the development of murine-human hybrid TCRs in which the constant region of the human TCR chains was replaced by their murine counterparts [15]. Compared with human TCR, the hybrid TCR exhibited enhanced expression leading to superior function of the engineered T cells including higher levels of cytokine release and cytolytic activity [15]. Most significantly, the chimeric TCR chains preferentially associated with themselves and not the endogenous TCR chains. Another approach to increase the specific pairing of TCR chains was to introduce additional cysteine residues within the constant region of the TCR α and β chains [16,17]. The additional disulfide bond formed by the extra cysteine residues was demonstrated to enhance TCR expression and therefore improve the functional activity. Alternatively, mutational inversions of the critical interacting amino acids in the TCR α and β chain constant regions favor the pairing of the introduced chains and also increase TCR reactivity [17]. As an alternative to protein engineering, Okamoto et al. have reported that small interfering RNA (siRNA) can be used to specifically down-regulate the endogenous TCR resulting in improved expression and reactivity of the transduced TCR [18]. Finally, a non-molecular approach to avoid mis-pairing is to use γδ T cells for αβ TCR gene engineering, however, the function and persistence of γδ T cells in adoptive cell therapy are not well studied.
The most critical property for a TCR used for engineering T cells for clinical application is the requirement for high affinity specific recognition of the tumor antigen peptide-HLA complex. Producing T cells with high functional avidity can be achieved by increasing the TCR affinity or increasing the expression of the TCR on the T cell surface. Several methods have been published recently to increase TCR affinity by modification of TCR genes. It is known that the third complementarity determining region (CDR3) of the TCR is critical for antigen recognition and binding. Single or dual amino acid substitutions in the CDR α or β chains have been shown to provide modest increase in TCR affinity that can enhance antigen-specific reactivity in T cells [12,19–21]. Some reports suggested that the removal of defined N-glycosylation motifs in the constant domains of TCR chains resulted in increased functional avidity and enhanced recognition of tumor cells [22]. At the level of mRNA, it has been demonstrated that improved expression of TCR genes on the T cell surface can be achieved by codon optimization [23]. In this approach rare codons used by the wild type human or murine derived TCR are replaced by those codons most frequently distributed in highly expressed human genes. During the optimization process cis-acting AT or GC rich sequence stretches, cryptic splicing and RNA instability motifs are also removed. An alternative biological strategy to generate high avidity TCRs is to immunize human-HLA-transgenic mice with human tumor antigen peptides. Murine CTL generated by this method is used to isolate murine TCR genes specific for HLA-restricted human peptides. This approach has been shown feasible for peptides from the human gp100 [13], p53 [24], CEA [20] and MAGE-A3 [12]. It has been reported that some patients treated with murine TCR gene-engineered T cells developed antibodies directed to murine TCR variable regions. However, the development of patient immune response did not affect the clinical outcome [25]. One potential concern regarding the generation of very high affinity TCRs, is the observed lack of peptide-specificity in some ultra-high affinity TCRs and, it is not clear what is the affinity threshold for optimal TCR function [26].
2.2. TCR gene delivery systems
The success of TCR gene transfer is built on the gene delivery system. It has been demonstrated that getting high efficiency and stable gene transfer in T cell using traditional chemical methods is difficult. To date, most of the reported TCR gene transfer studies are based on viral vector-based expression system. Gamma-retroviral vectors have been used as gene transfer reagents in human application for more than 20 years, including MFG/SFG-, MP71/SF91-, and MSGV1-based vector systems [27–29]. These vectors have been used in several TCR clinical studies in which they were shown to have good transduction efficiency and no vector-associated toxicity has been reported to date. Alternatives to gamma-retroviral vectors are lentiviral vectors’ expression systems. Lentiviral vectors can transduce non-dividing or minimal stimulated T cells, which may be beneficial as less-differentiated or naïve T cells have been reported to be superior for adoptive immunotherapy in animal models [30,31]. It is also reported that lentiviral vectors offer a larger gene insertion capacity and may be less prone to insertional mutagenesis due to their random integration unlike gamma-retroviral vectors that have preferential integration near the transcription start sites of genes [32]. Transposon-based non-viral gene delivery systems, such as Sleeping Beauty and PiggyBac, also have random integration profiles with acceptable gene transfer efficiency and are under development for potential clinical application [33–35]. It has been reported that electroporation/nucleofection yielded good gene transfer with RNA-based expression systems [36]. Although the short half-life of RNA expression post-transfer may limit its clinical application, RNA-based therapy would eliminate the safety concern of gene transfer caused by genome integration [37].
Design of the optimal TCR expression cassette is critical for efficient expression in viral vectors. In the early studies, TCR α and β chains were assembled in different configurations in retroviral vectors either alone or together. Initial bicistronic vectors were generated with dual promoters or the chains were linked with IRES sequences [9]. The gene inserted downstream of the IRES is expressed poorly compared to the gene positioned upstream, leading to suboptimal expression of the TCR chains and poor T cell reactivity. More recent vectors are constructed using picornavirus ribosomal skip peptides to link TCR α and β chain expression and this affords optimal stoichiometric expression of the TCR chains [13,30].
3. TCR gene transfer clinical studies
The first human clinical trial of melanoma TCR gene therapy was reported in 2006 from our group [14]. In this trial HLA-A2 positive metastatic melanoma patients were treated with retrovirally transduced autologous PBL expressing a TCR against the MART-1:27–35 epitope. Fifteen patients were treated with MART-1 TCR gene-engineered T cells plus IL-2 after nonmyeloablative chemotherapy and we observed durable tumor regression in 2 out of 15 patients [14]. This study provided the first proof-of-principle for this novel genetic immunotherapy involving TCR modified T cells. In an attempt to improve the efficacy of TCR based therapy, efforts were made to isolate higher avidity TCRs [13]. In a second reported TCR gene therapy trial targeting either MART-1 or gp100, objective cancer regression was observed in 6 of 20 (30%) and 3 of 16 (19%) patients respectively. However, patients also exhibited destruction of normal melanocytes in the skin, eye, and ear and required local steroid administration. This trial revealed that T cells expressing highly reactive TCRs might target cognate-antigen expression on the normal cells and cause on-target/off-tumor toxicity [13].
The approach to redirect effector cells using genetically modified T cells with tumor-specific T cell receptors (TCR) has opened the possibility to treat patients with a variety of cancer types other than melanoma. The first clinical trial utilizing lymphocytes transduced with a TCR targeting carcinoembryonic antigen (CEA) to treat metastatic colorectal cancer was reported by Parkhurst et al. recently [38]. CEA is a 180-kDa tumor-associated glycoprotein over-expressed in many epithelial cancers, most notably in colorectal adenocarcinoma. As reported by Parkhurst et al., three patients with metastatic colorectal cancer were treated; all patients experienced a decrease in serum CEA levels (74–99%), and one experienced a measurable response. Severe transient colitis was also observed in the patients presumptively due to targeting CEA, which is also expressed in normal intestinal epithelial cells [38]. This is another example of how targeting self-antigens with a high affinity TCR can mediate cancer regression, but can also result in recognition and destruction of normal tissue(s), which may be a limitation to this treatment.
The observation of on-target/off-tumor toxicities suggests that ideally, future TCR gene therapies should choose antigens that are only expressed by the tumor or nonessential organs. Cancer testis (CT) antigens are expressed in a wide variety of epithelial cancers including melanoma, and carcinomas of the bladder, liver, and lung [6,7]. Their expression is restricted in normal adult tissues to the testes, whose cells do not express HLA molecules, and are thus not susceptible to recognition by a TCR. The first clinical trial using adoptive transfer of autologous lymphocytes genetically engineered to express a TCR against CT antigen-NY-ESO-1 has recently been conducted at Surgery Branch, NCI. In this trial reported by Robbins et al., there was a measurable response rate in synovial cell sarcoma patients of 66% (4/6) and in melanoma patients of 45% (5/11) with two melanoma patients being ongoing complete responders [39]. In contrast to the vigorous on-target/off-tumor toxicity seen using TCRs targeting melanoma differentiation antigens and in the CEA TCR trial, none of the patients who received NY-ESO-1 TCR gene-modified T cells experienced toxicity related to the infused cells. These objective regressions with the concomitant lack of toxicity exemplify the use of CT antigens as targets in adoptive cell therapy to treat established solid tumors without damage to normal tissues. Other CT antigens, such as MAGE-A3 [12], are currently under clinical investigation (Table 1).
Table 1.
TCR gene therapy trials registered in the United States.
Data from: ClinicalTrial.gov 08/04/2011.
| Disease | TCR-target | Lymphodepletion | Gene transfer | ClinicalTrial.gov identifier |
|---|---|---|---|---|
| Melanoma | MART-1 | Y | RTV |
NCT00509288 NCT00910650 |
| Melanoma | gp100 | Y | RTV | NCT00509496 |
| Renal cancer | DR4-TRAIL | Y | RTV | NCT00923390 |
| p53 expressing metastatic cancer | p53 | Y | RTV | NCT00393029 |
| HIV infection | HIV-Gag | N | LTV | NCT00991224 |
| CEA expressing metastatic cancer | CEA | Y | RTV | NCT00923806 |
| NY-ESO-1 expressing metastatic cancer | NYE-ESO-1 | Y | RTV | NCT00670748 |
| MAGE-A3.12 expressing metastatic cancer | MAGE-A3/12 | Y | RTV | NCT01273181 |
| Melanoma | NY-ESO-1/MAGE-A3 | Y | LTV | NCT01350401 |
| Myeloma | MAGE-A3/NY-ESO | N | LTV | NCT01352286 |
Abbreviation: RTV, retrovirus; LTV, lentivirus.
These encouraging results point to the feasibility of transferring T cells engineered with TCR to treat solid tumors. However, the durable objective response rate of this approach is generally lower than the rate seen in TIL therapy. Several reasons could contribute to this including the fact that antigen targets may not be the same as TIL. Phenotype of the PBL for used TCR engineering may also be different from that of the TIL in terms of the expression of homing and other molecules necessary for the efficient tumor trafficking and/or effector function. Alternatively the immune response by the TIL against the tumor may be of polyclonal nature; targeting a variety of tumor antigens.
4. Strategies to improve TCR gene therapy
Though highly effective T cells can be generated against the desired tumor antigens ex vivo, the environment that these cells encounter at the tumor site can be a major determining factor in the outcome of therapy (Fig. 2). It has been shown in some cases that normal tumor growth proceeds despite high levels of circulating anti-tumor CD8 cells [40]. The mechanism of the local suppression of effector T cell function at the tumor is not fully understood. Tumors display multiple immune suppressive mechanisms, including the local presence of inhibitory cytokines such as IL-10 [41], TGF-β [42–44], recruitment of regulatory T cells [45] and myeloid derived suppressor cell [46–48], or the expression of ligands for inhibitory molecules on T cells such as PD-1 [49,50] and CTLA-4 [51–53].
Fig. 2.
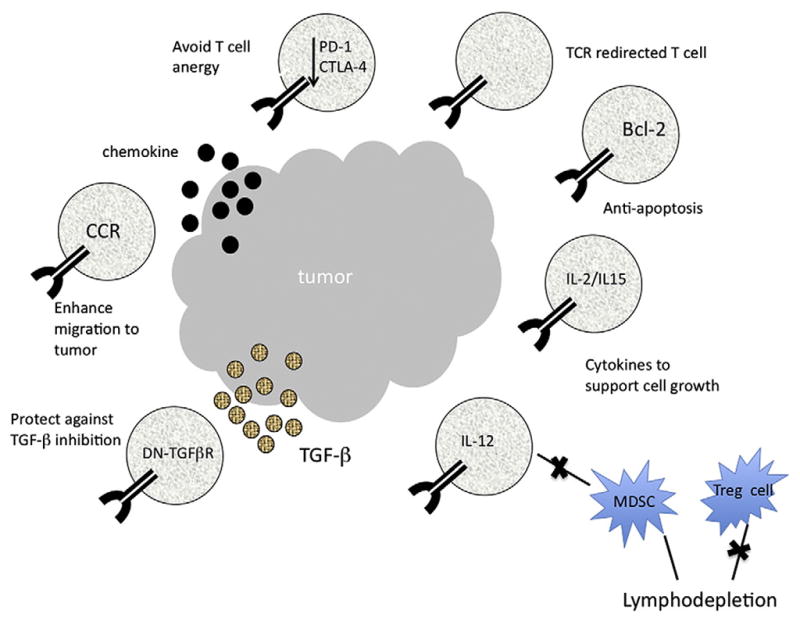
Strategies for improving ACT to treat human cancer. Shown are the potential ways to facilitate engraftment, persistence, and activity of adoptively transferred TCR gene-modified cells (see text for details). To potentially lessen T cell anergy, antibodies to CTLA-4 or PD-1 could be co-administered with TCR gene-engineered T cells. In addition TCR gene engineering, T cells could be further modified to resist apoptosis, to preferentially migrate to tumors, or to be resistant to inhibitory factors at the tumor environment and to deliver inflammatory cytokines to tumor site. DN-TGFβR indicates dominant-negative TGFβ receptor; CCR, chemokine receptor; MDSC, myeloid-derived suppressor cells; Treg, T regulatory cell.
Since the first report of the use of TCR engineered lymphocytes in human in 2006, efforts have been made to improve treatment efficacy of adoptive cell therapy. Strategies to address some of these issues are already incorporated in the current clinical trials involving TCR modified T cells, for example, lymphodepletion of the host increases the effectiveness of cell transfer therapy by eliminating cellular elements like regulatory T cells and myeloid derived suppressor cells and also by removing the lymphocyte pool in the body that might compete for available growth factors or cytokines [54]. In an attempt to blunt the inhibitory molecules expressed on the tumor-active T cell, strategies like the expression of inhibitory RNAs designed to suppress the expression of PD-1 or CTLA-4 may potentially improve the functionality of TCR engineered lymphocytes [55,56]. As reported recently, anti-CTLA-4 antibody (ipilimumab), with or without a gp100 vaccine, improves survival in patients with metastatic melanoma [57]. T cells can undergo apoptosis after infusion due to the lack of growth factors or co-stimulation. Survival of tumor-specific T cells may be enhanced by engineering them to co-express anti-apoptotic genes like Bcl-2 [58,59]. To block TGF-b signaling, T cells were modified to express a dominant-negative or soluble form of the TGF-β receptor, which resulted in the improvement of TCR transfer therapy in several mouse models [60–62].
Cytokine interleukin 12 (IL-12) was recognized as an important regulator of cell-mediated immunity, potentially beneficial for the treatment of infectious and malignant diseases by enhancing the cytotoxic activity of NK cells and cytotoxic T lymphocytes (CTL) [63,64], and mediating the differentiation of naïve CD4+ T cells to Th1 cells [65,66]. The anti-tumor activity of recombinant IL-12 was tested in a variety of murine tumor models where it caused tumor regression and prolonged the survival of tumor-bearing animals [67–70]. However, its clinical application has been hindered by the toxicity associated with its system administration. Kerkar et al. recently demonstrated that co-expression of IL-12 in TCR gene-engineered lymphocytes dramatically augmented tumor treatment efficacy of adoptive transfer in the B16 murine melanoma model without need the administration of IL-2 and vaccine [71]. To further limit potential toxicity related to constitutive express of IL-12, an NFAT responsive promoter was used to control human IL-12 expression at the tumor site. T cells genetically modified with a tumor-specific TCR and NFAT-IL12 demonstrated powerful therapeutic efficacy in mouse B16 tumor model [72]. Based on these results, a phase I/II clinical study using the administration of NFAT-IL12 vector-transduced TIL to patients with metastatic melanoma is in progress.
5. Summary
Recent TCR based clinical trials in melanoma patients have highlighted the promise as well as the challenges facing this therapy. Various factors will contribute towards the clinical efficacy of TCR gene therapy including, the affinity of the transgenic TCR, the maintenance of TCR gene expression over time, and the in vivo persistence of the TCR engineered T cells. Lessons learned from the clinical response of patients treated by TCR gene therapy demonstrated the importance of choice the tumor antigen in order to limit off-tumor/on-target toxicity. The identification of best subset of T cell to engineer and development of novel in vitro expansion protocols, such as using artificial APC, may benefit cell persistence in vivo [73]. These approaches to enhance TCR expression and recognition of tumor antigens may need to be coupled with mechanisms designed to overcome inhibitory elements within the tumor microenvironment. Alternative strategies to improve the adoptive cell therapy could be to co-administer TCR gene-engineered T cells with antibodies that block T cell negative regulatory activity, such as anti-CTLA-4 or anti-PD-1. Based on preliminary clinical results, it is likely that this therapy will continue to improve and potentially be of benefit to patients with a variety of cancers.
Footnotes
This review is part of the Advanced Drug Delivery Reviews theme issue on “Therapeutic Strategies for Controlling Metastasis and Recurrence of Cancers; Contribution of drug Delivery Technologies”.
References
- 1.Dudley ME, Wunderlich JR, Robbins PF, Yang JC, Hwu P, Schwartzentruber DJ, Topalian SL, Sherry R, Restifo NP, Hubicki AM, Robinson MR, Raffeld M, Duray P, Seipp CA, Rogers-Freezer L, Morton KE, Mavroukakis SA, White DE, Rosenberg SA. Cancer regression and autoimmunity in patients after clonal repopulation with antitumor lymphocytes. Science. 2002;298:850–854. doi: 10.1126/science.1076514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rosenberg SA, Restifo NP, Yang JC, Morgan RA, Dudley ME. Adoptive cell transfer: a clinical path to effective cancer immunotherapy. Nat Rev Cancer. 2008;8:299–308. doi: 10.1038/nrc2355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dudley ME, Yang JC, Sherry R, Hughes MS, Royal R, Kammula U, Robbins PF, Huang J, Citrin DE, Leitman SF, Wunderlich J, Restifo NP, Thomasian A, Downey SG, Smith FO, Klapper J, Morton K, Laurencot C, White DE, Rosenberg SA. Adoptive cell therapy for patients with metastatic melanoma: evaluation of intensive myeloablative chemoradiation preparative regimens. J Clin Oncol. 2008;26:5233–5239. doi: 10.1200/JCO.2008.16.5449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blattman JN, Greenberg PD. Cancer immunotherapy: a treatment for the masses. Science. 2004;305:200–205. doi: 10.1126/science.1100369. [DOI] [PubMed] [Google Scholar]
- 5.Romero P, Cerottini JC, Speiser DE. The human T cell response to melanoma antigens. Adv Immunol. 2006;92:187–224. doi: 10.1016/S0065-2776(06)92005-7. [DOI] [PubMed] [Google Scholar]
- 6.Simpson AJ, Caballero OL, Jungbluth A, Chen YT, Old LJ. Cancer/testis antigens, gametogenesis and cancer. Nat Rev Cancer. 2005;5:615–625. doi: 10.1038/nrc1669. [DOI] [PubMed] [Google Scholar]
- 7.Suri A. Cancer testis antigens—their importance in immunotherapy and in the early detection of cancer. Expert Opin Biol Ther. 2006;6:379–389. doi: 10.1517/14712598.6.4.379. [DOI] [PubMed] [Google Scholar]
- 8.Clay TM, Custer MC, Sachs J, Hwu P, Rosenberg SA, Nishimura MI. Efficient transfer of a tumor antigen-reactive TCR to human peripheral blood lymphocytes confers anti-tumor reactivity. J Immunol. 1999;163:507–513. [PubMed] [Google Scholar]
- 9.Morgan RA, Dudley ME, Yu YY, Zheng Z, Robbins PF, Theoret MR, Wunderlich JR, Hughes MS, Restifo NP, Rosenberg SA. High efficiency TCR gene transfer into primary human lymphocytes affords avid recognition of melanoma tumor antigen glycoprotein 100 and does not alter the recognition of autologous melanoma antigens. J Immunol. 2003;171:3287–3295. doi: 10.4049/jimmunol.171.6.3287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schaft N, Willemsen RA, de Vries J, Lankiewicz B, Essers BW, Gratama JW, Figdor CG, Bolhuis RL, Debets R, Adema GJ. Peptide fine specificity of anti-glycoprotein 100 CTL is preserved following transfer of engineered TCR alpha beta genes into primary human T lymphocytes. J Immunol. 2003;170:2186–2194. doi: 10.4049/jimmunol.170.4.2186. [DOI] [PubMed] [Google Scholar]
- 11.Zhao Y, Zheng Z, Robbins PF, Khong HT, Rosenberg SA, Morgan RA. Primary human lymphocytes transduced with NY-ESO-1 antigen-specific TCR genes recognize and kill diverse human tumor cell lines. J Immunol. 2005;174:4415–4423. doi: 10.4049/jimmunol.174.7.4415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chinnasamy N, Wargo JA, Yu Z, Rao M, Frankel TL, Riley JP, Hong JJ, Parkhurst MR, Feldman SA, Schrump DS, Restifo NP, Robbins PF, Rosenberg SA, Morgan RA. A TCR targeting the HLA-A*0201-restricted epitope of MAGE-A3 recognizes multiple epitopes of the MAGE-A antigen superfamily in several types of cancer. J Immunol. 2011;186:685–696. doi: 10.4049/jimmunol.1001775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Johnson LA, Morgan RA, Dudley ME, Cassard L, Yang JC, Hughes MS, Kammula US, Royal RE, Sherry RM, Wunderlich JR, Lee CC, Restifo NP, Schwarz SL, Cogdill AP, Bishop RJ, Kim H, Brewer CC, Rudy SF, VanWaes C, Davis JL, Mathur A, Ripley RT, Nathan DA, Laurencot CM, Rosenberg SA. Gene therapy with human and mouse T-cell receptors mediates cancer regression and targets normal tissues expressing cognate antigen. Blood. 2009;114:535–546. doi: 10.1182/blood-2009-03-211714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Morgan RA, Dudley ME, Wunderlich JR, Hughes MS, Yang JC, Sherry RM, Royal RE, Topalian SL, Kammula US, Restifo NP, Zheng Z, Nahvi A, de Vries CR, Rogers-Freezer LJ, Mavroukakis SA, Rosenberg SA. Cancer regression in patients after transfer of genetically engineered lymphocytes. Science. 2006;314:126–129. doi: 10.1126/science.1129003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cohen CJ, Zhao Y, Zheng Z, Rosenberg SA, Morgan RA. Enhanced antitumor activity of murine-human hybrid T-cell receptor (TCR) in human lymphocytes is associated with improved pairing and TCR/CD3 stability. Cancer Res. 2006;66:8878–8886. doi: 10.1158/0008-5472.CAN-06-1450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cohen CJ, Li YF, El-Gamil M, Robbins PF, Rosenberg SA, Morgan RA. Enhanced antitumor activity of T cells engineered to express T-cell receptors with a second disulfide bond. Cancer Res. 2007;67:3898–3903. doi: 10.1158/0008-5472.CAN-06-3986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Voss RH, Willemsen RA, Kuball J, Grabowski M, Engel R, Intan RS, Guillaume P, Romero P, Huber C, Theobald M. Molecular design of the Calphabeta interface favors specific pairing of introduced TCRalphabeta in human T cells. J Immunol. 2008;180:391–401. doi: 10.4049/jimmunol.180.1.391. [DOI] [PubMed] [Google Scholar]
- 18.Okamoto S, Mineno J, Ikeda H, Fujiwara H, Yasukawa M, Shiku H, Kato I. Improved expression and reactivity of transduced tumor-specific TCRs in human lymphocytes by specific silencing of endogenous TCR. Cancer Res. 2009;69:9003–9011. doi: 10.1158/0008-5472.CAN-09-1450. [DOI] [PubMed] [Google Scholar]
- 19.Robbins PF, Li YF, El-Gamil M, Zhao Y, Wargo JA, Zheng Z, Xu H, Morgan RA, Feldman SA, Johnson LA, Bennett AD, Dunn SM, Mahon TM, Jakobsen BK, Rosenberg SA. Single and dual amino acid substitutions in TCR CDRs can enhance antigen-specific T cell functions. J Immunol. 2008;180:6116–6131. doi: 10.4049/jimmunol.180.9.6116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Parkhurst MR, Joo J, Riley JP, Yu Z, Li Y, Robbins PF, Rosenberg SA. Characterization of genetically modified T-cell receptors that recognize the CEA:691–699 peptide in the context of HLA-A2.1 on human colorectal cancer cells. Clin Cancer Res. 2009;15:169–180. doi: 10.1158/1078-0432.CCR-08-1638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang QJ, Hanada K, Feldman SA, Zhao Y, Inozume T, Yang JC. Development of a genetically-modified novel T-cell receptor for adoptive cell transfer against renal cell carcinoma. J Immunol Methods. 2011;366:43–51. doi: 10.1016/j.jim.2011.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kuball J, Hauptrock B, Malina V, Antunes E, Voss RH, Wolfl M, Strong R, Theobald M, Greenberg PD. Increasing functional avidity of TCR-redirected T cells by removing defined N-glycosylation sites in the TCR constant domain. J Exp Med. 2009;206:463–475. doi: 10.1084/jem.20082487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Scholten KB, Kramer D, Kueter EW, Graf M, Schoedl T, Meijer CJ, Schreurs MW, Hooijberg E. Codon modification of T cell receptors allows enhanced functional expression in transgenic human T cells. Clin Immunol. 2006;119:135–145. doi: 10.1016/j.clim.2005.12.009. [DOI] [PubMed] [Google Scholar]
- 24.Cohen CJ, Zheng Z, Bray R, Zhao Y, Sherman LA, Rosenberg SA, Morgan RA. Recognition of fresh human tumor by human peripheral blood lymphocytes transduced with a bicistronic retroviral vector encoding a murine anti-p53 TCR. J Immunol. 2005;175:5799–5808. doi: 10.4049/jimmunol.175.9.5799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Davis JL, Theoret MR, Zheng Z, Lamers CH, Rosenberg SA, Morgan RA. Development of human anti-murine T-cell receptor antibodies in both responding and non-responding patients enrolled in TCR gene therapy trials. Clin Cancer Res. 2010;16:5852–5861. doi: 10.1158/1078-0432.CCR-10-1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhao Y, Bennett AD, Zheng Z, Wang QJ, Robbins PF, Yu LY, Li Y, Molloy PE, Dunn SM, Jakobsen BK, Rosenberg SA, Morgan RA. High-affinity TCRs generated by phage display provide CD4+ T cells with the ability to recognize and kill tumor cell lines. J Immunol. 2007;179:5845–5854. doi: 10.4049/jimmunol.179.9.5845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Riviere I, Brose K, Mulligan RC. Effects of retroviral vector design on expression of human adenosine deaminase in murine bone marrow transplant recipients engrafted with genetically modified cells. Proc Natl Acad Sci U S A. 1995;92:6733–6737. doi: 10.1073/pnas.92.15.6733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schambach A, Swaney WP, van der Loo JC. Design and production of retro- and lentiviral vectors for gene expression in hematopoietic cells. Methods Mol Biol. 2009;506:191–205. doi: 10.1007/978-1-59745-409-4_14. [DOI] [PubMed] [Google Scholar]
- 29.Hughes MS, Yu YY, Dudley ME, Zheng Z, Robbins PF, Li Y, Wunderlich J, Hawley RG, Moayeri M, Rosenberg SA, Morgan RA. Transfer of a TCR gene derived from a patient with a marked antitumor response conveys highly active T-cell effector functions. Hum Gene Ther. 2005;16:457–472. doi: 10.1089/hum.2005.16.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yang S, Cohen CJ, Peng PD, Zhao Y, Cassard L, Yu Z, Zheng Z, Jones S, Restifo NP, Rosenberg SA, Morgan RA. Development of optimal bicistronic lentiviral vectors facilitates high-level TCR gene expression and robust tumor cell recognition. Gene Ther. 2008;15:1411–1423. doi: 10.1038/gt.2008.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yang S, Rosenberg SA, Morgan RA. Clinical-scale lentiviral vector transduction of PBL for TCR gene therapy and potential for expression in less-differentiated cells. J Immunother. 2008;31:830–839. doi: 10.1097/CJI.0b013e31818817c5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wu X, Li Y, Crise B, Burgess SM. Transcription start regions in the human genome are favored targets for MLV integration. Science. 2003;300:1749–1751. doi: 10.1126/science.1083413. [DOI] [PubMed] [Google Scholar]
- 33.Singh H, Manuri PR, Olivares S, Dara N, Dawson MJ, Huls H, Hackett PB, Kohn DB, Shpall EJ, Champlin RE, Cooper LJ. Redirecting specificity of T-cell populations for CD19 using the Sleeping Beauty system. Cancer Res. 2008;68:2961–2971. doi: 10.1158/0008-5472.CAN-07-5600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hackett PB, Largaespada DA, Cooper LJ. A transposon and transposase system for human application. Mol Ther. 2010;18:674–683. doi: 10.1038/mt.2010.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nakazawa Y, Huye LE, Salsman VS, Leen AM, Ahmed N, Rollins L, Dotti G, Gottschalk SM, Wilson MH, Rooney CM. PiggyBac-mediated Cancer Immunotherapy Using EBV-specific Cytotoxic T-cells Expressing HER2-specific Chimeric Antigen Receptor. Mol Ther. 2011;12:2133–2143. doi: 10.1038/mt.2011.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhao Y, Zheng Z, Cohen CJ, Gattinoni L, Palmer DC, Restifo NP, Rosenberg SA, Morgan RA. High-efficiency transfection of primary human and mouse T lymphocytes using RNA electroporation. Mol Ther. 2006;13:151–159. doi: 10.1016/j.ymthe.2005.07.688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhao Y, Moon E, Carpenito C, Paulos CM, Liu X, Brennan AL, Chew A, Carroll RG, Scholler J, Levine BL, Albelda SM, June CH. Multiple injections of electroporated autologous T cells expressing a chimeric antigen receptor mediate regression of human disseminated tumor. Cancer Res. 2010;70:9053–9061. doi: 10.1158/0008-5472.CAN-10-2880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Parkhurst MR, Yang JC, Langan RC, Dudley ME, Nathan DA, Feldman SA, Davis JL, Morgan RA, Merino MJ, Sherry RM, Hughes MS, Kammula US, Phan GQ, Lim RM, Wank SA, Restifo NP, Robbins PF, Laurencot CM, Rosenberg SA. T cells targeting carcinoembryonic antigen can mediate regression of metastatic colorectal cancer but induce severe transient colitis. Mol Ther. 2011;19:620–626. doi: 10.1038/mt.2010.272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Robbins PF, Morgan RA, Feldman SA, Yang JC, Sherry RM, Dudley ME, Wunderlich JR, Nahvi AV, Helman LJ, Mackall CL, Kammula US, Hughes MS, Restifo NP, Raffeld M, Lee CC, Levy CL, Li YF, El-Gamil M, Schwarz SL, Laurencot C, Rosenberg SA. Tumor regression in patients with metastatic synovial cell sarcoma and melanoma using genetically engineered lymphocytes reactive with NY-ESO-1. J Clin Oncol. 2011;29:917–924. doi: 10.1200/JCO.2010.32.2537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rosenberg SA, Sherry RM, Morton KE, Scharfman WJ, Yang JC, Topalian SL, Royal RE, Kammula U, Restifo NP, Hughes MS, Schwartzentruber D, Berman DM, Schwarz SL, Ngo LT, Mavroukakis SA, White DE, Steinberg SM. Tumor progression can occur despite the induction of very high levels of self/tumor antigen-specific CD8+ T cells in patients with melanoma. J Immunol. 2005;175:6169–6176. doi: 10.4049/jimmunol.175.9.6169. [DOI] [PubMed] [Google Scholar]
- 41.O’Garra A, Barrat FJ, Castro AG, Vicari A, Hawrylowicz C. Strategies for use of IL-10 or its antagonists in human disease. Immunol Rev. 2008;223:114–131. doi: 10.1111/j.1600-065X.2008.00635.x. [DOI] [PubMed] [Google Scholar]
- 42.Flavell RA, Sanjabi S, Wrzesinski SH, Licona-Limon P. The polarization of immune cells in the tumour environment by TGFbeta. Nat Rev Immunol. 2010;10:554–567. doi: 10.1038/nri2808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Filippi CM, Juedes AE, Oldham JE, Ling E, Togher L, Peng Y, Flavell RA, von Herrath MG. Transforming growth factor-beta suppresses the activation of CD8+ T-cells when naive but promotes their survival and function once antigen experienced: a two-faced impact on autoimmunity. Diabetes. 2008;57:2684–2692. doi: 10.2337/db08-0609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gorelik L, Flavell RA. Immune-mediated eradication of tumors through the blockade of transforming growth factor-beta signaling in T cells. Nat Med. 2001;7:1118–1122. doi: 10.1038/nm1001-1118. [DOI] [PubMed] [Google Scholar]
- 45.Gabrilovich DI, Bronte V, Chen SH, Colombo MP, Ochoa A, Ostrand-Rosenberg S, Schreiber H. The terminology issue for myeloid-derived suppressor cells. Cancer Res. 2007;67:425. doi: 10.1158/0008-5472.CAN-06-3037. author reply 426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Filipazzi P, Valenti R, Huber V, Pilla L, Canese P, Iero M, Castelli C, Mariani L, Parmiani G, Rivoltini L. Identification of a new subset of myeloid suppressor cells in peripheral blood of melanoma patients with modulation by a granulocyte-macrophage colony-stimulation factor-based antitumor vaccine. J Clin Oncol. 2007;25:2546–2553. doi: 10.1200/JCO.2006.08.5829. [DOI] [PubMed] [Google Scholar]
- 47.Nagaraj S, Gabrilovich DI. Tumor escape mechanism governed by myeloid-derived suppressor cells. Cancer Res. 2008;68:2561–2563. doi: 10.1158/0008-5472.CAN-07-6229. [DOI] [PubMed] [Google Scholar]
- 48.Nagaraj S, Gabrilovich DI. Myeloid-derived suppressor cells in human cancer. Cancer J. 2010;16:348–353. doi: 10.1097/PPO.0b013e3181eb3358. [DOI] [PubMed] [Google Scholar]
- 49.Ahmadzadeh M, Johnson LA, Heemskerk B, Wunderlich JR, Dudley ME, White DE, Rosenberg SA. Tumor antigen-specific CD8 T cells infiltrating the tumor express high levels of PD-1 and are functionally impaired. Blood. 2009;114:1537–1544. doi: 10.1182/blood-2008-12-195792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Barber DL, Wherry EJ, Masopust D, Zhu B, Allison JP, Sharpe AH, Freeman GJ, Ahmed R. Restoring function in exhausted CD8 T cells during chronic viral infection. Nature. 2006;439:682–687. doi: 10.1038/nature04444. [DOI] [PubMed] [Google Scholar]
- 51.Sarnaik AA, Weber JS. Recent advances using anti-CTLA-4 for the treatment of melanoma. Cancer J. 2009;15:169–173. doi: 10.1097/PPO.0b013e3181a7450f. [DOI] [PubMed] [Google Scholar]
- 52.Wing K, Yamaguchi T, Sakaguchi S. Cell-autonomous and -non-autonomous roles of CTLA-4 in immune regulation. Trends Immunol. 2011;9:428–433. doi: 10.1016/j.it.2011.06.002. [DOI] [PubMed] [Google Scholar]
- 53.Qureshi OS, Zheng Y, Nakamura K, Attridge K, Manzotti C, Schmidt EM, Baker J, Jeffery LE, Kaur S, Briggs Z, Hou TZ, Futter CE, Anderson G, Walker LS, Sansom DM. Trans-endocytosis of CD80 and CD86: a molecular basis for the cell-extrinsic function of CTLA-4. Science. 2011;332:600–603. doi: 10.1126/science.1202947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gattinoni L, Finkelstein SE, Klebanoff CA, Antony PA, Palmer DC, Spiess PJ, Hwang LN, Yu Z, Wrzesinski C, Heimann DM, Surh CD, Rosenberg SA, Restifo NP. Removal of homeostatic cytokine sinks by lymphodepletion enhances the effi-cacy of adoptively transferred tumor-specific CD8+ T cells. J Exp Med. 2005;202:907–912. doi: 10.1084/jem.20050732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Borkner L, Kaiser A, van de Kasteele W, Andreesen R, Mackensen A, Haanen JB, Schumacher TN, Blank C. RNA interference targeting programmed death receptor-1 improves immune functions of tumor-specific T cells. Cancer Immunol Immunother. 2010;59:1173–1183. doi: 10.1007/s00262-010-0842-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bishop KD, Harris JE, Mordes JP, Greiner DL, Rossini AA, Czech MP, Phillips NE. Depletion of the programmed death-1 receptor completely reverses established clonal anergy in CD4(+) T lymphocytes via an interleukin-2-dependent mechanism. Cell Immunol. 2009;256:86–91. doi: 10.1016/j.cellimm.2009.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hodi FS, O’Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, Gonzalez R, Robert C, Schadendorf D, Hassel JC, Akerley W, van den Eertwegh AJ, Lutzky J, Lorigan P, Vaubel JM, Linette GP, Hogg D, Ottensmeier CH, Lebbe C, Peschel C, Quirt I, Clark JI, Wolchok JD, Weber JS, Tian J, Yellin MJ, Nichol GM, Hoos A, Urba WJ. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363:711–723. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Charo J, Finkelstein SE, Grewal N, Restifo NP, Robbins PF, Rosenberg SA. Bcl-2 overexpression enhances tumor-specific T-cell survival. Cancer Res. 2005;65:2001–2008. doi: 10.1158/0008-5472.CAN-04-2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kalbasi A, Shrimali RK, Chinnasamy D, Rosenberg SA. Prevention of interleukin-2 withdrawal-induced apoptosis in lymphocytes retrovirally cotransduced with genes encoding an antitumor T-cell receptor and an antiapoptotic protein. J Immunother. 2010;33:672–683. doi: 10.1097/CJI.0b013e3181e475cd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hu Z, Gerseny H, Zhang Z, Chen YJ, Berg A, Stock S, Seth P. Oncolytic Adeno-virus Expressing Soluble TGFbeta Receptor II-Fc-mediated Inhibition of Established Bone Metastases: A Safe and Effective Systemic Therapeutic Approach for Breast Cancer. Mol Ther. 2011;9:1609–1618. doi: 10.1038/mt.2011.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yang YA, Dukhanina O, Tang B, Mamura M, Letterio JJ, MacGregor J, Patel SC, Khozin S, Liu ZY, Green J, Anver MR, Merlino G, Wakefield LM. Lifetime exposure to a soluble TGF-beta antagonist protects mice against metastasis without adverse side effects. J Clin Invest. 2002;109:1607–1615. doi: 10.1172/JCI15333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lacuesta K, Buza E, Hauser H, Granville L, Pule M, Corboy G, Finegold M, Weiss H, Chen SY, Brenner MK, Heslop HE, Rooney CM, Bollard CM. Assessing the safety of cytotoxic T lymphocytes transduced with a dominant negative transforming growth factor-beta receptor. J Immunother. 2006;29:250–260. doi: 10.1097/01.cji.0000192104.24583.ca. [DOI] [PubMed] [Google Scholar]
- 63.Gately MK. Interleukin-12: a recently discovered cytokine with potential for enhancing cell-mediated immune responses to tumors. Cancer Invest. 1993;11:500–506. doi: 10.3109/07357909309018881. [DOI] [PubMed] [Google Scholar]
- 64.Mehrotra PT, Wu D, Crim JA, Mostowski HS, Siegel JP. Effects of IL-12 on the generation of cytotoxic activity in human CD8+ T lymphocytes. J Immunol. 1993;151:2444–2452. [PubMed] [Google Scholar]
- 65.Manetti R, Parronchi P, Giudizi MG, Piccinni MP, Maggi E, Trinchieri G, Romagnani S. Natural killer cell stimulatory factor (interleukin 12 [IL-12]) induces T helper type 1 (Th1)-specific immune responses and inhibits the development of IL-4-producing Th cells. J Exp Med. 1993;177:1199–1204. doi: 10.1084/jem.177.4.1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kennedy MK, Picha KS, Shanebeck KD, Anderson DM, Grabstein KH. Interleukin-12 regulates the proliferation of Th1, but not Th2 or Th0, clones. Eur J Immunol. 1994;24:2271–2278. doi: 10.1002/eji.1830241002. [DOI] [PubMed] [Google Scholar]
- 67.Brunda MJ, Luistro L, Warrier RR, Wright RB, Hubbard BR, Murphy M, Wolf SF, Gately MK. Antitumor and antimetastatic activity of interleukin 12 against murine tumors. J Exp Med. 1993;178:1223–1230. doi: 10.1084/jem.178.4.1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Tahara H, Zitvogel L, Storkus WJ, Robbins PD, Lotze MT. Murine models of cancer cytokine gene therapy using interleukin-12. Ann N Y Acad Sci. 1996;795:275–283. doi: 10.1111/j.1749-6632.1996.tb52677.x. [DOI] [PubMed] [Google Scholar]
- 69.Colombo MP, Vagliani M, Spreafico F, Parenza M, Chiodoni C, Melani C, Stoppacciaro A. Amount of interleukin 12 available at the tumor site is critical for tumor regression. Cancer Res. 1996;56:2531–2534. [PubMed] [Google Scholar]
- 70.Cavallo F, Di Carlo E, Butera M, Verrua R, Colombo MP, Musiani P, Forni G. Immune events associated with the cure of established tumors and spontaneous metastases by local and systemic interleukin 12. Cancer Res. 1999;59:414–421. [PubMed] [Google Scholar]
- 71.Kerkar SP, Muranski P, Kaiser A, Boni A, Sanchez-Perez L, Yu Z, Palmer DC, Reger RN, Borman ZA, Zhang L, Morgan RA, Gattinoni L, Rosenberg SA, Trinchieri G, Restifo NP. Tumor-specific CD8+ T cells expressing interleukin-12 eradicate established cancers in lymphodepleted hosts. Cancer Res. 2010;70:6725–6734. doi: 10.1158/0008-5472.CAN-10-0735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zhang L, Kerkar SP, Yu Z, Zheng Z, Yang S, Restifo NP, Rosenberg SA, Morgan RA. Improving adoptive T cell therapy by targeting and controlling IL-12 expression to the tumor environment. Mol Ther. 2011;19:751–759. doi: 10.1038/mt.2010.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Butler MO, Friedlander P, Milstein MI, Mooney MM, Metzler G, Murray AP, Tanaka M, Berezovskaya A, Imataki O, Drury L, Brennan L, Flavin M, Neuberg D, Stevenson K, Lawrence D, Hodi FS, Velazquez EF, Jaklitsch MT, Russell SE, Mihm M, Nadler LM, Hirano N. Establishment of antitumor memory in humans using in vitro-educated CD8+ T cells. Sci Transl Med. 2011;3:80ra34. doi: 10.1126/scitranslmed.3002207. [DOI] [PMC free article] [PubMed] [Google Scholar]