Abstract
Cysteinyl leukotriene receptor antagonist (LTRA) is a widely used medicine for asthma. Cysteinyl leukotrienes (cysLTs) are involved in the regulation of dendritic cell (DC) function. However, the effects of LTRA on DC-related antimicrobial immunity against harmful respiratory pathogens remain unknown. The purpose of this study was to examine the effects of LTRA administered in vivo on DC function against representative respiratory pathogens in vitro. Pulmonary DCs were isolated from four groups of mice: control, mite allergen sensitized (AS), and AS mice treated with the corticosteroid dexamethasone (Dex) or with the LTRA pranlukast (Prl). These DCs were incubated with mite allergen, lipopolysaccharide (LPS), Aspergillus fumigatus, or respiratory syncytial virus (RSV). IL-10 and IL-12 production was then determined. Dex treatment significantly inhibited lipopolysaccharide (LPS)-induced IL-10 and IL-12 production as well as baseline IL-12 production in AS mice. The Prl did not significantly inhibit LPS-induced IL-10 and IL-12 production in AS mice. More importantly, Prl significantly increased IL-10 and IL-12 in AS mice after RSV infection. This study shows that LTRA that is used for asthma potentially up-regulates antimicrobial immunity through modulation of DC function against some respiratory infections without immunosuppression.
Keywords: Allergic airway inflammation, Aspergillus fumigatus, asthma, corticosteroids, cysteinyl leukotrienes receptor antagonist, cytokines, dendritic cell, Dermatophagoides farinae, lipopolysaccharide, respiratory syncytial virus
Bronchial asthma is a chronic inflammatory disease of the airways that is characterized by reversible airway obstruction and bronchial hyperresponsiveness.1 Many types of inflammatory cells and mediators are involved in asthma pathogenesis. Of these inflammatory mediators, cysteinyl leukotrienes (cysLTs) have received attention as critical mediators of asthma since their discovery. A number of reports have established the clinical efficacy of cysLT receptor antagonists (LTRA) in the treatment of asthma.2–4 In fact, international guidelines recommend LTRA as a daily controller for both childhood and adult asthma.5 CysLTs cause bronchoconstriction, vascular hyperpermeability, and mucous hypersecretion and are thus important factors in the pathogenesis of asthma.6–8 In addition, we have reported distinct immunologic effects of cysLTs in asthma, such as modulation of allergen-specific cytokine production.9
Dendritic cells (DCs) represent specialized antigen presenting cells in the airway and play critical roles in the pathogenesis of both asthma and respiratory infections.10,11 Similarly, cysLTs are not only critically involved in the pathogenesis of asthma but are also produced in the airway during respiratory infection and are involved in protection against respiratory pathogens.12 Additionally, it has been reported that cysLTs can affect the migration and functions of DCs.13 We have also reported that LTRA can modulate immunity through DCs in the airway in a murine model of asthma.14 The modulation of immunity by cysLTs through an effect on DCs suggests that LTRA suppresses antimicrobial immunity. Indeed, a deficiency of cysLTs causes some infectious diseases in animals to worsen.15,16 This study examines the effects of LTRA administered in vivo on DC function against representative respiratory pathogens in vitro.
MATERIALS AND METHODS
Animals and Immunization Protocol
Four groups of female BALB/c mice (Charles River Japan, Inc., Yokohama, Japan), 4 weeks of age, were housed at the Laboratory Animal Center for Biochemical Research, Nagasaki University School of Medicine. All mice were immunized twice i.p. on days 1 and 14 with 0.5 mg/mouse of Dermatophagoides farinae (LG-5339; Cosmo Bio, Tokyo, Japan) precipitated in aluminum hydroxide. These mice were then challenged intranasally with 50 μL of phosphate-buffered saline (control group) or 50 μg/50 μL of D. farinae allergen (allergen sensitized [AS]group) on days 14, 16, and 18 as previously described.9 The selective cysLT receptor antagonist pranlukast (Prl; 0.5 mg/mouse; Ono Pharmaceutical Co., Osaka, Japan), or dexamethasone (Dex; 0.02 mg/mouse; Sigma, St. Louis, MO) was injected subcutaneously into D. farinae AS and challenged AS mice from days 13 to 19 (Prl and Dex groups, respectively). On day 21, all mice were killed by dislocation of the cervical vertebrae and lung tissues were obtained from each group for either lung pathology or DC isolation. These procedures were reviewed and approved by Nagasaki University School of Medicine Committee on Animal Research. All experiments were repeated at least three times.
Semiquantification of Pulmonary Inflammation
Hematoxylin and eosin–stained lung sections were coded and evaluated at least twice in a blinded fashion by three different observers as previously described.9 The number of eosinophils was determined in 10 perivascular areas per section under an oil immersion lens. These examined areas were selected randomly under a low-power magnification (×4), under which leukocyte subtypes were not easily distinguishable. Differences in the mean diameters of the selected blood vessels and bronchioles in each group were not statistically significant. Inter- and intraoperative variations were <10%. The results are expressed as the mean cell numbers of each group.
Isolation of Pulmonary DC
After dissection of the lungs from four groups of mice, the lung tissues were cut into small pieces, which were digested with continual agitation for 1 hour at 37°C in RPMI-1640 (Gibco, Grand Island, NY) containing 1% fetal bovine serum and 0.1% penicillin-streptomycin (Gibco; hereafter referred to as cRPMI) supplemented with 1.5 mg/mL of collagenase A (Boehringer-Mannheim, Mannheim, Germany), 0.02 mg/mL of DNase I (Boehringer-Mannheim), and 0.75 mg/mL of hyaluronidase (Sigma-Aldrich, St. Louis, MO). The lung tissues were mechanically filtered through a 250-μm mesh and resuspended and washed in cRPMI. Cells were further purified using a Ficoll gradient by suspending the cells in 10 mL 1.075 g/mL of high-density Ficoll (Amersham Biosciences, Uppsala, Sweden) and centrifugation at 1500 rpm for 10 minutes. Low-density cells were collected, resuspended, washed in cRPMI, and then incubated with CD11c MicroBeads (Miltenyl Biotec, Bergisch Gladbach, Germany) according to the manufacturer's guidelines. Purity was established using CD11c-FITC antibodies and enriched populations of pulmonary DCs (>95%) were obtained. Isolated pulmonary DCs were subjected to an in vitro infection assay.
Respiratory syncytial virus and Aspergillus fumigatus Preparation
The A2 strain of human respiratory syncytial virus (RSV; American Type Culture Collection [ATCC], Rockville, MD) was propagated in HEp-2 cells (ATCC) in monolayer culture. For preparation of a large stock of the virus, HEp-2 cells were infected with the ATCC stock virus, and the cell supernatant was collected at 5 or 6 days postinfection. The supernatant was centrifuged at 2000 rpm for 10 minutes at 4°C to remove cellular debris. The concentration of virus was adjusted as assessed by a quantitative plaque-forming assay. A. fumigatus MF-13, isolated from the sputum of a patient with pulmonary aspergilloma, was subcultured on Sabourand dextrose agar (Becton Dickinson, Cockeysville, MD) at 30°C for 7 days. The conidia were then harvested with sterile saline containing 0.02% Tween 80 (Wako Pure Chemical Industries, Tokyo, Japan). The suspension was filtered through a 40-μm pore-size cell strainer (Millipore, Molsheim, France) to separate conidia from the contaminating mycelium and verified microscopically (100% resting conidia). The suspension was then counted in a hemocytometer and diluted with sterile saline.
DC Function Analysis In Vitro
Pulmonary DCs isolated from four groups of mice were washed and cultured in cRPMI supplemented with either D. farinae allergen (100 μg/mL), lipopolysaccharide (LPS; 100 ng/mL), A. fumigatus (conidia/DC, 1:1), or RSV at a multiplicity of infection of 0.1 at 37°C in 5% CO2 overnight. Cell culture supernatants were analyzed for IL-10 and IL-12 by enzyme-linked immunosorbent assay (Quantikine; R & D Systems Inc., Minneapolis, MN), using the procedure described in the instruction manual.
Statistical Analysis
Results are expressed as the mean ± SEM. Data were evaluated using repeated-measures ANOVA with a Bonferroni multiple comparison test. A value of p < 0.05 was considered significant.
RESULTS
In Vivo Antagonism of cysLTs Attenuates Allergen-Induced Airway inflammation
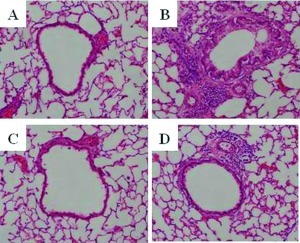
Representative pathological features of hematoxylin and eosin–stained lung tissue with allergen-induced airway inflammation are shown in Fig. 1. Eosinophils could not be determined in the lung tissues on control mice. AS mice exhibited goblet cell metaplasia and a cellular infiltrate with eosinophils. The mean number of infiltrating eosinophils per 10 perivascular areas in AS mice was significantly reduced in both Prl and Dex mice (AS, 342.6 ± 12.3 cells; Prl, 83.2 ± 22.4 cells; Dex, 5.2 ± 1.4 cells; p < 0.05 between AS and Prl; p < 0.01 between AS and Dex; p < 0.01 between Prl and Dex).
Figure 1.
Pulmonary pathology. Representative photomicrographs (×400) of the lung tissue from each mouse group (n = 8/group). (A) Control, (B) allergen sensitized (AS), (C) dexamethasone (Dex), and (D) pranlukast (Prl) groups are shown. AS mice show allergic airway inflammatory changes. Dex mice show almost complete inhibition of these inflammatory changes. These inflammatory changes are also inhibited in Prl mice but to a lesser extent than in Dex mice.
Pulmonary DC Functions against Harmful Pathogens
Cytokine production from pulmonary DCs of the four groups of mice is shown in Table 1. In agreement with our previous reports,14,17 compared with the control, pulmonary DCs from AS mice produced significantly more IL-10 and IL-12 in the presence of D. farinae allergen, wherein IL-10 dominated over IL-12. After LPS stimulation, DCs from AS mice produced significantly higher IL-10 compared with DCs from control mice. Dex significantly inhibited LPS-induced as well as D. farinae--induced IL-10 and IL1–2 production in AS mice. Neither D. farinae sensitization nor drug administration significantly affected cytokine production after A. fumigatus infection. Prl significantly enhanced D. farinae–specific IL-12 production and did not regulate LPS-induced IL-10 and IL-12 production in AS mice. Most importantly, Prl significantly enhanced IL-10 and IL-12 production in AS mice after RSV infection.
Table 1.
Cytokine production from pulmonary DCs
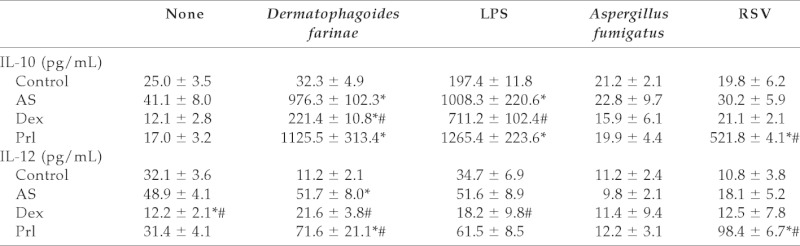
Data represents mean (n =8 for each) ± SEM.
*p < 0.01 vs control; #p < 0.05 vs AS. AS = allergen sensitized; DCs = dendritic cells; Dex = dexamethasone; LPS = lipopolysaccharide; Prl = pranlukast; RSV = respiratory syncytial virus.
DISCUSSION
Currently, LTRAs are widely used for the treatment of asthma, which is a highly prevalent disease and a major cause of morbidity. CysLTs (leukotrienes C4, D4, and E4) are central mediators in asthma pathophysiology, because they cause bronchoconstriction, vascular hyperpermeability, and mucous hypersecretion.6–8 In the present study, using a murine model of allergic asthma, the LTRA, Prl, significantly attenuated allergic airway inflammation to a lower extent than the corticosteroid Dex, consistent with our previous reports.9 Besides their proinflammatory effects, cysLTs potentially play an important role in antimicrobial defense against harmful infections. Infection caused by Trypanosoma cruzi in mice that are genetically deficient for 5-lipoxygenase (5-LO), a primary enzyme for LT synthesis, is more severe than that in wild-type mice.15,18 In contrast, cysLTs contribute to Escherichia coli penetration of the blood–brain barrier.19 Thus, the exact role of cysLTs in antimicrobial defense against harmful infections is still to be determined. The present study was performed to evaluate whether the use of LTRA for asthma might regulate the function of airway DCs against harmful pathogens. The major findings of the present study are that, in contrast to Dex, which inhibited immunoregulatory cytokine production regardless of infectious pathogens, LTRA selectively up-regulated immunoregulatory cytokine production against RSV infection.
There is increasing evidence of a critical interaction between cysLTs and DCs. Murine and human bone marrow–derived DCs express LT-synthesizing enzymes as well as receptors for cysLTs.14,20 Additionally, these DCs produce cysLTs after exposure to aeroallergens.14,20 LTs also induce DC migration to draining lymph nodes.13 We previously showed17 that in vitro LTRA treatment of DCs selectively regulated the production of immunoregulatory cytokines that suppress allergic airway inflammation in vivo. In the present study, we showed that in vivo treatment with LTRA results in the selective production of allergen-specific immunoregulatory cytokines from pulmonary DCs in vitro. The combined data indicate that not only do cysLTs play an important role in exacerbation of established asthma, but that they also interact with DCs in the development of asthma.
Interaction between viral infection and cysLTs has been well studied. The concentration of cysLTs in induced sputum is significantly increased in virus-proven exacerbated asthmatic patients.21 The LTRA montelukast significantly reduces virus-induced exacerbation in childhood asthma.22 We have also reported that Prl significantly inhibits RSV-induced exacerbation of allergic airway inflammation in a murine model of allergic asthma.23 Nevertheless, few studies have evaluated the effects of LTRA used for asthma on DC function in antimicrobial defense. In the present study, Dex significantly inhibited the induction of cytokine production from pulmonary DCs sensitized with mite allergen by LPS, a cellular component of Gram-negative bacteria. These findings agree with the fact that systemic corticosteroids suppress antimicrobial immunity. In contrast, LTRA did not significantly reduce cytokine production regardless of infection. Interestingly, the present study showed that LTRA significantly increased IL-10 and IL-12 production from mite allergen–sensitized pulmonary DC after RSV infection. The role of IL-10 in the interaction between allergy and infection is controversial. In the development of allergy, high amounts of IL-10, and low amounts of IL-12 induce naive CD4+ T cells to Th2 cells, whereas low amounts of IL-10 and high amounts of IL-12 induce Th1 cells.24 In antimicrobial immunity IL-10 reduces morbidity in an infection25 as a regulatory cytokine to broadly suppress immune responses. Thus, IL-10 seems to be a bad player in the development of allergy and a good player in the antimicrobial immunity. IL-12 is a Th1-like cytokine and inhibits Th2-dominant allergic airway inflammation.26 Thus, an increase in the production of IL-12 after D. farinae stimulation and an increase in both IL-10 and IL-12 from LPS and RSV exposed pulmonary DCs in LTRA-treated mice might suppress allergic airway inflammation in the former and also enhance antimicrobial immunity in the latter. In fact, to the best of our knowledge, no report has indicated that LTRA can cause severe infectious diseases in asthmatic patients.
In conclusion, LTRA that is used for asthma treatment potentially up-regulates antimicrobial immunity through the regulation of DC function against some respiratory infections without immune suppression. However, it should be also emphasized that the response of DCs to various agents in mice could not be always established in humans.20 Because viral respiratory tract infection represents a major cause of acute exacerbation in adult and childhood asthma27,28 and because systemic corticosteroids increase viral load in the airway,29 it will be an interesting issue for future clinical research to combine LTRA and corticosteroids for the treatment of virus infection–induced acute exacerbation of asthma.
Footnotes
Funded by Grants-in-Aid for Scientific Research (17607009 and 21590968) from the Japanese Ministry of Education, Culture, Sports, Science, and Technology
The authors have no conflicts of interest to declare pertaining to this article
REFERENCES
- 1. Busse WW, Lemanske RF. Asthma. N Engl J Med 344:350–362, 2001 [DOI] [PubMed] [Google Scholar]
- 2. Tomari S, Shimoda T, Kawano T, et al. Effects of pranlukast, a cysteinyl leukotriene receptor 1 antagonist, combined with inhaled beclomethasone in patients with moderate or severe asthma. Ann Allergy Asthma Immunol 87:156–161, 2001 [DOI] [PubMed] [Google Scholar]
- 3. Obase Y, Shimoda T, Tomari S, et al. Efficacy and safety of long-term treatment of asthmatic patients with pranlukast, a cysteinyl leukotriene receptor antagonist: Four-year follow-up study. Ann Allergy Asthma Immunol 87:43–47, 2001 [DOI] [PubMed] [Google Scholar]
- 4. Diamant Z, Mantzouranis E, Bjermer L. Montelukast in the treatment of asthma and beyond. Expert Rev Clin Immunol 5:639–658, 2009 [DOI] [PubMed] [Google Scholar]
- 5. Global Initiative for Asthma (GINA) Global strategy for asthma management and prevention. National Institutes of Health, National Heart, Lung, and Blood Institute, Bethesda, MD, 2006 [Google Scholar]
- 6. Dahlen SE, Hedqvist P, Hammarstrom S, et al. Leukotrienes are potent constrictors of human bronchi. Nature 288:484–486, 1980 [DOI] [PubMed] [Google Scholar]
- 7. Drasen JM, Austen KF. Leukotrienes and airway responses. Am Rev Respir Dis 136:368–372, 1987 [DOI] [PubMed] [Google Scholar]
- 8. Uunderwood DC, Osborn RR, Newsholme SJ, et al. Persistent airway eosinophilia after leukotriene (LT). D4 administration in the guinea pig: Modulation by the LTD4 receptor antagonist, pranlukast, or an interleukin-5 monoclonal antibody. Am J Respir Crit Care Med 154:850–857, 1996 [DOI] [PubMed] [Google Scholar]
- 9. Kawano T, Matsuse H, Kondo Y, et al. Cysteinyl leukotrienes induce nuclear factor kappa b activation and RANTES production in a murine model of asthma. J Allergy Clin Immunol 112:369–374, 2003 [DOI] [PubMed] [Google Scholar]
- 10. Van Rijt LS, Lambrecht BN. Role of dendritic cells and Th2 lymphocytes in asthma: Lessons from eosinophilic airway inflammation in the mouse. Microsc Res Tech 53:256–272, 2001 [DOI] [PubMed] [Google Scholar]
- 11. Klagge IM, Schneider-Schaulies S. Virus interactions with dendritic cells. J Gen Virol 80:823–833, 1999 [DOI] [PubMed] [Google Scholar]
- 12. Peters-Golden M, Canetti C, Mancuso P, et al. Leukotrienes: Underappreciated mediators of innate immune responses. J Immunol 173:589–594, 2004 [DOI] [PubMed] [Google Scholar]
- 13. Robbiani DF, Finch RA, Jager D, et al. The leukotriene C4 transporter MRP1 regulates CCL19 (MIP-3β, ECL)-dependent mobilization of dendritic cells to lymph nodes. Cell 103:757–768, 2000 [DOI] [PubMed] [Google Scholar]
- 14. Machida I, Matsuse H, Kondo Y, et al. Cysteinyl leukotrienes regulate dendritic cell functions in a murine model of asthma. J Immunol 172:1833–1838, 2004 [DOI] [PubMed] [Google Scholar]
- 15. Pavanelli WR, Gutierrez FR, Mariano FS, et al. 5-lipoxygenase is a key determinant of acute myocardial inflammation and mortality during Trypanosoma cruzi infection. Microbes Infect 12:587–597, 2010 [DOI] [PubMed] [Google Scholar]
- 16. Mancuso P, Gottschalk A, Phare SM, et al. Leptin-deficient mice exhibit impaired host defense in gram-negative pneumonia. J Immunol 168:4018–4024, 2002 [DOI] [PubMed] [Google Scholar]
- 17. Machida I, Matsuse H, Kondo Y, et al. Effects of various anti-asthmatic agents on mite allergen-pulsed murine bone marrow-derived dendritic cells. Clin Exp Allergy 35:884–888, 2005 [DOI] [PubMed] [Google Scholar]
- 18. Panis C, Mazzuco TL, Fernandes CZ, et al. Trypanosoma cruzi: Effect of the absence of 5-lipoxygenase (5-LO)-derived leukotrienes on levels of cytokines, nitric oxide and iNOS expression in cardiac tissue in the acute phase of infection in mice. Exp Parasitol 127:58–65, 2010 [DOI] [PubMed] [Google Scholar]
- 19. Zhu L, Maruvada R, Sapirstein A, et al. Arachidonic acid metabolism regulates E. coli penetration of the blood-brain barrier. Infect Immun 78:4302–4310, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Saeki S, Matsuse H, Kondo Y, et al. Effects of antiasthmatic agents on the functions of peripheral blood monocyte-derived dendritic cells from atopic patients. J Allergy Clin Immunol 114:538–544, 2004 [DOI] [PubMed] [Google Scholar]
- 21. Matsuse H, Kondo Y, Saeki S, et al. Naturally occurring parainfluenza virus 3 infection in adults induces mild exacerbation of asthma associated with increased sputum concentrations of cysteinyl leukotrienes. Int Arch Allergy Immunol 138:267–272, 2005 [DOI] [PubMed] [Google Scholar]
- 22. Bisgaard H, Zielen S, Garcia-Garcia ML, et al. Montelukast reduces asthma exacerbations in 2- to 5-year-old children with intermittent asthma. Am J Respir Crit Care Med 171:315–322, 2005 [DOI] [PubMed] [Google Scholar]
- 23. Matsuse H, Kondo Y, Machida I, et al. Effects of anti-inflammatory therapies on recurrent and low-grade respiratory syncytial virus infections in a murine model of asthma. Ann Allergy Asthma Immunol 97:55–60, 2006 [DOI] [PubMed] [Google Scholar]
- 24. Lambrecht BN. Allergen uptake and presentation by dendritic cells. Curr Opin Allergy Clin Immunol 1:52–59, 2001 [DOI] [PubMed] [Google Scholar]
- 25. Hesse M, Piccirillo CA, Beikaid Y, et al. The pathogenesis of schistosomiasis is controlled by cooperating IL10-producing innate effector and regulatory T cells. J Immunol 172:3157–3166, 2004 [DOI] [PubMed] [Google Scholar]
- 26. Matsuse H, Kong X, Hu J, et al. Intranasal IL-12 produces discreet pulmonary and systemic effects on allergic inflammation and airway reactivity. Int Immunopharmacol 3:457–468, 2003 [DOI] [PubMed] [Google Scholar]
- 27. Cypcar D, Stark J, Lemanske RF, et al. The impact of respiratory infections on asthma. Pediatr Clin North Am 39:1259–1276, 1992 [DOI] [PubMed] [Google Scholar]
- 28. Nicholson KG, Kent J, Ireland DC, et al. Respiratory viruses and exacerbations of asthma in adults. BMJ 307:982–986, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Gustafson LM, Proud D, Hendley JO, et al. Oral prednisone therapy in experimental rhinovirus infections. J Allergy Clin Immunol 97:1009–1014, 1996 [DOI] [PubMed] [Google Scholar]