Abstract
Clinical observations have suggested that there is an association of atopic conditions with hypersensitivity reactions to nonsteroidal anti-inflammatory drugs (NSAIDs). This relationship has been especially present in patients allergic to mites. This study was designed to review clinical and experimental evidence linking atopy, mite allergy, and hypersensitivity to aspirin and NSAIDs and discuss the possible mechanisms explaining this association. A review of the medical literature concerning the association of atopic diseases, mite hypersensitivity, and intolerance to NSAIDs using PubMed and other relevant articles is presented. NSAID-sensitive patients are frequently atopic and allergic to mites, and patients who develop oral mite anaphylaxis (OMA) show an increased prevalence of NSAID hypersensitivity. The study of atopic, mite-sensitive patients, who experience urticaria and angioedema when exposed to NSAIDs and patients with OMA suggests an interesting interaction between atopic allergy and disorders of leukotriene synthesis or metabolism. Various mechanisms that could be involved in this interaction are presented, including genetic factors, inhibition of cyclooxygenase-1, and other effects (not related to IgE sensitization) of mite constituents on the immune system. The association of mite hypersensitivity with aspirin/NSAIDs intolerance has been confirmed and provides additional clues to various nonallergic pathways that may contribute to the acute and chronic inflammatory process observed in atopic, mite-allergic, individuals. The clinical relevance of these observations is presently under investigation.
Keywords: Aspirin, acetylsalicylic acid, angioedema, cysteinyl-leukotrienes, Dermatophagoides, immunoglobulin E, mites, leukotriene C4 synthase, nonsteroidal anti-inflammatory drugs, NSAIDs
Allergic respiratory diseases induced by domestic mites, such as rhinitis and asthma, are highly prevalent in most regions of the world and represent a significant medical problem with heavy impact on sanitary budgets and people's quality of life.1 A number of mite allergens have been characterized and are currently being used for the diagnosis and treatment of respiratory allergic diseases.2
Although allergic inflammation induced by mites has received a great deal of attention in the literature, less interest has been devoted to additional pathogenic mechanisms due to mite constituents. In this article we discuss the pathophysiological implications of various types of reactions induced by mites based on information derived from the study of patients who developed the clinical picture of oral mite anaphylaxis (OMA), a recently recognized nature's experiment.
ORAL MITE ANAPHYLAXIS
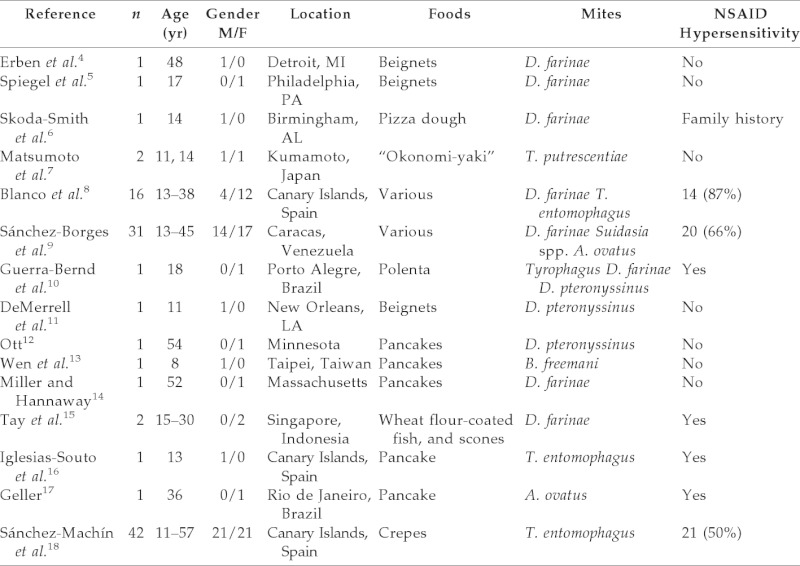
Allergens hidden in food represent a difficult and challenging issue for patients and allergists, as recently emphasized by Puglisi and Frieri.3 Hidden food proteins constitute a dangerous and even life-threatening source of allergen exposure for unsuspecting hypersensitive individuals and efforts to increase public awareness have led to such proposals as the Food Allergen Labeling and Consumer Protection.3 The first case of systemic anaphylaxis induced by the ingestion of foods (more often wheat flour) contaminated with mites was published by Erben et al. in 1993.3 Later on, a number of investigators have reported isolated cases or small series of patients with OMA. Table 1 summarizes the information presently available in the literature on 102 cases observed in the United States, Japan, Canary Islands (Spain), Venezuela, Brazil, Taiwan, and Singapore. There are also unpublished cases in the Dominican Republic, Israel, and Peru.4–18
Table 1.
Publications on oral mite anaphylaxis
Hypersensitivity to NSAIDs 59/102 (57.8%).
NSAIDs = nonsteroidal anti-inflammatory drugs.
Among precipitating foods, beignets, pizza dough, “okonomi-yaki,” polenta, flour-coated fish, and scones have been reported. However, the most frequently incriminated food has been pancakes, and therefore the term “pancake syndrome” was proposed.19,20 Various mite species have been associated with OMA, including domestic (Dermatophagoides farinae and Dermatophagoides pteronyssinus) and storage mites (Tyrophagus spp., Suidasia spp., Blomia freemani, and Aleuroglyphus ovatus). A young female patient who developed exercise-induced anaphylaxis after the ingestion of pancakes made with wheat flour containing mites of the species Suidasia was also reported by our group.21
As shown in Table 1, it is remarkable that a significant proportion of patients with OMA also showed hypersensitivity to nonsteroidal anti-inflammatory drugs (NSAIDs), especially NSAID-induced angioedema, which was present in 59 of the 102 published cases (57.8%). We proposed a “new aspirin triad” of allergic rhinoconjunctivitis, aspirin/NSAID hypersensitivity, and severe systemic reactions to mite-contaminated foods.22
The diagnosis of OMA is suspected through patient interrogation where a history of symptoms temporarily related to the ingestion of foods prepared with wheat flour is obtained. Microscopic identification of mites in the flour is required to confirm the diagnosis, together with positive skin-prick tests to extracts of contaminated flour and negative prick tests to commercial allergenic wheat extracts and to uncontaminated flour. These patients are tolerant to foods made with wheat flour that do not contain mites. The prevalence of OMA in the population has not been established, although in a recent series of 179 patients with anaphylaxis attending our institution 13 cases of OMA (7.2%) were observed.23 It is likely that OMA occurs more frequently in tropical/subtropical areas where climatic conditions (heat and humidity) are favorable for mite proliferation.24
Increased Prevalence of Atopy and Mite Allergy in Patients with NSAID Hypersensitivity
In 1992 we reported a high prevalence of atopic diseases in patients with cutaneous hypersensitivity to NSAIDs (urticaria and angioedema).25 This initial observation was confirmed in a study of cases and controls showing that an increased proportion of patients with urticaria/angioedema induced by NSAIDs had concomitant rhinitis, asthma, conjunctivitis, and positive prick tests to inhalant allergens.26
More recently, we observed that patients with cutaneous hypersensitivity to NSAIDs have significantly increased levels of total and mite-specific IgE in the serum when compared with control individuals.27 The relation of atopy and NSAID hypersensitivity has also been observed by a number of other investigators.28–37
POSSIBLE MECHANISMS OF THE RELATION BETWEEN ATOPY AND NSAID HYPERSENSITIVITY
Because atopic diseases are mediated by specific antibodies of IgE class, and most cross-reactive reactions to aspirin and other NSAIDs are likely caused by cyclooxygenase 1 (COX-1) inhibition,38,39 the relation between atopic diseases and aspirin/NSAID hypersensitivity constitutes an intellectual challenge. We have considered various pathogenic pathways that could explain this interaction.
Genetic factors. A critical enzyme for the synthesis of cysteinyl leukotrienes, leukotrienes C4 synthase (LTC4S), is controlled by a gene located in the long arm of human chromosome 5, close to genes related to atopy such as those determining the production of IL-4, IL-5, and IL-13.40 Then, it could be hypothesized that a genetic linkage of atopic genes and LTC4S gene could be responsible for the association between atopy and hypersensitivity to NSAIDs. In this regard, various genetic polymorphisms including LTC4S gene and other loci that are associated to NSAID hypersensitivity have been reported.41,42
Inhibition of COX-1. As previously mentioned, respiratory and cutaneous reactions to aspirin and NSAIDs are mediated through inhibition of COX-1 isoenzyme.38,39 Because OMA was observed with increased prevalence in atopic patients who concomitantly show NSAID intolerance, we investigated the presence of COX-1 inhibitory substances in commercial mite allergenic extracts using recombinant human COX-1 microsomal assays. COX-1 enzymic activity was inhibited by 76 and 70% with a 2000 AU/mL concentration of D. farinae and D. pteronyssinus extracts, respectively. It was proposed that inhibition of COX-1 by mite-derived constituents might contribute to the clinical picture of severe allergic reactions observed in some NSAID-sensitive atopic patients immediately after oral exposure to foods contaminated with mites.43
A related observation, recently published by Barret et al., is that extracts from Dermatophagoides spp. and Aspergillus fumigatus stimulate the production of cysteinyl-leukotrienes by bone marrow-derived dendritic cells. Furthermore, using the Fluorescence-activated cell sorter they showed that extracts from D. farinae, D. pteronyssinus, and A. fumigatus enhanced the generation of leukotrienes by bone marrow mast cells transfected with Dectin-2. A glycan–dectin-2 interaction was postulated as the mechanism responsible for this stimulatory activity.44
To investigate the association between OMA and aspirin hypersensitivity, Blanco et al. investigated the presence of salicylates in mite-contaminated wheat flour by means of high-pressure liquid chromatography. No salicylates were shown.7 However, Sato et al. have observed that opisthonotal gland secretion from D. pteronyssinus contains salicylaldehyde analog 2-formyl-3-hydrobenzyl formate.45
OTHER PATHOGENIC PATHWAYS
Other mite-activated mechanisms contributing to the inflammation and tissue damage observed in allergic diseases have been studied in recent years. Four of them are relevant in the context of nonallergic effects of mites.
Stimulation of innate immunity. It has been shown that antigen-presenting cells and cytokine production can be directly stimulated by mite products such as Der p2 and Der p7.46
Protease activity. Some allergenic proteins, mainly Der p1, Der p3, and Der p9, from mites are peptidases that are able to break mucosal barriers and activate dendritic cells.2,47 Group 1 mite allergens (Der p1 and Der f1) are cysteine proteases that can activate eosinophils to release proinflammatory mediators.48
Toll-like receptor (TLR) 4–mediated inflammation. Der p2, a member of the ML (MD-2–related lipid recognition) domain lipid-binding family, promotes TLR4 signaling and TH2-mediated inflammation in vivo in a TLR4-dependent manner.49
Epigenetic changes. The strong contribution of environmental factors in the induction of epigenetic modifications resulting in the increase of asthma prevalence observed in the last 30 years has recently received a great deal of attention. Dust-mite allergens are among these environmental agents able to produce epigenetic changes through the expression of miRNA-16, mi-RNA 21, and miRNA-126 and the activation of TLR4, leading to increased inflammation, a TH2 response, suppression of GATA-3, and airway hyperresponsiveness.50
Effects on remodeling of the airways. Various studies have investigated the possible participation of house-dust mites in airway remodeling. First, mite proteases can cleave cell adhesion, induce cell death, and increase the permeability of lung epithelium.51 Second, Dermatophagoides sp. peptidases can induce apoptosis in a bronchial cell model independent of tight junction perturbation.52 Third, dust-mite allergen may stimulate airway cells to increase vascular endothelial growth factor (VEGF) secretion, potentially contributing to edema in airway remodeling. Dermatophagoides sp. extract stimulates A549 cells to secrete factors that dysregulate mesenchymal cell growth in vitro, increase VEGF secretion and expression of cell-associated VEGF, and stimulate those cells to secrete mediators that stimulate normal human lung fibroblasts to increase secretion of VEGF.53,54 These investigators observed a dose-dependent increase of aggregation and decreased adhesion of human lung microvascular endothelial cells in response to transforming growth factor β in conditioned media from confluent alveolar epithelial cells treated with D. pteronyssinus extracts.53,55 Furthermore, D. pteronyssinus extract induced apoptosis and stimulated secretion of transforming growth factor β1 in A549 cells.56
These results led investigators to propose that dust-mite proteases and proteinase-activated receptors play a role in stimulating fibroblast-mediated events and reactivation of the epithelial-mesenchymal trophic unit involved in airway remodeling.57 This can also occur in rhinitis and possibly sinusitis and additional studies are needed to further understand the mechanisms of this process and to examine alternative airway, nasal models, and additional allergens.
CONCLUSIONS
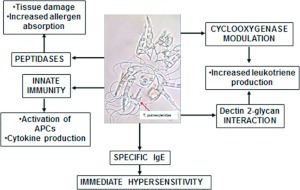
A subset of atopic individuals shows increased risk and severity of reactions to mites, aspirin, and NSAIDs. Various products released by domestic mites induce inflammatory processes through the activation of both immunologic (IgE-mediated) and nonimmunologic mechanisms, as depicted in Fig. 1. Although allergic reactions have been extensively studied, we are only beginning to understand additional nonallergic pathways that are activated by mite constituents. The relative clinical importance of these is presently unknown and deserves further consideration by basic and clinical investigators.
Figure 1.
Mechanisms of inflammation induced by mite constituents.
Footnotes
Funded by the investigator's funds
The authors have no conflicts of interest to declare pertaining to this article
REFERENCES
- 1. Pawankar R, Canonica GW, Holgate ST, Lockey RF. WAO White Book on Allergy 2011–2012. www.worldallergy.org/publications/wao_white_book.pdf; last accessed April 23, 2012
- 2. Thomas WR, Hales BJ, Smith WA. House dust mite allergens in asthma and allergy. Trends Mol Med 16:321–328, 2010 [DOI] [PubMed] [Google Scholar]
- 3. Puglisi G, Frieri M. Update on hidden food allergens and food labeling. Allergy Asthma Proc 28:634–639, 2007 [DOI] [PubMed] [Google Scholar]
- 4. Erben AM, Rodriguez JL, Mc Cullough J, Ownby DR. Anaphylaxis after ingestion of beignets contaminated with Dermatophagoides farina. J Allergy Clin Immunol 92:846–849, 1993 [DOI] [PubMed] [Google Scholar]
- 5. Spiegel WA, Anolik R, Jakabovics E, Arlian LG. Anaphylaxis associated with mite ingestion. Ann Allergy 72:56, 1994 [Google Scholar]
- 6. Skoda-Smith S, Mullen GR, Oi F, Atkinson TP. Angioedema following dust mite exposure presenting as suspected food allergy. J Allergy Clin Immunol 97:228, 1996 [Google Scholar]
- 7. Matsumoto T, Hisano T, Hamaguchi M, Miike T. Systemic anaphylaxis after eating storage mite-contaminated food. Int Arch Allergy Immunol 109:197–200, 1996 [DOI] [PubMed] [Google Scholar]
- 8. Blanco C, Quiralte J, Castillo R, et al. Anaphylaxis after ingestion of wheat flour contaminated with mites. J Allergy Clin Immunol 99:308–313, 1997 [DOI] [PubMed] [Google Scholar]
- 9. Sánchez-Borges M, Capriles-Hulett A, Fernández-Caldas E, et al. Mite-contaminated foods as a cause of anaphylaxis. J Allergy Clin Immunol 99:738–743, 1997 [DOI] [PubMed] [Google Scholar]
- 10. Guerra-Bernd LA, Arruda LK, Barros Antunes HB. Oral anaphylaxis to mites. Allergy 56:83–84, 2001 [DOI] [PubMed] [Google Scholar]
- 11. DeMerrell DG, Olmos CE, El-Dahr JM. Mites in the mix: Dust mite contamination of a flour product. J Allergy Clin Immunol 113(suppl):S235, 2004 [Google Scholar]
- 12. Ott NL. Anaphylaxis caused by dust mite ingestion. Ann Allergy Asthma Immunol 92:196a, 2004 [Google Scholar]
- 13. Wen DC, Shyur SD, Ho CM. Systemic anaphylaxis after the ingestion of pancake contaminated with the storage mite Blomia freemani. Ann Allergy Asthma Immunol 95:612–614, 2005 [DOI] [PubMed] [Google Scholar]
- 14. Miller JD, Hannaway PJ. The pancake syndrome. Allergy Asthma Proc 28:251–252, 2007 [Google Scholar]
- 15. Tay SY, Tham E, Yeo CT, et al. Anaphylaxis following the ingestion of flour contaminated by house dust mites—A report of two cases from Singapore. Asian Pac J Allergy Immunol 26:165–170, 2008 [PubMed] [Google Scholar]
- 16. Iglesias-Souto J, Sánchez-Machin I, Iraola V, et al. Oral mite anaphylaxis by Thyreophagus entomophagus in a child: A case report. Clin Mol Allergy 7:10, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Geller M, Ludwig Hahnstadt R, Malheiros Rego R, Fernández-Caldas E. Anafilaxia induzida por farinha de trigo contaminada por ácaros (Dust mite-contaminated wheat flour induced anaphylaxis). Rev Bras Alerg Imunopatol 32:199–201, 2009 [Google Scholar]
- 18. Sánchez-Machín I, Glez-Paloma Poza R, Iglesias-Souto J, et al. Oral mite anaphylaxis. Allergy 65:1345–1347, 2010 [DOI] [PubMed] [Google Scholar]
- 19. Sánchez-Borges M, Suárez-Chacón R, Capriles-Hulett A, Caballero-Fonseca F. An update on oral anaphylaxis from mite ingestion. Ann Allergy Asthma Immunol 94:216–220, 2005 [DOI] [PubMed] [Google Scholar]
- 20. Sánchez-Borges M, Suárez-Chacón R, Capriles-Hulett A, Caballero-Fonseca F. Pancake syndrome (oral mite anaphylaxis). WAO J 2:91–96, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Sánchez-Borges M, Iraola V, Fernández-Caldas E, et al. Dust mite ingestion-associated, exercise-induced anaphylaxis. J Allergy Clin Immunol 120:714–716, 2007 [DOI] [PubMed] [Google Scholar]
- 22. Sánchez-Borges M, Capriles-Hulett A, Capriles-Behrens E, Fernández-Caldas E. A new triad: Sensitivity to aspirin, allergic rhinitis, and severe allergic reaction to ingested aeroallergens. Cutis 59:311–314, 1997 [PubMed] [Google Scholar]
- 23. Sánchez-Borges M. Etiology and clinical picture of anaphylaxis in ambulatory patients from Caracas, Venezuela. J Investig Allergol Clin Immunol 20:623–624, 2010 [PubMed] [Google Scholar]
- 24. Sánchez-Borges M, Capriles-Hulett A, Suárez-Chacón R, Fernández-Caldas E. Oral anaphylaxis from mite ingestion. Allergy Clin Immunol Int: J World Allergy Org 13:33–35, 2001 [Google Scholar]
- 25. Sánchez-Borges M, Suárez-Chacón R. Additives in allergic and pseudoallergic reactions. In: Progress in Allergy Clin Immunol. Miyamoto T. (ed.). Toronto, Canada, 1992 [Google Scholar]
- 26. Sánchez-Borges M, Capriles-Hulett A. Atopy is a risk factor for nonsteroidal anti-inflammatory drug sensitivity. Ann Allergy 84:101–106, 2000 [DOI] [PubMed] [Google Scholar]
- 27. Sánchez-Borges M, Acevedo N, Caraballo L, et al. Increased total and mite-specific IgE in patients with aspirin-induced urticarial and angioedema. J Investig Allergol Clin Immunol 20:139–145, 2010 [PubMed] [Google Scholar]
- 28. Kupczyk M, Kuprýs I, Gorski P, Kuna P. Aspirin intolerance and allergy to house dust mites: Important factors associated with development of severe asthma. Ann Allergy Asthma Immunol 92:453–458, 2004 [DOI] [PubMed] [Google Scholar]
- 29. Rosario NA, Ribeiro AC. Achados clínicos da sensibilidades a analgésicos e anti-inflamatorios não hormonais. Rev Assoc Med Bras 46:201–206, 2000 [DOI] [PubMed] [Google Scholar]
- 30. Pastorello EA, Zara C, Riario-Sforza GG, et al. Atopy and intolerance of antimicrobial drugs increase the risk of reactions to acetaminophen and nimesulide in patients allergic to nonsteroidal anti-inflammatory drugs. Allergy 53:880–884, 1998 [DOI] [PubMed] [Google Scholar]
- 31. Quiralte J, Blanco C, Castillo R, et al. Intolerance to nonsteroidal anti-inflammatory drugs: Results of controlled drug challenges in 98 patients. J Allergy Clin Immunol 98:678–685, 1996 [DOI] [PubMed] [Google Scholar]
- 32. Hassani A, Ponvert C, Karila C, et al. Hypersensitivity to cyclooxygenase inhibitory drugs in children: A study of 164 cases. Eur J Dermatol 18:561–565, 2008 [DOI] [PubMed] [Google Scholar]
- 33. Berges-Gimeno MP, Simon RA, Stevenson DD. The natural history and clinical characteristics of aspirin-exacerbated respiratory disease. Ann Allergy Asthma Immunol 89:474–478, 2002 [DOI] [PubMed] [Google Scholar]
- 34. Szczeklik A, Nizankowska E, Duplaga M. Natural history of aspirin-induced asthma. AIANE Investigators. European Network on Aspirin-induced Asthma. Eur Respir J 16:432–436, 2000 [DOI] [PubMed] [Google Scholar]
- 35. Kowalski M, Makowska JS. Aspirin-exacerbated respiratory disease. Allergy Clin Immunol Int–J World Allergy Org 18:140–149, 2006 [Google Scholar]
- 36. Kemp SF, Lockey RF, Wolf BL, Lieberman P. Anaphylaxis. A review of 266 cases. Arch Intern Med 155:1749–1754, 1995 [DOI] [PubMed] [Google Scholar]
- 37. Frieri M. Anaphylaxis, Chap 68. In Manual of Critical Care. Philadelphia, PA: American College of Physicians, 721–730, 2009 [Google Scholar]
- 38. Szczeklik A. The cyclooxygenase theory of aspirin-induced asthma. Eur Respir J 3:588–593, 1990 [PubMed] [Google Scholar]
- 39. Mastalerz L, Setkowicz M, Sanak M, Szczeklik A. Hypersensitivity to aspirin: Common eicosanoid alterations in urticarial and asthma. J Allergy Clin Immunol 113:771–775, 2004 [DOI] [PubMed] [Google Scholar]
- 40. Penrose JF, Spector J, Baldasano M, et al. Molecular cloning of the gene for human leukotriene C4 synthase. Organization, nucleotide sequence and chromosomal localization to 5q35. J Biol Chem 271:11356–11361, 1996 [DOI] [PubMed] [Google Scholar]
- 41. Kim S-H, Park HS. Genetic markers for differentiating aspirin hypersensitivity. Yonsei Med J 47:15–21, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Sánchez-Borges M, Acevedo N, Vergara C, et al. The A-444C polymorphism in the leukotriene C4 synthase gene is associated with aspirin-induced urticarial. J Investig Allergol Clin Immunol 19:375–382, 2009 [PubMed] [Google Scholar]
- 43. Sánchez-Borges M, Ouellet M, Percival M, et al. Inhibition of human cyclooxygenase-1 by Dermatophagoides allergenic extracts. J Allergy Clin Immunol 115:S51, 2005 [Google Scholar]
- 44. Barret NA, Maekawa A, Rahman OM, et al. Dectin-2 recognition of house dust mite triggers cysteinyl leukotriene generation by dendritic cells. J Immunol 182:1119–1128, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Sato M, Kuwahara Y, Matsuyama S, Suzuki T. 2-Formyl-3hydrobenzyl formate (Rhizoglyphiny formate), a novel salicylaldehyde analog from the house dust mite Dermatophagoides pteronyssinus (Astigmata, pyroglyphidae). Biosci Biotechnol Biochem 57:1299–1301, 1993 [Google Scholar]
- 46. Wills-Karp M, Nathan A, Page K, Karp CL. New insights into innate immune mechanisms underlying allergenicity. Mucosal Immunol 3:104–110, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Shakib F. The molecular basis of allergenicity. Trends Immunol 29:633–642, 2008 [DOI] [PubMed] [Google Scholar]
- 48. Miike S, Kita H. Human eosinophils are activated by cysteine proteases and release inflammatory mediators. J Allergy Clin Immunol 111:704–713, 2003 [DOI] [PubMed] [Google Scholar]
- 49. Trompette A, Divanovic S, Visintin A, et al. Allergenicity resulting from functional mimicry of a toll-like receptor complex protein. Nature 457:585–588, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Ho SM. Environmental epigenetics of asthma: An update. J Allergy Clin Immunol 126:453–465, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Winton HL, Wan H, Cannell MB, et al. Class specific inhibition of house dust mite proteinases which cleave cell adhesion, induce cell death and which increase the permeability of lung epithelium. Br J Pharmacol 124:1048–1059, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Baker SP, Yin Y, Runswick SK, et al. Peptidase allergen Der p 1 initiates apoptosis of epithelial cells independently of tight junction proteolysis. Mol Membr Biol 20:71–81, 2003 [DOI] [PubMed] [Google Scholar]
- 53. Capetandes A, Hornes NS, Frieri M. Dermatophagoides extract-treated confluent type II epithelial cells (cA549) and human lung mesenchymal cell growth. Ann Allergy Asthma Immunol 95:381–388, 2005 [DOI] [PubMed] [Google Scholar]
- 54. Capetandes A, Zhuang M, Haque FN, et al. Vascular endothelial growth factor is increased by human pulmonary cells stimulated with Dermatophagoides sp. extract. Allergy Asthma Proc 28:324–330, 2007 [DOI] [PubMed] [Google Scholar]
- 55. Capetandes A, Zimmerman J, Frieri M. Adhesion of human lung microvascular endothelial cells (HLMVEC) is decreased by conditioned media from D. pteronyssinus-treated confluent human type II alveolar epithelial cells containing elevated TGF-β1. J Allergy Clin Immunol 115:S14, 2005 [Google Scholar]
- 56. Frisella PD, Silverberg J, Joks R, Frieri M. Transforming growth factor beta: A role in the upper airway and rhinosinusitis- Dermatophagoides pteronyssinus-induced apoptosis with pulmonary alveolar cells. Am J Rhinol Allergy 25:231–235, 2011 [DOI] [PubMed] [Google Scholar]
- 57. Phipps S, Benyahia F, Ou TT, et al. Acute allergen-induced airway remodeling in atopic asthma. Am J Respir Cell Mol Biol 31:626–632, 2004 [DOI] [PubMed] [Google Scholar]